Abstract
Background & Aims:
The long-term risks of colorectal cancer (CRC) and CRC-related death following adenoma removal are uncertain. Data are needed to inform evidence-based surveillance guidelines, which vary in follow-up recommendations for some polyp types. Using data from a large, community-based integrated health care setting, we examined the risks of CRC and related death by baseline colonoscopy adenoma findings.
Methods:
Participants at 21 medical centers underwent baseline colonoscopies from 2004 through 2010; findings were categorized as no adenoma, low-risk adenoma, or high-risk adenoma. Participants were followed until the earliest of CRC diagnosis, death, health plan disenrollment, or December 31, 2017. Risks of CRC and related deaths among the high- and low-risk adenoma groups were compared with the no adenoma group using Cox regression adjusting for confounders.
Results:
Among 186,046 patients, 64,422 met eligibility criteria (54.3% female; mean age, 61.6±7.1 y; median follow-up time, 8.1 y from the baseline colonoscopy). Compared with the no-adenoma group (45,881 patients), the high-risk adenoma group (7563 patients) had a higher risk of CRC (hazard ratio [HR], 2.61; 95% CI, 1.87–3.63) and related death (HR, 3.94; 95% CI, 1.90–6.56), whereas the low-risk adenoma group (10,978 patients) did not have a significant increase in risk of CRC (HR, 1.29; 95% confidence interval, 0.89–1.88) or related death (HR, 0.65; 95% CI, 0.19–2.18).
Conclusions:
With up to 14-years of follow-up, high-risk adenomas were associated with an increased risk of CRC and related death, supporting early colonoscopy surveillance. Low-risk adenomas were not associated with a significantly increased risk of CRC or related deaths. These results can inform current surveillance guidelines for high- and low-risk adenomas.
Keywords: Colorectal cancer, adenoma, colonoscopy, polyp
Lay Summary:
With up to 14-years of follow-up, high-risk adenomas were associated with an increased risk of colorectal cancer and related deaths. Low-risk adenomas were not associated with a significantly increased risk of colorectal cancer or related deaths.
Graphical Abstract
INTRODUCTION
Colonoscopy is an established screening method for decreasing colorectal cancer incidence and mortality1–15 through a combination of detection of early-stage cancers and removal of adenomas, the main precursor lesion to colorectal cancer. Many countries have implemented colorectal cancer screening programs, which have resulted in a growing cohort of patients with prior adenoma findings. In some settings, greater than 40% of patients receiving a colonoscopy have at least one adenoma detected; however, there have been few large, long-term follow-up studies to inform evidence-based management of these patients.14–17
To prevent development of colorectal cancer after adenoma removal, patients are advised to undergo colonoscopy surveillance. According to current United States Multi-Society Task Force guidelines, surveillance examination intervals are determined by the number, size, and histology of the adenomas detected.17 For example, after removal of 1–2 tubular adenomas each <10 mm in size (considered low-risk), guidelines call for colonoscopy surveillance in 5–10 years, a wide interval with marked implications for patients and societal cost.17 However, granular evidence supporting these surveillance recommendations for important endpoints such as cancer and related deaths, among patients who undergo adenoma removal, particularly low-risk adenomas, is limited.14–17 Ideally, surveillance recommendations are based on the risk of developing and dying from colorectal cancer after adenoma removal; yet most studies that serve as the basis for the guidelines have either been small in size, performed under highly standardized conditions (e.g., clinical trials) or with selected populations (e.g., referral centers, Veteran Affairs population), lacked long-term follow-up and colonoscopy quality data, evaluated broad time intervals, or focused on advanced adenomas rather than colorectal cancer incidence and mortality as the primary outcome.17–26 Also, no population-based studies have compared the annual incidence of colorectal cancer and related deaths in patients with adenoma findings to those from the same underlying population who had a colonoscopy with normal findings (no adenoma).
To inform guideline recommendations for surveillance after adenoma removal, we examined the annual, long-term risks of colorectal cancer and colorectal cancer-related deaths among colonoscopy patients with low- and high-risk adenomas, compared to patients from the same underlying population who had a colonoscopy with normal findings (no adenoma).
METHODS
Study Design, Setting, and Oversight
This retrospective cohort study was conducted among members of Kaiser Permanente Northern California (KPNC), an integrated health care organization that serves approximately 4.4 million members across 21 medical centers in urban, suburban, and semi-rural regions throughout Northern California. KPNC’s membership is diverse and similar in socioeconomic characteristics to the region’s census demographics, including the proportions with commercial insurance, Medicare, or Medicaid.27 The study was approved by the KPNC institutional review board.
Study Eligibility Criteria
Individuals were eligible for the study if they were KPNC health plan members, 50–75 years of age, underwent a baseline (first) colonoscopy between 2004–2010, and had >1 year of membership enrollment prior to cohort entry date. Individuals were excluded if, prior to the baseline colonoscopy, they had a diagnosis of colorectal cancer, a hereditary colorectal cancer syndrome, inflammatory bowel disease, colonic polyp or adenoma; a prior colectomy; a documented family history of colorectal cancer; or <3 months of follow-up after their baseline colonoscopy. Individuals were also excluded if the baseline colonoscopy detected colorectal cancer (within 3 months after the procedure), sessile or traditional serrated polyps, or proximal hyperplastic polyps; had an inadequate bowel preparation or was not complete to the cecum; or they had an adenoma diagnosis but without a pathology or colonoscopy report. Thus, only patients with confirmed normal findings or conventional adenomas at the baseline colonoscopy were included in the study.
Data Sources and Definitions
Demographic and clinical data were obtained from KPNC electronic health records and databases. Colonoscopies, including subsequent colonoscopies after baseline, were identified using Current Procedural Terminology codes. Colonoscopy quality measures (i.e., extent of the examination and bowel preparation quality) were ascertained from colonoscopy reports using a commercial natural language processing-based software (Linguamatics I2E, www.linguamatics.com; United Kingdom).28 Pathology findings (i.e., hyperplastic polyps, conventional adenomas, and colorectal cancer) were identified using Systematized Nomenclature of Medicine (SNOMED) codes.27 Conventional adenomas were defined as tubular adenomas, tubulovillous adenomas, or villous adenomas. Because high-grade dysplasia, sessile serrated adenomas/polyps, and traditional serrated adenomas have no specific SNOMED codes, they were identified using SAS-based natural language processing-based searches of pathology reports. We made an a priori decision to exclude serrated adenomas given the complexity of accurately assigning size to each polyp type (i.e., conventional adenomas, serrated polyps), the lack of a commonly accepted definition for sessile serrated polyps during the study interval (2004–2010), and likely variation in pathologists’ application of serrated adenoma diagnoses during the study’s period (2004–2010). The study interval was chosen to allow for extended follow-up. The query strategies were refined using an iterative process and negation phrases such that ‘no evidence of high-grade dysplasia’ or ‘no high-grade dysplasia seen’ would not be assigned as positive responses. The final validation on a separate set of 300 pathology reports of persons demonstrated the positive predictive value, sensitivity, and specificity of the query strategies were 92%, 92%, and 100%, respectively, for high-grade dysplasia, and 100%, 94%, and 100%, respectively, for sessile or traditional serrated adenomas.
Conventional adenomas detected at the baseline colonoscopy were categorized as low- or high-risk by an algorithm (Supplemental Figure 1) that incorporated a combination of SNOMED codes and findings from colonoscopy and pathology reports ascertained using natural language processing-based searches. Consistent with the United States Multi-Society Task Force guidelines, 17 low-risk adenomas were defined as 1–2 tubular adenomas each <10 mm in size. High-risk adenomas were defined as either 3 or more conventional adenomas, any conventional adenoma with high-grade dysplasia or villous component, or any conventional adenoma ≥10 mm in size. Polyp size was ascertained from colonoscopy reports using a commercial natural language processing software (Linguamatics I2E, www.linguamatics.com; United Kingdom).28 The total number of adenomas per patient was initially determined based on the number of pathology bottles with a conventional adenoma (as determined by SNOMED codes). Multiple polyps can be placed into a single pathology bottle, particularly if two or more polyps are excised from the same colon location, and this is typically recorded on the colonoscopy report and pathology submission form. Pathologists report whether a bottle contains an adenoma; however, if the colonoscopy report and/or pathology submission form notes, for example, “two polyps in bottle A,” the pathologist will typically report the number of adenomas in the pathology bottle (e.g., “bottle A: tubular adenoma x2”). Thus, for example, if two adenoma fragments were reported in a single pathology bottle, we assumed that they represented two different adenomas. For patients identified as having at least one adenoma who were not otherwise labelled as high risk by having ≥3 containers with adenomas, we developed and utilized a SAS-based natural language process query strategy to evaluate pathology reports for evidence of adenoma multiplicity in pathology bottles within a 300 patient derivation group. After our algorithm reached a high level of accuracy, we validated the algorithm using a separate 500 patient validation data set of patients with manually verified adenoma histology and adenoma number. The algorithm’s accuracy for classifying low- and high-risk adenomas demonstrated a positive predictive value, sensitivity, and specificity of 95%, 99%, and 92%, respectively, for low-risk adenomas, and 99%, 92%, and 99%, respectively, for high-risk adenomas.
The primary outcome was colorectal cancer (defined as a colorectal adenocarcinoma) following the baseline colonoscopy. The secondary outcome was colorectal cancer-related deaths following the baseline colonoscopy. Cancer diagnoses were obtained from the KPNC cancer registry, which reports to the Surveillance, Epidemiology and End-Results (SEER) program and captures >98% of cancers diagnosed among members compared with manual review.27 The underlying cause of death was obtained from state mortality files.
Statistical Analyses
Descriptive statistics were used to describe demographic and clinical characteristics of the cohort overall and by baseline colonoscopy findings (i.e., no adenoma group, low-risk adenoma group, and high-risk adenoma group). For our primary outcome (i.e., colorectal cancer), all individuals who met study eligibility criteria were followed from 3 months after their baseline colonoscopy to the earliest of colorectal cancer diagnosis, death, disenrollment from the health plan, or end of the study period (December 31, 2017). For our secondary outcome (i.e., colorectal cancer-related deaths), all individuals who met study eligibility criteria were followed from 3 months after their baseline colonoscopy to the earliest of colorectal cancer-related death, death from other causes, disenrollment from the health plan, or end of the study period (December 31, 2017). Overall and annual colorectal cancer incidence rates were calculated per 100,000 person-years of follow-up and were age-adjusted to the 2000 United States Standard Population (Census). Kaplan-Meier estimates were plotted for cumulative colorectal cancer incidence and related deaths. Cox regression was used to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for colorectal cancer and related deaths. Estimates were adjusted for age, sex, race/ethnicity, body mass index (BMI), Charlson co-morbidity index, year of baseline examination, baseline colonoscopy indication,29 and physician adenoma detection rate based on screening colonoscopies performed during the study interval.27 The no adenoma group served as the referent group.
The modifying effects of age at the baseline examination (50–64 vs.65–75), sex (male vs. female), race (African-American vs. others), BMI (<25 vs. ≥25 kg/m2), colonoscopy indication (screening vs. fecal immunochemical test (FIT) positive vs. non-screening), and physician adenoma detection rate (<25% vs. >25%) on associations of baseline colonoscopy findings with risks of colorectal cancer and related deaths were evaluated using regression models with additional multiplicative interaction terms and by stratified analyses for each covariate listed above. The statistical significance of effect modification was assessed by Wald tests of the cross-product terms between the main exposure (baseline colonoscopy findings) and the potential effect modifier.
In a sensitivity analysis, we entered patients into the analyses 2-years after the baseline colonoscopy as colorectal cancers diagnosed within 2 years of follow-up may reflect prevalent cancers missed at the baseline examination. In addition, subsequent examinations within 2 years may have been performed to clear the colon of additional polyps identified but not removed during the baseline examination and/or to ensure complete polyp resection stemming from the baseline colonoscopy. The restriction to ≥2 years still allowed for the earliest recommended surveillance interval, which per current guidelines is 3 years.
To examine the potential for bias associated with differential subsequent colonoscopy utilization between groups, we used Kaplan-Meier survival analysis to evaluate the cumulative probability of a subsequent colonoscopy among all cohort members, and the cumulative probability of detecting any adenoma or an advanced adenoma among patients who had a subsequent colonoscopy. All statistical analyses were two-sided and a p-value <0.05 was considered statistically significant. SAS software (version 9.4) was used for all statistical analyses.
RESULTS
Cohort characteristics and colorectal cancer outcomes
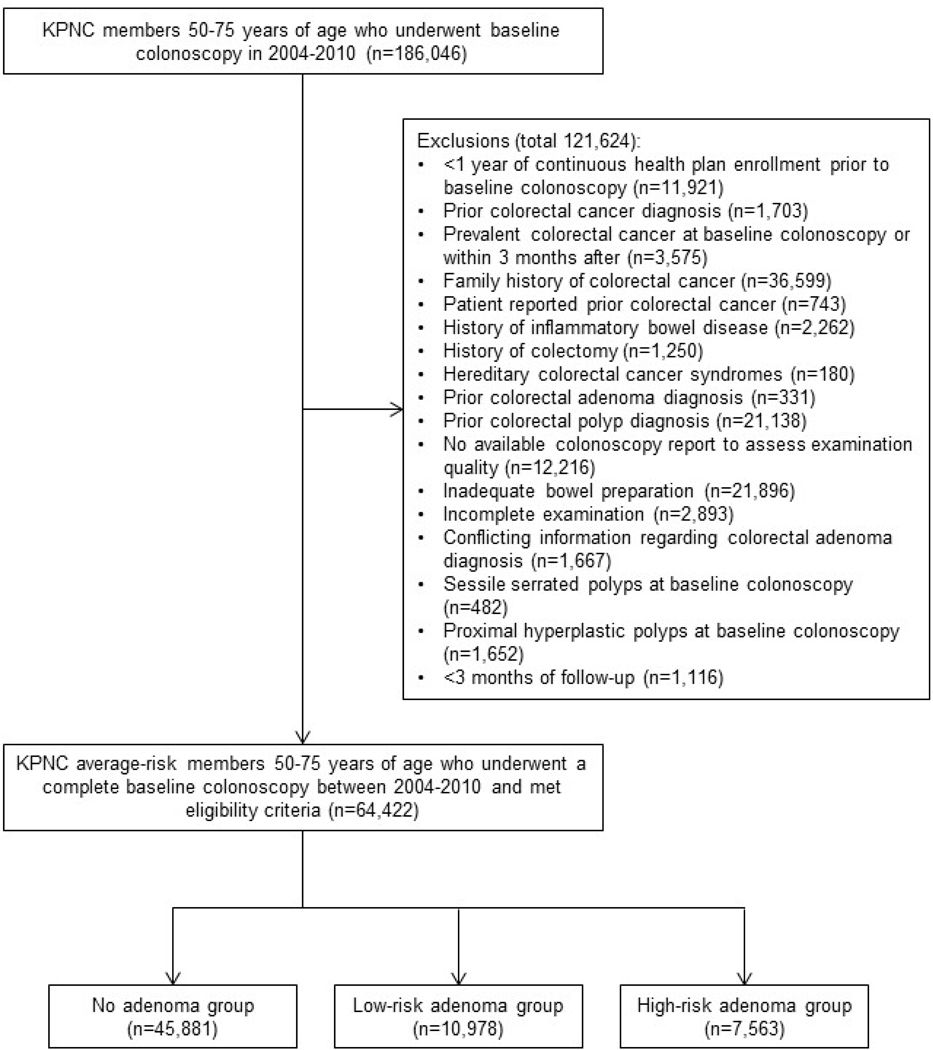
There were 64,422 patients who met eligibility criteria and underwent a baseline colonoscopy between 2004 and 2010 (Figure 1). Their mean (±standard deviation) age at baseline was 61.6±7.1 years, 54.3% were female, 45,881 (71.2%) were assigned to the no adenoma group (normal findings), 10,978 (17.0%) were assigned to the low-risk adenoma group, and 7,563 (11.7%) were assigned to the high-risk adenoma group (Table 1). Participants were followed from baseline for a median of 8.1 years (interquartile range: 6.8 – 9.6 years). By the end of the study period, 117 colorectal cancers were detected in the no-adenoma group, 37 in the low-risk adenoma group, and 60 in the high-risk adenoma group. Also, there were 22 deaths attributable to colorectal cancer in the no-adenoma group, 3 in the low-risk adenoma group, and 13 in the high-risk adenoma group.
Figure 1.
Flow diagram of adenoma study cohort
Table 1.
Characteristics of study cohort at baseline colonoscopy
| Group | ||||
|---|---|---|---|---|
| Characteristics | No Adenoma n (%) | Low-Risk Adenoma n (%) | High-Risk Adenoma n (%) | Overall Cohort n (%) |
| All Participants | 45881 (100.0) | 10978 (100.0) | 7563 (100.0) | 64422 (100.00) |
| Baseline Colonoscopy Age, years | ||||
| 50–59 | 21180 (46.2) | 4322 (39.4) | 2865 (37.9) | 28367 (44.0) |
| 60–69 | 18089 (39.4) | 4670 (42.5) | 3171 (41.9) | 25930 (40.3) |
| 70–75 | 6612 (14.4) | 1986 (18.1) | 1527 (20.2) | 10125 (15.7) |
| Mean (Standard Deviation) | 61.2 (7.1) | 62.4 (7.1) | 62.8 (7.2) | 61.6 (7.1) |
| Median (Interquartile Range) | 60.7 (55.2 – 66.7) | 62.2 (56.6 – 68.1) | 62.6 (56.8 – 68.7) | 61.2 (55.6 – 67.2) |
| Sex | ||||
| Male | 18874 (41.1) | 6001 (54.7) | 4582 (60.6) | 29457 (45.7) |
| Female | 27002 (58.9) | 4977 (45.3) | 2981 (39.4) | 34960 (54.3) |
| Race/Ethnicity | ||||
| White | 29780 (64.9) | 6864 (62.5) | 4752 (62.8) | 41396 (6436) |
| Hispanic | 4976 (10.9) | 1316 (12.0) | 855 (11.3) | 7147 (11.1) |
| Black | 2944 (6.4) | 693 (6.3) | 637 (8.4) | 4274 (6.6) |
| Asian/Pacific Islander | 7103 (15.5) | 1876 (17.1) | 1128 (14.9) | 10107 (15.7) |
| Other, Unknown | 1078 (2.4) | 229 (2.1) | 191 (2.5) | 1498 (2.3) |
| Charlson Comorbidity Score | ||||
| 0 | 29455 (64.2) | 6588 (60.0) | 4571 (60.4) | 40614 (63.0) |
| 1 | 8038 (17.5) | 2139 (19.5) | 1383 (18.3) | 11560 (17.9) |
| ≥2 | 8388 (18.3) | 2251 (20.5) | 1609 (21.3) | 12248 (19.0) |
| Body Mass Index, kg/m2 | ||||
| <25 | 15691 (34.2) | 3056 (27.8) | 1956 (25.9) | 20703 (32.1) |
| 25–29.9 | 16829 (36.7) | 4242 (38.6) | 2870 (38.0) | 23941 (37.2) |
| ≥30 | 13361 (29.1) | 3680 (33.5) | 2737 (36.2) | 19778 (30.7) |
| Missing | 73 (0.2) | 12 (0.1) | 14 (0.2) | 99 (0.2) |
| Mean (Standard Deviation) | 27.8 (5.9) | 28.5 (5.8) | 28.9 (6.0) | 28.1 (5.9) |
| Colonoscopy Indication | ||||
| Screening | 17219 (37.5) | 3465 (31.6) | 1396 (18.5) | 22080 (34.3) |
| FIT-positive | 8974 (19.6) | 3330 (30.3) | 3709 (49.0) | 16013 (24.9) |
| Non-screening | 19688 (42.9) | 4183 (38.1) | 2458 (32.5) | 26329 (40.9) |
| Follow-up Time, person-years | ||||
| Mean (Standard Deviation) | 7.6 (3.0) | 7.4 (3.0) | 7.3 (3.0) | 7.6 (3.0) |
| Median (Interquartile Range) | 8.1 (7.0 – 9.7) | 7.9 (6.6 – 9.4) | 7.9 (5.9 – 9.3) | 8.1 (6.8 – 9.6) |
| Outcomes | ||||
| Colorectal cancer cases | 117 (0.3) | 37 (0.3) | 60 (0.8) | 214 (0.3) |
| Colorectal cancer-related deaths | 22 (0.0) | 3 (0.0) | 13 (0.2) | 38 (0.1) |
| Provider’s Adenoma Detection Rate, % | ||||
| <25 | 26555 (57.9) | 5015 (45.7) | 3771 (49.9) | 35341 (54.9) |
| ≥25 | 19088 (41.6) | 5928 (54.0) | 3761 (49.7) | 28777 (44.7) |
| Missing | 238 (0.5) | 35 (0.3) | 31 (0.4) | 304 (0.5) |
| Mean (Standard Deviation) | 24.1 (7.9) | 26.5 (8.0) | 25.9 (8.3) | 24.7 (8.0) |
Risk of colorectal cancer and related deaths
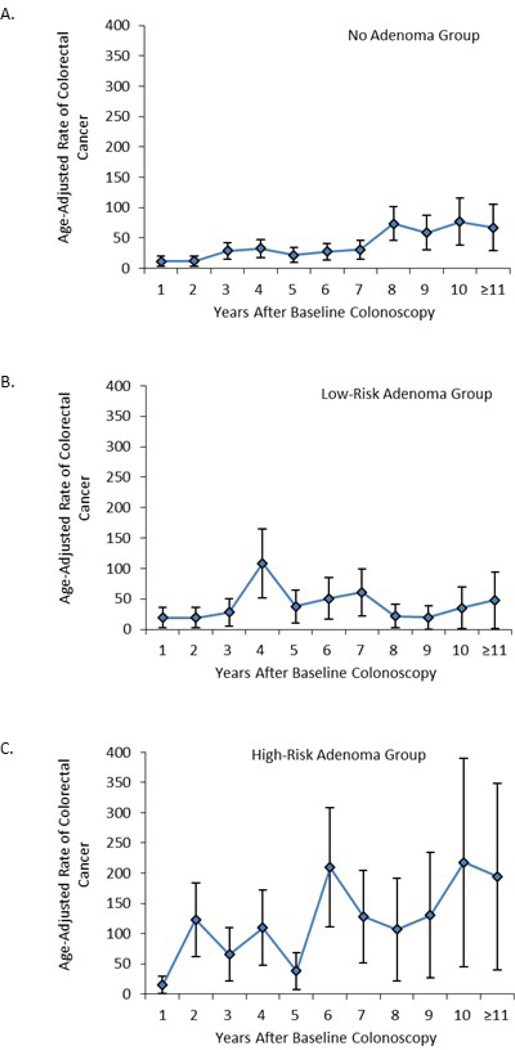
The annual, age-adjusted colorectal cancer incidence rates/100,000 person-years in the no adenoma and low-risk adenoma groups increased gradually over the >10-year study interval (Figure 2, Supplemental Table 1). In contrast, incidence rates per 100,000 person-years in the high-risk adenoma group rose sharply, from 15.0 (95% CI: 0.4, 29.6) in year 1 to 217.7 (95% CI: 44.9, 390.5) in year 10. Over the study interval, the average annual age-adjusted colorectal cancer incidence rates per 100,000 person-years were 31.1 (95% CI: 25.7, 36.5) in the no-adenoma group, 38.8 (95% CI: 27.3, 50.2) in the low-risk adenoma group, and 90.8 (95% CI: 69.3, 112.4) in the high-risk adenoma group (Figure 2, Supplemental Table 1).
Figure 2.
Age-adjusted colorectal cancer incidence rates per 100,000 person-years by year since baseline colonoscopy
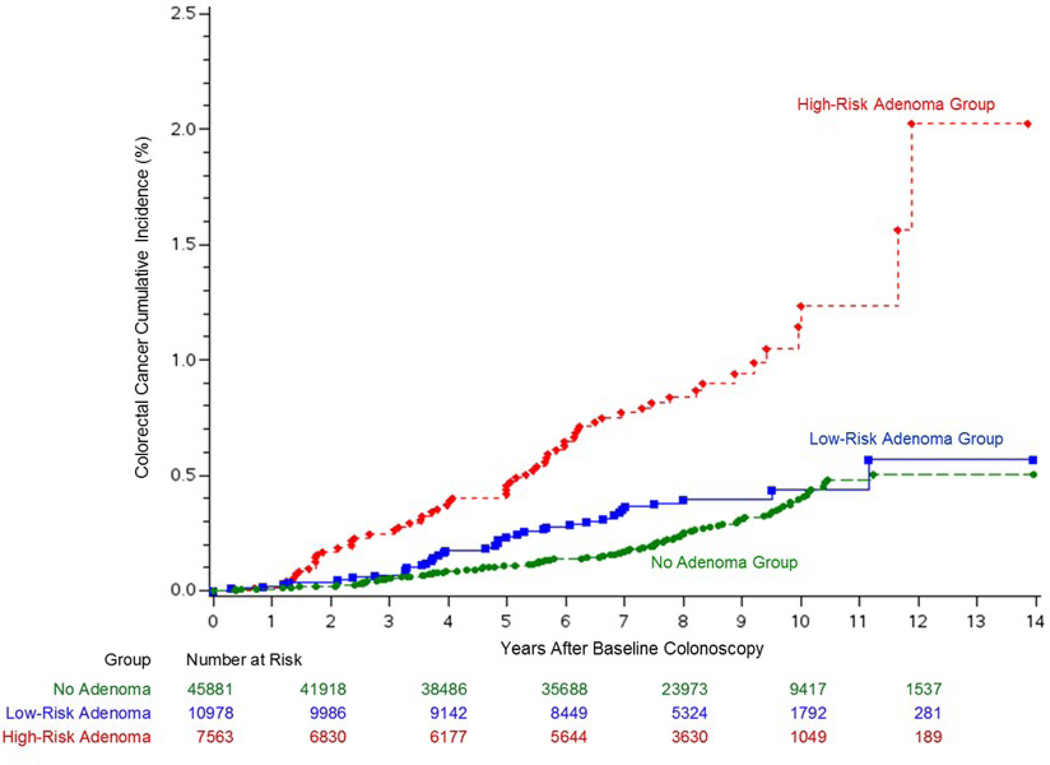
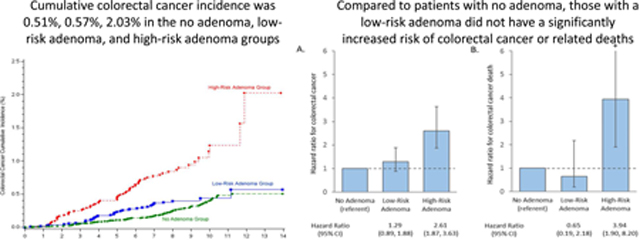
At year 10 (Figure 3 and Supplemental Table 2), the cumulative colorectal cancer incidence was 0.39% (95% CI: 0.33%, 0.49%) in the no-adenoma group, 0.44% (95% CI: 0.31%, 0.62%) in the low-risk adenoma group, and 1.24% (95% CI: 0.88%, 1.68%) in the high-risk adenoma group. At the end of follow-up (i.e., 14 years), the cumulative colorectal cancer incidence was 0.51% (95% CI: 0.40%, 0.64%) in the no-adenoma group, 0.57% (95% CI: 0.34%, 0.97%) in the low-risk adenoma group, and 2.03% (95% CI: 1.13%, 3.61%) in the high-risk adenoma group. There was no significant difference in overall colorectal cancer incidence between the no-adenoma and low-risk adenoma groups (P=0.082 by the log-rank test) at the end of follow-up. At year 10 (Supplemental Figure 2 and Supplemental Table 2), the cumulative mortality from colorectal cancer was 0.07% (95% CI: 0.04%, 0.12%) in the no-adenoma group, 0.03% (95% CI: 0.01%, 0.11%) in the low-risk adenoma group, and 0.25% (95% CI: 0.13%, 0.47%) in the high-risk adenoma group. The cumulative mortality from colorectal cancer at the end of follow-up was 0.07% (95% CI: 0.04%, 0.12%) in the no-adenoma group, 0.03% (95% CI: 0.01%, 0.11%) in the low-risk adenoma group, and 0.55% (95% CI: 0.18%, 1.69%) in the high-risk adenoma group. Most deaths from colorectal cancer in the no-adenoma and low-risk adenoma groups occurred within 7 years from the baseline colonoscopy (Supplemental Figure 2). There was no significant difference in overall colorectal cancer mortality between the no-adenoma and low-risk adenoma groups (P=0.35 by the log-rank test) at the end of follow-up.
Figure 3.
Cumulative colorectal cancer incidence by adenoma findings at the baseline colonoscopy
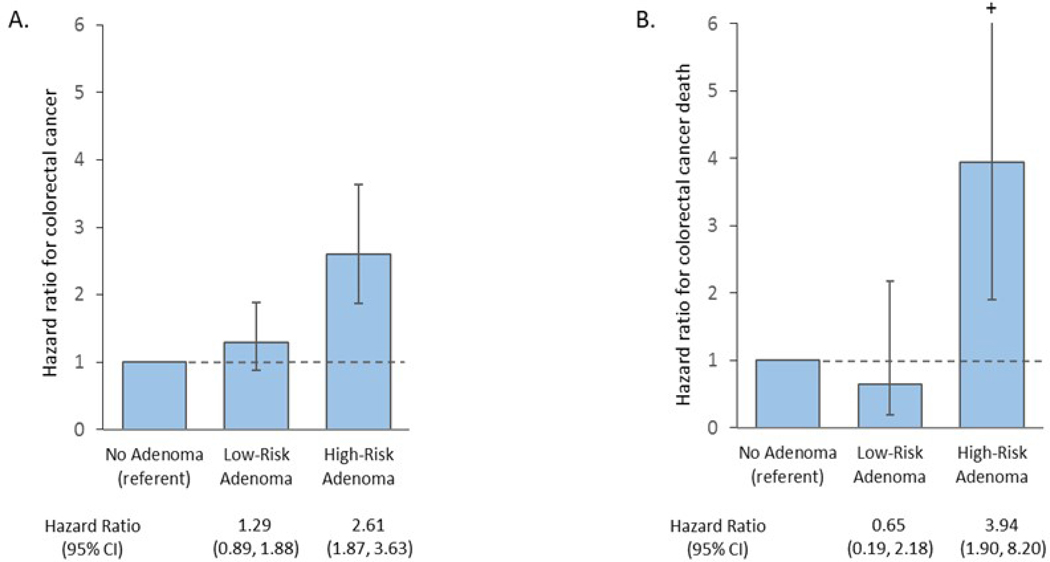
In covariate-adjusted Cox regression models, compared to the no-adenoma group, the low-risk adenoma group did not have a significantly higher risk of colorectal cancer (HR: 1.29; 95% CI: 0.89, 1.88) or related deaths (HR: 0.65; 95% CI: 0.19, 2.18, Figure 4). In contrast, compared to the no-adenoma group, participants in the high-risk adenoma group had a higher risk of both colorectal cancer (HR: 2.61; 95% CI: 1.87, 3.63) and related deaths (HR: 3.94, 95% CI: 1.90, 6.56).
Figure 4.
Covariate-adjusted* hazard ratios for colorectal cancer(A) and related deaths(B)
* Cox proportional hazards models adjusted for participant’s age, sex, race/ethnicity, Charlson comorbidity score at baseline, body mass index at baseline, baseline colonoscopy indication, physician adenoma detection rate, and year of baseline coionoscopy.
Cl, confidence interval
There was no evidence of effect modification by age, sex, race/ethnicity, BMI, colonoscopy indication, or physician adenoma detection rate on the association between baseline colonoscopy findings and colorectal cancer risk (Supplemental Table 3).
In a sensitivity analysis, exclusion of the first two years of follow-up after the baseline colonoscopy (potentially prevalent cancers) had little impact on the overall risk estimates for colorectal cancer in the low-risk (HR: 1.26; 95% CI: 0.85, 1.87) or high-risk adenoma groups (HR: 2.29; 95% CI: 1.59, 3.28).
Subsequent colonoscopy utilization and colonoscopy findings
Subsequent colonoscopy utilization starting 2 years after the baseline colonoscopy was consistently higher at each year of follow-up for the high- and low-risk adenoma groups compared to the no-adenoma group. At 6 years of follow-up, the cumulative incidence of subsequent colonoscopy use in the no-adenoma, low-risk adenoma, and high-risk adenoma groups was 9.3%, 40.5%, and 60.0%, respectively, and at 10 years it was 19.8%, 58.8%, and 72.7%, respectively (Supplemental Table 4). Among those who had a subsequent colonoscopy, the cumulative incidence of any adenoma or advanced adenoma was higher in the low-risk adenoma and high-risk adenoma groups compared to the no-adenoma group at nearly all years of follow-up. For example, at year 6 of follow-up, the cumulative incidence of advanced adenoma for the no-adenoma, low-risk adenoma, and high-risk adenoma group was 1.7%, 4.1%, and 10.5%, respectively; at 10 years it was 3.9%, 6.4%, and 13.3%, respectively.
DISCUSSION
In a population-based cohort study from a large community-based setting, the absolute risks of colorectal cancer, as measured by the cumulative incidence, at year 10 of follow-up after colonoscopy were 0.39%, 0.44%, and 1.12% in the no adenoma, low-risk adenoma, and high-risk adenoma groups, respectively. At the end of follow-up, absolute risks of colorectal cancer were 0.51%, 0.57%, and 2.03%, respectively. Compared to patients with no adenoma at baseline colonoscopy, those with a high-risk adenoma had a 2.6-fold increased risk of colorectal cancer during a median follow-up time of 8.1 years, along with a 3.9- fold higher risk of colorectal cancer-related death. In contrast, patients with a low-risk adenoma did not have a significantly increased risk of colorectal cancer or related deaths compared to those with no adenoma.
These findings expand our understanding of the risks of colorectal cancer and related deaths following removal of low-risk adenomas. Prior studies largely used comparisons to the general population of persons with unknown screening status, which precluded calculations of post-screening risk needed to inform surveillance guidelines. For example, in a population-based study from Norway, patients with low-risk adenomas had a significantly lower risk of colorectal cancer (standardized incidence ratio: 0.68, 95% CI: 0.44–0.99) and related deaths than the general population (standardized mortality ratio: 0.75, 95% CI: 0.63, 0.88) at a median 7.7 years of follow-up, but whether this risk differed from that of patients with a normal examination could not be calculated.15 A recent post-hoc analysis of a sigmoidoscopy trial found that participants with low-risk adenomas, as defined by the current guidelines,17 did not have a significantly elevated risk of colorectal cancer (relative risk (RR): 1.2, 95% CI: 0.8, 1.7) or related deaths (RR: 1.2, 95% CI: 0.5, 2.8) compared to participants with no adenoma.30 However, this study was limited because it only included participants who underwent a diagnostic colonoscopy after a positive sigmoidoscopy and was unable to adjust for important clinical factors related to the colonoscopy itself, which may have influenced colorectal cancer risk including the year of the colonoscopy examination (to account for secular trends) and operator/provider factors (e.g., adenoma detection rate). Our study extends the findings of these prior studies and strengthens our knowledge on the risks of colorectal cancer and related deaths following low-risk adenoma removal by making comparisons to patients from the same background population who had normal findings (i.e., no adenoma) at the baseline examination, only including colonoscopy examinations that were complete to the cecum and had an adequate bowel preparation, using United States Multi-Society Task Force’s definition for low-risk adenoma, and adjusting for covariates such as physician adenoma detection rate, an established quality metric for operator performance.17
Current guidelines recommend that patients with a low-risk adenoma finding, the most common finding on screening, receive surveillance colonoscopy in 5–10 years,17 although in practice, clinicians often use even more frequent surveillance (i.e., ≤5 years) in this low-risk group.31–34 The rationale for continued support of shorter-than-recommended surveillance intervals for patients with low-risk adenomas is unclear, but could stem from a lack of long-term population-based studies assessing colorectal cancer incidence and related deaths following low-risk adenoma removal or randomized trials evaluating optimal post-polypectomy surveillance intervals. While awaiting results of the European Polyp Surveillance randomized clinical trial (completion date 2028),35 our findings of similar absolute risks of colorectal cancer for the low-risk adenoma and no-adenoma groups at 10 years and at the end of follow-up; and no significant increased risk of colorectal cancer and related deaths among patients with low-risk adenomas compared to no adenoma offer some assurance that less intensive surveillance (i.e., >5 years) may be acceptable. However, any conclusions must be tempered by the potential biases introduced from differences in subsequent colonoscopy utilization and advanced adenoma removal between the no adenoma and low-risk adenoma groups, which may complicate interpretation of the results. For example, higher rates of subsequent colonoscopy utilization in the low-risk adenoma group compared to the no adenoma group could be expected to inflate colorectal cancer incidence through early detection of clinically silent, early-stage colorectal cancers, resulting in a higher relative risk estimate, and potentially introducing both lead-time bias and overdiagnosis bias. Conversely, adenoma removal during these subsequent colonoscopies could be expected to lower future colorectal cancer incidence and bias the relative risk estimate downwards. Colorectal cancer-related death was included as a secondary outcome because it largely reduces the potential biases (i.e., lead-time, overdiagnosis, and detection biases) introduced by differential rates of colonoscopy surveillance. Lastly, like the previous study,30 our risk estimated confidence interval upper bounds include potentially clinically important associations (HR 95% CI upper limit of 1.88 for colorectal cancer; HR 95% CI upper limit of 2.18 for colorectal cancer mortality) for the low-risk adenoma group in comparison to the no-adenoma group.
For advanced adenomas, to date, only one other study has compared risk of colorectal cancer and related deaths to an unexposed or no adenoma comparison group. In a post-hoc analysis of a randomized screening sigmoidoscopy trial of 15,935 patients, those with advanced adenomas found by diagnostic colonoscopy after a positive sigmoidoscopy had an increased risk of colorectal cancer (RR: 2.7, 95% CI: 1.9, 3.7) and related deaths (RR: 2.6, 95% CI: 1.2, 5.7) over a median of 12.9 years of follow-up compared to participants with no adenoma.30 These findings of an increased cancer risk following detection of an advanced adenoma are consistent with studies that found patients with an advanced adenoma had a greater risk of cancer than the general population (of variable screening status).14–16 Although these studies provide important long-term risk estimates for colorectal cancer and related deaths after high-risk adenoma removal, risk estimates may have been impacted by uncontrolled underlying differences between the study and general populations of mixed screening status.14–16 For example, patient-related risk factors for colorectal cancer despite adenoma removal include predisposing lifestyle, environmental, and host/genetic risk factors.36–38 Suboptimal adherence to surveillance colonoscopy, which has been shown across multiple healthcare systems and countries, and failure to completely remove high-risk adenomas during baseline or subsequent colonoscopies, can also influence risk of colorectal cancer and related deaths.39–41 Also, recent studies have shown that polypectomy competency varies significantly among colonoscopists and may not correlate with established colonoscopy quality metrics (e.g., adenoma detection rate, withdrawal time).42–44
Strengths of the study include colorectal cancer and related death risk estimates from a large, multi-medical center, community-based population, a median follow-up time of 8.1 years, comprehensive capture of adenoma and cancer diagnoses, use of a validated algorithm to categorize adenomas as low- or high-risk, the ability to adjust for important confounders including examination indication and quality (i.e., physician adenoma detection rate), use of a validated natural language processing tool to accurately and comprehensively detect high-quality complete colonoscopies,28 and comparisons to a no-adenoma group from the same population.
Study limitations include the possibility of residual confounding inherent to observational studies, particularly misclassification of adenomas as low- or high-risk. Our classification algorithm validation demonstrated a sensitivity of 93% for high-risk adenomas. Given almost perfect capture of pathology (any adenoma), this means about 7% of high-risk lesions were potentially misclassified into the low-risk adenoma group. Thus, our results may slightly overestimate the risks in the low-risk adenoma group and underestimate the risks in the high-risk adenoma group. Our adenoma counts per patient assumed that multiple adenomas reported in each pathology bottle represented different adenomas as opposed to a single adenoma in pieces due to piecemeal resection or suctioning. The study was conducted in a large integrated health care delivery setting and the findings may not be generalizable to other settings (e.g., non-health maintenance organization settings); for example, only 14.5% of low-risk adenoma patients had a surveillance colonoscopy within 5 years of the baseline examination, which is lower than that reported in general practice.34,45 However, the low surveillance colonoscopy rate among those with low-risk adenomas actually makes this population an ideal setting for decreasing potential biases introduced by these exams, compared with “high surveillance” settings. Also, while excluding patients with serrated polyps allowed for a focus on conventional adenomas, it limits generalizability, particularly relative to individuals with conventional adenomas and synchronous sessile serrated polyps who are at high risk for advanced neoplasia.46,47 While our cohort sample was large, the number of events still limited the precision of our risk estimates. Also, somewhat higher subsequent colonoscopy utilization in the low-risk adenoma group compared to the no-adenoma group may have impacted our colorectal cancer and colorectal cancer mortality risk estimates through the detection of cancer at an earlier and more treatable stage and the removal of precancerous adenomas. Lastly, California mortality files were used to ascertain colorectal cancer-related deaths and these files may miss patients who moved out of California.
In conclusion, our findings, in a setting with high colonoscopy quality, demonstrate that patients with high-risk adenomas at baseline had a significantly increased risk of colorectal cancer and related deaths compared to patients with no adenomas; these findings support guideline recommendations for intensive colonoscopy surveillance in these patients. Patients with low-risk adenomas did not have a significantly elevated risk of colorectal cancer or related deaths compared to those with no adenomas. This finding suggests that guidelines recommending comparable follow-up for low risk adenomas and normal examinations, such as lengthening the surveillance interval to >5 years and possibly 10 years, may provide comparable cancer incidence and mortality benefits for these two groups. However, imprecision of the estimates (i.e. wide confidence intervals) and the differential rates of surveillance (and advanced adenoma removal) between the no adenoma and low-risk adenoma groups preclude definitive conclusions regarding whether low-risk adenomas substantially increase the future risk of colorectal cancer. Additional studies, potentially including randomized trials, on the natural history of low-risk adenoma and normal findings without intervening surveillance exams before 10 years are needed to help guide future surveillance practices.
Supplementary Material
What you need to know:
BACKGROUND AND CONTEXT:
Using data from a large, community-based integrated health care plan, we examined the risks of CRC and related death based on baseline colonoscopy adenoma findings.
NEW FINDINGS:
In an analysis of 14-years of follow-up data from 64,422 patients, we found that high-risk adenomas are associated with an increased risk of CRC and related death. Low-risk adenomas were not associated with a significantly increased risk of CRC or related deaths.
LIMITATIONS:
This was a retrospective study
IMPACT:
High-risk adenomas have an increased risk of CRC and related death, supporting early colonoscopy surveillance. In contrast, low-risk adenomas do not have an increased risk of CRC and related death, providing assurance that less intensive surveillance (i.e., >5 years) may be acceptable.
Acknowledgments
Funding: This study was conducted within the National Cancer Institute-funded Population-Based Research Optimizing Screening Through Personalized Regimens consortium (U54 CA163262, UM1 CA222035), and was supported by a career development grant (K07 CA212057) from the National Cancer Institute (Dr. Lee) and American Gastroenterological Association Research Scholar Award. These funding institutions had no role of the study sponsor.
Acronyms:
- CI
confidence interval
- HR
hazard ratio
- KPNC
Kaiser Permanente Northern California
- PROSPR
Population-based Research Optimizing Screening through Personalized Regimens
- SEER
Surveillance Epidemiology and End Results
- AJCC
American Joint Committee on Cancer
Footnotes
Disclosures: No conflicts of interest exist for any of the authors.
Disclaimer: Dr. Doubeni is a member of the United States Preventive Services Task Force (USPSTF) and authors topics on UpToDate. This article does not necessarily represent the views and policies of the USPSTF or UpToDate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;316:545. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–2575. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993;329:1977–81. [DOI] [PubMed] [Google Scholar]
- 4.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deathss. N Engl J Med.2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30. [DOI] [PubMed] [Google Scholar]
- 6.Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30(21):2664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and deaths from colorectal cancer. Ann Intern Med. 2009;150(1):1–8. [DOI] [PubMed] [Google Scholar]
- 8.Doubeni CA, Weinmann S, Adams K, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med. 2013;158:312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samadder NJ, Curtin K, Pappas L, et al. Risk of incident colorectal cancer and deaths after colonoscopy: a population-based study in Utah. Clin Gastroenterol Hepatol. 2016;14(2):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeve F, van Ballegooijen M, Boer R, Kuipers EJ, Habbema JD. Colorectal cancer risk in adenoma patients: a nation-wide study. Int J Cancer. 2004; 111 (1):147–51. [DOI] [PubMed] [Google Scholar]
- 11.Coleman HG, Loughrey MB, Murray LJ, et al. Colorectal cancer risk following adenoma removal: A large prospective population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shergill AK, Conners EE, McQuaid KR, et al. Protective association of colonoscopy against proximal and distal colon cancer and patterns of interval cancer. Gastrointest Endosc. 2015;82(3):529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of deaths from right and left colon cancer: a large community-based study. Gut 2018;67(2):291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottet V, Jooste V, Fournel I, et al. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012;61 (8):1180–1186. [DOI] [PubMed] [Google Scholar]
- 15.Loberg M, Kalager M, Holme O, et al. Long-term colorectal cancer mortality after adenoma removal. N Engl J Med. 2014;371(9):799–807. [DOI] [PubMed] [Google Scholar]
- 16.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326(10):658–62. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143(3):844–57. [DOI] [PubMed] [Google Scholar]
- 18.Laiyemo AO, Pinsky PF, Marcus PM, Lanza E, Cross AJ, Schatzkin A, Schoen RE. Utilization and yield of surveillance colonoscopy in the continued follow-up study of the polyp prevention trial. Clin Gastro Hepatol. 2009;7(5):562–567. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenoma at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006;64(4):614–626. PMID: . [DOI] [PubMed] [Google Scholar]
- 20.Lieberman DA, Weiss DF, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J, Bond JH, Nelson DB, Triadafilopoulos G, Ramirez FC, Collins JF, Johnston TK, McQuaid KR, Garewal H, Sampliner RE, Esquivel R, Robertson D. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077–85. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 21.Martinez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, Zauber AG, Jiang R, Ahnen DJ, Bond JH, Church TR, Robertson DJ, Smith-Warner SA, Jacobs ET, Alberts DS, Greenberg ER. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136(3):832–841. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller H, Mukherjee R, Tian J, Nagar AB. Colonoscopy surveillance after polypectomy may be extended beyond five years. J Clin Gastroenterol. 2010;44(8):e162–6. PMID: . [DOI] [PubMed] [Google Scholar]
- 23.Miller J, Mehta N, Feldman M, Furth E, Ginsberg GG, Yang YX, Lewis JD. Findings on serial surveillance colonoscopy in patients with low-risk polyps on initial colonoscopy. J Clin Gastroenterol. 2010;44(3):e46–50. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung SJ, Kim YS, Yang SY, Song JH, Kim D, Park MJ, Kim SG, Song IS, Kim JS. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011;60(11):1537–1543. PMID: . [DOI] [PubMed] [Google Scholar]
- 25.Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, Waye JD, Bond J, Schapiro M, Stewart ET, et al. Randomized comparison of surveillance intervals after colonoscopy removel of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328(13):901–6. PMID: . [DOI] [PubMed] [Google Scholar]
- 26.van Heijningen EM, Lansdorp-Vogelaar I, Kuipers EJ, Dekker E, Lesterhuis W, Ter Borg F, Vecht J, de Jonge V, Spoelstra P, Engels L, Bolwerk CJ, Timmer R, Kleibeuker JH, Koornstra JJ, van Ballegooijen M, Steyerberg EW. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology. 2013;144(7) :1410–1418. PMID: . [DOI] [PubMed] [Google Scholar]
- 27.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and deaths. N Engl J Med. 2014;370(14):1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JK, Jensen CD, Levin TR, Zauber AG, Doubeni CA, Zhao WK, Corley DA. Accurate identification of colonoscopy quality and polyp findings using natural language processing. J Clin Gastroenterol. 2019;53(1):e25–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JK, Jensen CD, Lee A, et al. Development and validation of an algorithm for classifying colonoscopy indication. Gastrointest Endosc. 2015;81(3):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA. 2018;319(19):2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141(4):264–271. PMID: . [DOI] [PubMed] [Google Scholar]
- 32.Boolchand V, Olds G, Singh J, Singh P, Chak A, Cooper GS. Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med. 2006;145(9):654–659. PMID: . [DOI] [PubMed] [Google Scholar]
- 33.Patel N, Tong L, Ahn C, Singal AG, Gupta S. Post-polypectomy guideline adherence: importance of belief in guidelines, not guideline knowledge or fear of missed cancer. Dig Dis Sci. 2015;60(10):2937–45. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoen RE, Pinsky PF, Wessfeld JL, Yokochi LA, Reding DJ, Hayes RB. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138(1):73–81. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jover R, Bretthauer M, Dekker E, et al. Rationale and design of the European polyp surveillance (EPoS) trials. Endoscopy. 2016;48(6):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell SM, Zilz N, Beazer-Barclay Y, et al. (1992) APC mutations occur early during colorectal tumorigene- sis. Nature, 359, 235–237 [DOI] [PubMed] [Google Scholar]
- 37.Tsao JI and Shibata D. (1994) Further evidence that one of the earliest alterations in colorectal carcinogenesis involves APC. American Journal of Pathology, 145, 531–534. [PMC free article] [PubMed] [Google Scholar]
- 38.Strum WB. Colorectal adenomas. N Engl J Med. 2016;374:1065–1075. [DOI] [PubMed] [Google Scholar]
- 39.Chubak J, McLerran D, Zheng Y, et al. Receipt of colonoscopy following diagnosis of advanced adenomas: an analysis within integrated health delivery systems. Cancer Epidemiol Biomarkers Prev. 2019;28(1):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Heijningen EM, Landsdorp-Vogelaar I, Steyerberg EW, et al. Adherence to surveillance guidelines after removal of colorectal adenomas: a large, community-based study. Gut. 2015;64(10):1584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy CC, Sandler RS, Grubber JM, et al. Underuse and overuse of colonoscopy for repeat screening and surveillance in the Veterans Health Administration. Clin Gastroenterol Hepatol. 2016;14(3):436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pohl H, Srivastava A, Bensen SP. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144(1):74–80. [DOI] [PubMed] [Google Scholar]
- 43.Duloy AM, Kaltenbach TR, Keswani RN. Assessing colon polypectomy competency and its association with established quality metrics. Gastrointest Endosc. 2018;87(3):635–644. [DOI] [PubMed] [Google Scholar]
- 44.Gupta S, Anderson J, Bhandari P, et al. Development and validation of a novel method for assessing competency in polypectomy: direct observation of polypectomy skills. Gastrointest Endosc. 2011;73(6):1232–9. [DOI] [PubMed] [Google Scholar]
- 45.Anderson JC, Baron JA, Ahnen DJ, et al. Factors associated with shorter colonoscopy surveillance intervals for patients With low-risk colorectal adenomas and effects on outcome. Gastroenterology. 2017;152(8):1933–43 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson JC, Butterly LF, Robinson CM, et al. Risk of metachronous high-risk adenomas and large serrated polyps in individuals with serrated polyps on index colonoscopy: data from the New Hampshire colonoscopy registry. Gastroenterology. 2018;154(1):117–27 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Symonds E, Anwar S, Young G. Sessile serrated polyps with synchronous conventional adenomas increase risk of future advanced neoplasia. Dig Dis Sci 2019;64(6):1680–1685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.