Abstract
Background
Surgical resection is currently the only treatment with the potential for long‐term survival and cure of pancreatic cancer. Surgical resection is provided as distal pancreatectomy for cancers of the body and tail of the pancreas. It can be performed by laparoscopic or open surgery. In operations on other organs, laparoscopic surgery has been shown to reduce complications and length of hospital stay as compared with open surgery. However, concerns remain about the safety of laparoscopic distal pancreatectomy compared with open distal pancreatectomy in terms of postoperative complications and oncological clearance.
Objectives
To assess the benefits and harms of laparoscopic distal pancreatectomy versus open distal pancreatectomy for people undergoing distal pancreatectomy for pancreatic ductal adenocarcinoma of the body or tail of the pancreas, or both.
Search methods
We used search strategies to search the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index Expanded and trials registers until June 2015 to identify randomised controlled trials (RCTs) and non‐randomised studies. We also searched the reference lists of included trials to identify additional studies.
Selection criteria
We considered for inclusion in the review RCTs and non‐randomised studies comparing laparoscopic versus open distal pancreatectomy in patients with resectable pancreatic cancer, irrespective of language, blinding or publication status..
Data collection and analysis
Two review authors independently identified trials and independently extracted data. We calculated odds ratios (ORs), mean differences (MDs) or hazard ratios (HRs) along with 95% confidence intervals (CIs) using both fixed‐effect and random‐effects models with RevMan 5 on the basis of intention‐to‐treat analysis when possible.
Main results
We found no RCTs on this topic. We included in this review 12 non‐randomised studies that compared laparoscopic versus open distal pancreatectomy (1576 participants: 394 underwent laparoscopic distal pancreatectomy and 1182 underwent open distal pancreatectomy); 11 studies (1506 participants: 353 undergoing laparoscopic distal pancreatectomy and 1153 undergoing open distal pancreatectomy) provided information for one or more outcomes. All of these studies were retrospective cohort‐like studies or case‐control studies. Most were at unclear or high risk of bias, and the overall quality of evidence was very low for all reported outcomes.
Differences in short‐term mortality (laparoscopic group: 1/329 (adjusted proportion based on meta‐analysis estimate: 0.5%) vs open group: 11/1122 (1%); OR 0.48, 95% CI 0.11 to 2.17; 1451 participants; nine studies; I2 = 0%), long‐term mortality (HR 0.96, 95% CI 0.82 to 1.12; 277 participants; three studies; I2 = 0%), proportion of people with serious adverse events (laparoscopic group: 7/89 (adjusted proportion: 8.8%) vs open group: 6/117 (5.1%); OR 1.79, 95% CI 0.53 to 6.06; 206 participants; three studies; I2 = 0%), proportion of people with a clinically significant pancreatic fistula (laparoscopic group: 9/109 (adjusted proportion: 7.7%) vs open group: 9/137 (6.6%); OR 1.19, 95% CI 0.47 to 3.02; 246 participants; four studies; I2 = 61%) were imprecise. Differences in recurrence at maximal follow‐up (laparoscopic group: 37/81 (adjusted proportion based on meta‐analysis estimate: 36.3%) vs open group: 59/103 (49.5%); OR 0.58, 95% CI 0.32 to 1.05; 184 participants; two studies; I2 = 13%), adverse events of any severity (laparoscopic group: 33/109 (adjusted proportion: 31.7%) vs open group: 45/137 (32.8%); OR 0.95, 95% CI 0.54 to 1.66; 246 participants; four studies; I2 = 18%) and proportion of participants with positive resection margins (laparoscopic group: 49/333 (adjusted proportion based on meta‐analysis estimate: 14.3%) vs open group: 208/1133 (18.4%); OR 0.74, 95% CI 0.49 to 1.10; 1466 participants; 10 studies; I2 = 6%) were also imprecise. Mean length of hospital stay was shorter by 2.43 days in the laparoscopic group than in the open group (MD ‐2.43 days, 95% CI ‐3.13 to ‐1.73; 1068 participants; five studies; I2 = 0%). None of the included studies reported quality of life at any point in time, recurrence within six months, time to return to normal activity and time to return to work or blood transfusion requirements.
Authors' conclusions
Currently, no randomised controlled trials have compared laparoscopic distal pancreatectomy versus open distal pancreatectomy for patients with pancreatic cancers. In observational studies, laparoscopic distal pancreatectomy has been associated with shorter hospital stay as compared with open distal pancreatectomy. Currently, no information is available to determine a causal association in the differences between laparoscopic versus open distal pancreatectomy. Observed differences may be a result of confounding due to laparoscopic operation on less extensive cancer and open surgery on more extensive cancer. In addition, differences in length of hospital stay are relevant only if laparoscopic and open surgery procedures are equivalent oncologically. This information is not available currently. Thus, randomised controlled trials are needed to compare laparoscopic distal pancreatectomy versus open distal pancreatectomy with at least two to three years of follow‐up. Such studies should include patient‐oriented outcomes such as short‐term mortality and long‐term mortality (at least two to three years); health‐related quality of life; complications and the sequelae of complications; resection margins; measures of earlier postoperative recovery such as length of hospital stay, time to return to normal activity and time to return to work (in those who are employed); and recurrence of cancer.
Keywords: Humans, Laparoscopy, Laparoscopy/adverse effects, Laparoscopy/mortality, Case‐Control Studies, Pancreatectomy, Pancreatectomy/adverse effects, Pancreatectomy/methods, Pancreatectomy/mortality, Pancreatic Neoplasms, Pancreatic Neoplasms/surgery, Retrospective Studies
Plain language summary
Key‐hole (laparoscopic) versus standard access (open) abdominal operation for people with pancreatic cancer
Review question
How does key‐hole (laparoscopic) abdominal surgery compare with standard access (open) abdominal operation for people with pancreatic cancer?
Background
The pancreas is an organ in the abdomen that secretes pancreatic juice that aids digestion and contains cells that produce important hormones such as insulin. The pancreas can be divided into the head of the pancreas (right part of the pancreas) and the body and tail of the pancreas (left part or distal part of the pancreas). Distal pancreatic cancer is cancer of the body and/or tail of the pancreas. Removal of distal pancreatic cancer by surgery (distal pancreatectomy) is the preferred treatment for people with distal pancreatic cancers limited to the pancreas who are likely to withstand major surgery, because no other treatments have the potential to cure pancreatic cancer. Cancer can be removed through an abdominal operation, either laparoscopic distal pancreatectomy or open distal pancreatectomy. Laparoscopic distal pancreatectomy is a relatively new procedure as compared with the well‐established open distal pancreatectomy. In operations on other parts of the body, laparoscopic surgery has been shown to reduce complications and length of hospital stay as compared with open surgery. However, concerns remain about the safety of laparoscopic distal pancreatectomy in terms of complications after operation (postoperative complications). In addition, it is not clear whether laparoscopic distal pancreatectomy achieves the same amount of cancer clearance as is attained by open distal pancreatectomy. It also is not clear whether laparoscopic distal pancreatectomy is better than open distal pancreatectomy in terms of earlier recovery after operation. We sought to resolve this issue by searching the medical literature for studies on this topic until June 2015.
Study characteristics
No randomised controlled trials have examined this topic. Randomised controlled trials are the best studies for finding out whether one treatment is better or worse than another because they ensure that similar types of people are receiving the treatments being assessed. In the absence of randomised controlled trials, we sought information from non‐randomised studies. We identified 12 non‐randomised studies that compared laparoscopic versus open distal pancreatectomy in a total of 1576 patients. One of these studies did not provide results in a useable way. Thus, we included 11 studies in which a total of 1506 patients underwent distal pancreatectomy. Some 353 patients underwent laparoscopic distal pancreatectomy, and 1153 patients underwent open distal pancreatectomy. In all studies, historical information was collected from hospital records (retrospective studies). In general, historical information is less reliable than newly collected (prospective) information and findings of randomised controlled trials.
Key results
Differences in short‐term deaths, long‐term deaths, percentage of people with major complications, percentage of people with a pancreatic fistula (abnormal communication between the pancreas and other organs or the skin), recurrence of cancer at final time of follow‐up of participants, percentage of people with any complications and percentage of patients in whom cancer was not completely removed were imprecise. Average length of hospital stay was shorter in the laparoscopic group than in the open group by about two days. However, this is not relevant until we can be sure that cancer cures are similar between laparoscopic surgery and open surgery. No studies have reported quality of life at any point in time, short‐term recurrence of cancer, time to return to normal activity, time to return to work or blood transfusion requirements.
Quality of the evidence
The quality of the evidence was very low, mainly because it was not clear whether similar types of participants received laparoscopic and open distal pancreatectomy. In many studies, people with less extensive cancer received laparoscopic surgery, and those with more extensive cancer received open surgery. This makes study findings unreliable. Well‐designed randomised controlled trials are necessary if we are to obtain good quality evidence on this topic.
Summary of findings
Summary of findings for the main comparison. Laparoscopic distal pancreatectomy compared with open distal pancreatectomy for pancreatic cancer.
| Laparoscopic distal pancreatectomy compared with open distal pancreatectomy for pancreatic cancer | |||||
| Patient or population: patients with pancreatic cancer Settings: secondary or tertiary care centre Intervention: laparoscopic distal pancreatectomy Comparison: open distal pancreatectomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Open distal pancreatectomy | Laparoscopic distal pancreatectomy | ||||
| Short‐term mortality | 10 per 1000 | 5 per 1000 (1 to 22) | OR 0.48 (0.11 to 2.17) | 1451 (9 studies) | ⊕⊝⊝⊝ Very lowa,b |
| Long‐term mortality Follow‐up: 2 to 3 years | 549 per 1000 | 535 per 1000 (480 to 590) | HR 0.96 (0.82 to 1.12) | 277 (3 studies) | ⊕⊝⊝⊝ Very lowa,c |
| Serious adverse events (proportion) | 51 per 1000 | 88 per 1000 (28 to 247) | OR 1.79 (0.53 to 6.06) | 206 (3 studies) | ⊕⊝⊝⊝ Very lowa,b,c |
| Pancreatic fistula (grade B or C) | 66 per 1000 | 77 per 1000 (32 to 175) | OR 1.19 (0.47 to 3.02) | 246 (4 studies) | ⊕⊝⊝⊝ Very lowa,b,c,d |
| None of the studies reported quality of life at any time point. | |||||
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; HR: hazard ratio; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||||
a We found no randomised controlled trials. The non‐randomised studies included in this review were at unclear or high risk of bias for most domains bConfidence intervals were wide cSample size was small dI2 was high and little overlap of confidence intervals was evident.
Summary of findings 2. Laparoscopic distal pancreatectomy compared with open distal pancreatectomy for pancreatic cancer.
| Laparoscopic distal pancreatectomy compared with open distal pancreatectomy for pancreatic cancer | |||||
| Patient or population: patients with pancreatic cancer Settings: secondary or tertiary care centre Intervention: laparoscopic distal pancreatectomy Comparison: open distal pancreatectomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Open distal pancreatectomy | Laparoscopic distal pancreatectomy | ||||
| Recurrence at maximal follow‐up | 495 per 1000 | 363 per 1000 (239 to 507) | OR 0.58 (0.32 to 1.05) | 184 (2 studies) | ⊕⊝⊝⊝ Very lowa,b,c |
| Adverse events (proportion) | 328 per 1000 | 317 per 1000 (209 to 448) | OR 0.95 (0.54 to 1.66) | 246 (4 studies) | ⊕⊝⊝⊝ Very lowa,b,c |
| Length of hospital stay | Mean length of hospital stay in the control groups was 9.4 days | Mean length of hospital stay in the intervention groups was 2.43 lower (3.13 to 1.73 lower) | 1068 (5 studies) | ⊕⊝⊝⊝ Very lowa | |
| Positive resection margins | 184 per 1000 | 143 per 1000 (99 to 198) | OR 0.74 (0.49 to 1.10) | 1466 (10 studies) | ⊕⊝⊝⊝ Very lowa,b |
| None of the studies reported perioperative transfusion requirements, time to return to normal activity or time to return to work | |||||
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | |||||
aWe found no randomised controlled trials. The non‐randomised studies included in this review were at unclear or high risk of bias for most domains bConfidence intervals were wide cSample size was small
Background
Description of the condition
Adenocarcinoma of the pancreas is the most common malignancy of the exocrine pancreas. It is the tenth most common cancer in the United States, the fifth most common cause of cancer‐related mortality in the East and the fourth most common cause of cancer‐related mortality in the West (Parkin 2001; Parkin 2005; Yamamoto 1998). In 2012, 338,000 people were newly diagnosed with pancreatic cancer, and 330,000 deaths were the result of pancreatic cancer globally (IARC 2014). Global variation has been noted in the incidence of pancreatic cancer, with an age‐standardised annual incidence rate of 7.2 per 100,000 in more developed regions and an age‐standardised annual incidence rate of 2.8 per 100,000 in less developed regions (IARC 2014). A similar trend has been noted in an age‐standardised annual mortality rate of 6.8 per 100,000 population in more developed regions and 2.7 per 100,000 population in less developed regions due to pancreatic cancer (IARC 2014). Mortality rates due to pancreatic cancer are increasing in the United States (Ma 2013). Pancreatic adenocarcinoma has a poor prognosis for many reasons. It is a biologically aggressive cancer that is relatively resistant to chemotherapy and radiotherapy and has a high rate of local and systemic recurrence (Abrams 2009; Ghaneh 2007; Orr 2010). Surgical resection remains the only treatment with the potential for long‐term survival and cure. However, about half the people have metastatic disease at presentation, and one‐third have locally advanced unresectable disease, leaving only about 10% to 20% of people suitable for resection (Tucker 2008). Overall five‐year survival after radical resection ranges from 7% to 25% (Cameron 1993; Livingston 1991; Niederhuber 1995; Nitecki 1995; Orr 2010; Trede 1990), with median survival of 11 to 15 months (British Society of Gastroenterology 2005). With adjuvant chemotherapy, median survival after radical resection ranges between 14 and 24 months (Liao 2013).
Pancreatic cancer can occur in the head of the pancreas or in the body and tail of the pancreas. In early pancreatic cancer (with no invasion of adjacent structures such as the superior mesenteric vein, portal vein or superior mesenteric artery), surgical resection remains the primary treatment of choice for people likely to withstand major surgery.
Description of the intervention
Surgical resection is provided as pancreaticoduodenectomy for cancers of the head of the pancreas and as distal pancreatectomy for cancers of the body and tail of the pancreas (Park 2013). In open distal pancreatectomy, surgical access to the abdominal cavity (and hence the pancreas) is attained by upper midline incision, bilateral subcostal incision (roof‐top or Chevron incision) or transverse abdominal incision (Fernandez‐Cruz 2006). In laparoscopic distal pancreatectomy, surgical access to the abdominal cavity (and hence the pancreas) is typically attained by four small ports (holes) of about 1 cm each through which laparoscopic instruments can be inserted after the abdomen is distended using carbon dioxide pneumoperitoneum. For people with pancreatic cancer, the pancreas and the spleen are removed together (en bloc) after isolation and mobilisation of the distal pancreas, spleen and surrounding lymph nodes from surrounding structures such as the stomach, colon, diaphragm and kidneys by dividing attachments and blood vessels (Fernandez‐Cruz 2006). Although splenic preservation is possible in open or laparoscopic distal pancreatectomy (Fernandez‐Cruz 2006), the spleen is usually removed during distal pancreatectomy for cancers because of concern about cancer clearance in spleen preservation surgeries (Fernandez‐Cruz 2005). However, no evidence suggests that splenectomy improves cancer clearance.
After resection of the body and tail of the pancreas, the cut surface of the pancreatic remnant (pancreatic stump) is usually closed with staples or sutures (Diener 2011). Despite this, a high incidence of clinically significant pancreatic fistula (11%) has been reported (Diener 2011; Montorsi 2012), and various interventions including somatostatin analogues may be used to decrease pancreatic fluid secretion (Gurusamy 2013), and fibrin sealants (in the form of glue (Suzuki 1995) or patches (Montorsi 2012)) to seal the pancreatic stump.
Distal pancreatectomy can also be performed with the assistance of a robot (robot‐assisted distal pancreatectomy). In robot‐assisted distal pancreatectomy, laparoscopic instruments are controlled by a robot. This is generally considered distinct from laparoscopic distal pancreatectomy (Daouadi 2013). The term 'minimally invasive distal pancreatectomy' is usually used to describe both laparoscopic distal pancreatectomy and robot‐assisted distal pancreatectomy.
How the intervention might work
For many surgical procedures, laparoscopic surgery is currently preferred over open surgery. Laparoscopic surgery includes surgical procedures such as cholecystectomy (removal of gallbladder), colon cancer treatment and hysterectomy (Bijen 2009; Keus 2006; Reza 2006; Talseth 2014; Walsh 2009). Laparoscopic surgery is preferred over open surgery because it is associated with decreased pain, decreased blood loss, shorter hospital stay, earlier postoperative recovery, better cosmesis (physical appearance) and decreased costs (Bijen 2009; Keus 2006; Kooby 2008; Reza 2006; Rutz 2014; Talseth 2014; Walsh 2009).
Why it is important to do this review
A smaller incision and earlier postoperative recovery appear to be potential advantages of laparoscopic distal pancreatectomy; however, the safety of this approach for a procedure that has a high complication rate and cancer clearance after laparoscopic distal pancreatectomy must be ensured before the method can be widely recommended. Healthcare providers have expressed concerns about cancer clearance because port‐site metastases (recurrence of cancer at the laparoscopic port site) have been reported after laparoscopic surgery for many different cancers (Kais 2014; Palomba 2014; Song 2014). Animal research has shown that increased intra‐abdominal pressure during laparoscopy (pneumoperitoneum) may drive malignant cells into ports, resulting in seeding of the port site and port‐site metastases (Hopkins 1999). Also, malignant cells may be adherent to laparoscopic instruments that are introduced and removed through the ports, resulting in seeding of the port site and port‐site metastases (Hopkins 1999). Other issues include the adequacy of cancer clearance in terms of resection margins and the extent of lymph nodes removed through laparoscopy. Therefore, oncological efficacy (cancer clearance) is an important issue with laparoscopic distal pancreatectomy. No Cochrane review has examined this topic.
Objectives
To assess the benefits and harms of laparoscopic distal pancreatectomy versus open distal pancreatectomy for people undergoing distal pancreatectomy for pancreatic ductal adenocarcinoma of the body or tail of the pancreas, or both.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include only randomised controlled trials (RCTs) in this review. However, we found no RCTs on the topic, so we performed a meta‐analysis of observational studies clearly highlighting the bias involved in interpretation of results. We included studies reported as full text, studies published as abstract only and unpublished data.
Types of participants
We included adults undergoing distal pancreatectomy for pancreatic ductal adenocarcinoma. Although we excluded people undergoing distal pancreatectomy for neuroendocrine cancers (cancers that arise from neural and endocrine cells; Rindi 2011), when possible we included trials in which no separate outcome data were available for people undergoing distal pancreatectomy for pancreatic adenocarcinoma, provided that distal pancreatectomy for other causes including neuroendocrine cancer was performed in less than 10% of participants included in the trial.
Types of interventions
We included trials comparing laparoscopic distal pancreatectomy versus open distal pancreatectomy provided that the only difference between groups was the use of the laparoscopic or open method of access to the pancreas. We excluded studies that compared different methods of laparoscopic distal pancreatectomy, robotic distal pancreatectomy or open distal pancreatectomy.
Types of outcome measures
Primary outcomes
-
Mortality.
Short‐term mortality (in‐hospital mortality or mortality within three months).
Long‐term mortality.
-
Serious adverse events (within three months). We will accept the following definitions of serious adverse events.
Clavien‐Dindo classification (Clavien 2009; Dindo 2004): grade III or greater.
International Conference on Harmonisation ‐ Good Clinical Practice (ICH‐GCP) guideline (ICH‐GCP 1996): serious adverse events defined as any untoward medical occurrences that result in death, are life‐threatening, require hospitalisation or prolongation of existing hospitalisation or result in persistent or significant disability/incapacity.
Individual complications that can clearly be classified as grade III or greater with the Clavien‐Dindo classification (Clavien 2009; Dindo 2004), or as a serious adverse event with the ICH‐GCP classification.
Clinically significant pancreatic fistulas (type B or type C International Study Group on Pancreatic Fistula (ISGPF) definition) (Bassi 2005).
-
Health‐related quality of life (using any validated scale).
Short‐term (four weeks to three months).
Medium‐term (longer than three months to one year).
Secondary outcomes
-
Recurrence (local recurrence, surgical wound recurrence (also called port‐site metastasis in the laparoscopic group) or distal metastasis).
Short‐term recurrence (within six months).
Long‐term recurrence (recurrence at maximal follow‐up).
Adverse events (within three months). We will accept all adverse events reported by the study author irrespective of their severity.
-
Perioperative blood transfusion requirements (during surgery or within one week after surgery) (whole blood or red cell transfusion).
Proportion of people requiring blood transfusion.
Quantity of blood transfusion.
-
Measures of earlier postoperative recovery.
Length of hospital stay (including the index admission for distal pancreatectomy and any surgical complication‐related re‐admissions).
Time to return to normal activity (return to preoperative mobility with no additional carer support).
Time to return to work (for people who were employed previously).
Positive resection margins (presence of macroscopic or microscopic cancer tissue at the plane of resection) at histopathological examination after surgery.
We based our choice of clinical outcomes (above) on the necessity to assess whether laparoscopic surgery results in adequate cancer clearance, is safe and is beneficial in terms of decreased blood transfusion requirements; earlier postoperative recovery, allowing earlier discharge from hospital, return to normal activity and return to work; and improvement in health‐related quality of life. We highlighted that positive resection margins at histopathological examination after surgery represent a surrogate outcome, and we have included this to explore whether positive resection margins after surgery are responsible for any differences in survival or mortality.
We included studies that met the inclusion criteria irrespective of whether they reported our secondary outcomes.
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published and unpublished RCTs and non‐randomised studies and to identify potential studies in all languages. We translated non‐English language papers and assessed them for potential inclusion in the review as necessary.
We searched the following electronic databases to identify potential studies.
The Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 6) (Appendix 1).
MEDLINE (1966 to June 2015) (Appendix 2).
EMBASE (1988 to June 2015) (Appendix 3).
Science Citation Index (1982 to June 2015) (Appendix 4).
We also conducted a search of ClinicalTrials.gov (ClinicalTrials.gov; Appendix 5) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en/; Appendix 6) on 20 June 2015.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies.
We searched PubMed for errata or retractions from eligible trials (www.ncbi.nlm.nih.gov/pubmed) on 14 December 2015.
Data collection and analysis
Selection of studies
Two review authors (D Riviere and K Gurusamy) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports, and two review authors (D Riviere and K Gurusamy) independently screened these reports, identified studies for inclusion and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion and identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram and Characteristics of excluded studies table.
Data extraction and management
We used a standard data collection form that had been piloted on at least one study in the review to record study characteristics and outcome data. Two review authors (D Riviere and K Gurusamy) extracted study characteristics from included studies and detailed them in a Characteristics of included studies table. We extracted the following study characteristics.
Methods: study design, total study duration and run‐in, number of study centres and locations, study settings, withdrawals, date of study.
Participants: number, mean age, age range, gender, American Society of Anesthesiologists (ASA) status (ASA 2014), inclusion criteria, exclusion criteria.
Interventions: intervention, comparison, concomitant interventions.
Outcomes: primary and secondary outcomes specified and collected, time points reported.
Notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (D Riviere and K Gurusamy) independently extracted outcome data from included studies. If outcomes were reported multiple times for the same time frame, for example, if short‐term health‐related quality of life was reported at six weeks and at three months, we chose the later time point (i.e. three months) for data extraction. For time‐to‐event outcomes for which data were censored, we extracted data to calculate the natural logarithm of the hazard ratio (HR) and its standard error using the methods suggested by Parmar et al. (Parmar 1998).
We included all randomised participants for medium‐term and long‐term outcomes (e.g. mortality, quality of life), and this will not be conditional upon short‐term outcomes (e.g. being alive at three months, having a low or high quality‐of‐life index at three months).
We noted in the Characteristics of included studies table whether outcome data ware reported in an unuseable way. We resolved disagreements by consensus. One review author (D Riviere) copied data from the data collection form into Review Manager 5 (RevMan 2014). We double‐checked that the data were entered correctly by comparing study reports versus how the data were presented in the systematic review.
Assessment of risk of bias in included studies
Two review authors (D Riviere and K Gurusamy) independently assessed risk of bias for each study. We planned to use the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, because randomised controlled trials on the topic were insufficient, we used relevant risk of bias domains from 'A Cochrane Risk Of Bias Assessment Tool: for Non‐Randomized Studies of Interventions' (ACROBAT‐NRSI) (Sterne 2014).
We assessed risk of bias according to the following domains.
Bias due to confounding.
Bias due to selection of participants.
Bias due to departure from intended intervention.
Bias in measurement of outcomes.
Bias due to missing data.
Bias in selection of reported findings.
We resolved disagreements by discussion.
We graded each potential source of bias as critical, serious, moderate, low or no information and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from a participant‐reported pain scale). When information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to each outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratio (OR) and continuous data as mean difference (MD) when the outcome was reported or was converted to the same units in all trials (e.g. hospital stay). We planned to calculate standardised mean difference (SMD) when different scales were used for measuring the outcome (e.g. quality of life) and planned to ensure that higher scores for continuous outcomes have the same meaning for the particular outcome, explain the direction to the reader and report when the directions were reversed, if this was necessary. We planned to calculate the rate ratio (RaR) for outcomes such as adverse events and serious adverse events, when it was possible for the same person to develop more than one adverse event (or serious adverse event). If study authors had calculated the RaR of adverse events (or serious adverse events) in the intervention versus control based on Poisson regression, we planned to obtain the RaR by the Poisson regression method in preference to RaR calculated on the basis of the number of adverse events (or serious adverse events) that occurred during a certain period. We calculated the HR for time‐to‐event outcomes such as long‐term mortality.
We undertook meta‐analyses only when this was meaningful (i.e. when treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
Trialists commonly indicate when they have skewed data by reporting medians and interquartile ranges. When we encountered this, we planned to note that the data were skewed by following the rough guide for identifying skewed distribution available in the Cochrane Handbook for Systematic Reviews of Interventions and considered the implication of this.
When multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. laparoscopic distal pancreatectomy method 1 vs open pancreatectomy, laparoscopic distal pancreatectomy method 2 vs open pancreatectomy) must be entered into the same meta‐analysis, we planned to half the control group to avoid double‐counting. The alternative way of including such trials with multiple arms is to pool the results of laparoscopic distal pancreatectomy method 1 and laparoscopic distal pancreatectomy method 2 and compare these with open pancreatectomy. We planned to perform a sensitivity analysis to determine whether results of the two methods of dealing with multi‐arm trials led to different conclusions. However, we found no study with more than two arms that could be included in this review.
Unit of analysis issues
The unit of analysis was the individual participant undergoing distal pancreatectomy. As expected, we found no cluster‐randomised trials for this comparison.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). If we were not able to obtain the information from investigators or study sponsors, we imputed mean from median (i.e. considered median as the mean) and calculated standard deviation from standard error, interquartile range or P value according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), but we assessed the impact of including such studies as indicated in a sensitivity analysis. Standard deviation could be calculated from P values; therefore, we did not impute standard deviation as the highest standard deviation in remaining trials included in the outcome.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity as per the Cochrane Handbook for Systematic Reviews of Interventions (> 50% to 60%; Higgins 2011), we planned to explore this through prespecified subgroup analysis).
Assessment of reporting biases
We attempted to contact study authors to ask them to provide missing outcome data. When this was not possible, and when missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by using a sensitivity analysis.
If we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible publication biases. We used Egger's test to determine the statistical significance of the reporting bias (Egger 1997). We considered a P value less than 0.05 as statistically significant reporting bias.
Data synthesis
We performed analyses using Review Manager 5 (RevMan 2014). We calculated 95% confidence intervals for the treatment effect and used the Mantel‐Haenszel method for dichotomous data, the inverse variance method for continuous data and generic inverse variance for time‐to‐event data. We planned to use the inverse variance method for count data. We used both fixed‐effect (Demets 1987) and random‐effects models (DerSimonian 1986) for the analysis. In case of discrepancy between the two models, we reported both results; otherwise, we reported only results from the fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table by using all selected outcomes. We used the five GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to the meta‐analyses for prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and GRADEpro software. We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid the reader's understanding of the review when necessary. We considered whether any additional outcome information was provided that we were unable to incorporate into meta‐analyses, and we planned to note this in the comments and state whether it supports or contradicts information derived from the meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
People with different anaesthetic risk (ASA I (a healthy person) or II (a person with mild systemic disease) vs ASA III or greater (a person with severe systemic disease or worse)).
Different body mass index (BMI) (healthy weight (BMI 18.5 to 25) vs overweight or obese (BMI ≥ 25)).
Use of fibrin sealants versus no use of fibrin sealants.
Stapler closure versus suture closure of pancreatic stump.
We used all primary outcomes in the subgroup analyses.
We planned to use the formal Chi2 test for subgroup differences to test for subgroup interactions.
Sensitivity analysis
We planned to perform sensitivity analysis defined a priori to assess the robustness of our conclusions by:
excluding trials at unclear or high risk of bias (≥ 1 risk of bias domain (other than blinding of surgeon) classified as unclear or high);
excluding trials in which either mean or standard deviation or both are imputed;
excluding cluster RCTs in which adjusted effect estimates are not reported; and
using different methods of dealing with multi‐arm trials (see Measures of treatment effect).
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of studies included in this review. We avoided making recommendations for practice and believe that our implications for research will give the reader a clear sense of where the focus of any future research in the area should be and will reveal remaining uncertainties.
Results
Description of studies
Results of the search
We identified 2340 references through electronic searches of The Cochrane Library (Wiley) (n = 1), MEDLINE (OvidSP) (n = 650), EMBASE (OvidSP) (n = 1382), Science Citation Index Expanded (n = 488), ClinicalTrials.gov (n = 2) and the World Health Organization (WHO) Trials Register (n = 7). After duplicate references were removed, 1596 references remained. We excluded 1505 clearly irrelevant references by reading the abstracts. We retrieved from the full publication a total of 91 references for further detailed assessment. We excluded 76 references (62 studies) for the reasons listed in the Characteristics of excluded studies table. Fifteen references reporting 12 non‐randomised studies fulfilled the inclusion criteria (Characteristics of included studies). The reference flow is shown in Figure 1.
1.

Study flow diagram.
Included studies
We included a total of 12 non‐randomised studies (Braga 2015; Ceppa 2013; Dancea 2012; Hu 2014; Kooby 2010; Lee 2015; Rehman 2014; Sharpe 2015; Shin 2015; Stauffer 2015; Vijan 2010; Zhang 2014). All 12 were retrospective studies (Braga 2015; Ceppa 2013; Dancea 2012; Hu 2014; Kooby 2010; Lee 2015; Rehman 2014; Sharpe 2015; Shin 2015; Stauffer 2015; Vijan 2010; Zhang 2014). Nine studies were single institutional studies (Ceppa 2013; Dancea 2012; Hu 2014; Lee 2015; Rehman 2014; Shin 2015; Stauffer 2015; Vijan 2010; Zhang 2014). Two were multi‐centre studies (Kooby 2010; Sharpe 2015). It was not clear whether one study was a single‐centre or a multi‐centre study (Braga 2015). Nine were cohort studies (Ceppa 2013; Dancea 2012; Hu 2014; Lee 2015; Rehman 2014; Sharpe 2015; Shin 2015; Stauffer 2015; Zhang 2014), and the remaining three were case‐control studies (Braga 2015; Kooby 2010; Vijan 2010).
Only one study reported ASA status (Shin 2015). Most participants in this study belonged to ASA I and II. Only one participant with ASA IV was included in this study (Shin 2015). This study did not report outcome data separately by ASA status. None of the studies reported individuals with healthy weight versus overweight or obese participants. Fibrin sealant was not used routinely, or its use was not reported in any of the studies. Two studies routinely used stapler closure (Shin 2015; Zhang 2014). Information on stapler use was not available for the remaining studies.
Investigators in four studies used four ports to perform laparoscopic distal pancreatectomy (Hu 2014; Rehman 2014; Vijan 2010; Zhang 2014). Information on the number of ports was not available for the remaining studies. Four studies included participants who underwent distal pancreatectomy with or without splenectomy (Braga 2015; Hu 2014; Vijan 2010; Zhang 2014). The remaining studies did not state whether they included participants who underwent distal pancreatectomy with splenectomy. Two studies routinely placed one or more drains (Braga 2015; Hu 2014). One study reported selective drain use (Vijan 2010). Information on drain use was not available for the remaining studies.
The 12 studies included a total of 1593 participants. One study excluded 17 patients (metastatic disease (n = 12) and conversion to open procedure (n = 5)) (Shin 2015). After these 17 patients were excluded, a total of 1576 participants underwent laparoscopic distal pancreatectomy (n = 394) or open distal pancreatectomy (n = 1182). One study did not report any outcomes of interest for this review (Stauffer 2015). Upon exclusion of this study, a total of 1506 participants undergoing laparoscopic distal pancreatectomy (353 participants) or open distal pancreatectomy (1153 participants) contributed to one or more outcomes in this review. Mean or median age ranged from 50 years to 66 years in the five studies that reported this information (Hu 2014; Kooby 2010; Rehman 2014; Sharpe 2015; Shin 2015). The average proportion of females ranged from 36.7% to 72.7% in the four studies that reported this outcome (Hu 2014; Kooby 2010; Rehman 2014; Shin 2015).
The average follow‐up period was one month in one study (Braga 2015). In another study, the follow‐up period was 12 to 72 months (range) (Hu 2014). Information on the follow‐up period was not available for the remaining studies.
Outcomes reported in these studies are summarised in Characteristics of included studies.
Data were available for the entire cohort of participants who underwent laparoscopic and open distal pancreatectomy and for those who underwent laparoscopic distal pancreatectomy versus matched controls of open distal pancreatectomy in one study (Kooby 2010). We used data from the matched control analysis because long‐term mortality was available for this analysis only.
Excluded studies
We excluded 38 studies because separate data on patients with pancreatic cancer were not provided Abu Hilal 2012; Baker 2011; Baker 2013; Barrie 2014; Belli 2012; Cao 2014; Cheek 2014; Cho 2011; de Rooij 2015; DiNorcia 2010; Duran 2014; Durlik 2013; Ejaz 2014; Eom 2008; Ferrara 2014; Finan 2009; Fox 2012; Jayaraman 2010; Jeon 2014; Kang 2010; Kooby 2008; Lee 2014; Limongelli 2012; Magge 2013; Malde 2012; Matejak‐Gorska 2013; Mehta 2012; Nakamura 2009; Pieretti‐Vanmarcke 2014; Rooij 2014; Rosales‐Velderrain 2012; Sherwinter 2012; Soh 2012; Stauffer 2013; Tseng 2011; Velanovich 2006; Zhao 2010; Zibari 2014). We excluded nine studies because they excluded patients with benign or premalignant disease (Butturini 2011; Casadei 2010; Chen 2012; Chung 2014; Gumbs 2008; Matsumoto 2008; Morikawa 2012; Sahay 2011; Slepavicius 2014). We excluded seven studies because the indication for surgery was not stated (Kausar 2010; Liao 2014; Newman 2010; Parikh 2015; Stauffer 2012; Vicente 2013; Yoon 2012). Two studies did not include open distal pancreatectomy as control (Daouadi 2011; Tang 2007). One study did not include distal pancreatectomy (Langan 2014). We excluded five studies because they were reviews or provided comments (Ahmed 2015; Limongelli 2014; Mehrabi 2015; Nigri 2011; Ricci 2015).
Risk of bias in included studies
Bias due to confounding
Risk of bias due to confounding was critical in five studies (Ceppa 2013; Lee 2015; Rehman 2014; Sharpe 2015; Shin 2015) because the open distal pancreatectomy group had more extensive cancer. Risk of bias due to confounding was 'no information' for the seven remaining studies (Braga 2015; Dancea 2012; Hu 2014; Kooby 2010; Stauffer 2015; Vijan 2010; Zhang 2014). Although some studies reported no baseline differences between groups, these studies were not powered to measure baseline differences.
Bias due to selection of participants
In three studies, the decision to perform laparoscopic distal pancreatectomy or open distal pancreatectomy was based on surgeon preference (Ceppa 2013; Lee 2015; Rehman 2014). In two studies, the decision to perform laparoscopic distal pancreatectomy or open distal pancreatectomy was based on participant preference (Hu 2014; Shin 2015). One study excluded patients who underwent conversion to open surgery despite meeting inclusion criteria (Shin 2015). This study was considered to be at critical risk of bias related to selection of participants. Risk of bias was 'no information' for the remaining four of the five studies for which decisions to perform laparoscopic distal pancreatectomy or open distal pancreatectomy were based on surgeon or participant preference (Ceppa 2013; Hu 2014; Lee 2015; Rehman 2014). The criteria used to perform laparoscopic or open distal pancreatectomy were not stated in the remaining studies (Braga 2015; Dancea 2012; Kooby 2010; Sharpe 2015; Stauffer 2015; Vijan 2010; Zhang 2014), so risk of bias remains 'no information' in these studies.
Bias due to departures from intended intervention
Three studies were at moderate risk of bias; study authors replied that no differences were noted in postoperative management of participants (Ceppa 2013; Kooby 2010; Lee 2015). None of the remaining studies reported whether participant care other than laparoscopic or open procedure was identical in the two groups. These studies were classified as 'no information'.
Bias in measurement of outcomes
Three study authors replied that outcome assessors were not blinded (Ceppa 2013; Kooby 2010; Lee 2015). This might have introduced bias in measurement of outcomes other than mortality. So we classified these studies as 'no information'. Risk of bias was classified as 'no information' for the remaining studies because information on outcome assessor blinding was not reported.
Bias due to missing data
Two studies were at low risk of bias; all eligible participants were included in the study (Ceppa 2013), and a clear participant flow indicated that all participants who underwent laparoscopic or open distal pancreatectomy were included (Hu 2014). Two studies were at critical risk of bias because participants who underwent conversion to open surgery were excluded despite meeting inclusion criteria (Shin 2015), or because some participants in the open group were not matched for the laparoscopic group (Kooby 2010). It was not clear whether any participants were excluded from analysis in the remaining studies. Therefore, we classified these studies as 'no information'.
Bias in selection of reported findings
Four studies reported mortality and morbidity adequately and can be considered at low risk of bias for selective outcome reporting (Ceppa 2013; Hu 2014; Rehman 2014; Shin 2015). The remaining studies were considered to be at serious or critical risk of bias depending upon whether they did not report morbidity alone, or whether they did not report both mortality and morbidity, because one would expect that studies comparing laparoscopic distal pancreatectomy versus open distal pancreatectomy would report data on mortality and morbidity in a detailed manner.
Effects of interventions
The effect of intervention is summarised in Table 1 and Table 2.
Mortality
Nine studies reported short‐term mortality (perioperative mortality) (Braga 2015; Ceppa 2013; Hu 2014; Kooby 2010; Lee 2015; Rehman 2014; Sharpe 2015; Shin 2015; Zhang 2014). Investigators reported no statistically significant differences in short‐term mortality between the two groups (laparoscopic group: 1/329 (adjusted proportion based on meta‐analysis estimate: 0.5%) vs open group: 11/1122 (1%); OR 0.48, 95% CI 0.11 to 2.17; 1451 participants; nine studies; I2 = 0%) (Analysis 1.1). A random‐effects meta‐analysis revealed no change in results.
1.1. Analysis.

Comparison 1 Laparoscopic distal pancreatectomy versus open distal pancreatectomy, Outcome 1 Short‐term mortality.
Three studies reported long‐term mortality (Hu 2014; Kooby 2010; Shin 2015). Three‐year mortality was between 44% and 75% in these studies (Hu 2014;Kooby 2010; Shin 2015). Researchers noted no statistically significant differences in long‐term mortality between the two groups (HR 0.96, 95% CI 0.82 to 1.12; 277 participants; three studies; I2 = 0%) (Analysis 1.2). A random‐effects meta‐analysis revealed no change in results.
1.2. Analysis.

Comparison 1 Laparoscopic distal pancreatectomy versus open distal pancreatectomy, Outcome 2 Long‐term mortality.
Serious adverse events
Three studies reported the proportions of participants with serious adverse events (Hu 2014; Rehman 2014; Shin 2015). One study reported no serious adverse events (Hu 2014). Serious adverse events in the other studies included complications that required radiological or surgical re‐intervention and grade III pancreatic fistula (Rehman 2014;Shin 2015). Investigators reported no statistically significant differences in the proportions of people with serious adverse events between the laparoscopic group (7/89: adjusted proportion: 8.8%) and the open group (6/117: 5.1%) (OR 1.79, 95% CI 0.53 to 6.06; 206 participants; three studies; I2 = 0%) (Analysis 1.3). A random‐effects meta‐analysis revealed no change in results.
1.3. Analysis.
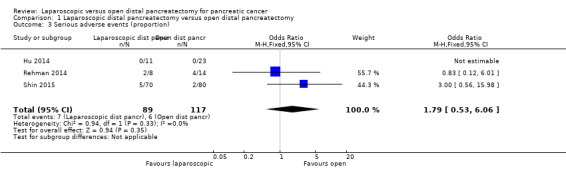
Comparison 1 Laparoscopic distal pancreatectomy versus open distal pancreatectomy, Outcome 3 Serious adverse events (proportion).
Pancreatic fistula
Four studies reported the proportions of participants with clinically significant pancreatic fistula (grade B or C) (Ceppa 2013; Hu 2014; Rehman 2014; Shin 2015). Researchers noted no statistically significant differences in the proportions of people with pancreatic fistula between the laparoscopic group (9/109: adjusted proportion: 7.7%) and the open group (9/137: 6.6%) (OR 1.19, 95% CI 0.47 to 3.02; 246 participants; four studies; I2 = 61%) (Analysis 1.4). The I2 statistic and visual inspection of forest plots provided evidence of heterogeneity, i.e. lack of overlap of confidence intervals. However, the Chi2 test for heterogeneity was not statistically significant (P value = 0.08). A random‐effects meta‐analysis revealed no change in results.
1.4. Analysis.

Comparison 1 Laparoscopic distal pancreatectomy versus open distal pancreatectomy, Outcome 4 Pancreatic fistula (grade B or C).
Quality of life
None of the studies reported quality of life at any point in time.
Recurrence
None of the studies reported recurrence within six months. Two studies reported recurrence at maximal follow‐up (Hu 2014; Shin 2015). In one study, two participants (18%) in the laparoscopic group versus 11 participants (48%) in the open group had recurrence at maximal follow‐up of 12 to 72 months (Hu 2014). In another study, 35 participants (49%) in the laparoscopic group versus 48 participants (60%) in the open group had recurrence at maximal follow‐up (follow‐up period not stated) (Shin 2015). Details were insufficient to permit calculation of the hazard ratio for recurrence. So we calculated the odds ratio of recurrence at maximal follow‐up. Results showed no statistically significant differences between groups (laparoscopic group: 37/81 (adjusted proportion based on meta‐analysis estimate: 36.3%) vs open group: 59/103 (49.5%); OR 0.58, 95% CI 0.32 to 1.05; 184 participants; two studies; I2 = 13%) (Analysis 1.5). A random‐effects meta‐analysis revealed no change in results.
1.5. Analysis.

Comparison 1 Laparoscopic distal pancreatectomy versus open distal pancreatectomy, Outcome 5 Recurrence at maximal follow‐up.
Adverse events
Four studies reported the proportions of participants with adverse events of any severity (Ceppa 2013; Hu 2014; Rehman 2014; Shin 2015). Researchers reported no statistically significant differences in the proportions of people with adverse events between the laparoscopic group (33/109: adjusted proportion: 31.7%) and the open group (45/137: 32.8%) (OR 0.95, 95% CI 0.54 to 1.66; 246 participants; four studies; I2 = 18%) (Analysis 1.6). A random‐effects meta‐analysis revealed no change in results.
1.6. Analysis.

Comparison 1 Laparoscopic distal pancreatectomy versus open distal pancreatectomy, Outcome 6 Adverse events (proportion).
Measures of earlier postoperative recovery
Five studies reported length of hospital stay (Hu 2014; Kooby 2010; Rehman 2014; Sharpe 2015; Shin 2015). The median of mean lengths of hospital stay in these studies was 9.4 days in the open distal pancreatectomy group. Mean length of hospital stay was statistically significantly shorter in the laparoscopic group than in the open group (MD ‐2.43 days, 95% CI ‐3.13 to ‐1.73; 1068 participants; five studies; I2 = 0%) (Analysis 1.7). We imputed mean and SD from median and P value for length of hospital stay for two studies (Rehman 2014; Shin 2015). No change in results occurred when we excluded these two studies (MD ‐2.25 days, 95% CI ‐3.03 to ‐1.47; 896 participants; three studies; I2 = 0%) (Analysis 3.1). A random‐effects meta‐analysis revealed no change in results.
1.7. Analysis.

Comparison 1 Laparoscopic distal pancreatectomy versus open distal pancreatectomy, Outcome 7 Length of hospital stay.
3.1. Analysis.

Comparison 3 Sensitivity analysis, Outcome 1 Length of hospital stay.
No studies reported any of the other measures of earlier postoperative recovery such as return to normal activity and return to work.
Blood transfusion requirements
None of the studies reported blood transfusion requirements.
Positive resection margins
Ten studies reported the proportions of participants with positive resection margins (Braga 2015; Dancea 2012; Hu 2014; Kooby 2010; Lee 2015; Rehman 2014; Sharpe 2015; Shin 2015; Vijan 2010; Zhang 2014). The fixed‐effect model revealed a statistically significantly lower proportion of people with positive resection margins between the two groups (laparoscopic group: 49/333 (adjusted proportion: 14.3%) vs open group: 208/1133 (18.4%); OR 0.69, 95% CI 0.48 to 1.00; 1466 participants; 10 studies; I2 = 6%) (Analysis 1.8). The random‐effects model revealed no statistically significant differences between groups in the proportions of people with positive resection margins (OR 0.74, 95% CI 0.49 to 1.10).
1.8. Analysis.
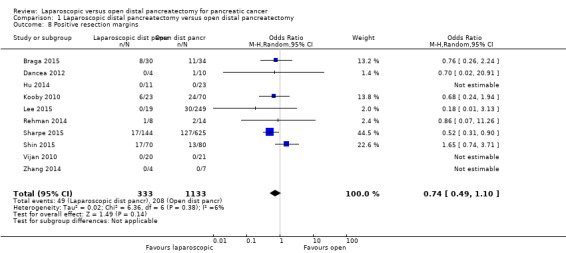
Comparison 1 Laparoscopic distal pancreatectomy versus open distal pancreatectomy, Outcome 8 Positive resection margins.
Assessment of reporting biases
We assessed reporting bias only for the positive resections margin because this was the only outcome included in 10 trials. We found no evidence of reporting bias upon visualisation of the funnel plot and completion of Egger's test (P value = 0.9798).
Subgroup analysis
Stapler closure
Stapler closure was standard procedure in two studies (Shin 2015; Zhang 2014). The remaining studies did not report whether stapler closure was performed or did not report outcome data separately for stapler closure. We found no change in the results of short‐term mortality, long‐term mortality, proportions of people with serious adverse events or clinically significant pancreatic fistula in this subgroup as compared with the main analysis (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4).
2.1. Analysis.

Comparison 2 Subgroup analysis (stapler only), Outcome 1 Short‐term mortality.
2.2. Analysis.

Comparison 2 Subgroup analysis (stapler only), Outcome 2 Long‐term mortality.
2.3. Analysis.
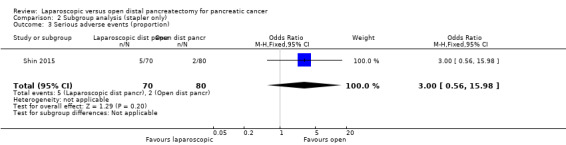
Comparison 2 Subgroup analysis (stapler only), Outcome 3 Serious adverse events (proportion).
2.4. Analysis.

Comparison 2 Subgroup analysis (stapler only), Outcome 4 Pancreatic fistula (grade B or C).
We examined no other subgroups. So we were not able to use the formal Chi2 test for differences in subgroup interactions.
Other subgroup analyses
We were not able to perform subgroup analyses of different anaesthetic risks or weights or fibrin sealants because the studies did not report this information or did not report outcome data separately for different categories.
Sensitivity analysis
We performed no other planned sensitivity analysis other than exclusion of studies in which standard deviation was calculated from the P value because no studies were at low risk of bias and we identified no cluster RCTs.
Discussion
Summary of main results
In this systematic review, we compared the benefits and harms of laparoscopic versus open distal pancreatectomy. We found no randomised controlled trials (RCTs) on this topic. We included in this review 12 observational studies that compared laparoscopic versus open distal pancreatectomy; 11 studies (1506 participants: 394 underwent laparoscopic distal pancreatectomy and 1182 open distal pancreatectomy) provided information for one or more outcomes. People with less extensive cancer underwent laparoscopic distal pancreatectomy, and those with more extensive cancer underwent open distal pancreatectomy in some studies (Ceppa 2013;Rehman 2014; Sharpe 2015). We found no statistically significant differences between laparoscopic and open distal pancreatectomy in terms of short‐term mortality, long‐term mortality, proportions of participants with serious adverse events, pancreatic fistula (grade B or C), recurrence at maximal follow‐up, proportions of participants with any adverse events and proportions of people with positive resection margins. None of the studies reported quality of life, short‐term recurrence, proportions of participants requiring blood transfusion, time to return to normal activity (return to preoperative mobility with no additional carer support) or time to return to work. Mean length of hospital stay was 2.4 days shorter in the laparoscopic distal pancreatectomy group than in the open distal pancreatectomy group. For other surgeries, laparoscopic procedures have been shown to be advantageous over open procedures in terms of fewer complications, shorter hospital stay or both (Bijen 2009; Keus 2006; Reza 2006; Walsh 2009). So the reduction in hospital stay may be due to quicker postoperative recovery resulting from the minimally invasive nature of laparoscopic surgery. It may also be due to bias to confounding, as people with less extensive cancer received laparoscopic distal pancreatectomy and those with more extensive cancer underwent open distal pancreatectomy. Differences in length of hospital stay are important only if laparoscopic distal pancreatectomy provides equivalent cancer clearance as open distal pancreatectomy. Although the confidence intervals were relatively narrow for long‐term mortality, it is not possible to conclude that laparoscopic distal pancreatectomy provides cancer clearance equivalent to that of open distal pancreatectomy because of bias due to confounding, as discussed in the Quality of the evidence section. In addition to bias, the relatively small sample size for most outcomes makes study findings unreliable on the basis of random error.
Overall completeness and applicability of evidence
The studies included in this review examined ductal adenocarcinoma of the distal pancreas and different stages (I to III) of pancreatic cancer. Hence, the findings of this review are applicable only to distal pancreatic ductal adenocarcinomas that are amenable to potentially curative surgery. One study clearly mentioned that investigators included participants classified as American Society of Anesthesiologists (ASA) stage I to IV (Shin 2015). Remaining studies did not state the ASA status of participants. In any case, all included studies examined only participants who could withstand major surgery. Hence, the findings of this review are applicable only to patients who can withstand major surgery.
Quality of the evidence
The overall quality of evidence was very low. Major reasons for this were that the studies were observational; consequently, the risk of confounding bias was unclear or high. Studies did not report baseline differences for all confounding factors, and the sample size was not sufficient to reveal differences in confounding factors. Even if the sample size was large and all confounding factors were reported, one cannot rule out the problem of residual confounding. It is not clear whether this would have introduced bias into the results.
In three studies, the decision to perform laparoscopic distal pancreatectomy or open distal pancreatectomy was based on surgeon preference (Ceppa 2013; Lee 2015; Rehman 2014). In two studies, the decision to perform laparoscopic distal pancreatectomy or open distal pancreatectomy was based on participant preference (Hu 2014; Shin 2015). Surgeon preference could be the result of the surgeon's experience with either technique, which one study author reported in the reply (Lee 2015). Also, it is quite possible that participants with less extensive cancer were operated laparoscopically or were given the choice between laparoscopic and open distal pancreatectomy, and those with more extensive cancer were operated by open surgery. Open distal pancreatectomy was associated with greater tumour size, lymph node sampling and the presence of lymph node metastasis in one study (Ceppa 2013). In another study, participants with large tumours (> 10 cm) considered difficult to mobilise laparoscopically were reserved for open resections (Rehman 2014). In a third study, more participants in the open group received neoadjuvant chemotherapy or radiation and had larger tumours (Sharpe 2015). All of these factors are associated with more advanced disease. This suggests that participants with more advanced disease had open distal pancreatectomy and those with less advanced disease underwent laparoscopic distal pancreatectomy.
Unless RCTs ensure that the same types of participants receive laparoscopic and open distal pancreatectomy, one cannot present reliable conclusions on the safety and effectiveness of laparoscopic versus open distal pancreatectomy because of residual confounding. In terms of other types of bias, many outcomes were subjective, and the retrospective nature of most of the studies means that blinding of outcome assessors is extremely unlikely, even though we have classified this risk as unclear because such information was not provided in the study reports. This may also introduce bias. Complications were not reported adequately in most studies, leading to selective outcome reporting bias.
Another factor that decreased the quality of evidence was the small sample size resulting in wide confidence intervals for many outcomes. Future studies should be adequately powered to measure differences in clinically important outcomes. Heterogeneity was not significant in the effect estimates for most outcomes despite differences in study design.
Potential biases in the review process
We planned to include only RCTs in this review. However, in the absence of any RCTs, we have reported the best available evidence on this topic. We removed the RCT filter to ensure that observational studies were not removed by electronic filters. Two review authors independently selected studies with no language restrictions and extracted data, decreasing potential errors in study selection and data extraction. However, this is a systematic review of non‐randomised studies. Mandatory registration was not required; therefore, studies showing that laparoscopic distal pancreatectomy had poorer results than open distal pancreatectomy may not have been submitted to the journals by study authors because laparoscopic distal pancreatectomy is a new procedure compared with the established treatment of open distal pancreatectomy. So we cannot rule out publication bias.
We imputed mean and calculated standard deviation from median and P values for length of hospital stay in two studies (Rehman 2014; Shin 2015). Exclusion of these two studies did not alter effect estimates for length of hospital stay, suggesting that this imputation of mean and calculation of standard deviation are unlikely to result in bias. We calculated the hazard ratio for long‐term mortality using methods suggested by Parmar et al (Parmar 1998), which assume constant proportional hazards. Kaplan‐Meier curves in these studies indicated that proportional hazards appeared constant.
Agreements and disagreements with other studies or reviews
This is the first systematic review on laparoscopic distal pancreatectomy versus open distal pancreatectomy with specific reference to pancreatic cancer. Seven study authors concluded that laparoscopic distal pancreatectomy is a safe and feasible surgical modality (Ceppa 2013; Hu 2014; Lee 2015; Rehman 2014; Sharpe 2015; Shin 2015; Zhang 2014). Four study authors suggested that laparoscopic distal pancreatectomy offers equivalent oncological outcomes (Hu 2014; Lee 2015; Rehman 2014; Sharpe 2015). Despite the statement made by one of the study authors that a randomised controlled trial comparing cancer outcomes for laparoscopic and open distal pancreatectomy for pancreatic ductal adenocarcinoma is likely to fail because of the small target patient population that would satisfy the criteria for enrolment (Kooby 2010), we agree with three study authors that a randomised controlled trial is necessary to assess the role of laparoscopic surgery in the treatment of people undergoing distal pancreatectomy (Ceppa 2013; Hu 2014; Rehman 2014).
Authors' conclusions
Implications for practice.
Currently, no randomised controlled trials have compared laparoscopic distal pancreatectomy versus open distal pancreatectomy for patients with pancreatic cancer. In observational studies, laparoscopic distal pancreatectomy is associated with shorter hospital stay as compared with open distal pancreatectomy. However, this association is unlikely to be causal. Currently no available information has revealed a causal association in the differences between laparoscopic versus open distal pancreatectomy.
Implications for research.
Future studies should try to address as many issues mentioned below as possible. The rationale for the study design is mentioned alongside.
Study design: randomised controlled trial (only a randomised controlled trial can establish a causal association in this situation).
Participants: people with potentially resectable distal pancreatic cancer (stages I and II adenocarcinoma of the pancreas) fit to undergo major surgery. Alternatively, people undergoing distal pancreatectomy for benign or malignant pancreatic disease but stratified according to benign or malignant pancreatic lesions.
Intervention: laparoscopic distal pancreatectomy.
Control: open distal pancreatectomy.
Outcomes: important patient‐oriented measures such as short‐term mortality and long‐term mortality (at least two to three years), health‐related quality of life, complications and the sequelae of complications, resection margins, measures of earlier postoperative recovery such as length of hospital stay, time to return to normal activity and time to return to work (for those who are employed) and recurrence of cancer. In addition, information on resource use can be collected if the purpose was cost‐effectiveness in addition to effectiveness.
Two to three years of follow‐up has been suggested because three‐year mortality was between 44% and 75% in these studies (Hu 2014;Kooby 2010; Shin 2015) .
Other aspects of study design:
observer‐blinded randomised controlled trial: to control for selection bias and detection bias;
identical care apart from laparoscopic versus open distal pancreatectomy: to control for performance bias; and
inclusion of all participants in the analysis and performance of an intention‐to‐treat analysis: to control for attrition bias.
Acknowledgements
We thank Karin Dearness, Managing Editor, Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group, for providing administrative and logistical support during the conduct of the current review, and Racquel Simpson, Trials Search Co‐ordinator, Cochrane UGPD Group, for developing and executing the search strategies.
We thank peer reviewers, copy editors and the Cochrane Editorial Unit for their comments.
We thank the study authors who provided further information.
Appendices
Appendix 1. CENTRAL search strategy
#1 (pancreas)
#2 (pancrea*)
#3 MeSH descriptor: [Pancreas] explode all trees
#4 #1 or #2 or #3
#5 MeSH descriptor: [Carcinoma] this term only
#6 MeSH descriptor: [Adenocarcinoma] this term only
#7 MeSH descriptor: [Carcinoma, Ductal] this term only
#8 MeSH descriptor: [Neoplasms] explode all trees
#9 (cancer* or carcin* or neoplas* or tumo* or cyst* or growth* or adenocarcin* or malig*)
#10 #5 or #6 or #7 or #8 or #9
#11 #4 and #10
#12 Pancreatectomy
#13 MeSH descriptor: [Pancreatectomy] explode all trees
#14 #12 or #13
#15 (laparoscopy or laparoscopic)
#16 MeSH descriptor: [Laparoscopy] explode all trees
#17 #15 or #16
#18 #11 and #14 and #17
Appendix 2. MEDLINE search strategy
1. (pancreas or pancrea*).mp.
2. exp Pancreas/
3. 1 or 2
4. Carcinoma/
5. Adenocarcinoma/
6. Carcinoma, Ductal/
7. exp Neoplasms/
8. (cancer* or carcin* or neoplas* or tumo* or cyst* or growth* or adenocarcin* or malig*).mp.
9. 4 or 5 or 6 or 7 or 8
10. 3 and 9
11. Pancreatectomy.mp.
12. exp Pancreatectomy/
13. 11 or 12
14. (laparoscopy or laparoscopic).mp.
15. exp Laparoscopy/
16. 14 or 15
17. 13 and 16
18. 10 and 17
Appendix 3. EMBASE search strategy
1. (pancreas or pancrea*).mp.
2. exp pancreas/
3. 1 or 2
4. carcinoma/ or adenocarcinoma/ or carcinoma, ductal/
5. exp neoplasms/
6. (cancer* or carcin* or neoplas* or tumo* or cyst* or growth* or adenocarcin* or malig*).mp.
7. 4 or 5 or 6
8. 3 and 7
9. Pancreatectomy.mp.
10. exp Pancreatectomy/
11. 9 or 10
12. (laparoscopy or laparoscopic).mp.
13. exp laparoscopy/
14. 12 or 13
15. 11 and 14
16. 8 and 15
Appendix 4. Science Citation Index search strategy
#1 TS=(pancreas or pancrea*)
#2 TS=(cancer* or carcin* or neoplas* or tumo* or cyst* or growth* or adenocarcin* or malig*)
#3 TS=(Pancreatectomy)
#4 TS=(laparoscopy or laparoscopic)
#5 #4 AND #3 AND #2 AND #1
Appendix 5. ClinicalTrials.gov search strategy
"Interventional" [STUDY‐TYPES] AND pancreatic cancer [DISEASE] AND laparoscopic distal pancreatectomy [TREATMENT] AND ( "Phase 2" OR "Phase 3" OR "Phase 4" ) [PHASE]
Appendix 6. WHO ICTRP search strategy
Distal pancreatectomy AND laparoscop*
Data and analyses
Comparison 1. Laparoscopic distal pancreatectomy versus open distal pancreatectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term mortality | 9 | 1451 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.11, 2.17] |
| 2 Long‐term mortality | 3 | 277 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.82, 1.12] |
| 3 Serious adverse events (proportion) | 3 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.53, 6.06] |
| 4 Pancreatic fistula (grade B or C) | 4 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.47, 3.02] |
| 5 Recurrence at maximal follow‐up | 2 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.05] |
| 6 Adverse events (proportion) | 4 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.54, 1.66] |
| 7 Length of hospital stay | 5 | 1068 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐3.13, ‐1.73] |
| 8 Positive resection margins | 10 | 1466 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.49, 1.10] |
Comparison 2. Subgroup analysis (stapler only).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term mortality | 2 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 9.38] |
| 2 Long‐term mortality | 1 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.67, 1.15] | |
| 3 Serious adverse events (proportion) | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.56, 15.98] |
| 4 Pancreatic fistula (grade B or C) | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.31 [0.84, 13.01] |
Comparison 3. Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | 3 | 896 | Mean Difference (IV, Random, 95% CI) | ‐2.25 [‐3.03, ‐1.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Braga 2015.
| Methods | Study design: case‐control study with propensity score matching | |
| Participants | Country: Italy
Number eligible: 64
Number excluded: not stated
Number analysed: 64
Average age: not stated
Females: not stated
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: not clear
Period of recruitment: 2010 to 2013
Follow‐up in months: 1 Inclusion criteria Patients with pancreatic cancer (adenocarcinoma) undergoing distal pancreatectomy Note: The study included patients without pancreatic adenocarcinoma who were excluded from the analysis Exclusion criteria 1. Borderline resectable cancer 2. Cardiovascular dysfunction 3. Respiratory dysfunction 4. BMI > 35 5. Refusal to consent to laparoscopy |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 30) Further details: number of ports: not stated; with or without splenectomy; 1 drain placed routinely Group 2: open distal pancreatectomy (n = 34) Further details: not stated | |
| Outcomes | Outcomes reported were mortality and resection margins | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Comment: Study authors used propensity score matching for matching laparoscopic and open groups. Although the presence of malignancy was considered a factor in the matching, the size of the tumour and involvement of adjacent structures were not considered in the matching |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: It was not clear whether eligible patients were excluded from the report |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: This information was not available |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: This information was not available |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available |
| Bias in selection of the reported findings | High risk |
Serious risk of bias Comment: Complications were not reported in participants with pancreatic cancer |
Ceppa 2013.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: USA
Number eligible: 40
Number excluded: not stated
Number analysed: 40
Average age: not stated
Females: not stated
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: single centre; Indiana University School of Medicine, Indianapolis, USA
Period of recruitment: 2005 to 2012
Follow‐up in months: not stated Inclusion criteria Patients with pancreatic adenocarcinoma undergoing distal pancreatectomy |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 20) Further details: not stated Group 2: open distal pancreatectomy (n = 20) Further details: not stated The choice of laparoscopic vs open method was based on surgeon preference | |
| Outcomes | Outcomes reported were short‐term mortality and complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | High risk |
Critical risk of bias Quote: "Open DP was associated with greater tumor size (4.3 +/‐ 0.4 cm vs. 2.9 +/‐ 0.3 cm), lymph node sampling (18 +/‐ 2 vs. 13 +/‐ 2) and presence of lymph node metastasis (80% vs. 25%)" Comment: Participants undergoing open distal pancreatectomy had more extensive cancer |
| Bias due to selection of participants to intervention and control | Low risk |
Moderate risk of bias Comment: All eligible participants were included |
| Bias due to differences in co‐interventions which were different between the groups | Low risk |
Moderate risk of bias Quote: "The postoperative care for these patients was and is identical. So no differences in how the patients are managed postoperatively (author replies)" |
| Bias in the measurement of outcomes | High risk |
Critical risk of bias Quote: "the assessors were not blinded (author replies)" |
| Bias due to missing data | Low risk |
Low risk of bias Comment: Participants with pancreatic cancer who underwent distal pancreatectomy were not excluded from the analysis (author replies) |
| Bias in selection of the reported findings | Low risk |
Low risk of bias Comment: Mortality and complications were reported |
Dancea 2012.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: USA
Number eligible: 14
Number excluded: not stated
Number analysed: 14
Average age: not stated
Females: not stated
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: single centre; Geisinger Medical Center, Danville, USA
Period of recruitment: 1999 to 2011
Follow‐up in months: not stated Inclusion criteria Patients with pancreatic malignancy undergoing distal pancreatectomy Notes: The study included patients without pancreatic malignancy who were excluded from the analysis |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 4) Further details: not stated Group 2: open distal pancreatectomy (n = 10) Further details: not stated | |
| Outcomes | The outcome reported was resection margins | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Comment: This information was not available |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: It was not clear whether eligible participants were excluded from the report |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: This information was not available |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: This information was not available |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available |
| Bias in selection of the reported findings | High risk |
Critical risk of bias Comment: Mortality and complications were not reported |
Hu 2014.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: China
Number eligible: 34
Number excluded: not stated
Number analysed: 34
Average age: 50 years
Females: 14 (41.2%)
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: single centre; The General Hospital of Chinese People’s Liberation Army, Beijing, China
Period of recruitment: 2007 to 2011
Follow‐up in months: 12 to 72 (range) Inclusion criteria 1. Patients with resectable distal pancreatic cancer undergoing distal pancreatectomy 2. Tumour size < 4 cm Exclusion criteria 1. Involvement of the superior mesenteric artery 2. Requirement for an extended resection 3. Previous history of upper abdominal surgery 4. Serious cardiopulmonary or hepatorenal insufficiency |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 11) Further details: 4 ports; with or without splenectomy; 2 drains were placed Group 2: open distal pancreatectomy (n = 23) Further details: not stated The choice of laparoscopic or open method was made at the sole discretion of the participant | |
| Outcomes | Outcomes reported were short‐term and long‐term mortality, complications, operating time, length of hospital stay, recurrence at maximal follow‐up | |
| Notes | Adjuvant treatment: Two‐cycle gemcitabine was given 1 month after distal pancreatectomy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Quote: "The choice of either technique was at the sole discretion of the patient....... The two groups were comparable in terms of age, sex, body mass index, American Society of Anesthesiology classification, Eastern Cooperative Oncology Group grading, tumor size, location and staging, CA 19‐9 levels, previous history of abdominal surgery, and concomitant medical/surgical conditions (all P[0.05))" Comment: The sample size was small and was not powered to identify baseline differences between groups |
| Bias due to selection of participants to intervention and control | Unclear risk |
Moderate risk of bias Comment: All eligible participants were included |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: This information was not available |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: This information was not available |
| Bias due to missing data | Low risk |
Low risk of bias Comment: A clear participant flow indicated that all participants who underwent laparoscopic or open distal pancreatectomy were included |
| Bias in selection of the reported findings | Low risk |
Low risk of bias Comment: Mortality and complications were reported |
Kooby 2010.
| Methods | Study design: retrospective case‐control study | |
| Participants | Country: USA
Number eligible: 93
Number excluded: not stated
Number analysed: 93
Average age: 65 years
Females: 55 (59.1%)
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: 26 (28%)
Study setting: multi‐centre USA; USA
Period of recruitment: 2000 to 2008
Follow‐up in months: not stated Inclusion criteria Patients undergoing distal pancreatectomy for pancreatic cancer Exclusion criteria 1. Adenocarcinoma with a background of intraductal papillary mucinous neoplasm or mucinous cystadenocarcinoma 2. Insufficient demographic, operative and outcomes data available for analysis and reporting |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 23) Further details: not stated Group 2: open distal pancreatectomy (n = 70) Further details: not stated Of 23 participants who underwent laparoscopic distal pancreatectomy, 4 underwent hand access procedures and another 4 procedures had to be converted to open procedures | |
| Outcomes | Outcomes reported were long‐term mortality, operating time, length of hospital stay and positive margins | |
| Notes | Adjuvant therapy (use of preoperative or postoperative chemotherapy with or without radiation therapy): laparoscopic distal pancreatectomy (n = 13), open distal pancreatectomy (n = 45) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Quote: "Three factors were used to select patients for the matched cohort comparison: age (years), ASA status (1 to 4), and tumor size (cm)". "We excluded patients who needed vascular resection and other organ resections (author replies)" Comment: Tumours were not matched for all known confounding factors, for example, lymph node status, body mass index |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: We were not able to assess this information because control participants were excluded because they did not match intervention participants |
| Bias due to differences in co‐interventions which were different between the groups | Low risk |
Moderate risk of bias Quote: "No difference in the care between patients apart from laparoscopic or open approach (author replies)" |
| Bias in the measurement of outcomes | High risk |
Critical risk of bias Outcome assessors were not blinded Quote: "..this was a retrospective study (author replies)" |
| Bias due to missing data | High risk |
Critical risk of bias Comment: Several participants were excluded because complete data were not available, and because some participants in the open group were not matched for the laparoscopic group |
| Bias in selection of the reported findings | High risk |
Critical risk of bias Comment: Short‐term mortality and complications were not reported |
Lee 2015.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: USA
Number eligible: 268
Number excluded: not stated
Number analysed: 268
Average age: not stated
Females: not stated
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: single centre; Memorial Sloan Kettering Cancer Center, New York, USA
Period of recruitment: 2000 to 2013
Follow‐up in months: not stated Inclusion criteria Patients with pancreatic malignancy undergoing distal pancreatectomy Notes: The study included patients without pancreatic malignancy who were excluded from the analysis Exclusion criteria Patients with additional organ resection |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 19) Further details: not stated Group 2: open distal pancreatectomy (n = 249) Further details: not stated | |
| Outcomes | The outcome reported was resection margins | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | High risk |
Critical risk of bias Quote: "Selection was purely by individual surgeon. Some us do more MIS, some do more open. In the MIS group some of us do robotics and some laparoscopy. The only exclusion criteria is portal vein involvement – none of us will do a case minimally invasive if we think this may be what we find (author replies)" Comment: It appears that the cancer involvement of adjacent tissues was greater in the open group than in the laparoscopic group |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: It was not clear whether eligible participants were excluded from the report |
| Bias due to differences in co‐interventions which were different between the groups | Low risk |
Moderate risk of bias Quote: "No other difference in the care – they all follow the same general pathways (author replies)" |
| Bias in the measurement of outcomes | High risk |
Critical risk of bias Quote: "Complications are filled into our database prospectively, so no one is blinded (author replies)" |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available |
| Bias in selection of the reported findings | High risk |
Serious risk of bias Comment: Complications were not reported |
Rehman 2014.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: UK
Number eligible: 22
Number excluded: not stated
Number analysed: 22
Average age: 64 years
Females: 16 (72.7%)
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: single centre; Freeman Hospital, Newcastle upon Tyne, UK
Period of recruitment: 2008 to 2011 (another report with a larger number of participants was included for resection margins and operating time; the period of recruitment was 2005 to 2012)
Follow‐up in months: not stated Inclusion criteria Patients undergoing distal pancreatectomy for pancreatic adenocarcinoma Exclusion criteria Patients with > 10 cm tumour were excluded in laparoscopic group and underwent open distal pancreatectomy |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 8) Further details: 4 ports; splenectomy: not stated; drains: not stated Group 2: open distal pancreatectomy (n = 14) Further details: not stated The choice of laparoscopic versus open method was based on the surgeon who operated on the participant | |
| Outcomes | Outcomes reported were short‐term mortality, complications, operating time, positive resection margins and length of hospital stay | |
| Notes | Additionally 5 participants in the laparoscopic distal pancreatectomy group and 8 in the open distal pancreatectomy group received adjuvant chemotherapy Significant overlap of participants was noted between the Rehman 2014 reference and the Rehman 2013 reference. We have obtained information from the Rehman 2014 reference in full text; the Rehman 2013 reference was a conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | High risk |
Critical risk of bias Quote: "In general patients with large tumours (> 10 cm) considered difficult to mobilise laparoscopically were reserved for open resections" |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: It was not clear whether eligible participants were excluded from the report |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: This information was not available |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: This information was not available |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available |
| Bias in selection of the reported findings | Low risk |
Low risk of bias Comment: Mortality and complications were reported |
Sharpe 2015.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: USA
Number eligible: 769
Number excluded: not stated
Number analysed: 769
Average age: 66 years
Females: not stated
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: National Cancer Data Base (USA) (Joint Project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society; captures information from approximately 1500 Commission on Cancer‐accredited hospitals and more than 70% of all newly diagnosed malignancies in the United States)
Period of recruitment: 2010 and 2011
Follow‐up in months: not stated Inclusion criteria Adults (18 years or older) undergoing distal pancreatectomy for pancreatic adenocarcinoma Exclusion criteria Metastatic disease or concomitant cancer diagnosis |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 144) Further details: not stated Group 2: open distal pancreatectomy (n = 625) Further details: not stated | |
| Outcomes | Outcomes reported were short‐term mortality, resection margins and length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | High risk |
Critical risk of bias Quote: "More patients in the open group received neoadjuvant chemotherapy (11% vs 2%, P < .001) or radiation (6% vs 2%, P =.049). Patients in the open group had larger tumours than those in the laparoscopic group (4.2 ± 3.2 vs 3.7 ± 1.9 cm, P = .018)" |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: It was not clear whether eligible participants were excluded from the report |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: This information was not available |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: This information was not available |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available |
| Bias in selection of the reported findings | High risk |
Serious risk of bias Comment: Complications were not reported |
Shin 2015.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: South Korea Number eligible: 167 Number excluded: 17 (10.2%) Number analysed: 150 Average age: 63 years Females: 55 (36.7%) ASA I or II: 133(88.7%) ASA III or IV: 17 (11.3%) Stapler closure: 150 (100%) Fibrin sealant: not stated Mean BMI: not stated Study setting: single centre; Asan Medical Center, Seoul, South Korea Period of recruitment: 2006 to 2013 Follow‐up in months: not stated | |
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 70) Further details: not stated Group 2: open distal pancreatectomy (n = 80) Further details: not stated The choice of laparoscopic or open method was made at the sole discretion of the participant | |
| Outcomes | Outcomes reported were short‐term and long‐term mortality, recurrence, complications, operating time, resection margins and hospital stay | |
| Notes | Adjuvant chemotherapy: laparoscopic distal pancreatectomy (n = 55) and open distal pancreatectomy (n = 55) Reasons for exclusions: metastatic disease (12) and conversion to open procedure (5) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | High risk |
Critical risk of bias Comment: Tumour size was smaller in laparoscopic group |
| Bias due to selection of participants to intervention and control | High risk |
Critical risk of bias Comment: People who met eligibility criteria were excluded because they underwent conversion to open surgery |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: This information was not available |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: This information was not available |
| Bias due to missing data | High risk |
Critical risk of bias Comment: Participants who met eligibility criteria were excluded. This could have affected the outcome |
| Bias in selection of the reported findings | Low risk |
Low risk of bias Comment: Mortality and complications were reported |
Stauffer 2015.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: USA
Number eligible: 70
Number excluded: not stated
Number analysed: 70
Average age: not stated
Females: not stated
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: single centre; Mayo Clinic Florida, Jacksonville, USA
Period of recruitment: not stated
Follow‐up in months: not stated Inclusion criteria Patients undergoing distal pancreatectomy for pancreatic adenocarcinoma |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 41) Further details: not stated Group 2: open distal pancreatectomy (n = 29) Further details: not stated | |
| Outcomes | None of the outcomes of interest were reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Comment: This information was not available |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: It was not clear whether eligible participants were excluded from the report |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: This information was not available |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: This information was not available |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available |
| Bias in selection of the reported findings | High risk |
Critical risk of bias Comment: Mortality and complications were not reported |
Vijan 2010.
| Methods | Study design: case‐control study | |
| Participants | Country: USA
Number eligible: 41
Number excluded: not stated
Number analysed: 41
Average age: not stated
Females: not stated
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: not stated
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: single centre; Mayo Clinic, Rochester, USA
Period of recruitment: 2004 to 2009
Follow‐up in months: not stated Inclusion criteria Patients with pancreatic malignancy undergoing distal pancreatectomy Notes: The study included participants without pancreatic malignancy who were excluded from the analysis |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 20) Further details: 4 ports; with or without splenectomy; selective closed suction drain Group 2: open distal pancreatectomy (n = 21) | |
| Outcomes | The outcome reported was resection margins | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Quote: "Following institutional review board approval, 100 patients undergoing LDP were matched by patient age (± 8 years), pathologic diagnosis (benign vs malignant), and pancreatic specimen length (± 2 cm) to a cohort (n = 100) undergoing ODP" Comment: Matching does not take all confounding factors into account, for example, stage of tumour |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: It was not clear whether eligible participants were excluded from the report |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: This information was not available |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: This information was not available |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available |
| Bias in selection of the reported findings | High risk |
Critical risk of bias Comment: Mortality and complications were not reported |
Zhang 2014.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: China
Number eligible: 11
Number excluded: not stated
Number analysed: 11
Average age: not stated
Females: not stated
ASA I or II: not stated
ASA III or IV: not stated
Stapler closure: 11 (100%)
Fibrin sealant: not stated
Mean BMI: not stated
Study setting: single centre; The Third Affiliated Hospital of Soochow University, Changzhou, China
Period of recruitment: 2009 to 2013
Follow‐up in months: not stated Inclusion criteria Patients with pancreatic malignancy undergoing distal pancreatectomy Notes: The study included participants without pancreatic malignancy who were excluded from the analysis |
|
| Interventions | Group 1: laparoscopic distal pancreatectomy (n = 4) Further details: 4 ports; with or without splenectomy; drain use not stated Group 2: open distal pancreatectomy (n = 7) | |
| Outcomes | Outcomes reported were short‐term mortality and resection margins | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Comment: This information was not available |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: It was not clear whether eligible participants were excluded from the report |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: This information was not available |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: This information was not available |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available |
| Bias in selection of the reported findings | High risk |
Serious risk of bias Comment: Complications were not reported |
Abbreviations:
ASA: American Society of Anethesiologists
BMI: body mass index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu Hilal 2012 | No separate data available on people with pancreatic cancer |
| Ahmed 2015 | Review |
| Baker 2011 | No separate data available on people with pancreatic cancer |
| Baker 2013 | No separate data available on people with pancreatic cancer |
| Barrie 2014 | Includes metastatic renal carcinoma |
| Belli 2012 | No separate data available on people with pancreatic cancer |
| Butturini 2011 | Study performed in people with benign or premalignant lesions |
| Cao 2014 | No separate data available on people with pancreatic cancer |
| Casadei 2010 | Study performed in people with benign or premalignant lesions |
| Cheek 2014 | No separate data available on people with pancreatic cancer |
| Chen 2012 | Study performed in people with benign or premalignant lesions |
| Cho 2011 | No separate data available on people with pancreatic cancer |
| Chung 2014 | Study performed in people with benign or premalignant lesions |
| Dancea 2012a | No separate data available on people with pancreatic cancer |
| Daouadi 2011 | No open distal pancreactectomy as control group |
| de Rooij 2015 | No separate data available on people with pancreatic cancer |
| DiNorcia 2010 | No separate data available on people with pancreatic cancer |
| Duran 2014 | No separate data available on people with pancreatic cancer |
| Durlik 2013 | No separate data available on people with pancreatic cancer |
| Ejaz 2014 | No separate data available on people with pancreatic cancer |
| Eom 2008 | No separate data available on people with pancreatic cancer |
| Ferrara 2014 | No separate data available on people with pancreatic cancer |
| Finan 2009 | No separate data available on people with pancreatic cancer |
| Fox 2012 | No separate data available on people with pancreatic cancer |
| Gumbs 2008 | Study performed in people with benign or premalignant lesions |
| Jayaraman 2010 | No separate data available on people with pancreatic cancer |
| Jeon 2014 | No separate data available on people with pancreatic cancer |
| Kang 2010 | No separate data available on people who underwent laparoscopic distal pancreatectomy |
| Kausar 2010 | Indication for surgery not stated |
| Kooby 2008 | No separate data available on people with pancreatic cancer |
| Langan 2014 | Not on distal pancreatectomy |
| Lee 2014 | No separate data available on people who underwent laparoscopic distal pancreatectomy |
| Liao 2014 | Indication for surgery not stated |
| Limongelli 2012 | No separate data available on people with pancreatic cancer |
| Limongelli 2014 | Comment on an excluded study (Cho 2011) |
| Magge 2013 | No separate data available on people who underwent laparoscopic distal pancreatectomy |
| Malde 2012 | No separate data available on people with pancreatic cancer |
| Matejak‐Gorska 2013 | No separate data available on people with pancreatic cancer |
| Matsumoto 2008 | Study performed in people with benign or premalignant lesions |
| Mehrabi 2015 | Review |
| Mehta 2012 | No separate data available on people with pancreatic cancer |
| Morikawa 2012 | Study performed in people with benign lesions |
| Nakamura 2009 | No separate data available on people with pancreatic cancer |
| Newman 2010 | Indication for surgery not stated |
| Nigri 2011 | Review |
| Parikh 2015 | Indication for surgery not stated |
| Pieretti‐Vanmarcke 2014 | No separate data available on people with pancreatic cancer |
| Ricci 2015 | Review |
| Rooij 2014 | No separate data available on people with pancreatic cancer |
| Rosales‐Velderrain 2012 | No separate data available on people with pancreatic cancer |
| Sahay 2011 | Study performed in people with non‐cancerous lesions |
| Sherwinter 2012 | No separate data available on people with pancreatic cancer |
| Slepavicius 2014 | Study performed in people with benign and borderline lesions |
| Soh 2012 | No separate data available on people with pancreatic cancer |
| Stauffer 2012 | Indication for surgery not stated |
| Stauffer 2013 | No separate data available on people with pancreatic cancer |
| Tang 2007 | No control group of open distal pancreatectomy |
| Tseng 2011 | No separate data available on people with pancreatic cancer |
| Velanovich 2006 | No separate data available on people with pancreatic cancer |
| Vicente 2013 | Indication for surgery not stated |
| Yoon 2012 | Indication for surgery not stated |
| Zhao 2010 | No separate data available on people with pancreatic cancer |
| Zibari 2014 | No separate data available on people who underwent laparoscopic distal pancreatectomy |
Differences between protocol and review
-
No randomised controlled trials were identified; therefore, we included non‐randomised studies to provide the current best available evidence. As a result, we made the following modifications to the protocol.
We did not use the filter for randomised controlled trials during electronic searches of the databases.
We used 'A Cochrane Risk Of Bias Assessment Tool: for Non‐Randomized Studies of Interventions' (ACROBAT‐NRSI) for assessment of risk of bias, rather than the standard Cochrane 'Risk of bias' tool for randomised controlled trials.
We planned to calculate the risk ratio for binary outcomes. However, because case‐control studies were included, we calculated the odds ratio because it is not possible to calculate the baseline risk in case‐control studies.
We planned to calculate the hazard ratio for long‐term recurrence. However, data were not available in the format required for calculating the hazard ratio. So we calculated the odds ratio.
Contributions of authors
Conceiving of the review: KG.
Designing the review: KG.
Co‐ordinating the review: KG, CVL.
Designing search strategies: KG.
Extracting data: DR, KG.
Analysing data: DR, KG.
Writing the review: DR, KG.
Providing critical comments on the review: DK, CV, MB, BRD, CVL.
Securing funding for the review: KG, BRD.
Performing previous work that served as the foundation of the current study: KG, BRD.
Sources of support
Internal sources
University College London, UK.
External sources
National Institute for Health Research, UK.
Declarations of interest
This report constitutes independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the review author(s) and are not necessarily those of the National Health Service (NHS), the National Institute for Health Research or the Department of Health.
DR: none known.
KSG: none known.
DAK: received partial sponsorship from Intuitive Surgical (makers of the da Vinci Surgical System) to attend a training course on robotic pancreatic surgery.
CMV: none known.
MGHB: none known.
BRD: none known.
CJHMvL: has written chapters for UpToDate, which are not related to this review.
New
References
References to studies included in this review
Braga 2015 {published data only}
- Braga M, Pecorelli N, Ferrari D, Balzano G, Zuliani W, Castoldi R. Results of 100 consecutive laparoscopic distal pancreatectomies: postoperative outcome, cost‐benefit analysis, and quality of life assessment. Surgical Endoscopy 2015;29(7):1871‐8. [DOI] [PubMed] [Google Scholar]
Ceppa 2013 {published data only}
- Ceppa EP, McCurdy RM, Parikh JA, Kilbane EM, Schmidt CM, Zyromski NJ, et al. Contemporary differences in postoperative outcomes following laparoscopic versus open distal pancreatectomy for pancreatic adenocarcinoma. HPB 2013;15:10. [Google Scholar]
Dancea 2012 {published data only}
- Dancea HC, Obradovic VN, Woll NL, Shabahang MM, Blansfield JA. Minimally invasive distal pancreatectomy improves outcomes compared to open at a rural tertiary care center. Surgical Endoscopy and Other Interventional Techniques 2012;26:S351. [Google Scholar]
Hu 2014 {published data only}
- Hu M, Zhao G, Wang F, Zhao Z, Li C, Liu R. Laparoscopic versus open distal splenopancreatectomy for the treatment of pancreatic body and tail cancer: a retrospective, mid‐term follow‐up study at a single academic tertiary care institution. Surgical Endoscopy and Other Interventional Techniques 2014;28(9):2584‐91. [DOI] [PubMed] [Google Scholar]
Kooby 2010 {published data only}
- Kooby DA, Hawkins WG, Schmidt CM, Weber SM, Bentrem DJ, Gillespie TW, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate?. Journal of the American College of Surgeons 2010;210(5):779‐85. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee SY, Allen PJ, Sadot E, D'Angelica MI, DeMatteo RP, Fong Y, et al. Distal pancreatectomy: a single institution's experience in open, laparoscopic, and robotic approaches. Journal of the American College of Surgeons 2015;220(1):18‐27. [DOI] [PubMed] [Google Scholar]
Rehman 2014 {published data only}
- Rehman S, John SK, Lochan R, Jaques BC, Manas DM, Charnley RM, et al. Oncological feasibility of laparoscopic distal pancreatectomy for adenocarcinoma: a single‐institution comparative study. World Journal of Surgery 2014;38(2):476‐83. [DOI] [PubMed] [Google Scholar]
- Rehman S, Lochan R, French JJ, Jaques BC, Manas DM, White SA. Oncological feasibility of laparoscopic distal pancreatectomy for adenocarcinoma of distal pancreas: a single‐institution comparative study. British Journal of Surgery 2013;100(S4):22. [DOI] [PubMed] [Google Scholar]
Sharpe 2015 {published data only}
- Sharpe SM, Talamonti MS, Wang E, Bentrem DJ, Roggin KK, Prinz RA, et al. The laparoscopic approach to distal pancreatectomy for ductal adenocarcinoma results in shorter lengths of stay without compromising oncologic outcomes. American Journal of Surgery 2015;209(3):557‐63. [DOI] [PubMed] [Google Scholar]
Shin 2015 {published data only}
- Nam J, Song KB, Lee YJ, Park KM, Lee JH, Hwang JW, et al. Comparison of outcomes between laparoscopic distal pancreatectomy and open distal pancreatectomy for pancreatic cancer (pdac). Pancreatology 2013;13(4 Suppl):S9‐S10. [Google Scholar]
- Nam JS, Kim SC, Song KB, Park KM, Lee JH, Hwang JW, et al. Comparison of short term outcomes between laparoscopic distal pancreatectomy and open distal pancreatectomy for pancreatic cancer. HPB 2013;15:64. [Google Scholar]
- Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Lee D, et al. A comparative study of laparoscopic vs. open distal pancreatectomy for left‐sided ductal adenocarcinoma: a propensity score‐matched analysis. Journal of the American College of Surgeons 2015;220(2):177‐85. [DOI] [PubMed] [Google Scholar]
Stauffer 2015 {published data only}
- Stauffer J, Coppola A, Asbun HJ. Pancreatic surgery for pancreatic adenocarcinoma: a comparison between the laparoscopic and open surgical approach. Gastroenterology 2015;148(4 Suppl):S1166‐S7. [Google Scholar]
Vijan 2010 {published data only}
- Vijan SS, Ahmed KA, Harmsen WS, Que FG, Reid‐Lombardo KM, Nagorney DM, et al. Laparoscopic vs open distal pancreatectomy: a single‐institution comparative study. Archives of Surgery 2010;145(7):616‐21. [DOI] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang Y, Chen XM, Sun DL. Laparoscopic versus open distal pancreatectomy: a single‐institution comparative study. World Journal of Surgical Oncology 2014;12:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abu Hilal 2012 {published data only}
- Abu Hilal M, Hamdan M, Fabio F, Pearce NW, Johnson CD. Laparoscopic versus open distal pancreatectomy: a clinical and cost‐effectiveness study. Surgical Endoscopy 2012;26(6):1670‐4. [DOI] [PubMed] [Google Scholar]
Ahmed 2015 {published data only}
- Ahmed R, Walsh CM, Makary MA. Laparoscopic distal pancreatectomy. Clinical Liver Disease 2015;5(3):51‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2011 {published data only}
- Baker MS, Bentrem DJ, Ujiki MB, Stocker S, Talamonti MS. Adding days spent in readmission to the initial postoperative length of stay limits the perceived benefit of laparoscopic distal pancreatectomy when compared with open distal pancreatectomy. American Journal of Surgery 2011;201(3):295‐9; discussion 299‐300. [DOI] [PubMed] [Google Scholar]
Baker 2013 {published data only}
- Baker M, Sherman KL, Hayman AV, Prinz R, Bentrem DJ, Talamonti M. Defining quality for distal pancreatectomy: does the laparoscopic approach protect patients from poor quality outcomes?. Gastroenterology 2012;1):S1078‐S9. [DOI] [PubMed] [Google Scholar]
- Baker MS, Sherman KL, Stocker S, Hayman AV, Bentrem DJ, Prinz RA, et al. Defining quality for distal pancreatectomy: does the laparoscopic approach protect patients from poor quality outcomes?. Journal of Gastrointestinal Surgery 2013;17(2):273‐80. [DOI] [PubMed] [Google Scholar]
Barrie 2014 {published data only}
- Barrie J, Deshpande R, O'Reilly D, Carino ND, Ammori B. Laparoscopic versus open distal pancreatectomy for the treatment of pre‐malignant and malignant lesions. British Journal of Surgery 2014;101(S6):22. [Google Scholar]
- Barrie J, Deshpande R, O'Reilly D, Liguori Carino N, Ammori BJ. Laparoscopic versus open distal pancreatectomy for the treatment of pre‐malignant and malignant lesions. Pancreas 2014;43(8):1343‐4. [Google Scholar]
Belli 2012 {published data only}
- Belli G, Fantini C, D'Agostino A, Limongelli P, Cioffi L, Belli A, et al. Laparoscopic and open distal pancreatectomy for left sided pancreatic lesions. Surgical Endoscopy and Other Interventional Techniques 2012;26:S41. [DOI] [PubMed] [Google Scholar]
- Belli G, Limongelli P, Gostino D, Fantini C, Cioffi L, Belli A, et al. Laparoscopic and open distal pancreatectomy for left‐sided pancreatic lesions. HPB 2011;13:105‐6. [Google Scholar]
Butturini 2011 {published data only}
- Butturini G, Partelli S, Crippa S, Malleo G, Casetti L, Melotti GL, et al. Resume of work after distal pancreatectomy: laparoscopic approach is better. A single institution comparative study on 114 patients. Pancreas 2009;38(8):986. [Google Scholar]
- Butturini G, Partelli S, Crippa S, Malleo G, Rossini R, Casetti L, et al. Perioperative and long‐term results after left pancreatectomy: a single‐institution, non‐randomized, comparative study between open and laparoscopic approach. Surgical Endoscopy 2011;25(9):2871‐8. [DOI] [PubMed] [Google Scholar]
Cao 2014 {published data only}
- Cao HST, Lopez N, Chang DC, Lowy AM, Bouvet M, Baumgartner JM, et al. Improved perioperative outcomes with minimally invasive distal pancreatectomy results from a population‐based analysis. JAMA Surgery 2014;149(3):237‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casadei 2010 {published data only}
- Casadei R, Ricci C, D'Ambra M, Marrano N, Alagna V, Rega D, et al. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: a case‐control study. Updates in Surgery 2010;62(3‐4):171‐4. [DOI] [PubMed] [Google Scholar]
Cheek 2014 {published data only}
- Cheek S, Patel S, Osman H, Jeyarajah D. The benefits of laparoscopic pancreatectomy: it does make a difference. HPB 2014;16(S1):71. [Google Scholar]
Chen 2012 {published data only}
- Chen J, Jin G, Shao C, Cai X, Shao Z, Hao J, et al. Comparative study of laparoscopic and open distal pancreatectomy. Academic Journal of Second Military Medical University 2012;33(9):996‐1001. [Google Scholar]
Cho 2011 {published data only}
- Cho CS, Kooby DA, Schmidt CM, Nakeeb A, Bentrem DJ, Merchant NB, et al. Laparoscopic versus open left pancreatectomy: can preoperative factors indicate the safer technique?. Annals of Surgery 2011;253(5):975‐80. [DOI] [PubMed] [Google Scholar]
Chung 2014 {published data only}
- Chung JC, Kim HC, Song OP. Laparoscopic distal pancreatectomy for benign or borderline malignant pancreatic tumors. Turkish Journal of Gastroenterology 2014;25(Suppl 1):S162‐6. [DOI] [PubMed] [Google Scholar]
Dancea 2012a {published data only}
- Dancea HC, Obradovic VN, Woll NL, Shabahang MM, Blansfield JA. Minimally invasive distal pancreatectomy improves outcomes compared to open at a rural tertiary care center. Surgical Endoscopy and Other Interventional Techniques 2012;26:S351. [Google Scholar]
Daouadi 2011 {published data only}
- Daouadi M, Steel JL, Choudry MH, Magge DR, Zureikat AH, Bartlett DL, et al. Outcomes of minimally‐invasive distal pancreatectomy: single institution comparison of robotic and laparoscopic methods. HPB 2011;13:15‐6. [Google Scholar]
de Rooij 2015 {published data only}
- Rooij T, Jilesen AP, Boerma D, Bonsing BA, Bosscha K, Dam RM, et al. A nationwide comparison of laparoscopic and open distal pancreatectomy for benign and malignant disease. Journal of the American College of Surgeons 2015;220(3):263‐70.e1. [DOI] [PubMed] [Google Scholar]
DiNorcia 2010 {published data only}
- DiNorcia J, Schrope BA, Lee MK, Reavey PL, Lee JA, Chabot JA, et al. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. Gastroenterology 2010;138(5 Suppl):S875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNorcia J, Schrope BA, Lee MK, Reavey PL, Rosen SJ, Lee JA, et al. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. Journal of Gastrointestinal Surgery 2010;14(11):1804‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duran 2014 {published data only}
- Duran H, Ielpo B, Caruso R, Ferri V, Quijano Y, Diaz E, et al. Does robotic distal pancreatectomy surgery offer similar results as laparoscopic and open approach? A comparative study from a single medical center. International Journal of Medical Robotics and Computer Assisted Surgery 2014;10(3):280‐5. [DOI] [PubMed] [Google Scholar]
Durlik 2013 {published data only}
- Durlik M, Matejak‐Gorska M, Jaworowski R, Kaszycka Z, Baumgart K. Laparoscopic distal pancreatectomy ‐ new standard in the pancreatic surgery. Polski Przeglad Chirurgiczny 2013;85(10):589‐97. [DOI] [PubMed] [Google Scholar]
Ejaz 2014 {published data only}
- Ejaz A, Sachs T, He J, Spolverato G, Hirose K, Ahuja N, et al. A comparison of open and minimally invasive surgery for hepatic and pancreatic resections using the nationwide inpatient sample. Surgery 2014;156(3):538‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eom 2008 {published data only}
- Eom BW, Jang JY, Lee SE, Han HS, Yoon YS, Kim SW. Clinical outcomes compared between laparoscopic and open distal pancreatectomy. Surgical Endoscopy 2008;22(5):1334‐8. [DOI] [PubMed] [Google Scholar]
Ferrara 2014 {published data only}
- Ferrara MJ, Croome KP, Farnell MB, Que FG, Reid Lombardo KM, Truty MJ, et al. Readmission and prolonged hospital stay after distal pancreatectomy: advantage laparoscopic approach. Gastroenterology 2014;146(5 Suppl):S‐1049. [Google Scholar]
Finan 2009 {published data only}
- Finan KR, Cannon EE, Kim EJ, Wesley MM, Arnoletti PJ, Heslin MJ, et al. Laparoscopic and open distal pancreatectomy: a comparison of outcomes. American Surgeon 2009;75(8):671‐9. [PubMed] [Google Scholar]
Fox 2012 {published data only}
- Fox AM, Pitzul K, Bhojani F, Kaplan M, Moulton CA, Wei AC, et al. Comparison of outcomes and costs between laparoscopic distal pancreatectomy and open resection at a single center. Surgical Endoscopy 2012;26(5):1220‐30. [DOI] [PubMed] [Google Scholar]
- Fox AM, Pitzul KB, Bhojani FD, Kaplan M, Moulton CA, Wei A, et al. Outcomes and costs of laparoscopic distal pancreatectomy: comparison to open resection in a single centre. Surgical Endoscopy and Other Interventional Techniques 2011;25:S218. [DOI] [PubMed] [Google Scholar]
Gumbs 2008 {published data only}
- Gumbs AA, Gres P, Madureira FA, Gayet B. Laparoscopic vs. open resection of noninvasive intraductal pancreatic mucinous neoplasms. Journal of Gastrointestinal Surgery 2008;12(4):707‐12. [DOI] [PubMed] [Google Scholar]
Jayaraman 2010 {published data only}
- Jayaraman S, Gonen M, Brennan MF, D'Angelica MI, DeMatteo RP, Fong Y, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. Journal of the American College of Surgeons 2010;211(4):503‐9. [DOI] [PubMed] [Google Scholar]
Jeon 2014 {published data only}
- Jeon J, Jung J, Kim D, Kim J, Chon S, Hwang J. Comparison of open with laparoscopic distal pancreatectomy in low volume center. HPB 2014;16(S2):618‐9. [Google Scholar]
Kang 2010 {published data only}
- Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left‐sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surgical Endoscopy 2010;24(7):1533‐41. [DOI] [PubMed] [Google Scholar]
Kausar 2010 {published data only}
- Kausar A, Ajala‐Agbo T, O'Reilly D, Sherlock D, Ammori BJ. Laparoscopic versus open distal pancreatectomy: a comparative case‐matched study. HPB 2010;12:201. [Google Scholar]
- Kausar A, Sherlock D, O'Reilly D, Ammori B. Laparoscopic versus open distal pancreatectomy: a comparative casematched study. Pancreatology 2010;10(2‐3):289‐90. [Google Scholar]
Kooby 2008 {published data only}
- Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, et al. Left‐sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Annals of Surgery 2008;248(3):438‐46. [DOI] [PubMed] [Google Scholar]
Langan 2014 {published data only}
- Langan RC, Graham JA, Chin AB, Rubinstein AJ, Oza K, Nusbaum JA, et al. Laparoscopic‐assisted versus open pancreaticoduodenectomy: early favorable physical quality‐of‐life measures. Surgery 2014;156(2):379‐84. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee SH, Hwang HK, Choi SH, Lee WJ, Kang CM. Minimally invasive ramps in well‐selected left‐sided pancreatic cancer with yonsei criteria: long‐term (>median 3 years) oncologic outcome. Pancreatology 2013;13(4 Suppl):S73. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kang CM, Hwang HK, Choi SH, Lee WJ. Minimally invasive ramps in well‐selected left‐sided pancreatic cancer with yonsei criteria: long‐term (>median 3 years) oncologic outcome. HPB 2014;16(S2):312. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kang CM, Hwang HK, Choi SH, Lee WJ, Chi HS. Minimally invasive ramps in well‐selected left‐sided pancreatic cancer within yonsei criteria: long‐term (>median 3 years) oncologic outcomes. Surgical Endoscopy 2014;28(10):2848‐55. [DOI] [PubMed] [Google Scholar]
Liao 2014 {published data only}
- Liao CH, Yeh CN, Yang SJ, Wang SY, Ouyang CH, Tsai CY, et al. Effectiveness and feasibility of laparoscopic distal pancreatectomy on patients at high anesthetic risk. Journal of Laparoendoscopic & Advanced Surgical Techniques 2014;24(12):865‐71. [DOI] [PubMed] [Google Scholar]
Limongelli 2012 {published data only}
- Limongelli P, Belli A, Russo G, Cioffi L, D'Agostino A, Fantini C, et al. Laparoscopic and open surgical treatment of left‐sided pancreatic lesions: clinical outcomes and cost‐effectiveness analysis. Surgical Endoscopy 2012;26(7):1830‐6. [DOI] [PubMed] [Google Scholar]
Limongelli 2014 {published data only}
- Limongelli P, Belli A, Belli G. Laparoscopic versus open left pancreatectomy: do risk factors for pancreatic fistula differ between the 2 techniques?. Annals of Surgery 2014;259(3):e39. [DOI] [PubMed] [Google Scholar]
Magge 2013 {published data only}
- Magge D, Choudry HA, Steel J, Zureikat A, Hughes S, Bartlett D, et al. Oncologic outcomes following minimally‐invasive distal pancreatectomy for pancreatic adenocarcinoma: matched comparison with open resection. Annals of Surgical Oncology 2011;18:S133‐S4. [Google Scholar]
- Magge D, Gooding W, Choudry H, Steve J, Steel J, Zureikat A, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surgery 2013;148(6):525‐31. [DOI] [PubMed] [Google Scholar]
Malde 2012 {published data only}
- Malde DJ, Khaled Y, Packer J, Liguori N, Deshpande R, O'Reilly D, et al. A comparative study of laparoscopic versus open distal pancreatectomy. Pancreatology 2013;13(1):e4. [DOI] [PubMed] [Google Scholar]
- Malde DJ, Khaled Y, Packer J, Liguori N, Deshpande R, O'Reilly D, et al. A comparative study of laparoscopic vs open distal pancreatectomy. Gut 2012;61:A113‐A4. [Google Scholar]
Matejak‐Gorska 2013 {published data only}
- Matejak‐Gorska M, Durlik M. Laparoscopic distal pancreatectomy ‐ "the new gold standard". Pancreatology 2013;13(3 Suppl):S50. [DOI] [PubMed] [Google Scholar]
Matsumoto 2008 {published data only}
- Matsumoto T, Shibata K, Ohta M, Iwaki K, Uchida H, Yada K, et al. Laparoscopic distal pancreatectomy and open distal pancreatectomy: a nonrandomized comparative study. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2008;18(4):340‐3. [DOI] [PubMed] [Google Scholar]
Mehrabi 2015 {published data only}
- Mehrabi A, Hafezi M, Arvin J, Esmaeilzadeh M, Garoussi C, Emami G, et al. A systematic review and meta‐analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: it's time to randomize. Surgery 2015;157(1):45‐55. [DOI] [PubMed] [Google Scholar]
Mehta 2012 {published data only}
- Mehta SS, Doumane G, Mura T, Nocca D, Fabre JM. Laparoscopic versus open distal pancreatectomy: a single‐institution case‐control study. Surgical Endoscopy 2012;26(2):402‐7. [DOI] [PubMed] [Google Scholar]
Morikawa 2012 {published data only}
- Morikawa T, Naitoh T, Kakyo M, Tanaka N, Watanabe K, Onogawa T, et al. Clinical outcomes compared between laparoscopic and open distal pancreatectomy: a retrospective cohort study. Surgical Endoscopy and Other Interventional Techniques 2012;26:S340. [Google Scholar]
Nakamura 2009 {published data only}
- Nakamura Y, Uchida E, Aimoto T, Matsumoto S, Yoshida H, Tajiri T. Clinical outcome of laparoscopic distal pancreatectomy. Journal of Hepato‐Biliary‐Pancreatic Surgery 2009;16(1):35‐41. [DOI] [PubMed] [Google Scholar]
Newman 2010 {published data only}
- Newman N, Edil BH, Makary MA. Lymph node resection in laparoscopic distal pancreatectomy is equivalent to the open operation. Annals of Surgical Oncology 2010;17:S27. [Google Scholar]
Nigri 2011 {published data only}
- Nigri GR, Rosman AS, Petrucciani N, Fancellu A, Pisano M, Zorcolo L, et al. Metaanalysis of trials comparing minimally invasive and open distal pancreatectomies. Surgical Endoscopy and Other Interventional Techniques 2011;25(5):1642‐51. [DOI] [PubMed] [Google Scholar]
Parikh 2015 {published data only}
- Parikh J, Anantha Sathyanarayana S, Bendix S, Jacobs MJ. The impact of minimally invasive distal pancreatectomy on 90‐day readmissions and cost: is it any better than open?. HPB 2015;17(S1):65. [Google Scholar]
Pieretti‐Vanmarcke 2014 {published data only}
- Pieretti‐Vanmarcke R, Wargo J, Fernandez‐Del Castillo C, Zheng H, Merrill A, Thayer S, et al. Outcomes in open versus laparoscopic distal pancreatectomy. HPB 2014;16(S1):72. [Google Scholar]
Ricci 2015 {published data only}
- Ricci C, Casadei R, Taffurelli G, Toscano F, Pacilio CA, Bogoni S, et al. Laparoscopic versus open distal pancreatectomy for ductal adenocarcinoma: a systematic review and meta‐analysis. Journal of Gastrointestinal Surgery 2015;19(4):770‐81. [DOI] [PubMed] [Google Scholar]
Rooij 2014 {published data only}
- Rooij TD, Jilesen A, Kazemier G, Boerma D, Bonsing B, Bosscha K, et al. Laparoscopic versus open distal pancreatectomy for benign and malignant disease: a multicenter retrospective analysis. Pancreatology 2014;14(3 Suppl 1):S9‐S10. [Google Scholar]
Rosales‐Velderrain 2012 {published data only}
- Rosales‐Velderrain A, Bowers SP, Goldberg RF, Clarke TM, Buchanan M, Stauffer J, et al. National trends in resection of the distal pancreas. Gastroenterology 2012;142(5 Suppl):S1061‐S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales‐Velderrain A, Bowers SP, Goldberg RF, Clarke TM, Buchanan MA, Stauffer JA, et al. National trends in resection of the distal pancreas. World Journal of Gastroenterology 2012;18(32):4342‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahay 2011 {published data only}
- Sahay SJ, Gonzalez HD, John J, Fusai G, Sharma D, Davidson BR, et al. Laparoscopic distal pancreatectomy ‐ an emerging gold standard. Pancreatology 2011;11(3):322. [Google Scholar]
Sherwinter 2012 {published data only}
- Sherwinter DA, Lewis J, Hidalgo JE, Arad J. Laparoscopic distal pancreatectomy. Journal of the Society of Laparoendoscopic Surgeons 2012;16(4):549‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slepavicius 2014 {published data only}
- Slepavicius A, Eismontas V. Clinical outcomes compered between laparoscopic and open distal pancreatectomy. HPB 2014;16(S2):688. [Google Scholar]
Soh 2012 {published data only}
- Soh YF, Kow A, Wong KY. Perioperative outcomes of laparoscopic distal pancreatectomy. Annals of the Academy of Medicine Singapore 2010;39(11 Suppl):S191. [Google Scholar]
- Soh YF, Kow AW, Wong KY, Wang B, Chan CY, Liau KH, et al. Perioperative outcomes of laparoscopic and open distal pancreatectomy: our institution's 5‐year experience. Asian Journal of Surgery 2012;35(1):29‐36. [DOI] [PubMed] [Google Scholar]
Stauffer 2012 {published data only}
- Stauffer J, Rosales‐Velderrain A, Goldberg R, Clarke T, Buchanan M, Bowers S, et al. Surrogate oncologic markers for long term survival are improved with the use of minimal access surgery for pancreatic cancer. HPB 2012;14(S2):231. [Google Scholar]
Stauffer 2013 {published data only}
- Stauffer JA, Rosales‐Velderrain A, Goldberg RF, Bowers SP, Asbun HJ. Comparison of open with laparoscopic distal pancreatectomy: a single institution's transition over a 7‐year period. HPB 2013;15(2):149‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2007 {published data only}
- Tang CN, Tsui KK, Ha JP, Wong DC, Li MK. Laparoscopic distal pancreatectomy: a comparative study. Hepato‐Gastroenterology 2007;54(73):265‐71. [PubMed] [Google Scholar]
Tseng 2011 {published data only}
- Tseng WH, Canter RJ, Bold RJ. Perioperative outcomes for open distal pancreatectomy: current benchmarks for comparison. Journal of Gastrointestinal Surgery 2011;15(11):2053‐8. [DOI] [PubMed] [Google Scholar]
Velanovich 2006 {published data only}
- Velanovich V. Case‐control comparison of laparoscopic versus open distal pancreatectomy. Journal of Gastrointestinal Surgery 2006;10(1):95‐8. [DOI] [PubMed] [Google Scholar]
Vicente 2013 {published data only}
- Vicente E. Robotic distal pancreatectomy: comparison with laparoscopic and open technique. HPB 2013;15:26. [Google Scholar]
Yoon 2012 {published data only}
- Yoon YS, Lee KH, Han HS, Cho JY, Hwang DW, Kang MJ, et al. The effect of operative approach on splenic vessel patency after spleen and splenic vessel‐preserving distal pancreatectomy in multi‐institutional study: laparoscopic versus open approach. Pancreas 2012;41(8):1415. [Google Scholar]
- Yoon YS, Lee KH, Han HS, Cho JY, Hwang DW, Kang MJ, et al. The effect of operative approach on splenic vessel patency after spleen and splenic vessel‐preserving distal pancreatectomy in multi‐institutional study: laparoscopic versus open approach. Pancreatology 2013;13(2):e6‐7. [Google Scholar]
Zhao 2010 {published data only}
- Zhao GD, Hu MG, Liu R. [A comparative study of laparoscopic fistal pancreatectomy and open distal pancreatectomy]. Nan Fang Yi Ke Da Xue Xue Bao = Journal of Southern Medical University 2010;30(12):2756‐8. [PubMed] [Google Scholar]
Zibari 2014 {published data only}
- Zibari GB, Fallahzadeh MK, Mokhtari N, Hamidian A, Chu Q, Abdehou ST, et al. Minimally invasive versus open distal pancreatectomy for treatment of pancreatic lesions: a single center experience. HPB 2014;16(S2):240‐1. [Google Scholar]
Additional references
Abrams 2009
- Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Annals of Surgical Oncology 2009;16(7):1751‐6. [DOI] [PubMed] [Google Scholar]
ASA 2014
- American Society of Anesthesiologists. ASA physical status classification system. www.asahq.org/Home/For‐Members/Clinical‐Information/ASA‐Physical‐Status‐Classification‐System (accessed 13 November 2014).
Bassi 2005
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138(1):8‐13. [DOI] [PubMed] [Google Scholar]
Bijen 2009
- Bijen CB, Vermeulen KM, Mourits MJ, Bock GH. Costs and effects of abdominal versus laparoscopic hysterectomy: systematic review of controlled trials. PLoS One 2009;4(10):e7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
British Society of Gastroenterology 2005
- Pancreatic Section British Society of Gastroenterology. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut 2005;54(Suppl 5):v1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 1993
- Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty‐five consecutive pancreaticoduodenectomies without mortality. Annals of Surgery 1993;217(5):430‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Annals of Surgery 2009;250(2):187‐96. [DOI] [PubMed] [Google Scholar]
Daouadi 2013
- Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, et al. Robot‐assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Annals of Surgery 2013;257(1):128‐32. [DOI] [PubMed] [Google Scholar]
Demets 1987
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Diener 2011
- Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, et al. Efficacy of stapler versus hand‐sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 2011;377(9776):1514‐22. [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez‐Cruz 2005
- Fernandez‐Cruz L, Orduna D, Cesar‐Borges G, Lopez‐Boado MA. Distal pancreatectomy: en‐bloc splenectomy vs spleen‐preserving pancreatectomy. HPB (Oxford) 2005;7(2):93‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez‐Cruz 2006
- Fernandez‐Cruz L. Distal pancreatic resection: technical differences between open and laparoscopic approaches. HPB (Oxford) 2006;8(1):49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghaneh 2007
- Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut 2007;56(8):1134‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2013
- Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008370.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hopkins 1999
- Hopkins MP, Dulai RM, Occhino A, Holda S. The effects of carbon dioxide pneumoperitoneum on seeding of tumor in port sites in a rat model. American Journal of Obstetrics and Gynecology 1999;181(6):1329‐34. [DOI] [PubMed] [Google Scholar]
IARC 2014
- International Agency for Research on Cancer. GLOBOCAN 2012. globocan.iarc.fr/Default.aspx (accessed 13 November 2014).
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996. [Google Scholar]
Kais 2014
- Kais H, Hershkovitz Y, Sandbank J, Halevy A. Port site metastases in squamous cell carcinoma of the gallbladder. Israel Medical Association Journal 2014;16(3):177‐9. [PubMed] [Google Scholar]
Keus 2006
- Keus F, Jong JA, Gooszen HG, Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006231] [DOI] [PubMed] [Google Scholar]
Liao 2013
- Liao WC, Chien KL, Lin YL, Wu MS, Lin JT, Wang HP, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta‐analysis. Lancet Oncology 2013;14(11):1095‐103. [DOI] [PubMed] [Google Scholar]
Livingston 1991
- Livingston EH, Welton ML, Reber HA. Surgical treatment of pancreatic cancer. The United States experience. International Journal of Pancreatology 1991;9:153‐7. [DOI] [PubMed] [Google Scholar]
Ma 2013
- Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women, 1970‐2009. Journal of the National Cancer Institute 2013;105(22):1694‐700. [DOI] [PubMed] [Google Scholar]
Montorsi 2012
- Montorsi M, Zerbi A, Bassi C, Capussotti L, Coppola R, Sacchi M, et al. Efficacy of an absorbable fibrin sealant patch (TachoSil) after distal pancreatectomy: a multicenter, randomized, controlled trial. Annals of Surgery 2012;256(5):853‐9. [DOI] [PubMed] [Google Scholar]
Niederhuber 1995
- Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer 1995;76(9):1671‐7. [DOI] [PubMed] [Google Scholar]
Nitecki 1995
- Nitecki SS, Sarr MG, Colby TV, Heerden JA. Long‐term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving?. Annals of Surgery 1995;221(1):59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orr 2010
- Orr RK. Outcomes in pancreatic cancer surgery. Surgical Clinics of North America 2010;90(2):219‐34. [DOI] [PubMed] [Google Scholar]
Palomba 2014
- Palomba S, Mandato VD, Sala GB. Isolated port‐site metastasis after robotic hysterectomy for stage IA endometrial adenocarcinoma. Obstetrics and Gynecology 2014;123(3):664. [DOI] [PubMed] [Google Scholar]
Park 2013
- Park JW, Jang JY, Kim EJ, Kang MJ, Kwon W, Chang YR, et al. Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. British Journal of Surgery 2013;100(8):1064‐70. [DOI] [PubMed] [Google Scholar]
Parkin 2001
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. European Journal of Cancer 2001;37(Suppl 8):S4‐66. [DOI] [PubMed] [Google Scholar]
Parkin 2005
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians 2005;55(2):74‐108. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reza 2006
- Reza MM, Blasco JA, Andradas E, Cantero R, Mayol J. Systematic review of laparoscopic versus open surgery for colorectal cancer. British Journal of Surgery 2006;93(8):921‐8. [DOI] [PubMed] [Google Scholar]
Rindi 2011
- Rindi G, Wiedenmann B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nature Reviews in Endocrinology 2011;8(1):54‐64. [DOI] [PubMed] [Google Scholar]
Rutz 2014
- Rutz DR, Squires MH, Maithel SK, Sarmiento JM, Etra JW, Perez SD, et al. Cost comparison analysis of open versus laparoscopic distal pancreatectomy. HPB 2014;16(10):907‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2014
- Song J, Kim E, Mobley J, Vemana G, Tanagho Y, Vetter J, et al. Port site metastasis after surgery for renal cell carcinoma: harbinger of future metastasis. Journal of Urology 2014;192(2):364‐8. [DOI] [PubMed] [Google Scholar]
Sterne 2014
- Sterne JAC, Higgins JPT, Reeves BC, on behalf of the Development Group for ACROBAT‐NRSI. A Cochrane Risk Of Bias Assessment Tool: for Non‐Randomized Studies of Interventions (ACROBAT‐NRSI), Version 1.0.0. http://www.riskofbias.info (accessed 25 November 2015).
Suzuki 1995
- Suzuki Y, Kuroda Y, Morita A, Fujino Y, Tanioka Y, Kawamura T, et al. Fibrin glue sealing for the prevention of pancreatic fistulas following distal pancreatectomy. Archives of Surgery 1995;130(9):952‐5. [DOI] [PubMed] [Google Scholar]
Talseth 2014
- Talseth A, Lydersen S, Skjedlestad F, Hveem K, Edna TH. Trends in cholecystectomy rates in a defined population during and after the period of transition from open to laparoscopic surgery. Scandinavian Journal of Gastroenterology 2014;49(1):92‐8. [DOI] [PubMed] [Google Scholar]
Trede 1990
- Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Annals of Surgery 1990;211(4):447‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tucker 2008
- Tucker ON, Rela M. Controversies in the management of borderline resectable proximal pancreatic adenocarcinoma with vascular involvement. HPB Surgery 2008;2008:839503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2009
- Walsh CA, Walsh SR, Tang TY, Slack M. Total abdominal hysterectomy versus total laparoscopic hysterectomy for benign disease: a meta‐analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2009;144(1):3‐7. [DOI] [PubMed] [Google Scholar]
Yamamoto 1998
- Yamamoto M, Ohashi O, Saitoh Y. Japan Pancreatic Cancer Registry: current status. Pancreas 1998;16(3):238‐42. [DOI] [PubMed] [Google Scholar]


