Summary
Objective:
We evaluated the impact of monitoring indication, early EEG, and clinical features on seizure risk in all neonates undergoing continuous EEG (cEEG) monitoring following a standardized monitoring protocol.
Methods:
All cEEGs from unique neonates 34–48 weeks postmenstrual age monitored from 1/2011–10/2017 (n=291) were included. We evaluated the impact of cEEG monitoring indication (acute neonatal encephalopathy [ANE], suspicious clinical events [SCE], or other high-risk conditions [OHR]), age, medication status, and early EEG abnormalities (including the presence of epileptiform discharges and abnormal background continuity, amplitude, asymmetry, asynchrony, excessive sharp transients, and burst suppression) on time to first seizure and overall seizure-risk using Kaplan-Meier survival curves and multivariable Cox-proportional hazards models.
Results:
Seizures occurred in 28% of high-risk neonates. Discontinuation of monitoring after 24 hours seizure-free would have missed 8.5% of neonates with seizures. Overall seizure risk was lower in neonates monitored for ANE compared to OHR (p=0.004) and trended lower compared to SCE (p=0.097). The time course of seizure presentation varied by group, where the probability of future seizure was less than 1% after 17 hours of seizure-free monitoring in the SCE group, but required 42 hours in the OHR group, and 73 hours in the ANE group. The presence of early epileptiform discharges increased seizure risk in each group (ANE: adjusted Hazard Ratio [aHR] 4.32, 95%CI 1.23–15.13, p=0.022; SCE: aHR 10.95, 95%CI 4.77–25.14, p <1e-07; OHR: aHR 56.90, 95%CI 10.32–313.72, p<1e-05).
Significance:
Neonates who undergo cEEG are at high risk for seizures and risk varies by monitoring indication and early EEG findings. Seizures are captured in nearly all neonates undergoing monitoring for SCE within 24 hours of cEEG monitoring. Neonates monitored for OHR and ANE can present with delayed seizures and require longer durations of monitoring. Early epileptiform discharges are the best early EEG feature to predict of seizure risk.
Keywords: continuous electroencephalogram, cEEG, critical care, NICU, electrographic seizure
Introduction
Seizures are common during the neonatal period, occurring in 1–3.5 per 1000 live births.1–4 Due to developing neurophysiology and immature movements, neonates are at high risk of subclinical seizures and misidentified seizures,5,6 necessitating use of continuous EEG (cEEG) monitoring to accurately diagnose seizures. The presence and burden of neonatal seizures predicts increased morbidity and mortality,7–11 and current standard of care is to treat seizures.5,12
In spite of the increased recognition of high seizure risk, the time course of seizure presentation among neonates undergoing cEEG is not well characterized, resulting in lack of clarity about risk and appropriate cEEG monitoring duration. Current consensus guidelines13 aim to balance the need to identify seizures in critically-ill neonates with the substantial personnel and equipment resources required to support cEEG,14 but are based on limited neonatal data. Among combined neonatal and pediatric critical care cohorts, 80–97% of seizures have been reported to occur in the first 24 hours.15–20 Delayed seizure onset beyond 24 hours has been reported in acute neonatal encephalopathy21–25 and congenital cardiac disease cohorts.26,27 In addition, younger age,28,29 and EEG background abnormalities22,30–33 are associated with increased seizure incidence; however, the impact of early EEG features on the time course of seizure presentation in high-risk neonates is unknown.
In this study, we characterize the seizure probability over time in relation to monitoring indication and early EEG features in a large population of neonates monitored following current consensus guidelines to identify features that stratify seizure risk.
Methods
Study population and study design
Standardized continuous EEG monitoring guidelines for neonates were developed by our service in 2010 consistent with the consensus based guidelines proposed in 2011.13 All neonates who underwent cEEG at Massachusetts General Hospital from January 2011 – October 2017 were reviewed (n=347). Patients were excluded if they were <34 weeks postmenstrual age (PMA) or ≥48 weeks PMA (n=8), if there was insufficient data in the medical record and the EEG was not available for primary review (n=1), or if patients were monitored for more than 1 monitoring indication (n=10). Additionally, in patients with more than one unique cEEG session during the neonatal period (n=37), only the first cEEG study was included. 291 total subjects were included for analysis. Two cEEG records had incomplete early EEG background information available due to technical reasons and were included only in applicable analyses.
Medical records were reviewed for PMA, monitoring indication, etiology, anti-seizure medication (ASM) administration prior to cEEG (including if used for sedation), and presence of therapeutic hypothermia. Preterm was defined as ≥ 34 and < 38 weeks PMA, term as ≥838 and < 44 weeks PMA, and postterm as ≥a44 and < 48 weeks PMA, in accordance with current neonatal EEG reporting standards.34 Indication for monitoring was assigned based on established neonatal monitoring guidelines1,34 and, to maintain adequate sample sizes, were grouped as: Acute neonatal encephalopathy (ANE, infants suspected of hypoxic-ischemic injury), suspicious clinical events (SCE), or all other high-risk conditions (OHR). Other high-risk conditions included all neonates monitored for any of the following risk factors: central nervous system (CNS) infection, CNS trauma, perinatal stroke, inborn error of metabolism, genetic/syndromic conditions, cardiac or pulmonary risk factors (such as extra-corporeal membrane oxygenation), sinovenous thrombosis, prematurity with additional risk factors (such as intraventricular hemorrhage), or ASM wean in a high-risk infant. As the number of neonates in each of the OHR categories was small, they were analyzed together.
Standard protocol approvals, registrations, and patient consents
This review of EEG recordings and associated medical records was carried out with the approval of the local institutional review board. Individual patient consent was not required.
EEG Data
All cEEG recordings were obtained using the international 10–20 scalp electrode convention. Recordings included electrooculogram (two channels), EEG (19 channels, Ag/AgCl electrodes placed according to the 10 –20 international system referred to a C2 spinous process reference: FP2, F4, C4, P4, O2, F8, T4, T6, Fz, Cz, Pz, Fp1, F3, C3, P3, O1, F7, T3, and T5), and electrocardiogram using a standard clinical recording system (Xltek, a subsidiary of Natus Medical). Following local practice, separate reports were generated for the initial 20–30 minutes of the EEG recording, henceforth referred to as the “early EEG”. Early EEG reports were reviewed for the presence of epileptiform discharges and each of the routinely reported neonatal EEG background features: continuity, amplitude, voltage asymmetry, asynchrony, excessive sharp transients, and burst suppression.34 As a minimum of one hour of cEEG is required to accurately identify sleep-wake cycling, this was not included in our analysis.13 Because the distinction between epileptiform discharges and normal or excessive sharp wave transients can be challenging in neonatal studies, for the purpose of this study, we defined any positive or negative sharp wave discharges that were fast (< 100 ms in duration) and / or consistently focal as epileptiform discharges. All other sharp wave discharges (e.g. multifocal and / or ≥ 100 ms in duration) were grouped as non-epileptiform sharp wave transients. In cases where there was insufficient detail or ambiguity to determine whether a discharge met criteria for a sharp transient or epileptiform activity, and for all cases when the clinical report reported epileptiform activity, the EEG tracing was reviewed by a board-certified pediatric clinical neurophysiologist and epileptologist for confirmation (C.J.C.). EEG background features were dichotomized as normal/abnormal (continuity, amplitude, and asynchrony), or present/absent (asymmetry, excessive sharp transients, and burst suppression) based on established criteria,13 corrected for PMA. The entire cEEG report was then reviewed and the presence and timing of the first electrographic seizure (rounded to the nearest minute) from the initiation of cEEG monitoring was recorded for each subject. The primary cEEG data were reviewed as necessary for incomplete data.
Statistical analysis
Data was analyzed using R Studio v.3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria).35 Descriptive statistics were used to summarize baseline characteristics. To account for variability in the duration of cEEG monitoring, seizure risk was analyzed using non-parametric Kaplan-Meier survival analysis. Patients were censored at the time of first seizure or when cEEG ended, whichever occurred sooner. Analyzing all neonates together in a single multivariable Cox proportional hazard model did not produce a good model fit, where the correlation of the scaled residuals for the global model using the Schoenfeld test was significant (p<0.05). Therefore, the impact of monitoring indication on seizure risk was evaluated using pair-wise comparisons via log-rank tests,36 and the impact of clinical and early EEG features on seizure risk were evaluated in a multivariable model for each monitoring indication.
For each monitoring indication, predictors were included in a multivariable Cox proportional hazard model to evaluate seizure risk over time using adjusted hazard ratios (aHR), with p-value significance set at <0.05. The following predictors were tested in each of the multivariable models: postmenstrual age group (preterm, term, or postterm), pretreatment with ASM, early EEG findings of abnormal amplitude, excessive discontinuity, asymmetry, asynchrony, excessive sharp transients, burst suppression, and epileptiform discharges. The appropriateness of each Cox proportional hazards model was confirmed by testing for correlations of scaled Schoenfeld residuals.
To test for collinearity of predictors in our multivariable models, we performed univariable analysis with a log rank test of each variable separately to predict seizure risk. Variables found to increase seizure risk were then systematically removed from the multivariable model to assess the impact on the predictor with the largest effect. Variables found to be collinear were removed from the multivariable model.
Because EEG features may be impacted by ASM treatment and age, we tested for possible interactions between these variables to predict seizure risk. To do so, we performed univariable analysis of the interaction between each EEG background feature and 1) age, and 2) pretreatment to predict seizure risk. Interaction variables found to increase seizure risk were then tested in the multivariable model and included if significant.
Probability curves were generated from the survival curves by computing, for each time point, the proportion of neonates that had a future seizure captured on cEEG using the following formula:
Where t is the time point at the duration of cEEG monitoring of interest.
Results
Study cohort
In this consecutive cohort of 291 patients, 101(34%) were monitored for ANE, 142 (49%) were monitored for SCE, and 48 (17%) were monitored for OHR. Early EEGs were a median of 23 minutes in duration (Interquartile range [IQR] 22–26 minutes) and the median total EEG monitoring duration was 42 hours (IQR 21–80 hours). Median cEEG monitoring duration was 84 hours (IQR 72–93) for the ANE group; 26 hours (IQR 18–44) for the SCE group, and 24 hours (IQR 19–43) for the OHR group. At the time of cEEG initiation, 82% of patients were term infants; gestational age at monitoring ranged from 34 1/7 – 47 4/7 weeks (median 40.0 weeks, IQR 38.6–41.3 weeks). Patient characteristics are summarized in Table 1. Early EEG features by monitoring indication are summarized in Table 2.
Table 1.
Patient demographics and baseline characteristics, N=291
| Female, n (%) | 126 (43%) |
| Gestational age at start of recording, median weeks (range) | 40.0 (34.1–47.6) |
| Preterma, n (%) | 33 (11%) |
| Termb, n (%) | 239 (82%) |
| Post-termc, n (%) | 19 (7%) |
| Therapeutic hypothermia, n (%) | 97 (33%) |
| Treatment with anti-seizure medication prior to cEEG, n (%) | 97 (33%) |
| Indication for cEEG | |
| Stereotyped clinical event (SCE), n (%) | 142 (49%) |
| Acute neonatal encephalopathy (ANE), n (%) | 101 (35%) |
| Other high-risk conditions (OHR), n (%) | 48 (16%) |
| Cardiac or pulmonary risk factors | 6 (2%) |
| CNS infection | 12 (4%) |
| CNS trauma | 7 (2%) |
| Genetic/syndromic disease | 9 (3%) |
| Inborn errors of metabolism (IEM) | 3 (1%) |
| Perinatal stroke | 3 (1%) |
| Premature infants with additional risk factors | 4 (1%) |
| Sinovenous thrombosis | 4 (1%) |
Preterm = 34- <37wk
Term = 37-<44 weeks
Post-term = 44- <48 weeks. cEEG = continuous EEG monitoring.
Table 2.
Early EEG background features by indication
| All | Suspicious Clinical Event | Acute Neonatal Encephalopathy | Other High-Risk Condition | |
|---|---|---|---|---|
| Total, N | 291* | 142 | 101 | 48 |
| Monitoring Duration in hr (median, range) | 42 (2–281) | 26 (3–281) | 84 (9–229) | 24 (2–212) |
| Early EEG characteristics, N (%)* | ||||
| Epileptiform Discharges | 54 (19) | 31 (22) | 11 (11) | 12 (25) |
| Abnormal Continuity | 94 (32) | 35 (25) | 47 (47) | 12 (25) |
| Abnormal Amplitude | 101 (35) | 27 (19) | 54 (53) | 20 (42) |
| Excessive Sharps | 71 (25) | 38 (27) | 21 (21) | 12 (25) |
| Voltage Asymmetry | 16 (6) | 5 (4) | 5 (5) | 6 (13) |
| Asynchrony | 29 (10) | 16 (11) | 6 (6) | 7 (15) |
| Burst Suppression | 8 (3) | 0 (0) | 6 (6) | 2 (4) |
N=290 with the exception of epileptiform discharges where N=291
Seizure risk and time course of seizure in all neonates
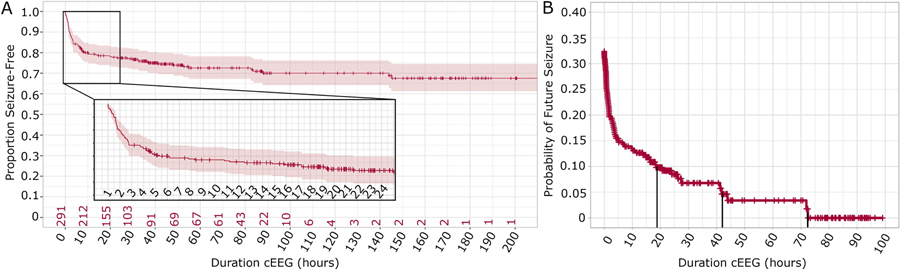
Seizures occurred in 28% (80/291) of neonates during cEEG monitoring. For all subjects, the probability of a future seizure decreased with increased duration of seizure-free cEEG monitoring (Figure 1). Consistent with prior pediatric cohorts,15–20 among all high-risk neonates, after 24 hours of seizure-free cEEG monitoring, the risk of future seizure was 8.5%. The risk of a future seizure was less than 5% only after 42 hours of seizure-free cEEG monitoring and less than 1% only after 73 hours.
Figure 1: Seizure risk and duration of continuous EEG.
Continuous (cEEG) monitoring among all high-risk neonates. (A) Results are displayed as a Kaplan-Meier survival curve (± 95% CI) with the cumulative probability of seizure plotted as a function of duration of cEEG monitoring. Hash marks indicate censored patients. Red numbers on the horizontal axis indicate the number of subjects on cEEG remaining seizure-free at the indicated time point. The first 24 hours of cEEG are shown in the inset. The proportion seizure-free decreases with duration of cEEG monitoring, but most of the neonates who will have a seizure are identified within the first few hours of monitoring. (B) The probability of future seizure is plotted as a function of duration of seizure-free cEEG monitoring. Vertical lines indicate seizure-free cEEG monitoring duration required to reach less than 10%, 5%, and 1% risk of a future seizure.
Seizure risk over time by monitoring indication
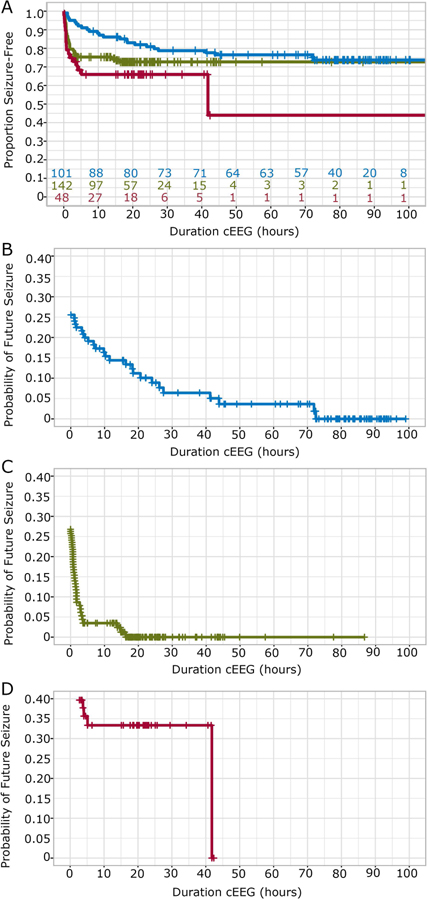
Seizure risk over time varied based on cEEG monitoring indication (Figure 2). Twenty-five of 101 (25%) neonates monitored for ANE, 38/142 (27%) neonates monitored for SCE, and 17/48 (35%) neonates monitored for OHR developed a seizure during cEEG. On pairwise comparison, accounting for monitoring duration, neonates monitored for ANE had a lower risk of seizure compared to those monitored for OHR (p=0.004) and trended towards lower risk compared to those monitored for SCE (p=0.097).
Figure 2: Seizure risk over time by monitoring indication.
(A) Kaplan-Meier survival curves for cumulative proportion seizure-free as a function of cEEG monitoring duration (hours), based on monitoring indication: acute neonatal encephalopathy (ANE: blue), suspicious clinical event (SCE; green), and other high-risk condition (OHR; red). Hash marks indicate censored patients. Colored numbers on the horizontal axis indicate the remaining neonates at risk in each group. (B-D) Corresponding probability of future seizure incidence within 100 hours of monitoring in ANE (B), SCE (C), and OHR (D) groups. Compared to other monitoring indication groups, those monitored for ANE have a slower reduction in seizure risk over time.
For each monitoring indication, the probability of future seizure decreased with increased duration of seizure-free cEEG monitoring, however the risk decayed at different rates (Figure 2B–D). Although neonates monitored due to ANE had the lowest overall seizure rate, seizures presented over a longer time-course, requiring 73 hours of seizure-free cEEG monitoring for the probability of future seizure to be only 1%. Among neonates with SCE, the probability of future seizure was less than 1% after 17 hours of seizure-free cEEG. Among neonates with OHR, the probability of future seizure was less than 1% after 42 hours of seizure-free cEEG monitoring.
Seizure risk over time by postmenstrual age, medication status and early EEG features
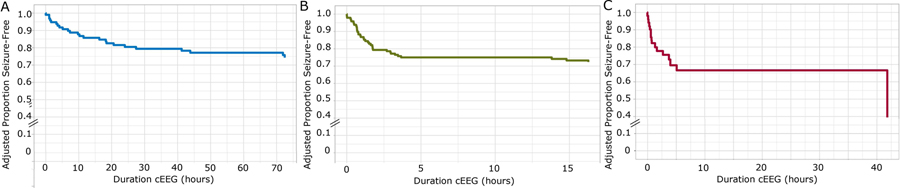
For each monitoring indication, we evaluated seizure risk over time as a dependent variable in a multivariable Cox proportional hazard model with the following predictors: post-menstrual age group (e.g., preterm, term, or postterm), pretreatment with ASM, early EEG findings of abnormal amplitude, excessive discontinuity, asymmetry, asynchrony, excessive sharp transients, burst suppression, and epileptiform discharges. The adjusted survival curves for cumulative proportion seizure-free as a function of cEEG monitoring duration and indication are shown in Figure 3.
Figure 3: Adjusted seizure risk over time by monitoring indication.
The adjusted Kaplan-Meier survival curves for cumulative proportion seizure-free as a function of cEEG monitoring duration (hours), adjusted for all covariates included in each model. (A) Acute neonatal encephalopathy (adjusted for covariates: abnormal amplitude, abnormal continuity, asymmetry, asynchrony, burst suppression, excessive sharp transients, and epileptiform activity); (B) Suspicious clinical event (adjusted for covariates: abnormal amplitude, asymmetry, burst suppression, excessive sharp transients, and epileptiform activity); (C) Other high-risk indications (adjusted for covariates: abnormal amplitude, abnormal continuity, asymmetry, asynchrony, burst suppression, excessive sharp transients, and epileptiform activity).
Model goodness of fit
On Schoenfeld test, In SCE, continuity had a significant correlation (p=0.02) and was therefore excluded from the SCE model. Subsequently, each variable included in each of the final models had a p ≥ 0.05 (ANE range [0.10–0.67], SCE range [0.15–1.0], OHR range [0.14–0.97]). For each indication, the global model did not show a significant correlation (ANE p=0.44, SCE p=0.65, OHR p=0.69), indicating acceptable model fit.
Age and medication status
Neither age nor medication status was a strong predictor of seizure risk in any of the indication groups. Univariable analyses of each predictor (Supplementary Table 1) identified pretreatment with ASM as a potential predictor of increased seizure risk (p<0.001) in neonates monitored for SCE. However, this variable did not remain significant in the multivariable model. Age did not predict seizure risk in the univariable or multivariable analyses in any of the indications groups.
EEG background features
In the multivariable models, early EEG background features only modestly influenced seizure risk, with different effects depending on group. Testing of interactions between clinical features and EEG background features did not identify any further predictors (Supplementary Table 2).
In the ANE group, in univariable analysis, no background features predicted increased seizure risk. In the multivariable model, neonates with burst suppression on early EEG had a 4.45-fold increased risk of seizure (aHR 4.45, 95%CI 1.03–19.26, p=0.046) compared to those without. In addition, in the ANE group, neonates with excessive discontinuity on early EEG had a 0.35-fold decreased risk of seizures (aHR 0.35, 95% CI 0.12–0.96, p=0.042 compared to those without.
In the SCE group, univariable analysis of EEG background features found that asynchrony (p<1e-06) and asymmetry (p=0.002) each increased risk of subsequent seizure (Supplementary Table 1). In the multivariable model, neither of these variables remained significant. Further analysis revealed collinearity of asynchrony with epileptiform discharges (impacting the aHR by 11.5%) and asynchrony was thus removed from the multivariable model (see below section, “epileptiform discharges”). Asymmetry did not demonstrate collinearity with epileptiform discharges (aHR was impacted by <0.05%). In the SCE group, neonates with excessive sharp transients on EEG had a 0.35-fold decreased risk of seizure (aHR 0.35, 95%CI 0.15–0.79, p=0.012) in the multivariable model.
In the OHR group, no background features predicted increased seizure risk in univariable analysis or in the multivariable model.
Epileptiform discharges
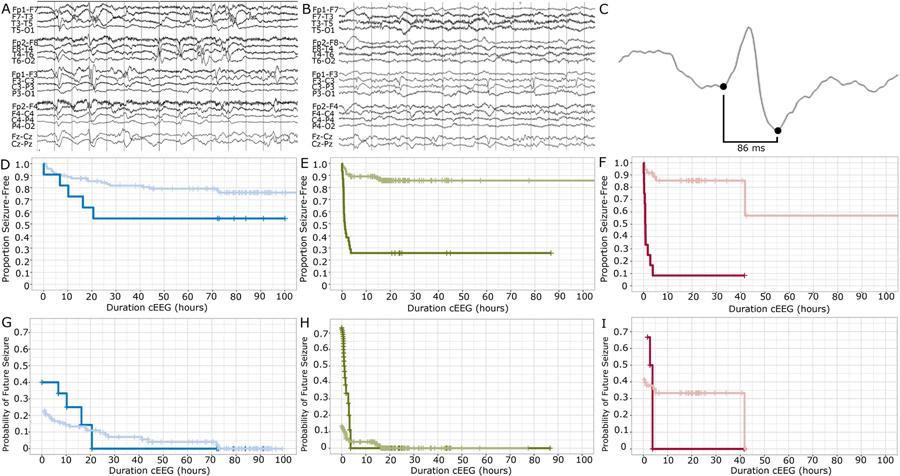
Patients with epileptiform discharges on early EEG had an overall higher risk of seizure than those without and required a shorter duration of seizure-free cEEG monitoring to be identified because all seizures presented early.
Among the ANE group, the risk of subsequent seizure was 4.31 times higher when epileptiform activity was present in the early EEG (aHR 4.31, 95% CI 1.23–15.13, p=0.022), compared to neonates without early epileptiform discharges. Among neonates monitored for SCE, the risk of seizure was 10.95 times higher (aHR 10.95, 95% CI 4.77–25.14, p <1e-07) in those with early epileptiform discharges. Among neonates monitored for OHR indications, the risk of seizure was 56.90 times higher (aHR 56.90, 95% CI 10.32–313.72, p<1e-05) in those with epileptiform discharges.
For each indication, the presence of epileptiform discharges on early EEG predicted seizures that presented early. When epileptiform discharges were absent, first seizures presented over a longer time course. Among neonates monitored for ANE with early epileptiform discharges, the risk of future seizure was less than 1% after 21 hours of seizure-free monitoring. When early epileptiform discharges were not present, the risk of future seizure reached less than 1% only after 73 hours of seizure-free monitoring (Figure 4A). Notably, the late occurring seizures were subclinical in each of the ANE neonates who presented at or after 72 hours. In one case, the late occurring seizure at 72 hours during rewarming heralded multiple subsequent subclinical electrographic seizures. Among neonates monitored for SCE with early epileptiform discharges, the risk of future seizure was less than 1% after just 4 hours of seizure-free monitoring. When early epileptiform discharges were not present, the risk of future seizure was less than 1% only after 17 hours of seizure-free monitoring (Figure 4B). Among neonates monitored for OHR with early epileptiform discharges, the risk of future seizure was less than 1% after 4 hours of seizure-free monitoring. When early epileptiform discharges were not present, the risk of future seizure reached less than 1% only after 42 hours of seizure-free monitoring (Figure 4C).
Figure 4: Probability of future seizure in the presence or absence of early epileptiform activity.
A) Example neonatal early EEG recording demonstrating broad, multifocal, excessive sharp transients. B) Example neonatal early EEG recording demonstrating consistent focal epileptiform discharges. C) Example fast epileptiform discharge (<100 ms) from an early neonatal EEG recording. D-F) Kaplan-Meier survival curves for D) Acute neonatal encephalopathy (blue), E) Suspicious clinical event (green), and F) Other high-risk indications (red) groups separated by the presence (dark) or absence (light) of epileptiform discharges on early EEG. G-I) Probability of future seizure separated by the presence (dark) or absence (light) of epileptiform discharges in the early EEG record, according to monitoring indication: G) Acute neonatal encephalopathy (blue) H) Suspicious clinical event (green) I) Other high-risk indications (red).
Discussion
We confirm that neonates who undergo cEEG following consensus criteria are each initially at high risk of seizure, however not all neonates require the same duration of monitoring to confidently identify those at continued risk. Rather, seizure risk over time can be accurately predicted by monitoring indication and early EEG features. These findings have immediate implications to inform clinical guidelines for monitoring duration and vigilance, to identify appropriate populations for clinical trials, and provide insights into the time course of insults that provoke seizures in this population.
Current consensus-based guidelines recommend monitoring most neonates for a minimum of 24 hours seizure-free across all high-risk indications.13 An appropriate monitoring duration is critical to capture seizures in at-risk neonates and minimize risk and cost of this technology. Excessive cEEG monitoring is resource intensive,14 and though largely safe, introduces risk of skin breakdown and infection.37 Conversely, prematurely terminating cEEG monitoring misses the opportunity for identification and treatment of a neurologically-ill neonate.3 Our data suggest that a shorter monitoring duration is sufficient for some neonates; while others require a longer duration than current guidelines. In the absence of early EEG information, stopping cEEG after 24 hours would have missed 8.5% of neonates with eventual seizure captured in our cohort. Although a recent study reported that 99% of neonates with seizure were captured by 25 hours of seizure-free cEEG, the median duration of monitoring was 33 hours in that cohort.38 Consistent with prior reports in mixed pediatric populations,15–22,39 when monitored for longer, we found that some neonates presented with first seizure after 40 hours. Further, 78% of neonates were monitored for SCE in the prior study.38 Here, we found that the time course of seizure presentation varies by monitoring indication. To capture 99% of neonates with seizures in our cohort, those monitored for SCE required the shortest duration of seizure-free monitoring and those monitored for ANE the longest.
Our observation that neonates with ANE have a prolonged period of seizure risk is consistent with prior observations. One prior study reported seizures in 17/26 undergoing therapeutic hypothermia, where the mean seizure onset was at 9.5 hours, and 4 subjects presented with their first seizure after 48 hours and one neonate as late as 98 hours.21 Another group reported seizures in 23/43 neonates monitored for ANE, with a median seizure onset at 13.1 hours, however one neonate presented with first seizure after 82 hours seizure-free.39 In the largest previous cohort reported, 43/90 neonates monitored for ANE had seizures, with a median seizure onset of 19.9 hours, however among these neonates, 4 had seizure onset after 72 hours during the rewarming period.22 Together with our data, these findings highlight the prolonged period of continued seizure risk in the ANE population suggesting the need for a longer duration of cEEG monitoring to identify those with seizures. Furthermore, because seizure risk decays slowly over time, this population would benefit from more frequent review than the current consensus-based recommendation of twice daily review.
For each monitoring indication, neonates with epileptiform activity on early EEG had an increased risk of seizure, and their seizures presented early in cEEG monitoring. Thus, neonates with early epileptiform activity may be considered for more frequent early cEEG review and for early enrollment in neonatal ASM clinical trials. In spite of epileptiform activity, if seizures do not occur early, they are very unlikely and prolonged cEEG is of low yield. The observation that neonates without early epileptiform activity require a longer monitoring duration is the opposite of what we previously observed in critically-ill adults, where the absence of early epileptiform discharges supported early cEEG termination.40 Thus, neonates present with unique physiological response and time course to injury. This data suggests that epileptiform activity is a reliable biomarker of seizure risk in the neonatal population. Future work is required to determine whether epileptiform activity on late EEG reliably precedes late presenting seizures in neonates.
We note that the identification of epileptiform discharges in neonatal EEGs can be challenging and is not uniformly implemented. Consensus guidelines on terminology identify several features to help distinguish pathologic negative sharp waves and positive sharp waves from normal sharp transients including fast duration (<100 ms duration), occurrence in the setting of an abnormal background, occurrence more often in trains, and concentration in one region or in atypical locations for normal sharp transients.34 We introduced a standardized operational definition to separate epileptiform discharges from excessive sharp waves, requiring the presence of at least one of two features: persistent focality or short duration (<100 ms duration). We chose these standardized rules because they can be easily and reliably implemented across practitioners and sites. Here we found that these simple rules successfully stratified seizure risk among neonates in all monitoring groups.
In contrast to several previous studies which have suggested that EEG background abnormalities predict seizure risk,22,30,31,38,41 when included in a multivariable model with epileptiform discharges, we found that early EEG background features had only a modest impact. On univariable analysis, asynchrony and asymmetry predicted increased seizure risk in SCE, but the effect of these variables was small compared to epileptiform activity. Burst suppression has consistently been found to be a significant predictor of seizure risk in prior studies.22,41–43 Here, we found that burst suppression increased risk in ANE, but not other groups, but our power to detect a difference was limited. The presence of excessive sharp transients in SCE and excessive discontinuity in ANE modestly decreased seizure risk. Excessive sharp transients and epileptiform activity can be difficult to distinguish at times, thus in the setting of excessive sharp transients, waveforms may be misidentified as epileptiform activity. Alternatively, an excess of these developmentally normal waveforms may indicate a robust response to injury. Similarly, excessive discontinuity in the absence of burst suppression in neonates with ANE may indicate more effective cooling or medication effect, each of which may protect against seizures.44
Advances in neonatal seizure care are limited by the time, expense, and challenges inherent in designing successful clinical trials in this population.45 Although prior studies have focused on the well-studied and homogenous ANE population, our data suggests that enrolling neonates across etiologies may be feasible, as seizure risk was high in all groups. When epileptiform activity was present, this risk was increased 4- to 56-fold and over 99% of neonates with seizures were captured within the first 24 hours of monitoring, suggesting that targeting this population may produce more powerful and shorter clinical trials.
This study is strengthened by its large and continuous cohort of neonates monitored according to standardized criteria; however, there remain limitations. The study is limited in its scope as a single center study and observational study design. Further, the majority of neonates are born in our hospital, reflecting a less acute population than would be selected for transfer to a tertiary facility. We are reassured that the overall incidence of seizures in our study is similar to what is reported in other centers for all combined indications.6 We note that patients monitored for ANE at our site had a slightly lower incidence of seizure compared to prior studies, which have reported seizures in 30–65% of children with ANE.21,22,43,46 This may because our hospital protocol includes cEEG monitoring in mild cases of neonatal encephalopathy given the favorable risk-benefit profile seen over time. Previous work evaluating the relationship between the severity of clinical encephalopathy and seizure risk have reported inconsistent results.11,22,47 We did not evaluate clinical encephalopathy here, but note that EEG background abnormalities correlate with clinical encephalopathy13 and we did not find a strong relationship between EEG background abnormalities after controlling for epileptiform activity. The presence of sleep wake cycling on EEG has previously been shown to predict developmental outcomes48–50 and one study reports that the presence of sleep-wake cycling is associated with decreased seizure risk38. As our early EEGs were too short to adequately assess sleep-wake cycling, we were unable to include this in our analysis. Lastly, over the sampled time period, we had limited cardiac surgeries for congenital heart diseases performed at our institution, limiting the contribution of this well-characterized group.27 Future studies can be done to validate the generalizability of our findings across centers. Additionally, due to our study sample sizes, we chose to group all neonates monitored for SCE and multiple subtypes of OHR conditions together. As both of these groups are heterogeneous, subgroups within these categories likely have different chronological patterns of seizure risk that was not captured here. Larger studies would enable classification of seizure risk based on each individual indication.
Neonates at risk for neurological injury are at high risk of seizure, though this risk varies by indication and early EEG features. Increased knowledge of the risk and time course of seizures in this population based on data readily available early in the clinical course can provide critical information to guide the management and care of these high-risk neonates. Further, understanding the time course of seizure risk in this population provides empirical insights to optimize research and clinical trials in this vulnerable population.
Supplementary Material
Key Points.
Continuous EEG monitoring of high risk neonates for only 24 hours, would miss a large percentage of neonates with seizure
Neonates who undergo cEEG are at high risk for seizures and risk varies by monitoring indication and early EEG findings.
Seizures are captured in nearly all neonates undergoing monitoring for SCE within 24 hours of cEEG monitoring.
Neonates monitored for OHR and ANE can present with delayed seizures and require longer durations of monitoring.
Epileptiform discharges are the best early EEG feature to predict seizure risk.
Acknowledgements
The authors would like to thank the team of EEG technologists at MGH who provide 24–7 support to monitor high-risk neonates. This study was supported in part by grants from the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NIH K23 NS092923 and NIH-NINDS 1K23NS090900, NINDS U01‐045911, 1R01NS102190, 1R01NS102574, 1R01NS107291).
Footnotes
Disclosure of Conflicts of Interest
Dr. Chu serves as a consultant Biogen, SleepMed, and Alliance Family of Companies. The remaining authors report no conflict of interests.
Ethical Publication Statement
The authors confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Lanska MJ, Lanska DJ, Baumann RJ, et al. A population-based study of neonatal seizures in Fayette County, Kentucky. Neurology. 1995; 45(4):724–32. [DOI] [PubMed] [Google Scholar]
- 2.Glass HC, Pham TN, Danielsen B, et al. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998–2002. J Pediatr. 2009; 154(1):24–28.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Semin Fetal Neonatal Med. 2013; 18(4):185–91. [DOI] [PubMed] [Google Scholar]
- 4.Hall DA, Wadwa RP, Goldenberg NA, et al. Maternal risk factors for term neonatal seizures: population-based study in Colorado, 1989–2003. J Child Neurol. 2006; 21(9):795–8. [DOI] [PubMed] [Google Scholar]
- 5.Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary Profile of Seizures in Neonates: A Prospective Cohort Study. J Pediatr. 2016; 174:98–103.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wietstock SO, Bonifacio SL, Sullivan JE, et al. Continuous Video Electroencephalographic (EEG) Monitoring for Electrographic Seizure Diagnosis in Neonates. J Child Neurol. 2016; 31(3):328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abend NS, Wusthoff CJ, Goldberg EM, et al. Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol. 2013; 12(12):1170–9. [DOI] [PubMed] [Google Scholar]
- 8.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000; 55(4):506–13. [DOI] [PubMed] [Google Scholar]
- 9.Pisani F, Cerminara C, Fusco C, et al. Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology. 2007; 69(23):2177–85. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007; 119(5):912–21. [DOI] [PubMed] [Google Scholar]
- 11.Glass HC, Glidden D, Jeremy RJ, et al. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009; 155(3):318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass HC, Kan J, Bonifacio SL, et al. Neonatal Seizures: Treatment Practices Among Term and Preterm Infants. Pediatr Neurol. 2012; 46(2):111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Societyʼs Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011; 28(6):611–7. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez-Colina AM, Topjian AA, Dlugos DJ, et al. Electroencephalogram monitoring in critically ill children: indications and strategies. Pediatr Neurol. 2012; 46(3):158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol. 2007; 37(3):165–70. [DOI] [PubMed] [Google Scholar]
- 16.Jette N, Claassen J, Emerson RG, et al. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006; 63(12):1750–5. [DOI] [PubMed] [Google Scholar]
- 17.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011; 52(6):1130–6. [DOI] [PubMed] [Google Scholar]
- 18.Greiner HM, Holland K, Leach JL, et al. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012; 129(3):e748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreiber JM, Zelleke T, Gaillard WD, et al. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012; 17(1):31–8. [DOI] [PubMed] [Google Scholar]
- 20.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011; 76(12):1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011; 26(6):724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass HC, Wusthoff CJ, Shellhaas RA, et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology. 2014; 82(14):1239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014; 99(3):F219–24. [DOI] [PubMed] [Google Scholar]
- 24.Yap V, Engel M, Takenouchi T, et al. Seizures are common in term infants undergoing head cooling. Pediatr Neurol. 2009; 41(5):327–31. [DOI] [PubMed] [Google Scholar]
- 25.Kendall GS, Mathieson S, Meek J, et al. Recooling for rebound seizures after rewarming in neonatal encephalopathy. Pediatrics. 2012; 130(2):e451–5. [DOI] [PubMed] [Google Scholar]
- 26.Clancy RR, Sharif U, Ichord R, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005; 46(1):84–90. [DOI] [PubMed] [Google Scholar]
- 27.Gaynor JW, Nicolson SC, Jarvik GP, et al. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J Thorac Cardiovasc Surg. 2005; 130(5):1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glass HC, Shellhaas RA, Tsuchida TN, et al. Seizures in Preterm Neonates: A Multicenter Observational Cohort Study. Pediatr Neurol. 2017; 72:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janackova S, Boyd S, Yozawitz E, et al. Electroencephalographic characteristics of epileptic seizures in preterm neonates. Clin Neurophysiol. 2016; 127(8):2721–7. [DOI] [PubMed] [Google Scholar]
- 30.Laroia N, Guillet R, Burchfiel J, et al. EEG backgrounds as predictor of electrographic seizures in high-risk neonates. Epilepsia. 1998; 39(5):545–51. [DOI] [PubMed] [Google Scholar]
- 31.Glauser TA, Clancy RR. Adequacy of routine EEG examinations in neonates with clinically suspected seizures. J Child Neurol. 1992; 7(2):215–20. [DOI] [PubMed] [Google Scholar]
- 32.Rowe JC, Holmes GL, Hafford J, et al. Prognostic value of the electroencephalogram in term and preterm infants following neonatal seizures. Electroencephalogr Clin Neurophysiol. 1985; 60(3):183–96. [DOI] [PubMed] [Google Scholar]
- 33.Awal MA, Lai MM, Azemi G, et al. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: A structured review. Clin Neurophysiol. 2016; 127(1):285–96. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous eeg monitoring in neonates: Report of the american clinical neurophysiology society critical care monitoring committee. J Clin Neurophysiol. 2013; 30(2):161–73. [DOI] [PubMed] [Google Scholar]
- 35.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 36.Bewick V, Cheek L, Ball J. Statistics review 12: Survival analysis. Crit Care. 2004; 8(5):389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drees C, Makic MB, Case K, et al. Skin Irritation during Video-EEG Monitoring. Neurodiagn J. 2016; 56(3):139–50. [DOI] [PubMed] [Google Scholar]
- 38.Sansevere AJ, Kapur K, Peters JM, et al. Seizure Prediction Models in the Neonatal Intensive Care Unit. J Clin Neurophysiol. 2019; 36(3):186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure. 2015; 33:60–5. [DOI] [PubMed] [Google Scholar]
- 40.Westover MB, Shafi MM, Bianchi MT, et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol. 2015; 126(3):463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009; 72(22):1931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang A, Arndt DH, Berg RA, et al. Development and validation of a seizure prediction model in critically ill children. Seizure. 2015; 25:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011; 76(6):556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obeid R, Tsuchida TN. Treatment effects on neonatal EEG. J Clin Neurophysiol. 2016; 33(5):376–81. [DOI] [PubMed] [Google Scholar]
- 45.Soul JS, Pressler R, Allen M, et al. Recommendations for the design of therapeutic trials for neonatal seizures. Pediatr Res. 2018; . [DOI] [PMC free article] [PubMed]
- 46.Glass HC, Nash KB, Bonifacio SL, et al. Seizures and Magnetic Resonance Imaging–Detected Brain Injury in Newborns Cooled for Hypoxic-Ischemic Encephalopathy. J Pediatr. 2011; 159(5):731–735.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon JM, Guillet R, Shankaran S, et al. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. J Child Neurol. 2011; 26(3):322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scher MS. Ontogeny of EEG-sleep from neonatal through infancy periods. Sleep Med. 2008; 9(6):615–36. [DOI] [PubMed] [Google Scholar]
- 49.Murray DM, Boylan GB, Ryan CA, et al. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009; 124(3):e459–67. [DOI] [PubMed] [Google Scholar]
- 50.Shellhaas RA, Burns JW, Hassan F, et al. Neonatal Sleep-Wake Analyses Predict 18-month Neurodevelopmental Outcomes. Sleep. 2017; 40(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.