SUMMARY
Background:
There has been limited evaluation of the association between vedolizumab trough concentration and clinical outcomes in patients with inflammatory bowel diseases (IBD).
Aim:
To perform a systematic review and meta-analysis to evaluate the potential role of therapeutic drug monitoring (TDM) for vedolizumab.
Methods:
Through a systematic literature search through February 28, 2019, we identified five cohort studies (558 patients, 42% with ulcerative colitis) reporting the association between vedolizumab trough concentration and clinical outcomes in patients with IBD. We calculated mean difference (MD) in vedolizumab trough concentration in patients achieving vs. not achieving clinical outcomes, and qualitatively synthesized thresholds associated with favorable outcomes.
Results:
In patients with UC, median vedolizumab trough concentrations were consistently higher in patients achieving clinical remission (median, 14.3μg/ml vs. 10.5μg/ml; MD, 5.1μg/ml, 95% CI, 2.8 to 7.4) or endoscopic remission (median, 13.0μg/ml vs. 9.7; MD, 5.1μg/ml, 95% CI, 2.2 to 7.9). In patients with CD, there was no significant difference in median vedolizumab trough concentrations in patients achieving vs. not achieving clinical remission (MD, 2.0μg/ml; 95% CI, −0.5 to 4.5) or endoscopic remission (MD, 3.6μg/ml; 95% CI, −1.4 to 8.6). In patients with UC, week 6 vedolizumab trough concentrations ≥18.5–20.8μg/ml, and maintenance trough concentrations ≥9.0–12.6μg/ml were associated with favorable clinical outcomes. Antibodies to vedolizumab were reported in 1.7–3.0% patients on maintenance therapy.
Conclusion:
Based on meta-analysis, patients with UC who achieve endoscopic and clinical remission have significantly higher vedolizumab trough concentration during maintenance therapy. Vedolizumab trough concentration >20μg/ml at week 6, and >12μg/ml during maintenance may be associated with better outcomes, though cause-effect relationship remain unclear. Prospective studies on reactive and proactive therapeutic drug monitoring of vedolizumab (vs. empiric dose escalation) are warranted.
Keywords: Therapeutic drug monitoring, anti-integrin, clinical remission, endoscopic remission
INTRODUCTION
Therapeutic drug monitoring (TDM) has been recognized as an important strategy to inform clinical decision-making in patients with inflammatory bowel diseases (IBD).1–3 The rationale for TDM is that a systematic and algorithmic assessment of drug concentration and anti-drug antibodies may help objectively evaluate potential reasons for failure of therapy and define next steps in management, and proactively provide opportunities for optimizing therapy. This is based on clinical observations including: (a) inter-individual variability in drug clearance through both immune-mediated (formation of neutralizing anti-drug antibodies) and non-immune-mediated mechanisms (associated with high inflammatory burden) which contribute to differences in drug concentration, (b) presence of an exposure-response relationship, wherein serum drug concentration is associated with magnitude of the clinical response, and (c) concept of mechanistic failure, wherein despite adequate drug exposure at site of receptor, some patients may not respond to a particular class of biologics due to differences in underlying disease pathophysiology.4, 5
While TDM has been extensively studied and implemented when using tumor necrosis factor-α (TNFα) antagonists, it’s role in the optimization of vedolizumab is unclear. Vedolizumab trough concentrations have been variably associated with clinical outcomes in patients with IBD, with some studies suggesting higher vedolizumab trough concentrations in patients responding to therapy, whereas others suggesting no differences in trough concentrations in responding vs. non-responding patients.6–10 In in vitro cell-based assays, complete α4β7 receptor saturation was reached at a vedolizumab serum concentration of approximately 1μg/mL, a concentration considered subtherapeutic.11, 12 This suggests that while receptor saturation may be necessary, it is not sufficient for clinical efficacy of vedolizumab. The immunogenicity of vedolizumab is low, such that rates of immune-mediated pharmacokinetic failure may be low. There has been limited guidance on the use of TDM with vedolizumab. The recent American Gastroenterological Association guidelines and the Sydney IBD Consensus statements on TDM focused only on TNFα antagonists.2, 13, 14 The BRIDGe group recommended use of TDM in vedolizumab-treated patients with primary non-response or secondary loss of response, primarily to determine the presence or absence of drug, but could not recommend optimal trough concentrations.3
Hence, we conducted a systematic review and meta-analysis to evaluate the association between vedolizumab trough concentrations and clinical outcomes in patients with IBD, and evaluated serum drug trough concentrations associated with superior efficacy. We synthesize this evidence to inform the use of TDM for vedolizumab in clinical practice.
METHODS
This systematic review followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) standards, and followed an a priori protocol (available upon request).15
Selection Criteria
We included retrospective and prospective cohort studies (including post-hoc analyses of clinical trials) that reported the association between vedolizumab trough concentrations during induction or maintenance therapy and clinical outcomes (clinical response or remission, endoscopic response or remission) in patients with IBD, stratified by ulcerative colitis (UC) and Crohn’s disease (CD). To estimate mean differences in vedolizumab trough concentrations between patients who achieved vs. did not achieve favorable clinical outcomes, studies had to report mean or median vedolizumab concentration (with measure of variability) in the two groups. We excluded studies that did not provide adequate information to allow estimation of mean differences. When multiple studies from the same cohort were reported, then the most comprehensive report providing information of interest was included.
Search Strategy
We conducted a comprehensive search of multiple electronic databases initially through March 18, 2018, with no language restrictions, with the help of an experienced medical librarian, as part the American Gastroenterological Association’s technical review on the pharmacological management of moderate to severe ulcerative colitis (details in clonline supplement). The databases included Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Elsevier EMBASE and Cochrane Central Register of Controlled Trials. Subsequently, a focused updated search of Medline was performed on February 28, 2019 by a study investigator using a combination of phrases indicating the diseases of interest [“Crohn(s) disease”, “Ulcerative colitis”, “inflammatory bowel disease”, “regional enteritis”] and drug of interest “vedolizumab”, “anti-integrin”]. Two study investigators independently reviewed the title and abstract of studies identified in the search to exclude studies that did not address the research question of interest on the basis of pre-specified inclusion and exclusion criteria. The full text of the remaining articles was examined to determine whether it contained relevant information. Conflicts in study selection at this stage were resolved by consensus, referring back to the original article, in consultation with a senior investigator. Second, we searched the bibliographies of these selected articles, systematic reviews and consensus documents to identify any additional studies. Third, we conducted a manual search of abstracts from major gastroenterology conferences (Digestive Disease Week, American College of Gastroenterology annual meeting, Advances in Inflammatory Bowel Diseases meeting organized by the Crohn’s and Colitis Foundation of America, European Crohn’s and Colitis Organization annual meeting and United European Gastroenterology Week) from 2014 to 2018 to identify additional abstracts on the topic.
Data Abstraction and Risk of Bias Assessment
Data on study-, participant-, disease- and treatment-related characteristics were abstracted onto a standardized form, by a single investigator, and a random subset of data elements were independently reviewed by a second investigator. Discrepancies in abstraction were resolved by consensus, referring to the original article, in consultation with a third reviewer if needed. Specifically, we abstracted data on vedolizumab trough concentrations (mean or median, standard deviation or range or interquartile range) in patients achieving vs. not achieving various clinical end points (clinical remission or response, endoscopic remission or response), definition of clinical and endoscopic outcomes, time point of assessment of outcomes and trough concentration measurements and assay used for assessing vedolizumab trough concentrations and anti-drug antibodies. We also abstracted reports of “optimal” trough concentration at specific time points associated with the presence of, or predictive of future favorable outcomes, including diagnostic performance, sensitivity and specificity, where reported.
A formal tool for assessing risk of bias was not used. Instead, studies were rated based on enrollment of consecutive patients, incomplete outcome reporting, measurement of true trough concentration and use of a validated tool for assessing clinical outcomes.
Outcomes Assessed
The primary outcome was the mean difference in vedolizumab trough concentrations during maintenance therapy in patients with ulcerative colitis and Crohn’s disease who achieved vs. did not achieve clinical remission and/or endoscopic remission. Secondary outcomes were: (a) estimation of vedolizumab trough concentrations maintenance therapy associated with the presence of favorable clinical outcomes in patients with IBD, and (b) estimation of vedolizumab trough concentrations during induction therapy predictive of future favorable outcomes during maintenance therapy. With primary analyses already stratified by type of IBD (ulcerative colitis vs. Crohn’s disease) and outcome (clinical remission or response vs. endoscopic remission or response), no a priori subgroup analyses were planned.
Statistical Analysis
We performed pairwise meta-analyses using a DerSimonian and Laird random effects approach to obtained mean difference (MD) and 95% confidence intervals (CI) in vedolizumab trough concentrations in patients with ulcerative colitis and Crohn’s disease who achieved vs. did not achieve clinical remission and/or endoscopic remission.16 For these calculations, mean and standard deviations were used where reported; if not reported, then median was considered equivalent to mean, and standard deviation was estimated from the interquartile range (IQR) (standard deviation = IQR/1.35). We examined statistical heterogeneity using the I2 statistic; due to limited number of studies, formal assessment for publication bias could not be reliably performed.17, 18 Quantitative synthesis of optimal vedolizumab trough concentrations was not feasible due to paucity of data; hence, to inform ‘optimal’ vedolizumab trough concentrations for outcomes of interest, we qualitatively reported median (and range) optimal concentrations reported in individual studies. All analysis was performed using Comprehensive Meta-Analysis (CMA) version 2 (Biostat, Englewood, NJ).
RESULTS
From 12,104 studies identified using the original search strategy, 5,655 unique studies were identified. From these, 32 studies focusing on vedolizumab were identified for full text review, and five studies were included in quantitative synthesis (Figure 1).8–10, 19, 20 Additionally, data from 5 studies was qualitatively synthesized to inform vedolizumab concentrations associated with favorable outcomes.7, 21–24 Multiple studies were reported using the GEMINI trials and unique aspects of each analyses were used to quantitatively or qualitatively inform evidence on role of TDM with vedolizumab. Table 1 reports study characteristics, and key findings from included studies, and supplementary Table 2 provides data on studies synthesized qualitatively.
Figure 1.
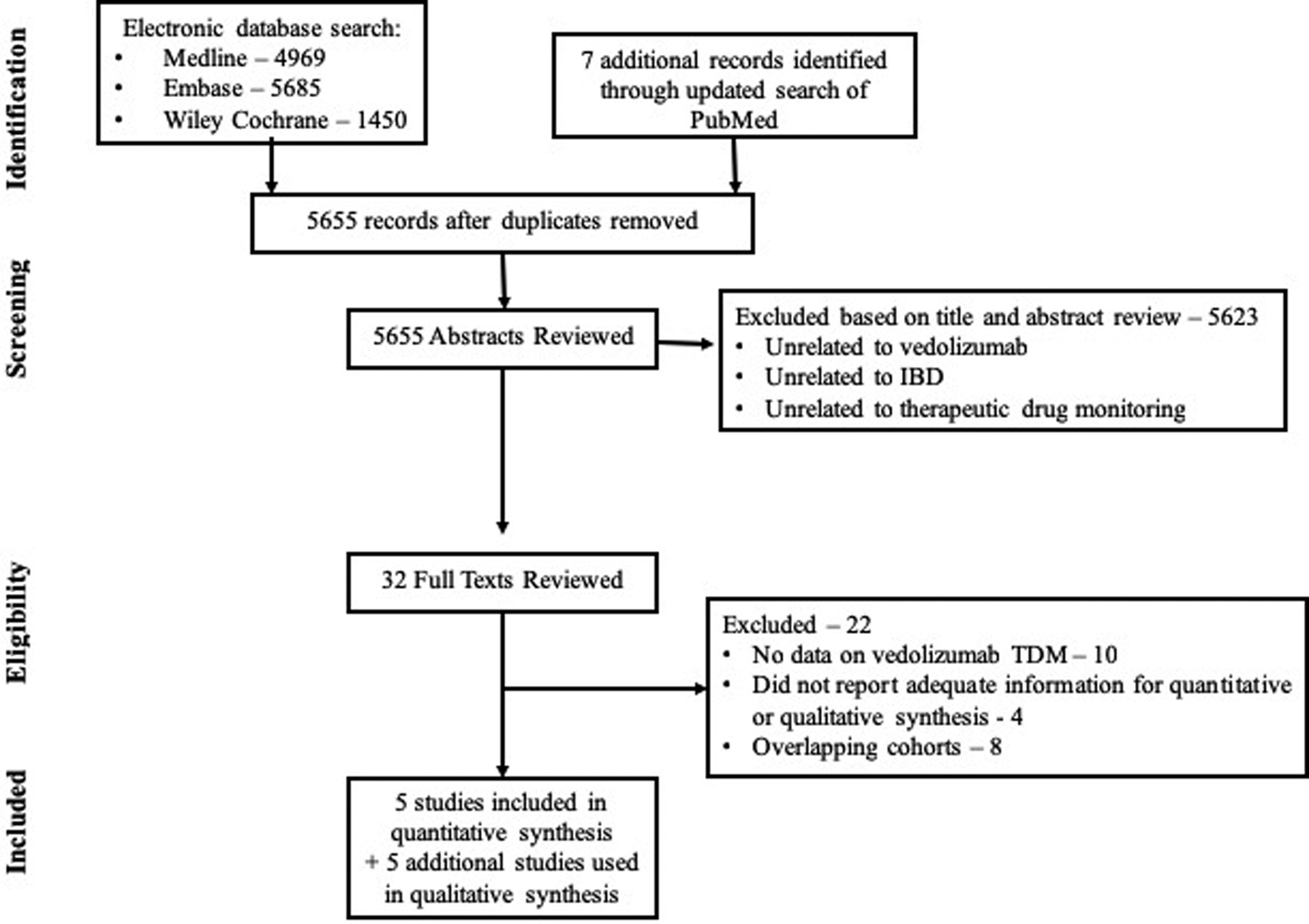
Study selection flowchart
Table 1.
Summary of included studies
| Author, Year | Location, Setting, Design | # of patients (CD, UC), VDZ regimen; prior biologic exposure | VDZ assay, # of VDZ measurements and time points; # with antibodies to VDZ | Clinical outcomes | Optimal VDZ trough concentrations; performance |
|---|---|---|---|---|---|
| Ungaro, Yarur; 201920 | Two centers, prospective cohort; USA | 258 (142 CD, 116 UC); median 5 VDZ infusions; 19% on q4w VDZ; 66% prior biologic exposure | HMSA drug-tolerant assay (Prometheus®); NR (single VDZ measurement per patient for analysis); 4/258 (1.6% patients) with ATV | Maintenance therapy:
|
Maintenance trough for
|
| Dreesen, 20189 | Single-center; retrospective cohort; Belgium | 179 (113 CD, 66 UC); 9% on q4w VDZ; 102/113 CD patients received extra week 10 dose; 85% prior biologic exposure | In-house ELISA, drug-sensitive assay; multiple samples per patient; 1/928 (0.1% samples) samples with ATV | Week 22 for CD; week 14 for UC:
|
Maintenance trough (week 14) for UC:
|
| Al-Bawardy, 201810 | Single-center; retrospective cohort; USA | 171 (106 CD, 65 UC+IC); 71% on VDZ for >6m; 33% on accelerated VDZ (q4w or q6w); 90% prior biologic exposure | ELISA drug-sensitive assay (Miraca®, similar to Theradiag®); 1/171 (0.6% patients) with ATV |
|
Not reported |
| Battat, 201819 | Single-center; prospective cohort study; USA | 32 (0 CD, 32 UC); median time of assessing outcomes, 26 weeks; escalation NR; 84% prior biologic exposure | HMSA drug-tolerant assay (Prometheus®); NR (single VDZ measurement per patient for analysis); 2/32 (5.9% patients) with ATV |
|
Not reported |
| Yacoub, 20188 | Multi-center; prospective cohort study; France | 82 (39 CD, 43 UC); median follow-up 30 weeks; escalation NR; 82% prior biologic exposure | ELISA drug-sensitive assay (Theradiag®); NR; NR | Maintenance therapy:
|
Week 6 VDZ trough predicting outcomes during maintenance:
|
| Ungar, 20186 | Two-center; prospective cohort; Israel | 106 (67 CD, 39 UC); 11.3% received week 10 VDZ, 11.5% on q4w VDZ; 86% prior biologic exposure | In-house ELISA drug-sensitive; multiple samples per patient (140 serial sera from 60 patients on VDZ maintenance >22w); 2/60 (3% patients) with ATV during maintenance therapy | Maintenance therapy:
|
NR |
Abbreviations: ATV-Antibodies to vedolizumab; BR-biochemical remission; CR-clinical remission; CRes-clinical response; CRP-C-reactive protein; CS-corticosteroid; CD-Crohn’s disease; ER-Endoscopic remission; UC-Ulcerative colitis; VDZ-vedolizumab
Overall, studies were at moderate risk of bias – TDM was applied selectively in patients failing therapy, rather than routinely on all vedolizumab-treated patients; endoscopic outcomes were selectively reported in patients where endoscopy was performed; clinical outcomes were assessed using validated indices for ulcerative colitis, but relied on physician global assessment and absence of ulcerations for Crohn’s disease (Supplementary Table 2).
Ulcerative Colitis
Five studies reported differences in vedolizumab trough concentrations between patients with ulcerative colitis achieving clinical and/or endoscopic remission during maintenance therapy.8–10, 19, 20
Clinical Remission:
On meta-analysis of 4 studies (n=216 patients, 47% achieving clinical remission), vedolizumab trough concentration during maintenance therapy (week 14 to 52) was significantly higher in patients achieving clinical remission (median of median vedolizumab trough concentration, 14.3μg/ml; range, 10.2–15.0) vs. patients who did not achieve remission (median of median vedolizumab trough concentration, 10.5μg/ml; range, 6.7–11.3) (MD, 5.1μg/ml; 95% CI, 2.8 to 7.4, p<0.01), with moderate heterogeneity (I2=36%) (Figure 2).
Figure 2.
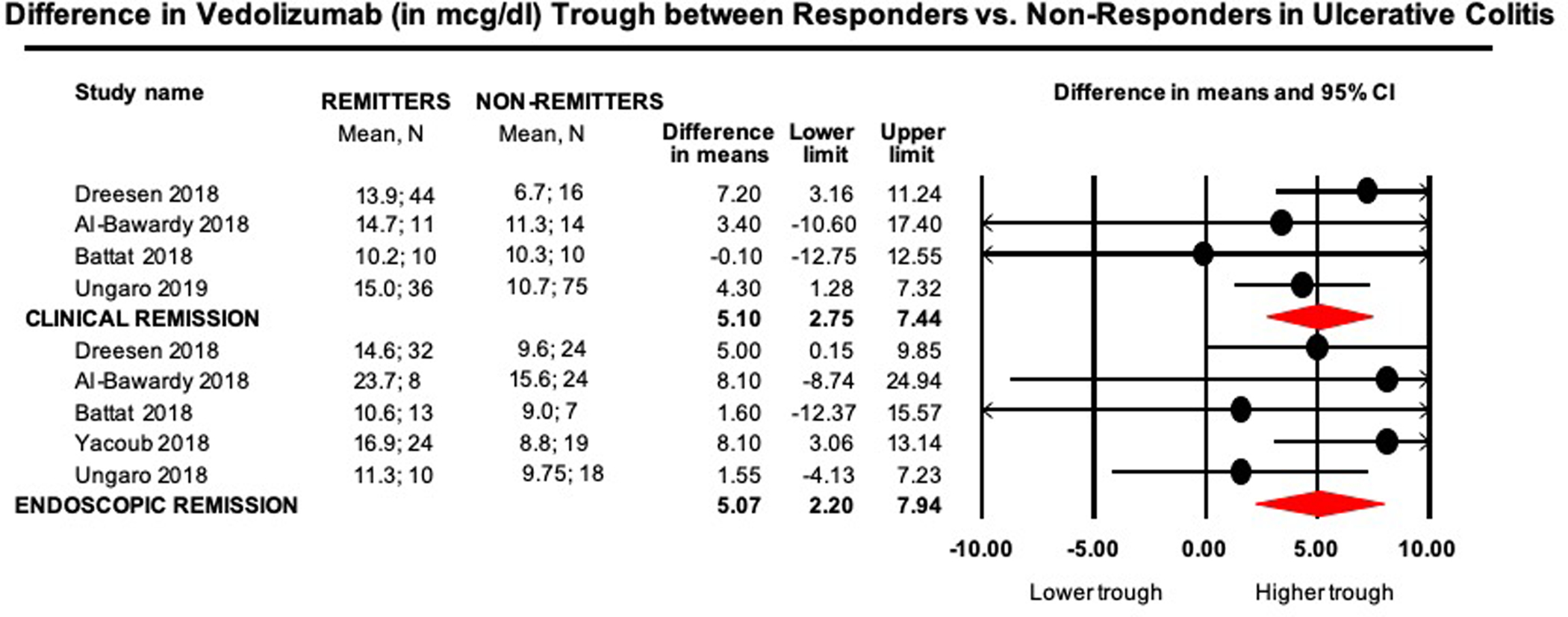
Mean difference in vedolizumab trough concentrations (in μg/ml) in remitters vs. non-remitters in patients with UC
Endoscopic Remission:
On meta-analysis of 5 studies (n=179 patients with endoscopic outcomes, 49% achieving endoscopic remission), vedolizumab trough concentration during maintenance therapy was significantly higher in patients achieving endoscopic remission (median of median vedolizumab trough concentration, 13.0μg/ml; range, 10.6–23.7) vs. patients who did not achieve remission (median of median vedolizumab trough concentration, 9.7μg/ml; range, 9.0–15.6) (MD, 5.1μg/ml; 95% CI, 2.2 to 7.9, p<0.01), with minimal heterogeneity (I2=0%) (Figure 2).
Crohn’s Disease
Four studies reported differences in vedolizumab trough concentrations between patients with Crohn’s disease achieving clinical and/or endoscopic remission during maintenance therapy.8–10, 20
Clinical Remission:
On meta-analysis of 2 studies (n=167 patients, 38% achieving clinical remission), vedolizumab trough concentration during maintenance therapy was numerically but not significantly different in patients achieving clinical remission (median of median vedolizumab trough concentration, 12.3μg/ml; range, 10.9–13.7) vs. patients who did not achieve remission (median of median vedolizumab trough concentration, 12.5μg/ml; range, 8.6–24.9) (MD, 2.0μg/ml; 95% CI, −0.5 to 4.5, p=0.11), with moderate heterogeneity (I2=40%) (Figure 3).
Figure 3.
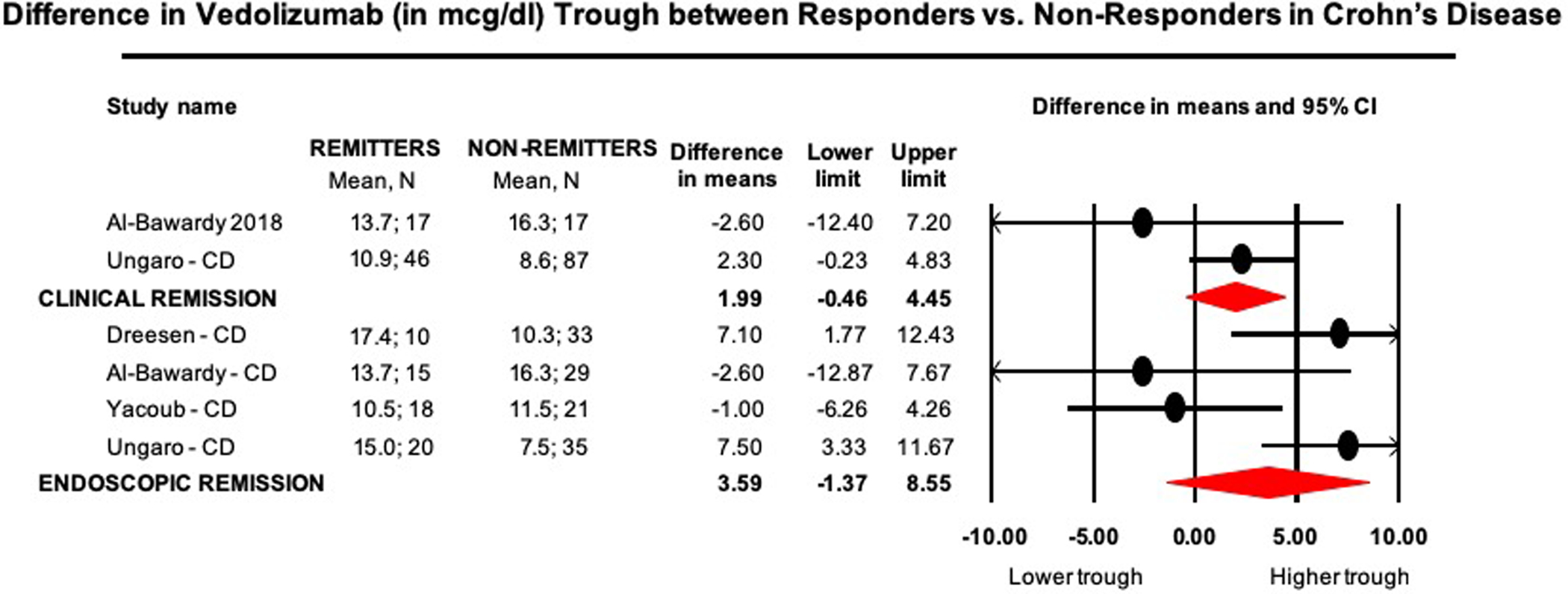
Mean difference in vedolizumab trough concentrations (in μg/ml) in remitters vs. non-remitters in patients with CD
Endoscopic Remission:
On meta-analysis of 4 studies (n=181 patients with endoscopic outcomes, 35% achieving endoscopic remission), vedolizumab trough concentration during maintenance (week 14 to 52) therapy was numerically but not significantly higher in patients achieving endoscopic remission (median of median vedolizumab trough concentration, 14.4μg/ml; range, 10.5–17.4) vs. patients who did not achieve remission (median of median vedolizumab trough concentration, 10.9μg/ml; range, 7.5–16.3) (MD, 3.6μg/ml; 95% CI, −1.4 to 8.6, p=0.16), with considerable heterogeneity (I2=74%) (Figure 3).
Vedolizumab Trough During Induction Predicting Future Outcomes
In three studies, week 6 vedolizumab trough concentrations were associated with favorable outcomes during maintenance therapy. In a post-hoc analyses of GEMINI-1 trial for ulcerative colitis, week 6 vedolizumab trough concentrations in quartiles 2–4 (≥20.6μg/ml) were associated with higher rates of clinical remission at week 14 (65–74% vs. quartile 1, 40%).23 Similarly, in a retrospective Belgian cohort, week 6 trough ≥20.8μg/ml was deemed optimal to predict clinical response at week 14 in patients with ulcerative colitis (area under receiver operating curve [AUROC], 0.72; sensitivity/specificity, 0.75/0.69).9 In a French cohort study, Yacoub and colleagues reported that in a cohort of 43 ulcerative colitis and 39 Crohn’s disease patients, vedolizumab trough ≥18μg/ml was associated with endoscopic remission during maintenance therapy (AUROC, 0.74; sensitivity/specificity, 0.88/0.67; AUROC in patients with ulcerative colitis and Crohn’s disease, 0.75 and 0.70, respectively).8 In an overlapping cohort, week 6 trough concentration <18.5μg/ml was associated with need for drug optimization within 6 months (AUROC, 0.72).7
Vedolizumab Trough During Maintenance Associated with Favorable Outcomes
Four studies reported vedolizumab trough concentrations during maintenance therapy associated with favorable outcomes. In a post-hoc analysis of GEMINI-1 trial in patients with ulcerative colitis, vedolizumab steady state trough concentrations in quartiles 2–4 (≥9.0μg/ml) was associated with higher rates of symptomatic remission and endoscopic improvement (52.5–57.5% vs. quartile 1, 35%).22 Similarly, in the GEMINI-2 trial in patients with Crohn’s disease, steady state vedolizumab trough concentration in quartiles 2–4 (≥7.6μg/ml) was associated with numerically higher rates of clinical remission (83–89% vs. quartile 1, 67%).21 In an observational study, Ungaro and colleagues reported that vedolizumab trough concentration ≥11.3μg/ml during maintenance therapy had the most optimal, yet modest, discriminatory performance for assessing the presence of corticosteroid-free clinical and biochemical remission in patients with IBD (AUROC, 0.62; sensitivity/specificity, 0.57/0.63); specifically, in patients with ulcerative colitis, the cut-off was ≥10.1μg/ml (sensitivity/specificity, 0.89/0.43) and in patients with Crohn’s disease, the cut-off was ≥6.8μg/ml (sensitivity/specificity 0.83/0.38).20 A cut-off of ≥10.7μg/ml was most discriminative for the presence of corticosteroid-free endoscopic remission. Dreesen and colleagues reported that cut-offs of ≥12.6μg/ml (sensitivity/specificity, 0.46/0.92) and ≥17.0μg/ml (sensitivity/specificity, 0.46/0.92) at week 14 were best associated with the presence of clinical response and endoscopic remission, respectively, in patients with ulcerative colitis.9 In patients with Crohn’s disease, they estimated that cut-offs of ≥13.6μg/ml (sensitivity/specificity, 0.71/0.69) and ≥12.6μg/ml (sensitivity/specificity, 0.75/0.79) at week 22 were best associated with the presence of endoscopic remission and biochemical remission, respectively.
Immunogenicity to Vedolizumab
In the GEMINI-1 and GEMINI-2 trial, using a drug-sensitive assay, 3.7–4.1% patients had antibodies to vedolizumab at any time, and 0.4–1% patients had persistently positive antibodies on ≥2 consecutive samples.21, 22 In observational studies, using drug-tolerant assays, antibodies to vedolizumab were observed in 1.7–3.0% of patients during maintenance therapy.6, 20 Using drug-sensitive assays, antibodies to vedolizumab were observed in 0.1–2.4% of samples.8, 9
DISCUSSION
Through a quantitative and qualitative synthesis of evidence on vedolizumab trough concentration and immunogenicity, we have attempted to ascertain optimal use of TDM with vedolizumab. We observed that, cross-sectionally, vedolizumab trough concentration is significantly higher in patients responding to therapy vs. those not responding to therapy, among patients with ulcerative colitis; in Crohn’s disease, there were similar numeric trends, but the results were not significant. During induction therapy, at week 6, vedolizumab trough concentration >18–20μg/ml is associated with higher likelihood of favorable outcomes on follow-up. During maintenance therapy, vedolizumab trough concentrations >10–12μg/ml are associated with the presence of clinical remission, and higher trough concentrations are associated with the presence of endoscopic remission, albeit with modest discriminative power. It is important to note that this correlation does not imply causative association. Finally, immunogenicity with vedolizumab is low with antibodies to vedolizumab detected in ~2–3% patients using a drug-tolerant assay, and 0.5–1% patients having persistent antibodies to vedolizumab using a drug-sensitive assay, suggesting the likelihood of immune-mediated pharmacokinetic failure may be low.
Across studies, we observed that among remitters (clinical and/or endoscopic), vedolizumab trough concentration is significantly higher than non-remitters, in patients with ulcerative colitis, with similar trends in Crohn’s disease that did not reach statistical significance. Several factors consistently associated with superior clinical outcomes (high albumin, low C-reactive protein, low body mass index) are also associated with higher vedolizumab trough concentration.9, 20 Hence, as observed with infliximab, it is likely that ulcerative colitis remitters have lower drug clearance and higher vedolizumab trough concentration than non-remitters. Vedolizumab label for Crohn’s disease in Europe allows for extra vedolizumab dose at week 10 among patients with inadequate response to therapy for week 6. These patients may have higher post-induction and steady state vedolizumab trough concentrations, but due to confounding by indication, may have inferior clinical outcomes (due to more severe disease leading to lack of response by week 6). In addition, the measurement of clinical and endoscopic disease activity in patients with ulcerative colitis is standardized and straightforward, whereas in Crohn’s disease, there are numerous problems with the accurate and reproducible measurement of clinical symptoms and endoscopy, and the correlation between clinical symptoms and endoscopic findings is poor. These factors may in turn add noise to the comparison and make it relatively harder to detect differences between remitters and non-remitters. This may explain lack of significant difference in vedolizumab trough concentrations in remitters vs. non-remitters in patients with Crohn’s disease. Given these factors, it may be reasonable to consider applying the significant results observed in patients with ulcerative colitis, to patients with Crohn’s disease since the mechanisms of drug clearance and drug efficacy are likely to be similar in the two conditions.
Based on a qualitative synthesis of thresholds when monitoring vedolizumab trough concentrations, we observed that at week 6 and during maintenance therapy, vedolizumab trough concentrations >20μg/ml and >12μg/ml, respectively were associated with favorable clinical outcomes. It is unclear whether “subtherapeutic” vedolizumab concentration (below these thresholds) is a consequence of rapid drug clearance or the cause of inadequate response. Mechanistically, unlike cytokine antagonists, vedolizumab binds to the α4β7 integrin and blocks lymphocyte interaction with mucosal addressin cell adhesion molecule-1 expressed on the endothelium of mesenteric lymph nodes and gastrointestinal mucosa, impairing the migration of gut-homing lymphocytes into gastrointestinal mucosa.24 Recent studies have suggested that vedolizumab may exert it’s clinical effect through additional mechanisms of action. Zeissig and colleagues observed that vedolizumab’s efficacy might be related to modulation of innate immunity with significant changes in macrophage population and altered expression of pattern recognition receptors, rather than only due to inhibition of T-cell trafficking.25 In these instances, higher vedolizumab serum concentrations, or potentially tissue drug concentrations, may be associated with superior efficacy which remains to be studied in prospective, interventional studies. Besides vedolizumab trough concentrations, studies have demonstrated that combining this with other biomarkers involved in leukocyte trafficking such as soluble MAdCAM-1, s soluble VCAM-1 and soluble ICAM-1 may help identify patients at higher likelihood of response to therapy.19
It remains unclear whether routine assessment of vedolizumab trough concentration in patients with primary non-response or secondary loss of response is warranted. With low immunogenicity, and lack of clear data on target levels for mechanistic efficacy, the role of vedolizumab TDM in decision-making (dose-escalation or switching therapies) is adjunctive, and would require accounting for multiple clinical factors besides trough concentration. In a systematic review of 10 cohorts, pooled incidence rates of loss of response were 47.9 per 100 person-years of follow up among patients with Crohn’s disease and 39.8 per 100 person-years of follow up in patients with ulcerative colitis; dose intensification was able to response to the drug in 53.8% patients with secondary non-responders; TDM was not routinely performed in these cohorts.26 Since this data was not blinded or controlled data, it is possible that magnitude of benefit with empiric dose escalation may be overestimated; pivotal GEMINI trials did not identify any difference in efficacy of every 4 vs. every 8 week dosing of vedolizumab. As a counter-argument, TDM may help identify a substantial minority of patients with a conceivable, yet poorly-defined threshold trough concentration above which escalation of therapy may not be effective, and likewise, identify a small subset of patients with immunogenicity to vedolizumab, where escalation may not be effective in case of high-titer anti-drug antibodies. Early TDM during induction may help more accurately identify patients where vedolizumab clearance is high and for whom early dose escalation may be helpful. Some studies have identified an independent association between early vedolizumab trough concentrations and future outcomes, beyond what may be predicted by conventional clinical parameters.20 Accounting for these findings, we have proposed an algorithmic approach when applying TDM for vedolizumab in clinical practice (Figure 4). It is important to note that upper limit of therapeutic target beyond which further escalation of therapy is very unlikely to be helpful, has not been well-identified. We propose that if TDM is used for vedolizumab, then a therapeutic target range might be between 20–30μg/ml for week 6 concentration, and between 12–20μg/ml for maintenance therapy for achieving clinical and/or endoscopic remission.
Figure 4.
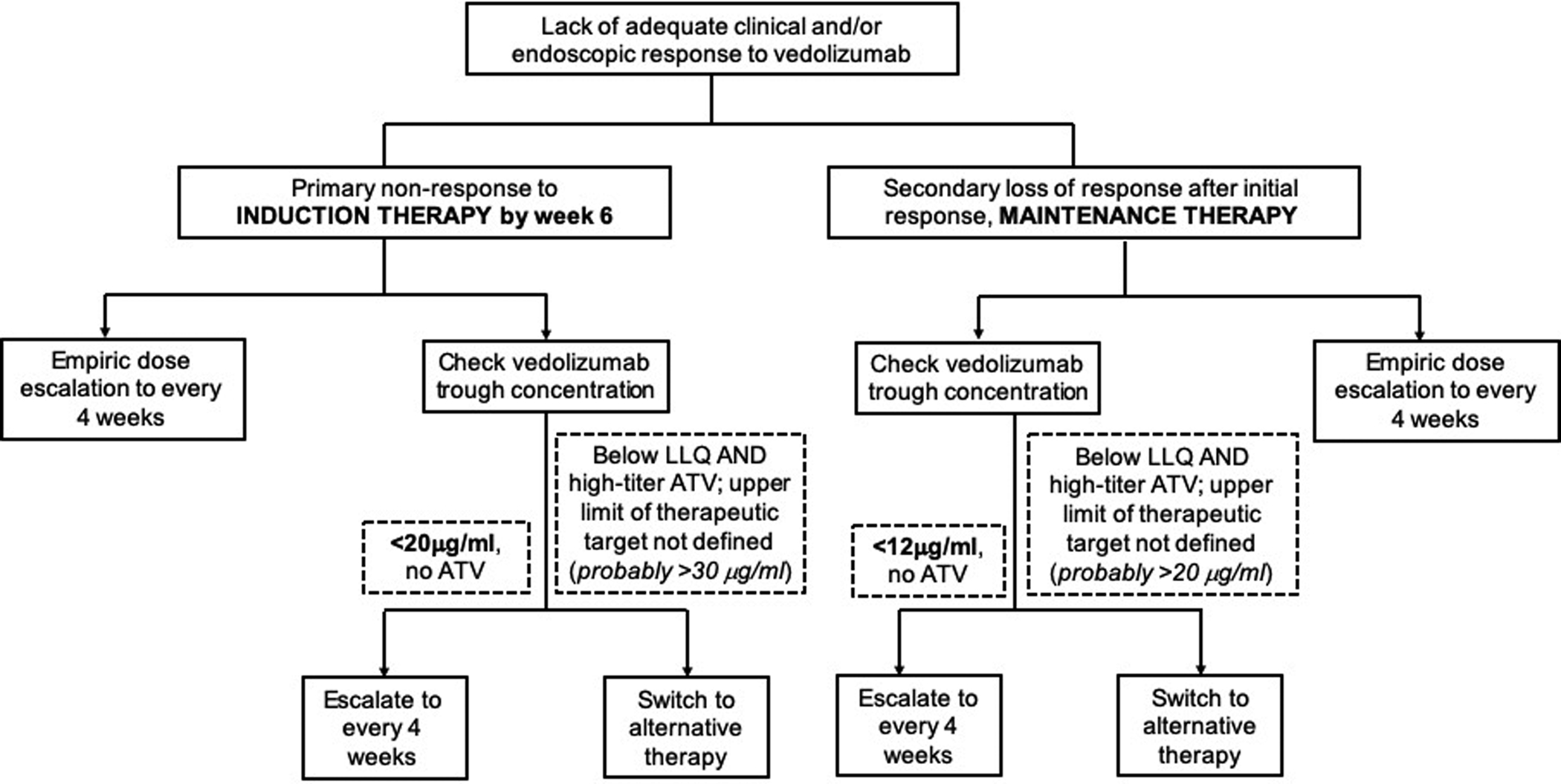
Proposed algorithm for applying therapeutic drug monitoring for vedolizumab. Please note, proposed trough concentrations may vary depending on what treatment endpoint is being targeted (clinical response, clinical remission, endoscopic remission, histologic remission, etc.). LLQ refers to lower limit of quantification of vedolizumab assay. Upper limit of therapeutic target beyond which further escalation of therapy is very unlikely to be helpful, has not been well-identified; based on expert opinion, this threshold is probably >30μg/ml for week 6 level and >20μg/ml for maintenance trough concentration.
The strengths of this review is a systematic, quantitative and qualitative synthesis of all available evidence on vedolizumab trough concentrations, across induction and maintenance therapy. The limitations of a study-level synthesis is the inability to account for individual patient level covariates, as well as inability to more accurately identify thresholds associated with high sensitivity or specificity of achieving clinical outcomes of interest. Individual studies used varying dosing schedules for vedolizumab (every 4 or 8 weeks) and time points of assessment, which may affect an association of exposure and response, and may have impacted proposed thresholds. Furthermore, given the lack of comparative studies of analytical assays, absolute concentrations across studies may differ. Some of these limitations may be overcome with an individual patient level synthesis, pooled exposure-response analysis and development of a dynamic prediction model which accounts for time-varying changes in factors associated with drug clearance.
In conclusion, based on a systematic evidence synthesis, there is a correlation between vedolizumab trough concentration and clinical outcomes, particularly in patients with ulcerative colitis. While appropriate use of TDM for vedolizumab is not entirely clear (unlike for TNFα antagonists), if TDM is used, then aiming for a minimum threshold vedolizumab trough concentrations >20μg/ml and >12μg/ml at week 6 and maintenance therapy, respectively, may help avoid premature treatment discontinuation in patients with suboptimal response; optimal therapeutic window remains unclear. While the field is evolving, we believe this synthesis will provide early evidence-based guidance on the use of TDM for vedolizumab in IBD. Prospective cohort and interventional studies are warranted to more inform the role of a treat-to-trough strategy for vedolizumab.
Supplementary Material
Funding:
This study did not receive any direct funding
Disclosures:
Siddharth Singh – Supported by the NIDDK K23DK117058, the American College of Gastroenterology Junior Faculty Development Award and the Crohn’s and Colitis Foundation Career Development Award (#404614). Research grant support from Pfizer and AbbVie; Consulting fees from AbbVie, Takeda, AMAG Pharmaceuticals; Honorarium from Pfizer for grant review
Parambir S. Dulai – Research grant support from Takeda, Pfizer, Janssen, Prometheus, Polymedco, and ALPCO; has served as a consultant for Takeda, Janssen, Prometheus Labs and Abbvie.
Niels Vande Casteele – Supported by a Research Scholar Award from the American Gastroenterological Association. Research grant support from R-Biopharm and Takeda. Consulting fees from Boehringer Ingelheim, Janssen, Pfizer, Progenity, Prometheus and Takeda.
Robert Battat - No actual or potential conflicts of interest to declare
Mathurin Fumery – Lecture fees or consultant fees from MSD, Abbvie, Takeda, Ferring, Hospira and Boehringer
Brigid S. Boland – Supported by the CTSA 1KL2TR001444; research grants from Janssen and Takeda; consulting fees from Prometheus Laboratories and Abbvie.
William J. Sandborn – research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos; consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Prizer, Precision IBD, Progenity, Prometheus Laboratories, Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Precision IBD, Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech - consultant, stock options; Progenity - consultant, stock; Oppilan Pharma - employee, stock options; Escalier Biosciences - employee, stock options; Precision IBD - employee, stock options; Ventyx Biosciences – employee, stock options; Vimalan Biosciences – employee, stock options.
REFERENCES
- 1.Khanna R, Sattin BD, Afif W, et al. Review article: a clinician’s guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment Pharmacol Ther 2013;38(5):447–59. [DOI] [PubMed] [Google Scholar]
- 2.Mitrev N, Vande Casteele N, Seow CH, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017;46(11–12):1037–1053. [DOI] [PubMed] [Google Scholar]
- 3.Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughn BP, Sandborn WJ, Cheifetz AS. Biologic concentration testing in inflammatory bowel disease. Inflamm Bowel Dis 2015;21(6):1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ordas I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10(10):1079–87; quiz e85–6. [DOI] [PubMed] [Google Scholar]
- 6.Ungar B, Kopylov U, Yavzori M, et al. Association of Vedolizumab Level, Anti-Drug Antibodies, and alpha4beta7 Occupancy With Response in Patients With Inflammatory Bowel Diseases. Clinical Gastroenterology & Hepatology 2017;07:07. [DOI] [PubMed] [Google Scholar]
- 7.Williet N, Boschetti G, Fovet M, et al. Association Between Low Trough Levels of Vedolizumab During Induction Therapy for Inflammatory Bowel Diseases and Need for Additional Doses Within 6 Months. Clinical Gastroenterology & Hepatology 2017;15(11):1750–1757.e3. [DOI] [PubMed] [Google Scholar]
- 8.Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Alimentary Pharmacology & Therapeutics 2018;47(7):906–912. [DOI] [PubMed] [Google Scholar]
- 9.Dreesen E, Verstockt B, Bian S, et al. Evidence to Support Monitoring of Vedolizumab Trough Concentrations in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2018;16(12):1937–1946 e8. [DOI] [PubMed] [Google Scholar]
- 10.Al-Bawardy B, Ramos GP, Willrich MAV, et al. Vedolizumab Drug Level Correlation With Clinical Remission, Biomarker Normalization, and Mucosal Healing in Inflammatory Bowel Disease. Inflamm Bowel Dis 2019;25(3):580–586. [DOI] [PubMed] [Google Scholar]
- 11.Feagan BG, Greenberg GR, Wild G, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol 2008;6(12):1370–7. [DOI] [PubMed] [Google Scholar]
- 12.Parikh A, Leach T, Wyant T, et al. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis 2012;18(8):1470–9. [DOI] [PubMed] [Google Scholar]
- 13.Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology 2017;153(3):835–857 e6. [DOI] [PubMed] [Google Scholar]
- 14.Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S, American Gastroenterological Association Institute Clinical Guidelines C. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017;153(3):827–834. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151(4):264–9, W64. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54(10):1046–55. [DOI] [PubMed] [Google Scholar]
- 19.Battat R, Dulai PS, Vande Casteele N, et al. Biomarkers Are Associated With Clinical and Endoscopic Outcomes With Vedolizumab Treatment in Ulcerative Colitis. Inflamm Bowel Dis 2019;25(2):410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungaro R, Yarur A, Jossen J, Phan BL, Chefitz E, Sehgal P, Kamal K, Bruss A, Patel PB, Fox C, Patel A, Bahur B, Jain A, Stein D, Naik S, Dubinsky MC. Higher Trough Vedolizumab Concentrations During Maintenance Therapy Are Associated with Corticosteroid-Free Remission in Inflammatory Bowel Disease. J Crohns Colitis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. New England Journal of Medicine 2013;369(8):711–721. [DOI] [PubMed] [Google Scholar]
- 22.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699–710. [DOI] [PubMed] [Google Scholar]
- 23.Rosario M, French JL, Dirks NL, et al. Exposure-efficacy Relationships for Vedolizumab Induction Therapy in Patients with Ulcerative Colitis or Crohn’s Disease. J Crohns Colitis 2017;11(8):921–929. [DOI] [PubMed] [Google Scholar]
- 24.Rosario M, Dirks NL, Milch C, et al. A Review of the Clinical Pharmacokinetics, Pharmacodynamics, and Immunogenicity of Vedolizumab. Clin Pharmacokinet 2017;56(11):1287–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeissig S, Rosati E, Dowds CM, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019;68(1):25–39. [DOI] [PubMed] [Google Scholar]
- 26.Peyrin-Biroulet L, Danese S, Argollo M, et al. Loss of Response to Vedolizumab and Ability of Dose Intensification to Restore Response in Patients With Crohn’s Disease or Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2019;17(5):838–846 e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


