Abstract
The current study was undertaken to investigate the effect of differentially formulated polyphenolic compound Essential Turmeric Oil-Curcumin (ETO-Cur), and Tocotrienol-rich fraction (TRF) of vitamin E isomers on colorectal cancer (CRC) cells that produce aggressive tumors. Combinations of ETO-Cur and TRF were used to determine the combinatorial effects of ETO-Cur and TRF-mediated inhibition of growth of CRC cells in vitro and HCT-116 cells xenograft in SCID mice. 16S rRNA gene sequence profiling was performed to determine the outcome of gut microbial communities in mice feces between control and ETO-Cur-TRF groups. Bacterial identifications were validated by performing SYBR-based Real Time (RT) PCR. For metagenomics analysis to characterize the microbial communities, multiple software/tools were used, including Quantitative Insights into Microbial Ecology (QIIME) processing tool. We found ETO-Cur and TRF to synergize and that the combination of ETO-Cur-TRF significantly inhibited growth of HCT-116 xenografts in SCID mice. This was associated with a marked alteration in microbial communities and increased microbial OTU (operation taxonomic unit) number. The relative abundance of taxa was increased and the level of microbial diversity after 34 days of combinatorial treatment was found to be 44% higher over the control. Shifting of microbial family composition was observed in ETO-Cur-TRF treated mice as evidenced by marked reductions in Bacteroidaceae, Ruminococcaceae, Clostridiales, Firmicutes and Parabacteroids families, compared to controls. Interestingly, during the inhibition of tumor growth in ETO-Cur treated mice, probiotic Lactobacillaceae and Bifidobacteriaceae were increased by 20-fold and 6-fold, respectively. The relative abundance of anti-inflammatory Clostridium XIVa was also increased in ETO-Cur-TRF treated mice when compared with the control. Our data suggest that ETO-Cur-TRF show synergistic effects in inhibiting colorectal cancer cell proliferation in vitro and in mouse xenografts in vivo, and might induce changes in microbial diversity in mice.
Introduction
Despite recent therapeutic advances, colorectal cancer (CRC) remains the third most common cancer in the US with nearly 50% of patients eventually developing recurrent tumors, which are resistant to routine chemotherapy. This underscores a need to develop novel strategies to combat chemo-resistance in cancer cells, which produce highly aggressive colon tumors.[1] The overall 5-year survival rate of CRC is approx. 60% and recurrent malignancy after initial treatment is 30–40% in patients with CRC.[2–4] Most recurrences happen during the first 2 to 3 years after initial treatment.[3, 5]
Curcumin, a lipophilic polyphenolic compound, with a broad medicinal value, is the major active ingredient of Curcuma Longa (turmeric).[6] Curcumin's pharmacological effects have been documented in various diseases including gastrointestinal and neurological disorders, as well as diabetes, hepatic, cardiovascular, Alzheimer’s disease, pancreatitis, cystic fibrosis, inflammatory bowel disease, arthritis, multiple sclerosis, and many types of cancer.[6–11] While numerous in vitro and animal models of colon cancer have found Curcumin to be a chemo-preventive and chemo-therapeutic agent, clinical trials have failed to duplicate Curcumin’s anti-cancer property.[11] Although Curcumin- induced sensitization of cancer cells to chemotherapy has been reported, there are major concerns regarding bioavailability of Curcumin based on in vivo studies.[12]
Due to its poor bioavailability;[12] efforts are underway to produce different analogs or formulations of Curcumin with increased bioavailability. ETO-Curcumin (ETO-Cur) is one such formulation, where Curcumin is complexed with essential turmeric oil (Dolcas Biotech; Chester, NJ). ETO-Cur has been shown to be 7–8 times more bioavailable with superior anti-cancer properties than standard Curcumin.[13–15]
The Vitamin E family consists of eight distinct isomers– 4 tocopherols and 4 tocotrienols [16] Tocotrienols display potent antioxidant, anticancer, anti-inflammatory, neuroprotective and cholesterol-lowering properties.[17–23] A tocotrienol-rich fraction (TRF), (containing a mixture of isomers) isolated from palm oil has been used as an anti-oxidant and anti-cancer agent.[24–27]
Given the sheer vastness of microflora in the gut and numerous arrays of species and metabolites produced, it is likely that bacteria plays a pivotal role in many gastrointestinal diseases including CRC.[28–33] Human colon harbors a complex microbial flora containing approximately 1014 bacteria consisting of 103 different microbial species that maintain homeostasis.[34]
The primary objectives of the current pre-clinical investigation were to determine whether synergistic ETO-Cur-TRF inhibits the growth of colorectal cancer cells in vitro and SCID mice xenograft and to elucidate the underlying regulatory process by focusing on the ETO-Cur-TRF mediated role of gut microbiome.
Methods
Cell lines and reagents
Human colon cancer cells HT-29 and HCT-116 were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Grand Island, NY) and 1% antibiotic/antimycotic in a humidified incubator at 37°C in an atmosphere of 95% air and 5% carbon dioxide. 5-Fluorouracil + Oxaliplatin (FuOx) resistant cells were generated in our laboratory as described earlier [35, 36]. These cells were maintained in DMEM supplemented with FBS containing 2 x FuOx (50μM 5 FU + 1.25 μM Ox) culture medium. The medium was changed twice a week, and cells were passaged using 0.05% trypsin/EDTA (Invitrogen).
ETO-Cur was obtained from Dolcas Biotech, Chester,NJ; (BCM-95, Lot # 1511–39). This product, in addition to Curcumin contained other constituents of turmeric, primarily essential turmeric oils (ETO) comprising of aromatic-tumerones (ar-tumerones), α-turmerones, β-turmerones, α-santalene and aromatic curcumene. TRF composed of 190 mg total tocotrienol (72 mg α-tocotrienol, 10 mg β-tocotrienol; 94 mg δ-tocotienol) + 57 mg α-tocopherol and 0.43 mg mixed carotenes was a product of Carotino, Sdn. Bhd., Malaysia. The capsules were manufactured by Kamata Co. Ltd., Tokyo, Japan (Lot # 161983170105).
Determination of cell growth
The growth of colon cancer cells was determined by mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma) to formazan as described previously.[37] Briefly, the cells (5x103) were seeded in quadruplicates onto 24 well culture dishes. After 24 hours, fresh medium containing various concentrations of ETO-Cur and TRF was added. After 72 h, cell survival was determined by MTT assay. The medium was removed from the dishes and the cells were incubated at 37°C with MTT (0.5 mg/mL in 1XPBS) for 4 hours. The medium was then aspirated and the cells were solubilized in 0.04 N HCL in isopropanol. The optical density (OD) was measured at both 570 and 630 nm.
Analysis of interaction between ETO-Curcumin and TRF
Combination indices (CI) method adapted for in vitro drug testing was employed to determine the nature of interaction between ETO-Cur and TRF. The method utilizes multiple drug effect equations which were originally derived from enzyme kinetic methods. The output is represented as CI and/or isobologram analysis. CI analysis was performed by using Calcusyn software (Biosoft, Ferguson, MO). Based on the CI values attained, the extent of synergism/antagonism is determined. Generally, CI values below 1 suggest synergy, whereas CI values above 1 indicate antagonism between the 2 drugs. CI values in the range of 0.9–1.1 would mostly indicate additive effects, values between 0.85–0.9 suggest slight synergy, and values between 0.3–0.7 are indicative of moderate synergy.[38] All values less than 0.3 would suggest strong synergy between the two drugs.[38]
Tumor growth in SCID mice
All animal experiments were performed according to Wayne State University’s Institutional Animal Care and Use Committee (IACUC) approved protocol #A02-02-13. Animal Welfare Assurance #A3310-01.
Tumors were generated in 4-week-old female SCID mice (Taconic Laboratory) by subcutaneous injections of 1 x 106 HCT-116 cells suspended in 100 μL Matrigel in the flank region on either side. To study the chemo-preventive efficacy of ETO-Cur and TRF combination, animals were given 5 mg/kg ETO-Cur and 2 mg/kG TRF in 100 μL sesame oil by oral gavage 7 days after inoculation of cells. ETO-Cur-TRF treatment, given 5 days a week (Monday to Friday) was continued until the animals were sacrificed. The animals in the control group were given only sesame oil by oral gavage. Tumor volumes were calculated as previously described [37]. Feces were collected at the start and during the experimental period to analyze changes in gut microbiome. Mice were monitored regularly for any signs of discomfort. At the end of the treatment period, all animals were sacrificed by CO2 inhalation. Assurance of death was performed by cervical dislocation.
DNA extraction for 16S rRNA microbiota community profiling
Genomic DNA was extracted from mice feces using QIAamp DNA Stool Mini Kit (Qiagen, CA, USA) according to the manufacturer’s instruction and as described.[39] Mice feces in ASL buffer were thoroughly vortexed and the suspension was heated for 15 min at 70 0C. DNA was isolated from this suspension according to manufacturer’s protocol. Purified DNA was used for analysis of 16S rRNA community profiling, which was performed by LC Sciences (Houston, Texas, USA) on fee per basis. The conserved regions of V3 and V4 of the 16S rRNA gene were amplified using PCR. After one cycle of PCR, sequencing adapters and barcodes were added for further amplification of the library for sequencing. Bacterial 16S rDNA universal primer (338F: 5’-ACTCCTACGGGAGGCAGCAG-3’; 806R: 5’-GGACTACHVGGGTWTCTAAT-3’) was used to generate amplicons of approximately 469 bp using Phusion Hot start Master Mix (New England Biolab, USA). PCR condition for 16S fragment amplification was consisted of initial denatuation 98°C for 30sec, followed by 35 cycles of 98°C for 10sec, 64°C for 30sec, 72°C for 45sec, with a final extension at 72°C for 10 min. PCR amplification products were detected by 2% agarose gel electrophoresis and target fragments were recovered using the AxyPrep PCR Cleanup Kit (Fisher Scientific, USA).
The PCR product was further purified using the Quant-iT PicoGreen dsDNA Assay kit (Therofisher Scientific, USA). The library was quantified using the Promega QuantiFluor Fluoresence quantification system. Each qualified sequencing library (above 2 nM) was diluted and pooled/multiplexed. The pooled library was loaded on Illumina MISeq system and MISeq Reagent Kit V3 (600 cycles) was used for pair-end sequencing (2x300 cycles). Raw reads were obtained from MiSeq in the FASTQ format and the Phred score was in the Sanger format (Illumina CASAVA version 1.8) was used for base calling. For raw data processing, the data analysis software FLASH (Fast Length Adjustment of Short reads) v1.2.8 was used to merge the pair-end reads and Vsearch was used to remove chimera sequences. The filtered sequences were clustered into operational taxonomic units (OTUs) based on 97% sequence similarity. The Biological Observation Matrix (BIOM) format, QIIME (Quantitative insights into microbial ecology) was used to save the abundance and taxa information for each sample. R (OTU) statistics was used for each sample statistics. For alpha and beta diversity analysis, OTUs abundance was used to assess the diversity of bacteria in each sample. For taxonomic analysis OTUs were mapped to RDP and NT-16S for bacterial taxonomy.
Real-Time PCR
For Real Time PCR, DNA was analyzed in triplicate using the 2× Power-Up SYBR Green PCR Master Mix (Applied Biosystems) and the ABI Prism 7500 sequence detection system. PCR consisted of 40 cycles of 95°C for 10 min and then 95°C for 15 sec, 60°C for 60 sec. The primer sequences of Bifidobacteria and Lactobacillus were used to evaluate the presence of specific types of bacteria [39]. Ct values were normalized to assess the relative concentration of specific DNA for each sample as described by the manufacturer. Each sample ΔΔCT values were calculated by normalizing to the CT value of total bacteria (Eubacteria) [40]. All primers were synthesized by Integrated DNA Technology Inc. (Coralville, IA, USA).
Statistical analysis
For microbiota data statistical analysis, Metastats software for metagenomics sequencing data from two groups to characterize the microbial communities was utilized. CD-HT and R statistical software was used for BIOM-formatted OTU communities clustering and OTU statistics. For examining alpha diversity, QIIME software was used for graphics and statistical purposes. RDP classifier, QIIME, TUIT GraPhlAn, MetaPhlAn, R software/Too were used for taxonomic classification and statistics.
Statistical analysis was performed using Microsoft Excel. Unless otherwise stated, data were expressed as mean +/- SD. For Real Time PCR data, standard deviation of mean between two groups and t-test were performed to determine the significance level between two groups. The p-values were calculated using 2 sample t-test, assuming unequal variance. Values <0.05 were considered statistically significant.
Results
ETO-Curcumin and TRF show synergistic effect
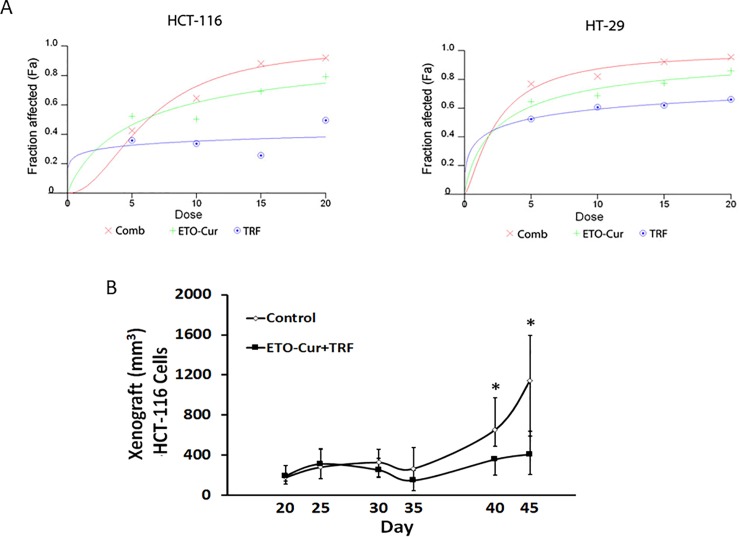
Two human colon cancer cells HT-29 and HCT-116 were used to study synergy effects between ETO-Cur and TRF. Data from in vitro studies revealed that although ETO-Cur and TRF, each alone inhibited the growth in both HCT-116 and HT-29 cells, the magnitude of inhibition was much greater when both agents were given together (Fig 1A). The effect of the combinatorial treatment was found to be synergistic in both HT-116 and HT-29 colon cancer cells but only at 5 μM and 10 μM levels as evidenced by CI values of ≤0.8 for both cell lines (Table 1). However, maximal synergism revealing a CI value of 0.42 for HCT-116 cells was observed with the lowest level (5 μM) of ETO-Cur and TRF (Table 1). We also found HT-116 cells to be more sensitive to both ETO-Cur and TRF (Fig 1A).
Fig 1.
Dose response curves for ETO-Cur and TRF in HCT-116 and HT-29 cells produced by fixed ratio method (A). The cells were treated with increasing doses of ETO- Cur and TRF for 72 h in quadruplicate. MTT assay was performed to calculate viable and the affected cells. Fa represents the fraction of cells affected in response to the treatment. Fa for combination treatment was higher than either agent alone. Fa values were used to calculate synergy using CalcuSyn software as described in Materials and Methods. CI values < 0.9 suggest synergy. Tumor growth in SCID mice injected with HCT-116 cells (B). The mice were given a mixture of 5 mg/kg ETO-Cur and 2 mg/kg TRF five days a week by oral gavage in 100 μl sesame oil for 6 weeks. Control animals were given sesame oil alone. Tumors were measured once a week and tumor volumes were calculated using the formula: Tumor volume = length x width x width/2. Each point represents average volume of 8 tumors ± standard deviation. *p value <0.05.
Table 1. Synergy analysis for ETO-Cur and TRF combination therapy in colon cancer cells.
| Combination Therapy | Combination Index (CI) | ||
|---|---|---|---|
| ETO-Cur (μM) | TRF (μM) | HCT116 | HT-29 |
| 5 | 5 | 0.4260 | 0.7690 |
| 10 | 10 | 0.6450 | 0.8200 |
| 15 | 15 | 0.8820 | 0.9230 |
| 20 | 20 | 0.9200 | 0.9560 |
Combination index values <1.0 are increasingly supra-additive, and Values >1.0 are increasing less than additive
In HCT-116 xenograft mouse model, combination of ETO-Cur and TRF significantly inhibited tumor growth compared to the control (Fig 1B). Significant inhibition of tumor growth in response to the combinatorial treatment of ETO-Cur and TRF became evident after 40 days (Fig 1B). The current observation suggests a synergistic effect of ETO-Cur and TRF in inhibiting the growth of CRC cells in vitro and in mouse xenograft in vivo.
Microbial 16S rRNA profiling of ETO-Cur-TRF treated mice
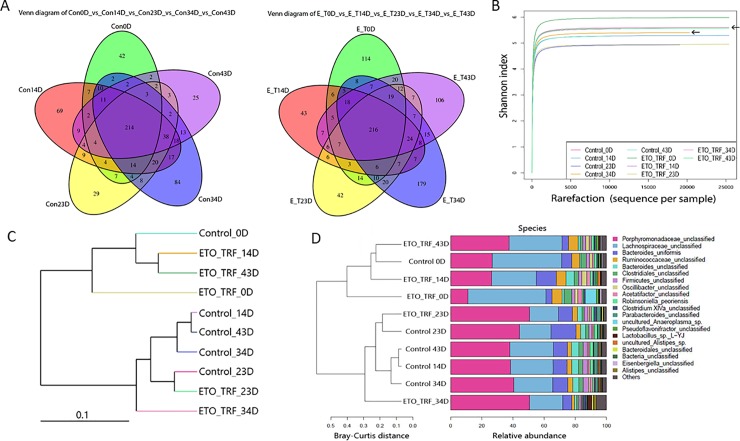
It is well known that gut microbiome plays a key role in modulating biological activities, bioavailability and bio-efficacy of polyphenols and their metabolites.[41–44] To elucidate changes in microbial communities associated with ETO-Cur-TRF treatment, we sequenced 16S rRNA genes isolated from feces of mice bearing HCT-116 xenograft tumors. Sequencing of the V3+V4 region was used for operation taxonomic unit (OTU) clustering based on 97% sequence similarity. The changes of microbial OTU exhibited during different periods of ETO-Cur-TRF treatment and respective controls revealed that microbial OTU number increased following 34 days of ETO-Cur-TRF treatment (Fig 2A), when tumor inhibition also began to show difference over the control and began to diverge (Fig 1B).
Fig 2.
Venn diagram showing the shifting of microbial OTUs between control and ETO-Cur-TRF treated mice feces from 0 days to 43 days (A). Values were obtained from purified DNA pooled from 3–4 mice in each group. Venn diagram shows the similarity and specificity of OTU in different samples/groups. Each circle in Venn diagram stands for a sample. The overlapped region shows the number of common OTUs and non–overlapped region showed the number of common OTUs and non overlapped region shows the number of unique OTUs. Alpha diversity shows that the combination of ETO-Cur-TRF increases species diversity and richness in treated mice. Rarefaction curves are plotted by Shannon index showing the OTUs/species abundance in different days and that the species richness increased on 34day as indicated by arrows (B). Alpha diversity was determined using QIIME. ETO-Cur-TRF treated mice shows the differential distribution of microbial communities. Beta diversity analysis shows the relative abundance of microbial communities using distant matrix as determined by UPGMA Weighted-UniFrac clustering of microbial OTU/species sequence in different samples. The distance suggests differences in microbial composition in samples (C) and Bray-Curtis plot shows the differences of microbial species abundance in community (D).
For alpha diversity, Shannon diversity index revealed the distribution of microbial taxa richness. Although the diversity index shows a similar index number between the two groups (Control = 5.40; ETO-Cur-TRF = 5.52), OTU of species revealed a marked increase in the ETO-Cur-TRF group compared to control at 34 day (Fig 2B). Beta diversity in phylogenetic tree showed the relative abundance of microbial composition diverse between ETO-Cur-TRF treated and the control after 34 day of treatment (Fig 2C) and Bray-Curtis plot revealed the dissimilarity of species among microbial communities in samples (Fig 2D).
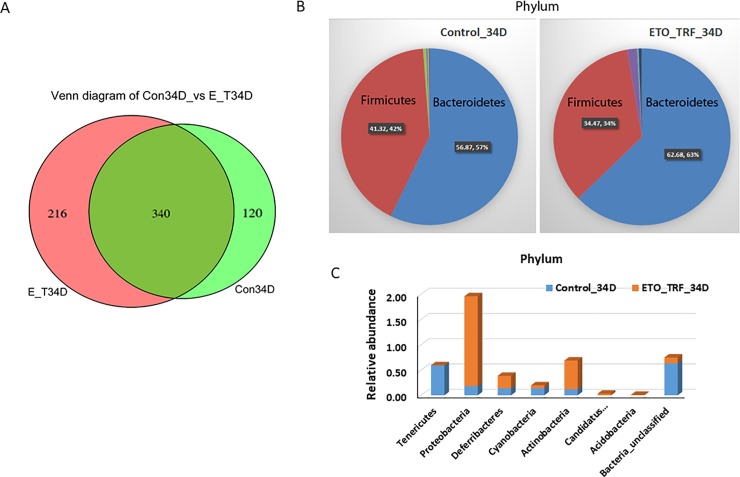
Further analysis of the data revealed that an overlap of 340 bacterial species between the control and ETO-Cur-TFR-treated SCID mice and the diversity of species number(n) increased 44% in ETO-Cur-TRF treated mice (216n) compared to control mice (120n) after 34 days (Fig 3A). Thus ETO-Cur-TRF treatment correlated with increased species richness and diversity. Relative abundance of microbial phylum was found to be predominantly Bacteroides and Firmicutes (97–99%) in both groups of mice (Fig 3B). However, ETO-Cur-TRF significantly increased the abundance of Proteobacteria (9.9-fold) and Actinobacteria (4.7-fold) (Fig 3C). In the controls, the abundance of Proteobacteria and Actinobacteria represented 2% and 0.57% of total microbiota phylum, (Fig 3B). Tenericute, whose abundance in control represented 0.59%, was totally eliminated in ETO-Cur-TRF treated mice (Fig 3B).
Fig 3.
Venn diagram showing shared microbial OTUs between 34 day of ETO-Cur-TRF treated and control mice; ETO-Cur-TRF increased microbial diversity (A) and altered microbial phylum (B and C). Genomic DNA from mouse fecal cells was isolated using QIAamp DNA Stool Mini Kit (Qiagen) according to the manufacturer’s instruction, subsequently used for analysis of 16S rRNA gene community profiling.
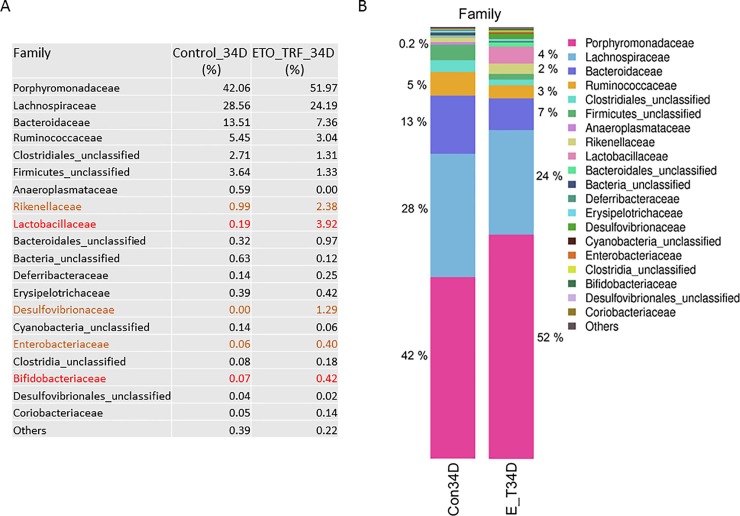
The composition of the microbial family was predominantly Porphydomonadaceae, Lachnospiraceae, Bacteroidaceae and Ruminococcaceae in both control and ETO-Cur-TRF treated mice. While 16s RNA gene sequencing revealed that ETO-Cur-TRF increased the abundance of Porphymonadaceae, Rickenellaceae, Lactobacillaeceae, Desulphovibrionaceae, Enterobactericeae and Bifidobacteriaceae families, it decreased Bacteroidaceae significantly that included Lachnospirace, Ruminococcaceae and Firmicutes (Fig 4A and 4B). Microbial diversity decreased by 45% in Bacteroidaceae family in ETO-Cur-TRF group after 34 days of treatment, but Rickenellaceae increased in the treated group (Fig 4B). Interestingly, probiotic and anti-inflammatory Lactobacillaceae and Bifidobactericeae was increased 20-fold and 6-fold, respectively in the ETO-Cur-TRF treated mice compared to the corresponding control (Fig 4B).
Fig 4. 16S rRNA microbial community profiling showing the relative abundance of Family.
The changes of microbial family after ETO-Cur-TRF treatment at 34 days when compared with the corresponding control (A). Percent changes in different microbial family between ETO-Cur-TRF treated and the corresponding control (B). Genomic DNA from mouse fecal cells was used to analyze microbial OTU abundance.
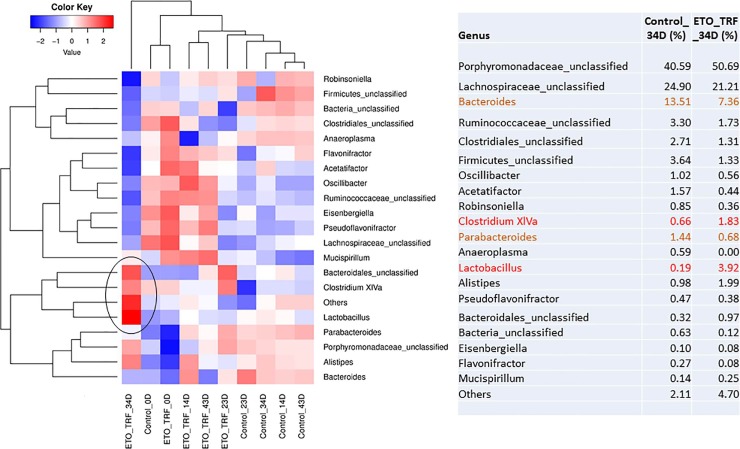
16S gene profiling revealed that the abundance of Bacteroides and Parabacteroides was decreased in ETO-Cur-TRF treated mice while genus Clostridium XIVa was increased significantly (Fig 5). Lactobacillus also increased significantly with ETO-Cur-TRF as well as Alistipes. At species level, Bacteroides Uniformis were reduced in ETO-Cur-TRF treated mice.
Fig 5. Heatmap showed the effect of ETO-Cur-TRF on relative abundance of microbial genus level in genomic DNA from mice feces.
Red indicates high abundance and blue indicates less abundance. Marked increases in beneficial bacteria could be noted as indicated by red color after 34 days of ETO-Cur-TRF treatment. Black circle indicates increased abundance of specific microbial genus following ETO-Cur-TRF treatment.
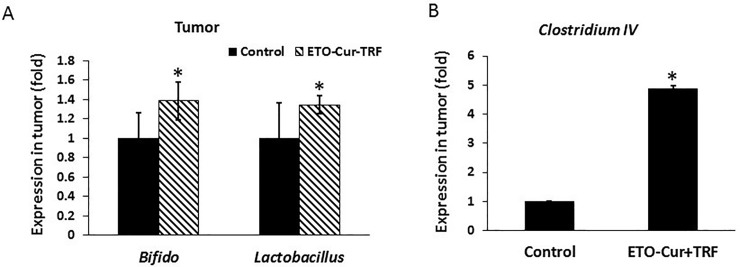
To determine whether gut microbial changes are reflected in the tumor, relative abundance of a limited number of bacteria in the mouse xenograft was performed by qPCR. Genomic DNA extracted from the tumors of SCID mice that were treated with ETO-Cur-TRF or vehicle (controls) for 34 days. DNA was assayed for Bifidobacteriaceae and Lactobacilaceae by qPCR with specific primers. Abundance of probiotic bacteria, Bifidobateria, and Lactobacillus in the tumor was found to be significantly higher in the ETO-Cur-TRF treated mice than their control (Fig 6A). Likewise, the relative abundance of beneficial bacteria Clostridium IV was also increased, compared to the control (Fig 6B). Clostridium IV is known to possess anti-inflammatory effects.[45–47]
Fig 6.
Changes in the relative abundance of (A) Bifidobacteria and Lactobacillus and (B) Clostridium IV in the xenograft of SCID mice 34 days following administration of Cur-ETO-TRF or vehicle (controls) as determined by qPCR (50 ng DNA/sample) with specific primers and each sample ΔΔCT values were calculated by normalizing to the CT value of total Eubacteria. Bars represent mean of three reading standard deviation; *p<0.01, compared to the corresponding control.
Discussion
While vitamin E isomers and Curcumin exhibit poor bioavailability, ETO-Cur, which is found to be 7–8 times more bioavailable than standard Curcumin synergized with TRF and together they greatly inhibited the growth of colon cancer cells in vitro and in vivo. It has been reported that the anti-inflammatory property of ETO-Cur is superior than standard Curcumin as evidenced by the improved disease activity index in dextran sodium sulfate–induced ulcerative colitis in mice.[15] Earlier studies also demonstrated that treatment with Curcumin or dietary polyphenols causes alterations in microbial composition accompanied by induction of mucosal immune cells.[6, 43, 48, 49] Our current data also demonstrate that administration of ETO-Cur-TRF modulates fecal microbial composition and increases microbial OTU biodiversity.
Analysis of 16S rRNA genes in cells isolated from feces from control and ETO-Cur-TRF treated mice revealed the phylogenetic structure of active microbial communities. Our current data show that ETO-Cur-TRF mediated alterations in microbial composition exhibited an increased abundance of Porphymonadaceae, Firmicutes, Rickenellaceae, Lactobacillaeceae and Bifidobacteriaceae; while Bacteroidaceae, Lachnospirace and Ruminococcaceae were decreased. These changes in bacterial populations in mice following ETO-Cur-TRF treatment clearly show that the current combinatorial treatment exerts a profound effect on the gut microbiome and suggest that the changes in microbial diversity are linked to changes in CRC proliferation. Future work will examine whether they have a role in inhibiting the growth of colon cancer cells in vitro and in vivo.
It has been reported that enrichment of Bacteriodes and reduction of members of the Porphydomonaceae family are associated with tumor growth in mice.[50] Our current observation that administration of ETO-Cur-TRF decreased Bacteroides by about 45% suggests that inhibition of tumor growth in response to the current combinatorial treatment could partly be attributed to reduction in Bacteroides. It has also been reported that Bacteroides, Parabacteroides and Alistipes are strongly associated with tumor growth, whereas several members of Clostridium cluster including XIVa are associated with reduction in CRC tumor.[44] In the current investigation, we also found a similar trend in that ETO-Cur-TRF induced inhibition of tumor growth was associated with a greater abundance of Clostridium XIVa.
The gut microbiome plays a pivotal role in maintaining epithelial health and immune homeostasis.[34] Certain bacterial populations serve a protective role and are considered to be important mediators of immune health. Consequently, loss of them can induce inflammation and tumorigenesis. Our study shows that the microbial community in the gut changes in response to ETO-Cur and TRF treatment. In addition, we show that the combination of ETO-Cur and TRF leads inhibits proliferation of colorectal cancer cells in vitro and in mouse xenografts in vivo.
Acknowledgments
This study was supported by grants to Dr. Majumdar from the NIH (1R21CA175916), the Department of Veteran Affairs (I101BX001927) and the Metropolitan Detroit Research and Education Fund (MDREF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data have been deposited to NCBI, DOI: PRJNA600246 (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA600246).
Funding Statement
This study was funded by grants to Dr. Adhip Majumdar from the National Institute of Health, USA (NIH) (1R21CA175916), the Department of Veteran Affairs, USA (I101BX001927) and the Metropolitan Detroit Research and Education Fund, Detroit, Michigan, USA (MDREF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yu Y, Nangia-Makker P, Farhana L, S GR, Levi E, Majumdar AP. miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Molecular cancer. 2015;14:98 10.1186/s12943-015-0372-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duineveld LA, van Asselt KM, Bemelman WA, Smits AB, Tanis PJ, et al. Symptomatic and Asymptomatic Colon Cancer Recurrence: A Multicenter Cohort Study. Annals of family medicine. 2016;14(3):215–20. 10.1370/afm.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. The Cochrane database of systematic reviews. 2007;(1):CD002200. [DOI] [PubMed] [Google Scholar]
- 4.Sargent DJ, Conley BA, Allegra C, Collette L. Clinical trial designs for predictive marker validation in cancer treatment trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(9):2020–7. [DOI] [PubMed] [Google Scholar]
- 5.van Gestel YR, de Hingh IH, van Herk-Sukel MP, van Erning FN, Beerepoot LV, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer epidemiology. 2014;38(4):448–54. 10.1016/j.canep.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 6.Zam W. Gut Microbiota as a Prospective Therapeutic Target for Curcumin: A Review of Mutual Influence. Journal of nutrition and metabolism. 2018;2018:1367984 10.1155/2018/1367984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantzorou M, Pavlidou E, Vasios G, Tsagalioti E, Giaginis C. Effects of curcumin consumption on human chronic diseases: A narrative review of the most recent clinical data. Phytotherapy research: PTR. 2018;32(6):957–75. 10.1002/ptr.6037 [DOI] [PubMed] [Google Scholar]
- 8.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clinical and experimental pharmacology & physiology. 2012;39(3):283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Sureda A, Devkota HP, Pittala V, Barreca D, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnology advances. 2019. [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clinical and experimental pharmacology & physiology. 2006;33(10):940–5. [DOI] [PubMed] [Google Scholar]
- 11.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnology advances. 2014;32(6):1053–64. 10.1016/j.biotechadv.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 12.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Molecular pharmaceutics. 2007;4(6):807–18. 10.1021/mp700113r [DOI] [PubMed] [Google Scholar]
- 13.Shishu, Gupta N, Aggarwal N. Bioavailability enhancement and targeting of stomach tumors using gastro-retentive floating drug delivery system of curcumin—"a technical note". AAPS PharmSciTech. 2008;9(3):810–3. 10.1208/s12249-008-9096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian journal of pharmaceutical sciences. 2008;70(4):445–9. 10.4103/0250-474X.44591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toden S, Theiss AL, Wang X, Goel A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Scientific reports. 2017;7(1):814 10.1038/s41598-017-00812-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans HM, Bishop KS. On the Existence of a Hitherto Unrecognized Dietary Factor Essential for Reproduction. Science. 1922;56(1458):650–1. 10.1126/science.56.1458.650 [DOI] [PubMed] [Google Scholar]
- 17.Abubakar IB, Lim KH, Loh HS. Alkaloid extracts of Ficus species and palm oil-derived tocotrienols synergistically inhibit proliferation of human cancer cells. Natural product research. 2015;29(22):2137–40. 10.1080/14786419.2014.991927 [DOI] [PubMed] [Google Scholar]
- 18.Lim SW, Loh HS, Ting KN, Bradshaw TD, Zeenathul NA. Cytotoxicity and apoptotic activities of alpha-, gamma- and delta-tocotrienol isomers on human cancer cells. BMC complementary and alternative medicine. 2014;14:469 10.1186/1472-6882-14-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim SW, Loh HS, Ting KN, Bradshaw TD, Zeenathul NA. Antiproliferation and induction of caspase-8-dependent mitochondria-mediated apoptosis by beta-tocotrienol in human lung and brain cancer cell lines. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2014;68(8):1105–15. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Xiao H, Jin H, Koo PT, Tsang DJ, Yang CS. Synergistic actions of atorvastatin with gamma-tocotrienol and celecoxib against human colon cancer HT29 and HCT116 cells. International journal of cancer Journal international du cancer. 2010;126(4):852–63. 10.1002/ijc.24766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127–42. 10.1196/annals.1331.013 [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80(11):1613–31. 10.1016/j.bcp.2010.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mocchegiani E, Costarelli L, Giacconi R, Malavolta M, Basso A, et al. Vitamin E-gene interactions in aging and inflammatory age-related diseases: implications for treatment. A systematic review. Ageing research reviews. 2014;14:81–101. 10.1016/j.arr.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 24.Kaileh M, Sen R. Role of NF-kappaB in the anti-inflammatory effects of tocotrienols. Journal of the American College of Nutrition. 2010;29(3 Suppl):334S–9S. [DOI] [PubMed] [Google Scholar]
- 25.Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. The Journal of biological chemistry. 2007;282(1):809–20. 10.1074/jbc.M610028200 [DOI] [PubMed] [Google Scholar]
- 26.Rajendran P, Li F, Manu KA, Shanmugam MK, Loo SY, et al. gamma-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. British journal of pharmacology. 2011;163(2):283–98. 10.1111/j.1476-5381.2010.01187.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachawal SV, Wali VB, Sylvester PW. Enhanced antiproliferative and apoptotic response to combined treatment of gamma-tocotrienol with erlotinib or gefitinib in mammary tumor cells. BMC cancer. 2010;10:84 10.1186/1471-2407-10-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keku TO, McCoy AN, Azcarate-Peril AM. Fusobacterium spp. and colorectal cancer: cause or consequence? Trends Microbiol. 2013;21(10):506–8. 10.1016/j.tim.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulal S, Keku TO. Gut microbiome and colorectal adenomas. Cancer journal. 2014;20(3):225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang LL, Liu JX, Yu XJ, Si JL, Zhai YX, Dong QJ. Microbial community reshaped in gastric cancer. European review for medical and pharmacological sciences. 2018;22(21):7257–64. 10.26355/eurrev_201811_16260 [DOI] [PubMed] [Google Scholar]
- 31.Yan Q, Gu Y, Li X, Yang W, Jia L, et al. Alterations of the Gut Microbiome in Hypertension. Frontiers in cellular and infection microbiology. 2017;7:381 10.3389/fcimb.2017.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGarr SE, Ridlon JM, Hylemon PB. Diet, anaerobic bacterial metabolism, and colon cancer: a review of the literature. Journal of clinical gastroenterology. 2005;39(2):98–109. [PubMed] [Google Scholar]
- 33.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Current opinion in gastroenterology. 2014;30(3):332–8. 10.1097/MOG.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dethlefsen L, Eckburg PB, Bik EM, Relman DA. Assembly of the human intestinal microbiota. Trends in ecology & evolution. 2006;21(9):517–23. [DOI] [PubMed] [Google Scholar]
- 35.Nautiyal J, Kanwar SS, Yu Y, Majumdar AP. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J Mol Signal. 2011;6:7 10.1186/1750-2187-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Translational oncology. 2009;2(4):321–8. 10.1593/tlo.09193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nangia-Makker P, Yu Y, Vasudevan A, Farhana L, Rajendra SG, et al. Metformin: a potential therapeutic agent for recurrent colon cancer. PloS one. 2014;9(1):e84369 10.1371/journal.pone.0084369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majumdar AP, Banerjee S, Nautiyal J, Patel BB, Patel V, et al. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutrition and cancer. 2009;61(4):544–53. 10.1080/01635580902752262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farhana L, Antaki F, Murshed F, Mahmud H, Judd SL, et al. Gut microbiome profiling and colorectal cancer in African Americans and Caucasian Americans. World journal of gastrointestinal pathophysiology. 2018;9(2):47–58. 10.4291/wjgp.v9.i2.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farhana L, Dawson MI, Das JK, Murshed F, Xia Z, et al. Adamantyl Retinoid-Related Molecules Induce Apoptosis in Pancreatic Cancer Cells by Inhibiting IGF-1R and Wnt/beta-Catenin Pathways. Journal of oncology. 2012;2012:796729 10.1155/2012/796729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerda B, Tomas-Barberan FA, Espin JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. Journal of agricultural and food chemistry. 2005;53(2):227–35. 10.1021/jf049144d [DOI] [PubMed] [Google Scholar]
- 42.Gross G, Jacobs DM, Peters S, Possemiers S, van Duynhoven J, et al. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. Journal of agricultural and food chemistry. 2010;58(18):10236–46. 10.1021/jf101475m [DOI] [PubMed] [Google Scholar]
- 43.Cardona F, Andres-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuno MI. Benefits of polyphenols on gut microbiota and implications in human health. The Journal of nutritional biochemistry. 2013;24(8):1415–22. 10.1016/j.jnutbio.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 44.Baxter NT, Zackular JP, Chen GY, Schloss PD. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome. 2014;2:20 10.1186/2049-2618-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50. 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- 47.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohno M, Nishida A, Sugitani Y, Nishino K, Inatomi O, et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PloS one. 2017;12(10):e0185999 10.1371/journal.pone.0185999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Espin JC, Gonzalez-Sarrias A, Tomas-Barberan FA. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol. 2017;139:82–93. 10.1016/j.bcp.2017.04.033 [DOI] [PubMed] [Google Scholar]
- 50.Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, et al. The gut microbiome modulates colon tumorigenesis. mBio. 2013;4(6):e00692–13 10.1128/mBio.00692-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data have been deposited to NCBI, DOI: PRJNA600246 (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA600246).