Abstract
(a). Purpose:
Extracellular vesicles (EV), which include exosomes and microvesicles, are membrane-bound particles shed by most cell types and are important mediators of cell-cell communication by delivering their cargo of proteins, miRNA and mRNA to target cells and altering their function. Here, we provide an overview of what is currently known about EV composition and function in bone and muscle cells and discuss their role in mediating crosstalk between these two tissues as well as their role in musculoskeletal aging.
(b). Recent Findings:
Recent studies have shown that muscle and bone cells produce EV, whose protein, mRNA and miRNA cargo reflects the differentiated state of the parental cells. These EV have functional effects within their respective tissues but evidence is accumulating that they are also shed into the circulation and can have effects on distant tissues. Bone and muscle-derived EV can alter the differentiation and function of bone and muscle cells. Many of these effects are mediated via small microRNAs that regulate target genes in recipient cells.
(c). Summary:
EV-mediated signaling in muscle and bone is an exciting and emerging field. While considerable progress has been made, much is still to be discovered about the mechanisms regulating EV composition, release, uptake and function in muscle and bone. A key challenge is to understand more precisely how exosomes function in truly physiological settings.
Keywords: Bone, Muscle, Exosomes, miRNA, Crosstalk, Aging
Introduction
Extracellular vesicles (EV) are increasingly being recognized as new players in cell-to-cell communication, leading to changing paradigms in our understanding of how cells communicate with their neighbors and how they crosstalk with cells in distant tissues (reviewed in[1–7]). EV are membrane-bound particles shed from cells which carry their cargo of proteins, mRNAs and microRNAs (miRNAs). These EV dock with a target cell, delivering their cargo to the cell and thereby altering its differentiation and/or function. Several types of EV have been described, with the most widely studied being exosomes (20–140nm), which are released from multivesicular bodies, and microvesicles (100nm-1μm), which bud from the plasma membrane (figure 1)[8, 9]. Although initially studied in the cancer field, EV are now known to be shed by most normal cell types, with their cargo reflecting the differentiated state of the parent cells. Accumulating evidence suggests that exosomes are essential for cell-to-cell, organ-to-organ, and whole-body communication and have pleiotropic effects on the functions of neighboring or distant cells, tissues and organs[10, 11, 9, 12, 13]. For example, EVs released by cells of one tissue or organ can travel in the circulation and may bind to cells of another tissue or organ through receptor-ligand interactions[14]. They can also spread through the body and anchor and/or fuse with the membrane to deliver cargos to distant recipient cells[15, 16].
Figure 1: Biogenesis and uptake of extracellular vesicles:
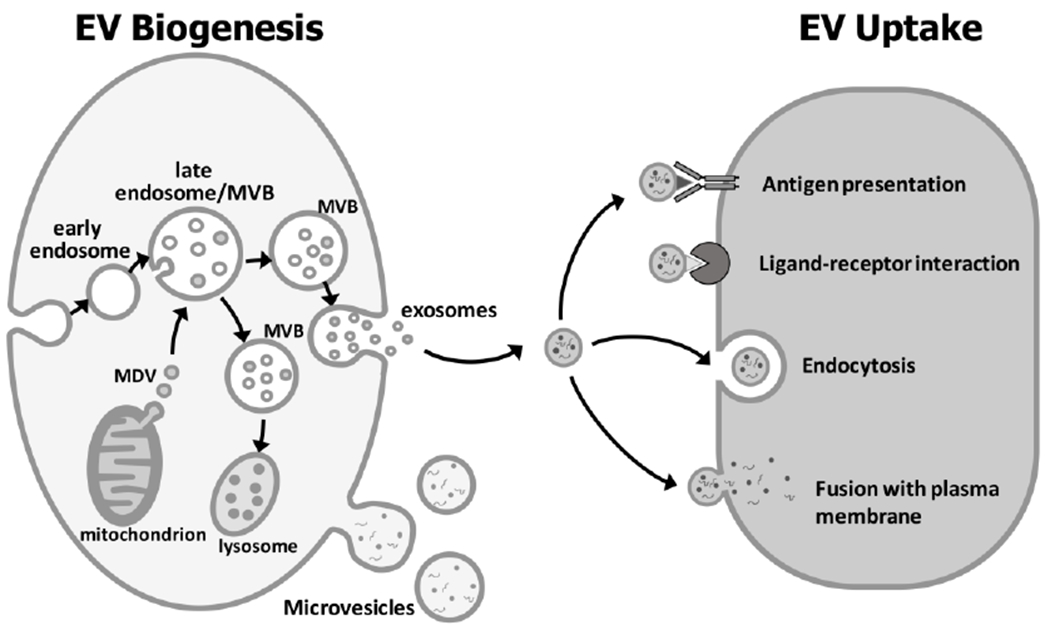
Exosomes form by inward budding of the late endosomal membrane to generate smaller internal vesicles within a multi-vesicular body (MVB). The MVBs can fuse with the lysosome for degradation of their cargo or fuse with the plasma membrane, releasing the internal vesicles, termed exosomes (20-140nm), extracellularly. Mitochondrial derived vesicles (MDV) (70-150 nm) can enter the endosome and its derivative MVBs and can also contribute to the exosomal population. Microvesicles (100nm-1μm) form by outward budding of the plasma membrane. EV can interact with recipient cells by signaling via antigen-antibody or ligand-receptor interactions. The most common mechanism of EV uptake involves endocytosis or the EV can fuse with the plasma membrane. Upon fusion or uptake, the EV cargo is released into the target cell (Diagram modified from[19, 69]).
The EV protein cargo can theoretically have direct effects in target cells and the mRNAs can be expressed in target cells, thereby altering their function. EVs also contain miRNA cargo, which are single stranded non-protein-coding RNAs of about 22–26 nucleotides in length. These function mainly as gene repressors at the transcriptional or post-transcriptional level by binding to the 3’-untranslated regions of mRNA transcripts and suppressing mRNA translation and/or stimulating mRNA degradation[17]. If delivered to cells via EV, miRNAs could regulate gene expression in the target cell. It is thought that the packaging of protein, mRNA and miRNA cargos into EV protects them from degradation by extracellular proteases and nucleases. By expressing specific cell surface proteins, ligands and receptors that may interact with proteins and/or receptors on target cells, the EV may also have increased specificity (i.e. a “molecular address”) to deliver their cargo to a defined target cell. In theory, this provides a more efficient mechanism for cell-cell communication compared to signaling via diffusible ligands/soluble mediators. Not only are EV involved in cell-cell communication, but increasing evidence suggests they play an important role in pathological and disease processes and that analysis of EV as circulating biomarkers may be a useful tool for diagnosis and monitoring of disease. Furthermore, numerous studies have examined potential applications of EVs for treatment of disease and as a drug delivery mechanism. In this review, we provide a brief overview of EV biogenesis and uptake and then focus on recent studies that have revealed important functions for exosomes in bone and muscle cells and their role in mediating crosstalk between these two tissues. We also review their role in musculoskeletal aging and discuss current limitations in the field.
EV Biogenesis and Uptake
Exosomes are derived from the endosomal cell sorting machinery (reviewed in[1, 18, 19]). An early endosome forms via inward budding of the cell membrane. After this, the endosomal membrane undergoes a second inward (intraluminal) budding to generate smaller vesicles within the late endosome lumen to form a multivesicular body (MVB) (figure 1). The MVB then either fuses with a lysosome for degradation or fuses with the plasma membrane, thereby releasing the intraluminal vesicles, termed exosomes, into the extracellular space. Mitochondrial-derived vesicles may also enter the MVBs and can contribute to the final exosome population. Formation of intraluminal vesicles is thought to be initiated by enrichment of microdomains on the late endosomal membrane with tetraspanins (e.g. CD9, CD63). Proteins of the Endosomal Sorting Complex Required for Transport (ESCRT) family also play a key role. ESCRT I and II are recruited to the site and may initiate the intraluminal budding process. Other ESCRT proteins (e.g. ESCRT III) are then recruited through accessory proteins, ALIX, TSG101 and others to complete the budding process. Proteins involved in exosome biogenesis are therefore commonly identified in exosome proteomic profiles. Alternative pathways for exosome biogenesis have also been proposed and are described in detail in other excellent review articles[1, 18, 19]. Release of exosomes from the cell surface is thought to be a cytoskeleton-dependent p53-controlled process involving ESCRTIII and GTPases of the Rab family (e.g. Rab 5, Rab 27, Rab35).
The mechanisms for microvesicle biogenesis are less well defined, but this type of vesicle forms via outward budding and fission of the plasma membrane of the cell (figure 1). Outward membrane budding may be induced by translocation of phosphatidyl serine from the inner to the outer membrane leaflet in localized domains. The budding process appears to be finalized by contraction of cytoskeletal structures through actin-myosin interactions, mediated via a signaling cascade involving ARF6, phospholipase D and ERK (reviewed in[1, 18, 19]).
Three main mechanisms for EV interaction with target cells have been proposed (figure 1) (reviewed in[19, 1]). The EV may bind to the cell surface via antigen-antibody interaction or ligand-receptor interactions and may potentially trigger signaling via surface receptors, even without EV entry into the cell. The most common mode of EV uptake into target cells involves internalization via endocytotic processes, such as clathrin, caveolin or lipid raft-mediated endocytosis, micropinocytosis or phagocytosis. For EVs entering by endocytosis, the mechanism for transfer out of the endosomal compartment remains unclear, but they may be discharged via lysosomes or released into the cytoplasm. A third proposed mechanism for EV uptake is through direct fusion of EV with the plasma membrane of the cell and release of cargo directly into the cytoplasm. Whether an exosome will bind to a particular cell depends on the EV content and characteristics of its parent cell and the EV uptake mechanism(s) may also be dependent on the cell types of the donor and recipient cells.
EV Function in Bone Cells
Although EV were initially studied in the cancer field, they are now known to be released by virtually all cell types and bone cells are no exception. EV are released by the major bone cell types, osteocytes, osteoblasts and osteoclasts and also by bone marrow stromal (stem) progenitor cells (BMSCs). Accumulating evidence suggests that EV play important roles in communication between these cell types to regulate bone formation and resorption, thereby regulating bone modeling and remodeling. Although we have used terminologies as reported in the various cited articles, readers should keep in mind that many studies describing “exosomes” or “microvesicles” are likely looking at mixed populations and it may be more appropriate to consider them under the generic term “extracellular vesicles” (EV).
Osteoblastic EVs:
Osteoblast-derived exosomes were characterized by Ge et al. (2015), who examined the proteomic profile of exosomes from culture supernatants of mouse MC3T3 osteoblast-like cells[20]. They identified a proteome of 1069 proteins, consisting predominantly of plasma membrane and cytoplasmic proteins. Pathway analysis showed that the exosome cargo included proteins involved in exosome biogenesis, formation and uptake and several proteins involved in osteogenesis. Proteins associated with the eukaryotic initiation factor-2 pathway were enriched in MC3T3-exosomes. This could potentially play a role in osteogenesis, since EIF2α plays a role in BMP2-induced osteoblast differentiation[21], although functional assays were not carried out in this study.
Cui and colleagues performed functional studies with exosomes from mineralizing pre-osteoblastic MC3T3-E1 cells, which they termed mineralizing osteoblasts (MOBs), and showed that they promoted differentiation of ST2 bone marrow stromal cells into osteoblast-like cells[22]. Microarray analysis showed that MOB-exosomes contained 457 miRNAs, of which 43 were highly expressed, including several “osteo-miRNAs” known to be expressed in osteoblasts (miR-1192, miR-680 and miR-302a). Treatment of ST2 cells with MOB-exosomes upregulated 91 and downregulated 182 miRNAs. Several of the upregulated miRNAs were also detected in MOB-exosomes, including miR-3084-3p, miR-680, miR-677-3p and miR-5100, which were highly expressed. These miRNAs target gene pathway networks converging on the β-catenin gene (Ctnnb1), a key transcription factor for osteoblast differentiation. Other miRNAs upregulated in ST2 cells (miR-667-3p, miR-6769b-5p, miR-7044-5p, miR-7668-3p and miR-874-3p) are predicted to inhibit Axin 1, an inhibitor of Wnt/β-catenin signaling. Their work confirmed that Axin 1 was repressed and β-catenin was enhanced in ST2 cells treated with MOB-exosomes, thereby promoting osteogenesis, and raised the possibility that osteoblast-EV could play a role in recruitment of marrow stromal cells into the osteogenic pathway. The findings also highlight the possibility of using exosomes as a pro-osteogenic therapy in bone diseases and bone tissue engineering.
In addition to their osteogenic properties, osteoblast-EVs can contain RANKL protein, suggesting they may regulate osteoclast formation. Deng and colleagues showed that RANKL was present in EV from the UAMS-32P stromal/osteoblastic cell line[23]. EV from these cells promoted formation of TRAP-positive multinucleated cells in RAW264.7 osteoclast precursor cell cultures, presumably through RANKL on the surface of the EVs interacting with its receptor, RANK, on osteoclast precursors. In support of this, these authors showed that the EV bound to the surface of cells that expressed RANK. However, they were internalized in cells that did not express RANK or when RANK-RANKL interactions were inhibited. These studies suggest that release of RANKL-containing EV from osteoblasts may represent a novel mechanism for osteoblast-osteoclast cell-cell communication and regulation of bone resorption.
In addition to their role in bone, osteoblasts play important roles in other biologic processes, such as providing stem cell niches for hematopoiesis and modulating bone metastasis of various types of cancer. A recent study using the human osteoblast cell line, SV-HFO, showed that EV from both non-mineralizing human osteoblasts (NMOBs) and MOB contained an extensive proteome and were taken up by the human prostate cancer cell line, PC3[24]. Bioinformatic analysis of the proteomes of the osteoblast-EV and the RNA expression profiles of PC3 cells treated with osteoblast-EV showed that they converged on pathways related to cell growth and survival. Functional studies confirmed that osteoblast-EV stimulated PC3 prostate cancer cell growth. This highlights the importance of EV-mediated signaling in crosstalk between normal and cancer cells in the metastatic site, and particularly in the bone microenvironment.
Osteoclastic EVs:
Osteoclast-enriched cultures generated from M-CSF-dependent mouse marrow precursors produce exosome-like EVs[25]. Interestingly, EVs from osteoclast precursors enhanced 1,25-dihydroxyvitamin D3-dependent formation of osteoclasts in mouse marrow cultures. In contrast, EVs from differentiated osteoclasts inhibited osteoclast formation in the same assay. These data suggest that osteoclast-EVs can locally regulate osteoclastogenesis, either positively or negatively, depending on the differentiation state of the producer cell. Mechanistically, this study showed that EVs from mature osteoclasts contained the RANKL receptor, RANK, which may compete with RANK on osteoclast surfaces for binding to RANKL. Thus, the EV may function like a decoy RANKL receptor, similar to the naturally occurring RANKL inhibitor, osteoprotegerin[25].
Li and co-workers showed that osteoclast-derived EV can transfer miR-214-3p to osteoblasts and inhibit osteogenesis in vitro and in vivo[26]. Their work showed that increased expression of miR-214-3p in human bone biopsies correlated with increased serum exosomal miR-214-3p and that serum miR-21-3p levels were higher in elderly patients with fractures compared to age-matched controls without fracture. They also found that miR-214-3p serum levels increased with aging in females, regardless of fracture status. Osteoclasts rather than osteoblasts provided the major source of serum exosomal miR-214-3p. Increased osteoclastic and serum miR-214-3p were also associated with decreased bone formation rates in an aged mouse model of ovariectomy-induced bone loss. Targeting of miR-214-3p overexpression to osteoclasts inhibited bone formation in mice and inhibition of miR-214-3p using an osteoclast-targeted antagomir promoted bone formation.
In a related study, Sun and colleagues showed that osteoclasts release exosomes enriched in miR-214[27]. These were taken up into osteoblasts via interaction of ephrinA2 on the exosomes with its receptor EphA2 on osteoblasts. They also showed that bone formation was reduced in transgenic mice overexpressing miR-214 in osteoclasts and confirmed that osteoclast-derived exosomes are present in the circulation and can regulate osteoblast function. The serum exosome miR-214 levels were increased in osteoporotic patients and ovariectomized mice. They further showed that osteoblasts incubated with exosomes from osteoporotic compared to non-osteoporotic patients had higher levels of miR-214, leading to reduced expression of osteogenesis-related genes. Inhibition of exosome release in ovariectomized mice using in vivo delivery of Rab27a siRNA improved the bone mass. Together, these studies provide compelling in vivo evidence for an important role of osteoclast-EV in regulating bone formation. A proposed mechanism by which miR-214–3p regulates osteogenesis is through targeting of ATF4, an osteogenic transcriptional factor, to inhibit bone formation[28]. Additionally, miR-214–3p enhances osteoclast formation through activation of the PI3K/Akt pathway, mediated via inhibition of phosphatase and tensin homologue (PTEN)[29].
Osteocytic EVs:
Signaling molecules produced by osteocytes are widely believed to serve as soluble factors that contribute to bone remodeling, as well as signaling to distant organs (reviewed in [30, 31]). However, whether osteocytes communicate via EV release is less clear. Osteocyte-derived vesicles have been observed in the bone extracellular matrix in transgenic mice expressing a membrane-bound GFP in osteocytes[32]. Furthermore, release of osteocyte-derived microvesicles was observed by Veno et al. using live-cell imaging in osteogenic calvarial cell cultures from the same mouse model[33].
Recently, Qin et al. characterized the morphology and biological features of exosomes from Ocy454 osteocyte-like cells[13]. These vesicles have a diameter of 50–100 nm, a cup-shaped appearance, and express CD63 as shown by immunogold-labeling (figure 2A), consistent with previous descriptions of exosomes[13, 11]. The microRNA, miR-218, stimulates Wnt/β-catenin signaling and promotes osteogenic differentiation of bone marrow stromal cells by inactivation of Wnt inhibitors, SOST, DKK2 and SFRP2[34]. Interestingly, Qin et al showed that in response to the muscle-released myokine, myostatin, osteocytes produced greater amounts of SOST, RANKL and DKK1, which was associated with reduced miR-218 in the parent Ocy454 cells and their exosomes[13]. They further showed that the myostatin-modified osteocyte-EV were internalized by MC3T3 cells, inhibiting osteoblastic differentiation via inactivation of Wnt signaling[13]. These studies show that osteocytes and their exosome cargo can be influenced by muscle-related factors, suggesting that exosomes can provide a mechanism for crosstalk between muscle and bone. This topic is further discussed in later sections of this review.
Figure 2:
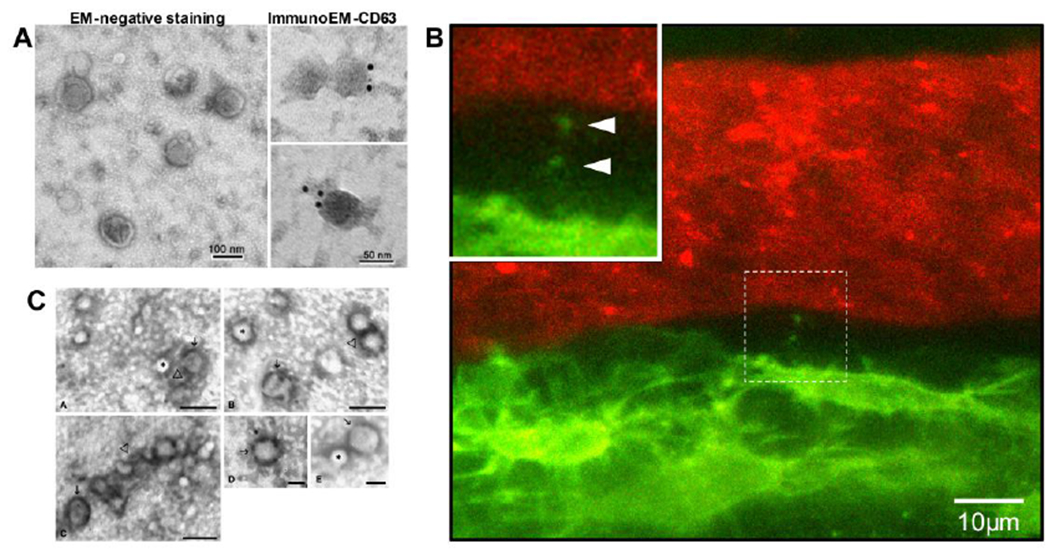
A) Morphology and structure of exosomes obtained from Ocy454 osteocyte-like cells by electron microscopy analysis. The exosomes were stained with uranyl acetate (EM-negative staining) or labeled with 10-nm immunogold using antibody against the exosomal membrane marker CD63 and stained with uranyl acetate (ImmunoEM-CD63). Exosomes were visualized using a Hitachi H7000 electron microscope (reproduced from Qin et al, 2017[13], with permission from the American Society for Biochemistry and Molecular Biology). B) Still frame from intravital confocal imaging in calvarial bone of a Dmp1-mGFP transgenic mouse. Osteocytes are GFP-positive (green) and the vascular lumen is labeled with Texas Red-conjugated high MW dextran. Note an osteocyte that is releasing small vesicle-like particles adjacent to the lumen of the vascular channel (also see enlarged inset, arrowheads). C) TEM images of microvesicles released from C2C12 cells. The microvesicles were isolated from culture supernatants by 110,000 x g centrifugation, then fixed, negatively stained, and observed by TEM. The images show small vesicles of 50–80 nm in diameter. Some of these microvesicles apparently contain a diffuse electron-dense material (→) while others appear completely electron transparent (*). A tendency to aggregate (Δ) occasionally appears. Images A, B and C, bar = 100 nm; Image D and E bar = 50nm (Reproduced from Guescini et al, 2010[48] with permission from Elsevier).
It is currently unclear whether osteocyte-EV can signal to distant organs, but recent work from Sato et al. provides suggestive evidence that osteocyte-EV are present in the circulation[35]. They showed that ablation of osteocytes in mice modifies miRNA levels in circulating exosomes and that many of the miRNAs downregulated are ones that are expressed at high levels in MLO-Y4 osteocyte-like cells. Exosomes are too large to travel down the canalicular fluid space and therefore it seems logical that osteocyte-EV in the circulation must arise from osteocytes located adjacent to a blood vessel and/or bordering on the marrow space. Support for this comes from the work of Wang et al., using mice expressing a membrane-targeted GFP in osteocytes[36]. They showed using intravital imaging that osteocytes adjacent to blood vessels shed vesicle-like particles from their dendrite tips (see Figure 2B). They further showed that GFP-positive EV were present in the blood and in the bone marrow.
It is currently unclear whether there are specific cell surface markers that can be used to identify the cell-specific origin of bone cell-derived EV (e.g. osteocyte, osteoblast, osteoclast) and/or to enrich for EV of specific bone cell types. Potential markers expressed on the osteocyte cell surface include E11/gp38 and PHEX. However, expression of these marker genes is not uniquely restricted to osteocytes and may be limited to early rather than mature osteocytes. Dentin matrix protein-1 and Sclerostin are expressed in early and mature osteocytes, respectively, but is is not clear whether they are cell surface markers. Fluorescence activated cell sorting of osteoblasts has been performed using antibodies to alkaline phosphatase, which could potentially be applied to circulating EV[37]. Surface markers that could potentially be used to identify EV of osteoclast origin include DC-STAMP, OSCAR and calcitonin receptor. Future studies are therefore needed to define which markers or sets of markers are optimal for distinguishing subpopulations of circulating EV derived from different bone cells.
EVs from Bone Marrow Stromal Cells (BMSCs)
Human BMSCs are an attractive cell source for stem cell therapy treatments, including potential applications in bone regeneration. Recent studies have shown that exosomes from BMSC may play a role in osteoblastic differentiation, bone healing and regeneration. One such study demonstrated that EVs from human BMSC upregulated expression of osteogenic marker genes and promoted osteoblastic differentiation in a human osteoblast-like cell line, mediated by exosomal miR-196a[38]. These BMSC-derived EVs were used to treat critical sized calvarial defects in rats and promoted bone formation and bone healing. Similarly, Martins et al. showed that hBMSCs grown under osteogenic conditions shed EVs that have osteoinductive potential by promoting expression of BMP2, SP7, SPP1, BGLAP/IBSP, and alkaline phosphatase in uncommitted BMSC cells[39]. They proposed that these BMSC-derived EV may have potential application in bone regenerative therapies. In further support of this, exosomes from MSC from human induced pluripotent stem cells were combined with scaffolds of tricalcium phosphate and found to promote bone regeneration[40].
Interestingly, Furuta et al. showed impairment of bone healing in CD9 null mice, which make reduced amounts of exosomes[41]. Injection with MSC-derived exosomes accelerated fracture healing in this model. In another translational study, it was reported that MSC transplantation rescues impaired bone marrow MSC function and improves osteopenia in a mouse model of Lupus [42]. The likely mechanism was through the MSCs releasing exosomes, which transferred Fas to the host bone marrow MSCs, thereby reducing miR-29b levels. This in turn promotes epigenetic regulation of Notch signaling. It is clear from the above studies that BMSC-derived EVs are emerging as novel cell-free-based therapies with potential applications in regenerative medicine, including in bone regeneration.
EV Function in Muscle
Pederson et al first proposed that muscle may function in an endocrine manner through “myokine” release[43, 44]. Several muscle-secreted peptide hormones and cytokines have been identified that may mediate muscle communication with distant organs, including liver, pancreas, adipose tissue, brain and bone or may regulate myogenesis[43–47]. More recently, several groups have shown that skeletal and cardiac muscle cells secrete EV[48–54]. Accumulating evidence suggests that exosomes released by myogenic cells can transfer their cargo of proteins, mRNA and miRNA to recipient cells and regulate muscle cell function in an autocrine or paracrine manner. They can also be shed into the circulation[55, 56] and may have effects on distant tissues. Guescini et al. showed that muscle-derived EVs (defined as EVs positive for alpha sarcoglycan) represent about 1–5% of the total circulating EVs[55].
Characteristics of Muscle Exosomes
The production of EVs by muscle cells was first reported in 2010 by Guescini et al.[48], who showed that C2C12 myoblasts produced EVs of ~50–80 nm in diameter (figure 2C), consistent with the size range of exosomes[57]. These EV contained known exosome markers, Tsg101 and Alix, demonstrated by immunogold labeling, and also carried mitochondrial DNA in their cargo. Their proteome included many exosome-associated proteins and proteins involved in signal transduction, such as small GTP-binding proteins and 14-3-3 proteins[48]. In a related study, proteomic comparison of EV from C2C12 myoblasts and myotubes showed distinct subsets of proteins that were reflective of the differentiation state of the parent cells[53]. Interestingly, the C2C12-EVs expressed molecules including ITGB1, CD9, CD81, NCAM, CD44 and Myoferlin on their surfaces, which play a role in myoblast fusion by facilitating recognition and subsequent adhesion of competent myoblasts[53].
miRNAs are present in muscle-EV and over 170 miRNAs have been found in exosomes from C2C12 myoblasts and myotubes[58], including miR-181a, miR-146a, miR-145, miR-378, miR-1, miR-133a, miR-133b and miR-206, which regulate muscle differentiation [59–61, 58]. In particular, miR-133a, miR-133b, miR-1, and miR-206 showed the greatest increase in expression in the cytoplasm and in exosomes during C2C12 differentiation[58] and during development of human skeletal muscle[62]. Fry and colleagues showed that skeletal muscle myogenic progenitor cells also produce exosomes containing miR-206[63]. These exosomes may regulate extracellular matrix production by fibrogenic cells, preventing excessive extracellular matrix deposition and ensuring optimal early myofiber growth.
Muscle Exosomes in Cell-Cell Communication
As mentioned previously, exosomes are released by many cell types and provide a protected environment for transportation of protein, miRNA and mRNA to distant target cells[64]. Evidence is accumulating that muscle-EV play a role in crosstalk between myoblasts and myotubes as well as with other tissues. Incubation of C2C12 myoblasts with exosomes from myotubes suppressed myoblast proliferation and promoted differentiation[53]. The cell-to-cell transfer of myotube-derived exosomal cargo was confirmed by labeling the exosomes with GFP and showing transfer of GFP fluorescence into myoblasts. These data support a “feed forward” role for exosomes from differentiated myofibrils by promoting differentiation of more myofibrils. In a follow up study, the same authors showed that induction of myotube differentiation may occur via exosomal transfer of miRNAs that silenced expression of Sirtl, a regulator of myogenesis-related gene expression[58].
Muscle-EV can be taken up into other organs, where they may have functional effects. For example, fluorescently labeled muscle-derived exosomes injected into the tail vein of C57Bl/6 mice were taken up into most tissues within 24h, with predominant labeling in lung, liver and spleen and uptake also seen in brain, heart, pancreas and GI tract (bone was not analyzed)[65]. These authors further showed that mice on a high fat diet were obese and insulin-resistant and secreted more exosomes compared to mice on standard chow. Muscle-derived exosomes from mice on the high fat diet induced myoblast proliferation and altered expression of cell cycle-related genes and genes involved in muscle differentiation. These findings suggest that muscle-EV have a paracrine signaling function by altering muscle homeostasis in response to high fat diet and may have an endocrine function by targeting other tissues in vivo[65]. In a related study, the same group showed that EVs from muscle of mice on a high fat diet increased the size of isolated islets in vitro and induced proliferation of murine insulin secreting MIN6B1 cells[66]. These effects appeared to be mediated in part through transfer of miR-16, which regulates expression of Ptch1, a regulator of pancreas development. Not only do muscle cells release EV, but they respond to EV from other cells. For example, EV from MSC enhanced muscle regeneration[67] and endothelial-derived EV protected cardiomyocytes from hypertrophy[68].
Exercise-Induced Release of Muscle Exosomes
Exercise has beneficial effects on multiple organ systems, has protective effects on age-related diseases and attenuates aging effects on mitochondrial function. Release of nucleic acids and peptides from skeletal muscle and other tissues (termed “exerkines”) has been proposed to mediate these effects[69]. However, since many growth factors, miRNAs and nucleic acids would be unstable in the circulation, it has recently been suggested that exosomes and microvesicles are important carriers of “exerkines” that may mediate the systemic effects of exercise[69]. Circulatory EV increase with endurance exercise[70] and the term “exersomes” has been coined to denote these exercise-induced EV[69]. Guescini et al. showed that acute aerobic exercise in young men is associated with increased numbers of circulating exosomes positive for the muscle marker alpha sarcoglycan[55]. Their study also showed upregulation of miR-181a-5p and miR133b in these muscle-derived circulating exosomes following exercise. In a related study, both acute and chronic exercise reduced circulating levels of the muscle-enriched miR-486 in young men[71]. Overall, accumulating evidence supports the idea that EVs play an important role in muscle crosstalk with other organs and that they may also function to mediate the beneficial effects of exercise on muscle and other tissues.
Muscle Exosomes in Pathological Conditions
Several studies suggest that pathological states, including injury, atrophy and aging, can alter the cargo of muscle-derived exosomes. One such study showed that muscle denervation by transection of the sciatic nerve resulted in an increase in miR-206 and a decrease in miRs 1, 133a, and 133b in exosomes from cultured myofibers[72]. miR-206 may play a role in the activation of satellite cells that occurs after nerve injury and potentially in regulating muscle mass and new synapse formation during re-innervation. Madison et al[54] proposed that muscle-EV may play a role in regeneration of motor neurons after nerve injury. Their work showed that exosomes from C2C12 myoblasts promoted cell survival and neurite outgrowth in NSC-34 motor neuron cells. Hudson et al reported that miR-1 and miR-23a, two miRNAs involved in muscle atrophy, were enriched in exosomes from cultured myotubes in which atrophy was induced by dexamethasone treatment[73]. Their work also showed downregulation of miR-23a in rats under conditions that induced muscle atrophy, suggesting a potential pathogenic role.
Another example of alteration of muscle exosomes under pathological conditions was shown in mice fed on a high fat diet, as mentioned above[65]. These mice are obese and become insulin-resistant. Muscle-derived exosomes from these mice induced myoblast proliferation and downregulated Myog and Myodl, two markers of terminal muscle cell differentiation[65], suggesting that exosomes can transfer the effects of high fat diet to other myoblasts. EV may also function in muscle regeneration in response to injury and mechanical stress. This topic has been reviewed by Murphy et al.[7] and the reader is referred to their excellent review for a more in depth discussion.
Overall, evidence is accumulating that the cargo of muscle-derived exosomes can be changed under pathological conditions and that exosomes contribute to pathogenic processes and/or the propagation of pathogenic responses to neighboring cells as well as distant cells. These findings also suggest that circulating levels of muscle-related miRs could be used as biomarkers of muscle health or disease states and potentially to indicate response to therapies.
Role of Exosomes in Muscle-Bone Crosstalk and Their Potential Role in Musculoskeletal Aging
Mechanical loading through muscle contraction was previously thought to be the main mechanism by which muscle influences bone mass. Pederson et al first proposed that muscle functions in an endocrine manner through “myokine” release[43, 44] and evidence is emerging for signaling crosstalk between bone and muscle via circulating and local mediators[74–82] (reviewed in[83–86]). Examples of factors proposed to functionally couple muscle and bone include growth hormone/IGF-l[87], FGF-2[78], osteocalcin[81], irisin[88], PGE2[74], Wnt3a[82], and β-aminoisobutyric acid (BAIBA)[89]. Myostatin inhibitors also have beneficial effects on bone[90]. An obvious question is whether extracellular vesicles are also mediators of muscle-bone crosstalk.
Exosomes from C2C12 myoblasts were shown to promote differentiation of pre-osteoblastic MC3T3-E1 cells to mature osteoblasts[91]. This was attributed to the function of the exosomes to increase miR-27a-3p levels in the recipient cells, which decreases expression of its target, adenomatous polyposis coli (APC). This in turn activates β-catenin signaling, promoting osteogenesis. Our own recent studies have shown that exosomes from C2C12 myotubes, but not myoblasts, enhance luciferase activity in TOPflash-MLOY4 osteocyte-like cells, which express a luciferase reporter as a readout for Wnt/β-catenin signaling[36]. Previous studies have shown that Wnt/β-catenin signaling enhances osteocyte survival and protects them from apoptotic stimuli and oxidative stress[76, 92, 89].
The microRNA, miR-218, stimulates Wnt/β-catenin signaling and promotes osteogenic differentiation of bone marrow stromal cells by inactivation of Wnt inhibitors, SOST, DKK2 and SFRP2[34]. Qin et al showed that in response to the muscle released myokine, myostatin, Ocy454 osteocyte-like cells produced greater amounts of SOST, RANKL and DKK1, which was associated with decreased miR-218 in the parent Ocy454 cells and their exosomes[13]. The myostatin-modified osteocyte-EV were internalized by MC3T3 cells, inhibiting osteoblastic differentiation via inactivation of Wnt signaling[13]. These studies show that osteocytes and their exosome cargo can be influenced by muscle-related factors, providing more evidence of EV-mediated crosstalk between muscle and bone.
Osteoporosis, a disease of reduced bone density and quality, is a major clinical problem in the aging population and is associated with a high fracture risk[93, 94]. Sarcopenia, a disease of age-related loss of muscle mass and strength, often accompanies osteoporosis and the muscle weakness contributes to falls that lead to fractures. Since these diseases go hand in hand, common molecular pathogenic mechanism(s) seem likely. This has led to the concept that molecular crosstalk between these two tissues may coordinate age-related degenerative changes. Aging is associated with changes in the cargo of circulating EV, which are likely secondary to cellular changes in the producer cells in response to age-associated stimuli, such as inflammation and oxidative stress[95–98]. Recent evidence suggests that EV may play a role in musculoskeletal aging.
Davis et al. compared miRNA profiles of EVs from the bone marrow interstitial fluid of aged and young mice[99]. They showed increased expression of the miR-183 cluster (miR-96/-182/-183) in EV from aged mice, which could be mimicked in vitro by experimentally inducing oxidative stress, a hallmark of aging. Treatment of BMSCs from young mice with bone marrow-EV from aged mice inhibited their osteogenic differentiation and these effects were mimicked by transfection of BMSCs with miR-183-5p, which also induced BMSC senescence. Their data show that the cargo of bone marrow-EVs can be altered by aging and by oxidative stress, and suggest that these EV may play a role in propagating aging effects on stem cell senescence and on impaired osteogenic differentiation.
In a follow up study, the same group showed that increased oxidative stress in skeletal muscle with aging was associated with increased expression of the senescence-associated microRNA, miR-34a, in skeletal muscle and in muscle-derived circulating EVs[56]. miR-34a was also induced in exosomes from C2C12 myoblasts subjected to oxidative stress. This miRNA was previously shown to be increased with aging in skeletal muscle[100] and in the circulation[101] and has been associated with muscle atrophy[100, 102]. In addition to negative effects on muscle, BMSC treated with these exosomes showed increased senescence. Their data suggest that aged skeletal muscle produces circulating EV’s that can induce senescence in stem cell populations in bone and other tissues via their miRNA cargo.
Summary and Conclusions
This review has highlighted exosome-mediated cell-cell communication as an exciting and paradigm-shifting field, with emerging new discoveries providing ever more insight into this previously underappreciated and important mechanism for cell-cell signaling. There is a clear need for future studies to further delineate the role of exosomes in muscle-to-bone crosstalk in aging as well as studies aimed at understanding the mechanisms governing their release and uptake by muscle and bone cells. One key limitation in the field is in showing whether EV-mediated miRNA transfer can alter cell function in a truly physiological setting, which still remains controversial. In a stoichiometric analysis of circulating exosomes, Chevillet et al. estimated that even miRNAs that are fairly abundant are actually present at less than one copy per exosome[103]. It is therefore valid to ask whether miRNAs present in such low amounts per exosome can have effects under completely physiological settings as opposed to controlled experimental studies where large amounts of purified exosomes are added to cells or injected into animals. An argument in favor of a physiological role is that, under in vivo conditions, the producer cells can continually shed EV. Therefore, over time, target cells could accumulate “hits” from multiple EV, even if each vesicle only delivers a relatively small amount of miRNA to the target cell. Challenges still remain in quantifying and separating different EV subpopulations, as most studies are examining “whole population” effects of EV preparations containing mixtures of exosomes and microvesicles. Future studies using intravital imaging and mouse genetic approaches targeting mechanisms of release and uptake of EV should help to confirm the importance of EV signaling under in vivo physiological and pathological conditions. Such studies will help to not only enhance understanding of EV function, but also to exploit the exciting possibilities for their use in therapeutic applications as well as in diagnosis and monitoring of musculoskeletal disease and aging.
Acknowledgements
We thank Dr. LeAnn Tiede-Lewis in the UMKC Confocal Imaging Core for her expert assistance with the confocal inftravital imaging data shown in Figure 2B.
Funding Information
SLD was supported by NIH grants P01-AG039355 and R21-AR071563. WQ was supported by Veterans Health Administration, Rehabilitation Research and Development Service Merit Review Award 1 I01 RX002089-01A2 and NIH R21 NS111393-01A1. We acknowledge use of the UMKC Confocal Microscopy Core supported by NIH grants S10RR027668 and S10OD021665, the UMKC Office of Research Services and UMKC Center of Excellence in Dental and Musculoskeletal Tissues.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: Sarah Dallas and Weiping Qin declare no conflict of interest.
Human and Animal Rights and Informed Consent: All reported studies/experiments with animal subjects performed by the authors have complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
••Of major importance
- 1.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Andaloussi S, Lakhal S, Mager I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65(3):391–7. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164(6): 1226–32. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 6.Withrow J, Murphy C, Liu Y, Hunter M, Fulzele S, Hamrick MW. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18(1):286. doi: 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy C, Withrow J, Hunter M, Liu Y, Tang YL, Fulzele S et al. Emerging role of extracellular vesicles in musculoskeletal diseases. Mol Aspects Med. 2018;60:123–8. doi: 10.1016/j.mam.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23(2):91–7. doi: 10.1097/MOL.0b013e328350a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3(5):321–30. doi:tra030502 [pii]. [DOI] [PubMed] [Google Scholar]
- 11.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi:pr.112.005983 [pii] 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 12.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 13.Qin Y, Peng Y, Zhao W, Pan J, Ksiezak-Reding H, Cardozo C et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J Biol Chem. 2017;292(26):11021–33. doi: 10.1074/jbc.M116.770941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168(7):3235–41. [DOI] [PubMed] [Google Scholar]
- 15.Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18(9):977–9. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 16.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–66. doi: 10.1182/blood-2004-03-08242004-03-0824 [pii]. [DOI] [PubMed] [Google Scholar]
- 17.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117(9):2369–76. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause M, Samoylenko A, Vainio SJ. Exosomes as renal inductive signals in health and disease, and their application as diagnostic markers and therapeutic agents. Front Cell Dev Biol. 2015;3:65. doi: 10.3389/fcell.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge M, Ke R, Cai T, Yang J, Mu X. Identification and proteomic analysis of osteoblast-derived exosomes. Biochem Biophys Res Commun. 2015;467(1):27–32. doi: 10.1016/j.bbrc.2015.09.135. [DOI] [PubMed] [Google Scholar]
- 21.Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR et al. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem. 2011;286(6):4809–18. doi: 10.1074/jbc.M110.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui Y, Luan J, Li H, Zhou X, Han J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590(1):185–92. doi: 10.1002/1873-3468.12024. [DOI] [PubMed] [Google Scholar]
- 23.Deng L, Wang Y, Peng Y, Wu Y, Ding Y, Jiang Y et al. Osteoblast-derived microvesicles: A novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;79:37–42. doi: 10.1016/j.bone.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Morhayim J, van de Peppel J, Demmers JA, Kocer G, Nigg AL, van Driel M et al. Proteomic signatures of extracellular vesicles secreted by nonmineralizing and mineralizing human osteoblasts and stimulation of tumor cell growth. FASEB J. 2015;29(1):274–85. doi: 10.1096/fj.14-261404. [DOI] [PubMed] [Google Scholar]
- 25.Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J et al. Characterization of Regulatory Extracellular Vesicles from Osteoclasts. J Dent Res. 2016;95(6):673–9. doi: 10.1177/0022034516633189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **26.Li D, Liu J, Guo B, Liang C, Dang L, Lu C et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872. doi: 10.1038/ncomms10872. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used mouse genetic approaches combined with analysis of patient samples to show that osteoclasts release EV into the circulation and that elevated osteoclast-derived EV containing miR-214-3p are associated with low bone mass in elderly women. The work suggests that inhibiting miR-214-3p in osteoclasts could be a potential therapeutic strategy for treating diseases of low bone mass.
- 27.Sun W, Zhao C, Li Y, Wang L, Nie G, Peng J et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016;2:16015. doi: 10.1038/celldisc.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Guo B, Li Q, Peng J, Yang Z, Wang A et al. miR-214 targets ATF4 to inhibit bone formation. Nat Med. 2013;19(1):93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- 29.Zhao C, Sun W, Zhang P, Ling S, Li Y, Zhao D et al. miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol. 2015;12(3):343–53. doi: 10.1080/15476286.2015.1017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34(5):658–90. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamel-ElSayed SA, Tiede-Lewis LM, Lu Y, Veno PA, Dallas SL. Novel approaches for two and three dimensional multiplexed imaging of osteocytes. Bone. 2015;76:129–40. doi: 10.1016/j.bone.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veno P, Prideaux M, Dusevich V, Bonewald L, Dallas S Osteocytes release microvesicles that regulate osteoblast function. J Bone Miner Res 2013;28 (Suppl 1) FR0280 and SA. [Google Scholar]
- 34.Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang W, Jafferji M, Gordon JA et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem. 2012;287(50):42084–92. doi:M112.377515 [pii] 10.1074/jbc.M112.377515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato M, Suzuki T, Kawano M, Tamura M. Circulating osteocyte-derived exosomes contain miRNAs which are enriched in exosomes from MLO-Y4 cells. Biomed Rep. 2017;6(2):223–31. doi: 10.3892/br.2016.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K, Tiede-Lewis LM, McCormick LA, Lara N, Keightley A, Farina N et al. Extracellular Vesicle-Mediated Cell-Cell Communication in Bone and Potential Role in Muscle-Bone Crosstalk. J Bone and Miner Res. 2017;32((suppl1)):S50. [Google Scholar]
- 37.Arpornmaeklong P, Brown SE, Wang Z, Krebsbach PH. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev. 2009;18(7):955–68. doi: 10.1089/scd.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961. doi: 10.1038/srep21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins M, Ribeiro D, Martins A, Reis RL, Neves NM. Extracellular Vesicles Derived from Osteogenically Induced Human Bone Marrow Mesenchymal Stem Cells Can Modulate Lineage Commitment. Stem Cell Reports. 2016;6(3):284–91. doi: 10.1016/j.stemcr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Liu X, Li H, Chen C, Hu B, Niu X et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7(1):136. doi: 10.1186/s13287-016-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N et al. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med. 2016;5(12):1620–30. doi: 10.5966/sctm.2015-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, Liu D, Chen C, Hamamura K, Moshaverinia A, Yang R et al. MSC Transplantation Improves Osteopenia via Epigenetic Regulation of Notch Signaling in Lupus. Cell Metab. 2015;22(4):606–18. doi: 10.1016/j.cmet.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 44.Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B et al. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537(Pt 2):633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouzakri K, Plomgaard P, Berney T, Donath MY, Pedersen BK, Halban PA. Bimodal effect on pancreatic beta-cells of secretory products from normal or insulin-resistant human skeletal muscle. Diabetes. 2011;60(4):1111–21. doi: 10.2337/db10-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haugen F, Norheim F, Lian H, Wensaas AJ, Dueland S, Berg O et al. IL-7 is expressed and secreted by human skeletal muscle cells. Am J Physiol Cell Physiol. 2010;298(4):C807–16. doi: 10.1152/ajpcell.00094.2009. [DOI] [PubMed] [Google Scholar]
- 47.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113(4):483–94. [DOI] [PubMed] [Google Scholar]
- 48.Guescini M, Guidolin D, Vallorani L, Casadei L, Gioacchini AM, Tibollo P et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res. 2010;316(12):1977–84. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Le Bihan MC, Bigot A, Jensen SS, Dennis JL, Rogowska-Wrzesinska A, Laine J et al. Indepth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts. J Proteomics. 2012;77:344–56. doi: 10.1016/j.jprot.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Romancino DP, Paterniti G, Campos Y, De Luca A, Di Felice V, d’Azzo A et al. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett. 2013;587(9):1379–84. doi: 10.1016/j.febslet.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2015;24(4):199–206. doi: 10.1016/j.carpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Waldenstrom A, Genneback N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7(4):e34653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forterre A, Jalabert A, Berger E, Baudet M, Chikh K, Errazuriz E et al. Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: a new paradigm for myoblast-myotube cross talk? PLoS One. 2014;9(1):e84153. doi: 10.1371/journal.pone.0084153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madison RD, McGee C, Rawson R, Robinson GA. Extracellular vesicles from a muscle cell line (C2C12) enhance cell survival and neurite outgrowth of a motor neuron cell line (NSC-34). J Extracell Vesicles. 2014;3. doi: 10.3402/jev.v3.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guescini M, Canonico B, Lucertini F, Maggio S, Annibalini G, Barbieri E et al. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying miRNAs in the Bloodstream. PLoS One. 2015;10(5):e0125094. doi: 10.1371/journal.pone.0125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **56.Fulzele S, Mendhe B, Khayrullin A, Johnson M, Kaiser H, Liu Y et al. Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging (Albany NY). 2019;11(6):1791–803. doi: 10.18632/aging.101874. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper showed that increased oxidative stress in skeletal muscle with aging was associated with increased expression of senescence-associated microRNAs. The data further showed that circulating EV derived from muscle in aged mice can induce senescence in stem cell populations in bone and other tissues via their miRNA cargo.
- 57.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 58.Forterre A, Jalabert A, Chikh K, Pesenti S, Euthine V, Granjon A et al. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle. 2014;13(1):78–89. doi: 10.4161/cc.26808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228–33. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Townley-Tilson WH, Callis TE, Wang D. MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol. 2010;42(8):1252–5. doi: 10.1016/j.biocel.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCarthy JJ. MicroRNA-206: the skeletal muscle-specific myomiR. Biochim Biophys Acta. 2008;1779(11):682–91. doi: 10.1016/j.bbagrm.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koutsoulidou A, Mastroyiannopoulos NP, Furling D, Uney JB, Phylactou LA. Expression of miR-1, miR-133a, miR-133b and miR-206 increases during development of human skeletal muscle. BMC Dev Biol. 2011;11:34. doi: 10.1186/1471-213X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy. Cell Stem Cell. 2017;20(1):56–69. doi: 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–32. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Aswad H, Forterre A, Wiklander OP, Vial G, Danty-Berger E, Jalabert A et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 2014;57(10):2155–64. doi: 10.1007/s00125-014-3337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jalabert A, Vial G, Guay C, Wiklander OP, Nordin JZ, Aswad H et al. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia. 2016;59(5):1049–58. doi: 10.1007/s00125-016-3882-y. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11):1257–65. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 68.Gu S, Zhang W, Chen J, Ma R, Xiao X, Ma X et al. EPC-derived microvesicles protect cardiomyocytes from Ang II-induced hypertrophy and apoptosis. PLoS One. 2014;9(1):e85396. doi: 10.1371/journal.pone.0085396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016;12(9):504–17. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- 70.Fruhbeis C, Helmig S, Tug S, Simon P, Kramer-Albers EM. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015;4:28239. doi: 10.3402/jev.v4.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aoi W, Ichikawa H, Mune K, Tanimura Y, Mizushima K, Naito Y et al. Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front Physiol. 2013;4:80. doi: 10.3389/fphys.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Gasperi R, Hamidi S, Harlow LM, Ksiezak-Reding H, Bauman WA, Cardozo CP. Denervation-related alterations and biological activity of miRNAs contained in exosomes released by skeletal muscle fibers. Sci Rep. 2017;7(1):12888. doi: 10.1038/s41598-017-13105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hudson MB, Woodworth-Hobbs ME, Zheng B, Rahnert JA, Blount MA, Gooch JL et al. miR-23a is decreased during muscle atrophy by a mechanism that includes calcineurin signaling and exosome-mediated export. Am J Physiol Cell Physiol. 2014;306(6):C551–8. doi: 10.1152/ajpcell.00266.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mo C, Zhao R, Vallejo J, Igwe O, Bonewald L, Wetmore L et al. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle. 2015;14(10):1507–16. doi: 10.1080/15384101.2015.1026520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang J, Hsu YH, Mo C, Abreu E, Kiel DP, Bonewald LF et al. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-kappaB signaling pathway. J Bone Miner Res. 2014;29(7):1531–40. doi: 10.1002/jbmr.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jahn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M et al. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. European cells & materials. 2012;24:197–209; discussion -10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gorski JP, Huffman NT, Vallejo J, Brotto L, Chittur SV, Breggia A et al. Deletion of Mbtps1 (Pcsk8, S1p, Ski-1) Gene in Osteocytes Stimulates Soleus Muscle Regeneration and Increased Size and Contractile Force with Age. J Biol Chem. 2016;291(9):4308–22. doi: 10.1074/jbc.M115.686626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. Journal of musculoskeletal & neuronal interactions. 2010;10(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- 79.Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin E2: from clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiation. Recent patents on biotechnology. 2012;6(3):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Regan JN, Waning DL, Guise TA. Skeletal muscle Ca(2+) mishandling: Another effect of bone-to-muscle signaling. Seminars in cell & developmental biology. 2016;49:24–9. doi: 10.1016/j.semcdb.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. 2016;5(10): 1042–7. doi: 10.1016/j.molmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-catenin pathway (ePub ahead of print). JBMRPlus. 2017. doi: 10.1002/jbm4.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamrick MW. The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. BoneKEy reports. 2012; 1:60. doi: 10.1038/bonekey.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cianferotti L, Brandi ML. Muscle-bone interactions: basic and clinical aspects. Endocrine. 2014;45(2): 165–77. doi: 10.1007/sl2020-013-0026-8. [DOI] [PubMed] [Google Scholar]
- 85.Ferrucci L, Baroni M, Ranchelli A, Lauretani F, Maggio M, Mecocci P et al. Interaction between bone and muscle in older persons with mobility limitations. Current pharmaceutical design. 2014;20(19):3178–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brotto M, Bonewald L. Bone and muscle: Interactions beyond mechanical. Bone. 2015;80:109–14. doi: 10.1016/j.bone.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. The Journal of endocrinology. 2010;205(3):201–10. [DOI] [PubMed] [Google Scholar]
- 88.Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P et al. The myokine irisin increases cortical bone mass. Proceedings of the National Academy of Sciences of the United States of America. 2015; 112(39): 12157–62. doi: 10.1073/pnas,1516622112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jahn K, Yi J et al. beta-aminoisobutyric Acid, 1-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018;22(6): 1531–44. doi: 10.1016/j.celrep.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamrick MW, Arounleut P, Kellum E, Cain M, Immel D, Liang LF. Recombinant Myostatin (GDF-8) Propeptide Enhances the Repair and Regeneration of Both Muscle and Bone in A Model of Deep Penetrant Musculoskeletal Injury. The Journal of trauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu Q, Cui Y, Luan J, Zhou X, Li H, Han J. Exosomes from C2C12 myoblasts enhance osteogenic differentiation of MC3T3-E1 pre-osteoblasts by delivering miR-27a-3p. Biochem Biophys Res Commun. 2018;498(l):32–7. doi: 10.1016/j.bbrc.2018.02.144. [DOI] [PubMed] [Google Scholar]
- 92.Kitase Y, Barragan L, Qing H, Kondoh S, Jiang JX, Johnson ML et al. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25(12):2657–68. doi: 10.1002/jbmr.l68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haleem S, Lutchman L, Mayahi R, Grice JE, Parker MJ. Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury. 2008;39(10): 1157–63. [DOI] [PubMed] [Google Scholar]
- 94.Pedersen SJ, Borgbjerg FM, Schousboe B, Pedersen BD, Jorgensen HL, Duus BR et al. A comprehensive hip fracture program reduces complication rates and mortality. Journal of the American Geriatrics Society. 2008;56(10):1831–8. [DOI] [PubMed] [Google Scholar]
- 95.Bertoldi K, Cechinel LR, Schallenberger B, Corssac GB, Davies S, Guerreiro ICK et al. Circulating extracellular vesicles in the aging process: impact of aerobic exercise. Mol Cell Biochem. 2018;440(1-2): 115–25. doi: 10.1007/s11010-017-3160-4. [DOI] [PubMed] [Google Scholar]
- 96.Kangas R, Tormakangas T, Fey V, Pursiheimo J, Miinalainen I, Alen M et al. Aging and serum exomiR content in women-effects of estrogenic hormone replacement therapy. Sci Rep. 2017;7:42702. doi: 10.1038/srep42702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Terlecki-Zaniewicz L, Lammermann I, Latreille J, Bobbili MR, Pils V, Schosserer M et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging (Albany NY). 2018;10(5):1103–32. doi: 10.18632/aging.101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie Y, Gao Y, Zhang L, Chen Y, Ge W, Tang P. Involvement of serum-derived exosomes of elderly patients with bone loss in failure of bone remodeling via alteration of exosomal bone-related proteins. Aging Cell. 2018;17(3):e12758. doi: 10.1111/acel.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **99.Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM et al. MicroRNA-183–5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue Eng Part A. 2017;23(21-22):1231–40. doi: 10.1089/ten.TEA.2016.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compared miRNA profiles of EVs derived from bone marrow of young and aged mice. They showed that the cargo of EVs was altered by aging and that this could be mimicked experimentally by inducing oxidative stress. The study also showed that these EV may play a role in propagating aging effects on stem cell senescence and impaired osteogenic differentiation.
- 100.Zheng Y, Kong J, Li Q, Wang Y, Li J. Role of miRNAs in skeletal muscle aging. Clin Interv Aging. 2018;13:2407–19. doi: 10.2147/CIA.S169202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X, Khanna A, Li N, Wang E. Circulatory miR34a as an RNAbased, noninvasive biomarker for brain aging. Aging (Albany NY). 2011;3(10):985–1002. doi: 10.18632/aging.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greco S, Perfetti A, Fasanaro P, Cardani R, Capogrossi MC, Meola G et al. Deregulated microRNAs in myotonic dystrophy type 2. PLoS One. 2012;7(6):e39732. doi: 10.1371/journal.pone.0039732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(41):14888–93. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]


