Abstract
Aims
The definition of sarcopenia based on appendicular lean mass/height (2) (ALM/height (2)) is often used, although it can underestimate the prevalence of sarcopenia in overweight/obese patients with heart failure. Therefore, new methods have been proposed to overcome this limitation. We aimed to evaluate the prevalence of sarcopenia by three methods and compare body composition in this population.
Methods and results
We enrolled 168 male patients with heart failure (left ventricular ejection fraction <40%). Sixty‐six patients (39.3%) were identified with sarcopenia by at least one method. The lower 20th percentile defined as the cut‐off point for sarcopenia was 7.03 kg/m2, −2.32 and 0.76 for Baumgartner's (20.8%), Newman's (21.4%), and Studenski's methods (21.4%), respectively. Patients with body mass index (BMI) <25 kg/m2 were more likely to be identified by Baumgartner's than Studenski's method (P < 0.001). However, in patients with BMI ≥ 25 kg/m2, Studenski's and Newman's methods were more likely to detect sarcopenia than Baumgartner's method (both P < 0.005). Patients were further divided into three subgroups: (i) patients classified in all indexes (n = 8), (ii) patients classified in Baumgartner's (sarcopenic; n = 27), and (iii) patients classified in both Newman's and Studenski's methods (sarcopenic obesity; n = 31). Comparing body composition among groups, all sarcopenic groups presented lower total lean mass compared with non‐sarcopenic patients, whereas sarcopenic obese patients had higher total lean mass than lean sarcopenic patients.
Conclusions
Our results demonstrate that the prevalence of sarcopenia in overweight/obese patients is similar to lean sarcopenic patients when other methods are considered. In patients with higher BMI, Studenski's method seems to be more feasible to detect sarcopenia.
Keywords: Heart failure, Sarcopenia, Sarcopenic obesity, Body composition
1. Introduction
Sarcopenia has been defined as the age‐related decline in skeletal muscle mass, strength, and physical performance.1 Sarcopenia affects about 20% of patients suffering from heart failure (HF) and is a strong predictor of reduced exercise capacity and mortality in these patients.2, 3
Despite the large‐scale use of the term ‘sarcopenia', precise criteria to predict clinically relevant reduction in muscle mass and function have not been established yet. In fact, the classic cut‐off point of 7.26 kg/m2 for men, calculated as appendicular lean mass (ALM) in kilograms divided by height in metres squared (ALM/height2), is still frequently used to classify patients with sarcopenia.4 Nonetheless, this definition does not include the amount of fat mass to predict sarcopenia in overweight and obese patients.5, 6
Moreover, the combination of reduced muscle mass and fat accumulation, a phenomenon known as sarcopenic obesity (SO), leads to a higher‐risk group with both disorders,6, 7 and men may have a higher prevalence of SO than women.8 Paradoxically, although obesity increases the risk of HF, obese patients with HF with reduced ejection fraction (HFrEF) may have lower mortality rate than patients with healthy standard body mass index (BMI; 18.5–24.99 kg/m2).9 However, the protective effect of higher BMI is abolished in the presence of reduced lean mass and increased fat mass.10, 11
To overcome the limitation of ALM/height2 (Baumgartner's method) to define sarcopenia in overweight/obese patients, other definitions have been suggested. For instance, the residuals of a linear regression derived from adjusting lean mass for height and fat mass (Newman's method) seem to be more accurate in patients with high BMI, mainly composed of fat mass.6, 12 In addition, adjusting ALM by BMI (Studenski's method) has recently been proposed as an alternative to detect SO.13, 14 Even though both methods may be similar to identify patients with SO, the latter may have a better applicability.
Therefore, the aim of this study was to compare the prevalence of sarcopenia and SO using Baumgartner's, Newman's, and Studenski's methods and to compare body composition features in patients with HFrEF classified by these methods.
2. Methods
2.1. Study population
We prospectively included 168 ambulatory male patients with stable HFrEF. This is an unicentric, cross‐sectional study conducted between (May) 2016 and November 2018. Patients were eligible to participate if they had (i) age from 18 to 65 years old; (ii) at least 1 year of diagnosed HF; (iii) left ventricular ejection fraction (<)40% measured by echocardiography; (iv) non‐ischaemic and ischaemic aetiologies; (v) New York Heart Association Functional Classes I to IV; and (vi) compensated HF with optimal medication for at least 1 month prior to the study.
Patients with autonomic diabetic neuropathy, chronic renal failure in haemodialysis, heart transplantation, cardiac implantable devices, muscular dystrophy (i.e. Duchenne muscular dystrophy), any hormonal treatment, history of cancer, ongoing infection, and myocardial infarction with percutaneous coronary intervention or revascularization 6 months prior to the study entry were not included.
Written informed consent was obtained from all patients before any study‐related procedure if they agreed to participate. The study was approved by the local ethics committee (No. 0892/07) and fulfilled all principles of the Declaration of Helsinki. This protocol is registered at http://ClinicalTrials.gov under the Unique Identifier Number NCT03463226.
2.2. Maximal cardiopulmonary exercise test
Symptom‐limited cardiopulmonary exercise test (Vmax Encore 29 System; VIASYS Healthcare Inc., Palm Springs, CA, USA) was performed on a cycle ergometer by all patients (Ergometer 800S; Sensor Medics, Yorba Linda, CA, USA), using a progressive protocol with workload increments of 5 or 10 W/min. Ventilation (VE), oxygen consumption (VO2), and carbon dioxide output (VCO2) were acquired on a breath‐by‐breath basis and expressed as 30 s averages. The patients were initially monitored for 2 min at rest when seated on the ergometer, and after that, they were instructed to pedal at a pace of 60–70 rpm, and the completion of the test occurred when, despite verbal encouragement, the patient reached maximal volitional fatigue. Respiratory exchange ratio higher than 1.10 was reached by 82% of the patients. Heart rate was monitored continuously at rest, during the test and recovery phase, using a 12‐lead digital electrocardiogram (Cardio Soft 6.51 ECG/CAM‐14, GE Medical Systems Information Technologies, Wisconsin, WI, USA).
2.3. Body composition and muscle strength
Body composition measurements were performed using dual‐energy X‐ray absorptiometry (DXA; Lunar iDXA; GE Medical Systems Lunar, Madison, WI, USA). Whole‐body DXA scans were used to measure total and regional lean and fat mass. The height of each patient was measured to the nearest 0.1 cm with a wall‐mounted stadiometer. DXA measurements were performed by the same experienced technician.
First, sarcopenia was calculated as the sum of ALM of both arms and legs in kilograms divided by height in metres squared (ALM/height2), following the method proposed by Baumgartner.4 Second, a linear regression was performed using lean mass as dependent variable and height and total fat mass as independent variables, using the residuals to define sarcopenia as proposed by Newman.12 In our sample, the equation was ALM (kg) = −32.94 + 31.00 × height (m) + 0.18 × total fat mass (kg). Third, ALM was divided by BMI as proposed by Studenski.13 The cut‐off values for sarcopenia in this sample were defined as the lower 20th percentile of the distribution of each method.
After adjusting handle position, muscle strength was assessed by handgrip dynamometer (Model J00105; Jamar Hydraulic Hand Dynamometer, Sammons preston rolyan, Bolingbrook, Illionis, USA) using the dominant hand in a supinated position with elbow flexed at 90°. There was 1 min rest interval between efforts, and the maximum value of three attempts was used.15 The handgrip cut‐off point for sarcopenia was defined according to the BMI categories as proposed by the European Working Group on Sarcopenia in Older People.16 We defined sarcopenia as having a cut‐off value below the 20th percentile and reduced handgrip strength according to BMI ranges.
2.4. Laboratory measurements
Blood samples were drawn in the morning after 12 h overnight fasting. The laboratory tests included B‐type natriuretic peptide (pg/mL) plasma level, serum sodium (mEq/L), serum potassium (mEq/L), creatinine (mg/dL), haemoglobin level (g/dL), high‐sensitivity C‐reactive protein (hs‐CRP; mg/L), and fasting glucose (mg/dL).
2.5. Statistical analysis
Data are presented as mean ± standard deviation, median with lower and upper quartiles (95% confidence interval), and categorical variables as frequencies and percentages. One sample Kolmogorov–Smirnov test was used to assess the normal distribution of all variables. Independent Student's t‐test and Mann–Whitney U test were used to compare parametric and nonparametric variables, respectively. One‐way analysis of variance, Kruskal–Wallis, and χ 2 tests were used as appropriate. Data were analysed using the Statistical Package for the Social Sciences Version 23 for Windows (SPSS Inc., Chicago, IL, USA). A P‐value lower than 0.05 was considered statistically significant for all analyses.
3. Results
We enrolled 168 male patients with stable HFrEF (Table 1). Sixty‐six patients (39.3%) were identified with sarcopenia by at least one method, using the 20th percentile defined as the cut‐off point of 7.03 kg/m2, −2.32 and 0.76 for Baumgartner's, Newman's, and Studenski's methods, respectively. Of those 66 sarcopenic patients, there were eight patients exclusively classified by Baumgartner's method, six patients by Newman's method, and 19 patients by Studenski's method (Figure 1 ). Twenty‐five patients were classified in two of the three methods, which included three patients in Baumgartner's and Studenski's methods, 16 patients in Baumgartner's and Newman's methods, and six patients in Newman's and Studenski's methods (Figure 1 ). The remaining eight patients were classified in all methods (Figure 1). When using only one of the methods to detect sarcopenia, there were 35 patients in Baumgartner's method (20.8%), 36 patients in Newman's method (21.4%), and 36 patients in Studenski's method (21.4%) (Figure 1 ).
Table 1.
Baseline characteristics of the patients with and without sarcopenia
| Variable | All patients (n = 168) | Without sarcopenia (n = 102) | With sarcopeniaa (n = 66) | P‐value |
|---|---|---|---|---|
| Age (years) | 58 (51–62) | 56 (50–61) | 60 (55–63) | <0.001 |
| Weight (kg) | 71.5 ± 13.1 | 74.5 ± 10.5 | 66.8 ± 14.9 | <0.001 |
| Height (m) | 1.67 ± 0.07 | 1.68 ± 0.06 | 1.64 ± 0.07 | <0.001 |
| BMI (kg/m2) | 25.7 ± 4.2 | 26.2 ± 3.0 | 24.9 ± 5.3 | 0.04 |
| Aetiology (ischaemic/non‐ischaemic) | 48/120 | 26/76 | 22/44 | 0.30 |
| Aetiology (Chagas/no Chagas) | 40/128 | 26/76 | 14/52 | 0.58 |
| NYHA class (I/II/III/IV) | 54/57/46/11 | (39/31/24/8) | (15/26/22/3) | 0.20 |
| LVEF (%) | 27 (22–33) | 27 (22–33) | 25 (21–34) | 0.82 |
| BNP (pg/mL) | 438 (147–1050) | 363 (123–993) | 461 (154–1336) | 0.54 |
| hs‐CRP (mg/L) | 2.8 (1.0–7.1) | 2.4 (0.9–5.9) | 3.3 (1.2–12.7) | 0.049 |
| Creatinine (mg/dL) | 1.1 (1.0–1.5) | 1.2 (1.0–1.5) | 1.1 (1.0–1.4) | 0.12 |
| Sodium (mEq/L) | 140 (138–141) | 140 (138–141) | 139 (138–141) | 0.75 |
| Potassium (mEq/L) | 4.5 (4.3–4.8) | 4.5 (4.3–4.7) | 4.6 (4.3–4.9) | 0.16 |
| Haemoglobin (g/dL) | 13.9 ± 1.8 | 14.0 ± 1.8 | 14.0 ± 1.5 | 0.84 |
| Fasting glucose (mg/dL) | 104 (95–115) | 104 (95–114) | 104 (95–119) | 0.87 |
| Medication | ||||
| Beta‐blocker, n (%) | 164 (98) | 99 (97) | 65 (98) | 1.00 |
| ACE‐I/ARB, n (%) | 153 (91) | 95 (93) | 58 (88) | 0.28 |
| Thiazide/loop diuretics, n (%) | 132 (79) | 80 (78) | 52 (79) | 1.00 |
| Aspirin, n (%) | 66 (39) | 34 (33) | 32 (48) | 0.06 |
| Statins, n (%) | 91 (54) | 51 (50) | 40 (61) | 0.21 |
| MRA, n (%) | 118 (70) | 71 (70) | 47 (71) | 0.86 |
| Warfarin, n (%) | 44 (26) | 32 (31) | 12 (18) | 0.07 |
| Vasodilators, n (%) | 49 (29) | 32 (31) | 17 (26) | 0.49 |
| Metformin, n (%) | 22 (13) | 11 (11) | 11 (17) | 0.35 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; hs‐CRP, high‐sensitivity C‐reactive protein; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Data are presented as mean ± SD, median (with lower and upper quartiles), or frequencies and percentages.
Patients classified by all methods.
Figure 1.
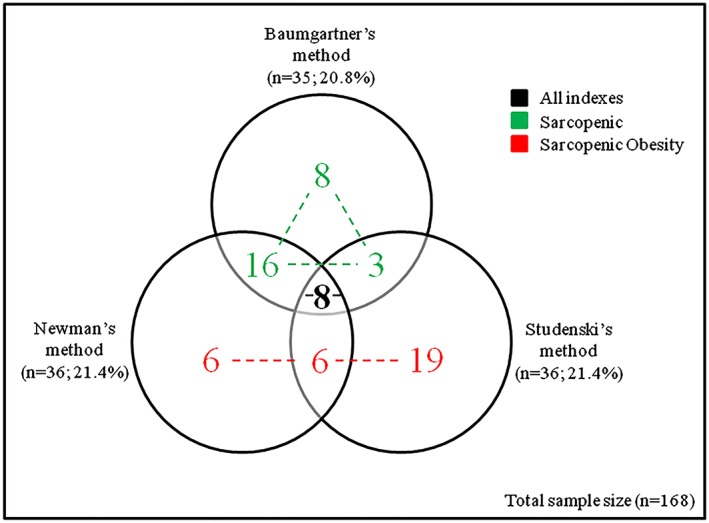
Prevalence of sarcopenia according to Baumgartner's method (circle 1), Newman's method (circle 2), and Studenski's method (circle 3). Patients were further divided into three subgroups based on body composition. Number in black intersected by all circles refers to patients included in all indexes (n = 8). Numbers in green represent patients in the sarcopenic group (n = 27). Numbers in red represent patients in the sarcopenic obesity group (n = 31).
The distribution of individuals according to the BMI categories is presented in Figure 2 . Patients with BMI < 25 kg/m2 were more likely to be identified as sarcopenic by Baumgartner's method (n = 31; 18.5%) when compared with Studenski's method (n = 12; 7.1%; P < 0.001), and there was a trend towards higher number of cases in Baumgartner's method compared with Newman's method (n = 20; 11.9%; P = 0.053). Patients with BMI ≥ 25 kg/m2 were more likely to be identified as sarcopenic by Studenski's (n = 24; 14.3%) and Newman's methods (n = 16; 9.5%) when compared with Baumgartner's method (n = 4; 2.4%; both P < 0.005). There was no difference between Studenski's and Newman's methods in both categories of BMI (P = 0.11; P = 0.16).
Figure 2.
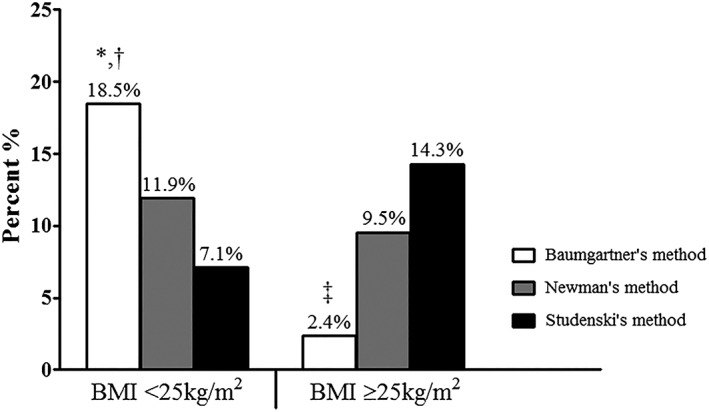
Prevalence of sarcopenia by method in patients with body mass index (BMI) <25 and ≥25 kg/m2. * P = 0.053 vs. Newman's method. † P < 0.001 vs. Studenski's method. ‡ P < 0.005 vs. both Newman's and Studenski's methods.
Regardless of method used for definition, patients with sarcopenia were older and had higher hs‐CRP when compared with non‐sarcopenic patients. In addition, weight, BMI, and height were lower in sarcopenic patients compared with non‐sarcopenic. There was no statistical difference in medication between groups (Table 1).
We further divided the 66 sarcopenic patients into three subgroups (Table 2 and Figure 1 ) according to body composition features, which consisted of patients classified in all indexes (n = 8), patients classified in Baumgartner's method (sarcopenic; n = 27) and patients classified in Newman's plus Studenski's method (sarcopenic obesity; n = 31).
Table 2.
Demographic and clinical characteristics in subgroups of sarcopenia
| Variable | Without sarcopenia (n = 102) | All indexes (n = 8) | Sarcopenic (n = 27) | Sarcopenic obesity (n = 31) |
|---|---|---|---|---|
| Age (years) | 56 (50–61) | 63 (56–68)* | 61 (57–63)* | 59 (55–63)* |
| Weight (kg) | 74.5 ± 10.5 | 56.3 ± 9.6*,† | 58.4 ± 7.8*,† | 76.8 ± 14.5 |
| Height (m) | 1.68 ± 0.06 | 1.59 ± 0.05* , ‡ | 1.67 ± 0.06† | 1.62 ± 0.07* |
| BMI (kg/m2) | 26.2 ± 3.0 | 22.3 ± 3.1* , † | 20.9 ± 2.3* , † | 29.1 ± 4.4* |
| Aetiology (ischaemic/non‐ischaemic) | 26/76 | 2/6 | 6/21 | 14/17 |
| Aetiology (Chagas/no Chagas) | 26/76 | 1/7 | 8/19 | 5/26 |
| NYHA class (I/II/III/IV) | (39/31/24/8) | 1/3/4/0 | 8/11/7/1 | 6/12/11/2 |
| LVEF (%) | 27 (22–33) | (25) (21–37) | 25 (20–31) | 28 (21–33) |
| BNP (pg/mL) | 363 (123–993) | 986 (299–2073)† | 647 (207–1635)† | 201 (99–784) |
| hs‐CRP (mg/L) | 2.4 (0.9–5.9) | 9.2 (3.1–29.2)* | 3.0 (1.2–12.4) | 3.8 (0.9–9.1) |
| Creatinine (mg/dL) | 1.2 (1.0–1.5) | 1.4 (1.1–2.0)† , ‡ | 1.1 (0.9–1.3) | 1.1 (1.0–1.4) |
| Sodium (mEq/L) | 140 (138–141) | 140 (138–142) | 139 (137–141) | 140 (138–142) |
| Potassium (mEq/L) | 4.5 (4.3–4.7) | 4.5 (4.2–4.9) | 4.6 (4.2–4.7) | 4.7 (4.4–4.9) |
| Haemoglobin (g/dL) | 14.0 ± 1.8 | 13.2 ± 1.2 | 13.8 ± 1.5 | 14.3 ± 1.5 |
| Fasting glucose (mg/dL) | 104 (95–114) | 97 (91–110)* , † | 98 (88–108)* , † | 114 (102–130)* |
| Functional capacity and strength | ||||
| Peak VO2 (L/min) | 1.41 (1.01–1.81) | 0.96 (0.90–1.06)* , † | 0.98 (0.85–1.31)* , † | 1.25 (1.09–1.73) |
| Peak VO2 (mL/min/kg) | 19.3 (13.9–24.7) | 16.0 (14.7–17.5) | 17.0 (14.3–23.9) | 16.6 (14.1–22.0) |
| VE/VCO2 slope | 34 (29–39) | 33 (26–36) | 36 (32–40) | 36 (31–44) |
| Peak workload (W) | 110 (70–150) | 70 (38–88)* | 70 (60–90)* | 90 (55–115)* |
| Time (s) | 556 (475–664) | 590 (469–783) | 510 (379–569)* , † | 572 (509–663) |
| Handgrip strength (kg) | 34.6 ± 7.6 | 25.4 ± 5.5*,(†) | 29.6 ± 6.8* | 33.2 ± 6.1 |
BMI, body mass index; BNP, B‐type natriuretic peptide; hs‐CRP, high‐sensitivity C‐reactive protein; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; VE/VCO2, ventilatory equivalent for carbon dioxide; VO2, oxygen consumption.
Data are presented as mean ± SD or median (with lower and upper quartiles). Symbols in brackets denote a trend with P < 0.10.
P < 0.05 (vs. without sarcopenia).
P < 0.05 (vs. sarcopenic obesity).
P < 0.05 (vs. sarcopenic).
Table 2 shows clinical characteristics of patients included in the subgroups of sarcopenia. In general, we observed that lean sarcopenic patients presented lower exercise tolerance (reduced absolute peak VO2 but not relative peak VO2) and higher B‐type natriuretic peptide and hs‐CRP when compared with non‐sarcopenic and sarcopenic obese patients. On the other hand, lean sarcopenic patients showed normal glucose levels when compared with both non‐sarcopenic and sarcopenic obese patients. Left ventricular ejection fraction was not different among groups (Table 2).
3.1. Body composition
Patients included in the sarcopenic group had a lower total fat mass, fat percentage, fat mass in arms, legs, and trunk when compared with non‐sarcopenic patients and sarcopenic obese patients. In addition, sarcopenic obese patients presented higher total fat mass, fat percentage, fat mass in arms, legs, and trunk than those without sarcopenia and classified in all indexes (Table 3).
Table 3.
Body composition in subgroups of sarcopenia
| Variable | Without sarcopenia (n = 102) | All indexes (n = 8) | Sarcopenic (n = 27) | Sarcopenic obesity (n = 31) |
|---|---|---|---|---|
| Fat mass (kg) | ||||
| Arms | 1.9 ± 0.6 | 1.7 ± 0.4† | 1.4 ± 0.5* , † | 2.7 ± 1.1* |
| Legs | 5.5 ± 2.3 | 4.3 ± 1.0(*),† | 4.2 ± 1.2* , † | 6.6 ± 1.9* |
| Trunk | 10.6 ± 4.9 | 8.5 ± 2.8† | 6.5 ± 4.0* , † | 15.9 ± 5.4* |
| Total | 18.4 ± 7.0 | 15.2 ± 4.1† | 12.8 ± 5.6* , † | 26.2 ± 8.1* |
| Total fat (%) | 25 ± 7 | 28 ± 5† , ‡ | 22 ± 7* , † | 35 ± 5* |
| Lean mass (kg) | ||||
| Arms | 6.5 ± 0.9 | 4.2 ± 0.6* , † | 5.0 ± 0.6* , † | 5.9 ± 0.8* |
| Legs | 17.4 ± 2.7 | 11.3 ± 1.4* , † | 13.2 ± 1.4* , † | 15.4 ± 2.5* |
| Trunk | 26.0 ± 4.3 | 20.3 ± 4.6* | 21.7 ± 2.2* | 23.4 ± 4.3* |
| Total | 52.4 ± 5.8 | 38.0 ± 5.9* , † | 42.6 ± 4.1*,(†) | 46.3 ± 4.0* |
| Total lean (%) | 71 ± 6 | 68 ± 4(*),†,‡ | 73 ± 6† | 63 ± 5* |
| Lean mass/fat mass | 3.3 ± 1.6 | 2.7 ± 0.8† , ‡ | 3.9 ± 1.5* , † | 2.0 ± 0.5* |
Data are presented as mean ± SD or median (with lower and upper quartiles). Symbols in brackets denote a trend with P < 0.10.
P < 0.05 (vs. without sarcopenia).
P < 0.05 (vs. sarcopenic obesity).
P < 0.05 (vs. sarcopenic).
Despite the method used to define sarcopenia, all sarcopenic groups presented lower total lean mass and reduced lean mass in arms, legs, and trunk compared with non‐sarcopenic patients, whereas sarcopenic obese patients had higher total lean mass and lean mass in arms and legs than patients classified in all indexes and in the sarcopenic group. In addition, lean percentage and lean/fat mass ratio were lower in sarcopenic obese patients when compared with their sarcopenic counterparts and non‐sarcopenic patients. Patients classified in all indexes showed lower lean percentage and lean/fat mass ratio than lean sarcopenic patients (Table 3).
4. Discussion
The main findings of this study are that the criteria based on ALM adjusted by height plus fat mass (Newman's method) or adjusted by BMI (Studenski's method) can be used to determine sarcopenia in overweight/obese patients with HFrEF, but the latter seems to be more appropriate to apply in the clinical setting. The prevalence of sarcopenia alone and SO is similar in patients with HFrEF when using methods that consider fat mass and body size in its equation. Moreover, as expected, patients with HFrEF and SO present higher lean mass than lean sarcopenic patients, although the amount of lean mass is still lower than non‐sarcopenic patients.
Sarcopenia has become a major public health issue as the population ages. It is well recognized as an independent marker of worse prognosis in patients with HF,2 and has been related to reduced functional capacity and sympathovagal imbalance.17, 18 The last revised European consensus about sarcopenia states that the cut‐off point for sarcopenia must be lower than 7.0 kg/m2 for men using Baumgartner's method.1 In our study, using the lower 20th percentile of Baumgartner's method to define sarcopenia, we found a similar cut‐off of 7.03 kg/m2. In addition, the cut‐off of Newman's method and Studenski's method was −2.32 and 0.76, respectively. They were also very close to what have been proposed by others.12, 13
In a sample of 200 patients with HF, Fülster and colleagues found that 39 patients (19.5%) were classified as sarcopenic, and about 37 of these patients (94.9%) were men. In our study, the prevalence of sarcopenia was 20.8% for Baumgartner's method and 21.4% for Newman's and Studenski's methods, showing a very similar prevalence among the three methods and also close to the percentage described in some studies19, 20 but a little higher than in other populations.14
In addition, obesity, defined as BMI ≥ 30 kg/m2, has reached epidemic proportions and is known to increase the risk of developing HF.21 However, once HF is established, obese patients may have an improved outcome because of the obesity paradox.10 Although BMI is high, this protective effect of overweight/obesity in these patients may be abolished in the presence of decreased lean mass and increased fat mass.10, 11 Moreover, increases in BMI cannot detect the regional body fat mass distribution, especially visceral fat that has been associated with mortality in these patients.22 Furthermore, the protective effect of higher BMI should be considered with markers of functional capacity, once the obesity paradox has been described to be more significant in patients with lower cardiorespiratory fitness.23
In spite of the limitations involved with BMI, we can still use it to determine the relation between lean and fat mass, once ALM is measured by a gold standard method to determine body composition (DXA).24 In our study, we found that using Studenski's method to determine SO was alike Newman's method, which uses a linear regression and adds fat mass to the index proposed by Baumgartner to diagnose sarcopenia. Furthermore, considering the similarities between Newman's and Studenski's methods in predicting SO in patients with HFrEF, BMI can be an easy and applicable tool to adjust ALM in patients with higher body weight, although neither of these methods is superior to the other.
The combination of sarcopenia and obesity has been described as a higher‐risk group that can potentiate the adverse effects of these conditions alone.6, 7 Low‐grade inflammation seems to be a common pathway in the progression of these disorders and leads to alterations in body composition.25, 26 Regardless of the definition of sarcopenia, we found that sarcopenic patients presented increased hs‐CRP. Although we only measured one inflammatory marker, previous studies have shown that hs‐CRP enables detection of even mild alterations in inflammation.27
Moreover, muscle mass seems to be increased in patients with SO because of a higher body weight they have to carry, and it leads to a better maintenance of lean mass. In our study, although patients with SO presented higher lean mass compared with lean sarcopenic patients, percentage lean mass and lean/fat mass ratio, used to correct muscle mass in relation to body weight and fat, were reduced in these patients. On the other hand, even though peak workload was lower in all sarcopenic patients, strength and peak VO2 were similar between patients with SO and non‐sarcopenic. These findings imply that some components of functional capacity and body composition have already been compromised in these patients, whilst others are still preserved. Physical activity has extensively shown to improve health status in patients with HF, and it may play a role in reducing the risk of sarcopenia and SO in the general population.28, 29
4.1. Limitations
Despite the novel findings presented in this study, some limitations must be addressed. First, we only included male patients with HFrEF, so we are not able to extrapolate these results to women and patients with HF with preserved ejection fraction. Second, the cut‐off value of 20th percentile was arbitrary, although it has been used in larger trials than ours. Finally, there was no follow‐up to evaluate the impact of these alterations on prognosis in these patients, and the cross‐sectional analysis of this study does not allow conclusions about cause‐and‐effect relationships.
5. Conclusions
In summary, our results demonstrate that the prevalence of sarcopenia in overweight/obese patients is similar to lean sarcopenic patients when appropriate definitions are used to determine sarcopenia in male patients with HFrEF. In patients with higher BMI, Studenski's method seems to be more feasible to detect sarcopenia in these patients.
Conflict of interest
None declared.
Funding
This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2015/22814‐5). G.W.P.d.F. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 148758/2016‐9); M.R.d.S. by FAPESP (2016/24306‐0); and C.E.N. by FAPESP (2015/22814‐5) and CNPq (303573/2015‐5). FAPESP is from São Paulo, Brazil. CNPq is a national institution that supports research in Brazil.
Fonseca, G. W. P. D. , dos Santos, M. R. , de Souza, F. R. , Takayama, L. , Rodrigues Pereira, R. M. , Negrão, C. E. , and Alves, M.‐J. N. N. (2020) Discriminating sarcopenia in overweight/obese male patients with heart failure: the influence of body mass index. ESC Heart Failure, 7: 84–91. 10.1002/ehf2.12545.
References
- 1. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013; 34: 512–519. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) atEGfE. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cicoira M, Zanolla L, Franceschini L, Rossi A, Golia G, Zamboni M, Tosoni P, Zardini P. Skeletal muscle mass independently predicts peak oxygen consumption and ventilatory response during exercise in noncachectic patients with chronic heart failure. J Am Coll Cardiol 2001; 37: 2080–2085. [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763. [DOI] [PubMed] [Google Scholar]
- 5. Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, Tylavsky FA, Newman AB, Health AiaBCS . Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007; 55: 769–774. [DOI] [PubMed] [Google Scholar]
- 6. Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 2009; 33: 885–892. [DOI] [PubMed] [Google Scholar]
- 7. Tian S, Xu Y. Association of sarcopenic obesity with the risk of all‐cause mortality: a meta‐analysis of prospective cohort studies. Geriatr Gerontol Int 2016; 16: 155–166. [DOI] [PubMed] [Google Scholar]
- 8. Batsis JA, Mackenzie TA, Barre LK, Lopez‐Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 2014; 68: 1001–1007. [DOI] [PubMed] [Google Scholar]
- 9. Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc 2017; 92: 266–279. [DOI] [PubMed] [Google Scholar]
- 10. Thomas E, Gupta PP, Fonarow GC, Horwich TB. Bioelectrical impedance analysis of body composition and survival in patients with heart failure. Clin Cardiol 2019; 42: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Schutter A, Lavie CJ, Kachur S, Patel DA, Milani RV. Body composition and mortality in a large cohort with preserved ejection fraction: untangling the obesity paradox. Mayo Clin Proc 2014; 89: 1072–1079. [DOI] [PubMed] [Google Scholar]
- 12. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB, Investigators HAS. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003; 51: 1602–1609. [DOI] [PubMed] [Google Scholar]
- 13. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tyrovolas S, Koyanagi A, Olaya B, Ayuso‐Mateos JL, Miret M, Chatterji S, Tobiasz‐Adamczyk B, Koskinen S, Leonardi M, Haro JM. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi‐continent study. J Cachexia Sarcopenia Muscle 2016; 7: 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011; 40: 423–429. [DOI] [PubMed] [Google Scholar]
- 16. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, People EWGoSiO . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fonseca GWPD, Santos MRD, Souza FR, Costa MJAD, Haehling SV, Takayama L, Pereira RMR, Negrão CE, Anker SD, Alves MJNN. Sympatho‐vagal imbalance is associated with sarcopenia in male patients with heart failure. Arq Bras Cardiol 2019; 112: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suzuki T, Palus S, Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018; 5: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamada M, Nishiguchi S, Fukutani N, Tanigawa T, Yukutake T, Kayama H, Aoyama T, Arai H. Prevalence of sarcopenia in community‐dwelling Japanese older adults. J Am Med Dir Assoc 2013; 14: 911–915. [DOI] [PubMed] [Google Scholar]
- 20. Fukuda T, Bouchi R, Takeuchi T, Tsujimoto K, Minami I, Yoshimoto T, Ogawa Y. Sarcopenic obesity assessed using dual energy X‐ray absorptiometry (DXA) can predict cardiovascular disease in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol 2018; 17: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, Kitzman DW, Kanaya AM, Pahor M, Harris TB. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc 2006; 54: 413–420. [DOI] [PubMed] [Google Scholar]
- 22. Streng KW, Voors AA, Hillege HL, Anker SD, Cleland JG, Dickstein K, Filippatos G, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Zannad F, Damman K, van der Meer P, Lang CC. Waist‐to‐hip ratio and mortality in heart failure. Eur J Heart Fail 2018; 20: 1269–1277. [DOI] [PubMed] [Google Scholar]
- 23. Clark AL, Fonarow GC, Horwich TB. Impact of cardiorespiratory fitness on the obesity paradox in patients with systolic heart failure. Am J Cardiol. 2015; 115: 209–213. [DOI] [PubMed] [Google Scholar]
- 24. Clark BC, Tavoian D, Goodpaster BH, Cawthon PM, Hansen RD, Manini TM. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018; 9: 1269–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park CH, Do JG, Lee YT, Yoon KJ. Sarcopenic obesity associated with high‐sensitivity C‐reactive protein in age and sex comparison: a two‐center study in South Korea. BMJ Open 2018; 8: e021232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res 2012; 2012: 629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ridker PM. High‐sensitivity C‐reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001; 103: 1813–1818. [DOI] [PubMed] [Google Scholar]
- 28. Ryu M, Jo J, Lee Y, Chung YS, Kim KM, Baek WC. Association of physical activity with sarcopenia and sarcopenic obesity in community‐dwelling older adults: the Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2013; 42: 734–740. [DOI] [PubMed] [Google Scholar]
- 29. Saitoh M, Ishida J, Springer J. Physical activity for the prevention and treatment of sarcopenic obesity. J Cachexia Sarcopenia Muscle 2017; 8: 518–519. [DOI] [PMC free article] [PubMed] [Google Scholar]


