Abstract
Background
To investigate the risk factors and changes in serum inflammatory factors in puerperal infection, and propose clinical prevention measures.
Methods
A total of 240 subjects with suspected puerperal infection treated in our hospital from January 2017 to December 2017 were collected, among which puerperal infection was definitely diagnosed in 40 cases, and it was excluded in 40 cases. Levels of interleukin‐6 (IL‐6), tumor necrosis factor‐α (TNF‐α), and high‐sensitivity C‐reactive protein (hs‐CRP) were compared between the two groups, and the change trends of IL‐6 and hs‐CRP were recorded.
Results
Levels of IL‐6, hs‐CRP, and TNF‐α in puerperal infection group were higher than those in non‐infection group (P < .05). Levels of IL‐6 and hs‐CRP at enrollment and 1‐3 days after enrollment in infection group were higher than those in non‐infection group (P < .05). The body mass index >25, placenta previa, placenta accreta, postpartum hemorrhage, premature rupture of membrane, gestational diabetes mellitus, and anemia during pregnancy were relevant and independent risk factors for puerperal infection. Puerperal infection occurred in uterine cavity, vagina, pelvic peritoneum, pelvic tissue, incision, urinary system, etc, and gram‐negative (G+) bacteria were dominated in pathogens.
Conclusion
The inflammatory response of patients with puerperal infection is significantly enhanced.
Keywords: inflammatory factors, prevention measures, puerperal infection, risk factors
1. INTRODUCTION
In clinic, puerperal infection mainly refers to the reproductive tract infection occurring after delivery, which, as a kind of complication seriously threatening delivery quality and life safety of puerperae, has a certain influence on postpartum recovery and even neonatal feeding.1 Birth canal injury is caused due to fetal delivery via reproductive tract during the puerperium, and the body's immunity of pregnant women significantly declines during the puerperium. As a result, pathogenic microorganisms invade the human body, leading to infection, and even septicopyemia and threatening the maternal life.2 At the same time, the drug resistance of pathogenic bacteria of puerperal infection has significantly increased nowadays, and the incidence rate of puerperal infection caused by uncommon pathogenic bacteria in the past also increases, so the traditional antibacterial drugs, such as penicillin, fail to control the infection effectively in clinical experiential medication.3 Meanwhile, levels of inflammation‐related cytokines in patients with puerperal infection are also significantly increased. Interleukin‐6 (IL‐6), as the most important inflammation‐related cytokine in the body, mainly regulates the cell function and is involved in the body immunity.4 High‐sensitivity C‐reactive protein (hs‐CRP), as the most widely used inflammation‐related cytokine currently, has a definite correlation with the severity of tissue damage and infection, which is an important index in clinical observation in acute infection stage.5
In this study, in order to better investigate the occurrence of puerperal infection, the analysis of common pathogenic factors of puerperal infection started with changes in the body's common inflammatory factors after puerperal infection, and common infection sites and pathogen distribution were explored, hoping to provide corresponding guidance for clinical diagnosis, treatment, and prevention.
2. DATA AND METHODS
2.1. General data
A total of 240 subjects with suspected puerperal infection treated in our hospital from January 2017 to December 2017 were collected. Another 40 subjects without puerperal infection during the same period were selected as control group. Before enrollment, all subjects signed and agreed to be enrolled, and this survey research was approved by the Ethics Committee of hospital. Inclusion criteria: primipara who displayed signs of infection in any part of the body within 48 hours before enrollment, underwent vaginal delivery, and had relevant medical data. Subject who was associated with pain abdomen, malodorous lochia, abdominal distention, uterine tenderness, pelvic abscess, peritonitis, mechanical or foreign body injury. Exclusion criteria: subjects who took antibacterial drugs or immunosuppressors within 48 hours before enrollment, or complicated with malignant tumor, severe dysfunction in the heart, lung, liver, kidney, mental diseases, fever due to medical causes, wound/surgical site infection, mastitis, urinary tract infection or thrombophlebitis.
2.2. Investigation methods
Clinical relevant data of subjects with puerperal infection were analyzed, and all clinical data were collected by obstetricians and related investigators receiving unified training. Patients and their authorizers should be fully cooperated in the collection of investigation data, the investigation was conducted anonymously, the patients' privacy should be protected during the process, and the question raised by patients was answered. The relevant investigation results obtained should not be disclosed to any organization or individual without the permission of subjects enrolled and their authorizers. At enrollment and 30 days after the first investigation, investigators filled out the same questionnaire and completed the data of subjects, and the correlation coefficients obtained in the two times were set as the stability coefficients. In this study, the reliability coefficient (α = 0‐1) was used to evaluate the reliability level, and it was 0.921 after calculation. Clinical data obtained were checked alternatively by two people and entered into the EpiData software data analysis system, followed by statistical processing via Statistical Product and Service Solutions (SPSS) 21.0.
2.3. Detection of inflammatory factor levels and standard reference values
IL‐6 was detected via enzyme‐linked immunosorbent assay, whose standard reference value is 0.37‐0.46 ηg/L. TNF‐α was detected via double‐antibody single‐step sandwich method, whose standard reference value is 5‐100 ng/L, and hs‐CRP was detected via latex‐enhanced immunoturbidimetry, whose standard reference value is ≤10 mg/L.
2.4. Identification of pathogen species of puerperal infection
Pathogen culture and identification were based on the National Clinical Laboratory Procedures, and all operations were performed in strict accordance with the above operating procedures. Bacteria were cultured using the Kirby‐Bauer (K‐B) diffusion method, while fungi were cultured using the glucose peptone agar medium. All operations were conducted strictly according to the CLSI 2008‐2010 procedures, and data were analyzed using WHONET (version 5.3‐5.4). All the above operations were performed by the medical laboratory technicians with working experience in clinical detection for 5 years or above strictly according to the operation procedures and regulations.
2.5. Observation indexes
Levels of inflammatory cytokines (IL‐6, TNF‐α and hs‐CRP) in subjects with and without puerperal infection were compared, and the change trends of IL‐6 and hs‐CRP in both groups were recorded at enrollment and 1‐3 days after enrollment. Univariate and multivariate analyses were performed for relevant clinical data, such as age, body mass index, gestational week, placenta previa, placenta accreta, postpartum hemorrhage, premature rupture of membrane, gestational hypertension, gestational diabetes mellitus, and anemia during pregnancy, and the infection sites and pathogen distribution in subjects with puerperal infection were recorded.
2.6. Statistical analysis
SPSS 21.0 (IBM) statistical software was used. Univariate analysis was performed first for puerperal infection, followed by multivariate logistic regression analysis. Measurement data were presented as mean ± standard deviation (), and chi‐square test was used for the intergroup comparison of rate. P < .05 suggested that the difference was statistically significant.
3. RESULTS
3.1. Comparisons of inflammatory factor levels between the two groups
A total of 240 subjects with suspected puerperal infection treated in our hospital from January 2017 to December 2017 were collected and 40 women with severe maternal sepsis were finally enrolled for the study according to the inclusion and exclusion criteria and result of pathogen culture. They were aged 19‐42 years old ([28.5 ± 1.2] years old on average) with the gestational week of 34‐41 weeks ([38.1 ± 0.6] weeks on average), including 21 cases receiving lateral episiotomy. Another 40 subjects in control group were aged 19‐42 years old ([28.6 ± 1.1] years old on average) with the gestational week of 34‐41 weeks ([38.0 ± 0.6] weeks on average), including 20 cases receiving lateral episiotomy. Levels of inflammatory factors (IL‐6, hs‐CRP and TNF‐α) were detected in both groups and the levels in puerperal infection group were significantly higher than those in non‐infection group (P < .05; Table 1).
Table 1.
Comparisons of inflammatory factor levels between the two groups ()
| IL‐6 (ηg/L) | hs‐CRP (mg/L) | TNF‐α (ng/L) | |
|---|---|---|---|
| Infection group | 1.05 ± 0.15 | 16.5 ± 1.0 | 130.0 ± 7.4 |
| Non‐infection group | 0.41 ± 0.03 | 4.1 ± 0.1 | 71.3 ± 4.0 |
| t | 26.461 | 78.035 | 44.134 |
| P | .000 | .000 | .000 |
3.2. Change trends of main inflammatory factor (IL‐6) level in the two groups
At enrollment and 1‐3 days after enrollment, the IL‐6 level was (1.05 ± 0.15) ηg/L, (0.89 ± 0.06) ηg/L, (0.63 ± 0.05) ηg/L, and (0.51 ± 0.03) ηg/L, respectively, in infection group, and (0.41 ± 0.03) ηg/L, (0.39 ± 0.03) ηg/L, (0.28 ± 0.02) ηg/L, and (0.26 ± 0.02) ηg/L, respectively, in non‐infection group. Levels of IL‐6 in infection group were evidently higher than those in non‐infection group at different time points (t = 26.461, 47.140, 41.105, and 43.853, P < .05; Figure 1A).
Figure 1.
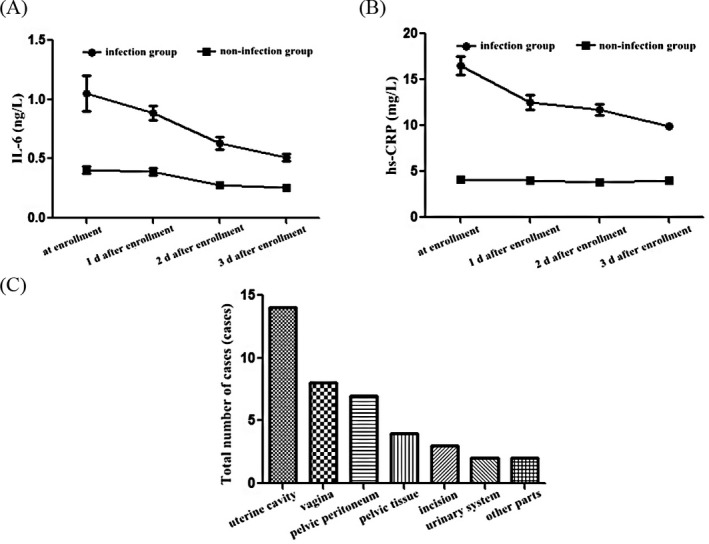
Levels of inflammatory factors and infection sites. Change trends of main inflammatory factors IL‐6 (A) and hs‐CRP (B) levels in the two groups by ELISA. C, Main infection sites in patients with puerperal infection include uterine cavity, vagina, pelvic peritoneum, pelvic tissue, incision, and urinary system
3.3. Change trends of main inflammatory factor (hs‐CRP) level in the two groups
At enrollment and 1‐3 days after enrollment, the hs‐CRP level was (16.5 ± 1.0) mg/L, (12.5 ± 0.8) mg/L, (11.7 ± 0.6) mg/L, and (9.9 ± 0.3) mg/L, respectively, in infection group, and (4.1 ± 0.1) mg/L, (4.0 ± 0.1) mg/L, (3.8 ± 0.1) mg/L, and (4.0 ± 0.1) mg/L, respectively, in non‐infection group. Levels of hs‐CRP in infection group were obviously higher than those in non‐infection group at different time points (t = 78.035, 66.679, 82.140, and 118.0000, P < .05; Figure 1B).
3.4. Univariate analysis of relevant risk factors in puerperal infection group
According to the univariate analysis, the incidence rate of puerperal infection was significantly increased in subjects with the body mass index >25, placenta previa, placenta accreta, postpartum hemorrhage, premature rupture of membrane, gestational diabetes mellitus, and anemia during pregnancy (P < .05), and they were relevant risk factors for puerperal infection (Table 2).
Table 2.
Univariate analysis of relevant risk factors in puerperal infection group (n)
| Case surveyed | Case of puerperal infection | χ 2 | P | |
|---|---|---|---|---|
| Age | ||||
| >30 y old | 115 | 19 | 0.003 | .954 |
| <30 y old | 125 | 21 | ||
| Body mass index | ||||
| >25 | 135 | 31 | 8.808 | .003 |
| <25 | 105 | 9 | ||
| Gestational week | ||||
| >38 wk | 130 | 21 | 0.054 | .817 |
| <38 wk | 110 | 19 | ||
| Placenta previa | ||||
| Yes | 116 | 35 | 29.486 | .000 |
| No | 124 | 5 | ||
| Placenta accreta | ||||
| Yes | 112 | 34 | 31.228 | .000 |
| No | 128 | 4 | ||
| Postpartum hemorrhage | ||||
| Yes | 125 | 30 | 10.101 | .001 |
| No | 115 | 10 | ||
| Premature rupture of membrane | ||||
| Yes | 130 | 29 | 6.498 | .011 |
| No | 110 | 11 | ||
| Gestational hypertension | ||||
| Yes | 110 | 19 | 0.054 | .817 |
| No | 130 | 21 | ||
| Gestational diabetes mellitus | ||||
| Yes | 112 | 32 | 18.552 | .000 |
| No | 128 | 8 | ||
| Anemia during pregnancy | ||||
| Yes | 123 | 30 | 10.837 | .001 |
| No | 117 | 10 | ||
3.5. Multivariate Logistic regression analysis of relevant risk factors in puerperal infection group
According to the multivariate logistic regression analysis, the body mass index >25, placenta previa, placenta accreta, postpartum hemorrhage, premature rupture of membrane, gestational diabetes mellitus, and anemia during pregnancy were independent risk factors for puerperal infection (Table 3).
Table 3.
Multivariate Logistic regression analysis of relevant risk factors in puerperal infection group
| β | Standard error (SE) | W | OR | P | 95% CI | |
|---|---|---|---|---|---|---|
| Body mass index | 2.34 | 0.46 | 9.92 | 12.81 | .01 | 1.21‐34.40 |
| Placenta previa | 2.92 | 0.54 | 4.46 | 6.86 | .04 | 1.59‐49.24 |
| Placenta accreta | 1.82 | 0.50 | 10.82 | 4.81 | .01 | 1.58‐14.38 |
| Postpartum hemorrhage | 1.83 | 0.57 | 4.82 | 1.96 | .04 | 1.03‐7.45 |
| Premature rupture of membrane | 0.89 | 0.53 | 9.47 | 2.44 | .00 | 1.38‐4.31 |
| Gestational diabetes mellitus | 1.12 | 15.50 | 6.38 | 3.07 | .00 | 1.76‐5.34 |
| Anemia during pregnancy | 1.71 | 33.60 | 7.11 | 5.52 | .00 | 3.10‐9.83 |
3.6. Analysis of main infection sites
Among 40 cases of puerperal infection, there were 14 cases (35.0%) of uterus cavity infection, 8 cases (20.0%) of vaginal infection, 7 cases (17.5%) of pelvic peritoneal infection, 4 cases (10.0%) of pelvic tissue infection, 3 cases (7.5%) of incision infection, 2 cases (5.0%) of urinary system infection, and 2 cases (5.0%) of infection in other sites (Figure 1C).
3.7. Pathogen distribution in subjects with puerperal infection
Among 40 cases of puerperal infection, G− bacteria were detected in 24 cases (60.0%), G+ bacteria in 14 cases (35.0%), and fungi in two cases (5.0%). Specifically, Staphylococcus aureus accounts for 12.5%, Staphylococcus epidermidis 2.5%, Enterococcus 15.0%, Streptococcus 5.0%, Gardnerella vaginalis 12.5%, Escherichia coli 27.5%, Pseudomonas aeruginosa 10.0%, Acinetobacter baumannii 5.0%, Klebsiella pneumonia 5.0%, Fungi 5.0%, Candida albicans 2.5%, and Candida tropicalis 2.5%.
4. DISCUSSION
Puerperal infection is a common and frequently occurring disease in clinic,6 which is also a common postpartum complication in puerperae. Its clinical manifestations mainly include elevated postpartum temperature accompanied by chills, lower abdominal pain, and tenderness, and severe infection displayed in biochemical examination.7 During the delivery process, changes in the physiological structure of pregnant women, hemorrhage, delivery injury, etc, lead to the decline in the body's immunity, resulting in puerperal infection under the action of some risk factors.8 Subjects with mild inflammatory response mostly suffer from the local infection in the reproductive system, while those with severe inflammatory response may have sepsis and even septicopyemia, threatening the life.9 Therefore, effective measures should be actively taken in clinic to ensure the clinical therapeutic effect once puerperal infection occurs, thereby improving the prognosis of pregnant women, and minimizing the impact of puerperal infection on puerperae and neonates.10
In this study, in order to better investigate the occurrence, development, and prevention of puerperal infection, levels of inflammatory factors were compared between subjects with and without puerperal infection. It was found that levels of inflammatory factors (IL‐6, hs‐CRP, and TNF‐α) in puerperal infection group were significantly higher than those in non‐infection group, and levels of IL‐6 and hs‐CRP at enrollment and 1‐3 days after enrollment in infection group were also obviously higher than those in non‐infection group, indicating that levels of inflammatory cytokines in the body of subjects with puerperal infection are remarkably increased, and the body's inflammatory response is significant. At the same time, analyses of risk factors for puerperal infection showed that the body mass index >25, placenta previa, placenta accreta, postpartum hemorrhage, premature rupture of membrane, gestational diabetes mellitus, and anemia during pregnancy were relevant and independent risk factors for puerperal infection, suggesting that subjects with the body mass index >25, placenta previa, placenta accreta, postpartum hemorrhage, premature rupture of membrane, gestational diabetes mellitus, and anemia during pregnancy should be paid attention to in clinic, and related measures should be taken to prevent puerperal infection. Finally, the study on puerperal infection sites and pathogenic bacteria manifested that the infection mainly occurred in reproductive tract, such as uterine cavity and vagina, followed by pelvic cavity. Gram‐negative bacteria were dominated in the pathogenic bacteria, followed by gram‐positive bacteria (about 30%). In terms of antibacterial drugs selected in experiential therapy, therefore, it is recommended that gram‐negative bacteria be the main target with consideration to gram‐positive bacteria.
Prevention measures of puerperal infection should be taken before pregnancy and during pregnancy, delivery, and puerperium. First, before pregnancy, it is suggested that women of child‐bearing age strengthen physical exercise, pay attention to nutritional regulation, actively prevent and control the reproductive system diseases, especially inflammatory diseases, reduce the frequency of uterine curettage, and make good preparation for pregnancy.11 During pregnancy, it is suggested that early pregnancy be determined in time, health management during pregnancy be actively implemented,12 the standard maternal manual be created before 13 weeks of gestation, the comprehensive physical examination be given,13 and high‐risk pregnancy and pathologic pregnancy be screened to improve the safety of pregnancy. During delivery, sterile operations should be strictly performed, the digital anal examination is strictly forbidden,14 the frequency of vaginal examination should be reduced as far as possible to reduce the incidence rate of retrograde infection,15 indications of cesarean section and lateral episiotomy should be strictly grasped, antibacterial drugs can be used for a short time for those undergoing cesarean section to prevent the infection, and the maternal management should be strengthened 16 to improve the hospital delivery rate,17 prevent postpartum hemorrhage,18 and reduce the injury of birth canal due to delivery as far as possible.19 During puerperium, it is recommended that puerperae get enough sleep, strengthen nutrition reasonably, and improve the body's immunity, the health management be strengthened, and prevention measures be actively taken for puerperae with high‐risk factors, thereby improving the prognosis of patients and reducing the incidence of puerperal infection.20 The limitation in our study still exists that a relatively larger study population from different regions requires to be included to confirm our finding and even reflect data at national level, which may provide fundamental leads for the diagnosis and treatment of puerperal infection.
5. CONCLUSION
In conclusion, the inflammatory response of patients with puerperal infection is significantly enhanced, with elevation of IL‐6 and hs‐CRP levels. Subjects with the body mass index >25, placenta previa, placenta accreta, postpartum hemorrhage, premature rupture of membrane, gestational diabetes mellitus, and anemia during pregnancy should be paid attention to, and prevention measures for puerperal infection should be actively taken. In experiential medication, it is recommended that gram‐negative bacteria be the main target with consideration to gram‐positive bacteria.
ACKNOWLEDGMENTS
This work was supported by special fund for clinical research of Wu Jieping medical fund (320.6750.17173).
Song H, Hu K, Du X, Zhang J, Zhao S. Risk factors, changes in serum inflammatory factors, and clinical prevention and control measures for puerperal infection. J Clin Lab Anal. 2020;34:e23047 10.1002/jcla.23047
REFERENCES
- 1. Nenke MA, Zeng A, Meyer EJ, et al. Differential effects of estrogen on corticosteroid‐binding globulin forms suggests reduced cleavage in pregnancy. J Endocr Soc. 2017;1:202‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonet M, Ota E, Chibueze CE, Oladapo OT. Routine antibiotic prophylaxis after normal vaginal birth for reducing maternal infectious morbidity. Cochrane Database Syst Rev. 2017;13:21‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mascarello KC, Horta BL, Silveira MF. Maternal complications and cesarean section without indication: systematic review and meta‐analysis. Rev Saude Publica. 2017;51:116‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gudu W, Abdulahi M. Labor, delivery and postpartum complications in nulliparous women with female genital mutilation admitted to karamara hospital. Ethiop Med J. 2017;55:11‐17. [PubMed] [Google Scholar]
- 5. Bonet M, Ota E, Chibueze CE, Oladapo OT. Antibiotic prophylaxis for episiotomy repair following vaginal birth. Cochrane Database Syst Rev. 2017;2:11‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tardieu SC, Schmidt E. Group A streptococcus septic shock after surgical abortion: a case report and review of the literature. Case Rep Obstet Gynecol. 2017;20:63‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Orazulike NC, Alegbeleye JO, Obiorah CC, Nyengidiki TK, Uzoigwe SA. A 3‐year retrospective review of mortality in women of reproductive age in a tertiary health facility in Port Harcourt, Nigeria. Int J Womens Health. 2017;16:769‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu X, Wang C, Li Y, et al. Cervical dilation balloon combined with intravenous drip of oxytocin for induction of term labor: a multicenter clinical trial. Arch Gynecol Obstet. 2018;297:77‐83. [DOI] [PubMed] [Google Scholar]
- 9. Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gon G, Ali SM, Towriss C, et al. Unpacking the enabling factors for hand, cord and birth‐surface hygiene in Zanzibar maternity units. Health Policy Plan. 2017;32:1220‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Easter SR, Molina RL, Venkatesh KK, Kaimal A, Tuomala R, Riley LE. Clinical risk factors associated with peripartum maternal bacteremia. Obstet Gynecol. 2017;130:710‐717. [DOI] [PubMed] [Google Scholar]
- 12. Cobo F, Rodríguez‐Granger J, Sampedro A, Navarro‐Mari JM. Infected breast cyst due to Prevotella buccae resistant to metronidazole. Anaerobe. 2017;48:177‐178. [DOI] [PubMed] [Google Scholar]
- 13. Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Antibiotic prophylaxis for operative vaginal delivery. Cochrane Database Syst Rev. 2017;5:8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jena P, Sheela CN, Venkatachala RP, Devarbhavi H. Obstetric outcome in women with chronic liver disease. J Obstet Gynaecol India. 2017;67:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marwah S, Topden SR, Sharma M, Mohindra R, Mittal P. Severe puerperal sepsis‐a simmering menace. J Clin Diagn Res. 2017;11: QC04‐QC08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kulkarni GB, Mirza AM, Ramakrishnan S, Mustare V. Preliminary data on utility of subcutaneous unfractionated heparin in patients with deep cerebral venous thrombosis. J Thromb Thrombolysis. 2017;44:247‐253. [DOI] [PubMed] [Google Scholar]
- 17. Cobo F, Rodríguez‐Granger J, Sampedro A, Navarro‐Mari JM. Breast abscess due to Finegoldia magna in a non‐puerperal women. Anaerobe. 2017;47:183‐184. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi N, Ahmed S, Sumi A, Urushibara N, Kawaguchiya M, Aung MS. Collaborative research on puerperal infections in Bangladesh. Nihon Eiseigaku Zasshi. 2017;72:106‐111. [DOI] [PubMed] [Google Scholar]
- 19. Subramaniam A, Owen J, Campbell SB, Harper LM, Fitzwater JL, Edwards RK. Maternal body mass index and oxytocin exposure in nulliparous women: is there an interaction associated with maternal and neonatal morbidities? J Matern Fetal Neonatal Med. 2017;6:1‐6. [DOI] [PubMed] [Google Scholar]
- 20. Ehrmann Feldman D, Vinet É, Sylvestre MP, et al. Postpartum complications in new mothers with juvenile idiopathic arthritis: a population‐based cohort study. Rheumatology (Oxford). 2017;56:1378‐1385. [DOI] [PubMed] [Google Scholar]


