Abstract
Aims
Spironolactone has been shown to improve outcomes in patients with heart failure (HF) with reduced ejection fraction (EF). We investigated whether the discharge use of spironolactone could be associated with better long‐term outcomes among patients with HF with mid‐range EF (HFmrEF).
Methods and results
We analysed HFmrEF (left ventricular EF 40–49%) patients enrolled in the Japanese Cardiac Registry of Heart Failure in Cardiology, which prospectively studied the clinical characteristics, treatments, and long‐term outcomes of patients hospitalized due to HF. Patients were divided into two groups according to the use of spironolactone at discharge. The primary outcome was a composite of all‐cause death or HF rehospitalization. A total of 457 patients had HFmrEF. The mean age was 69.3 years and 286 (62.6%) were male. Among them, spironolactone was prescribed at discharge in 158 patients (34.6%). Chronic kidney disease (7.6% vs. 16.8%, P = 0.007) was less prevalent and loop diuretics (89.2% vs. 70.2%, P < 0.001) were more often prescribed in patients with spironolactone. During a mean follow‐up of 2.2 years, patients with spironolactone had a lower incidence rate of the primary outcome than those without it (171.5 vs. 278.8 primary outcome per 1000 patient‐years, incidence rate ratio 0.61, 95% confidence interval 0.44–0.86; P = 0.004). After multivariable adjustment, spironolactone use at discharge was associated with a significant reduction in the composite of all‐cause death or HF rehospitalization (adjusted hazard ratio 0.63, 95% confidence interval 0.44–0.90, P = 0.010).
Conclusions
Among patients with HF hospitalized for HFmrEF, spironolactone use at discharge was associated with better long‐term outcomes.
Keywords: Spironolactone, Heart failure with mid‐range ejection fraction, Outcomes, Rehospitalization
1. Introduction
Heart failure (HF) with left ventricular ejection fraction (LVEF) of 40–49% was first proposed in 2013 American College of Cardiology/American Heart Association guidelines as an intermediate group.1 In 2016, the European Society of Cardiology HF guidelines defined this distinct group as HF with mid‐range ejection fraction (HFmrEF)2 and indicated the need for more research on this clinical entity because patients with LVEF of 40–49% were traditionally excluded from most of the previous randomized controlled clinical trials. Recent studies have investigated the clinical characteristics, outcomes, and prognostic factors in HFmrEF in comparison with HF with preserved EF (HFpEF) or HF with reduced EF (HFrEF).3, 4 However, an effective therapeutic strategy for HFmrEF remains to be established.
Several prospective randomized controlled clinical trials have demonstrated effects of mineralocorticoid receptor antagonists (MRA) including spironolactone and eplerenone on outcomes in patients with HFrEF. Spironolactone reduced morbidity and mortality in patients with HFrEF.5 Eplerenone, a more selective MRA, was also shown to reduce the risk of mortality and hospitalization among patients with LV systolic dysfunction after myocardial infarction6 and HFrEF.7 TOPCAT trial, a multinational randomized controlled trial of spironolactone, demonstrated that spironolactone reduced the composite outcome of cardiovascular mortality or HF rehospitalization in patients who were diagnosed with HFpEF by elevated brain natriuretic peptide (BNP) levels and had a higher event rate.8, 9 To date, it has not been determined whether spironolactone could improve outcomes of patients with HFmrEF. The aim of the present study was to analyse the prognostic impact of spironolactone on mortality and rehospitalization due to worsening HF among patients with HFmrEF by using the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE‐CARD) database.
2. Methods
2.1. Patient selection
The JCARE‐CARD is a multicentre registry of patients hospitalized for the worsening HF in Japan.10 Baseline data were collected during the episode of index hospitalization from January 2004 to June 2005. Follow‐up data were collected at least 1 year after the index admission. The baseline data include (i) demography; (ii) cause of HF; (iii) precipitating cause; (iv) co‐morbidities; (v) complications; (vi) clinical status; (vii) electrocardiographic and echocardiographic findings; and (viii) treatment including discharge medications.
From the database of JCARE‐CARD, those with LVEF of ≥40% and <50% were registered in this study. Of these, patients who died during the index hospitalization were excluded. These patients were divided into two groups according to the use of spironolactone at discharge from the index hospitalization.
2.2. Outcomes
The primary outcome of this study was a composite of all‐cause death or HF rehospitalization. Secondary outcomes were a composite of cardiovascular death or HF rehospitalization, all‐cause death, and cardiovascular death.
2.3. Statistical analysis
Patients with spironolactone were compared with those without it. The patient characteristics, including age, gender, New York Heart Association (NYHA) functional class at discharge, previous HF admission, echocardiographic data, co‐morbidities, previous procedures, and therapeutic agents, were compared with Pearson χ2 test for categorical variables, Student's t‐test, or Wilcoxon rank sum test for continuous variables where applicable and were presented as mean ± SD or median with interquartile range (IQR). For analysis of associations between spironolactone and outcomes, incidence rates per 1000 patient‐years and incidence rate ratio were calculated for each outcome. Cumulative incidence of outcomes was estimated by using regression estimates from a Cox proportional hazards model including covariates that were either statistically significant on univariate analysis or clinically relevant. Adjusted hazard ratios (HRs) were estimated by Cox regression model and were presented with 95% confidence interval (CI) and P value. Patient characteristics and prognostic factors in those with LVEF ≥50% were tested by same methods for comparison. For the purpose of the sensitivity analysis, a propensity score was estimated by fitting a logistic‐regression model that adjusted for age (≥70 years vs. <70 years), sex, previous HF admission, NYHA functional class (I–II vs. III–IV), ischaemic heart disease, hypertension, diabetes mellitus, chronic kidney disease, stroke, anaemia, history of percutaneous coronary intervention, chronic atrial fibrillation or atrial flutter, ventricular tachycardia or fibrillation, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, beta‐blockers, loop diuretics, oral inotropes, and warfarin. One‐to‐one pair matching between the two groups was performed by nearest neighbor matching without replacement. The same analysis was performed for the propensity matched cohorts. All tests were two tailed, and P < 0.05 was considered to be statistically significant. All analyses were performed with the SAS statistical package (version 9.4, SAS Institute, Cary, North Carolina).
The JCARE‐CARD protocol conforms with the principles outlined in the Declaration of Helsinki and the Guidelines for the Epidemiological Research published by the Japanese Ministry of Health, Labour and Welfare. The original study protocol was approved by the Institutional Review Board at Kyushu University. Institutional Review Board approval from each participating hospital was also required. Informed consent was given by each patient. The authors had full access to and take full responsibility for the integrity of the data.
3. Results
3.1. Patient characteristics
Figure 1 shows the method of patient selection in this study. Of the 2675 patients in this registry, 2499 patients were assessed with echocardiography during the index hospitalization, of whom 477 patients were defined as HFmrEF. Of them, 457 patients who survived to discharge were included in this study. Among them, 158 patients were treated with spironolactone. Baseline characteristics are shown in Table 1. The mean age was 69.3 years and 286 (62.6%) were male. Patient demographics, cause of HF, rates of previous HF diagnosis, previous HF hospitalization, and smoking were comparable between groups. Co‐morbidities were also comparable except for lower prevalence of chronic kidney disease in the spironolactone group (7.6% vs. 16.8%, P = 0.007). The echocardiography demonstrated that LVEF was significantly but slightly lower in the spironolactone group (44.0 [IQR 41.0–46.0] vs. 44.7 [IQR 42.0–47.0]%; P = 0.031). Although BNP value at discharge was lower in the spironolactone group (190.4 [IQR 73.9–393.0] vs. 258.5 [IQR 127.2–545.0] pg/mL; P = 0.005), it was available only in 49.7% of the study cohort. Loop diuretics were more frequently prescribed in the spironolactone group (89.2% vs. 70.2%, P < 0.001), while nitrate was less frequently prescribed in the spironolactone group (19.6% vs. 29.4%, P = 0.023).
Figure 1.
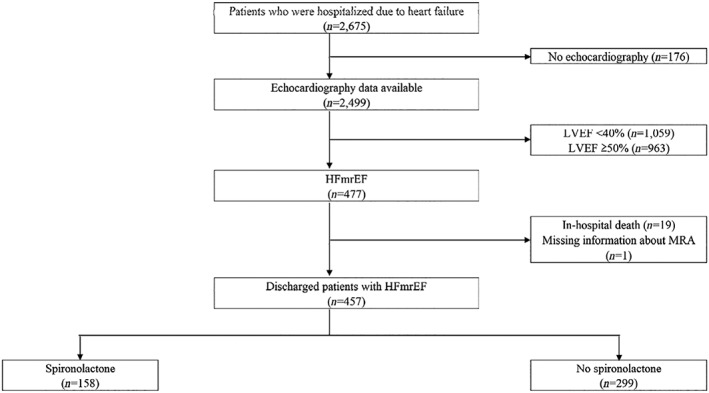
Patient selection. HFmrEF, heart failure with mid‐range ejection fraction; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists.
Table 1.
Patient characteristics
| Variables | Spironolactone (n = 158) | No spironolactone (n = 299) | P value |
|---|---|---|---|
| Demographics | |||
| Age, years | 68.1 ± 15.0 | 70.0 ± 13.1 | 0.17 |
| Male | 99 (62.7) | 187 (62.5) | 0.98 |
| BMI, kg/m2 | 22.9 ± 4.6 | 22.3 ± 4.2 | 0.17 |
| Previous HF diagnosis | 91 (57.6) | 169 (56.5) | 0.83 |
| Previous HF admission | 66 (43.4) | 123 (43.9) | 0.92 |
| NYHA III–IV at discharge | 7 (4.4) | 12 (4.0) | 0.83 |
| Smoking | 63 (42.6) | 113 (40.4) | 0.66 |
| Causes of HF | |||
| Ischaemic | 58 (36.7) | 119 (39.8) | 0.52 |
| Hypertensive | 37 (23.4) | 75 (25.1) | 0.69 |
| Cardiomyopathic, dilated | 25 (15.8) | 45 (15.1) | 0.83 |
| Cardiomyopathic, hypertrophic | 0 (0.0) | 4 (1.3) | 0.14 |
| Other or unknown etiology | 48 (30.4) | 85 (28.4) | 0.66 |
| Co‐morbidities | |||
| Hypertension | 81 (51.6) | 160 (54.1) | 0.62 |
| Diabetes mellitus | 49 (31.0) | 110 (36.8) | 0.22 |
| Dyslipidaemia | 39 (24.8) | 92 (31.0) | 0.17 |
| Chronic kidney disease | 12 (7.6) | 50 (16.8) | 0.007 |
| Hyperuricemia | 73 (48.3) | 136 (46.9) | 0.77 |
| Stroke | 22 (14.0) | 42 (14.2) | 0.95 |
| Anaemia | 24 (15.2) | 63 (21.2) | 0.12 |
| COPD | 9 (5.8) | 19 (6.4) | 0.78 |
| Previous heart disease | |||
| Myocardial infarction | 54 (34.2) | 100 (34.1) | 0.99 |
| Previous PCI | 25 (16.0) | 67 (22.7) | 0.094 |
| Previous CABG | 18 (11.5) | 34 (11.5) | 0.996 |
| Pacemaker implantation | 2 (1.3) | 3 (1.0) | 0.80 |
| CRT implantation | 3 (1.9) | 2 (0.7) | 0.23 |
| ICD implantation | 6 (3.8) | 4 (1.3) | 0.087 |
| Chronic AF, AFL | 55 (34.8) | 95 (32.2) | 0.57 |
| Sustained VT, VF | 9 (5.8) | 15 (5.2) | 0.77 |
| ECG | 0.11 | ||
| Left bandle branch block | 11 (7.0) | 35 (11.7) | |
| Pacing | 10 (6.3) | 10 (3.3) | |
| Echocardiography | |||
| LVDd, mm | 55.8 ± 8.7 | 55.1 ± 7.9 | 0.44 |
| LVDs, mm | 43.7 ± 7.4 | 42.7 ± 7.2 | 0.19 |
| IVS, mm | 10.1 ± 2.2 | 10.6 ± 2.5 | 0.025 |
| LVPW, mm | 10.0 ± 2.0 | 10.7 ± 2.3 | 0.001 |
| LVEF, % | 44.0 (IQR: 41.0–46.0) | 44.7 (IQR: 42.0–47.0) | 0.031 |
| MR (moderate–severe) | 30 (19.2) | 52 (17.9) | 0.74 |
| BNP (admission), pg/mL | 609.5 (IQR: 313.5–1230.0) | 616.5 (IQR: 302.0–1202.0) | 0.82 |
| BNP (discharge), pg/mL | 190.4 (IQR: 73.9–393.0) | 258.5 (IQR: 127.2–545.0) | 0.005 |
| Medications | |||
| ACE‐I | 66 (41.8) | 117 (39.1) | 0.58 |
| ARB | 79 (50.0) | 135 (45.2) | 0.32 |
| Beta‐blocker | 80 (50.6) | 157 (52.5) | 0.70 |
| Thiazide | 5 (3.2) | 6 (2.0) | 0.44 |
| Loop diuretics | 141 (89.2) | 210 (70.2) | <0.001 |
| CCB | 34 (21.5) | 88 (29.4) | 0.069 |
| Nitrate | 31 (19.6) | 88 (29.4) | 0.023 |
| Digitalis | 49 (31.0) | 85 (28.4) | 0.56 |
| Oral inotrope | 10 (6.3) | 17 (5.7) | 0.78 |
| Aspirin | 83 (52.5) | 148 (49.5) | 0.54 |
| Anti‐platelet | 19 (12.0) | 53 (17.7) | 0.11 |
| Warfarin | 63 (39.9) | 108 (36.1) | 0.43 |
| Statin | 30 (19.0) | 69 (23.1) | 0.31 |
Data are shown as n (per cent), median (IQR), or mean ± SD. ACE‐I, angiotensin‐converting enzyme inhibiter; AF, atrial fibrillation; AFL, atrial flutter; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter defibrillator; IVS, interventricular septum thickness; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; LVPW, left ventricular posterior wall thickness; MR, mitral regurgitation; PCI, percutaneous coronary intervention; VF, ventricular fibrillation; VT, ventricular tachycardia.
3.2. Clinical outcomes
During a mean follow‐up of 2.2 years, the incidence rate of primary outcome was lower in patients with spironolactone than those without it (48 [30.4%] vs. 123 [41.1%]). The incidence rates of composite outcome of all‐cause death or HF rehospitalization, composite outcome of cardiovascular death or HF rehospitalization, all‐cause death, and cardiovascular death per 1000 patient‐years were 171.5 vs. 278.8, 150.0 vs. 249.4, 65.0 vs. 91.5, and 27.8 vs. 53.8 for the spironolactone and no spironolactone groups, respectively (Table 2). The incidence rate ratios were 0.61 (95% CI 0.44–0.86; P = 0.004), 0.60 (95% CI 0.42–0.86; P = 0.005), 0.71 (95% CI 0.43–1.18; P = 0.19), and 0.52 (95% CI 0.25–1.09; P = 0.083) for each outcome (Table 2). Univariate analysis for the composite of all‐cause death or HF rehospitalization by Cox regression hazard model demonstrated that spironolactone use at discharge, age, previous HF admission, NYHA functional class III–IV at discharge, chronic kidney disease, anaemia, ischaemic heart disease, the history of sustained ventricular tachycardia or ventricular fibrillation, oral inotrope, and warfarin was significantly associated with the primary outcome. Multivariate analysis including these factors showed that spironolactone was independently associated with better prognosis (adjusted HR 0.63, 95% CI 0.44–0.90; P = 0.010). Age (≥70 years) (adjusted HR 1.56, 95% CI 1.10–2.21; P = 0.012), prior HF admission (adjusted HR 2.59, 95% CI 1.84–3.65; P < 0.001), chronic kidney disease (adjusted HR 1.98, 95% CI 1.29–3.04; P = 0.002), and sustained ventricular tachycardia or fibrillation (adjusted HR 2.12, 95% CI 1.24–3.62; P = 0.006) were independently associated with poor prognosis (Table 3). Figure 2 demonstrates the cumulative incidence of each outcome adjusted by covariates. The covariates were selected from the factors that were either statistically significant on univariate analysis (age, previous HF admission, NYHA functional class, chronic kidney disease, anaemia, ischaemic heart disease, sustained ventricular tachycardia or fibrillation, oral inotrope, and warfarin in Table 3) or clinically relevant (sex). The cumulative incidence of the composite of all‐cause death or HF rehospitalization (P = 0.012; Figure 2 A), the composite of cardiovascular death or HF rehospitalization (P = 0.011; Figure 2 B), and cardiovascular death (P = 0.030; Figure 2 D) were significantly decreased in the spironolactone group, except for all‐cause death P = 0.24; Figure 2 C). Patient characteristics before and after propensity score matching as a sensitivity analysis were shown in Supporting Information, Tables S1 and S2. Spironolactone reduced the composite of all‐cause death or HF rehospitalization also in propensity score‐matched cohorts (P = 0.048; Supporting Information, Figure S1).
Table 2.
Incidence rate and incidence rate ratio
| Outcomes | Spironolactone (n = 158) | No spironolactone (n = 299) | ||||
|---|---|---|---|---|---|---|
| Patients with event, n (%) | Incidence/1000 person‐years at risk | Patients with event, n (%) | Incidence/1000 person‐years at risk | Rate ratio (95% CI) | P value | |
| Primary outcome | ||||||
| All‐cause death or HF rehospitalization | 48 (30.4) | 171.5 | 123 (41.1) | 278.8 | 0.61 (0.44–0.86) | 0.004 |
| Secondary outcomes | ||||||
| CV death or HF rehospitalization | 42 (26.6) | 150.0 | 110 (36.8) | 249.4 | 0.60 (0.42–0.86) | 0.005 |
| All‐cause death | 21 (13.3) | 65.0 | 51 (17.1) | 91.5 | 0.71 (0.43–1.18) | 0.19 |
| CV death | 9 (5.7) | 27.8 | 30 (10.0) | 53.8 | 0.52 (0.25–1.09) | 0.083 |
CV, cardiovascular; HF, heart failure.
Table 3.
Unadjusted and adjusted hazard ratio for primary outcome
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| Spironolactone | 0.62 | 0.44–0.87 | 0.005 | 0.63 | 0.44–0.90 | 0.010 |
| Age (≥70 years) | 1.73 | 1.26–2.36 | <0.001 | 1.56 | 1.10–2.21 | 0.012 |
| Male | 1.02 | 0.74–1.38 | 0.93 | 0.95 | 0.67–1.34 | 0.76 |
| Previous HF admission | 3.20 | 2.30–4.39 | <0.001 | 2.59 | 1.84–3.65 | <0.001 |
| NYHA III–IV at discharge | 2.22 | 1.20–4.10 | 0.011 | 1.70 | 0.90–3.23 | 0.10 |
| Smoking | 0.96 | 0.70–1.32 | 0.79 | |||
| SBP (per 10 mmHg), mmHg | 0.99 | 0.91–1.08 | 0.84 | |||
| LVEF (per 10%), % | 1.52 | 0.89–2.60 | 0.13 | |||
| LVDd (per 10 mm), mm | 0.89 | 0.73–1.07 | 0.21 | |||
| LVDs (per 10 mm), mm | 0.89 | 0.72–1.11 | 0.30 | |||
| Hypertension | 1.05 | 0.77–1.42 | 0.77 | |||
| Diabetes mellitus | 1.16 | 0.85–1.59 | 0.35 | |||
| Dyslipidaemia | 1.04 | 0.75–1.45 | 0.82 | |||
| Chronic kidney disease | 2.70 | 1.89–3.86 | <0.001 | 1.98 | 1.29–3.04 | 0.002 |
| Hyperuricemia | 1.35 | 0.99–1.83 | 0.055 | |||
| Stroke | 1.34 | 0.91–1.98 | 0.14 | |||
| Anaemia | 1.67 | 1.18–2.36 | 0.004 | 0.95 | 0.63–1.45 | 0.82 |
| COPD | 1.71 | 0.99–2.96 | 0.054 | |||
| Ischaemic heart disease | 1.45 | 1.07–1.97 | 0.015 | 0.84 | 0.60–1.17 | 0.30 |
| Previous PCI | 1.24 | 0.87–1.78 | 0.24 | |||
| Previous CABG | 1.31 | 0.85–2.01 | 0.23 | |||
| Chronic AF, AFL | 1.08 | 0.79–1.49 | 0.64 | |||
| Sustained VT, VF | 2.36 | 1.41–3.97 | 0.001 | 2.12 | 1.24–3.62 | 0.006 |
| ACE‐I or ARB | 0.71 | 0.50–1.02 | 0.065 | |||
| Beta‐blocker | 0.92 | 0.68–1.24 | 0.58 | |||
| Thiazide | 1.07 | 0.40–2.87 | 0.90 | |||
| Loop diuretics | 1.16 | 0.80–1.68 | 0.43 | |||
| Nitrate | 1.24 | 0.89–1.74 | 0.21 | |||
| Digitalis | 0.91 | 0.65–1.28 | 0.60 | |||
| Oral inotrope | 1.76 | 1.05–2.95 | 0.032 | 1.64 | 0.93–2.89 | 0.087 |
| Aspirin | 0.98 | 0.73–1.33 | 0.90 | |||
| Warfarin | 0.66 | 0.48–0.92 | 0.014 | 0.79 | 0.55–1.14 | 0.21 |
| Statin | 0.91 | 0.63–1.31 | 0.60 | |||
Abbreviations as in Table 1. CI, confidence interval; HR, hazard ratio.
Figure 2.
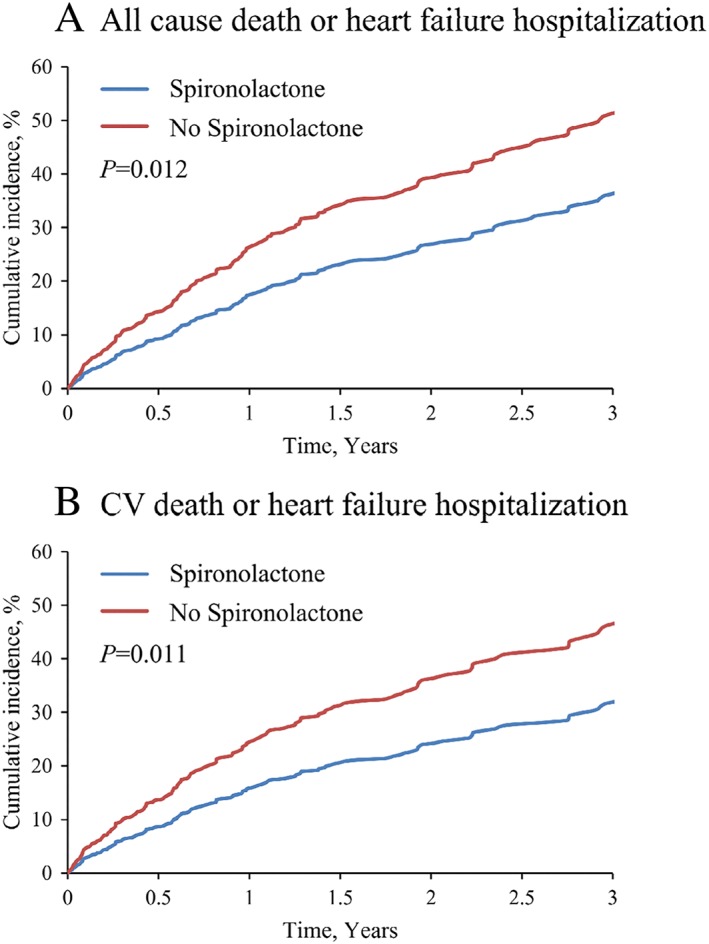
Covariate‐adjusted cumulative incidence of each outcome. Covariate‐adjusted cumulative incidence of composite of all‐cause death or heart failure rehospitalization (A), composite of cardiovascular death or heart failure rehospitalization (B), all‐cause death (C), and cardiovascular death (D). The covariates were selected from the factors that were either statistically significant on univariate analysis (age, previous heart failure admission, New York Heart Association functional class, chronic kidney disease, anaemia, ischaemic heart disease, sustained ventricular tachycardia or fibrillation, oral inotrope, and warfarin in Table 3) or clinically relevant (sex).
For comparison, the primary outcome of patients with LVEF ≥50%, so called HFpEF, in the JCARE‐CARD cohort was also tested. Patient characteristics of HFpEF were shown in Supporting Information, Table S3. Spironolactone was not associated with the better outcome among patients with HFpEF (P = 0.20; Supporting Information, Table S4).
4. Discussion
The major finding of the present study was that spironolactone use at discharge was independently associated with a significant reduction in the composite of all‐cause death or HF rehospitalization among the patients with HFmrEF. This is the first report to demonstrate the beneficial effects of spironolactone on long‐term outcomes in HFmrEF.
The 2016 European Society of Cardiology HF guidelines defined HFmrEF in patients with EF of 40–49%.2 Recent observational studies extensively described characteristics of patients with HFmrEF in comparison with HFpEF and HFrEF3, 4, 11, 12, 13, 14, 15, 16; however, it remains unknown whether patients with HFmrEF represent a transitional phenotype between HFpEF and HFrEF or a distinct pathophysiological entity. Furthermore, effective treatment strategies for these patients have not been established.
In the present study, patients with HFmrEF accounted for 19.1% of the total cohort in JCARE‐CARD, which was consistent with previous studies.3, 4, 11, 12, 13, 14, 15, 16 We have previously reported characteristics of HFrEF and HFpEF.17 In comparison with the present study and our previous study, the characteristics of patients with HFmrEF were, in part, similar to those of patients with HFrEF. Patients with HFmrEF or HFrEF are younger than those with HFpEF (HFmrEF vs. HFrEF vs. HFpEF; 69.3 ± 13.8 vs. 66.6 ± 13.8 vs. 73.6 ± 12.6 years old), more frequent in men (62.6% vs. 72.2% vs. 52.7%), and more likely to have a history of ischaemic heart disease (38.7% vs. 39.8% vs. 25.4%). On the other hand, the HFmrEF occupied an intermediate position between the other two categories regarding chronic atrial fibrillation or atrial flutter (HFmrEF vs. HFrEF vs. HFpEF; 33.1% vs. 24.5% vs. 38.3%) These features were compatible with a recent study.18 Prognosis in these distinct groups is controversial. The composite outcome of all‐cause death or HF rehospitalization was not different among these distinct groups.17, 19 On the other hand, a meta‐analysis demonstrated that HFmrEF had a lower rate of cardiac mortality than HFrEF.20 This discrepancy might be due to the difference in patient characteristics and treatment in each cohort.
The efficacy of MRA for patients with HFrEF has been already established based on previous randomized controlled clinical trials such as RALES and EMPHASIS‐HF. The RALES demonstrated that spironolactone significantly improved outcomes in patients with LVEF less than 35% and NYHA functional class III or IV.5 In the EPHESUS, eplerenone on top of the conventional treatment including angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker and beta‐blocker significantly reduced the risk of death.6 EMPHASIS‐HF and J‐EMPHASIS‐HF demonstrated that eplerenone reduced the risk of death and rehospitalization in patients with LVEF no more than 35% and NYHA functional class II.7, 21
The beneficial effects of spironolactone for patients with HFpEF have been also reported. TOPCAT trial showed that spironolactone reduced the composite outcome of hospitalization for HF or cardiovascular mortality among patients with LVEF ≥45% and elevated BNP who were enrolled in North and South America but not in Eastern Europe.8, 9, 22 Given the stratified nature of TOPCAT, this result is randomized evidence.23 The regional difference in clinical outcomes was explained as follows. Patients from Eastern Europe, who did not have BNP measured, had a very low event rate similar to the healthy population of the same age. Canrenone (an active metabolite of spironolactone) concentrations were undetectable in 30% of patients in Eastern Europe, compared with 3% of patients in North and South America.24
Another subgroup analysis of the TOPCAT trial demonstrated that spironolactone tended to be associated with better outcomes among patients with LVEF 45–50%, implicating that the potential efficacy of spironolactone was greatest at the lower end of the LVEF spectrum.25 Consistent with these findings, the present study confirmed the clinical efficacy of spironolactone in HFmrEF (Figure 2 and Tables 2 and 3).
Renin–angiotensin system inhibitors and beta‐blockers have been reported to be associated with a reduced risk of all‐cause mortality in HFmrEF.26, 27 In the present study, renin–angiotensin–aldosterone system inhibitors tended to lower all‐cause death and HF rehospitalization in patients with HFmrEF and beta‐blockers was not associated with better outcomes (Table 2). A meta‐analysis of 11 randomized controlled trials, which investigated the effect of beta‐blockers on LVEF and prognosis by stratifying according to the baseline LVEF and heart rhythm, showed that beta‐blockers significantly reduced all‐cause death and cardiovascular death in patients with HFmrEF only if the patient did not have atrial fibrillation.28 In our study, 32.8% of the patients had chronic atrial fibrillation or atrial flutter. Thus, the high rate of atrial arrhythmias might explain why beta‐blockers were ineffective. Importantly, even after adjusted for several factors including atrial fibrillation, spironolactone use was independently associated with a better prognosis (Table 2).
HFmrEF is composed of the following three types: HFmrEF recovered (previously HFrEF with LVEF <40%), HFmrEF deteriorated (previously HFpEF with LVEF ≥50%), and HFmrEF unchanged (previously HFmrEF with LVEF 40–50%).11 HFmrEF is known to transit into HFpEF or HFrEF, by 44% or 16% at 1 year, respectively.3 Those who transited into HFrEF had worse outcomes similar to HFrEF at registry. The present study did not have follow‐up echocardiographic data. The types of HFmrEF benefited by spironolactone and its impact on changes in LVEF cannot be inferred from our study.
We found that age, the history of HF hospitalization, chronic kidney disease, and the history of sustained ventricular tachycardia or fibrillation were independently associated with a poor prognosis. These factors are well known as poor prognostic predictors of HFrEF and HFpEF, suggesting the overlap in predictors of HFmrEF with those of other HF categories.
Taken together, our data suggest that the characteristics of patients with HFmrEF were, in part, similar to those of patients with HFrEF and spironolactone was independently associated with better outcomes in HFmrEF.
4.1. Study limitations
Several limitations in this study using the JCARE‐CARD database should be acknowledged. First, the dose of spironolactone and the information whether spironolactone was initiated before or during index hospitalization were not collected. In addition, the number of patients who stopped spironolactone during the follow‐up period was not recorded. Second, a recent study showed discrepancies of treatment effect on HF patients between non‐randomized studies and randomized controlled trials.29 The present study suggests the efficacy of spironolactone in patients with HFmrEF; however, it is based on the subgroup analysis of an observational study. Thus, stronger evidence by randomized controlled trials is definitely needed to recommend the use of spironolactone for patients with HFmrEF. Finally, there may be unmeasured biases that affect the results.
5. Conclusions
Among patients with HF hospitalized for HFmrEF, discharge use of spironolactone was independently associated with better long‐term outcomes. Spironolactone might be useful for the patients with HFmrEF.
Conflict of interest
None declared.
Funding
This work was supported by grants from Health Sciences Research Grants from the Japanese Ministry of Health, Labour and Welfare (Comprehensive Research on Cardiovascular Diseases) (H.T.), the Japan Heart Foundation, and Japan Arteriosclerosis Prevention Fund and in part by grants from Health Sciences Research Grants for Research on Intractable Diseases of the Japanese Ministry of Health, Labour and Welfare and from the Japanese Agency for Medical Research and Development (AMED) (H.T.: grant number 19ek0210080h0002).
Supporting information
Table S1. Patient characteristics before propensity score matching
Table S2. Patient characteristics after propensity score matching
Table S3. Patient characteristics of HFpEF
Table S4. Unadjusted hazard ratio for primary outcome according to spironolactone use in HFpEF patients
Figure S1. Cumulative incidence curve for primary outcome in propensity matched cohorts
Acknowledgements
The JCARE‐CARD investigators and participating cardiologists are listed in the Appendix of our previous publication.10 This study could not have been carried out without the help, cooperation, and support of the cardiologists in the survey institutions. We thank them for allowing us to obtain the data. The JCARE‐CARD was supported by the Japanese Circulation Society and the Japanese Society of Heart Failure.
Enzan, N. , Matsushima, S. , Ide, T. , Kaku, H. , Higo, T. , Tsuchihashi‐Makaya, M. , and Tsutsui, H. (2020) Spironolactone use is associated with improved outcomes in heart failure with mid‐range ejection fraction. ESC Heart Failure, 7: 339–347. 10.1002/ehf2.12571.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, Foundation ACoC, Guidelines AHATFoP . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Members ATF, Reviewers D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 3. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S, Shimokawa H. Investigators C‐. Characterization of heart failure patients with mid‐range left ventricular ejection fraction—a report from the CHART‐2 study. Eur J Heart Fail 2017; 19: 1258–1269. [DOI] [PubMed] [Google Scholar]
- 4. Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom U, Savarese G, Lam CSP, Lund LH. A comprehensive population‐based characterization of heart failure with mid‐range ejection fraction. Eur J Heart Fail 2017; 19: 1624–1634. [DOI] [PubMed] [Google Scholar]
- 5. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 6. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Investigators EP‐AMIHFEaSS. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 7. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Group E‐HS . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21.21073363 [Google Scholar]
- 8. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 2011; 162: 966‐972.e10. [DOI] [PubMed] [Google Scholar]
- 9. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131: 34–42. [DOI] [PubMed] [Google Scholar]
- 10. Tsutsui H, Tsuchihashi‐Makaya M, Kinugawa S, Goto D, Takeshita A, Investigators J‐C. Clinical characteristics and outcome of hospitalized patients with heart failure in Japan. Circ J 2006; 70: 1617–1623. [DOI] [PubMed] [Google Scholar]
- 11. Rastogi A, Novak E, Platts AE, Mann DL. Epidemiology, pathophysiology and clinical outcomes for heart failure patients with a mid‐range ejection fraction. Eur J Heart Fail 2017; 19: 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 13. Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014; 168: 721–730. [DOI] [PubMed] [Google Scholar]
- 14. Löfman I, Szummer K, Dahlström U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid‐range, and reduced ejection fraction. Eur J Heart Fail 2017; 19: 1606–1614. [DOI] [PubMed] [Google Scholar]
- 15. Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail 2016; 4: 464–472. [DOI] [PubMed] [Google Scholar]
- 16. Guisado‐Espartero ME, Salamanca‐Bautista P, Aramburu‐Bodas Ó, Conde‐Martel A, Arias‐Jiménez JL, Llàcer‐Iborra P, Dávila‐Ramos MF, Cabanes‐Hernández Y, Manzano L, Montero‐Pérez‐Barquero M, group Ri . Heart failure with mid‐range ejection fraction in patients admitted to internal medicine departments: findings from the RICA Registry. Int J Cardiol 2018; 255: 124–128. [DOI] [PubMed] [Google Scholar]
- 17. Tsuchihashi‐Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, Kato N, Takeshita A, Tsutsui H, Investigators J‐C . Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE‐CARD). Circ J 2009; 73: 1893–1900. [DOI] [PubMed] [Google Scholar]
- 18. Lauritsen J, Gustafsson F, Abdulla J. Characteristics and long‐term prognosis of patients with heart failure and mid‐range ejection fraction compared with reduced and preserved ejection fraction: a systematic review and meta‐analysis. ESC Heart Fail 2018; 5: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Altaie S, Khalife W. The prognosis of mid‐range ejection fraction heart failure: a systematic review and meta‐analysis. ESC Heart Fail 2018; 5: 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho JH, Choe WS, Cho HJ, Lee HY, Jang J, Lee SE, Choi JO, Jeon ES, Kim MS, Hwang KK, Chae SC, Baek SH, Kang SM, Choi DJ, Yoo BS, Kim KH, Cho MC, Kim JJ, Oh BH. Comparison of characteristics and 3‐year outcomes in patients with acute heart failure with preserved, mid‐range, and reduced ejection fraction. Circ J 2019; 83: 347–356. [DOI] [PubMed] [Google Scholar]
- 21. Tsutsui H, Ito H, Kitakaze M, Komuro I, Murohara T, Izumi T, Sunagawa K, Yasumura Y, Yano M, Yamamoto K, Yoshikawa T, Tsutamoto T, Zhang J, Okayama A, Ichikawa Y, Kanmuri K, Matsuzaki M, Group J‐E‐HS . Double‐blind, randomized, placebo‐controlled trial evaluating the efficacy and safety of eplerenone in Japanese patients with chronic heart failure (J‐EMPHASIS‐HF). Circ J 2017; 82: 148–158. [DOI] [PubMed] [Google Scholar]
- 22. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 23. Girerd N, Ferreira JP, Rossignol P, Zannad F. A tentative interpretation of the TOPCAT trial based on randomized evidence from the brain natriuretic peptide stratum analysis. Eur J Heart Fail 2016; 18: 1411–1414. [DOI] [PubMed] [Google Scholar]
- 24. de Denus S, O'Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376: 1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA, Investigators T. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016; 37: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi KH, Choi JO, Jeon ES, Lee GY, Choi DJ, Lee HY, Kim JJ, Chae SC, Baek SH, Kang SM, Yoo BS, Kim KH, Cho MC, Park HY, Oh BH. Guideline‐directed medical therapy for patients with heart failure with midrange ejection fraction: a patient‐pooled analysis from the Kor HF and Kor AHF registries. J Am Heart Assoc 2018; 7: e009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lund LH. Heart failure with mid‐range ejection fraction: lessons from CHARM. Card Fail Rev 2018; 4: 70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson Å, Wikstrand J, Kotecha D. Group B‐biHFC. Beta‐blockers for heart failure with reduced, mid‐range, and preserved ejection fraction: an individual patient‐level analysis of double‐blind randomized trials. Eur Heart J 2018; 39: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rush CJ, Campbell RT, Jhund PS, Petrie MC, McMurray JJV. Association is not causation: treatment effects cannot be estimated from observational data in heart failure. Eur Heart J 2018; 39: 3417–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient characteristics before propensity score matching
Table S2. Patient characteristics after propensity score matching
Table S3. Patient characteristics of HFpEF
Table S4. Unadjusted hazard ratio for primary outcome according to spironolactone use in HFpEF patients
Figure S1. Cumulative incidence curve for primary outcome in propensity matched cohorts


