Abstract
Aims
We hypothesized that grading of diastolic dysfunction (DDF) according to two DDF grading algorithms and strain imaging yields prognostic information on all‐cause mortality in patients with heart failure with reduced ejection fraction (HFrEF).
Methods and results
We enrolled ambulatory HFrEF (left ventricular ejection fraction < 45%; N = 1 065) patients who underwent echocardiography and speckle tracking assessment of global longitudinal strain (GLS). Patients were stratified according to DDF grades (Grades I–III) according to two contemporary DDF grading algorithms. Prognostic performance was assessed by C‐statistics. Of the originally 1 065 enrolled patients, a total of 645 (61%) patients (age: 67 ± 11 years, male: 72%, ejection fraction: 27 ± 9%) were classified according to both DDF grading algorithms. Concordance between the algorithms was moderate (kappa = 0.48) and the reclassification rate was 33%. During a median follow‐up of 3.3 years (1.9, 4.7 years), 101 (16%) died from all causes. When comparing DDF Grade I vs. Grade III, both algorithms provided prognostic information [Nagueh: (hazard ratio) HR 2.09, 95% confidence interval (CI),1.32–3.31, P = 0.002; Johansen: HR 2.47, 95% CI, 1.57–3.87, P < 0.001]. However, when comparing DDF Grade II vs. Grade III, only the Johansen algorithm yielded prognostic information (Nagueh: HR 1.04, 95% CI, 0.60–1.77, P = 0.90; Johansen: HR 2.26, 95% CI, 1.35–3.77, P = 0.002). We found no difference in prognostic performance between the two algorithms (C‐statistics: 0.604 vs. 0.623, P = 0.24). Assessed by C‐statistics, the most powerful predictors of the endpoint from the two algorithms were E/e'‐ratio (C‐statistics: 0.644), tricuspid regurgitation velocity (C‐statistics: 0.625) and E/A‐ratio (C‐statistics: 0.602). When adding GLS to a combination of these predictors, the prognostic performance increased significantly (C‐statistics: 0.705 vs. C‐statistics: 0.634, P = 0.028).
Conclusions
Evaluation of DDF in patients with HFrEF yields prognostic information on all‐cause mortality. Furthermore, adding GLS to contemporary algorithms of DDF adds novel prognostic information.
Keywords: Diastolic dysfunction, Speckle tracking, Prognosis
Introduction
Approximately 50% of patients with congestive heart failure (HF) do not exhibit left ventricular (LV) systolic dysfunction.1 Studies have suggested that these patients suffer from HF caused by LV diastolic dysfunction (DDF) and have increased LV filling pressures.2, 3 Hence, DDF is recognized as a paramount entity in the HF syndrome. Several prognostic markers have been examined in HF with reduced ejection fraction (HFrEF), however, the prognostic role of DDF in HFrEF patients is poorly investigated.4 Recently, studies have indicated that assessment of strain parameters, involving global longitudinal strain (GLS), may correlate with LV filling pressure.5 Despite GLS commonly is linked to systolic function, it may be reduced in patients with HF with preserved ejection fraction (HFpEF), and furthermore, studies have argued that longitudinal deformation should be regarded as a determinant of diastolic function.6, 7 Based on this, GLS may potentially offer guidance on grading of DDF in HFrEF patients.8
The guideline for echocardiographic assessment of DDF was recently updated by the American Society of Echocardiography and the European Association of Cardiovascular Imaging. This guideline suggests that the ratio between peak transmitral early (E) and late (A) diastolic inflow velocity (E/A‐ratio) should be the initial step in evaluating DDF in HFrEF. By contrast, a recent study proposed that average peak early diastolic mitral annular velocity (e') was a more precise parameter to evaluate impaired LV relaxation.9 Considering that assessment of DDF mostly relies upon empirically derived cut‐off values and consensus statements, only few studies have investigated and compared algorithms for classification of DDF with outcome.
The aim of the present study was to evaluate echocardiographic classification of DDF in HFrEF patients according to two contemporary algorithms and to investigate the prognostic potential for all‐cause mortality. We hypothesized that increasing degree of DDF, as assessed in each algorithm, was associated with a worse prognosis. Furthermore, we hypothesized that GLS offers novel information on grading of DDF and adds prognostic information to grading of DDF in patients with HFrEF.
Methods
Study population
This was a retrospective study of 1 102 nonacute patients referred to the HFrEF clinic at Gentofte University Hospital, Copenhagen, Denmark. Upon referral, all patients had a LV ejection fraction (LVEF) of 45% or less. Baseline clinical data including medical history and previous cardiac procedures were collected at first visit in the clinic, and we retrospectively assessed this from electronic medical records. The diagnosis of HFrEF was given by an experienced clinician. A cross reference with the hospital's echocardiographic database allowed us to retrieve echocardiographic examinations. Patients were included if their echocardiographic examination was performed at a maximum of 1 year from the first admittance [median 30 days before admittance; interquartile range (IQR) 6, 56 days]. All echocardiograms were conducted from December 2005 to July 2013. We excluded patients who had no echocardiogram performed within 1 year of the first admittance (N = 22), poor image quality or inadequate echocardiography (N = 15), atrial fibrillation/flutter (N = 172), no assessment of E/A (N = 29), and e' (N = 42). Additionally, we excluded 119 patients who were classified as normal by Johansen et al., 51 patients who were categorized as indeterminate by Nagueh et al. and seven patients who were deemed both normal and indeterminate by the two algorithms. A total of 645 patients were eligible for analysis (Figure 1). The study was approved by the Danish Data Protection Agency (j: 03240, GEH‐2014‐047) and complied with the Declaration of Helsinki.
Figure 1.
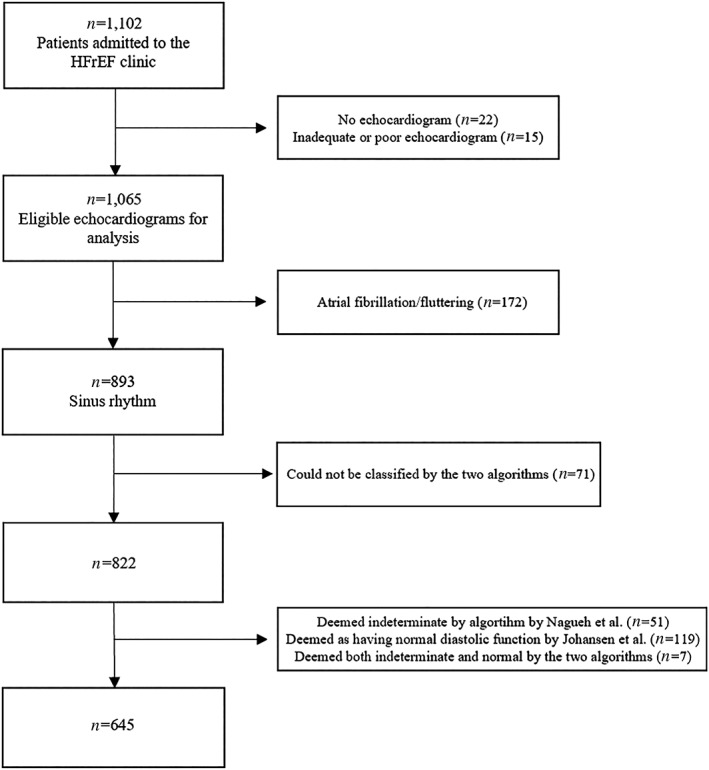
Study population. Flowchart showing the inclusion of patients eligible for grading of diastolic dysfunction by the two algorithms. HFrEF, heart failure with reduced ejection fraction.
Echocardiography
The echocardiographic examinations were performed using Vivid 7 or 9 machines (GE Healthcare, Little Chalfont, United Kingdom). A GE Healthcare image vault was used for storing the images. All images were then analyzed offline using EchoPac version 12 (GE Healthcare) by a single investigator blinded to all baseline data.
Conventional Echocardiography
The Simpson's biplane method was used to assess LVEF.10 LV end‐diastolic measures, including interventricular septum thickness, LV posterior wall dimension, and LV internal dimension, were measured in the parasternal long‐axis view at the tip of mitral valve leaflets.10 The Devereux formula was used to estimate LV mass.11 Accordingly, the LV mass index was calculated as LV mass divided by body surface area. The area length method was used to measure the left atrial volume, and the left atrial volume index (LAVI) was obtained by dividing with body surface area.12 We also calculated parameters of minimum and maximum LAVI. In the apical four‐chamber view, the tricuspid annular plane systolic excursion was measured using M‐mode echocardiography. The mitral valve inflow pattern was measured using pulsed‐wave Doppler imaging in the apical four‐chamber view and used to obtain the peak velocities of early (E) and late (A) diastolic LV filling. From these, the E/A‐ratio was calculated and deceleration time of the E‐wave was measured. In the apical four‐chamber view, by use of the continuous wave Doppler imaging, tricuspid regurgitation (TR) velocity was determined. By use of tissue Doppler imaging (TDI), the peak longitudinal early diastolic (e') tissue velocity was measured with the sample volume placed at the septal and lateral mitral annular sites in the apical four‐chamber view. The mean value was calculated as the average of the lateral and septal velocities. The ratio between E‐wave and e' (E/e') was then calculated to assess the LV filling pressure.13 Mitral regurgitation was classified as mild/moderate/severe according to the clinician performing the echocardiographic examination.
Diastolic dysfunction classification
We categorized all patients according to two contemporary algorithms of DDF.
Algorithm by Nagueh et al. (Figure 2 A)
Figure 2.
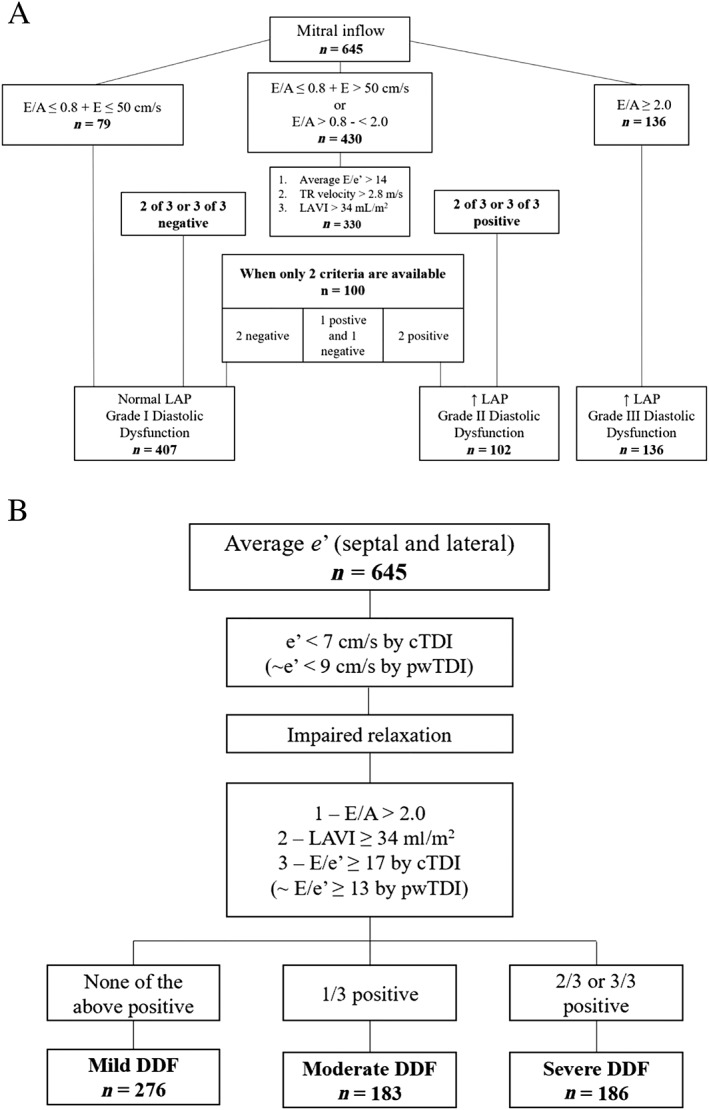
Flowcharts of algorithms for grading of diastolic dysfunction.
Study population divided into categories of diastolic dysfunction by Nagueh et al. algorithm (A) and Johansen et al. algorithm (B). E, peak transmitral early diastolic inflow velocity; e', peak early diastolic mitral annular velocity; E/A, ratio between peak transmitral early and late diastolic inflow velocity; E/e', ratio between peak transmitral early diastolic inflow velocity and peak early diastolic mitral annular velocity; TR, tricuspid regurgitation; LAVI, left atrial volume index; LAP, left atrial pressure; cTDI, color tissue Doppler imaging; pwTDI, pulsed‐wave tissue Doppler imaging; DDF, diastolic dysfunction.
The initial step included evaluation of the E/A‐ratio and E‐wave. If the E/A‐ratio was ≤0.8 and E > 50 cm/s or E/A‐ratio was between 0.8 and 2.0, three parameters had to be evaluated. These parameters were (i) E/e'‐ratio > 14, (ii) TR velocity > 2.8 m/s, and (iii) LAVI > 34 mL/m2. If two or three of these criteria were negative, patients were assigned as having DDF Grade I (N = 407). If two or three of these were positive, patients had DDF Grade II (N = 102). When patients only had two of the above‐mentioned parameters available, two negative criteria were categorized as DDF Grade I, two positives as DDF Grade II, and one positive and one negative as indeterminate. If the E/A‐ratio was ≥ 2.0, patients were assigned DDF Grade III (N = 136).
Algorithm by Johansen et al. (Figure 2 B)
The initial criterion for evaluation of DDF was e' < 9 cm/s by pulsed‐wave tissue Doppler imaging. If a patient had an e' below this, three criteria had to be evaluated: (i) E/A‐ratio > 2.0, (ii) LAVI ≥34 ml/m2 and (iii) E/e'‐ratio ≥ 13 by pulsed‐wave tissue Doppler imaging. If none of the above were positive, patients were assigned DDF Grade I (mild DDF; N = 276). If one criterion was positive, patients were assigned DDF Grade II (moderate DDF; N = 183). If two or three of the criteria were positive, patients were assigned DDF Grade III (severe DDF; N = 186).
Speckle tracking echocardiography
Two‐dimensional speckle tracking analysis was performed in the apical two‐chamber, three‐chamber, and four‐chamber views with an average of 74 frames per second (standard deviation: 18 frames per second). A region of interest (ROI) was defined by a semiautomated function that traced the endocardial border at end systole after placing three samples at the LV base and apex. The detected ROI was visually confirmed by the responsible investigator and, if necessary, the investigator manually modified the ROI to ensure correct tracking of the bright coherent speckles. A satisfactory tracking had to cover the entire cardiac wall from the endocardium to the myoepicardial border. Furthermore, visible motion of the speckles had to be present. In cases of poor tracking, the ROI was manually readjusted. Segments were excluded when they did not fulfill these criteria or were compromised by shadows or artifacts. GLS was assessed as the average value obtained from from the three apical projections. If speckle tracking could not be obtained from a chamber view, GLS was averaged from the two remaining views (total four‐chamber view: 614; two‐chamber view: 619; and three‐chamber view: 606).
Statistics
All statistical analyses were made using stata version SE 13.1 (StataCorp, College Station, Texas). P < 0.05 in two‐sided tests were regarded significant. Concordance between the two algorithms for grading of DDF was assessed using Kappa statistics (see Supporting Information, Table S1). The reclassification percentage was calculated as 100%—proportion of agreement. According to grades of DDF, we examined baseline characteristics in Table 1A,1B. For approximately normally distributed variables, we used linear regression algorithms to calculate P for trend, with the exception of systolic and diastolic blood pressure that were non‐Gaussian distributed. For these variables, we used Cuzick's nonparametric test for trend.14 We performed Cox proportional hazards regression algorithms yielding hazard ratios (HRs) with 95% confidence intervals (CIs) for grades of DDF (Table 2). Accordingly, we constructed survival curves using the Kaplan–Meier method (Figure 3 A–B). Furthermore, a sensitivity analysis was carried out where patients categorized as ‘normal’ by Johansen et al. were used as reference group in our survival models. We examined if mitral valve insufficiency modified the association between DDF and outcome and conducted a sensitivity analysis in which the subset of the population with mitral valve disease were stratified according to mild and moderate/severe insufficiency. The prognostic performance of the two DDF grading algorithms was assessed using Harrell's C‐statistics. The performance for individual parameters of the two algorithms was tested (Table 3). The three strongest single parameters, assessed as the highest C‐statistics, were combined to determine the greatest prognostic performance. Additionally, the algorithms were compared to see if one was statistically stronger than the other using the somersd method.
Table 1A.
Baseline characteristics according to diastolic dysfunction grades by Nagueh et al.
| I | II | III | ||
|---|---|---|---|---|
| DDF grades Nagueh et al. | (N = 407) | (N = 102) | (N = 136) | P trend |
| Clinical | ||||
| Age, years | 67 ± 11 | 71 ± 10 | 67 ± 11 | 0.006 |
| Heart rate, bpm | 72 ± 13 | 74 ± 16 | 75 ± 15 | 0.016 |
| Male, n (%) | 290 (71%) | 66 (65%) | 107 (79%) | 0.056 |
| BMI, kg/m2 | 26 ± 5 | 26 ± 5 | 27 ± 5 | 0.33 |
| NIDDM, n (%) | 37 (9%) | 15 (15%) | 15 (11%) | 0.24 |
| IDDM, n (%) | 14 (3%) | 1 (1%) | 3 (2%) | 0.36 |
| Total cholesterol, mmol/L | 4.5 ± 1.2 | 4.4 ± 1.1 | 4.3 ± 1.1 | 0.058 |
| Mean arterial pressure, mmHg | 94 ± 13 | 93 ± 14 | 91 ± 14 | 0.15 |
| Systolic blood pressure, mmHg | 130 (120, 140) | 130 (120, 150) | 130 (110, 140) | 0.12 |
| Diastolic blood pressure, mmHg | 80 (60, 80) | 73 (60, 80) | 70 (60, 80) | 0.30 |
| Hypertension, n (%) | 64 (16%) | 17 (17%) | 19 (14%) | 0.83 |
| History of MI, n (%) | 215 (53%) | 43 (42%) | 67 (49%) | 0.15 |
| Angina Pectoris, n (%) | 96 (24%) | 27 (26%) | 32 (24%) | 0.82 |
| PTCA, n (%) | 137 (34%) | 34 (33%) | 34 (25%) | 0.16 |
| CABG, n (%) | 74 (18%) | 21 (21%) | 33 (24%) | 0.30 |
| Ischemic cardiomyopathy, n (%) | 256 (63%) | 58 (57%) | 82 (60%) | 0.51 |
| No. with mitral insufficiency, n (%) | 106 (26%) | 52 (51%) | 76 (56%) | <0.001 |
| mild, n (%) | 96 (91%) | 39 (75%) | 48 (63%) | |
| moderate, n (%) | 9 (9%) | 12 (23%) | 25 (33%) | |
| severe, n (%) | 1 (1%) | 1 (2%) | 3 (4%) | |
| Medication | ||||
| RAS blockade, n (%) | 313 (77%) | 79 (77%) | 113 (83%) | 0.31 |
| Beta blockers, n (%) | 259 (64%) | 62 (61%) | 93 (68%) | 0.45 |
| Spironolactone, n (%) | 60 (15%) | 18 (18%) | 19 (14%) | 0.71 |
| Diuretics, n (%) | 202 (50%) | 52 (51%) | 72 (53%) | 0.80 |
| Anticoagulants, n (%) | 66 (16%) | 16 (16%) | 31 (23%) | 0.19 |
| Antiarrhythmics, n (%) | 20 (5%) | 3 (3%) | 9 (7%) | 0.43 |
| Calcium channel blockers, n (%) | 4 (1%) | 0 | 2 (1%) | 0.50 |
| Echocardiography | ||||
| LV ejection fraction, % | 29 ± 9 | 27 ± 10 | 22 ± 8 | <0.001 |
| LV mass index, g/m2 | 112 ± 38 | 135 ± 42 | 124 ± 32 | <0.001 |
| E, m/s | 0.6 ± 0.2 | 0.9 ± 0.3 | 1.0 ± 0.3 | <0.001 |
| A, m/s | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.4 ± 0.1 | <0.001 |
| e', cm/s | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.2 | <0.001 |
| Deceleration time, ms | 212 ± 85 | 196 ± 86 | 149 ± 46 | <0.001 |
| E/e’ | 11.4 ± 3.9 | 18.5 ± 5.7 | 19.1 ± 7.6 | <0.001 |
| E/A | 0.9 ± 0.3 | 1.2 ± 0.4 | 3.1 ± 1.0 | <0.001 |
| TR velocity, m/s | 2.3 ± 1.0 | 2.9 ± 1.2 | 2.9 ± 0.5 | <0.001 |
| Left atrial volume index, mL/m2 | 23.9 ± 7.5 | 37.4 ± 11.6 | 35.9 ± 11.5 | <0.001 |
| Max. left atrial volume index, mL/m2 | 26.1 ± 9.6 | 40.4 ± 16.1 | 37.2 ± 13.9 | <0.001 |
| Min. left atrial volume index, mL/m2 | 15.8 ± 7.4 | 27.7 ± 13.5 | 29.1 ± 12.9 | <0.001 |
| TAPSE, m/s | 2.0 ± 0.5 | 2.0 ± 0.6 | 1.7 ± 0.6 | <0.001 |
| Global longitudinal strain, % | −10.2 ± 3.2 | −9.1 ± 3.2 | −7.3 ± 2.5 | <0.001 |
A, peak late diastolic transmitral velocity; BMI, body mass index; CABG, coronary artery bypass graft; DDF, diastolic dysfunction; E, peak early diastolic transmitral velocity; e', peak early diastolic annular mitral velocity; IDDM, insulin dependent diabetes mellitus; LV, left ventricular; MI, myocardial infarction; NIDDM, non‐insulin dependent diabetes mellitus; PTCA, percutaneous transluminal coronary angioplasty; RAS, Renin‐Angiotensin system; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Table 1B.
Baseline characteristics according to diastolic dysfunction grades by Johansen et al.
| I | II | III | ||
|---|---|---|---|---|
| DDF grades Johansen et al. | (N = 276) | (N = 183) | (N = 186) | P trend |
| Clinical | ||||
| Age, years | 66 ± 11 | 68 ± 11 | 69 ± 11 | 0.013 |
| Heart rate, bpm | 72 ± 14 | 72 ± 13 | 74 ± 16 | 0.15 |
| Male, n (%) | 200 (72%) | 119 (65%) | 144 (77%) | 0.029 |
| Body mass index, kg/m2 | 27 ± 4 | 26 ± 6 | 26 ± 5 | 0.77 |
| NIDDM, n (%) | 27 (10%) | 13 (7%) | 27 (15%) | 0.060 |
| IDDM, n (%) | 11 (4%) | 2 (1%) | 5 (3%) | 0.18 |
| Total cholesterol, mmol/L | 4.5 ± 1.2 | 4.3 ± 1.1 | 4.4 ± 1.1 | 0.13 |
| Mean arterial pressure, mmHg | 94 ± 13 | 93 ± 13 | 92 ± 14 | 0.49 |
| Systolic blood pressure, mmHg | 130 (120, 140) | 130 (115, 150) | 130 (110, 145) | 0.84 |
| Diastolic blood pressure, mmHg | 80 (65, 80) | 70 (60, 80) | 70 (60, 80) | 0.14 |
| Hypertension, n (%) | 44 (16%) | 28 (15%) | 28 (15%) | 0.96 |
| History of MI, n (%) | 149 (54%) | 94 (51%) | 82 (44%) | 0.11 |
| Angina Pectoris, n (%) | 74 (27%) | 37 (20%) | 44 (24%) | 0.27 |
| PTCA, n (%) | 101 (37%) | 63 (34%) | 41 (22%) | 0.003 |
| CABG, n (%) | 48 (17%) | 30 (16%) | 50 (27%) | 0.017 |
| Ischemic cardiomyopathy, n (%) | 178 (64%) | 110 (60%) | 108 (58%) | 0.35 |
| No. with mitral insufficiency, n (%) | 62 (22%) | 74 (40%) | 98 (53%) | 0.006 |
| mild, n (%) | 57 (92%) | 60 (81%) | 66 (67%) | |
| moderate, n (%) | 5 (8%) | 12 (16%) | 29 (30%) | |
| severe, n (%) | 0 | 2 (1%) | 3 (2%) | |
| Medication | ||||
| RAS blockade, n (%) | 218 (79%) | 141 (77%) | 146 (78%) | 0.88 |
| Beta blocker, n (%) | 174 (63%) | 121 (66%) | 119 (64%) | 0.80 |
| Spironolactone, n (%) | 44 (16%) | 24 (13%) | 29 (16%) | 0.69 |
| Diuretics, n (%) | 129 (47%) | 100 (55%) | 97 (52%) | 0.22 |
| Anticoagulants, n (%) | 44 (16%) | 32 (17%) | 37 (20%) | 0.55 |
| Antiarrhythmics, n (%) | 11 (4%) | 10 (5%) | 11 (6%) | 0.60 |
| Calcium channel blocker, n (%) | 2 (1%) | 2 (1%) | 2 (1%) | 0.90 |
| Echocardiography | ||||
| LV ejection fraction, % | 30 ± 9 | 26 ± 8 | 24 ± 9 | <0.001 |
| LV mass index, g/m2 | 109 ± 37 | 120 ± 38 | 130 ± 35 | <0.001 |
| E, m/s | 0.6 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.3 | <0.001 |
| A, m/s | 0.8 ± 0.2 | 0.8 ± 0.3 | 0.5 ± 0.3 | <0.001 |
| e', cm/s | 0.6 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.2 | <0.001 |
| Deceleration time, ms | 213 ± 84 | 196 ± 80 | 171 ± 78 | <0.001 |
| E/e’ | 9.5 ± 2.0 | 15.3 ± 4.7 | 19.7 ± 6.8 | <0.001 |
| E/A | 0.8 ± 0.3 | 1.1 ± 0.7 | 2.4 ± 1.3 | <0.001 |
| TR velocity, m/s | 2.3 ± 1.1 | 2.5 ± 0.8 | 2.8 ± 1.0 | <0.001 |
| Left atrial volume index, mL/m2 | 22.1 ± 5.8 | 27.7 ± 9.0 | 39.2 ± 11.1 | <0.001 |
| Max. left atrial volume index, mL/m2 | 24.4 ± 8.2 | 29.4 ± 11.2 | 41.7 ± 14.6 | <0.001 |
| Min. left atrial volume index, mL/m2 | 14.5 ± 6.2 | 18.9 ± 8.7 | 31.1 ± 13.2 | <0.001 |
| TAPSE, m/s | 2.0 ± 0.5 | 2.0 ± 0.5 | 1.8 ± 0.6 | <0.001 |
| Global longitudinal strain, % | −10.6 ± 3.3 | −9.2 ± 2.7 | −7.9 ± 3.0 | <0.001 |
A, peak late diastolic transmitral velocity; BMI, body mass index; CABG, coronary artery bypass graft; DDF, diastolic dysfunction; E, peak early diastolic transmitral velocity; e', peak early diastolic annular mitral velocity; IDDM, insulin dependent diabetes mellitus; LV, left ventricular; MI, myocardial infarction; NIDDM, non‐insulin dependent diabetes mellitus; PTCA, percutaneous transluminal coronary angioplasty; RAS, Renin‐Angiotensin system; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Table 2.
Association between diastolic dysfunction algorithms by Nagueh et al. and Johansen et al. and all‐cause mortality
| Nagueh et al. | Johansen et al. | |||
|---|---|---|---|---|
| DDF grade | HR (95% CI) | P value | HR (95% CI) | P value |
| I | Ref. | Ref. | ||
| II | 2.02 (1.24–3.31) | 0.005 | 1.09 (0.63–1.90) | 0.76 |
| III | 2.09 (1.32–3.31) | 0.002 | 2.47 (1.57–3.87) | <0.001 |
| I | 0.49 (0.30–0.81) | 0.005 | 0.92 (0.53–1.59) | 0.76 |
| II | Ref. | Ref. | ||
| III | 1.04 (0.60–1.77) | 0.90 | 2.26 (1.35–3.77) | 0.002 |
| I | 0.47 (0.30–0.76) | 0.002 | 0.41 (0.26–0.64) | <0.001 |
| II | 0.97 (0.56–1.65) | 0.90 | 0.44 (0.27–0.74) | 0.002 |
| III | Ref. | Ref. | ||
Associations are assessed with different grades of diastolic dysfunction as reference.
CI, confidence intervals; DDF, diastolic dysfunction; HR, hazard ratio.
Figure 3.
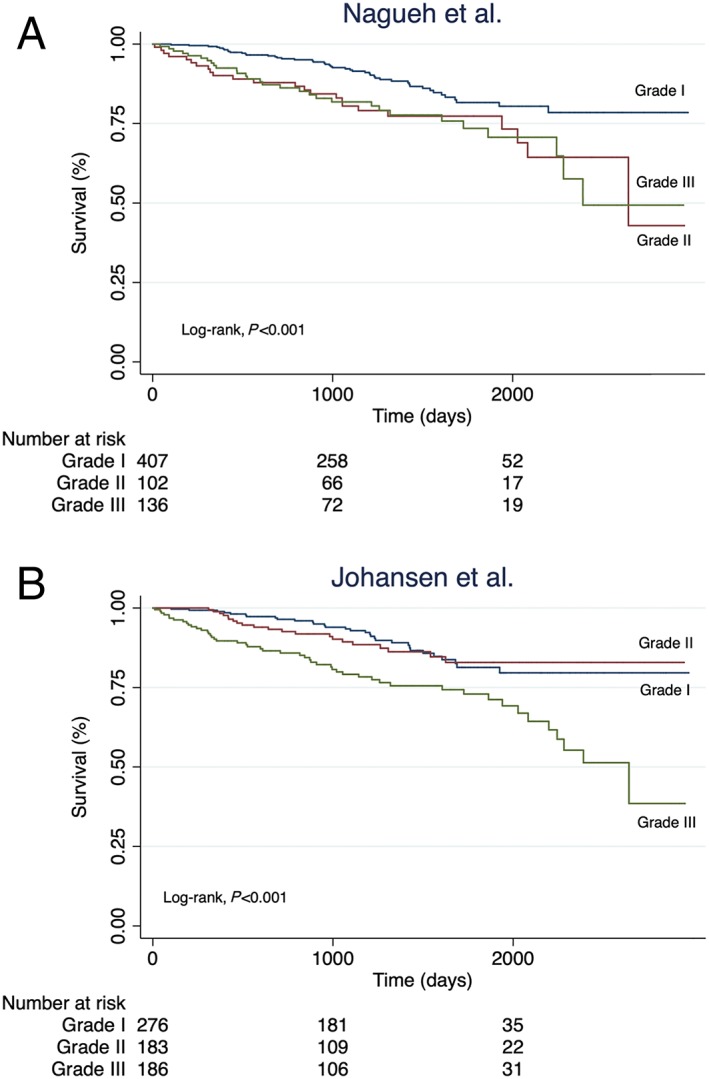
Kaplan–Meier survival curves. Kaplan–Meier curves showing the cumulated survival of patients divided into the three different DDF grades by Nagueh et al. (A) and Johansen et al. (B).
Table 3.
Prognostic performance, assessed as C‐statistics, for diastolic parameters included in the grading algorithms by Nagueh et al. and Johansen et al.
| C‐statistics | P value | |
|---|---|---|
| Single parameters | ||
| E/A | 0.602 | |
| E/e' | 0.644 | |
| LAVI | 0.598 | |
| E | 0.585 | |
| TR | 0.625 | |
| e' | 0.594 | |
| GLS | 0.708 | |
| Combined parameters | ||
| Model 1: E/e' | 0.644 | |
| Model 2: E/e' + GLS | 0.721 | 0.018* |
| Model 3: E/e' + TR | 0.644 | |
| Model 4: E/e' + TR + GLS | 0.705 | 0.060† |
| Model 5: E/e' + TR + E/A | 0.634 | |
| Model 6: E/e' + TR + E/A + GLS | 0.705 | 0.028‡ |
Difference between Models 1 and 2.
Difference between Models 3 and 4.
Difference between Models 5 and 6.
A, peak late diastolic transmitral velocity; E, peak early diastolic transmitral velocity; e', peak early diastolic mitral annular velocity; GLS, global longitudinal strain; LAVI, left atrial volume index; TR, tricuspid regurgitation velocity.
Results
Diastolic dysfunction grading
The study population (N = 645) was graded according to two DDF algorithms: Nagueh et al. (Figure 2 A) and Johansen et al. (Figure 2 B). When examining the discrepancy for grading of DDF in the two algorithms (see Supporting Information Table S1), the Kappa coefficient was 0.48 and the concordance was regarded as moderate. The reclassification rate was 33%. Baseline characteristics according to grades of DDF are displayed in Table 1A–B. Increasing grades of DDF were associated with higher age and mitral valve insufficiency in both algorithms. In the algorithm by Nagueh et al., increasing grades of DDF were associated with higher resting heart rate, whereas in the algorithm by Johansen et al., the proportion of male patients and a history of coronary revascularization increased with grades of DDF. All echocardiographic parameters differed across grades of DDF in both algorithms.
Nagueh et al.
The algorithm by Nagueh et al. yielded significant prognostic information on all‐cause mortality (Log‐rank P < 0.001; Figure 3 A). When using DDF Grade I as reference, patients assigned to Grade II (HR 2.02, 95% CI, 1.24–3.31, P = 0.005) and Grade III (HR 2.09, 95% CI, 1.32–3.31, P = 0.002; Table 2) had an approximate two‐fold increased risk of the endpoint. However, no significant difference was observed for Grade II vs. Grade III (HR 1.04, 95% CI, 0.60–1.77, P = 0.90). When restricting the analysis to the subset of patients with mitral valve disease, stratified according to mild and moderate/severe insufficiency, no significant associations were found (see Supporting Information Table S2A–B ).
Johansen et al.
The Johansen et al. algorithm displayed a significant log‐rank association for all‐cause mortality (Log‐rank P < 0.005; Figure 3 B). When using Grade I as reference, only patients in Grade III (HR 2.47, 95% CI, 1.57–3.87, P < 0.001; Table 2) had a significantly increased risk of the endpoint, whereas the risk remained nonsignificant for Grade II (HR 1.09, 95% CI, 0.63–1.90, P = 0.76). As opposed to Nagueh et al., a significant difference was observed when comparing Johansen et al. Grade II vs. Grade III (HR 2.26, 95% CI, 1.35–3.77, P = 0.002). In sensitivity analyses where patients with no DDF were used as reference group, the risk of the endpoint increased incrementally, such that patients in DDF Grade III had a significantly increased risk of the end point (HR 2.92, 95% CI, 1.61–5.30, P < 0.001). In patients with mild mitral valve insufficiency, patients in Grade III had a significantly increased risk of the endpoint compared with Grade II (HR 2.81, 95% CI, 1.02–7.96, P = 0.045) (see Supporting Information Table S2A–B ).
Prognostic performance
We found no difference in prognostic performance between the two algorithms (C‐statistics 0.604 vs 0.623, P = 0.244). Additionally, prognostic performance was assessed for all diastolic parameters in the algorithms, yielding C‐statistics ranging from 0.585 (E‐wave) to 0.644 (E/e') (Table 3). When combining the three most powerful diastolic parameters (E/e', TR velocity and E/A‐ratio), as assessed by the highest C‐statistics, the prognostic performance decreased to C‐statistics of 0.634, although this was not statistically significant (P = 0.478). When GLS was added to the single strongest and the three strongest combined parameters, the prognostic performance increased significantly (Model 2 vs. 1: C‐statistics 0.721 vs. 0.644, P = 0.018; Model 6 vs. 5: C‐statistics 0.705 vs. 0.634, P = 0.028; Table 3). We also examined LVEF (C‐statistics 0.687), however, no additional prognostic information was found when it was added to diastolic parameters. In a sensitivity analysis, where patients classified as ‘normal’ by Johansen et al. were included, no significant difference between the algorithms by Johansen and Nagueh were found (C‐statistics 0.627 vs. 0.614, P = 0.634).
Discussion
In this study, we demonstrate that grading of DDF, according to two contemporary algorithms, provides prognostic information in patients with HFrEF. Furthermore, we show that GLS potentially contributes incremental information on prognosis when added to DDF grading in patients with HFrEF. Only scarce information exists on the role of DDF in HFrEF, and to the authors' knowledge, this is the first study to compare the prognostic potential of two contemporary DDF algorithms in a large cohort of HFrEF patients.4
We demonstrated that increasing grades of DDF were associated with higher risk of all‐cause mortality in patients with HFrEF. This indicates that although patients with HFrEF have decreased myocardial contractility, the addition of more impaired diastolic function may still lead to a worse prognosis. We found that the algorithm by Johansen et al. provided a more equal distribution of patients across grades of DDF as compared with the algorithm by Nagueh et al. In the algorithm by Nagueh et al., both DDF Grade II and Grade III yielded an approximate two‐fold increased hazard ratio of all‐cause mortality. Noteworthy, the model lacked the ability to provide different risk estimates between DDF Grade II and Grade III (P difference = 0.90). In the algorithm by Johansen et al., patients assigned Grade III had a significantly increased risk of the endpoint compared with Grade II (P = 0.002). However, the Johansen algorithm lacked the ability to clearly delineate prognosis between Grade I and Grade II (P difference = 0.76).
The guideline from 2016 by Nagueh et al. was developed as the former guideline from 2009 and was deemed difficult to evaluate by clinicians.8, 15 Consequently, new guidelines were published in 2016, allowing an easier approach for clinicians to assess DDF. Almeida et al. showed that this algorithm contains greater specificity compared with older algorithms of DDF, but the sensitivity of the algorithm was too low for grading of less severe cases of DDF.16 It was proposed that the addition of TR velocity to the 2016 guidelines by Nagueh et al. could explain why the specificity of the guidelines dropped.16 This parameter was not included by Johansen et al. The TR velocity provides a measure of chronic pressure overload in HFpEF patients, indicating why it should be useful to characterize increased LV filling pressure in moderate to severe DDF.17 However, TR velocity may also be affected by lung diseases (e.g. chronic obstructive lung disease), explaining why the specificity dropped.18 In a clinical setting, the TR velocity gradient may sometimes be difficult to evaluate, although it is more accessible in HF patients. Noteworthy, the algorithms acquired different entry criteria such that Nagueh et al. used E/A, whereas Johansen et al. used e’. Patients with HF more often suffer from mitral valve disease, which may impact correct estimation of E/A.
An important consideration is that the algorithm by Nagueh et al. is an expert consensus statement, which is based on empirical evidence, for example, a study demonstrated how E/e' was correlated with increased filling pressures.8 Opposed to this, the algorithm by Johansen et al. is based on a risk analysis of different diastolic parameters in patients with impaired relaxation of the myocardium. This does not necessarily mean that they correctly identify patients with increased LV filling pressures.9 Despite the gold standard for diagnosing DDF is timely and costly—namely invasively assessed LV pressure measurements—there is a need for relating these measurements to clinical outcomes.19
When testing the prognostic strength of the individual parameters in the two algorithms, E/e' was found to provide the strongest prognostic information. This is in accordance with previous studies in patients with HFrEF.20 We observed no significant difference in terms of prognostic strength between the two algorithms (P = 0.24), indicating that both algorithms contain the same prognostic performance for all‐cause mortality. The greatest prognostic performance was observed when GLS was added to the prediction model.
Global longitudinal strain has, in numerous studies, been found to be a better predictor of cardiovascular outcome than any other single echocardiographic parameter.21, 22 Furthermore, GLS has been shown to be a superior predictor for all‐cause mortality in HFrEF patients.23 Although GLS is regarded to be a measure of systolic function6, we found that GLS added incremental and novel information when assessing prognostic performance of diastolic parameters. One study found that GLS correlated more with invasively assessed filling pressures, as compared with the conventional echocardiographic parameter such as E/e'.5 Traditionally, the cardiac cycle has been divided into systole and diastole, where LVEF has been the main determinant of systolic function, and echocardiographic parameters, such as TDI velocities and left atrial volume, have represented diastolic function. This approach builds upon a theoretical belief in which longitudinal deformational measurements do not necessarily fit.24 Contraction of myocardial fibers during systole affects the diastole through a coil effect, which results in the LV quickly expanding and thereby decreasing the intraventricular pressure.25 Myocardial longitudinal fibers undergo deformational changes during the entirety of this process, explaining why GLS may be regarded as both a determinant of systolic and diastolic function. Recent studies have also shown that in patients with HFpEF, GLS may be significantly decreased, although systolic function assessed by LVEF is maintained.6
Study limitations
The echocardiographic examination was performed within 1 year of first arrival and was for some patients relatively long, although the vast majority had the examination close to admittance (median: 30 before admittance; interquartile range: 6 to 56 days before admittance). Ideally, the echocardiographic examination was performed on the day of admittance to the clinic, such that it reflected the patients' current LV status.
The algorithm by Johansen et al. for grading of DDF is constructed for all patients regardless of LVEF, whereas the algorithm used by Nagueh et al. is specifically made for patients with a reduced LVEF. Therefore, HFrEF patients in the algorithm by Johansen et al. could be assigned normal diastolic function, which was not the case in the algorithm by Nagueh et al. In a clinical setting, it may be easier to evaluate DDF if only one algorithm is to be applied. The two algorithms applied different cut‐off levels for E/e' (13 and 14, respectively), which may be regarded as misleading as the aim for this parameter in both algorithms is to determine increased left atrial pressure. It is a limitation that the study cohort was reduced from 1 065 to 645 patients, as the ideal goal of an algorithm to assess DDF must be to make it applicable to most patients with HFrEF. In the presence of regional variations in wall thickness or asymmetric ventricles caused by hypertrophy or dilatation, assessment of LV mass by the Devereux formula may be inaccurate.10 Similarly, regional wall motion abnormalities may affect estimation of LVEF when using Simpson's biplane method.
Conclusions
We demonstrated that evaluation of DDF adds prognostic value in HFrEF patients when applying two contemporary DDF algorithms: Nagueh et al. and Johansen et al. Furthermore, we found that GLS could offer a novel addition to DDF algorithms, although larger and future studies are required to confirm this finding.
Conflicts of interest
Peter Godsk Jørgensen reports lecture fee from Novo Nordisk.
Gunnar Gislason reports research grants from Bayer, Boehringer Ingelheim, Pfizer, and Bristol Myers Squibb.
Sune Hansen, Philip Brainin, Morten Sengeløv, Niels Eske Bruun, Flemming Javier Olsen, Thomas Fritz‐Hansen, Morten Schou, and Tor Biering‐Sørensen report no conflicts of interest.
Funding
None.
Supporting information
Table S1. Distribution and reclassification between the two DDF algorithms by Nagueh et al. and Johansen et al.
Table S2. Association between diastolic dysfunction algorithms by Nagueh et al. and Johansen et al. and all‐cause mortality stratified according to patients with mild mitral insufficiency (A) and moderate/severe mitral insufficiency (B).
Hansen, S. , Brainin, P. , Sengeløv, M. , Jørgensen, P. G. , Bruun, N. E. , Olsen, F. J. , Fritz‐Hansen, T. , Schou, M. , Gislason, G. , and Biering‐Sørensen, T. (2020) Prognostic utility of diastolic dysfunction and speckle tracking echocardiography in heart failure with reduced ejection fraction. ESC Heart Failure, 7: 147–157. 10.1002/ehf2.12532.
References
- 1. Andersen MJ, Borlaug BA. Heart failure with preserved ejection fraction: current understandings and challenges. Curr Cardiol Rep 2014; 16: 501–511. [DOI] [PubMed] [Google Scholar]
- 2. Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol 1995; 26: 1565–1574. [DOI] [PubMed] [Google Scholar]
- 3. Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) . The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta‐analysis. Eur Heart J 2012; 33: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 4. Lüers C, Edelmann F, Wachter R, Pieske B, Mende M, Angermann C, Ertl G, Düngen HD, Störk S. Prognostic impact of diastolic dysfunction in systolic heart failure—a cross‐project analysis from the German Competence Network Heart Failure. Clin Cardiol 2017; 40: 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayashi T, Yamada S, Iwano H, Nakabachi M, Sakakibara M, Okada K, Murai D, Nishino H, Kusunose K, Watanabe K, Ishizu T, Wakami K, Yamada H, Dohi K, Seo Y, Ohte N, Mikami T, Tsutsui H. Left ventricular global strain for estimating relaxation and filling pressure—a multicenter study. Circ J 2016; 80: 1163–1169. [DOI] [PubMed] [Google Scholar]
- 6. Kraigher‐Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJV, Solomon SD. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014; 63: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yip GW, Zhang Y, Tan PY, Wang M, Ho P‐Y, Brodin L‐A, Sanderson JE. Left ventricular long‐axis changes in early diastole and systole: impact of systolic function on diastole. Clin Sci 2002; 102: 512–522. [PubMed] [Google Scholar]
- 8. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Elsevier Inc 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 9. Johansen ND, Biering‐Sørensen T, Jensen JS, Mogelvang R. Diastolic dysfunction revisited: a new, feasible, and unambiguous echocardiographic classification predicts major cardiovascular events. Am Heart J Elsevier Inc. 2017; 188: 136–146. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Victor MA, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Retzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Hear J ‐ Cardiovasc Imaging 2015; 16: 233–271. [DOI] [PubMed] [Google Scholar]
- 11. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977; 55: 613–618. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiograph. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 13. Park J‐H, Marwick TH. Use and limitations of E/e' to assess left ventricular filling pressure by echocardiography. J Cardiovasc Ultrasound 2011; 19: 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cuzick J. A wilcoxon‐type test for trend. Stat Med 1985; 4: 87–90. [DOI] [PubMed] [Google Scholar]
- 15. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr Mosby 2009; 22: 107–133. [DOI] [PubMed] [Google Scholar]
- 16. Almeida JG, Fontes‐Carvalho R, Sampaio F, Ribeiro J, Bettencourt P, Flachskampf FA, Leite‐Moreira A, Azevedo A. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging 2017; 0: 1–7. [DOI] [PubMed] [Google Scholar]
- 17. Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction prevalence, pathophysiology, and clinical perspectives. Circ Hear Fail 2014; 7: 367–377. [DOI] [PubMed] [Google Scholar]
- 18. Parasuraman S, Walker S, Loudon BL, Gollop ND, Wilson AM, Lowery C, Frenneaux MP. Assessment of pulmonary artery pressure by echocardiography—a comprehensive review. IJC Hear Vasc 2016; 12: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edvardsen T, Smiseth OA. Evaluation of diastolic function by echocardiography: important progression, but issues to be resolved. Eur Heart J Cardiovasc Imaging 2017; 0: 1–2. [DOI] [PubMed] [Google Scholar]
- 20. Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha JW, Xu J, Klein AL, Nagueh SF. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 2017; 69: 1937–1948. [DOI] [PubMed] [Google Scholar]
- 21. Biering‐Sørensen T, Biering‐Sørensen SR, Olsen FJ, Sengeløv M, Jørgensen PG, Mogelvang R, Shah AM, Jensen JS. Global longitudinal strain by echocardiography predicts long‐term risk of cardiovascular morbidity and mortality in a low‐risk general population. Circ Cardiovasc Imaging 2017; 10: e005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 2016; 37: 1196–1207b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz‐Hansen T, Nochioka K, Biering‐Sørensen T. Global longitudinal strain is a superior predictor of all‐cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 2015; 8: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 24. Biering‐Sørensen T, Solomon SD. Assessing contractile function when ejection fraction is normal. Circ Cardiovasc Imaging 2015; 8: e004181. [DOI] [PubMed] [Google Scholar]
- 25. Sengupta PP, Krishnamoorthy VK, Korinek J, Narula J, Vannan MA, Lester SJ, Tajik JA, Seward JB, Khandheria BK, Belohlavek M. Left Ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr 2007; 20: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Distribution and reclassification between the two DDF algorithms by Nagueh et al. and Johansen et al.
Table S2. Association between diastolic dysfunction algorithms by Nagueh et al. and Johansen et al. and all‐cause mortality stratified according to patients with mild mitral insufficiency (A) and moderate/severe mitral insufficiency (B).


