Abstract
Background
This study aimed to explore the potential of soluble urokinase plasminogen activator receptor (suPAR) as a biomarker for severe acute pancreatitis (SAP) risk prediction and disease management in SAP patients.
Methods
Totally 225 acute pancreatitis (AP) patients (including 75 SAP, 75 moderate‐severe acute pancreatitis [MSAP], and 75 mild acute pancreatitis [MAP] patients) were recruited based on the Atlanta classification, and their serum samples were obtained within 24 hours after admission. Meanwhile, 75 health controls (HCs) were recruited with their serum samples collected at the enrollment. The serum suPAR was then detected using enzyme‐linked immunosorbent assay.
Results
The suPAR level was increased in SAP patients compared with MSAP patients (P = .023), MAP patients (P < .001), and HCs (P < .001). Receiver operating characteristic (ROC) curve presented that suPAR could not only differentiate SAP patients from HCs (AUC: 0.920, 95%CI: 0.875‐0.965) but also differentiate SAP patients from MSAP (AUC: 0.684, 95%CI: 0.600‐0.769) and MAP patients (AUC: 0.855, 95%CI: 0.797‐0.912). In SAP patients, suPAR was positively correlated with Ranson score (P < .001), acute physiology and chronic healthcare evaluation II score (P = .001), sequential organ failure assessment score (P < .001), and C‐reaction protein (P = .002). Further ROC curve exhibited that suPAR (AUC: 0.806, 95%CI: 0.663‐0.949) was of good value in predicting increased inhospital mortality of SAP patients.
Conclusion
Soluble urokinase plasminogen activator receptor is of good predictive value for SAP risk and may serve as a potential biomarker for disease severity, inflammation, and inhospital mortality in SAP patients.
Keywords: disease severity, inflammatory, mortality, severe acute pancreatitis, soluble urokinase plasminogen activator receptor
1. INTRODUCTION
Acute pancreatitis (AP), one of the most common gastrointestinal diseases, is a rapidly developing inflammatory process of the pancreas, which can cause local injury, systemic inflammatory response syndrome (SIRS), and organ failure.1 The global incidence of AP ranges from 5 to 30 cases per 100 000 individuals per year and is still increasing in recent years.2 Although the majority of AP cases are mild and with acceptable prognosis, approximately 20% of AP patients develop moderate‐to‐severe AP (MSAP) and even severe AP (SAP), who present with severe local or systemic complications and poor prognosis.3, 4 Therefore, it is of significant importance to explore novel biomarkers which can identify SAP risk, monitor disease condition, and predict prognosis, which thereby improve clinical outcomes and optimize therapy effect in SAP patients.
Urokinase plasminogen activator receptor (uPAR) is a membrane‐bound receptor that mainly expresses on immunologically active cell membrane and participates in various physiological and pathological processes, such as inflammation and immune responses.5, 6 Circulating suPAR originates from shedding of the uPAR and expressed on a variety of cells, such as neutrophils, lymphocytes, macrophages, and endotheliocytes.7 Emerging evidence indicates that suPAR is involved in various biological functions, including cell adhesion, migration, and chemotaxis, and its increased level is associated with poor clinical outcomes in various inflammatory diseases, such as sepsis, bacteremia, and SIRS.7, 8, 9, 10, 11, 12 In addition, excessive inflammation cytokine production activates the coagulation system, which further promotes the expression of suPAR, and suPAR may serve as a diagnostic and prognostic biomarker in these diseases.5, 13 As for the role of suPAR in SAP, only one previous study with a small sample size reports that suPAR may serve as a biomarker for disease severity and risk of severe complications in SAP patients; however, in this previous study, MSAP patients are not separately classified, and SAP patients recruited are with advanced disease severity, and the sample size is small.14 Therefore, the ability of suPAR in predicting SAP risk and prognosis needs further exploration.
In the present study, we determined suPAR level in 75 SAP, 75 MSAP, 75 mild AP (MAP) patients, and 75 healthy controls (HCs) and aimed to investigate the value of suPAR in predicting SAP susceptibility, as well as to further explore the potential of suPAR as a biomarker for SAP management and prognosis prediction.
2. MATERIALS AND METHODS
2.1. Participants
From January 2016 to December 2018, 75 SAP patients, 75 MSAP patients, and 75 MAP patients treated in The First Affliated Hospital of Harbin Medical University were consecutively enrolled in this study. All patients were diagnosed as AP according to the 2012 revision of the Atlanta classification and definitions of acute pancreatitis,15 with age above 18 years. The SAP patients were defined as AP patients who presented with persistent organ failure >48 hours (single organ failure or multiple organ failure). The MSAP patients were defined as AP patients who met at least one of the following items: a) organ failure that resolves within 48 hours (transient organ failure); b) local or systemic complications without persistent organ failure; the MSAP patients were defined as AP patients who had no organ failure and no local or systemic complications. The exclusion criteria were as follows: (a) accompanied with pancreatic carcinoma or cholangiocarcinoma; (b) asthma, inflammatory bowel disease, arthritis, or other chronic inflammatory diseases; (c) serious infection (human immunodeficiency virus); (d) history of hematological disorders or other malignancies; (e) pregnant or lactating woman. In addition, 75 healthy age‐ and sex‐matched subjects who underwent physical examination at The First Affliated Hospital of Harbin Medical University were consecutively enrolled as HCs, between January 2019 and March 2019. All HCs had no history of pancreatic or bile duct diseases or other obvious abnormalities by physical examination.
2.2. Ethics approval
This study was approved by the Ethics Committee of The First Affliated Hospital of Harbin Medical University and was conducted according to the Declaration of Helsinki. All participants signed the informed consents before enrollment.
2.3. Collection of basic characteristics and blood samples
After the confirming the eligibility of AP patients, the demographic and clinical characteristics were collected including age, gender, etiology of AP (biliary acute pancreatitis (BAP), alcohol‐induced acute pancreatitis (AAP), hypertriglycemic acute pancreatitis (HTGAP), or others), and C‐reaction protein (CRP). And the Ranson score, Acute Physiology and Chronic Health Evaluation (APACHE) II score and sequential organ failure assessment (SOFA) score were calculated based on biochemical indexes and clinical symptoms. Meanwhile, the demographic characteristics (age and gender) of HCs were recorded at physical examination. Peripheral blood samples of AP patients were collected within 24 hours after enrollment, and peripheral blood samples of HCs were collected on the enrollment; then, the serum was isolated from peripheral blood samples by centrifugation, at the condition of 2000 × g, 10 minutes (4°C). After separation, the serum was stored at −80°C until determination.
2.4. Measurement of suPAR
The levels of serum suPAR in AP patients and HCs were determined by Enzyme‐linked immunosorbent assay (ELISA) with Human suPARnostic AUTO Flex enzyme‐linked immunosorbent assay (ELISA) Kit (ViroGates, Blokken), which were ready‐to‐use, according to the instructions of manufacturer. In brief, firstly, samples or standards were added to the 96‐well plate, followed by the antibody mix. After incubation, the wells were washed to remove unbound material. Tetramethylbenzidine (TMB) substrate (tetramethylbenzidine, TMB) was added, generating blue coloration. This reaction was then stopped by addition of Stop Solution completing any color change from blue to yellow. Signal was generated proportionally to the amount of bound analyte (Biotek), and the intensity was measured at 450 nm. Each reaction was run in triplicate by the same operator. The plates were run on the same kit lot. Standard curves were prepared before the antibody reaction.
2.5. Treatment and follow‐up
The AP patients received appropriate treatments based on the etiology of pancreatitis and usual practice of the hospital according to American College of Gastroenterology guideline: management of acute pancreatitis.1 For SAP patients, intensive follow‐up was conducted during hospital treatment, which was continued until the patients died in hospital or were discharged from hospital. At the same time, inhospital mortality of SAP patients was documented; then, the SAP patients were further divided into inhospital deaths and survivors.
2.6. Statistical analysis
All the statistical analyses were performed using SPSS 24.0 statistical software (SPSS Inc.), and figures were made using GraphPad Prism 7.00 software (GraphPad Software Inc). Continuous variables were presented as mean ± standard deviation (SD) or median and interquartile range (IQR); categorical variables were presented as count (percentage). Comparisons of demographic and clinical characteristics among different subjects were carried out by one‐way analysis of variance (ANOVA), chi‐square test, or Kruskal‐Wallis H rank sum test. The comparisons of suPAR level between SAP group and other groups were conducted by Benjamini‐Krieger‐Yekutieli test. The difference of suPAR level between inhospital deaths and survivors was determined by Wilcoxon rank sum test. Correlation of suPAR with Ranson score, APACHE II score, SOFA Score, and CRP was analyzed using Spearman's rank correlation test. Receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC) with 95% confidence interval (CI) was calculated to assess the ability of suPAR in discriminating different subjects or discriminating inhospital deaths and survivors. P value <.05 was considered significant.
3. RESULTS
3.1. Demographic and clinical characteristics
The mean age of SAP patients (N = 75), MSAP patients (N = 75), MAP patients (N = 75), and HCs (N = 75) was 59.9 ± 13.6 years, 56.6 ± 13.3 years, 56.2 ± 12.9 years, and 58.9 ± 13.3 years, respectively (Table 1). There were 46 (61.3%) males and 29 (38.7%) females in SAP patients, 50 (66.7%) males and 25 (33.3%) females in MSAP patients, 44 (58.7%) males and 31 (41.3%) females in MAP patients, and 40 (53.3%) males and 35 (46.7%) females in HCs. No difference of age (P = .248) or gender (P = .409) was observed among SAP, MSAP, MAP patients, and HCs. In all AP patients, Ranson score (P < .001), APACHE II score (P < .001), SOFA score (P < .001), and CRP (P < .001) were different among SAP patients, MSAP patients, and MAP patients, and these indexes were the highest in SAP patients, followed by MSAP patients, and the lowest in MAP patients. Furthermore, there were 56 (74.7%), 59 (78.7%), and 61 (81.3%) patients who used antibiotics treatment in SAP, MSAP, and MAP patients, respectively. However, SAP, MSAP, and MAP patients had no difference in etiology (P = .703) and antibiotics treatment (P = .610). However, SAP, MSAP, and MAP patients had no difference in etiology (P = .703). The detailed information of baseline characteristics was presented in Table 1.
Table 1.
Demographic and clinical characteristics of participants
| Items | SAP (N = 75) | MSAP (N = 75) | MAP (N = 75) | HCs (N = 75) | P value |
|---|---|---|---|---|---|
| Age (y), mean ± SD | 59.9 ± 13.6 | 56.6 ± 13.3 | 56.2 ± 12.9 | 58.9 ± 13.3 | .248 |
| Gender, No. (%) | |||||
| Male | 46 (61.3) | 50 (66.7) | 44 (58.7) | 40 (53.3) | .409 |
| Female | 29 (38.7) | 25 (33.3) | 31 (41.3) | 35 (46.7) | |
| Etiology, No. (%) | |||||
| BAP | 41 (54.7) | 34 (45.3) | 31 (41.3) | – | .703 |
| AAP | 7 (9.3) | 7 (9.3) | 6 (8.0) | – | |
| HTGAP | 19 (25.3) | 26 (34.7) | 29 (38.7) | – | |
| Others | 8 (10.7) | 8 (10.7) | 9 (12.0) | – | |
| Ranson score, mean ± SD | 3.7 ± 1.0 | 1.8 ± 0.7 | 1.1 ± 0.4 | – | <.001 |
| APACHE II score, mean ± SD | 14.3 ± 6.3 | 6.7 ± 3.2 | 4.1 ± 2.0 | – | <.001 |
| SOFA score, mean ± SD | 6.6 ± 2.0 | 4.3 ± 1.4 | 2.0 ± 0.6 | – | <.001 |
| CRP (mg/L), median (IQR) | 138.6 (95.5‐171.2) | 92.2 (61.6‐122.7) | 36.2 (23.9‐50.9) | – | <.001 |
| Antibiotics treatment, No. (%) | 56 (74.7) | 59 (78.7) | 61 (81.3) | – | .610 |
Comparison was determined by one‐way analysis of variance (ANOVA), chi‐square test, or Kruskal‐Wallis H rank sum test.
Abbreviations: AAP, alcohol‐induced acute pancreatitis; APACHE II, Acute Physiology and Chronic Health Evaluation II; BAP, biliary acute pancreatitis; CRP, C‐reaction protein; HCs, healthy controls; HTGAP, hypertriglycemic acute pancreatitis; MAP, mild acute pancreatitis; MSAP, moderate‐severe acute pancreatitis; SAP, severe acute pancreatitis; SD, standard deviation; SOFA, sequential organ failure assessment.
3.2. Comparison of suPAR among SAP patients, MSAP patients, MAP patients, and HCs
The level of suPAR was increased in SAP patients (16.048 [12.633‐24.190]) compared with MSAP patients (12.255 [9.624‐17.036]) (P = .023), MAP patients (9.410 [6.903‐12.577]) (P < .001), and HCs (5.166 [1.950‐8.221]) (P < .001) (Figure 1A). Soluble urokinase plasminogen activator receptor could differentiate SAP patients from MSAP (AUC: 0.684, 95%CI: 0.600‐0.769) (Figure 1B) and MAP patients (AUC: 0.855, 95%CI: 0.797‐0.912) (Figure 1C), and it was especially good at differentiating SAP patients from HCs (AUC: 0.920, 95%CI: 0.875‐0.965) (Figure 1D). These data indicated that suPAR could serve as a good biomarker for predicting SAP risk.
Figure 1.
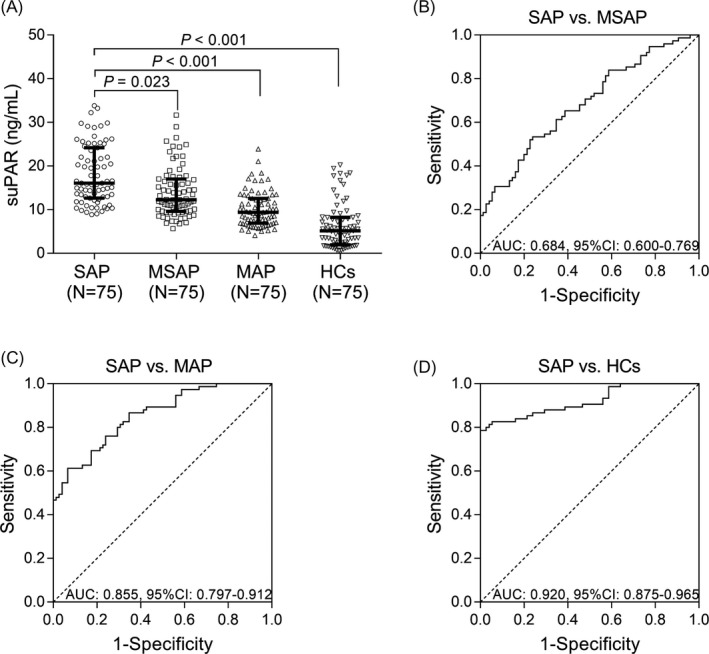
SuPAR level in SAP patients, MASP patients, MAP patients, and HCs. The comparison of suPAR level among SAP patients, MSAP patients, MAP patients, and HCs (A). The performance of suPAR in distinguishing SAP patients from MSAP patients (B), MAP patients (C), and HCs (D). The comparisons of suPAR level between SAP group and other groups were determined by Benjamini‐Krieger‐Yekutieli test. And ROC curves and the AUC with 95% CI were used to assess the ability of suPAR in discriminating different subjects. P < .05 was considered significant. AP, acute pancreatitis; SAP, severe acute pancreatitis; MSAP, moderate‐severe acute pancreatitis; MAP, mild acute pancreatitis; HCs, healthy controls; suPAR, soluble urokinase plasminogen activator receptor; AUC, area under the curve; CI, confidence interval
3.3. Correlation of suPAR with clinical characteristics in SAP patients
In SAP patients, suPAR was positively correlated with Ranson score (P < .001, r = .601) (Figure 2A), APACHE II score (P = .001, r = .361) (Figure 2B), SOFA score (P < .001, r = .496) (Figure 2C), and CRP (P = .002, r = .356) (Figure 2D). These data suggested that suPAR positively correlated with disease severity and inflammation in SAP patients.
Figure 2.
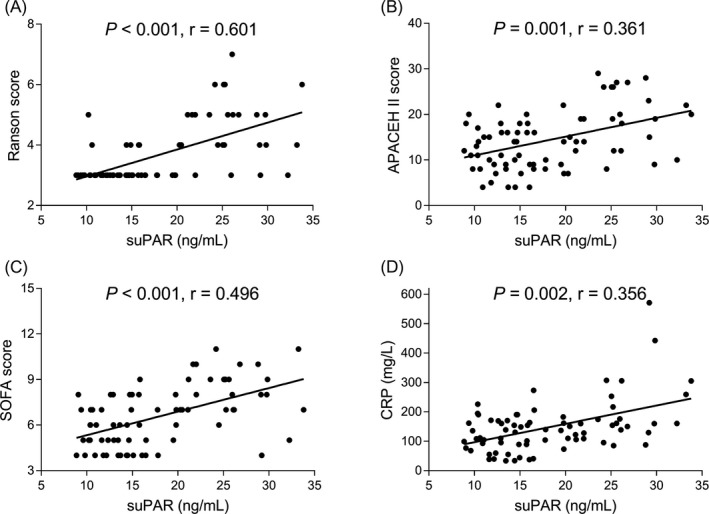
Association of suPAR with disease severity and inflammation in SAP patients. The association of suPAR with Ranson score (A), APACHE II score (B), SOFA score (C), and CRP (D). Association of suPAR with Ranson score, APACHE II score, SOFA Score, and CRP was analyzed using Spearman's rank correlation test. P < .05 was considered significant. SAP, severe acute pancreatitis; suPAR, soluble urokinase plasminogen activator receptor; APACHE II, Acute Physiology and Chronic Health Care Evaluation II; SOFA, sequential organ failure assessment; CRP, C‐reaction protein
3.4. Correlation of suPAR with inhospital mortality in SAP patients
Among 75 SAP patients, there were 16 inhospital deaths (21.3%) and 59 survivors (78.7%) (Figure 3A). Soluble urokinase plasminogen activator receptor level was elevated in inhospital deaths (25.792 [22.298‐28.302]) compared with survivors (14.899 [12.155‐19.824]) (P < .001) (Figure 3B). Further, ROC curve exhibited that suPAR (AUC: 0.806, 95%CI: 0.663‐0.949) was of good value in predicting inhospital mortality (Figure 3C). As for several common prognostic predictors in SAP, Ranson score (AUC: 0.853, 95%CI: 0.740‐0.966) was of great value in predicting inhospital mortality; APACHE II score (AUC: 0.787, 95%CI: 0.665‐0.909), SOFA score (AUC: 0.771, 95%CI: 0.645‐0.898), and CRP (AUC: 0.649, 95%CI: 0.4999‐0.800) were of acceptable value in predicting inhospital mortality. Numerically, the value of suPAR in predicting inhospital mortality was non‐inferior to Ranson score, APACHE II score, SOFA score, and CRP. The above data suggested that suPAR had a good value for predicting increased inhospital mortality of SAP patients.
Figure 3.
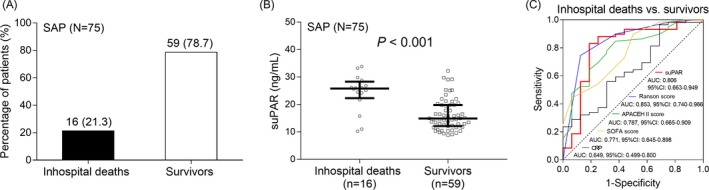
Correlation of suPAR with prognosis in SAP patients. The percentage of inhospital deaths and survivors in SAP patients (A). Comparison of suPAR level between inhospital deaths and survivors in SAP patients (B). The performance of suPAR, Ranson score, APACHE II score, SOFA score, CRP in distinguishing inhospital deaths and survivors in SAP patients (C). The difference of suPAR level between inhospital deaths and survivors was determined by Wilcoxon rank sum test. ROC curves and the AUC with 95% CI were used to assess the ability of suPAR, Ranson score, APACHE II score, SOFA score, CRP in distinguishing inhospital deaths and survivors. P < .05 was considered significant. SAP, severe acute pancreatitis; suPAR, soluble urokinase plasminogen activator receptor; APACHE II, Acute Physiology and Chronic Health Care Evaluation II; SOFA, sequential organ failure assessment; CRP, C‐reaction protein; AUC, area under the curve; CI: confidence interval
3.5. The ability of combined biomarkers in predicting inhospital mortality
The combination of suPAR, Ranson score, APACHE II score, SOFA score, and CRP exhibited good value in predicting inhospital mortality (AUC: 0.896, 95%CI: 0.806‐0.986), which suggesting that the predictive value of combination was better compared with single biological markers/ disease severity score (suPAR, Ranson score, APACHE II score, SOFA score, and CRP) (Figure 4).
Figure 4.
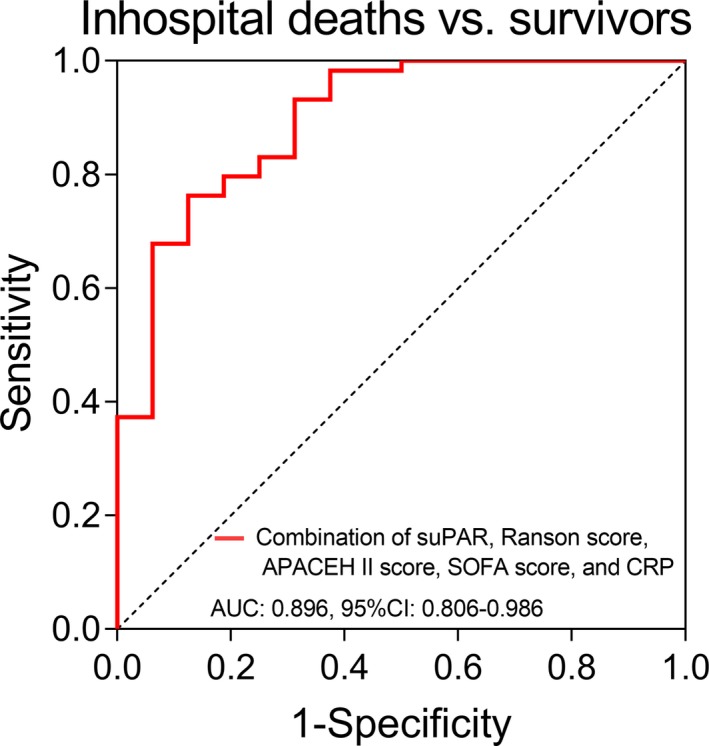
The performance of the combination (suPAR, Ranson score, APACHE II score, SOFA score, CRP) in predicting inhospital mortality in SAP patients. Receiver operating characteristic curves and the AUC with 95% CI were used to assess the ability of combination (suPAR, Ranson score, APACHE II score, SOFA score, CRP) in distinguishing inhospital deaths and survivors. P < .05 was considered significant. SAP, severe acute pancreatitis; suPAR, soluble urokinase plasminogen activator receptor; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, sequential organ failure assessment; CRP, C‐reaction protein; AUC, area under the curve; CI, confidence interval
4. DISCUSSION
In the present study, we found that (a) suPAR level was increased in SAP patients compared with MSAP patients, MAP patients, and HCs, and further, ROC curve exhibited that suPAR was especially good at differentiating SAP patients from HCs and could differentiate SAP patients from MSAP as well as MAP patients; suPAR level was positively correlated with disease severity and inflammation in SAP patients. (b) suPAR could predict higher inhospital mortality in SAP patients.
Soluble urokinase plasminogen activator receptor is a receptor released from cell membrane‐bound uPAR, and existing evidence illustrates that suPAR is involved in various inflammatory‐related diseases such as acute liver failure, sepsis, systemic inflammatory response syndrome, and lupus nephritis.5, 7, 12, 16, 17, 18 For example, suPAR is increased, and its concentration is closely correlated with higher risk of liver cell injury, poor liver function, and end‐stage liver disease in patients with acute liver failure.17 Another study indicates that suPAR level is increased in patients with SIRS, and ROC curve illustrates that suPAR is of great value in differentiating SIRS patients from HCs with AUC of 0.978.12 As for the role of suPAR in SAP, one previous study reveals that suPAR is elevated in SAP patients compared with MAP patients and HCs, and increased level of suPAR is associated with the advanced disease severity, risk of pancreatic infection, and multiple organ dysfunction syndrome in SAP patients.14 However, this previous study includes a small sample size, which might lead to decreased validation, and it also lacks the exploration for the value of suPAR in differentiating SAP patients from MSAP patients. Thus, with the purpose to further validate the predictive value of suPAR for SAP risk as well as the correlation of suPAR with disease severity and inflammation in SAP patients, we performed this study with 75 SAP, 75 MSAP, 75 MAP patients, and 75 HCs. We observed that suPAR was not only good at differentiating SAP patients from HCs but also could distinguish SAP patients from MSAP and MAP patients. Furthermore, suPAR was positively associated with disease severity and inflammation in SAP patients. Considering the role of suPAR in inflammatory diseases, suPAR might promote the release of inflammatory cytokines, contributing to extremely increased activation of inflammatory cytokines‐induced immune responses in SAP patients compared to MSAP, MAP patients as well as HCs. That might explain why suPAR was closely associated with higher risk of SAP.5 In addition, SuPAR might promote plasminogen‐activating signaling pathways and enhance the modulation of cell adhesion, migration, and proliferation, which led to organ injuries and further increased the occurrence and severity of multiple organ dysfunctions, thereby suPAR could predict higher risk of SAP. Since suPAR was correlated with increased possibility of inflammation, necrotizing pancreatitis, and the occurrence of multiple organ dysfunctions, it was positively associated with disease severity and inflammation in SAP patients. Besides, based on the classification of AP, SAP was characteristic of more persistent organ failure compared with MSAP which was defined by the presence of transient organ failure.15 Furthermore, suPAR level was activated by inflammation‐induced coagulation system, and systematic inflammation cascade might aggravate the severity of organ failure; therefore, AP patients with higher suPAR level had more severe organ failure, which was associated with higher risk of SAP.
In agreement with the correlation of suPAR with the severity of some inflammatory‐related diseases including sepsis, SIRS, and bacteremia, suPAR is shown to be of prognostic value for unfavorable survival in patients with these diseases.7, 9, 12 For example, in SIRS patients, the suPAR levels are significantly higher in non‐survivors compared with survivors, and persistent elevated suPAR level predicts higher morality in SIRS patients.12 As for in SAP, the prognostic value of suPAR is reported by only one previous study; however, this study exhibits no difference of suPAR between non‐survivors and survivors in SAP patients, and the possible reasons may include that (a) small sample size leads to the enlargement effect of extreme value; (b) the SAP patients recruited are with exacerbated SAP; therefore, their survivals are poor, which weakens the difference of suPAR between non‐survivors and survivors.14 We conducted this present study with larger sample size to further explore the value of suPAR in predicting inhospital mortality in SAP patients. The result revealed that suPAR level was elevated in inhospital deaths compared with survivors, and ROC curve presented that suPAR was of good value in predicting inhospital mortality of SAP patients with AUC of 0.806. Notably, the value of suPAR in predicting inhospital mortality was non‐inferior to Ranson score, APACHE II score, SOFA score, and CRP, and these predictive indexes were the most widely used routine examination of clinical practice and predictors for mortality in SAP patients.19 In addition, we also found that the combination of suPAR, Ranson score, APACHE II score, SOFA score, and CRP exhibited good value in predicting inhospital mortality, which suggested that the predictive value of combination was better compared with single biological markers/disease severity score (suPAR, Ranson score, APACHE II score, SOFA score, and CRP). The possible reasons might include the following: (a) According to the findings of our study, suPAR was positively associated with Ranson score, APACHE II score, SOFA score, and CRP; therefore, suPAR might indirectly influence inhospital mortality of SAP patients via interacting with these indexes. (b) SuPAR might promote the pro‐inflammatory immune response and increase the risk and severity of multiple organ dysfunctions, which directly increased inhospital mortality of SAP patients.
Our study still limited by the following shortcomings: (a) The underlying mechanism of suPAR in SAP was not explored in our study; therefore, further animal studies were still needed. (b) As the present study was single centered, which might lead to regional selective bias, therefore, patients from more geographic regions were needed for validation. (c) The follow‐up was conducted until the patients were discharged from hospital, while the long‐term effect of suPAR for disease recovery needed to be observed in a longer period. (d) Considering that antibiotics treatment might affect the suPAR level, however, we could not assess the influence of antibiotics on suPAR level as the peripheral blood samples were not collected after the antibiotics treatment.20
In summary, suPAR is of good predictive value for SAP risk and may serve as a potential biomarker for disease severity, inflammation, and inhospital mortality in SAP patients.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
This study supported by National Nature Scientific Foundation of China (81670583) and National Nature Scientific Foundation of China (81871974).
Zhang Q, Li L, Chen H, et al. Soluble urokinase plasminogen activator receptor associates with higher risk, advanced disease severity as well as inflammation, and might serve as a prognostic biomarker of severe acute pancreatitis. J Clin Lab Anal. 2020;34:e23097 10.1002/jcla.23097
REFERENCES
- 1. Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology . American College of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400‐1415; 1416. [DOI] [PubMed] [Google Scholar]
- 2. Tee YS, Fang HY, Kuo IM, Lin YS, Huang SF, Yu MC. Serial evaluation of the SOFA score is reliable for predicting mortality in acute severe pancreatitis. Medicine (Baltimore). 2018;97(7):e9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou H, Mei X, He X, Lan T, Guo S. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: a retrospective study. Medicine (Baltimore). 2019;98(16):e15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simsek O, Kocael A, Kocael P, et al. Inflammatory mediators in the diagnosis and treatment of acute pancreatitis: pentraxin‐3, procalcitonin and myeloperoxidase. Arch Med Sci. 2018;14(2):288‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thuno M, Macho B, Eugen‐Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27(3):157‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dinesh P, Rasool M. uPA/uPAR signaling in rheumatoid arthritis: shedding light on its mechanism of action. Pharmacol Res. 2018;134:31‐39. [DOI] [PubMed] [Google Scholar]
- 7. Donadello K, Scolletta S, Covajes C, Vincent JL. suPAR as a prognostic biomarker in sepsis. BMC Med. 2012;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schenk M, Eichelmann F, Schulze MB, et al. Reproducibility of novel immune‐inflammatory biomarkers over 4 months: an analysis with repeated measures design. Biomark Med. 2019;13(8):639‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Backes Y, van der Sluijs KF, Mackie DP, et al. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38(9):1418‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aronen A, Aittoniemi J, Huttunen R, et al. Plasma level of soluble urokinase plasminogen activator receptor (suPAR) predicts long‐term mortality after first acute alcohol‐induced pancreatitis. Eur J Intern Med. 2019;64:72‐75. [DOI] [PubMed] [Google Scholar]
- 11. Sidenius N, Sier CF, Ullum H, et al. Serum level of soluble urokinase‐type plasminogen activator receptor is a strong and independent predictor of survival in human immunodeficiency virus infection. Blood. 2000;96(13):4091‐4095. [PubMed] [Google Scholar]
- 12. Sirinoglu M, Soysal A, Karaaslan A, et al. The diagnostic value of soluble urokinase plasminogen activator receptor (suPAR) compared to C‐reactive protein (CRP) and procalcitonin (PCT) in children with systemic inflammatory response syndrome (SIRS). J Infect Chemother. 2017;23(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 13. Murciano JC, Higazi AA, Cines DB, Muzykantov VR. Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro‐urokinase and alters its functional profile. J Control Release. 2009;139(3):190‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long D, Wang Y, Wang H, Wu X, Yu L. Correlation of serum and ascitic fluid soluble form urokinase plasminogen activator receptor levels with patient complications, disease severity, inflammatory markers, and prognosis in patients with severe acute pancreatitis. Pancreas. 2019;48(3):335‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102‐111. [DOI] [PubMed] [Google Scholar]
- 16. Hamie L, Daoud G, Nemer G, et al. SuPAR, an emerging biomarker in kidney and inflammatory diseases. Postgrad Med J. 2018;94(1115):517‐524. [DOI] [PubMed] [Google Scholar]
- 17. Koch A, Zimmermann HW, Gassler N, et al. Clinical relevance and cellular source of elevated soluble urokinase plasminogen activator receptor (suPAR) in acute liver failure. Liver Int. 2014;34(9):1330‐1339. [DOI] [PubMed] [Google Scholar]
- 18. Zhao JJ, Lou XL, Chen HW, Zhu FT, Hou YQ. Diagnostic value of decoy receptor 3 combined with procalcitonin and soluble urokinase‐type plasminogen activator receptor for sepsis. Cell Mol Biol Lett. 2018;23:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juneja D, Gopal PB, Ravula M. Scoring systems in acute pancreatitis: which one to use in intensive care units? J Crit Care. 2010;25(2):358.e9‐358.e15. [DOI] [PubMed] [Google Scholar]
- 20. Tsai PK, Tsao SM, Yang WE, Yeh CB, Wang HL, Yang SF. Plasma soluble urokinase‐type plasminogen activator receptor level as a predictor of the severity of community‐acquired pneumonia. Int J Environ Res Public Health. 2019;16(6):E1035. [DOI] [PMC free article] [PubMed] [Google Scholar]


