Abstract
Aims
Proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as a therapeutic target for reducing plasma low‐density lipoprotein cholesterol. Beyond lipid control, recent findings suggest a deleterious effect of this protein in the pathogenesis of postmyocardial infarction left ventricle remodelling and heart failure‐related complications. The aim of this study was to assess the relationship between circulating PCSK9 and 6 month cardiac magnetic resonance imaging‐derived left ventricular ejection fraction (LVEF) after a first ST‐segment elevation myocardial infarction (STEMI).
Methods and results
We prospectively evaluated 40 patients with a first STEMI, LVEF < 50% and treated with primary percutaneous coronary intervention in which PCSK9 was measured 24 h postreperfusion. All patients underwent cardiac magnetic resonance imaging 1 week and 6 months after STEMI. Baseline characteristics were compared across median values of PCSK9. The association between PCSK9 levels and LVEF at 6 months was evaluated by analysis of covariance. The mean age of the sample was 60 ± 12 years and 33 (82.5%) were male patients. The infarct location was anterior in 27 patients (67.5%), and 9 patients (22.5%) were Killip class ≥ II. The mean 1 week and 6 month LVEF were 41 ± 7% and 48 ± 10%, respectively. The mean PCSK9 was 1.93 ± 0.38 U/mL. Testing the association between serum PCSK9 and 6 month LVEF with analysis of covariance revealed an inverse relationship (r = −0.35, P = 0.028). After multivariate adjustment, circulating PCSK9 remained significant and inversely associated with 6 month LVEF (P = 0.002).
Conclusions
In patients with a first STEMI with reduced ejection fraction at index admission and treated with primary percutaneous coronary intervention, circulating PCSK9 was associated with lower LVEF at 6 months.
Keywords: PCSK9, Left ventricular ejection fraction, Cardiac magnetic resonance, ST‐segment elevation myocardial infarction
Introduction
Inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as a novel and effective therapy for treating hypercholesterolemia.1 Some experimental evidence suggests that PCSK9 is up‐regulated in vascular smooth muscle cells and ischemic hearts, playing a potential pathogenic role in infarct size, cardiac function, and autophagy.2, 3 In addition, recent clinical evidence indicates that plasma PCSK9 is positively related to a higher risk of adverse events in a large cohort of patients with acute heart failure with predominantly left ventricular systolic dysfunction and ischemic heart disease.4
In this study, we sought to evaluate the relationship between PCSK9 levels 24 h after primary percutaneous coronary intervention and 6 month left ventricular ejection fraction (LVEF) assessed by cardiac magnetic resonance (CMR).
Material and methods
Study population
This study stems from a prospective observational study carried out from June 2009 to December 2010 that included 203 consecutive patients with a first ST‐segment elevation acute myocardial infarction (STEMI) in which a CMR at 1 week and 6 months was performed.5, 6 In a subgroup of this cohort (those with LVEF < 50% at 1 week CMR), PCSK9 levels were analysed from frozen samples. After applying the exclusion criteria (Figure 1 ), the final sample included 40 patients.
Figure 1.
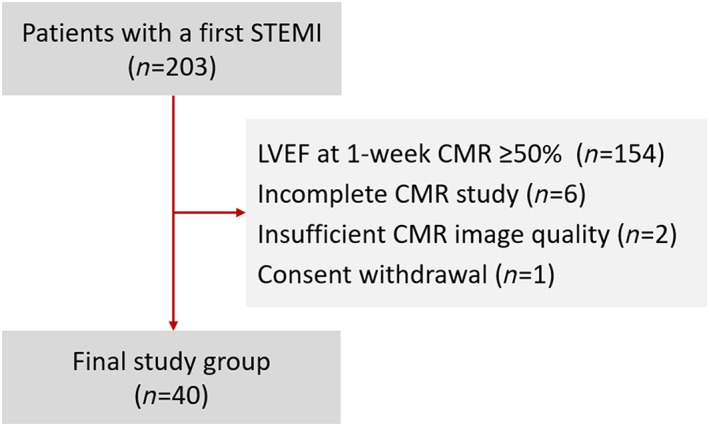
Flow chart.CMR, cardiac magnetic resonance; LVEF, left ventricular ejection fraction; STEMI, ST‐segment elevation acute myocardial infarction.
Baseline characteristics were recorded prospectively in all cases at admission. Informed consent was obtained from each patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Biomarker assays
Manipulation and storage of samples were performed for expert investigators. Blood samples were isolated 24 h after coronary revascularization, centrifuged at 2300 rpm for 15 min, and serum was immediately refrigerated at −80°C under the strict control of the temperature. PCSK9 and soluble interleukin‐1 receptor‐like 1 (ST2) were measured using the Proseek Multiplex CVD III panel (Olink Proteomics AB, Uppsala, Sweden)7 and a high‐sensitivity monoclonal sandwich immunoassay (Critical Diagnostic Presage ST2 assay), respectively, according to the manufacturers' instructions.
Cardiac magnetic resonance imaging
Detailed information about the CMR technique is described in Supporting Information, File S1 .5 Briefly, 1 week and 6 month LVEF were calculated using manual planimetry of endocardial and epicardial borders on short‐axis cine images. Infarct size was assessed as the percentage of left ventricular mass with late gadolinium enhancement. Intraobserver and interobserver variability for all CMR indices analysed in the present study were previously evaluated and reported by our group as being less than 5%.5
Endpoint
The CMR‐derived LVEF at 6 months was selected as the endpoint of this study.
Statistical analysis
Continuous variables were expressed as the mean ± 1 SD or median (interquartile range) when appropriate. Discrete variables were summarized as percentages. Baseline characteristics were compared across median values of PCSK9. The association between PCSK9 levels and LVEF at 6 months was evaluated by analysis of covariance. For multivariate regression analyses, candidate covariates were chosen based on previous medical knowledge, independent of their P value. A reduced and parsimonious model was derived using backward stepwise selection. During this selection process, the linearity assumption for continuous variables was tested and transformed, if appropriate, with fractional polynomials.8
The following covariates were included in the final analysis of covariance model: 1 week CMR‐derived LVEF, age, gender, infarct size at 1 week CMR, ST2, and low‐density lipoprotein (LDL) cholesterol. Moreover, the regression model was clustered on statin treatment in order to capture potential differences in the association between PCSK9 and LVEF at 6 months driven by this treatment.
A two‐sided P value < 0.05 was considered significant for all analyses, which were performed using STATA 15.1.
Results
The mean age of the sample was 60 ± 12 years, and 33 (82.5%) were male patients. The infarct location was anterior in 27 patients (67.5%), and 9 patients (22.5%) were Killip class ≥ II. The mean LVEF at 1 week was 41 ± 7%. Mean PCSK9 was 1.93 ± 0.38 U/mL.
The baseline characteristics of patients with PCSK9 values below and above the median (1.94 U/mL) are presented in Table 1. Patients with PCSK9 above the median exhibited no relationship with a worse baseline risk profile except for higher rates of dyslipidemia and prescription of statins.
Table 1.
Baseline characteristics across PCSK9 values
| Variables |
PCSK9 below median (<1.94 U/mL) (n = 20) |
PCSK9 above median (>1.94 U/mL) (n = 20) |
P |
|---|---|---|---|
| Demographics and medical history | |||
| Age (years) | 59 ± 12 | 60 ± 12 | 0.638 |
| Male, n (%) | 17 (85) | 16 (80) | 0.677 |
| Hypertension, n (%) | 12 (60) | 10 (50) | 0.525 |
| Diabetes mellitus, n (%) | 4 (20) | 4 (20) | 1 |
| Dyslipidemia, n (%) | 4 (20) | 11 (55) | 0.022 |
| Smoker, n (%) | 13 (65) | 11 (55) | 0.519 |
| Anterior infarction, n (%) | 15 (75) | 12 (60) | 0.311 |
| GRACE score | 137 ± 28 | 147 ± 38 | 0.321 |
| TIMI risk scorea | 3 (1.5–4) | 2 (1.5–3.5) | 0.499 |
| Laboratory data | |||
| Killip class ≥ II, n (%) | 6 (30) | 3 (15) | 0.256 |
| Peak hsTnT (ng/mL)a | 3631 (1772–4987) | 3702 (2892–7627) | 0.159 |
| ST2 (pg/mL)a | 86.7 (37.9–113.8) | 103.9 (63.2–135.9) | 0.083 |
| PCSK9 (U/mL) | 1.64 ± 0.25 | 2.22 ± 0.23 | <0.001 |
| CMR at 1 week | |||
| LVEF (%) | 42 ± 7 | 41 ± 7 | 0.641 |
| LVEDVI (mL/m2) | 84 ± 18 | 78 ± 20 | 0.445 |
| LVESVI (mL/m2) | 48 ± 11 | 48 ± 17 | 0.953 |
| Infarct size (%)a | 28 (19–34) | 30 (26–41) | 0.206 |
| MVO (%) | 2.1 ± 0.4 | 2.6 ± 0.6 | 0.482 |
| Medical treatment at discharge | |||
| Aldosterone receptor blockers, n (%) | 8 (40) | 4 (20) | 0.168 |
| ACEI/ARB, n (%) | 13 (65) | 18 (90) | 0.058 |
| Beta blockers, n (%) | 13 (65) | 13 (65) | 1 |
| Statins, n (%) | 13 (65) | 19 (95) | 0.018 |
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; CMR, cardiac magnetic resonance; GRACE, Global Registry of Acute Coronary Events; hsTnT, high‐sensibility troponin T; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end‐systolic volume index; MVO, microvascular obstruction; PCSK9, proprotein convertase subtilisin/kexin type 9; ST2, soluble interleukin‐1 receptor‐like 1; TIMI, Thrombolysis in Myocardial Infarction.
Values for continuous variables are expressed as mean ± SD unless otherwise specified.
Values expressed as median (interquartile range).
Proprotein convertase subtilisin/kexin type 9 and 1 week cardiac magnetic resonance parameters
For the CMR imaging variables at 1 week, no significant differences were found across median PCSK9 values and LVEF, left ventricular end‐diastolic volume index, left ventricular end‐systolic volume index, microvascular obstruction, and infarct size (Table 1). PCSK9 values did not correlate with LVEF (r = 0.20, P = 0.206), left ventricular end‐diastolic volume index (r = 0.02, P = 0.895), left ventricular end‐systolic volume index (r = 0.14, P = 0.384), microvascular obstruction (r = 0.20, P = 0.207), or infarct size (r = 0.29, P = 0.073).
Proprotein convertase subtilisin/kexin type 9 and 6 month left ventricular ejection fraction
Compared with baseline, the mean LVEF significantly increased from 41 ± 7% to 48 ± 10% (P < 0.001) at 6 months. PCSK9 values were inversely related to 6 month LVEF (r = −0.35, P = 0.028). The mean PCSK9 values were significantly higher in patients with LVEF < 50% at 6 months (2.06 ± 0.29 vs. 1.80 ± 0.41 U/mL, P = 0.028). After multivariate adjustment including well‐established determinants of left ventricle remodelling (1 week LVEF, infarct size, and ST2 levels) and potential confounders (age, gender, lipoprotein status, and statin treatment), circulating PCSK9 remained significant and inversely associated with 6 month LVEF (P = 0.002; Figure 2 ).
Figure 2.
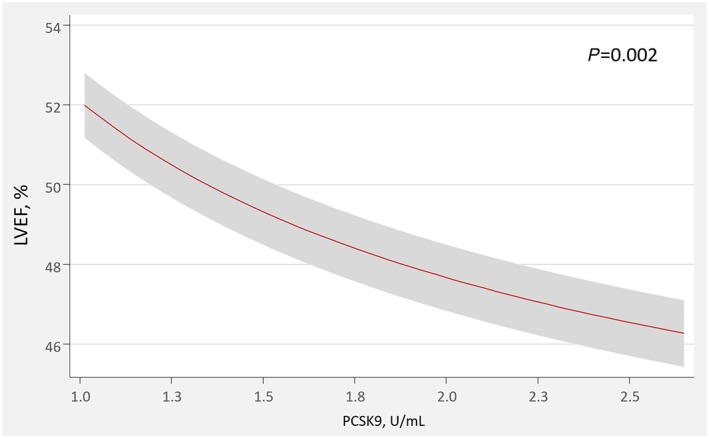
Adjusted effect of PCSK9 on 6 month left ventricular ejection fraction.LVEF, left ventricular ejection fraction; PCSK9, proprotein convertase subtilisin/kexin type 9.
Discussion
In this cohort of patients with a first STEMI and reduced ejection fraction at 1 week, we found an independent association between PCSK9 levels and 6 month CMR‐derived LVEF. This association was independent of crucial determinants of left ventricle remodelling (1 week CMR–LVEF, infarct size, and ST2) and important potential confounders.
Proprotein convertase subtilisin/kexin type 9 as a marker of left ventricular remodelling and heart failure‐related complications
PCSK9 is a well‐established target for treating hypercholesterolemia and atherosclerosis progression.1, 9 Although the major source of PCSK9 is the liver, in a recent experimental study in mice and explanted human hearts, Ding et al. reported that PCSK9 is up‐regulated in the zone bordering the infarct area and determines, at least in part, infarct size, cardiac function, and autophagy.3 The same group reported that PCSK9 is highly expressed in vascular smooth muscle cells, and its expression and development of autophagy are regulated by well‐known inflammation mediators, such as lipopolysaccharide, tumour necrosis factor α (TNFα), and reactive oxygen species.2, 10, 11
Other authors have also reported PCSK9 is associated with the inflammatory response.12 Ricci et al. showed, in a recent report, that human recombinant PCSK9 drives an inflammatory response on macrophages by inducing the pro‐inflammatory cytokines TNFα, interleukin‐1, and interleukin‐6, and the chemokines monocyte chemoattractant protein‐1 and C‐X‐C Motif Chemokine Ligand 2 (CXCL2). In addition, they reported a positive correlation between PCSK9 and TNFα plasma levels of healthy subjects. These authors suggest the pro‐inflammatory action of PCSK9 on macrophages is mainly dependent on the LDL receptor .12 Moreover, a role of PCSK9 on pathogen lipids (such as lipopolysaccharide) removal regulation has also been suggested; thus, reduced PCSK9 function should be associated with increased pathogen lipid clearance via the LDL receptor, a decreased inflammatory response, and improved septic shock outcome.13 In agreement with prior postulates, plasma PCSK9 was significantly associated with a higher risk of mortality and/or heart failure‐related readmission beyond well‐known prognosticators in a large European cohort including 2174 patients with acute heart failure with predominant ischaemic aetiology and left ventricular systolic dysfunction.4
Thus, and in agreement with our findings, we postulate up‐regulation of PCSK9 during an acute myocardial infarction may be implicated on pathophysiological processes causally linked to adverse remodelling, such as inflammation and/or autophagy.
Proprotein convertase subtilisin/kexin type 9 as a potential therapeutic target for preventing left ventricular remodelling
Several effects of PCSK9 antibodies have been postulated in patients with acute coronary syndromes. In addition to the reduction of LDL cholesterol, PCSK9 antibodies would contribute to plaque stabilization via several mechanisms.9, 14 However, a direct myocardial effect can also be envisioned. Glick et al. found that treatment of primary cardiomyocytes with recombinant mPCSK9 resulted in prompt expression of well‐known markers of autophagy. Interestingly, PCSK9 inhibition was associated with the attenuation of these markers.15 Moreover, Ding et al. reported that PCSK9 inhibition in mice by chemical inhibitors or gene deletion results in a significant improvement in infarct size and cardiac function, with a significant decrease in autophagy activity.3
In view of the available data, and accordingly to our results, we hypothesize that, in patients with STEMI, treatment with PCSK9 inhibitors might play a role by limiting adverse left ventricular postmyocardial infarction remodelling beyond its beneficial effects on the lipid profile and plaque stabilization. These myocardial effects could also explain part of the beneficial clinical effects found with PCSK9 inhibitors.1 Further studies are warranted to test this hypothesis.
Limitations
This study has some limitations. First, as this is an observational study, we cannot rule out selection bias or the absence of unmeasured confounding factors. Second, as no serial PCSK9 measurements were performed, we could not evaluate the significance of changes over time. However, available data in mice in the context of coronary artery disease show that the plasma PCSK9 concentration is mostly elevated in the early hours after onset of acute coronary syndrome.3
Conclusion
In patients with a first STEMI with LVEF < 50% and treated with primary percutaneous coronary intervention, PCSK9 levels in the acute phase predict the LVEF at 6 months. Further studies are warranted to confirm these findings and to explore the causative role of PCSK9 in post‐STEMI ventricular remodelling and its potential as a therapeutic target.
Conflict of interest
J.N. received board membership fees and travel expenses from AMGEN. J.S. reports grants from Biotronik, Prosmedica, and Bayer outside of the submitted work.
Funding
This work was supported in part by grants from CIBER CV [grant numbers 16/11/00420, 16/11/00486, and 16/11/00403], Instituto de Salud Carlos III‐FEDER [grant numbers (PIE15/00013 and, PI17/01836)], Madrid, Spain, and Generalitat Valenciana [GV/2018/116]. The authors have no other funding, financial relationships, or conflicts of interest to disclose relative to this work.
Abbreviation list
- CMR
cardiac magnetic resonance
- LDL
low‐density lipoprotein
- LVEDVI
left ventricular end‐diastolic volume index
- LVEF
left ventricular ejection fraction
- LVESVI
left ventricular end‐systolic volume index
- MVO
microvascular obstruction
- PCSK9
proprotein convertase subtilisin/kexin type 9
- ST2
soluble interleukin‐1 receptor‐like 1
- STEMI
ST‐segment elevation myocardial infarction
- TNFα
tumour necrosis factor α
Supporting information
Data S1. Supporting information
Miñana, G. , Núñez, J. , Bayés‐Genís, A. , Revuelta‐López, E. , Ríos‐Navarro, C. , Núñez, E. , Chorro, F. J. , López‐Lereu, M. P. , Monmeneu, J. V. , Lupón, J. , Sanchis, J. , and Bodí, V. (2020) Role of PCSK9 in the course of ejection fraction change after ST‐segment elevation myocardial infarction: a pilot study. ESC Heart Failure, 7: 117–122. 10.1002/ehf2.12533.
Gema Miñana and Julio Núñez contributed similarly in the present study.
“All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation”.
Contributor Information
Antoni Bayés‐Genís, Email: abayesgenis@gmail.com.
Vicent Bodí, Email: vicentbodi@hotmail.com.
References
- 1. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; Steering Committee FOURIER and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 2. Ding Z, Liu S, Wang X, Deng X, Fan Y, Sun C, Wang Y, Mehta JL. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid Redox Signal 2015; 22: 760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding Z, Wang X, Liu S, Shahanawaz J, Theus S, Fan Y, Deng X, Zhou S, Mehta JL. PCSK9 expression in the ischemic heart and its relationship to infarct size, cardiac function and development of autophagy. Cardiovasc Res 2018; 114: 1738–1751. [DOI] [PubMed] [Google Scholar]
- 4. Bayes‐Genis A, Núñez J, Zannad F, Ferreira JP, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Metra M, Lupón J, Voors AA. The PCSK9‐LDL receptor axis and outcomes in heart failure: BIOSTAT‐CHF subanalysis. J Am Coll Cardiol 2017; 70: 2128–2136. [DOI] [PubMed] [Google Scholar]
- 5. Bodi V, Monmeneu JV, Ortiz‐Perez JT, Lopez‐Lereu MP, Bonanad C, Husser O, Minana G, Gomez C, Nunez J, Forteza MJ, Hervas A, de Dios E, Moratal D, Bosch X, Chorro FJ. Prediction of reverse remodeling at cardiac MR imaging soon after first ST‐segment‐elevation myocardial infarction: results of a large prospective registry. Radiology 2016; 278: 54–63. [DOI] [PubMed] [Google Scholar]
- 6. Miñana G, Núñez J, Bayés‐Genís A, Revuelta‐López E, Ríos‐Navarro C, Núñez E, Chorro FJ, López‐Lereu MP, Monmeneu JV, Lupón J, Bodí V. ST2 and left ventricular remodeling after ST‐segment elevation myocardial infarction: a cardiac magnetic resonance study. Int J Cardiol 2018; 270: 336–342. [DOI] [PubMed] [Google Scholar]
- 7. Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Bucht Thorsen S, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson AC, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S. Homogenous 96‐plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014; 9: e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Z. Multivariable fractional polynomial method for regression model. Ann Transl Med 2016; 4: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Navarese EP, Kolodziejczak M, Kereiakes DJ, Tantry US, O'Connor C, Gurbel PA. Proprotein convertase subtilisin/kexin type 9 monoclonal antibodies for acute coronary syndrome: a narrative review. Ann Intern Med 2016; 164: 600–607. [DOI] [PubMed] [Google Scholar]
- 10. Ding Z, Liu S, Wang X, Mathur P, Dai Y, Theus S, Deng X, Fan Y, Mehta JL. Crosstalk between PCSK9 and damaged mtDNA in vascular smooth muscle cells: role in apoptosis. Antioxid Redox Signal 2016; 25: 997–1008. [DOI] [PubMed] [Google Scholar]
- 11. Ding Z, Liu S, Wang X, Deng X, Fan Y, Shahanawaz J, Shmookler Reis RJ, Varughese KI, Sawamura T, Mehta JL. Cross‐talk between LOX‐1 and PCSK9 in vascular tissues. Cardiovasc Res 2015; 107: 556–567. [DOI] [PubMed] [Google Scholar]
- 12. Ricci C, Ruscica M, Camera M, Rossetti L, Macchi C, Colciago A, Zanotti I, Lupo MG, Adorni MP, Cicero AFG, Fogacci F, Corsini A, Ferri N. PCSK9 induces a pro‐inflammatory response in macrophages. Sci Rep 2018; 8: 2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF, Christie JD, Nakada TA, Fjell CD, Thair SA, Cirstea MS, Boyd JH. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med 2014; 6: 258ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang Z, Jiang L, Peng J, Ren Z, Wei D, Wu C, Pan L, Jiang Z, Liu L. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF‐kappa B activation in THP‐1‐derived macrophages. Int J Mol Med 2012; 30: 931–938. [DOI] [PubMed] [Google Scholar]
- 15. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 2010; 221: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information


