Abstract
Aims
Heart failure with preserved ejection fraction (HFpEF) poses a substantial challenge for clinicians, but there is little guidance for effective management. The aim of this systematic review was to determine if there was evidence that disease management programmes (DMPs) improved outcomes for patients with HFpEF.
Methods and results
A systematic review of controlled studies in English or Greek of DMPs including patients with HFpEF from 2008 to 2018 was conducted using CINAHL, Cochrane, MEDLINE, and Embase. Interventions were assessed using a DMP taxonomy and scored for complexity and intensity. Bias was assessed using the Cochrane Collaboration tool. Initial and updated searches found 6089 titles once duplicates were removed. The final analysis included 18 studies with 5435 HF patients: 1866 patients (34%, study ranges 18–100%) had potential HFpEF (limited by variable definitions). Significant heterogeneity in terms of the population, intervention, comparisons, and outcomes prohibited meta‐analysis. Statistically significant or positive trends were found in mortality, hospitalization rates, self‐care ability, quality of life, anxiety, depression, and sleep, but findings were not robust or consistent. Four studies reported results separately for study‐defined HFpEF, with two finding less positive effect on outcomes.
Conclusions
Varying definitions of HFpEF used in studies are a substantial limitation in interpretation of findings. The reduced efficacy noted in contemporary HF DMP studies may not only be due to improvements in usual care but may also reflect inclusion of heterogeneous patients with HFpEF or HF with mid‐range EF who may not respond in the same way as HFrEF to individual components. Given that patients with HFpEF are older and multi‐morbid, DMPs targeting HFpEF should not rely on a single‐disease focus but provide care that addresses predisposing and presentation phenotypes and draws on the principles of comprehensive geriatric assessment. Other components could also be more targeted to HFpEF such as modification of lifestyle factors for which there is emerging evidence, rather than simply continuing the model of care used in HFrEF. Based on current evidence, HF DMPs may improve mortality, hospitalization rates, self‐care, and quality of life in patients with HFpEF; however, further research specifically tailored to appropriately defined HFpEF is required.
Keywords: Heart failure, Heart failure with preserved ejection fraction, Disease management, Systematic review
1. Introduction
Heart failure with preserved ejection fraction (HFpEF) is an increasingly prevalent condition that poses a substantial challenge for clinicians. Despite composing half of all patients with heart failure (HF), it remains less well recognized and understood.1, 2 Patients with HFpEF are more likely to be older, female, and have multiple co‐morbid conditions, and no drugs have yet been shown to improve morbidity and mortality.3, 4 Symptom burden and adverse outcomes are similar to patients with HF with a reduced ejection fraction (HFrEF).5, 6 Analysis of a large cohort of hospitalized patients with HFpEF (n = 53 065) found a 30 day and 1 year all‐cause readmission rate of 22% and 67% respectively and a composite all‐cause readmission and mortality rate of 74.5% at 1 year.7 Current recommendations for management of HFpEF are to control cardiovascular and non‐cardiovascular co‐morbidities and use diuretics to manage fluid status.8 Although multi‐disciplinary team (MDT) disease management programmes to reduce the risk of hospitalization and mortality are recommended for patients with HF, there is little information about their effectiveness specifically in HFpEF.8
Disease management programmes (DMP) are designed to ‘improve outcomes through structured follow‐up with patient education, optimization of medical treatment, psychosocial support and improved access to care’.9 Most HF DMPs in the 1990s–early 2000s focused on patients with HFrEF10 usually after an HF hospitalization. Outcomes for patients with HFrEF were improved through multi‐component DMPs that included the following: optimization of evidence‐based treatment (emphasis on medications for HFrEF), education, behaviour change, supported self‐management, and clinician monitoring. In previous systematic reviews, HF DMPs were found to significantly reduce HF hospitalizations, and those with continued specialized follow‐up reduced all‐cause mortality and all‐cause hospitalization.10, 11 However, some reviews have found limited or no benefit, especially in studies after 2008, in studies with <3 months of follow‐up, or in patients without a recent hospitalization.12, 13
In HF DMPs, it can be challenging to ascertain if the sample included patients with HFpEF, given relatively recent use of the term and controversies over diagnostic criteria. HFrEF is a more tempting target because of robust evidence for specific pharmacological therapies in reducing mortality and morbidity. Thus, little is known about the use and effectiveness of HF DMPs in patients with HFpEF in improving outcomes. The aim of this analysis was to determine if there was evidence that HF DMPs improved outcomes specifically for patients with HFpEF.
The review questions were as follows:
Do MDT or nurse‐led DMPs for patients with HFpEF result in better outcomes for patients compared with usual care or another intervention?
What are the components and processes of successful MDT or nurse‐led DMPs for patients with HFpEF?
2. Methods
The review protocol was registered on Prospero (http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017067980). The systematic review was conducted and reported in accordance to the PRISMA guidelines (http://www.prisma-statement.org/). The years 2008 to 2018 were chosen to reflect contemporary management of HF, increasing attention to HFpEF, and recommendations for HF DMPs in guidelines for management of HF.14 Language was restricted to English or Greek. Studies of community‐dwelling adults with HFpEF were included, as were studies with a mixed HF population if the proportion of patients with HFpEF was discernible, and represented approximately 20% or greater of the total sample. The intervention had to be an MDT or nurse‐led outpatient DMP with a minimum of 3 months of follow‐up and a control group for comparison. Single interventions composed only of pharmacotherapy, exercise, invasive monitoring, end‐of‐life care, or telemonitoring alone were excluded. Interventions were assessed using a taxonomy of DMPs9 and scored for intensity and complexity.15 Final consensus on findings, interpretation, and text were agreed by all authors.
2.1. Information sources, search strategy, and study selection
The following databases were searched from January 2017 to May 2018: CINAHL through EBSCO, Cochrane, MEDLINE, and Embase through Ovid. References in included articles were hand searched. The following terms along with synonyms and relevant terms were applied: HF, primary care, randomized controlled trials, disease management, nurse, and multi‐disciplinary. Titles and abstracts were reviewed by two authors (F. K. and C. D.), and full‐text papers were reviewed by at least two of the authors.
2.2. Data process
The Cochrane data extraction form was revised to align with the aims of the current review and pilot tested. Data were abstracted and cross‐checked by at least two authors independently. Bias was assessed using the Cochrane Collaboration tool16 by at least two authors on the following fields: (i) random sequence generation, (ii) allocation concealment, (iii) blinding of participants and personnel, (iv) blinded outcome assessment, (v) selective outcome reporting, (vi) incomplete outcome data, and (vii) other bias.
3. Results
Initial and updated searches found 7617 titles, with 6089 titles once duplicates were removed. The majority (5791) were excluded following title review. Abstracts (192) were screened in detail for eligibility, and 95 full‐text papers were reviewed. Reasons for exclusion of papers can be found in Figure 1 . An additional 20 papers from references were reviewed. The final analysis included 18 studies in 18 papers with 5435 patients with HF, 1866 of whom were considered by the study to have HFpEF (34%).
Figure 1.
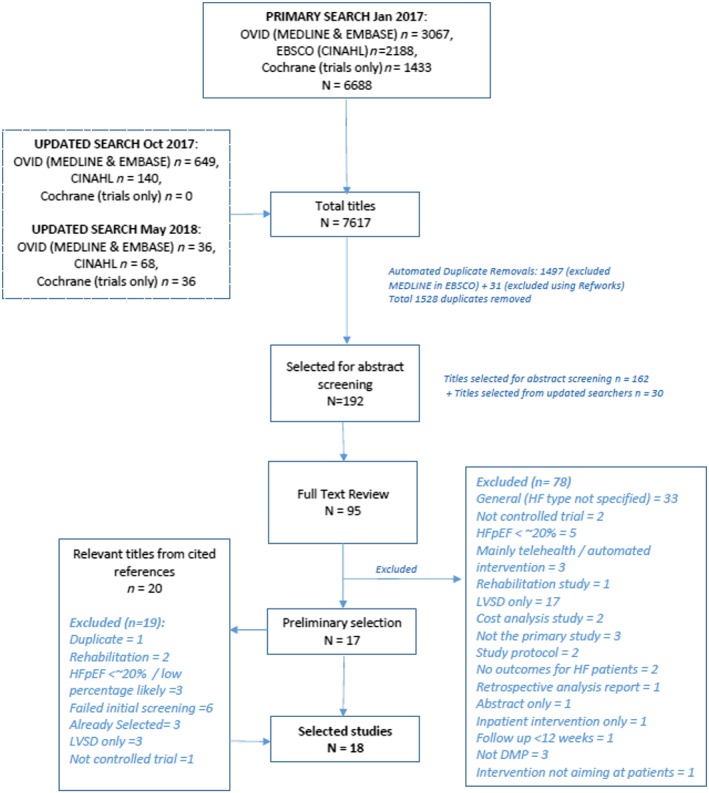
Results of the systematic search strategy and study selection process. DMP, disease management programme; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LVSD, left ventricular systolic dysfunction.
3.1. Inclusion of patients with heart failure with preserved ejection fraction
Only one study focused exclusively on patients with HFpEF; 17 other studies included and documented numbers of patients characterized as HFpEF based on study criteria. When studies stated that they included patients with HFpEF without documenting percentage or number, authors were contacted for information. The proportion of patients with HFpEF varied from 18% to 100% and was variably defined in the studies (Table 1). No studies defined HFpEF in line with the current European Society of Cardiology (ESC) guidelines, which include the following criteria: signs and symptoms of HF, a left ventricular EF ≥ 50%, elevated levels of natriuretic peptides and either relevant structural heart disease (left ventricular hypertrophy and/or left atrial enlargement), and/or diastolic dysfunction on echocardiogram.8 Four studies included some analysis specific to patients with HFpEF, with three of these in comparison with HFrEF. Five studies had samples that were predominantly patients with HFpEF (64–84%) as defined by the study, although only one included discussion of issues specific to HFpEF.
Table 1.
Identification of HF and HFpEF in selected studies
| Study | Country | Sample size | Identification of patients with HF | HFpEF definition/criteria | Proportion HFpEF (%) | Separate results given for HFpEF |
|---|---|---|---|---|---|---|
| Andryukhin et al. (2010) | Russia | N = 100 | Patients included if had signs and symptoms of HF, EF ≥ 50%, and echo evidence of DD, LV stiffness, or abnormal LV relaxation | Signs and symptoms of HF, EF ≥ 50%, and echo evidence of DD, LV stiffness, or abnormal LV relaxation | 100% | Yes |
| Bekelman et al. (2015) | USA | N = 392 | Inpatient or outpatient diagnosis of HF, any type | EF ≥ 50% | 47% (163 of 348 patients with EF measured) | No |
| Bekelman et al. (2018) | USA | N = 314 | Symptomatic outpatients with HF, used dx + data on meds, EF, and BNP | EF ≥ 50% | 40% (n = 121) | Intervention effect on KCCQ differed by EF, with less effect in HFpEF |
| Brotons et al. (2009) | Spain | N = 283 | Hospitalized for suspected HF, with HF as primary or second discharge diagnosis | EF ≥ 50% | 41% (n = 117) | No |
| Chang et al. (2016) | Taiwan | N = 84 | Recruited from cardiology outpatient department, with cardiology confirmed dx of HF | EF ≥ 50% | 55% (n = 46) | No |
| Dracup et al. (2014) | USA | N = 602 | Recruited from clinics and hospitals, needed hospitalization for HF within last 6 months | EF ≥ 40% | 49% (n = 295) | HF group (by EF < 40% or ≥40%) added as a covariate; no difference between HFpEF and HFrEF |
| Freedland et al. (2015) | USA | N = 158 | Recruited from single medical centre, dx with HF within last 3 months | EF ≥ 45% | 46% (n = 73) | No |
| Gonzalez‐Guerrero et al. (2015) | Spain | N = 117 | Hospitalized with acute HF (ESC criteria) in a single centre | Not specifically defined | 67% (n = 77) | No |
| Jaarsma et al. (2008) | The Netherlands | N = 1023 | Recruited during hospital admission for HF (signs and symptoms of HF, plus evidence of structural underlying heart disease on imaging) | Not specified in original paper, but secondary analysis used EF ≥ 40% for HFpEF | A secondary analysis of 661 patients found 33% with HFpEF (n = 218) | No |
| Kalter‐Leibovici et al. (2017) | Israel | N = 1360 | Recruited from public hospitals, primary care, and community cardiologists within 2 months after HF hospitalization; dx based on signs and symptoms, echo evidence | EF ≥ 50% | 18% (n = 247) | Yes, less effect of DMP and very wide CI for HFpEF compared with HFrEF; no significant difference by composite outcome, HF hospitalization, or all‐cause mortality |
| Kwok et al. (2008) | China | N = 105 | Recruited during hospital admission for HF | Differentiated between those with and without EF ≥ 40% | 77% (n = 81) | No |
| Leventhal et al. (2011) | Switzerland | N = 42 | Recruited based on hospitalization for decompensated HF | EF ≥ 45% | 49% (n = 20) | No |
| Masterson Creber et al. (2016) | USA | N = 100 | Recruited during HF hospitalization at 1 urban hospital | Defined as ‘diastolic HF’ with no EF specified | 25% (17 of the 67 who completed) | No |
| Shao et al. (2013) | Taiwan | N = 108 | Patients attending heart clinics with dx HF | EF > 40% | 22% (n = 24) | No |
| Srisuk et al. (2015) | Thailand | N = 100 dyads | Primary dx HF confirmed by MD with objective evidence by echo if possible | Not specifically defined; mean EF was 50–51% ± 13 | Unclear | No |
| Stewart et al. (2014) | Australia | N = 280 | Cardiologist confirmed dx HF | EF > 45% | 27% (n = 76) | Yes, no difference in % with HFpEF by survived or died |
| Tsuchihashi‐Makaya et al. (2013) | Japan | N = 168 | Recruited from 3 cardiology hospitals | EF > 40% | 64% (n = 107) | No |
| Young et al. (2016) | USA | N = 100 | Hospitalized with HF (HF discharge diagnosis) | EF ≥ 50% | 84% (n = 84) | No |
CI, confidence interval; DD, diastolic dysfunction; DMP, disease management programme; EF, ejection fraction; ESC, European Society of Cardiology; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricle.
3.2. Heart failure with preserved ejection fraction definition
The EF cut‐off point for defining HFpEF ranged between ≥40% and ≥50%. It is noticeable that the ESC recommended cut‐off point of EF ≥ 50% was used only in seven studies.17, 18, 19, 20, 21, 22, 23 Three studies included patients labelled as HFpEF but without explicitly defining this population.24, 25, 26 The remaining nine studies used either >45%27, 28, 29 or 40%30, 31, 32, 33, 34 as EF criterion to differentiate patients with HFpEF. The percentage of patients with HFpEF defined by the studies ranged between 22% and 77%. By ESC criteria, these would be samples of patients with both HF with mid‐range EF (HFmrEF, EF 40–49%) and HFpEF, which are considered two distinct clinical entities in the guidelines.8
3.3. Interventions and study characteristics
Interventions varied by components, duration, methods of delivery, intensity, complexity, and outcomes (Tables 2 and 3). All of the interventions were directed to patients, with three including carers. Eleven of the study interventions were delivered primarily by nurses with relevant experience or additional training,17, 20, 21, 25, 26, 28, 29, 30, 31, 33, 34 six were multi‐disciplinary,18, 19, 22, 27, 32, 35 and in one, it was unclear.23 All studies included some component of education, behavioural or psychosocial support, and self‐management support. Only one study included an exercise component delivered as part of the study,17 although advice or referral for increasing physical activity was frequently a component in other studies. Telemonitoring was included in the intervention in two studies,18, 22 and five provided medication adjustment by nurses or via general practitioners.18, 19, 20, 24, 28 Patient assessment was included in all but two studies,17, 18 although the extent, frequency, and type of assessment varied.
Table 2.
Intervention characteristics of selected studies
| Study | Components | Mode of delivery | Complexity, duration, intensity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assessment | Education/>behavioural/>SM | Exercise | Telemonitoring | Clinician >review | Medication >adjustment | Education/>assessment/>DC plan | Home >visits | Telephone | Outpatient >or clinic >session | Multi‐>media >resources | Complexity | Duration | Frequency of contact | |
| Andryukhin et al. (2010) | ✓ | ✓ | ✓ | ✓ | High | 6 months | Weekly F2F education/skills sessions × 4; weekly exercise × 4; weekly phone calls, Months 2–6 | |||||||
| Bekelman et al. (2015) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | 12 months | Monthly × 12 | |||
| Bekelman et al. (2018) | ✓ | ✓ | ✓ | ✓ | ✓ | High | 3 months | 1–2 phone calls per month planned; mean calls by RN 13 (5.7), SW 10 (4) | ||||||
| Brotons et al. (2009) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | 12 months+ | Home 1× per month × 12; phone 2× per month × 12 | |||
| Chang et al. (2016) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Mod | 12 weeks | ×1 monthly face to face and ×6 biweekly phone calls | |||||
| Dracup et al. (2014) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Mod | 1 month then PRN |
LITE: 2 phone calls biweekly PLUS: biweekly 1 phone call |
|||||
| Freedland et al. (2015) | ✓ | ✓ | ✓ | Not >specified | ✓ | ✓ | High | 12 months | Weekly 1 h sessions for 6 months, then biweekly and after monthly | |||||
| Gonzalez‐Guerrero et al. (2015) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | 12 months | Comprehensive hospital DC planning and close follow‐up at a geriatric day hospital (GDH), from a multidisciplinary team; phone contacts and face‐to‐face visits at the GDH | ||||
| Jaarsma et al. (2008) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | 18 months | Basic support group received UC + 9 HFSN clinic visits. Intensive support was UC + 18 HFSN clinic visits, phone calls weekly first month, MDT support, 2 home visits | ||||
| Kalter‐Leibovici et al. (2017) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | Mean = 2.7 years | Initial remote contact 1× per week, adjusted based on need. Clinic every 6 months or more if needed | |||||
| Kwok et al. (2008) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | 6 months | Weekly × 4 first month and then monthly for 6 months | |||||
| Leventhal et al. (2011) | ✓ | ✓ | ✓ | ✓ | ✓ | High | 12 months | Initially home visit, followed by 17 structured telephone calls (weekly × 4, bimonthly × 4, monthly × 6) plus additional calls when needed | ||||||
| Masterson Creber et al. (2016) | ✓ | ✓ | Not >specified | ✓ | ✓ | ✓ | ✓ | Moderate | 3 months | 1 F2F contact and 3–4 phone calls over 90 days | ||||
| Shao et al. (2013) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Moderate | 12 weeks | Home visit within 3 days and then telephone follow‐up at 1, 3, 7, and 11 weeks | |||||
| Srisuk et al. (2017) | ✓ | ✓ | ✓ | ✓ | ✓ | Moderate | 6 months | 1 F2F education/counselling session. Phone calls 15 min/week in first month, per fortnight in the second month and once a month in Months 3–6 | ||||||
| Stewart et al. (2014) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | 6 months | MDT comprehensive care by clinic or home visits | ||||
| Tsuchihashi et al. (2013) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | 6 months | Home visits by nurse within 14 days post‐DC, then q 2 weeks for 2 months. Then monthly telephone until 6 months | |||||
| Young et al. (2016) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | 3 months | Telephone contact twice a week, Weeks 1–2; once weekly, Weeks 3–6; every other week, Weeks 7–12 | ||||
Table 3.
Outcomes
| STUDY | TPs | OM | Morbidity and Mortality | Psychological | Physical | Other | RESULTS (impact of intervention on outcome measure) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MORT. | HOSP. | HF‐QOL | GENERIC QOL | ANX / DEPR. | SELF‐CARE | ACTIV. | BIOCHEM | ECHO | OTHER | |||||
| 1. Brotons et al. 2009 | Monthly for 12 months | USED | YES – AC* | YES – HF* | YES | NR | NR | YES | NR | NR | NR | YES | Positive | Neutral/ Negative |
| DETAILS | Assessed by review of hospital discharge records | Assessed by review of hospital discharge records | MLHFQ | NR | NR | MMAS‐8 | NR | NR | NR | NYHA Class, weight, HR, oedema, clinical warning signs, lifestyle change compliance | The aggregate of all‐cause mortality and HF hospital readmissions improved (but not significantly) in the intervention arm. There was a significant improvement in QoL between the control and intervention group at 1 year. | At 12 months, 86.1% of patients in the intervention group and 75.5% of the control group were adhering to prescribed pharmacological treatments, not significant. | ||
| 2. Chang et al. 2016 | BL, 4wks, 8wks, 12wks | USED | NR | NR | NR | NR | YES | NR | NR | NR | NR | YES* | Positive | Neutral/ Negative |
| DETAILS | NR | NR | NR | NR | HADS | NR | NR | NR | NR | Sleep quality measured by: Pittsburgh Sleep Quality Index, Epworth Sleepiness Scale | Significant improvement in level of sleep quality and significant decreases in levels of daytime sleepiness in the intervention group, no improvement or decrease observed in the control arm. | Anxiety and depression scores unchanged, when compared with controls, the intervention arm had a significantly greater improvement in both anxiety and depression. | ||
| 3. Dracup et al. 2014 | BL, 3, 12, 24 mobnths | USED | YES – CV* | YES – HF* | NR | NR | NR | YES | NR | NR | NR | YES | Positive | Neutral/ Negative |
| DETAILS | Assessed by medical record review, family /physician interview, death certificate and Social Security Death Index check | Assessed by questioning patients and physicians and medical record review | NR | NR | NR | EHFSCBQ | NR | NR | NR | Heart Failure Knowledge Scale, Short Test of Functional Health Literacy in Adults | No significant difference in the combined clinical outcome of cardiac death and HF hospitalization across groups. At 3 and 12 months both intervention groups had significantly lower (better) self‐care scores than the control group with no difference between the intervention groups. | At 24 months self‐care improvement observed in the intervention groups were no longer significantly different from the control group. | ||
| 4. Freedland et al. 2015 | BL, 3, 6, 9, 12 mts | USED | YES‐AC | YES‐AC | YES | YES | YES* | YES | YES | NR | NR | YES | Positive | Neutral/ Negative |
| DETAILS | Not defined | Not defined | KCCQ | SF‐12 | BDI‐II, BAI, Depression Interview, Structured Hamilton Rating Scale | SCHFI | 6MWT, average daily activity level on Actigraphy (1wk wear) | NR | NR | NIH PROMIS Measures | Six‐month depression scores were lower in the CBT than the usual care arm on the BDI‐II. Six‐month outcomes were superior in the CBT relative to the usual care arm on secondary measures of depression, anxiety, HF‐related quality of life, mental health‐related quality of life, fatigue and social functioning. | The groups did not differ on the Self‐Care Maintenance or Confidence subscales. The groups did not differ on any of the physical functioning measures. There was no statistically significant difference in the time to the first all‐cause hospitalization or death between the usual care and CBT groups | ||
| 5. Gonzalez‐Guerrero et al. 2014 | BL,, 12mts | USED | YES‐AC* | YES‐AC* | YES | YES | NR | NR | NR | YES | NR | YES | Positive | Neutral/ Negative |
| DETAILS | Assessed by discussion with patients /relatives, hospital record and the National Death Index review | Assessed by discussion with patients /relatives, hospital record and the National Death Index review | MLHFQ | EQ‐5D | NR | NR | NR | Hb, urea, creatinine, uric acid, Na+, K+, albumin, TC, troponin T, CRP, NT‐proBNP | NR | Global Deterioration Scale | In the intervention group, the probability of having an event (either hospitalisation or mortality) between BL and 1 year was significantly lower. Those receiving the intervention had a significant reduction in mortality risk. | Those receiving the intervention had a non‐significant reduction in HF readmissions but an increase in non‐HF related hospitalisations. | ||
| 9. Jaarsma e t al. 2008 | BL, 18 mts | USED | YES – AC* | YES – HF* | NR | NR | NR | NR | NR | NR | NR | YES* | Positive | Neutral/ Negative |
| DETAILS | Assessed by medical record review and patient interview. Cause was adjudicated by a central ‘endpoint committee’ | Assessed by medical record review and patient interview. Cause was adjudicated by a central ‘endpoint committee’ | NR | NR | NR | NR | NR | NR | NR | Number of days lost due to death or hospitalisation | A non‐significant 15% reduction in mortality, and shorter hospitalizations were observed in both intervention groups. | Neither the moderate nor intensive intervention reduced the combined end points of HF related death and hospitalization compared with standard follow‐up. | ||
| 10. Kalter‐Leibovici1 et al. 2017 | BL, 6, 12, 18, 24 mts | USED | YES – AC* | YES – HF* | NR | YES | YES | YES | YES | YES | NR | YES | Positive | Neutral/ Negative |
| DETAILS | Assessed by review of discharge summaries. Adjudicated by two independent investigators blinded to assignment. | Assessed by review of discharge summaries. Adjudicated by two independent investigators blinded to assignment. | NR | SF‐36 | PHQ‐9 | Purchase of recommended medications | 6MWT | BNP | NR | Total number of hospital admissions and in‐hospital days for heart failure and for all causes | The intervention arm was associated with prolonged time to first hospital admission for HF, especially among patients enrolled after recent hospitalization or with ischemia. Non‐significant trend towards lower total hospital admissions and LOS. Clinically important improvements in QoL and depression observed. | Based on primary endpoint, time to first hospital admission for heart failure or death from any cause, the intervention was not superior to usual care. | ||
| 11. Kwok et al. 2007 | BL, 6 mts | USED | NR | YES – AC* | NR | NR | YES | NR | YES | NR | NR | YES | Positive | Neutral/ Negative |
| DETAILS | NR | Electronic database review; readmission reason assessed by geriatrician or cardiologist and categorized | NR | NR | General Health Questionnaire | NR | 6MWT | NR | NR | LHS, Abbreviated Mental Test | The median number of unplanned admission was significantly lower in the intervention group. Intervention group significantly less limited in independence as assessed by LHS compared to control group. | No significant difference in six month readmission rates between intervention and control group. No change in functional status as assessed by 6MWT. | ||
| 12. Leventhal et al. 2011 | BL, 3, 6, 9, 12 mts | USED | YES AC* | YES HF & AC* | YES | YES | YES | NR | NR | NR | NR | YES | Positive | Neutral/ Negative |
| DETAILS | Assessed by collection of death certificates. | Assessed by review of medical records by blinded researcher | MLHFQ | EQ‐5D | Geriatric Depression Scale | NR | NR | NR | NR | Specific Activity Scale | QoL improved in the intervention group compared to the control group. | No statistically significant difference in mortality rates between control and intervention group. Hospitalisations more frequent in the intervention group. | ||
| 13. Masterson‐Creber et al. 2015 | BL, 90 days | USED | NR | NR | YES | NR | NR | YES* | NR | NR | NR | YES | Positive | Neutral/ Negative |
| DETAILS | NR | NR | KCCQ | NR | NR | SCHFI | NR | NR | NR | HF Symptoms via Heart Failure Somatic Perception Scale | Although not statistically significant, the improvement in self‐care maintenance was numerically greater in the intervention group and a statistical and clinically significant 8.7‐point increase in SCHFI was observed when adjusting for confounding factors. | There were no statistically significant differences in improvement in self‐care confidence, HF symptoms or QoL between the groups. | ||
| 14. Shao et al. 2013 | BL, 4, 12 wks | USED | NR | YES AC | NR | NR | NR | YES* | NR | NR | NR | YES | Positive | Neutral/ Negative |
| DETAILS | NR | Assessed by review of medical records. | NR | NR | NR | Self‐Efficacy for Salt & Fluid Control & modified EHFSCBS | NR | NR | NR | Heart Failure Symptom Distress Scale | Participants in the intervention group were significantly more likely to perform self‐management behaviours including controlling salt and fluid intake, and had a significant decrease in HF symptom distress. | There was no reduction in health service use between the groups. | ||
| 15. Srisuk et al. 2014 | BL, 3, 6 mth | USED | NR | NR | YES | NR | NR | YES | NR | NR | NR | YES* | Positive | Neutral/ Negative |
| DETAILS | NR | NR | Patients: MLHFQ | NR | NR | SCHFI | NR | NR | NR | Patients: HF Knowledge assessed by DHFKS. | Patients and carers in the family‐based intervention group had higher (better) scores than those in the control group on HF knowledge. Patient in the intervention had significantly better self‐care maintenance and self‐care confidence scores. Carers in the intervention had significantly better perceived HF management control. | Negative or neutral results not reported. | ||
| Carers: SF12 | Carers: Perceived control and knowledge assessed via CAS & DHFKS | |||||||||||||
| 16. Stewart et al. 2014 | BL, 12 ‐ 18 mth, 3‐4 yrs | USED | YES AC* | YES AC* | YES | YES | NR | YES | NR | NR | NR | YES | Positive | Neutral/ Negative |
| DETAILS | A blinded endpoint committee adjudicated on the type and cause of mortality | A blinded endpoint committee adjudicated on the type and cause of hospitalization | MLHFQ | EQ‐5D | NR | SCHFI | NR | NR | NR | Length of hospital stay; uptake of gold‐standard pharmacotherapy | Home‐based intervention was associated with significantly fewer all‐cause deaths and significantly fewer days of hospital stay. Favourable trends towards home‐based intervention were strengthened in the long‐term (16% fewer deaths and ⅓ reduction in AC mortality.) | Home‐based intervention group had a higher non‐significant number of all cause admission. Relative to clinic‐based intervention, home‐based intervention was not associated with prolonged event‐free survival | ||
| 17. Tsuchihashi‐Makaya et al. 2013 | BL, 2, 6, 12 mths | USED | YES AC | YES HF | NR | YES | YES* | NR | NR | NR | NR | NR | Positive | Neutral/ Negative |
| DETAILS | Not defined | Not defined | NR | SF‐8 | HADS | NR | NR | NR | NR | NR | The home based intervention significantly improved both anxiety and depression compared to usual care group. The physical and mental health QOL score significantly increased from baseline at all follow‐up time points in the intervention but not in the control. HF hospitalizations were reduced in the intervention group. | There was no difference in AC mortality between the groups. | ||
| 18. Young et al. 2016 | BL, 3 and 6 mths | USED | NR | YES ‐ AC | NR | NR | NR | YES* | YES | YES | NR | YES | Positive | Neutral/ Negative |
| DETAILS | NR | All‐cause readmission and emergency department visits at 30, 90 and 180 days assessed by both self‐report and primary care provider record review | NR | NR | NR | Self‐reported adherence to daily weights, low Na diet, medication, exercise and appointments | 7 day accelerometer wear at BL, 3 and 6 months to establish: average daily activity, expended energy, estimated energy expenditure, average activity intensity assessed by | BNP and urine sodium/creatinine ratio | NR | Atlanta HF Knowledge Test | The intervention group showed significantly greater improvement compared to usual care in patient‐reported SM adherence at 3 and 6 months after discharge. On average, the intervention group had a significantly greater increase in self‐efficacy for heart failure self‐management, self‐management strategies and patient activation. | No significant differences were observed between groups in the pattern of change across time on any of the actigraphy variables collected. There was no significant difference in clinical biomarkers. The 30‐day readmission rate was significantly higher in the intervention group than in the control group with no difference between groups at 90 and 180 days. No group differences were found for self‐management knowledge. | ||
| 2 x Medication Adherence Scales | ||||||||||||||
| SCHFI | ||||||||||||||
| Patient Activation Measure | ||||||||||||||
| Modified EHFSCBS | ||||||||||||||
Abbreviations: NR = Not reported, CV = Cardiovascular, AC= All cause, HF = heart Failure, MLHFQ = Minnesota Living with Heart Failure Questionnaire, KCCQ = Kansas City Cardiomyopathy Questionnaire, PHQ‐9 = Patient Health Questionnaire 9‐item, GAD‐7 = Generalised Anxiety and Depression Scale 7‐item, HDAS = Hospital Anxiety and Depression Scale, 6MWT = Six Minute Walk Test, TC = Total Cholesterol, LDL = Low Density Lipoprotein, HS‐CRP = High Specificity CRP, NTproBNP = N‐terminal pro b‐type natriuretic peptide, LA = Left Atrium, LVEDV = Left ventricular end‐diastolic volume, LV = Left ventricle, Hb = Haemaglobin, K+ = potassium, Na+ = Sodium, NYHA = New York Heart Association, BMI = Body Mass Index, WC = waist circumference, SF = Short Form, MMAS‐8 Moriskey Medication Adherence Scale, EHFSCBQ = European Heart Failure Self‐Care Behaviour Questionnaire, Hr = Heart rate, BDI = Beck Depression Inventory, BAI = Brief Anxiety Index, LHS = London Handicap Scale, SCHFI = Self Care of Heart Failure Index
The duration of the interventions ranged from 3 months to over 2 years, and interventions in seven of the studies were ≥12 months.18, 20, 22, 24, 27, 29, 32 All studies included at least one face‐to‐face encounter with patients, but telephone contact was used in all to deliver some of the intervention. Home visits were used in 11 studies.20, 21, 23, 25, 28, 29, 30, 31, 32, 33, 34 Outpatient or clinical visits were included in all but two studies.26, 27 The majority of studies were judged to be high in intensity and complexity based on delivery of multiple components using different methods of delivery and high frequency of contact, and five were judged to be moderate.
3.4. Comparison
Seventeen of the 18 studies compared an intervention with usual care, although two of these also included two intervention arms varying by intensity and complexity.30, 32 Usual care was variably described across studies, and efforts to standardize usual care were made in only four studies.25, 26, 29, 33 Controlling for patient contact as a confounding variable was only described in one study.26 Stewart et al.28 tested multi‐disciplinary comprehensive care delivered by either outpatient clinic or home visits.
3.5. Outcomes
3.5.1. The effect of disease management programmes on mortality
The most common primary outcome measure employed was a composite of mortality and hospitalization, either all‐cause/all‐cause (n = 4), all‐cause/HF (n = 3), cardiovascular/cardiovascular (n = 1), or cardiovascular/HF (n = 1). All‐cause mortality and/or all‐cause hospitalizations were secondary outcome measures in four studies respectively, and one study employed an all‐cause/HF composite as a secondary objective. In the studies measuring mortality, three reported a significant improvement.18, 24, 28, 35 The proportion of HFpEF patients in these studies was 67%,35 47%,18 and 27%,28 respectively. Of these studies, only Stewart et al.28 reported separate HFpEF statistics (HFpEF defined as those with EF > 45%) and found no difference in percentage with HFpEF by survived or died. Nine studies reported no significant difference of their respective interventions on mortality between either intervention groups or intervention and usual care.17, 19, 20, 22, 27, 29, 30, 32, 34 Kalter‐Leibovici and colleagues22 dichotomized findings by HF group and found no significant difference by composite outcome (all‐cause mortality/HF hospitalization), or all‐cause mortality alone, and much wider confidence intervals in those with EF ≥ 50%. Four of these nine studies without statistical significance did report positive trends in mortality in favour of the intervention.17, 20, 30, 32 Dracup et al.30 added HF group (by EF < 40% or >40%) as a covariate and found no difference in outcomes between groups.
3.5.2. The effect of disease management programmes on hospitalizations
Most studies (88%) employed hospitalization(s) as an outcome measure; the most common was all‐cause hospitalizations (n = 8), HF‐only hospitalizations (n = 5), HF and all‐cause (n = 2), or cardiovascular (n = 1). Only three reported a statistically significant result.24, 34, 35 The remainder reported either no impact17, 18, 19, 20, 27, 30, 33; positive trends in favour of the intervention such as shorter hospital stays, prolonged time to hospitalization, and lower total numbers hospitalized22, 32; or trends towards higher hospitalizations in intervention groups.23, 28, 29
3.5.3. The effect of disease management programmes on self‐care
Nine studies evaluated the effect of the intervention on self‐care. There was significant variability in the self‐care outcome measures employed: the most frequently employed (n = 5) was the Self‐Care of Heart Failure Index15 followed by the European Heart Failure Self‐Care Behaviours Questionnaire (n = 3).36 Of the studies assessing effect on self‐care (n = 9), four reported a statistically significant positive effect,23, 26, 33 two reported improvements that were not significant,20, 25, 30 and one reported no impact,27 and in two studies, the effect could not be ascertained from the publication.22, 28
3.5.4. The effect of disease management programmes on condition‐specific quality of life
Heart failure quality of life was measured by either the Minnesota Living with Heart Failure Questionnaire37 (n = 5) or the Kansas City Cardiomyopathy Questionnaire (KCCQ)38 (n = 4). Three studies failed to demonstrate significant improvement18, 25, 28; one study19 did not reach statistical significance but demonstrated a clinically meaningful improvement in quality of life as measured by KCCQ; and five studies reported a statistically significant improvement in the intervention arm.17, 20, 27, 29, 35
3.5.5. The effect of disease management programmes on anxiety, depression, and sleep quality
Anxiety and depression were commonly measured and featured in nine of the 18 studies, and these were the primary outcome in two studies.27, 34 Freedland et al.27 demonstrated that cognitive behavioural therapy plus an HF education programme was superior to usual care plus an HF education programme only, and this improvement was sustained over time. Tsuchihashi‐Makaya et al.34 similarly found that their home‐based DMP significantly improved psychological status compared with usual care and was also sustained over time. Of the seven studies examining anxiety or depression as a secondary outcome measure, impact of the intervention on either variable, anxiety or depression, was undeterminable in two publications29, 31 and improved either significantly or clinically in the intervention arm in five studies.17, 18, 19, 21, 22 One study specifically focussed on improving sleep in HF patients21 and found both sleep quality and day‐time sleepiness levels significantly improved in the intervention arm.
3.6. Outcomes by heart failure with preserved ejection fraction with ejection fraction ≥50%
If we use the ESC criterion that HFpEF includes an EF ≥ 50%, then seven studies are of interest. The outcomes of mortality and/or hospitalization were measured in six studies.17, 18, 19, 20, 22, 23 Of these, only one had significantly fewer events in the intervention group, specifically in all‐cause mortality.18 Kalter‐Leibovici et al.22 did not find a significant difference in outcomes for DMP vs. control, and as noted previously, the confidence intervals around the hazard ratios for HF hospitalization and all‐cause mortality were much wider for patients with HFpEF.
The single study that assessed self‐care reported significant improvement favouring the intervention group. The intervention was efficacious in terms of health‐related quality of life (HRQoL) in two studies.17, 20 In two studies, there was no significant difference between intervention and control groups in 3 and 6 months of HRQoL measured by the KCCQ.18, 19 Additionally, Bekelman et al.19 reported a lower effect size of the intervention on the KCCQ for HFpEF compared with HFrEF (−0.03 vs. 0.28). All five studies measuring the outcomes of anxiety and/or depression demonstrated significant improvement in favour of the intervention arm.17, 18, 19, 21, 22
3.7. Outcomes by intervention provider
Six of the 11 studies with mainly nurse‐led interventions had outcomes related to mortality and/or hospitalization; one of them demonstrated significant improvement in all‐cause mortality28 and another one in HF hospitalizations.34 Half of the six nurse‐led studies that assessed HRQoL reported improved HRQoL in the intervention group as compared with control.17, 26, 29 The majority of the nurse‐led studies evaluating self‐care changes achieved a significantly positive result at least once during follow‐up (including adjusted results) when comparing the intervention to the control group.25, 26, 30, 33 Among the nurse‐led studies, the intervention group yielded superior results in relation to anxiety and/or depression in three out of five studies.17, 21, 34 Five studies employed a multidisciplinary approach in their intervention: two reduced mortality and/or hospitalization18, 35 in the intervention group vs. control group, one improved HRQoL35 in comparison with control group, and all three studies measuring depression and/or anxiety had better results in the intervention group in this regard.18, 19, 22 None of the multidisciplinary studies assessed self‐care. In the study of Freedland et al.,27 the intervention was delivered by behavioural therapists and was effective in improving HF quality of life, anxiety, and depression. One study did not specify the provider of the intervention.23
3.8. Assessment of bias
Most studies were rated as low risk in terms of random sequence generation. However, bias varied considerably across other aspects with most studies being unable to conceal allocation to intervention or usual care arms after randomization from research team and patients. Almost all studies had blinded outcome assessment, and most reported complete outcome results (Table 4).
Table 4.
Bias
| First author and year | Random sequence generation | Allocation concealment | Blinded investigators/patients | Blinded outcome assessment | Selective outcome reporting | Incomplete outcome data | Other bias |
|---|---|---|---|---|---|---|---|
| Andryukhin (2010) | Unclear | Low | High | Low | High | High | Higha |
| Bekelman (2015) | Low | Low | High | Lowb | Low | Low | Low |
| Bekelman (2018) | Low | Low | High | Low | Low | High | Low |
| Brotons (2009) | Low | Low | High | Low | Low | Low | Low |
| Chang (2016) | Unclear | Low | High | Low | Low | Low | High |
| Dracup (2014) | Low | Low | Low | Low | Low | Low | Low |
| Freedland (2015) | Unclear | Low | High | Low | Low | Low | Low |
| González‐Guerrero (2014) | Low | Low | High | Lowc | Low | Low | Low |
| Jaarsma (2008) | Low | Unclear | High | Low | Low | Low | Low |
| Kalter‐Leibovici (2017) | Low | Unclear | High | High | Low | Low | High |
| Kwok (2008) | Low | Low | High | Low | Low | Low | Low |
| Leventhal (2011) | Low | Low | High | Low | Low | Low | High |
| Masterson Creber (2016) | Unclear | Unclear | High | Low | Low | High | Low |
| Shao (2013) | Low | Low | High | Low | Low | Low | High |
| Srisuk (2015) | Low | Low | High | Low | Low | Low | Low |
| Stewart (2014) | Low | Unclear | High | Low | Low | Low | Low |
| Tsuchihashi‐Makaya (2014) | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
| Young (2016) | Low | Low | High | Low | Low | Low | High |
Positive change included no change from baseline.
Positive change included improvement for 3 and 6 months.
Positive change included improvement for primary outcome.
4. Discussion
In this systematic review, we found a limited number of contemporary studies of DMPs in HF that included or sufficiently described patients with HFpEF and only one that was specifically designed for this group. Across the studies, there was significant heterogeneity in terms of the population, intervention, comparisons, and outcomes that prohibited meta‐analysis. Definitions for HFpEF were variable with only seven of the 18 studies employing an EF of ≥50%. This reflects the lack of a universal approach in defining HFpEF even among recent trials,39 despite the guidance provided by the ESC. Interventions were similarly heterogeneous with extensive variability in the components included, mode of delivery, complexity, and duration. Comparison groups received scant attention; few studies sufficiently described attempts to standardize or account for potential confounding in ‘usual care’ control arms. In terms of outcomes, the most commonly measured were hospitalizations (83%) and mortality (66%), and a minority demonstrated that the DMP had a statistically significant impact on either outcome.
Four studies with both HFrEF and HFpEF reported results separately for patients identified as HFpEF in the study, but only two defined HFpEF using the recommended ESC criterion of EF > 50%.19, 22 Bekelman et al.19 (40% HFpEF, n = 121) found that the intervention had less effect on quality of life in those with HFpEF compared with HFrEF. Kalter‐Leibovici et al.22 (18% HFpEF, n = 247) also found less effect of the DMP and wide confidence intervals for HFpEF compared with HFrEF; however, overall, there was no significant difference by composite outcome, HF hospitalization, or all‐cause mortality by HF group. In the study exclusively HFpEF (using EF ≥ 50%),15 the intervention group had improvement or no deterioration in several cardiovascular risk factors, quality of life, depression, and left ventricular end‐diastolic volume index compared with control. There was no statistically significant difference between intervention and control on cardiovascular events or mortality at 6 and 18 months. This trial was innovative in including exercise sessions and measuring specific echocardiographic parameters but nonetheless included recommendations for HFrEF medications that have not been shown to improve event‐free survival in patients with HFpEF. Bias was also assessed as high on some components (Table 4).
Programme components offer a useful framework to explore reasons for variable impact on outcomes assessed in included studies.
4.1. Clinical assessment
Sixteen studies included either MDT or nurse‐led clinical assessment that then formed the basis of individualized HF‐specific treatment plans. None detailed exploration of co‐morbidity, clinical phenotyping, or comprehensive geriatric assessment, although one DMP was delivered through a geriatric day‐care hospital.35 Data from clinical trials have clearly demonstrated the high incidence of co‐morbidities in HFpEF and effects of this has on outcome. The CHARM trial found that demographic risk factors (age and sex) and non‐cardiac risk factors contributed more to mortality and morbidity outcomes in patients with HFpEF (n = 1086 defined as EF > 40%), while cardiac disease burden contributed more to outcomes in those with HFrEF.40 If co‐morbidities drive the development of HFpEF through a systemic pro‐inflammatory state as currently postulated,41 then the focus on appropriate control of cardiovascular and non‐cardiovascular co‐morbid conditions is essential.3, 8, 42
The ARISE‐HF investigators recently presented a pragmatic framework that includes profiling to determine concurrent co‐morbidities, identifying individualized priorities and patient‐centred goals, supporting multi‐professional home‐based case management, coordinating care, and emphasizing self‐care.43 Shah et al.3 devised an HFpEF treatment grid organized by predisposition phenotype (e.g. hypertension and metabolic syndromes) and clinical presentation phenotype (e.g. lung congestion and atrial fibrillation) to determine management based on the patient's phenotypic features and co‐morbid conditions. Upadhya et al.44 have called for HFpEF to be recognized as a true geriatric condition and suggested that geriatric principles should be used in the treatment of HFpEF.
4.2. Educational/behavioural/self‐management interventions
Self‐management interventions have previously been found to reduce risk of the composite endpoint of HF‐related hospitalization and all‐cause death, HF‐related hospitalization alone, and result in a small improvement in HRQoL compared with usual care.45 In this review, all studies incorporated educational, behavioural, or self‐care components designed to improve self‐management. Only three of nine studies measuring self‐care as an outcome demonstrated a statistically significant improvement in self‐care. Programme characteristics, mechanisms of effect, and evidence for efficacy may explain this finding. In an individual patient data meta‐analysis of 20 trials of self‐management support in patients with HF (n = 5624), no specific programme characteristics were identified that consistently had a positive effect on multiple outcomes. A 2016 systematic review and realist synthesis of the main mechanisms of HF DMPs found that to be effective, programmes should contain components that increase patient understanding of HF, self‐care, self‐efficacy, family/caregiver involvement, psychosocial well‐being, health professional support, and technology use.46 Although many studies encompassed components that harnessed one or more of these mechanisms, no single programme comprehensively covered all. Finally, our knowledge of optimal life‐style behaviours and self‐care in HFpEF is limited, and self‐care interventions for patients with HFpEF lack evidence of effectiveness.
4.3. Exercise
Being physically active was advocated in most studies, but only one study delivered an exercise intervention.17 Other studies encouraged exercise as part of self‐management education and support, referred to formal exercise programmes (although uptake not reported), and one23 measured activity as part of a primarily telephone‐delivered self‐management intervention. No significant difference between the groups was found in activity, which was low in both groups.23 Exercise is a promising but underutilized intervention in patients with HFpEF. While data are limited, a meta‐analysis of six trials (n = 276 patients) showed that cardiorespiratory fitness and quality of life were significantly improved with exercise compared with control. Clinical outcomes were not reported, and the studies were of short duration (12–24 weeks).47 A small study tested the effect of a calorie restricted diet, aerobic exercise training (primarily walking), a combination of diet and exercise, or an attention control on 100 obese patients with HFpEF. At 24 weeks, the diet, exercise, and diet + exercise groups had significant improvements in exercise capacity by peak VO2 (greatest increase in diet + exercise) but no significant improvement on quality of life measured by the MLHFQ. Diet significantly improved KCCQ scores, and diet and exercise decreased body weight and improved New York Heart Association class.48
4.4. Telemonitoring
Two studies in this review included telemonitoring as a component of a DMP.18, 22 Multiple studies of non‐invasive telemonitoring as the primary intervention in HF have been conducted with inconsistent results. The latest guidelines on management of HF have no recommendations for non‐invasive telemonitoring in management.8 A recent paper tested a holistic and structured remote management intervention involving a multi‐disciplinary team (nurses, primary care physicians, cardiologists, other providers, and the patient), telemonitoring, risk assessment, and tailored support and management available 24 h or 7 days/week. The intervention resulted in fewer days lost to unplanned cardiovascular hospitalizations and all‐cause mortality compared with usual care over 1 year. However, the sub‐group analysis of patients with EF > 45% (n = 537) showed no benefit between intervention and usual care.49
4.5. Limitations
This systematic review has a number of limitations. Importantly, the varying and inconsistent definitions of HFpEF (some samples included HFmrEF), heterogeneity of studies with significant variation in the aims, interventions and outcomes measured, ascertainment of the condition, and proportion of patients with HFpEF limited our ability to compare the studies directly, employ a meta‐analysis, and draw clear conclusions for this group of patients. In only six studies, the percentage of patients with HFpEF exceeded 50%, and just four studies reported separate results for patients with HFpEF (including patients not meeting ESC guideline criteria for HFpEF). The search strategy may have failed to retrieve relevant studies, as grey literature or reports in languages other than English and Greek were not included. Time restrictions were applied, and the search was not extended to all available databases. In DMPs, blinding of the research team and participants is not feasible, which may bias results in favour of the intervention group. Caution should be used in the interpretation of findings and the results of the current review especially given the lack of data for appropriately defined HFpEF.
5. Conclusions
Varying definitions of HFpEF (including patients with HFmrEF) used in studies are a substantial limitation in interpretation of findings, which may not reflect the effect of DMPs in HFpEF patients. Although statistically significant or positive trends in the primary outcomes were found in mortality, hospitalization rates, self‐care ability, HF knowledge, quality of life, anxiety, depression, and sleep, the evidence is not sufficiently robust or consistent to draw substantive conclusions. We have used programme components as a way of exploring how impact may have been attenuated. Given that patients with HFpEF are older and multi‐morbid, DMPs targeting HFpEF should not rely on a single‐disease focus but provide care that addresses predisposing and presentation phenotypes of well‐defined HFpEF and draws on the principles of comprehensive geriatric assessment. Other components could also be more targeted to HFpEF such as modification of lifestyle factors for which there is emerging evidence, rather than simply continuing the model of care used in HFrEF. The reduced efficacy noted in contemporary HF DMP studies may not only be due to improvements in usual care but may reflect inclusion of heterogeneous patients with HFmrEF and HFpEF who may not respond in the same way as HFrEF to individual components. Based on current evidence, HF DMPs may improve mortality, hospitalization rates, self‐care, and quality of life in patients with HFpEF; however, further research specifically tailored to appropriately defined HFpEF is required.
Conflict of interest
None declared.
Funding
They acknowledge funding from the National Institute of Health Research School for Primary Care Research and European Society of Cardiology Nursing Training Grant.
Acknowledgements
This systematic review presents independent research funded by the National Institute for Health Research School for Primary Care Research (NIHR SPCR). The views expressed are those of the author(s) and not necessarily those of the NIHR, the NHS, or the Department of Health. The authors acknowledge funding received from the European Society of Cardiology in the form of European Society of Cardiology Nursing Training Grant 2017 NTG‐010.
Kalogirou, F. , Forsyth, F. , Kyriakou, M. , Mantle, R. , and Deaton, C. (2020) Heart failure disease management: a systematic review of effectiveness in heart failure with preserved ejection fraction. ESC Heart Failure, 7: 194–212. 10.1002/ehf2.12559.
References
- 1. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol, Vol 2014; 11: p 507–515. [DOI] [PubMed] [Google Scholar]
- 2. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2013; 10: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016; 134: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med 2017; 376: 897. [DOI] [PubMed] [Google Scholar]
- 5. Gallacher K, May CR, Montori VM, Mair FS. Understanding patients' experiences of treatment burden in chronic heart failure using normalization process theory. Ann Fam Med 2011; 9: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoekstra T, Lesman‐Leegte I, van Veldhuisen DJ, Sanderman R, Jaarsma T. Quality of life is impaired similarly in heart failure patients with preserved and reduced ejection fraction. Eur J Heart Fail 2011; 13: 1013–1018. [DOI] [PubMed] [Google Scholar]
- 7. Ziaeian B, Heidenreich PA, Xu H, DeVore AD, Matsouaka RA, Hernandez AF, Bhatt DL, Yancy CW, Fonarow GC. Race/ethnic differences in outcomes among hospitalized Medicare patients with heart failure and preserved ejection fraction. JACC Heart Fail 2017; 5: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 9. Krumholz HM, Currie PM, Riegel B, Phillips CO, Peterson ED, Smith R, Yancy CW, Faxon DP, American Heart Association Disease Management Taxonomy Writing Group . A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation 2006; 114: 1432–1445. [DOI] [PubMed] [Google Scholar]
- 10. Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis. JAMA 2004; 291: 1358–1367. [DOI] [PubMed] [Google Scholar]
- 11. McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810–819. [DOI] [PubMed] [Google Scholar]
- 12. Clark AM, Savard LA, Thompson DR. What is the strength of evidence for heart failure disease‐management programs? J Am Coll Cardiol 2009; 54: 397–401. [DOI] [PubMed] [Google Scholar]
- 13. Gandhi S, Mosleh W, Sharma UC, Demers C, Farkouh ME, Schwalm JD. Multidisciplinary heart failure clinics are associated with lower heart failure hospitalization and mortality: systematic review and meta‐analysis. Can J Cardiol 2017; 33: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 14. Dickstein K, Authors/Task Force Members , Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008; 10: 933–989. [DOI] [PubMed] [Google Scholar]
- 15. Riegel B, Lee CS, Sochalski J. Developing an instrument to measure heart failure disease management program intensity and complexity. Circ Cardiovasc Qual Outcomes 2010; 3: 324–330. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andryukhin A, Frolova E, Vaes B, Degryse J. The impact of a nurse‐led care programme on events and physical and psychosocial parameters in patients with heart failure with preserved ejection fraction: a randomized clinical trial in primary care in Russia. Eur J Gen Pract 2010; 16: 205–214. [DOI] [PubMed] [Google Scholar]
- 18. Bekelman DB, Plomondon ME, Carey EP, Sullivan MD, Nelson KM, Hattler B, McBryde CF, Lehmann KG, Gianola K, Heidenreich PA, Rumsfeld JS. Primary results of the patient‐centered disease management (PCDM) for heart failure study: a randomized clinical trial. JAMA Intern Med 2015; 175: 725–732. [DOI] [PubMed] [Google Scholar]
- 19. Bekelman DB, Allen LA, McBryde CF, Hattler B, Fairclough DL, Havranek EP, Turvey C, Meek PM. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA randomized clinical trial. JAMA Intern Med 2018; 178: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brotons C, Falces C, Alegre J, Ballarín E, Casanovas J, Catà T, Martínez M, Moral I, Ortiz J, Pérez E, Rayó E, Recio J, Roig E, Vidal X. Randomized clinical trial of the effectiveness of a home‐based intervention in patients with heart failure: the IC‐DOM study. Rev Esp Cardiol 2009; 62: 400–408. [DOI] [PubMed] [Google Scholar]
- 21. Chang YL, Chiou AF, Cheng SM, Lin KC. Tailored educational supportive care programme on sleep quality and psychological distress in patients with heart failure: a randomised controlled trial. Int J Nurs Stud 2016; 61: 219–229. [DOI] [PubMed] [Google Scholar]
- 22. Kalter‐Leibovici O, Freimark D, Freedman LS, Kaufman G, Ziv A, Murad H, Benderly M, Silverman BG, Friedman N, Cukierman‐Yaffe T, Asher E. Disease management in the treatment of patients with chronic heart failure who have universal access to health care: a randomized controlled trial. BMC Med 2017; 15: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Young L, Hertzog M, Barnason S. Effects of a home‐based activation intervention on self‐management adherence and readmission in rural heart failure patients: the PATCH randomized controlled trial. BMC Cardiovasc Disord 2016; 16: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. González‐Guerrero JL, Alonso‐Fernández T, García‐Mayolín N, Gusi N, Ribera‐Casado JM. Effect of a follow‐up program in elderly adults with heart failure with cognitive impairment after hospital discharge. J Am Geriatr Soc 2015; 63: 1950–1951. [DOI] [PubMed] [Google Scholar]
- 25. Masterson Creber R, Patey M, Lee CS, Kuan A, Jurgens C, Riegel B. Motivational interviewing to improve self‐care for patients with chronic heart failure: MITI‐HF randomized controlled trial. Patient Educ Couns 2016; 99: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Srisuk N, Cameron J, Ski CF, Thompson DR. Randomized controlled trial of family‐based education for patients with heart failure and their carers. J Adv Nurs 2017; 73: 857–870. [DOI] [PubMed] [Google Scholar]
- 27. Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive behavior therapy for depression and self‐care in heart failure patients: a randomized clinical trial. JAMA Intern Med 2015; 175: 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart S, Carrington MJ, Horowitz JD, Marwick TH, Newton PJ, Davidson PM, Macdonald P, Thompson DR, Chan YK, Krum H, Reid C, Scuffham PA. Prolonged impact of home versus clinic‐based management of chronic heart failure: extended follow‐up of a pragmatic, multicentre randomized trial cohort. Int J Cardiol 2014; 174: 600–610. [DOI] [PubMed] [Google Scholar]
- 29. Leventhal ME, Denhaerynck K, Brunner‐La Rocca HP, Burnand B, Conca‐Zeller A, Bernasconi AT, Mahrer‐Imhof R, Sivarajan Froelicher E, De Geest S. Swiss Interdisciplinary Management Programme for Heart Failure (SWIM‐HF): a randomised controlled trial study of an outpatient inter‐professional management programme for heart failure patients in Switzerland. Swiss Med Wkly 2011; 141: w13171. [DOI] [PubMed] [Google Scholar]
- 30. Dracup K, Moser DK, Pelter MM, Nesbitt TS, Southard J, Paul SM, Robinson S, Cooper LS. Randomized, controlled trial to improve self‐care in patients with heart failure living in rural areas. Circulation 2014; 130: 256–264. [DOI] [PubMed] [Google Scholar]
- 31. Kwok T, Lee J, Woo J, Lee DT, Griffith S. A randomized controlled trial of a community nurse‐supported hospital discharge programme in older patients with chronic heart failure. J Clin Nurs 2008; 17: 109–117. [DOI] [PubMed] [Google Scholar]
- 32. Jaarsma T, van der Wal M, Lesman‐Leegte I, Luttik ML, Hogenhuis J, Veeger NJ, Sanderman R, Hoes AW, van Gilst W, Lok DJ, Dunselman PH, Tijssen JG, Hillege HL, van Veldhuisen D, Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Investigators . Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med 2008; 168: 316–324. [DOI] [PubMed] [Google Scholar]
- 33. Shao JH, Chang AM, Edwards H, Shyu YI, Chen SH. A randomized controlled trial of self‐management programme improves health‐related outcomes of older people with heart failure. J Adv Nurs 2013; 69: 2458–2469. [DOI] [PubMed] [Google Scholar]
- 34. Tsuchihashi‐Makaya M, Matsuo H, Kakinoki S, Takechi S, Kinugawa S, Tsutsui H, for the J‐HOMECARE Investigators . Home‐based disease management program to improve psychological status in patients with heart failure in Japan. Circ J 2013; 77: 926–933. [DOI] [PubMed] [Google Scholar]
- 35. González‐Guerrero JL, Alonso‐Fernández T, García‐Mayolín N, Gusi N, Ribera‐Casado JM. Effectiveness of a follow‐up program for elderly heart failure patients after hospital discharge. A randomized controlled trial. Eur Geriatr Med 2014; 5: 252–257. [DOI] [PubMed] [Google Scholar]
- 36. Jaarsma T, Strömberg A, Mårtensson J, Dracup K. Development and testing of the European Heart Failure Self‐Care Behaviour Scale. Eur J Heart Fail 2003; 5: 363–370. [DOI] [PubMed] [Google Scholar]
- 37. Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J 1992; 124: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 38. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 39. Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, Fiuzat M, Zannad F, Pitt B, O'Connor CM, Lam CSP. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol 2015; 65: 1668–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolsk E, Claggett B, Køber L, Pocock S, Yusuf S, Swedberg K, McMurray JJV, Granger CB, Pfeffer MA, Solomon SD. Contribution of cardiac and extra‐cardiac disease burden to risk of cardiovascular outcomes varies by ejection fraction in heart failure. Eur J Heart Fail 2018; 20: 504–510. [DOI] [PubMed] [Google Scholar]
- 41. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 42. Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA 2008; 300: 431–433. [DOI] [PubMed] [Google Scholar]
- 43. Stewart S, Riegel B, Boyd C, Ahamed Y, Thompson DR, Burrell LM, Carrington MJ, Coats A, Granger BB, Hides J, Weintraub WS, Moser DK, Dickson VV, McDermott CJ, Keates AK, Rich MW. Establishing a pragmatic framework to optimise health outcomes in heart failure and multimorbidity (ARISE‐HF): a multidisciplinary position statement. Int J Cardiol 2016; 212: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol 2015; 12: 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jonkman NH, Westland H, Groenwold RHH, Ågren S, Atienza F, Blue L, Bruggink‐André de la Porte PWF, DeWalt DA, Hebert PL, Heisler M, Jaarsma T, Kempen GIJM, Leventhal ME, Lok DJA, Mårtensson J, Muñiz J, Otsu H, Peters‐Klimm F, Rich MW, Riegel B, Strömberg A, Tsuyuki RT, van Veldhuisen DJ, Trappenburg JCA, Schuurmans MJ, Hoes AW. Do self‐management interventions work in patients with heart failure? An individual patient data meta‐analysis. Circulation 2016; 133: 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clark AM, Wiens KS, Banner D, Kryworuchko J, Thirsk L, McLean L, Currie K. A systematic review of the main mechanisms of heart failure disease management interventions. Heart 2016; 102: 707–711. [DOI] [PubMed] [Google Scholar]
- 47. Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD. Exercise training in patients with heart failure and preserved ejection fraction: meta‐analysis of randomized control trials. Circ Heart Fail 2015; 8: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016; 315: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Störk S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM‐HF2): a randomised, controlled, parallel‐group, unmasked trial. Lancet 2018; 392: 1047–1057. [DOI] [PubMed] [Google Scholar]


