Abstract
Background
Serum ferritin (SF) test has been widely used in clinical practice. However, its reference intervals (RIs) vary depending on the analytical method and ethnic origin. This study was to establish the RIs using indirect method for SF in Chinese adults.
Methods
SF was assayed on Abbott i2000SR analyzer. The SF test results of all health examinees (8913 males aged 18‐93 years and 5397 females aged 18‐90 years) between December 2010 and April 2019 were obtained from our laboratory information system. After Box‐Cox transformation of raw data and exclusion of outliers, parametric and non‐parametric approaches were used to calculate 95% RIs. The correlation between SF levels and ages, and the differences in SF levels between subgroups were also analyzed.
Results
SF levels in females were significantly different from those in males (Z = 88.96, Z* = 23.17; Z > Z*) and showed a weak positive correlation with age (r = .466, P < .0001). The RIs based on parametric approach in males were 66.12‐561.58 µg/L, whereas in all females were 3.59‐269.59 µg/L, females aged <50 years 3.26‐148.02 µg/L and those aged ≥50 years 17.28‐303.27 µg/L. The RIs based on non‐parametric approach in males were 65.00‐571.37 µg/L whereas in all females were 4.00‐254.00 µg/L, females aged <50 years 4.00‐152.00 µg/L and those aged ≥50 years 16.00‐304.05 µg/L.
Conclusions
Our indirect RIs for SF were markedly different from the manufacturer's recommended RIs and might be more suitable for Chinese adults, which would be helpful in interpreting laboratory data and clinical decision‐making.
Keywords: adults, Chinese, indirect method, reference intervals, serum ferritin
1. INTRODUCTION
Ferritin, a soluble 450 kDa protein, is a major iron storage protein presenting in the intracellular compartments.1, 2, 3 As the most abundant transition metal element in the body, iron is widely involved in various physiological processes such as oxygen transport, electron transport, cell cycle regulation, energy metabolism, and DNA synthesis.4, 5 Ferritin provides intracellular storage of bio‐available iron in a safe and readily accessible form and protects cells from iron‐mediated free radical formation and toxicity1, 3. Normally, a small amount of ferritin exists in the peripheral circulating blood and is called serum ferritin (SF). Under normal circumstances, the amount of ferritin synthesized and secreted into the serum is proportional to the amount of cellular ferritin produced in the internal iron storage pathway, so that SF concentration is usually related to the quantity of body iron stores. Clinically, SF has been reported to link with iron‐deficiency anemia or a risk of iron overload.6, 7 For these reasons, SF is widely used as a surrogate marker of iron stores. In addition to this, SF has been found to be elevated in patient with inflammation, liver disease, and malignancy.8, 9, 10, 11 Moreover, previous studies have suggested that high SF is associated with poor prognosis in pancreatic cancer, colorectal cancer, lung cancer, peripheral T‐cell lymphoma, and hepatocellular carcinoma.12, 13, 14, 15, 16 Therefore, ferritin may not only function as a marker of iron stores, but may also aid in clinical decision‐making, including diagnosis, prognosis, treatment, and/or patient management. As a result, the number of tests for ferritin increase rapidly year by year.17
As the foundation for the interpretation of medical laboratory data, reference intervals (RIs) for analytes are one of the most widely used clinical decision‐making tool.18 Nevertheless, although measurement of SF has largely replaced laboratory assays of serum iron and transferrin or total iron‐binding capacity in clinical practice, there is no gold‐standard method for measuring SF. At present, SF can be measured using immunoassays, for example, enzyme‐linked immunosorbent assay (ELISA), electrochemiluminescent immunoassay (ECLIA), chemiluminescent immunoassay (CLIA), or immunoturbidometric assay.3, 19 Because of diversity and heterogeneity of ferritin antigens, and issues related to the reagent design in different assay methods, the RIs or normal ranges for SF in individuals vary depending on the assay method used.19, 20, 21 This makes it difficult for physicians to interpret the measurement results. In addition, studies have addressed there are significant ethnic‐specific differences in SF levels.3, 22 Hence, it is necessary to establish their own RIs for use in clinical laboratories with their population and analytical methods.18
According to international recommendations by the International Federation of Clinical Chemistry (IFCC) and the Clinical and Laboratory Standards Institute (CLSI),23 the so‐called “direct method,” in which healthy individuals representing the reference population are selected and sampled and the specimens from reference population are analyzed, is preferred for establishing RIs. In the “direct” method, a major problem that the laboratory faces is to obtain a sufficient number of specimens from healthy individuals representative of the reference population that the laboratory serves.24 Thus, as an alternative method, the “indirect” method via large collections of laboratory data stored in laboratory information systems (LIS) and statistical program is widely used to establish the RIs in recent years.24, 25, 26, 27, 28, 29, 30 Relative to the direct method, the “indirect” method is much simpler, faster, and cheaper25 and therefore is a convenient way for the clinical laboratory to set up their own RIs. In previous studies, however, most of which focused on the data obtained from hospital patient records and a group of “healthy” subjects was selected as the reference population from these hospital patients based on some statistical criteria,24, 27, 28, 29, 30 very few of which determined the RIs for analytes using the data from hospital database of health examination records. Although these data may not represent the general population, subjects were those who visited the health examination center for regular check‐ups, but not for treatment of a confirmed disease, which may lower the selection bias compared with previous hospital‐based studies. Therefore, the objective of the present study was to establish the RIs for SF, which is assayed by chemiluminescent microparticle immunoassay (CMIA) on the Abbott Architect i2000SR analyzer, by an indirect method using the data from our LIS records of health examinees.
2. MATERIALS AND METHODS
2.1. Data source
All raw data were obtained from the database of the LIS of Shaoxing Hospital of China Medical University (Shaoxing, Zhejiang, China PR). Except for pregnant women, all subjects aged ≥18 years who had undergone SF testing during their visiting the health examination center for routine check‐up between December 2010 and April 2019 would be considered eligible for this study. Data were excluded from the study without any statistical analysis if the SF levels >2000 or <1 µg/L because these values exceeding the detection limits of the assay methods. Data with missing demographic information were also removed. In addition, undesirable duplicate test results were removed by data cleaning technology, only the first result for each subject (a unique code, eg, medical record number, is preferred to use recognizable identifiers) was retained for further analysis.
This study was approved by the institutional ethics review board of the Shaoxing Hospital of China Medical University (Shaoxing, Zhejiang, China PR, ethical approval number: 20190429) and was in accordance with the Helsinki Declaration of 1975 (revision 1983).
2.2. SF measurement and quality control
Venous blood samples were obtained from each study participant after a 12‐hours fasting period and collected into BD vacutainer tubes (Becton Dickinson). Serum was separated from the blood by centrifugation at 3000 rpm for 5 min at room temperature (18‐25°C). Specimens with hemolysis, icterus, and lipemia were excluded according to the rules of the laboratory. All specimens were quantitatively determined within 2 hours of collection by CMIA on Abbott Architect i2000SR analyzer (Abbot Diagnostics) according to the manufacturer's specifications and following the laboratory standard operating procedures. The detection kits and calibrators were provided by Abbott Diagnostics. To ensure the accuracy and reliability of the analysis, assays were traceable to the 3rd international recombinant standard for ferritin (National Institute for Biological Standards and Control Code 94/572) and subject to participate in internal quality control (IQC) at different levels every day and inter‐laboratory external proficiency testing (PT) schemes from local external quality assessment (EQA) provider (Zhejiang, China) every year. Throughout this study period, the total analytical coefficient of variation (CV) was <10% and the PT/EQA score was more than 80% for SF, which met the quality assurance requirements as described in the Clinical and Laboratory Standards Institute's (CLSI) EP15‐A3 guidance document.31
2.3. Establishment of RIs for SF
All data were analyzed using EXCEL and MedCalc Statistical Software version 15.2.2 (MedCalc Software; http://www.medcalc.org; 2015). The establishment of RIs for SF in the present study would involve outlier removal, either before or after transformation of data, followed by calculation of the mean and standard deviation (SD) or median and relevant percentiles. Briefly, the steps are as follows:
2.3.1. Evaluation of test results’ distribution and transformation of data
Skewness‐Kurtosis test was used to evaluate whether the given test results are distributed symmetrically or normally or whether the distribution is asymmetrical or skewed.32 When the data were significantly skewed, Box‐Cox would be used to transform the data to approximately normal or symmetrical distribution. The Box‐Cox transformation is defined as:
where λ is an undetermined parameter, which is obtained by the maximum likelihood method.
2.3.2. Deletion of outliers
In this study, Tukey methods were used to check for multiple outliers at either side. In this method, an outlier value is defined as a value that is smaller than the lower quartile minus 1.5 times the interquartile range (IQR, the range from the 25th to the 75th percentile), or larger than the upper quartile plus 1.5 times the IQR. The calculation formulas are below:
With Tukey's criterion, an outlier will be eliminated and this process is repeated on the remaining data until all outliers are eliminated. When the data are significantly skewed, the outliers are removed after Box‐Cox transformation. Following the outlier removal, the remaining data are re‐transformed and the outliers are removed again (iteration) until no outlier after the final data transformation. Then, the data were back‐transformed to the original values for presentation.
2.3.3. Determination of RIs
Parametric or non‐parametric percentile statistical approach as described in the CLSI guidelines EP‐A3 guidelines can be used to calculate RIs in indirect method.21, 24, 25, 33 For parametric approach, the 95% RIs (double‐sided) was defined mean ± 1.96 SD; for non‐parametric approach, the values of the 2.5th and 97.5th percentile were used as lower and upper limits of RIs following the guideline EP28‐A3,23 respectively. Meanwhile, the 90% confidence intervals (CI) for lower and upper limits were also calculated. The RIs were also established for the various age‐ and sex‐specific subgroups when there are significant differences between the subgroups. Rank correlation was used to analyze the correlation between the SF levels and age. The standard normal deviation test (Z test) was performed to reveal the significance of differences in SF levels between the subgroups,23, 34 and the calculated Z > critical Z (Z*) was considered to be different from each other between subgroups.
3. RESULTS
A total of 15 949 raw data for males and 8141 for females were obtained from our LIS database, respectively. Of which, 7036 data for males (44.12%) and 2744 for females (33.70%) were excluded either because of the SF levels exceeding the detection limit of the methodology or because of missing information or repeated results. Finally, 8913 males aged 18‐93 years and 5397 females aged 18‐90 years were included in this study for further analysis.
The statistical significance of the difference between SF in males and in females was tested by the Z test, and significant difference was found between males and females (Z = 88.96, Z* = 23.17; Z > Z*). Thus, 95% RIs and their 90% CIs were established separately for males and females. Rank correlation analysis showed SF levels had a weak positive correlation with age in females (Spearman's coefficient of rank correlation (ρ) = 0.466, P < .0001) but no significant correlation between SF and age was found in males (Spearman's coefficient of rank correlation (ρ) = 0.0193, P = .0682) as shown in Figure 1. Z test revealed there was a significant difference in SF levels between females aged <50 years and those aged ≥50 years (Z = 34.71, Z* = 14.22; Z > Z*). So, we established the age‐specific RIs and their CIs for females finally.
Figure 1.
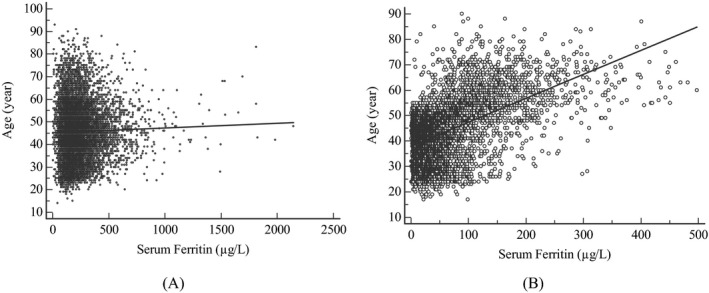
Correlation between age and serum ferritin levels. A, Males; B, Females
In this study, the SF in either males or females or their subgroups showed a significant skew distribution when Skewness‐Kurtosis test was used. After Box‐Cox was used to transform the data, all of them were approximately normal or symmetrical. The SF level distributions of sex‐ and age‐specific subgroups are detailed in Table 1, and the representative histograms are shown in Figures 2 and 3. After at least 2 iterations, the outliers in each group or subgroups were removed completely. The number of iterations and outlets in each group or subgroups is presented in Table 2. Then, we established 95% RIs for males and females and their age‐specific subgroups based on parametric and non‐parametric approach (Table 3). Compared with the manufacturer's recommended RIs, the RIs established for all females showed a slight difference; however, the RIs established for the other subgroups, whether based on parametric approach or based on non‐parametric approach, were markedly different (Table 3).
Table 1.
Skewness‐Kurtosis test before or after Box‐Cox transformation in various subgroups
| n | Before transformation | After transformation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CS | P value | CK | P value | CS | P value | CK | P value | ||
| Outliers undeleted | |||||||||
| Males | 8913 | 2.7256 | <.0001 | 15.7791 | <.0001 | 0.03184 | .2195 | 1.1574 | <.0001 |
| All females | 5397 | 2.6969 | <.0001 | 12.4985 | <.0001 | 0.009027 | .7864 | −0.389 | <.0001 |
| Females aged <50 y | 3758 | 2.4131 | <.0001 | 8.9486 | <.0001 | −0.01413 | .7232 | −0.2771 | .0001 |
| Females aged ≥50 y | 1639 | 1.9839 | <.0001 | 7.3882 | <.0001 | 0.01375 | .8196 | 0.5268 | .0004 |
| Outliers deleted | |||||||||
| Males | 8704 | 1.2173 | <.0001 | 1.6184 | <.0001 | 0.000449 | .9863 | −0.2635 | <.0001 |
| All females | 5385 | 2.0562 | <.0001 | 5.527 | <.0001 | −0.02546 | .4451 | −0.4779 | <.0001 |
| Females aged <50 y | 3746 | 1.9337 | <.0001 | 4.9303 | <.0001 | −0.02589 | .5171 | −0.3855 | <.0001 |
| Females aged ≥50 y | 1558 | 0.898 | <.0001 | 0.629 | .0001 | −0.04441 | .4728 | −0.309 | .0036 |
Abbreviations: CK, Coefficient of Kurtosis; CS, Coefficient of Skewness.
Figure 2.
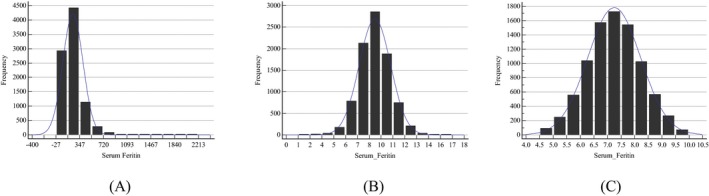
Frequency distribution graphs of serum ferritin (SF) for males. A, Data prior to transformation. B, Data after Box‐Cox transformation. C, Data excluding outliers after Box‐Cox transformation
Figure 3.
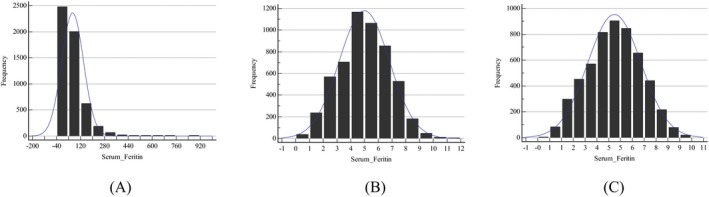
Frequency distribution graphs of serum ferritin (SF) for all females. A, Data prior to transformation. B, Data after Box‐Cox transformation. C, Data excluding outliers after Box‐Cox transformation
Table 2.
Number of iterations and outliers in different subgroups
| Subgroups | Sample size | Number of iterations | Number of outliers |
|---|---|---|---|
| Males | 8913 | 3 | 209 |
| All females | 5397 | 2 | 12 |
| Females aged <50 y | 3758 | 2 | 12 |
| Females aged ≥50 y | 1639 | 2 | 81 |
Table 3.
95% Reference intervals of serum ferritin for males and females
| Established RIs based on parametric method | Established RIs based on non‐parametric method | Recommended Rls (µg/L) | |||||
|---|---|---|---|---|---|---|---|
| RIs (µg/L) | 90% CI for LL | 90% CI for UL | RIs (µg/L) | 90% CI for LL | 90% CI for UL | ||
| Males | 66.12‐561.58 | 64.87‐67.39 | 553.38‐569.89 | 65.00‐571.37 | 63.00‐67.00 | 561.00‐582.00 | 21.18‐274.66 |
| All females | 3.59‐269.59 | 3.38‐3.81 | 261.22‐278.19 | 4.00‐254.00 | 4.00‐4.00 | 244.13‐268.00 | 4.63‐204 |
| Females aged <50 y | 3.26‐148.02 | 3.05‐3.48 | 143.24‐152.94 | 4.00‐152.00 | 3.00‐4.00 | 143.00‐156.00 | |
| Females aged ≥50 y | 17.28‐303.27 | 15.60‐19.07 | 293.94‐312.77 | 16.00‐304.05 | 14.00‐17.00 | 291.00‐320.00 | |
Abbreviations: LL, lower limits; RIs, Reference intervals; UL, upper limits.
4. DISCUSSION
In this study, we established the RIs for SF in Chinese adults using the data obtained from our LIS and found that the RIs for SF in Chinese adults were markedly different from the manufacturer's recommended RIs.
Reliable and accurate RIs for laboratory analyses are an integral part of the process of correct interpretation of clinical laboratory test results. RIs given in laboratory reports have an important role in assisting the clinical decision‐making, including diagnosis, prognosis, treatment, and/or patient management.18 However, to produce high‐quality RIs according to the recommendations of IFCC for all relevant analytes is far beyond the capacity of a single laboratory. Therefore, many laboratories adopt RIs from other laboratories or use manufacturer's recommended RIs directly. Nevertheless, the RIs for most analytes are to some degree method‐ and instrument‐dependent.19, 20 In addition, differences between populations are also an issue and can reduce the validity of RIs.3, 35 For example, it was reported that one method can give 1.63 times higher values than the other method for SF19; mean SF values are found to be higher at all ages in adult black males than in adult white males.35 For these reasons, scientists have investigated the possibility via large collections of laboratory data for the goal of determining population RIs by an indirect method. So far, various indirect methods for establishing RIs have been developed.24, 27, 28, 29, 30 These methods may be used for the selection of a group of healthy subjects from a general hospital population, and RIs are calculated from hospital data using statistical approaches. In the present study, we attempted to establish the RIs for SF in Chinese adults using the data available from our LIS records. Unlike the data in most of the previous studies, the data in the present study were obtained from the subjects during their visit for the health examination center. The very low probability of disease makes these subjects obviously more desirable as the reference population than hospital patients since exclusion of extreme results, which are those most likely to be affected by disease, is a vital issue in establishing RIs.24, 27, 28, 29, 30
The present study demonstrated the SF levels had a significant difference between females and males, and had a positive correlation with age in females. Nevertheless, the manufacturer simply recommends 21.18‐274.66 µg/L as the RIs for adult males and 4.63‐204 µg/L for adult females but does not recommend the age‐specific RIs. To gain insight into the effect of age on SF levels, we further tested whether there was a significant difference between various age‐specific subgroups by Z test and found that the SF levels in females aged ≥50 years were significantly higher than those in those aged <50 years. These findings are in good agreement with those by Ogilvie et al36 and Adams et al.37 Indeed, SF values in adult females start to rise after 50 years of age as a result of iron loss from menstruation and pregnancies.3 In view of this, it is necessary to establish the sex‐ and age‐specific RIs for SF in our own laboratory.
In the indirect method, RIs can be calculated using various statistical approaches.25 Of these statistical approaches, the standard parametric or non‐parametric percentile approach is usually used in the direct method for RIs as described in the CLSI EP‐A3 guidelines.23 Jones et al25 suggested that the standard parametric or non‐parametric statistical approach can be used in the indirect method for RIs when the data were collected at a community screening project or similar. In fact, the parametric approach has been used in the analysis of data from NHANES,21 and the non‐parametric approach has been used in a Chinese and a Turkish study, respectively.24, 33 Of course, when the standard parametric approach is used, this will involve data transformation if the data are non‐normal or asymmetric distribution. In this study, our data showed a significant skew distribution but were symmetrical or approximately normal distribution after transformation. Therefore, we established the RIs for SF based on parametric and non‐parametric approach, respectively, and found that there was no significant difference between these two approaches for establishing RIs. Interestingly, our study demonstrated both the lower and the upper limits of RIs in Chinese adult males were markedly higher than those of manufacturer's recommended Rls. In Chinese females, those aged over 50 years also had elevated lower and upper limits of RIs for SF. Even in whole Chinese adult females, the upper limits of RIs for SF are also elevated. Similar findings were observed in the Canadian Health Measures Survey by Khosrow et al.38 As mentioned above, these findings might be related to considerable variation in SF levels in ethnic origin.3, 22, 35 In fact, in multi‐ethnic population studies in the USA, Adams et al39 had proved that elevated SF levels are more frequent in Afro‐Caribbean and Asian subjects than in whites or Hispanics. In a previous Chinese study on Cobas® 6000 system E601 (Roche) analyzer, Li et al40 also found that Chinese adult males had higher RIs for SF. However, compared with that study,40 our study had slightly but significantly lower RIs for SF in Chinese adult males. This may be related to the different assay methods used, as reported in previous studies.19, 20, 21 Taken together, we believe that the sex‐ and age‐specific RIs for SF established in this study are appropriate.
In conclusion, the present study has provided evidence that the RIs for SF in Chinese adults are markedly different from the manufacturer's recommended Rls. In Chinese adult females, the age‐specific RIs must be also taken into account. As a result, the establishment of the sex‐ and age‐specific RIs by indirect method using the data from health examination records for SF in our own laboratory would give a better chance to aid the physician in differentiating or considering treatment of iron‐deficiency anemia or a risk of iron overload than using manufacturer's recommended RIs or adopting RIs from other laboratories.
ACKNOWLEDGMENT
We thank Li‐ming Mao and Hui Xie for critical reading of the study and numerous valuable comments.
Wang Q‐P, Guo L‐Y, Lu Z‐Y, Gu J‐W. Reference intervals established using indirect method for serum ferritin assayed on Abbott Architect i2000SR analyzer in Chinese adults. J Clin Lab Anal. 2020;34:e23083 10.1002/jcla.23083
Contributor Information
Lin‐ying Guo, Email: 272969068@qq.com.
Jian‐wen Gu, Email: ylnyln2003@163.com.
REFERENCES
- 1. Arosio P, Elia L, Poli M. Ferritin, cellular iron storage and regulation. IUBMB Life. 2017;69(6):414‐422. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Geneva, Switzerland: World Health Organization; 2011. https://apps.who.int/iris/handle/10665/85843. Accessed April 25, 2019. [Google Scholar]
- 3. Cullis JO, Fitzsimons EJ, Griffiths WJ, Tsochatzis E, Thomas DW. Haematology BSf. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181(3):331‐340. [DOI] [PubMed] [Google Scholar]
- 4. Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285‐297. [DOI] [PubMed] [Google Scholar]
- 5. Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci: Off J Isfahan Univ Med Sci. 2014;19(2):164‐174. [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia‐Casal MN, Pasricha SR, Martinez RX, Lopez‐Perez L, Peña‐Rosas JP. Serum or plasma ferritin concentration as an index of iron deficiency and overload. Cochrane Database Syst Rev. 2015;2015(7). Art. No.: CD011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daru J, Allotey J, Peña‐Rosas J, Khan K. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review. Transfusion Med. 2017;27(3):167‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(4):748‐773. [DOI] [PubMed] [Google Scholar]
- 9. Tran KT, Coleman HG, McCain RS, Cardwell CR. Serum biomarkers of iron status and risk of primary liver cancer: a systematic review and meta‐analysis. Nutr Cancer. 2019;71(8):1‐9. [DOI] [PubMed] [Google Scholar]
- 10. Shah RA, Kowdley KV. Serum ferritin as a biomarker for NAFLD: ready for prime time? Hep Intl. 2019;13(13):110‐112. [DOI] [PubMed] [Google Scholar]
- 11. Truffi M, Fiandra L, Sorrentino L, Monieri M, Corsi F, Mazzucchelli S. Ferritin nanocages: a biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol Res. 2016;107:57‐65. [DOI] [PubMed] [Google Scholar]
- 12. Song A, Eo W, Kim S, Shim B, Lee S. Significance of serum ferritin as a prognostic factor in advanced hepatobiliary cancer patients treated with Korean medicine: a retrospective cohort study. BMC Complement Altern Med. 2018;18(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alkhateeb AA, Connor JR. The significance of ferritin in cancer: anti‐oxidation, inflammation and tumorigenesis. Biochimica et Biophysica Acta (BBA) ‐ Rev Cancer. 2013;1836(2):245‐254. [DOI] [PubMed] [Google Scholar]
- 14. Feng Z, Chen J‐W, Feng J‐H, et al. The association between serum ferritin with colorectal cancer. Int J Clin Exp Med. 2015;8(12):22293. [PMC free article] [PubMed] [Google Scholar]
- 15. Kalousová M, Krechler T, Jáchymová M, Kuběna AA, Žák A, Zima T. Ferritin as an independent mortality predictor in patients with pancreas cancer. Results of a pilot study. Tumor Biol. 2012;33(5):1695‐1700. [DOI] [PubMed] [Google Scholar]
- 16. Yamazaki E, Tomita N, Koyama S, et al. Serum ferritin level is prognostic of patient outcome in extranodal NK/T cell lymphoma, nasal type. Med Oncol. 2014;31(9):149‐155. [DOI] [PubMed] [Google Scholar]
- 17. Health Quality Ontario . Ferritin testing: a rapid review. Toronto, Australia: Health Quality Ontario; 2012:16 http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/rapid-reviews. Accessed May 10, 2019. [Google Scholar]
- 18. International Organization for Standardization . Medical laboratories: requirements for quality and competence [ISO 15189:2012, 3rd ed.]. Geneva, Switzerland: International Organization for Standardization; 2012. http://www.iso.org/iso/catalogue_detail?csnumber=56115. Accessed April 28, 2016. [Google Scholar]
- 19. Kamei D, Mineshima M, Tsukada M, Miwa N, Hanafusa N, Tsuchiya K. Ferritin: diversity and management of ferritin measurement methods. Recent Adv Dialysis Ther Jpn. 2018;196:83‐87. [DOI] [PubMed] [Google Scholar]
- 20. Kamei D, Tsuchiya K, Miura H, Nitta K, Akiba T. Inter‐method variability of ferritin and transferrin saturation measurement methods in patients on hemodialysis. Ther Apher Dial. 2017;21(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 21. Lacher DA, Hughes JP, Carroll MD. Biological variation of laboratory analytes based on the 1999–2002 National Health and Nutrition Examination Survey. Natl Health Stat Rep. 2010;21:1‐7. [PubMed] [Google Scholar]
- 22. Tahmasebi H, Trajcevski K, Higgins V, Adeli K. Influence of ethnicity on population reference values for biochemical markers. Crit Rev Clin Lab Sci. 2018;55(5):359‐375. [DOI] [PubMed] [Google Scholar]
- 23. CLSI . Defining, establishing, and verifying reference intervals in the clinical laboratory. 3rd ed Wayne, PA: Clinical & Laboratory Standards Institute; 2010. [Google Scholar]
- 24. Inal TC, Serteser M, Coşkun A, Özpinar A, Ünsal I. Indirect reference intervals estimated from hospitalized population for thyrotropin and free thyroxine. Croatian Med J. 2010;51(2):124‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones GRD, Haeckel R, Loh TP, et al. Indirect methods for reference interval determination – review and recommendations. Clin Chem Lab Med (CCLM). 2018;57(1):20‐29. [DOI] [PubMed] [Google Scholar]
- 26. Ozarda Y. Reference intervals: current status, recent developments and future considerations. Biochem Med. 2016;26(1):5‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones G, Horowitz G, Katayev A, et al. Reference intervals data mining: getting the right paper. Am J Clin Pathol. 2015;144(3):526‐527. [DOI] [PubMed] [Google Scholar]
- 28. Ferré‐Masferrer M, Fuentes‐Arderiu X, Puchal‐Añé R. Indirect reference limits estimated from patients' results by three mathematical procedures. Clin Chim Acta. 1999;279(1–2):97‐105. [DOI] [PubMed] [Google Scholar]
- 29. Katayev A, Fleming JK, Luo D, Fisher AH, Sharp TM. Reference intervals data mining: no longer a probability paper method. Am J Clin Pathol. 2015;143(1):134‐142. [DOI] [PubMed] [Google Scholar]
- 30. Bhattarai K, Manandhar N, Shrestha P, et al. Estimation of indirect reference intervals for serum thyrotropin using hospital records. Asian J Med Sci. 2017;8(3):41‐48. [Google Scholar]
- 31. Wayne P.Clinical and Laboratory Standards Institute (CLSI): User verification of precision and estimation of bias; approved guideline ‐ 3rd ed CLSI document EP15‐A3, USA; 2014.
- 32. Cortina‐Borja M. Handbook of parametric and nonparametric statistical procedures, 5th ed. J R Stat Soc: Ser A (Stat Soc). 2012;175(3):829‐829. 10.1111/j.1467-985X.2012.01045_13.x. [DOI] [Google Scholar]
- 33. Wang Y, Yx Z, Yl Z, Xia J. Establishment of reference intervals for serum thyroid‐stimulating hormone, free and total thyroxine, and free and total triiodothyronine for the Beckman Coulter DxI‐800 analyzers by indirect method using data obtained from Chinese population in Zhejiang Province, China. J Clin Lab Anal. 2017;31(4):e22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Horn PS, Pesce AJ. Reference intervals: an update. Clin Chim Acta. 2003;334(1–2):5‐23. [DOI] [PubMed] [Google Scholar]
- 35. Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20(4):406‐416. [DOI] [PubMed] [Google Scholar]
- 36. Ogilvie C, Fitzsimons K, Fitzsimons EJ. Serum ferritin values in primary care: are high values overlooked? J Clin Pathol. 2010;63(12):1124‐1126. [DOI] [PubMed] [Google Scholar]
- 37. Adams PC, McLaren CE, Speechley M, McLaren GD, Barton JC, Eckfeldt JH. HFE mutations in Caucasian participants of the Hemochromatosis and Iron Overload Screening study with serum ferritin level <1000 μg/L. Can J Gastroenterol Hepatol. 2013;27(7):390‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adeli K, Higgins V, Nieuwesteeg M, et al. Complex reference values for endocrine and special chemistry biomarkers across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem. 2015;61(8):1063‐1074. [DOI] [PubMed] [Google Scholar]
- 39. Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and iron‐overload screening in a racially diverse population. N Engl J Med. 2005;352(17):1769‐1778. [DOI] [PubMed] [Google Scholar]
- 40. Li S, Lin L, Mo Z, et al. Reference values for serum ferritin in Chinese Han ethnic males: results from a Chinese male population survey. Clin Biochem. 2011;44(16):1325‐1328. [DOI] [PubMed] [Google Scholar]


