Abstract
Aims
The prognostic implication of left ventricular outflow tract velocity time integral (LVOT‐VTI) on admission in hospitalized heart failure with preserved ejection fraction (HFpEF) patients has not been determined. We sought to investigate whether LVOT‐VTI on admission is associated with worse clinical outcomes in hospitalized patients with HFpEF.
Methods and results
We studied consecutive 214 hospitalized HFpEF patients who had accessible LVOT‐VTI data on admission, from a prospective HFpEF‐specific multicentre registry. The primary outcome of interest was the composite of all‐cause death and readmission due to heart failure. During a median follow‐up period of 688 (interquartile range 162–810) days, the primary outcome occurred in 83 patients (39%). The optimal cut‐off value of LVOT‐VTI for the primary outcome estimated by receiver operating characteristic analysis was 15.8 cm. Lower LVOT‐VTI was significantly associated with the primary outcome compared with higher LVOT‐VTI (P = 0.005). Multivariable Cox regression analyses revealed that lower LVOT‐VTI was an independent determinant of the primary outcome (hazard ratio 0.94, 95% confidence interval 0.91–0.98). In multivariable linear regression, haemoglobin level was the strongest independent determinant of LVOT‐VTI among clinical parameters (β coefficient = −0.61, P = 0.007). Furthermore, patients with lower LVOT‐VTI and anaemia had the worst clinical outcomes among the groups (P < 0.001).
Conclusions
Lower admission LVOT‐VTI was an independent determinant of worse clinical outcomes in hospitalized HFpEF patients, indicating that LVOT‐VTI on admission might be useful for categorizing a low‐flow HFpEF phenotype and risk stratification in hospitalized HFpEF patients.
Keywords: Heart failure with preserved ejection fraction, Left ventricular outflow tract velocity time integral, Prognosis risk stratification
1. Introduction
Approximately half of heart failure (HF) patients have preserved left ventricular (LV) ejection fraction (LVEF), and this proportion has increased over time.1 There are few effective treatment strategies for HF with preserved ejection fraction (HFpEF) to reduce mortality,1, 2, 3 although advanced medication and devices have been proven to improve poor clinical outcomes in HF patients with reduced ejection fraction in randomized clinical trials. It has been emphasized that identifying HFpEF phenotypes is useful for selecting phenotype‐specific treatment to improve the clinical outcome, because the pathophysiology of HFpEF is very heterogeneous.4 Several clinical phenotypes of HFpEF, such as obesity, coronary artery disease, right‐side HF/pulmonary hypertension, atrial fibrillation, and hypertrophic cardiomyopathy‐like HFpEF, have been identified.5 Recently, a low‐flow HFpEF phenotype was identified, which was associated with severity of HF.6
The left ventricular outflow tract velocity time integral (LVOT‐VTI) is a representative and non‐invasive echocardiographic parameter for the evaluation of stroke volume. A previous report showed that lower LVOT‐VTI could predict mortality in patients with acute or chronic HF.7, 8 Nevertheless, the prognostic implication of LVOT‐VTI on admission in hospitalized patients with HFpEF in relation to long‐term outcomes is unclear.
Accordingly, the aim of this study was to investigate whether LVOT‐VTI on admission is associated with subsequent adverse events in hospitalized HFpEF patients.
2. Methods
2.1. Study design
Data from the Japanese Heart Failure Syndrome with Preserved Ejection Fraction (JASPER) registry, obtained between November 2012 and March 2015, were analysed. Details of the JASPER registry have been described previously.9 Briefly, the study is a multicentre, observational, prospective cohort that includes consecutive patients aged ≥20 years requiring hospitalization with a diagnosis of acute decompensated HF according to the Framingham criteria10 by at least two experienced cardiologists, with preserved LV systolic function defined as LVEF ≥50% by the modified Simpson method or LV fractional shortening ≥25% by echocardiography. Patients with acute coronary syndrome, receiving haemodialysis, or with a history of heart transplantation or severe valvular heart disease were excluded. This study was approved by the institutional review board of each site and registered under the Japanese UMIN Clinical Trials Registration (UMIN000010601).
2.2. Study population
From the 535 patients enrolled in the JASPER registry, those without LVOT‐VTI accessible data on admission were excluded. Ultimately, 214 patients were examined (Figure 1 ).
Figure 1.
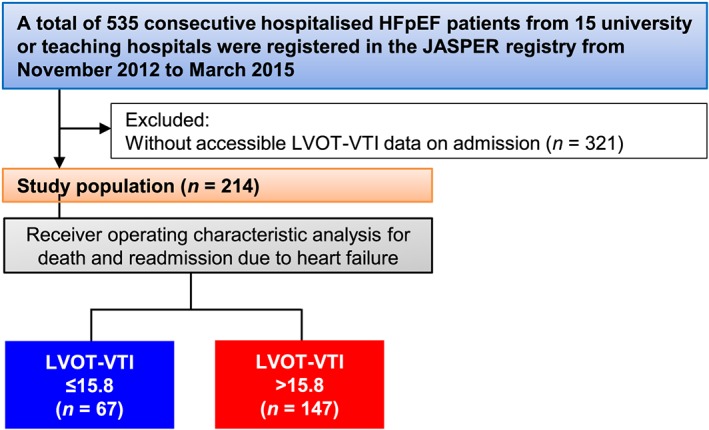
Flow diagram of the present study. HFpEF, heart failure with preserved ejection fraction; JASPER, Japanese Heart Failure Syndrome with Preserved Ejection Fraction; LVOT‐VTI, left ventricular outflow tract velocity time integral.
2.3. Echocardiography measurements
Echocardiographic examination was usually performed within 6 h of admission, and the results were digitally recorded. LVOT‐VTI was obtained by placing a pulsed‐wave Doppler sample volume in the LVOT immediately proximal to the aortic valve in the anteriorly angulated apical three‐chamber or four‐chamber view and tracing the outer boundaries of the peak spectral Doppler signal to obtain LVOT‐VTI. Proper location in the LVOT was confirmed by visualization of the aortic valve closure signal (Figure 2 ). For participants in atrial fibrillation, echocardiographic measurements were averaged over 3–5 beats at the time of examination. LVEF was calculated from apical two‐chamber and four‐chamber views using the biplane method of disks.11 LV end‐diastolic dimension, LV end‐systolic dimension, and left atrial dimension were measured. LV interventricular septum diameter and LV posterior wall diameter were measured at end diastole. LV inflow (E) and atrial‐systolic peak velocity (A) were measured using pulsed‐wave Doppler. Using the apical four‐chamber view, e′ was measured at the interventricular septum. Tricuspid regurgitation pressure gradient was estimated using Doppler echocardiography by calculating the right ventricular to right atrial pressure gradient during systole; the modified Bernoulli equation (∆P = 4 v2) was used to calculate gradients from the velocities.12
Figure 2.
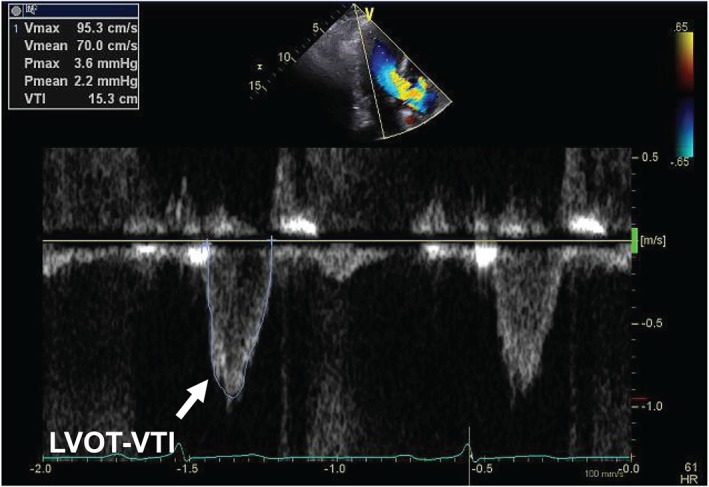
Representative echocardiographic findings of left ventricular outflow tract velocity time integral (LVOT‐VTI).
2.4. Clinical outcome
The primary outcome of interest was the composite of all‐cause death and readmission due to HF.
2.5. Statistical analyses
Continuous variables are presented as mean ± standard deviation when normally distributed and as median and interquartile range when non‐normally distributed. Comparisons of differences between two groups were made by unpaired t‐test or Mann–Whitney U test for continuous variables and by chi‐squared test or Fisher's exact test for dichotomous variables, when appropriate. We performed receiver operating characteristic curve analysis to evaluate the discriminatory value of LVOT‐VTI for all‐cause death and readmission due to HF. The optimal cut‐off value was chosen as the value maximizing sensitivity plus specificity. The cumulative incidence of the primary outcome was estimated by Kaplan–Meier analysis, and log‐rank test was performed to assess significance according to the LVOT‐VTI cut‐off value and the different groups of anaemia (anaemia was defined according to the World Health Organization criteria as a haemoglobin level <13.0 g/dL in men and <12.0 g/dL in women) and LVOT‐VTI. To evaluate the influence of LVOT‐VTI on the primary outcome, we constructed a multivariable Cox proportional hazard model. Adjustment for pre‐specified covariates derived from our previous study9 and renal function (Model 1) and adding haemoglobin level to Model 1 (Model 2) were performed. Multivariable linear regression analysis was performed based on the variables achieving P < 0.10 in univariable linear regression analysis, to explore the strongest independent determinants of LVOT‐VTI. All tests were two tailed, and a value of P < 0.05 was considered statistically significant. All analyses were performed with Stata MP64 version 15 (StataCorp, College Station, TX, USA).
3. Results
The baseline characteristics of the total 214 studied patients are shown in Table 1. We divided them into two groups according to the optimal cut‐off value of LVOT‐VTI (15.8 cm) based on receiver operating characteristic analysis. Patients with lower LVOT‐VTI (≤15.8 cm) had higher heart rate, prevalence of atrial fibrillation, and haemoglobin level. There were no significant differences between the two groups in terms of age, sex, body mass index, New York Heart Association functional class, prevalence of prior myocardial infarction and coronary artery disease, serum sodium, albumin, blood urea nitrogen, and plasma brain natriuretic peptide (BNP) levels, prevalence of use of medication before admission, and initial treatment. Patients with lower LVOT‐VTI had lower LVEF, LV end‐diastolic dimension, and tricuspid regurgitation pressure gradient and higher left atrial dimension, whereas there were no significant differences between the two groups regarding LV posterior wall diameter, LV interventricular septum diameter, LV mass index, E/A, E/e′, and inferior vena cava diameter.
Table 1.
Baseline characteristics on admission categorized by LVOT‐VTI
| Variable | Overall | LVOT‐VTI ≤15.8 | LVOT‐VTI >15.8 | P‐value |
|---|---|---|---|---|
| Number | 214 | 67 | 147 | |
| Age (years) | 78 ± 11 | 78 ± 11 | 79 ± 11 | 0.54 |
| Male | 100 (47) | 36 (54) | 64 (44) | 0.166 |
| Body mass index (kg/m2) | 24.0 ± 4.6 | 23.6 ± 4.7 | 24.1 ± 4.5 | 0.43 |
| NYHA functional class III or IV | 167 (83) | 54 (84) | 113 (82) | 0.189 |
| Heart rate (bpm) | 77 (61–97) | 96 (76–118) | 70 (58–85) | <0.001 |
| Systolic blood pressure (mmHg) | 151 ± 38 | 151 ± 39 | 153 ± 37 | 0.24 |
| Past history | ||||
| Prior heart failure admission | 82 (39) | 27 (41) | 55 (38) | 0.140 |
| Prior myocardial infarction | 27 (13) | 5 (8) | 22 (15) | 0.149 |
| Coronary artery disease | 64 (30) | 18 (28) | 46 (32) | 0.58 |
| Prior PCI | 38 (18) | 10 (15) | 28 (19) | 0.47 |
| Prior CABG | 16 (8) | 6 (9) | 10 (7) | 0.58 |
| Atrial fibrillation | 130 (62) | 54 (82) | 76 (53) | <0.001 |
| Diabetes mellitus | 78 (37) | 24 (36) | 54 (37) | 0.90 |
| Laboratory data | ||||
| Sodium (mEq/L) | 140 ± 4.3 | 139 ± 3.7 | 140 ± 4.5 | 0.31 |
| BUN (mg/dL) | 23 (17–35) | 24 (17–32) | 22 (17–37) | 0.98 |
| Haemoglobin (g/dL) | 11.1 ± 2.0 | 11.8 ± 2.0 | 10.7 ± 2.0 | <0.001 |
| Plasma BNP (pg/mL) | 400 (222–708) | 366 (183–715) | 422 (231–705) | 0.37 |
| Albumin (g/dL) | 3.6 ± 0.5 | 3.7 ± 0.5 | 3.6 ± 0.5 | 0.78 |
| Medications before admission | ||||
| ACE‐Is or ARBs | 122 (57) | 34 (51) | 88 (60) | 0.21 |
| Beta‐blockers | 87 (41) | 29 (43) | 58 (39) | 0.60 |
| Loop diuretics | 125 (58) | 40 (60) | 85 (58) | 0.80 |
| MRAs | 53 (25) | 18 (27) | 35 (24) | 0.63 |
| Initial treatment | ||||
| Intravenous diuretics | 166 (78) | 51 (76) | 115 (78) | 0.73 |
| Vasodilators | 132 (62) | 43 (64) | 89 (61) | 0.61 |
| Echocardiographic parameter | ||||
| LVEF (%) | 60 ± 8.1 | 57 ± 7.6 | 61 ± 7.9 | <0.001 |
| LAD (mm) | 45 ± 8.1 | 47 ± 8.4 | 45 ± 7.9 | 0.029 |
| LVDD (mm) | 47 ± 6.5 | 45 ± 6.7 | 48 ± 6.2 | 0.003 |
| LVPWD (mm) | 10 ± 2.1 | 11 ± 2.4 | 10 ± 2.0 | 0.30 |
| LVIVSD (mm) | 11 ± 2.5 | 10 ± 2.3 | 11 ± 2.6 | 0.77 |
| LVMI (g/m2) | 159 ± 49 | 141 ± 42 | 163 ± 52 | 0.064 |
| E/A | 1.2 (0.8–1.7) | 1.4 (0.7–2.7) | 1.1 (0.8–1.6) | 0.41 |
| E/e′ (cm/s) | 17 (13–22) | 15 (13–19) | 18 (13–22) | 0.166 |
| TRPG (mmHg) | 37 ± 12 | 33 ± 12 | 39 ± 11 | 0.002 |
| IVCD (mm) | 20 ± 6.6 | 21 ± 6.3 | 20 ± 6.7 | 0.29 |
Continuous variables are presented as mean ± standard deviation if normally distributed and median (interquartile range) if not normally distributed. Categorical variables are presented as number of patients (%). ACE‐Is, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; IVCD, inferior vena cava diameter; LAD, left atrial dimension; LVDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVIVSD, left ventricular interventricular septum diameter; LVMI, left ventricular mass index; LVOT‐VTI, left ventricular outflow tract velocity time integral; LVPWD, left ventricular posterior wall diameter; MRAs, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TRPG, tricuspid regurgitation pressure gradient.
The results of univariable and multivariable linear regression analyses to determine LVOT‐VTI are presented in Table 2. Multivariable linear regression analysis revealed that LVEF, LV mass index, heart rate, and haemoglobin level were independent determinants of LVOT‐VTI. Among these, haemoglobin level was the strongest determinant.
Table 2.
Linear regression analyses of admission left ventricular outflow tract velocity time integral
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| β coefficient | P‐value | β coefficient | P‐value | |
| Age | 0.20 | 0.62 | Not selected | |
| Body mass index (kg/m2) | 0.16 | 0.091 | 0.10 | 0.28 |
| LVEF (%) | 0.22 | <0.001 | 0.19 | <0.001 |
| LVDD (mm) | 0.16 | 0.023 | −0.05 | 0.48 |
| LAD (mm) | −0.53 | 0.36 | Not selected | |
| LVMI (g/m2) | 0.03 | 0.001 | 0.04 | <0.001 |
| TRPG (mmHg) | 0.11 | 0.008 | 0.02 | 0.63 |
| Systolic blood pressure (mmHg) | 0.01 | 0.21 | Not selected | |
| Heart rate (bpm) | −0.10 | <0.001 | −0.08 | <0.001 |
| BUN (mg/dL) | 0.02 | 0.30 | Not selected | |
| Sodium (mEq/L) | 0.05 | 0.63 | Not selected | |
| Haemoglobin (g/dL) | −0.85 | <0.001 | −0.61 | 0.007 |
| Log BNP (pg/mL) | 0.23 | 0.63 | Not selected | |
BNP, brain natriuretic peptide; BUN, blood urea nitrogen; LAD, left atrial dimension; LVDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; TRPG, tricuspid regurgitation pressure gradient.
During a median follow‐up period of 688 (interquartile range 162–810) days, adverse events occurred in 83 patients (39%), including 47 (22%) all‐cause deaths and 51 (24%) readmissions due to HF. Lower LVOT‐VTI was significantly associated with a higher incidence of adverse events compared with higher LVOT‐VTI (Figure 3 ). In addition, patients with lower LVOT‐VTI and anaemia had the worst clinical outcomes among the groups (Figure 4 ). Multivariable Cox regression analyses revealed that lower LVOT‐VTI was an independent determinant of adverse events even after adjustment for pre‐specified confounders and renal function (Model 1) and addition of haemoglobin level to Model 1 (Model 2) (Table 3).
Figure 3.
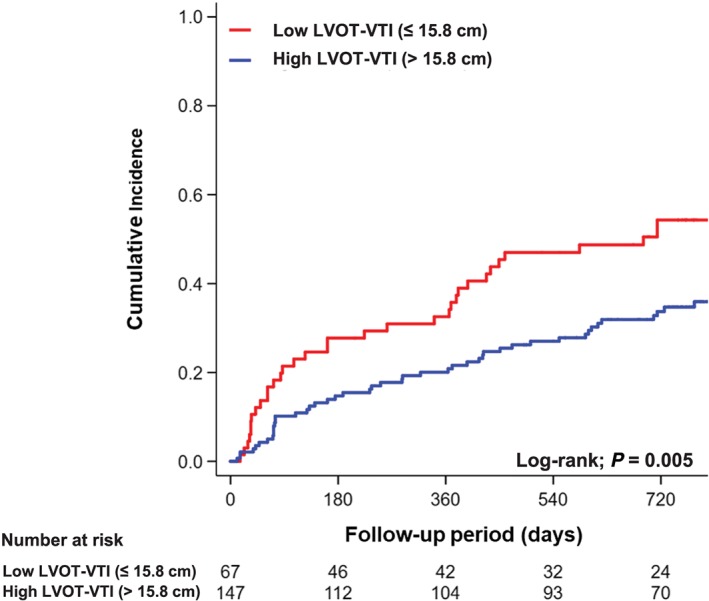
Kaplan–Meier analysis of composite of all‐cause death and readmission due to heart failure categorized by left ventricular outflow tract velocity time integral (LVOT‐VTI).
Figure 4.
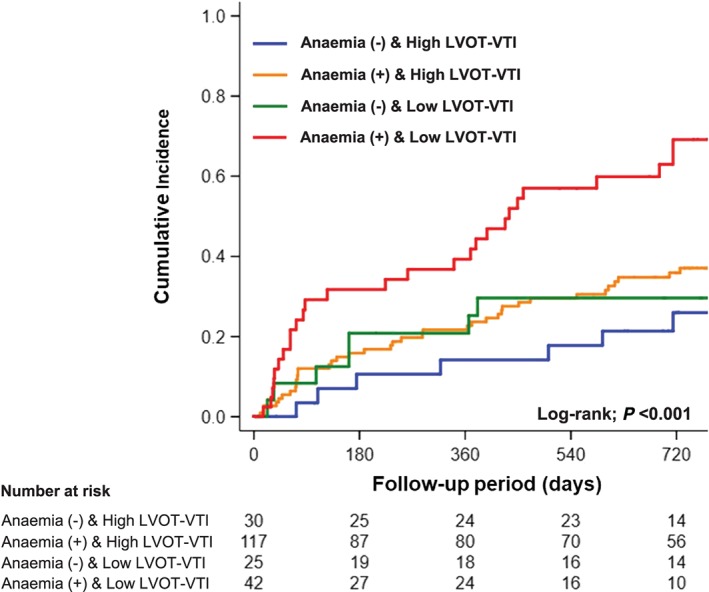
Kaplan–Meier analysis of composite of all‐cause death and readmission due to heart failure categorized by left ventricular outflow tract velocity time integral (LVOT‐VTI) and anaemia.
Table 3.
Multivariable Cox proportional hazard model for all‐cause death and readmission due to heart failure
| Variable | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| LVOT‐VTI, 1 cm | 0.95 | 0.91–0.98 | 0.005 | 0.94 | 0.90–0.98 | 0.029 |
| Age, 1 year | 1.01 | 0.99–1.04 | 0.184 | 1.01 | 0.99–1.04 | 0.27 |
| Male, sex | 0.83 | 0.53–1.30 | 0.42 | 0.81 | 0.51–1.27 | 0.36 |
| Systolic blood pressure, 1 mmHg | 1.00 | 0.99–1.003 | 0.37 | 1.00 | 0.99–1.004 | 0.54 |
| Sodium, 1 mEq/L | 0.94 | 0.89–0.98 | 0.004 | 0.94 | 0.90–0.98 | 0.006 |
| Log BNP, 1 pg/mL | 1.11 | 0.86–1.45 | 0.44 | 1.09 | 0.84–1.43 | 0.53 |
| Albumin, 1 g/dL | 0.41 | 0.26–0.65 | <0.001 | 0.44 | 0.28–0.70 | <0.001 |
| BUN, 1 mg/dL | 1.01 | 0.998–1.02 | 0.102 | 1.01 | 0.996–1.01 | 0.185 |
| Haemoglobin, 1 g/dL | 0.92 | 0.80–1.04 | 0.183 | |||
Model 1: adjustment for pre‐specified covariates and renal function. Model 2: addition of haemoglobin level to Model 1. BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CI, confidence interval; HR, hazard ratio; LVOT‐VTI, left ventricular outflow tract velocity time integral.
4. Discussion
The major finding of the present study was that lower LVOT‐VTI on admission was a significant independent determinant of all‐cause death and readmission due to HF in patients with decompensated HFpEF. Haemoglobin level was the strongest independent determinant of LVOT‐VTI among clinical parameters including LVEF. Notably, HFpEF patients categorized as having lower LVOT‐VTI and anaemia had a markedly higher incidence of subsequent adverse events, indicating that patients with an impaired increase in stroke volume in response to anaemia might be a specific phenotype of HFpEF with worse outcomes.
Heart failure with preserved ejection fraction presentation is heterogeneous due to environmental factors, co‐morbidity, and genetic susceptibility. There are several clinical presentation and predisposition phenotypes, including pulmonary hypertension, atrial fibrillation, skeletal muscle weakness, obesity, atrial hypertension, renal dysfunction, and coronary artery disease.5, 13 Importantly, a sub‐analysis of the RELAX trial demonstrated that 37% of patients were categorized as low‐flow HFpEF, defined as stroke volume index ≤35 mL/m2 estimated by pulsed‐wave Doppler technique.14 In the analysis, low‐flow HFpEF was associated with severity of HF represented by higher plasma N terminal pro BNP level and impaired peak oxygen consumption assessed by cardiopulmonary exercise testing6; however, the prognostic implication of low flow in HFpEF patients has not been clarified. Interestingly, our present study showed that lower LVOT‐VTI on admission was an independent determinant of the composite of all‐cause death and readmission due to HF in hospitalized HFpEF patients, even after adjustment for well‐known strong prognostic factors for acute decompensated HF such as age, systolic blood pressure, serum sodium, albumin, and plasma BNP levels and renal function. Identifying patients with specific HFpEF phenotypes and modifiable treatment targets may be important for developing novel effective therapies to improve clinical outcomes.
Despite the Fick or thermodilution method using invasive right heart catheterization being the gold standard for measuring stroke volume, routine use of invasive haemodynamic tests shows little evidence of favourable outcomes because of procedure‐related complications, such as bleeding and infection, and high health care cost.15 Thus, non‐invasive assessment of Doppler‐estimated stroke volume could overcome these issues. It is noteworthy that LVOT‐VTI has several advantages in that it is a non‐invasive, simple, rapid, repeatable, and common measurement at the bedside, with low cost compared with invasive haemodynamic monitoring. Previous studies demonstrated a very strong correlation between Doppler‐estimated stroke volume and that measured by right heart catheterization.16, 17, 18 Indeed, stroke volume estimated by pulsed‐wave Doppler technique correlated well with that measured by thermodilution (r = 0.83, n = 95).18 LVOT‐VTI is also useful for evaluating haemodynamics and cardiac function in patients with cardiovascular disease.19 It is often used in current practice to optimize biventricular pacing settings in cardiac resynchronization therapy or calculate aortic valve area using a continuity equation in patients with aortic stenosis. Moreover, lower LVOT‐VTI is known to have prognostic value in patients with various cardiovascular diseases. In fact, lower LVOT‐VTI was an independent determinant of worse clinical outcomes in patients with stable coronary artery disease,20, 21 acute myocardial infarction,22, 23, 24 chronic advanced HF,7 and hospitalized HF.8 In these studies, LVOT‐VTI multiplied by LVOT area simultaneously was not necessary to provide clinically relevant data for stratifying the subsequent risk of adverse events. Our present findings are consistent with these studies and provide additional information regarding the long‐term prognostic significance of admission LVOT‐VTI in hospitalized patients with HFpEF.
The presence of anaemia is a common co‐morbid condition and is independently associated with a significant risk of mortality in patients with HFpEF.25, 26 Both haemoglobin concentration and cardiac output are known to be major determinants of oxygen delivery to systemic organs. Severe anaemia decreases systemic vascular resistance by reduced inhibition of basal endothelium‐derived relaxing factor activity and leads to generalized vasodilatation27, 28 and increases heart rate and LV contractility to compensate oxygen delivery to systemic organs.29 In such cases, myocardial oxygen consumption and cardiac energy expenditure are increased, resulting in deterioration of cardiac structure and function. Indeed, a previous study demonstrated that patients with anaemia due to sickle cell disease have a unique form of cardiomyopathy that is characterized by diastolic dysfunction, left atrial dilation, and normal LV systolic function.30 If cardiac output could not increase in response to anaemia because of longitudinal changes in diastolic LV function, the tissue of systemic organs would become markedly hypoperfused, which would lead to a worse clinical outcome in hospitalized patients with HFpEF. In the present study, patients who had both low LVOT‐VTI and anaemia showed the worst clinical outcomes among the groups, suggesting that low‐flow HFpEF might be characterized by deterioration of cardiac structure and diastolic function, resulting in impaired cardiac output beyond compensation for hypoxia due to anaemia.
4.1. Study limitations
There are several potential limitations of the present study that should be acknowledged. First, the sample size of this study was relatively small, thereby limiting the ability to generalize the findings and the statistical power for detecting differences in negative data. Therefore, a larger‐scale study is warranted to confirm the relationship between impaired LVOT‐VTI and worse clinical outcomes in hospitalized patients with HFpEF. Second, there was unavoidable selection bias in our study, although variables other than the echocardiographic findings and the rate of adverse events were comparable between the excluded patients and the study population (Supporting Information, Tables S1 and S2 ). Third, stroke volume determined by the LVOT diameter multiplied by the LVOT‐VTI calculation is susceptible to error. Especially, the overestimation of stroke volume could occur with anatomic obstruction in the LVOT such as sigmoid septum or asymmetric ventricular septal hypertrophy.31 Unfortunately, we had no echocardiographic data about the presence of sigmoid septum and asymmetric ventricular septal hypertrophy. Moreover, in case of using 2D Doppler method, the LVOT shape was assumed to be a perfect circle because the LVOT area was estimated from the LVOT diameter. However, 3D echocardiography, computed tomography, and cardiac magnetic resonance imaging can overcome this issue because these modalities are able to measure LVOT area as elliptical. Thus, the estimation of the LVOT area by the 2D Doppler method may lead to an underestimation of the LVOT area.32 Unfortunately, we had no data of these new modalities in our registry. Fourth, we had no data about the treatment for anaemia and/or iron deficiency although these factors are known to be key determinants of worse clinical outcomes in hospitalized patients with HF.33 Finally, we could not demonstrate the tracked changes in LVOT‐VTI during hospitalization and the prognostic value of discharge LVOT‐VTI in relation to long‐term outcomes, because there was a large amount of missing data of LVOT‐VTI at the time of discharge [n = 94 (44%)]. Accordingly, a further study that can confirm the relationship between tracked changes in LVOT‐VTI from admission to discharge and long‐term clinical outcomes in patients with HFpEF is warranted.
5. Conclusions
Lower admission LVOT‐VTI was an independent determinant of worse clinical outcomes in hospitalized patients with HFpEF. Lower LVOT‐VTI concomitant with anaemia might strengthen its prognostic significance. Our findings indicated that LVOT‐VTI on admission might be useful for categorizing a low‐flow HFpEF phenotype and risk stratification in hospitalized patients with HFpEF.
Conflict of interest
None declared.
Funding
This work was supported by a grant from the Japan Cardiovascular Research Foundation (T.A., 24‐4‐2).
Supporting information
Table S1. Baseline characteristics of study population and patients excluded.
Table S2. Adverse events between study population and patients excluded.
Acknowledgements
The authors are grateful for the contributions of all the investigators, clinical research coordinators, and data managers involved in the JASPER registry.
Omote, K. , Nagai, T. , Iwano, H. , Tsujinaga, S. , Kamiya, K. , Aikawa, T. , Konishi, T. , Sato, T. , Kato, Y. , Komoriyama, H. , Kobayashi, Y. , Yamamoto, K. , Yoshikawa, T. , Saito, Y. , and Anzai, T. (2020) Left ventricular outflow tract velocity time integral in hospitalized heart failure with preserved ejection fraction. ESC Heart Failure, 7: 167–175. 10.1002/ehf2.12541.
References
- 1. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med 2016; 375: 1868–1877. [DOI] [PubMed] [Google Scholar]
- 2. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011; 32: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam CSP, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J 2018; 39: 2780–2792. [DOI] [PubMed] [Google Scholar]
- 4. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016; 134: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel KV, Mauricio R, Grodin JL, Ayers C, Fonarow GC, Berry JD, Pandey A. Identifying a low‐flow phenotype in heart failure with preserved ejection fraction: a secondary analysis of the RELAX trial. ESC Heart Fail 2019; 6: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan C, Rubenson D, Srivastava A, Mohan R, Smith MR, Billick K, Bardarian S, Thomas HJ. Left ventricular outflow tract velocity time integral outperforms ejection fraction and Doppler‐derived cardiac output for predicting outcomes in a select advanced heart failure cohort. Cardiovasc Ultrasound 2017; 15: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhong Y, Almodares Q, Yang J, Wang F, Fu M, Johansson MC. Reduced stroke distance of the left ventricular outflow tract is independently associated with long‐term mortality, in patients hospitalized due to heart failure. Clin Physiol Funct Imaging 2018; 38: 881–888. [DOI] [PubMed] [Google Scholar]
- 9. Nagai T, Yoshikawa T, Saito Y, Takeishi Y, Yamamoto K, Ogawa H, Anzai T, Investigators J. Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction—a report from the Japanese Heart Failure Syndrome with Preserved Ejection Fraction (JASPER) registry. Circ J 2018; 82: 1534–1545. [DOI] [PubMed] [Google Scholar]
- 10. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 11. Schiller NB, Acquatella H, Ports TA, Drew D, Goerke J, Ringertz H, Silverman NH, Brundage B, Botvinick EH, Boswell R, Carlsson E, Parmley WW. Left ventricular volume from paired biplane two‐dimensional echocardiography. Circulation 1979; 60: 547–555. [DOI] [PubMed] [Google Scholar]
- 12. Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol 1985; 6: 359–365. [DOI] [PubMed] [Google Scholar]
- 13. Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014; 113: 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309: 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merrer J, De Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, Rigaud JP, Casciani D, Misset B, Bosquet C, Outin H, Brun‐Buisson C, Nitenberg G. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 2001; 286: 700–707. [DOI] [PubMed] [Google Scholar]
- 16. Mowat DH, Haites NE, Rawles JM. Aortic blood velocity measurement in healthy adults using a simple ultrasound technique. Cardiovasc Res 1983; 17: 75–80. [DOI] [PubMed] [Google Scholar]
- 17. Haites NE, McLennan FM, Mowat DH, Rawles JM. How far is the cardiac output? Lancet 1984; 2: 1025–1027. [DOI] [PubMed] [Google Scholar]
- 18. Huntsman LL, Stewart DK, Barnes SR, Franklin SB, Colocousis JS, Hessel EA. Noninvasive Doppler determination of cardiac output in man. Clinical validation Circulation 1983; 67: 593–602. [DOI] [PubMed] [Google Scholar]
- 19. Goldman JH, Schiller NB, Lim DC, Redberg RF, Foster E. Usefulness of stroke distance by echocardiography as a surrogate marker of cardiac output that is independent of gender and size in a normal population. Am J Cardiol 2001; 87: 499–502 a498. [DOI] [PubMed] [Google Scholar]
- 20. Ristow B, Na B, Ali S, Whooley MA, Schiller NB. Left ventricular outflow tract and pulmonary artery stroke distances independently predict heart failure hospitalization and mortality: the Heart and Soul Study. J Am Soc Echocardiogr 2011; 24: 565–572. [DOI] [PubMed] [Google Scholar]
- 21. Stevens SM, Farzaneh‐Far R, Na B, Whooley MA, Schiller NB. Development of an echocardiographic risk‐stratification index to predict heart failure in patients with stable coronary artery disease: the Heart and Soul study. JACC Cardiovasc Imaging 2009; 2: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delemarre BJ, Visser CA, Bot H, de Koning HJ, Dunning AJ. Predictive value of pulsed Doppler echocardiography in acute myocardial infarction. J Am Soc Echocardiogr 1989; 2: 102–109. [DOI] [PubMed] [Google Scholar]
- 23. Metcalfe MJ, Rawles JM. Stroke distance in acute myocardial infarction: a simple measurement of left ventricular function. Lancet 1989; 1: 1371–1373. [DOI] [PubMed] [Google Scholar]
- 24. Trent RJ, Rawles JM. Risk stratification after acute myocardial infarction by Doppler stroke distance measurement. Heart 1999; 82: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Latado AL, Passos LC, Darze ES, Lopes AA. Comparison of the effect of anemia on in‐hospital mortality in patients with versus without preserved left ventricular ejection fraction. Am J Cardiol 2006; 98: 1631–1634. [DOI] [PubMed] [Google Scholar]
- 26. Felker GM, Shaw LK, Stough WG, O'Connor CM. Anemia in patients with heart failure and preserved systolic function. Am Heart J 2006; 151: 457–462. [DOI] [PubMed] [Google Scholar]
- 27. Anand IS, Chandrashekhar Y, Wander GS, Chawla LS. Endothelium‐derived relaxing factor is important in mediating the high output state in chronic severe anemia. J Am Coll Cardiol 1995; 25: 1402–1407. [DOI] [PubMed] [Google Scholar]
- 28. Anand IS, Chandrashekhar Y, Ferrari R, Poole‐Wilson PA, Harris PC. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J 1993; 70: 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harada T, Obokata M, Kurosawa K, Sorimachi H, Yoshida K, Ishida H, Ito K, Ogawa T, Ando Y, Kurabayashi M, Negishi K. Relationships of high cardiac output with ventricular morphology, myocardial energetics, and energy costs in hemodialysis patients with preserved ejection fraction. Int J Cardiovasc Imaging 2019; 35: 469–479. [DOI] [PubMed] [Google Scholar]
- 30. Niss O, Quinn CT, Lane A, Daily J, Khoury PR, Bakeer N, Kimball TR, Towbin JA, Malik P, Taylor MD. Cardiomyopathy with restrictive physiology in sickle cell disease. JACC Cardiovasc Imaging 2016; 9: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poh KK, Levine RA, Solis J, Shen L, Flaherty M, Kang YJ, Guerrero JL, Hung J. Assessing aortic valve area in aortic stenosis by continuity equation: a novel approach using real‐time three‐dimensional echocardiography. Eur Heart J 2008; 29: 2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saitoh T, Shiota M, Izumo M, Gurudevan SV, Tolstrup K, Siegel RJ, Shiota T. Comparison of left ventricular outflow geometry and aortic valve area in patients with aortic stenosis by 2‐dimensional versus 3‐dimensional echocardiography. Am J Cardiol 2012; 109: 1626–1631. [DOI] [PubMed] [Google Scholar]
- 33. Nakano H, Nagai T, Sundaram V, Nakai M, Nishimura K, Honda Y, Honda S, Iwakami N, Sugano Y, Asaumi Y, Aiba T, Noguchi T, Kusano K, Yokoyama H, Ogawa H, Yasuda S, Chikamori T, Anzai T, DEFi Na. Impact of iron deficiency on long‐term clinical outcomes of hospitalized patients with heart failure. Int J Cardiol 2018;261:114‐118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of study population and patients excluded.
Table S2. Adverse events between study population and patients excluded.


