Abstract
Aims
Severely elevated pre‐transplant pulmonary vascular resistance (PVR) has been linked to adverse effects after heart transplantation (HTX). The impact of a moderately increased PVR before HTX on post‐transplant outcomes remains uncertain. The aim of this study was to investigate the effects of an elevated pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) on outcomes after HTX.
Methods and results
This observational retrospective single‐centre study included 561 patients receiving HTX at Heidelberg Heart Center between 1989 and 2015. Patients were stratified by degree of pre‐transplant PVR. Analyses covered demographics, post‐transplant medication, mortality and causes of death after HTX, early post‐transplant atrial fibrillation (AF), and length of the initial hospital stay after HTX. Ninety‐four patients (16.8%) had a PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units). These patients had a higher rate of early post‐transplant AF [20.2 vs. 10.7%, difference: 9.5%, 95% confidence interval (CI): 0.9–18.1%, P = 0.01] and an increased 30 day post‐transplant mortality (25.5 vs. 6.4%, hazard ratio: 4.4, 95% CI: 2.6–7.6, P < 0.01), along with a higher percentage of death due to transplant failure (21.2 vs. 4.1%, difference: 17.1%, 95% CI: 8.7–25.5%, P < 0.01). Multivariate analysis revealed a PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) as a significant risk factor for increased 30 day mortality after HTX (hazard ratio: 4.4, 95% CI: 2.5–7.6, P < 0.01). Kaplan–Meier estimator showed a lower 2 year survival after HTX (P < 0.01) in patients with a PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units).
Conclusions
Elevated pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) is associated with early post‐transplant AF and increased mortality after HTX.
Keywords: Atrial fibrillation, Heart transplantation, Mortality, Length of initial hospital stay, Pulmonary vascular resistance
1. Introduction
Right‐sided heart failure is a common complication in the early stage after heart transplantation (HTX) and remains—despite all medical and technical advances—a main cause for early post‐transplant mortality accounting for up to 20% of early deaths after HTX.1, 2, 3, 4 Already at the beginning of the HTX era, Dr Shumway and his team at Stanford University Medical Center postulated an association between an elevated pre‐transplant pulmonary vascular resistance (PVR) and a higher risk for mortality after HTX due to right‐sided heart failure.5 Assessment of PVR is therefore a regular part of the HTX evaluation process and can be determined during right heart catheterization (RHC).6, 7 According to the International Society of Heart and Lung Transplantation (ISHLT) listing criteria for HTX, a PVR ≥ 400 dyn·s·cm−5 (≥5.0 Wood units) should be considered as a relative contraindication for HTX.8, 9
Several studies have investigated the influence of an elevated pre‐transplant PVR on outcomes after HTX.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 However, results regarding post‐transplant mortality were inconsistent as studies varied in study design, sample size, follow‐up, and definition of an elevated pre‐transplant PVR. Thus, the detrimental degree of a moderately increased PVR before HTX on post‐transplant results remains uncertain. As patients awaiting HTX routinely undergo RHC to measure cardiac haemodynamics, the extent of the very latest PVR before HTX may be more reliable to reflect current physiological properties than the assessed PVR during the HTX evaluation process and may therefore be used as a prognostic marker for early post‐transplant survival.
The objective of this study was to analyse the effects of an elevated pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) on outcomes after HTX with a focus on post‐transplant mortality including causes of death, duration of the initial post‐transplant hospital stay, and occurrence of early atrial fibrillation (AF) after HTX.
2. Patients and methods
2.1. Patients
The ethical principles of the Declaration of Helsinki were followed in this study. Approval was given by the ethics committee of the University of Heidelberg (ethical approval number: S‐286/2015; date of ethical approval: 22/06/15). This study included all adult patients (≥18 years) receiving HTX at the Heidelberg Heart Center between 06/1989 and 12/2015 except for patients with repeated cardiac transplantation or ventricular assist device (VAD) due to altered haemodynamics. No patient was excluded because of loss to follow‐up or incomplete data as 2 year follow‐up data could be obtained in all patients requiring no censoring. Data were taken from medical records and analysed in pseudonymized form. Written informed consent was obtained from patients for inclusion in the Heidelberg HTX registry allowing the clinical and scientific use of data. According to the ethical approval, no additional written informed consent was required for this observational retrospective single‐centre study as only routine clinical data were used.28, 29, 30, 31, 32, 33, 34
The latest RHC before HTX was used to stratify patients based on their pre‐transplant PVR, which was classified as follows: normal pre‐transplant PVR < 200 dyn·s·cm−5 (<2.5 Wood units); mildly elevated pre‐transplant PVR = 200 to 299 dyn·s·cm−5 (2.5 to 3.74 Wood units); moderately elevated pre‐transplant PVR = 300 to 399 dyn·s·cm−5 (3.75 to 4.99 Wood units); and severely elevated pre‐transplant PVR ≥ 400 dyn·s·cm−5 (≥5.0 Wood units). In an attempt to find the optimal pre‐transplant PVR discrimination threshold, we performed a receiver operating characteristic analysis for 30 day all‐cause mortality after HTX, which indicated a pre‐transplant PVR of 285 dyn·s·cm−5 (3.56 Wood units) as an optimal cut‐off for HTX listing. In order to provide a prudential cut‐off for routine clinical practice, we decided to use a pre‐transplant PVR of 300 dyn·s·cm−5 (3.75 Wood units). All patients were consequently divided into two groups: patients with a native PVR < 300 dyn·s·cm−5 (<3.75 Wood units) and patients with a native PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units). Then, patients with a native PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) were further stratified into patients with a declining PVR < 300 dyn·s·cm−5 (<3.75 Wood units) and patients with a remaining PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) after application of a vasodilator. Of importance, all patients had a PVR < 400 dyn·s·cm−5 (<5 Wood units) after application of a vasodilator in accordance with the ISHLT listing criteria for HTX.8, 9
2.2. Follow‐up
Patients after HTX were continuously cared for by the medical team of the Heidelberg Heart Center. Patient follow‐up was performed in accordance with the standard of care at the Heidelberg Heart Center. Post‐transplant treatment of patients with an increased PVR before HTX routinely included volume management, application of oxygen, nitric oxide, iloprost, sildenafil, and inotropes. Patients with beginning transplant failure were routinely treated with an extracorporeal membrane oxygenation, an intra‐aortic balloon pump, or a temporary right ventricular assist device to prevent graft failure. During the initial hospital stay, heart rhythm was continuously assessed by monitor telemetry. Additionally, 12‐lead electrocardiography (ECG) was performed on a regular basis and in case of any noted arrhythmia on telemetry. Before discharge, 24 h Holter recording was performed to detect arrhythmic disorders. Diagnosis of early post‐transplant AF (≤30 days after HTX) was based upon all available records pertaining to heart rhythm in the early post‐transplant period. After discharge, patients were followed up monthly within the first 6 months after HTX, then bimonthly between months 6 and 12 after HTX, and thereafter usually three to four times annually.28, 29, 30, 31, 32, 33, 34, 35, 36
2.3. Post‐transplant medication
Cyclosporine A (CsA) and azathioprine were used as the initial immunosuppressive drug regimen at the beginning of the study period. From 2001 onward, azathioprine was subsequently substituted by mycophenolate mofetil, and CsA was consecutively replaced by tacrolimus from 2006 onward.
Steroids (prednisolone) were tapered incrementally during the first post‐transplant months and were finally discontinued (if clinically possible) 6 months after HTX.28, 29, 30, 31, 32, 33, 34, 35, 36
2.4. Statistical analysis
Data were statistically analysed with SAS (Version 9.4, SAS Institute, Cary, NC, USA) and expressed as mean ± standard deviation or as count (n) with percentage (%). In addition, measures of association [hazard ratio (HR) or difference] and 95% confidence interval (CI) were applied. Student's t‐test was used for continuous variables and χ 2 test for categorical variables. Post‐transplant survival was graphically displayed by Kaplan–Meier estimator. Extensive univariate analyses were performed to test for differences between groups including recipient data, previous open‐heart surgery, principal diagnosis for HTX, donor data, transplant sex mismatch, perioperative data, and post‐transplant medication including immunosuppressive drug therapy.28, 29, 30, 31, 32, 33, 34
The influence of an elevated native PVR before HTX ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) on post‐transplant mortality was further analysed and adjusted for specific predetermined risk factors by performing a multivariate analysis (Cox regression model). The following six clinically relevant parameters were included based on a predetermined model: native PVR before HTX ≥ 300 dyn·s·cm−5 (≥3.75 Wood units), recipient age (>60.0 years), coronary artery disease, previous open‐heart surgery, donor age (>40.0 years), and ischaemic time (≥240 min). In order to avoid biased regression coefficients and to ensure a stable number of events (deceased patients) per analysed variable, no further clinically less relevant parameters were included in this multivariate analysis.28, 29, 30, 31, 32, 33, 34
Given the long study period, a sensitivity analysis was carried out to test the robustness of the study results and to investigate a possible era effect. Therefore, as the initial standard immunosuppressive drug therapy was changed during the study period, a subgroup analysis was performed with patients receiving CsA and azathioprine. Additionally, potential differences in outcomes between patients with and without a native PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) were investigated using data from the RHC at the time of HTX listing vs. the latest RHC before HTX.
The primary outcome of this study was post‐transplant all‐cause mortality. Secondary outcomes included length of the initial hospital stay and occurrence of AF within 30 days after HTX (30 day post‐transplant incidence), which was defined as ECG/Holter proven AF lasting 30 s or longer. Additionally, the causes of death within 30 days after HTX were investigated in the following categories: transplant failure (defined as failure of transplant from any cause), acute rejection, infection/sepsis, malignancy, and thromboembolic event/bleeding.
3. Results
3.1. Baseline characteristics
A total of 561 patients with a mean time interval of 52.3 ± 46.3 days between the latest RHC before HTX and HTX were included in this study. The selection process is displayed in Figure 1 . Hereof, 467 patients (83.2%) had a pre‐transplant PVR < 300 dyn·s·cm−5, and 94 patients (16.8%) had a pre‐transplant PVR ≥ 300 dyn·s·cm−5. No statistically significant differences between both groups were found concerning recipient data, previous open‐heart surgery, principal diagnosis for HTX, transplant sex mismatch, or perioperative data. Patients with a PVR ≥ 300 dyn·s·cm−5 had a significantly higher mean donor age (43.9 ± 12.5 vs. 39.7 ± 13.4 years, difference: 4.2 years, 95% CI: 1.4–7.0 years, P < 0.01) and a higher percentage of donors >40 years (70.2 vs. 53.3%, difference: 16.9%, 95% CI: 6.6–27.2%, P < 0.01). Both groups showed no significant differences in donor male sex or donor body mass index. Baseline characteristics are shown in Table 1.
Figure 1.
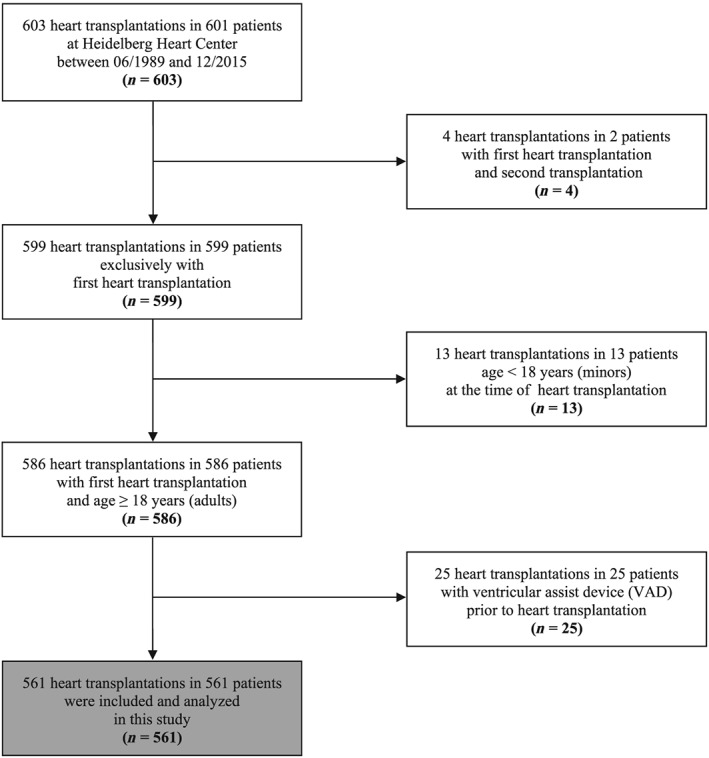
Flow diagram of selection process. A total of 601 patients with 603 heart transplantations (HTX) were assessed for eligibility. Patients with repeated HTX (two patients with four HTX), patients <18 years (13 patients), and patients with ventricular assist device (VAD) prior to HTX (25 patients) were excluded. Five hundred sixty‐one patients were finally included and analysed in this study.
Table 1.
Baseline characteristics
| All | PVR < 300 dyn·s·cm−5 (<3.75 WU) | PVR ≥ 300 dyn·s·cm−5 (≥3.75 WU) | Difference | 95% CI | P‐value | |
|---|---|---|---|---|---|---|
| (n = 561) | (n = 467) | (n = 94) | ||||
| Recipient data | ||||||
| PVR (dyn·s·cm−5), mean ± SD | 209.3 ± 103.0 | 171.8 ± 59.3 | 395.8 ± 65.0 | 224.0 | 209.7 to 238.4 | <0.01 |
| Age (years), mean ± SD | 52.1 ± 10.3 | 51.8 ± 10.1 | 53.6 ± 11.0 | 1.8 | −0.6 to 4.2 | 0.15 |
| Age (>60 years), n (%) | 117 (20.9%) | 91 (19.5%) | 26 (27.7%) | 8.2 | −1.5 to 17.9 | 0.08 |
| Male sex, n (%) | 436 (77.7%) | 362 (77.5%) | 74 (78.7%) | 1.2 | −7.9 to 10.3 | 0.80 |
| Body mass index (kg·m−2), mean ± SD 24.8 ± 3.9 | 24.9 ± 3.9 | 24.5 ± 4.1 | 0.4 | −0.5 to 1.3 | 0.38 | |
| Coronary artery disease, n (%) | 233 (41.5%) | 187 (40.0%) | 46 (48.9%) | 8.9 | −2.1 to 19.9 | 0.11 |
| Arterial hypertension, n (%) | 309 (55.1%) | 257 (55.0%) | 52 (55.3%) | 0.3 | −10.7 to 11.3 | 0.96 |
| Dyslipidaemia, n (%) | 361 (64.3%) | 301 (64.5%) | 60 (63.8%) | 0.7 | −9.9 to 11.3 | 0.91 |
| Diabetes mellitus, n (%) | 192 (34.2%) | 157 (33.6%) | 35 (37.2%) | 3.6 | −7.1 to 14.3 | 0.50 |
| Renal insufficiencya, n (%) | 327 (58.3%) | 274 (58.7%) | 53 (56.4%) | 2.3 | −8.7 to 13.3 | 0.68 |
| eGFR (mL·min−1·1.73 m−2), mean ± SD | 59.6 ± 20.8 | 59.7 ± 20.4 | 59.3 ± 22.6 | 0.4 | −4.6 to 5.4 | 0.87 |
| Previous open‐heart surgery | ||||||
| Overall open‐heart surgery, n (%) | 136 (24.2%) | 106 (22.7%) | 30 (31.9%) | 9.2 | −1.0 to 19.4 | 0.06 |
| CABG surgery, n (%) | 75 (13.4%) | 58 (12.4%) | 17 (18.1%) | 5.7 | −2.6 to 14.0 | 0.14 |
| Congenital, valvular, or | 67 (11.9%) | 52 (11.1%) | 15 (16.0%) | 4.9 | −3.1 to 12.9 | 0.19 |
| ventricular surgery, n (%) | ||||||
| Principal diagnosis for HTX | ||||||
| Ischaemic CMP, n (%) | 191 (34.0%) | 153 (32.8%) | 38 (40.5%) | 7.7 | −3.1 to 18.5 | 0.15 |
| Non‐ischaemic CMP, n (%) | 292 (52.1%) | 248 (53.1%) | 44 (46.8%) | 6.3 | −4.8 to 17.4 | 0.26 |
| Valvular heart disease, n (%) | 34 (6.1%) | 27 (5.8%) | 7 (7.4%) | 1.6 | −4.1 to 7.3 | 0.54 |
| Cardiac amyloidosis, n (%) | 44 (7.8%) | 39 (8.3%) | 5 (5.3%) | 3.0 | −2.2 to 8.2 | 0.32 |
| Donor data | ||||||
| Age (years), mean ± SD | 40.4 ± 13.3 | 39.7 ± 13.4 | 43.9 ± 12.5 | 4.2 | 1.4 to 7.0 | <0.01 |
| Age (>40 years), n (%) | 315 (56.1%) | 249 (53.3%) | 66 (70.2%) | 16.9 | 6.6 to 27.2 | <0.01 |
| Male sex, n (%) | 243 (43.3%) | 207 (44.3%) | 36 (38.3%) | 6.0 | −4.8 to 16.8 | 0.28 |
| Body mass index (kg·m−2), mean ± SD 24.6 ± 4.0 | 24.7 ± 3.9 | 24.1 ± 4.3 | 0.6 | −0.3 to 1.5 | 0.19 | |
| Transplant sex mismatch | ||||||
| Mismatch, n (%) | 246 (43.8%) | 200 (42.8%) | 46 (49.0%) | 6.2 | −4.9 to 17.3 | 0.28 |
| Donor (m) to recipient (f), n (%) | 26 (4.6%) | 22 (4.7%) | 4 (4.3%) | 0.4 | −4.1 to 4.9 | 0.85 |
| Donor (f) to recipient (m), n (%) | 220 (39.2%) | 178 (38.1%) | 42 (44.7%) | 6.6 | −4.4 to 17.6 | 0.23 |
| Perioperative data | ||||||
| Ischaemic time (min), mean ± SD | 215.4 ± 66.4 | 215.3 ± 65.8 | 216.1 ± 69.6 | 0.8 | −14.7 to 16.3 | 0.91 |
| Ischaemic time ≥240 min, n (%) | 203 (36.2%) | 168 (36.0%) | 35 (37.2%) | 1.2 | −9.5 to 11.9 | 0.82 |
| Biatrial HTX, n (%) | 163 (29.0%) | 138 (29.5%) | 25 (26.6%) | 2.9 | −6.9 to 12.7 | 0.56 |
| Bicaval HTX, n (%) | 144 (25.7%) | 119 (25.5%) | 25 (26.6%) | 1.1 | −8.7 to 10.9 | 0.82 |
| Total orthotopic HTX, n (%) | 254 (45.3%) | 210 (45.0%) | 44 (46.8%) | 1.8 | −9.2 to 12.8 | 0.74 |
CABG, coronary artery bypass graft; CI, confidence interval; CMP, cardiomyopathy; dyn, g·cm·s−2; eGFR, estimated glomerular filtration rate; f, female; HTX, heart transplantation; m, male; PVR, pulmonary vascular resistance; SD, standard deviation; VAD, ventricular assist device; WU, Wood unit (≙ 80 dyn·s·cm−5).
eGFR < 60 mL·min−1·1.73 m−2.
3.2. Initial medication after heart transplantation
A comparison of the immunosuppressive drug therapy indicated no statistically significant differences between groups concerning the administration of CsA, tacrolimus, azathioprine, or mycophenolate mofetil. Additionally, no statistically significant differences between both groups were found in the use of acetylsalicylic acid, beta‐blockers, ivabradine, calcium channel blockers, angiotensin‐converting enzyme inhibitors/sartans, or statins. The initial immunosuppressive drug regimen and medication after HTX is shown in Table 2.
Table 2.
Initial medication after heart transplantation
| All | PVR < 300 | PVR ≥ 300 | Difference | 95% CI | P‐value | |
|---|---|---|---|---|---|---|
| dyn·s·cm−5 | dyn·s·cm−5 | |||||
| (<3.75 WU) | (≥3.75 WU) | |||||
| (n = 561) | (n = 467) | (n = 94) | ||||
| Cyclosporine A, n (%) | 337 (60.1%) | 281 (60.2%) | 56 (59.6%) | 0.6% | −10.3 to 11.5% | 0.91 |
| Tacrolimus, n (%) | 224 (39.9%) | 186 (39.8%) | 38 (40.4%) | 0.6% | −10.3 to 11.5% | 0.91 |
| Azathioprine, n (%) | 263 (46.9%) | 226 (48.4%) | 37 (39.4%) | 9.0% | −1.8 to 19.8% | 0.11 |
| Mycophenolate mofetil, n (%) | 298 (53.1%) | 241 (51.6%) | 57 (60.6%) | 9.0% | −1.8 to 19.8% | 0.11 |
| Steroids, n (%) | 561 (100.0%) | 467 (100.0%) | 94 (100.0%) | 0.0% | n.a. | n.a. |
| Acetylsalicylic acid (ASA), n (%) | 52 (9.3%) | 45 (9.6%) | 7 (7.4%) | 2.2% | −3.7 to 8.1% | 0.50 |
| Beta‐blocker, n (%) | 92 (16.4%) | 78 (16.7%) | 14 (14.9%) | 1.8% | −6.1 to 9.7% | 0.67 |
| Ivabradine, n (%) | 38 (6.8%) | 31 (6.6%) | 7 (7.4%) | 0.8% | −4.9 to 6.5% | 0.78 |
| Calcium channel blocker, n (%) | 140 (25.0%) | 117 (25.1%) | 23 (24.5%) | 0.6% | −8.9 to 10.1% | 0.90 |
| ACE inhibitor/sartan, n (%) | 248 (44.2%) | 214 (45.8%) | 34 (36.2%) | 9.6% | −1.1 to 20.3% | 0.09 |
| Diuretic, n (%) | 561 (100.0%) | 467 (100.0%) | 94 (100.0%) | 0.0% | n.a. | n.a. |
| Statin, n (%) | 215 (38.3%) | 177 (37.9%) | 38 (40.4%) | 2.5% | −8.3 to 13.3% | 0.65 |
| Gastric protection (PPI/H2 blocker), n (%) | 561 (100.0%) | 467 (100.0%) | 94 (100.0%) | 0.0% | n.a. | n.a. |
ACE, angiotensin‐converting enzyme; CI, confidence interval; dyn, g·cm·s−2; H2 blocker, histamine receptor blocker; n.a., not applicable; PPI, proton pump inhibitor; PVR, pulmonary vascular resistance; WU, Wood unit (≙ 80 dyn·s·cm−5).
3.3. Outcomes after heart transplantation
3.3.1. Primary outcome
Regarding the primary outcome of the study, patients with a native PVR ≥ 300 dyn·s·cm−5 had a significantly higher 30 day post‐transplant mortality (25.5 vs. 6.4%, HR: 4.4, 95% CI: 2.6–7.6, P < 0.01), 1 year post‐transplant mortality (52.1 vs. 16.1%, HR: 4.2, 95% CI: 2.9–6.0, P < 0.01), and 2 year post‐transplant mortality (58.5 vs. 19.3%, HR: 4.1, 95% CI: 3.0–5.8, P < 0.01). All‐cause mortality after HTX is shown in Table 3A.
Table 3A.
Outcomes after heart transplantation: primary outcome—all‐cause mortality after heart transplantation
| PVR < 300 | PVR ≥ 300 | Hazard ratio | 95% CI | P‐value | |
|---|---|---|---|---|---|
| dyn·s·cm−5 | dyn·s·cm−5 | ||||
| (<3.75 WU) | (≥3.75 WU) | ||||
| (n = 467) | (n = 94) | ||||
| 30 day all‐cause mortality, n (%) | 30 (6.4%) | 24 (25.5%) | 4.4 | 2.6 to 7.6 | <0.01 |
| 1 year all‐cause mortality, n (%) | 75 (16.1%) | 49 (52.1%) | 4.2 | 2.9 to 6.0 | <0.01 |
| 2 year all‐cause mortality, n (%) | 90 (19.3%) | 55 (58.5%) | 4.1 | 3.0 to 5.8 | <0.01 |
CI, confidence interval; dyn, g·cm·s−2; PVR, pulmonary vascular resistance; WU, Wood unit (≙ 80 dyn·s·cm−5).
Kaplan–Meier survival analysis showed an inferior survival after HTX in patients with a native PVR ≥ 300 dyn·s·cm−5 (P < 0.01). Additionally, patients with a native PVR ≥ 300 dyn·s·cm−5 and a remaining PVR ≥ 300 dyn·s·cm−5 after application of a vasodilator had an inferior survival after HTX compared with patients with a native PVR ≥ 300 dyn·s·cm−5 and a declining PVR < 300 dyn·s·cm−5 after application of a vasodilator (P < 0.01). Kaplan–Meier estimators are displayed in Figures 2 and 3 .
Figure 2.
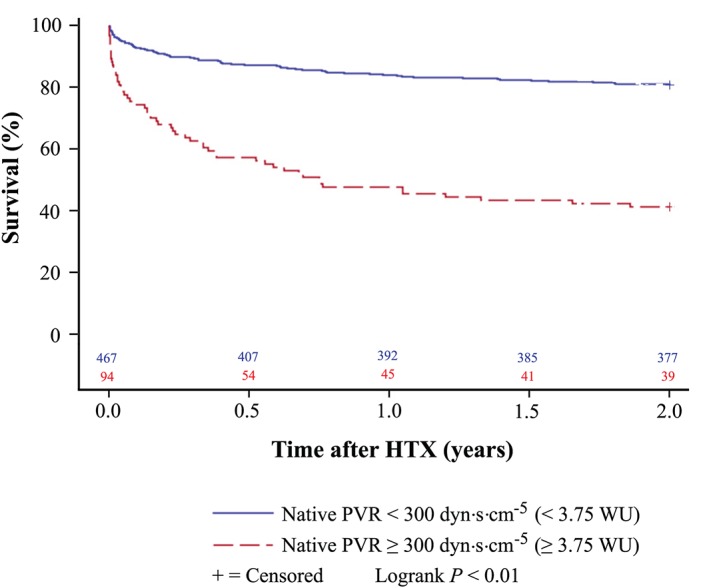
Survival after heart transplantation (HTX) (Kaplan–Meier estimator) stratified by native pre‐transplant pulmonary vascular resistance (PVR). Patients with a native pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 WU) showed a worse 2 year survival after HTX (P < 0.01) in comparison with patients with a native pre‐transplant PVR < 300 dyn·s·cm−5 (<3.75 WU). dyn, g·cm·s−2; WU, Wood unit (≙ 80 dyn·s·cm−5).
Figure 3.
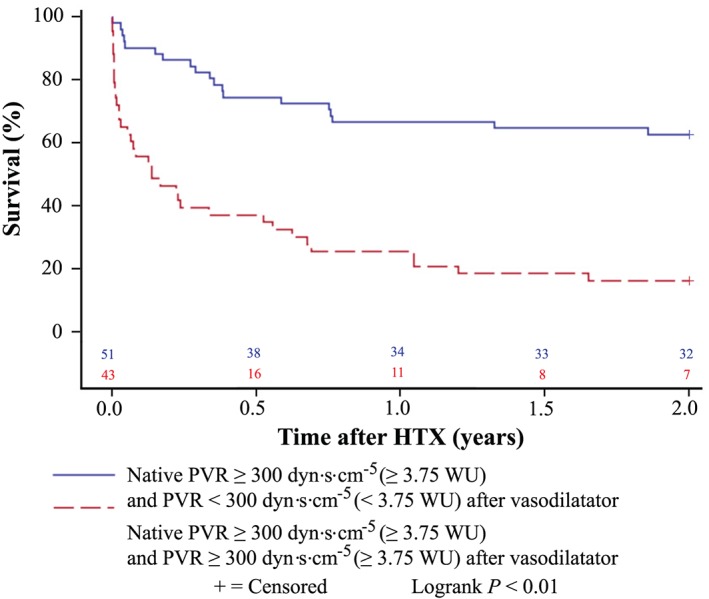
Survival after heart transplantation (HTX) (Kaplan–Meier estimator) stratified by pre‐transplant pulmonary vascular resistance (PVR) after application of a vasodilator in patients with a native pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 WU). Patients with a native pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 WU) and a remaining pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 WU) after application of a vasodilator had a worse 2 year survival after HTX than patients with a native pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 WU) and a declining pre‐transplant PVR < 300 dyn·s·cm−5 (<3.75 WU) after application of a vasodilator (P < 0.01). dyn, g·cm·s−2; WU, Wood unit (≙ 80 dyn·s·cm−5).
A stratified survival analysis showed that patients with a native PVR < 300 dyn·s·cm−5 had the highest 30 day post‐transplant survival (93.6%), followed by patients with a native PVR ≥ 300 dyn·s·cm−5 and a declining PVR < 300 dyn·s·cm−5 after application of a vasodilator (90.2%). Patients with a native PVR ≥ 300 dyn·s·cm−5 and a remaining PVR ≥ 300 dyn·s·cm−5 after application of a vasodilator had the lowest 30 day post‐transplant survival of all groups (55.8%). Survival after HTX by native PVR and PVR after application of a vasodilator is provided in Figure 4 .
Figure 4.
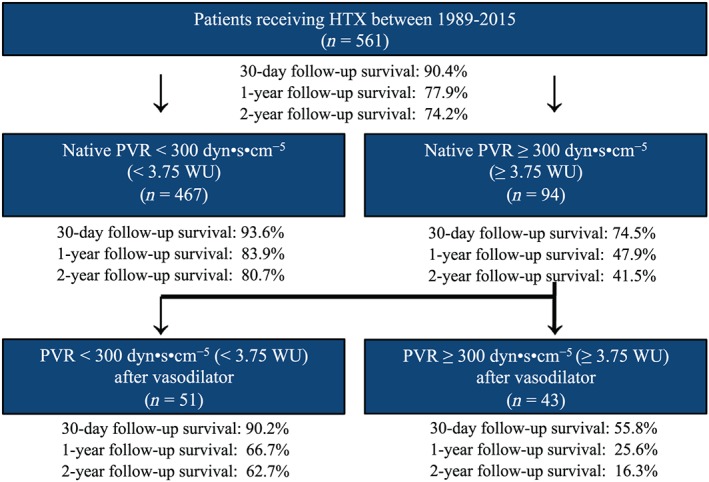
Overview of post‐transplant survival stratified by native pre‐transplant pulmonary vascular resistance (PVR) and pre‐transplant PVR after application of a vasodilator. Patients with a native pre‐transplant PVR < 300 dyn·s·cm−5 (<3.75 WU) had the best 30 day and 1 and 2 year survival after HTX, whereas patients with a native pre‐transplant PVR ≥ 300 dyn·s·cm−5 and a remaining pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 WU) after application of a vasodilator had the worst 30 day and 1 and 2 year survival after HTX. dyn = g·cm·s−2; WU, Wood unit (≙ 80 dyn·s·cm−5).
Fifty‐four patients (9.6%) died within 30 days after HTX. In the PVR < 300 dyn·s·cm−5 group, 30 patients (6.4%) died, while 24 patients (25.5%) passed away in the PVR ≥ 300 dyn·s·cm−5 group (difference: 19.1%, 95% CI: 10.0–28.2%, P < 0.01). Concerning the causes of death after HTX, significantly more patients died from transplant failure in the PVR ≥ 300 dyn·s·cm−5 group within 30 days after HTX (21.2 vs. 4.1%, difference: 17.1%, 95% CI: 8.7–25.5%, P < 0.01). This significant difference remained unchanged 1 year after HTX (27.7 vs. 5.4%, difference: 22.3%, 95% CI: 13.0–31.6%, P < 0.01) and was still found 2 years after HTX (28.7 vs. 6.4%, difference: 22.3%, 95% CI: 12.9–31.7%, P < 0.01). There were no significant differences between groups regarding the causes of death within 30 days after HTX in terms of acute rejection, infection/sepsis, malignancy, or thromboembolic event/bleeding. Causes of death within 30 days after HTX are shown in Table 3B.
Table 3B.
Outcomes after heart transplantation: primary outcome—causes of death within 30 days after heart transplantation
| PVR < 300 | PVR ≥ 300 | Difference | 95% CI | P‐value | |
|---|---|---|---|---|---|
| dyn·s·cm−5 | dyn·s·cm−5 | ||||
| (<3.75 WU) | (≥3.75 WU) | ||||
| (n = 467) | (n = 94) | ||||
| 30 day all‐cause mortality, n (%) | 30 (6.4%) | 24 (25.5%) | 19.1% | 10.0 to 28.2% | <0.01 |
| Transplant failure, n (%) | 19 (4.1%) | 20 (21.2%) | 17.1% | 8.7 to 25.5% | <0.01 |
| Acute rejection, n (%) | 1 (0.2%) | 1 (1.1%) | 0.9% | −1.3 to 3.1% | 0.21 |
| Infection/sepsis, n (%) | 7 (1.5%) | 2 (2.1%) | 0.6% | −2.5 to 3.7% | 0.66 |
| Malignancy, n (%) | 0 (0.0%) | 0 (0.0%) | 0.0% | n.a. | n.a. |
| Thromboembolic event/bleeding, n (%) | 3 (0.6%) | 1 (1.1%) | 0.5% | −1.7 to 2.7% | 0.66 |
CI, confidence interval; dyn, g·cm·s−2; n.a., not applicable; PVR, pulmonary vascular resistance; WU, Wood unit (≙ 80 dyn·s·cm−5).
3.3.2. Secondary outcomes
In terms of secondary outcomes, 69 of 561 patients (12.3%) had early post‐transplant AF within 30 days after HTX. Patients with a PVR ≥ 300 dyn·s·cm−5 had a higher percentage of AF within 30 days after HTX (20.2%) in comparison with patients with a PVR < 300 dyn·s·cm−5 (10.7%, difference: 9.5%, 95% CI: 0.9–18.1%, P = 0.01). Before HTX, 248 of 561 patients (44.2%) had diagnosed AF. There was no significant difference between patients with or without a PVR ≥ 300 dyn·s·cm−5 in the rate of diagnosed AF before HTX (P = 0.14).
Regarding the length of the initial hospital stay after HTX, there was no statistically significant difference between both groups (43.3 ± 19.6 vs. 40.7 ± 27.7 days, difference: 2.6 days, 95% CI: −3.4 to 8.6 days, P = 0.38). Likewise, the median length of the initial hospital stay was comparable between both groups (39 vs. 38 days with an inter‐quartile range of 34–49 vs. 29–51 days). Secondary outcomes after HTX are given in Table 3C.
Table 3C.
Outcomes after heart transplantation: secondary outcomes
| PVR < 300 | PVR ≥ 300 | Difference | 95% CI | P‐value | |
|---|---|---|---|---|---|
| dyn·s·cm−5 | dyn·s·cm−5 | ||||
| (<3.75 WU) | (≥3.75 WU) | ||||
| (n = 467) | (n = 94) | ||||
| Length of initial hospital stay (days), mean ± SD | 43.3 ± 19.6 | 40.7 ± 27.7 | 2.6 days | −3.4 to 8.6 days | 0.38 |
| 30 day follow‐up occurrence of AF, n (%) | 50 (10.7%) | 19 (20.2%) | 9.5% | 0.9 to 18.1% | 0.01 |
AF, atrial fibrillation; CI, confidence interval; dyn, g·cm·s−2; PVR, pulmonary vascular resistance; SD, standard deviation; WU, Wood unit (≙ 80 dyn·s·cm−5).
3.4. Multivariate analysis for post‐transplant survival
A multivariate analysis for mortality within 30 days after HTX was performed with the following six clinically relevant variables: native PVR before HTX ≥ 300 dyn·s·cm−5 (HR: 4.4, 95% CI: 2.5–7.6, P < 0.01), recipient age >60.0 years (HR: 1.2, 95% CI: 0.6–2.2, P = 0.61), coronary artery disease (HR: 0.7, 95% CI: 0.4–1.2, P = 0.20), previous open‐heart surgery (HR: 1.8, 95% CI: 0.9–3.3, P = 0.08), donor age >40 years (HR: 1.1, 95% CI: 0.6–1.9, P = 0.76), and ischaemic time ≥240 min (HR: 0.6, 95% CI: 0.3–1.2, P = 0.16). Multivariate analysis for mortality within 30 days after HTX is provided in Table 4.
Table 4.
Multivariate analysis for mortality within 30 days after HTX
| Hazard ratio | 95% confidence interval | P‐value | |
|---|---|---|---|
| Native PVR before HTX (≥300 dyn·s·cm−5 ≙ 3.75 WU) | 4.4 | 2.5–7.6 | <0.01 |
| Recipient age (>60 years) | 1.2 | 0.6–2.2 | 0.61 |
| Coronary artery disease (in total) | 0.7 | 0.4–1.2 | 0.20 |
| Previous open‐heart surgery (in total) | 1.8 | 0.9–3.3 | 0.08 |
| Donor age (>40 years) | 1.1 | 0.6–1.9 | 0.76 |
| Ischaemic time (≥240 min) | 0.6 | 0.3–1.2 | 0.16 |
dyn, g·cm·s−2; HTX, heart transplantation; PVR, pulmonary vascular resistance; WU, Wood unit (≙ 80 dyn·s·cm−5).
3.5. Sensitivity analysis
A sensitivity analysis to test the robustness of the study results and to investigate a possible era effect was carried out with a subgroup of patients receiving CsA and azathioprine [263 of 561 patients (46.9%)]. This analysis showed similar results in terms of the primary outcome (post‐transplant mortality) and the secondary outcomes (length of the initial hospital stay and occurrence of AF within 30 days after HTX) confirming the robustness of the study results and minimizing the likelihood of an era effect.
In regard to potential differences in outcomes between patients with and without a native PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units), data from the RHC at the time of HTX listing vs. the latest RHC before HTX were compared. Overall conclusions did not change, but effects were more pronounced in the RHC group close to HTX, which is in line with our initial assumptions.
4. Discussion
4.1. Influence of an elevated pre‐transplant pulmonary vascular resistance on outcomes after heart transplantation
In heart transplant recipients with an elevated pre‐transplant PVR, the right donor ventricle that was formerly used to a normal PVR is now exposed to an increased PVR.15 It requires a great effort from the right donor ventricle to pump against a high‐resistance pulmonary circulation and can potentially lead to acute right ventricular dilatation and ultimately right‐sided heart failure.5, 15 Adequate assessment of pre‐transplant cardiopulmonary haemodynamics including PVR is therefore essential to analyse the suitability of patients for HTX and to reduce the risk of right‐sided heart failure after HTX.15
4.2. Length of the initial hospital stay and early atrial fibrillation after heart transplantation
The presence of an elevated PVR has been associated with an increased risk for hospitalization and prolonged hospital stays in non‐transplant patients.37, 38 We could not detect a statistically significant difference between patients with and without a PVR ≥ 300 dyn·s·cm−5 in the length of the initial hospital stay after HTX. This may be due to the fact that patients with a PVR ≥ 300 dyn·s·cm−5 had a considerably higher 30 day mortality after HTX. An increased in‐hospital mortality causes a decrease in length of the initial hospital stay and could have therefore affected this outcome.
Cardiac arrhythmias, including AF, have been linked to an increased PVR.39, 40, 41 Patients with AF show a higher risk for morbidity especially for thromboembolic complications such as transient ischaemic attack or stroke.42 Several studies have even reported an inferior survival in patients with AF after HTX.32, 43, 44
In the initial period after HTX, the right donor ventricle in patients with an elevated pre‐transplant PVR is subject to considerable changes in the cardiopulmonary physiology including pulmonary hypertension, hypoxemia, hypercarbia, and acidosis. Especially the concomitance of an elevated PVR and increased oxidative stress can provoke the occurrence of AF.34, 45, 46
We found a significantly higher percentage of early AF after HTX in patients with a PVR ≥ 300 dyn·s·cm−5. Given the increased mortality after HTX in patients with early post‐transplant AF and the AF‐associated risk for thromboembolic complications, patients with a PVR ≥ 300 dyn·s·cm−5 should be closely watched with ECG and Holter monitoring for the occurrence of early AF after HTX.32, 34, 42, 43, 44
4.3. Mortality and causes of death after heart transplantation
The influence of an elevated pre‐transplant PVR on outcomes after HTX has been evaluated by multiple studies.4, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 However, the association between an elevated PVR and increased mortality after HTX remains indeterminate as some studies reported an increased post‐transplant mortality in patients with an elevated pre‐transplant PVR,4, 11, 13, 14, 19, 22, 27 whereas other studies showed no difference.17, 18, 23, 24, 25, 26 Murali et al.15 reported an increased 2 day mortality after HTX in patients with a pre‐transplant PVR ≥ 400 dyn·s·cm−5; however, no difference in 30 day mortality after HTX was found. Many of these studies were rather small single‐centre studies and varied in study design, sample size, follow‐up, and definition of an elevated pre‐transplant PVR.
A recent large multi‐centre study of the United Network for Organ Sharing registry by Vakil et al.27 showed an elevated pre‐transplant PVR ≥ 200 dyn·s·cm−5 as a significant predictor of 30 day mortality after HTX. Furthermore, analysis of subgroups revealed a significantly inferior 30 day post‐transplant survival in patients with severely elevated pre‐transplant PVR ≥ 400 dyn·s·cm−5 in comparison with patients with normal pre‐transplant PVR < 200 dyn·s·cm−5. However, the authors did not detect a significant difference in 30 day mortality after HTX when collating the results of patients having normal pre‐transplant PVR < 200 dyn·s·cm−5 with patients having mildly/moderately elevated PVR between 200 and 400 dyn·s·cm−5. Unfortunately, there was no reported comparison in terms of short‐term mortality between patients with normal or mildly elevated pre‐transplant PVR < 300 dyn·s·cm−5 and patients with a moderately elevated pre‐transplant PVR ≥ 300 dyn·s·cm−5. Therefore, the influence of a moderately elevated pre‐transplant PVR on mortality after HTX remains uncertain.10, 22, 27
Chang et al.22 showed in a single‐centre study an increased risk of mortality after HTX with each 80 dyn·s·cm−5 increase in pre‐transplant PVR. In contrast, Addonizio et al.10 found in another single‐centre study no correlation between the degree of an elevated pre‐transplant PVR and mortality after HTX. A large multi‐centre study of the ISHLT registry reported a linear correlation between pre‐transplant PVR and 1 year mortality after HTX.4 These findings suggest that even moderately increased levels of pre‐transplant PVR may be associated with reduced survival after HTX.
Univariate and multivariate analysis showed a significantly increased 30 day mortality after HTX (25.5 vs. 6.4%) in patients with a pre‐transplant PVR ≥ 300 dyn·s·cm−5 along with a higher percentage of death due to transplant failure. Furthermore, Kaplan–Meier survival analysis showed an inferior 2 year survival after HTX in patients with a pre‐transplant PVR ≥ 300 dyn·s·cm−5. This is in line with findings by Delgado et al.19 reporting an increased 30 day mortality after HTX (24.4 vs. 5.6%) and by Costard‐Jäckle et al.14 describing an increased 3 month mortality after HTX (17.9 vs. 6.9%) in patients with an increased pre‐transplant PVR > 200 dyn·s·cm−5.
Our study did not include patients with implanted VAD before HTX as those cause a change in haemodynamic parameters.3, 47, 48, 49, 50 Previous studies reported a decrease of PVR in patients with VAD implantation who were initially rejected for HTX listing due to severely elevated PVR eventually enabling candidacy for HTX.3, 47, 48, 49, 50 However, results regarding an improved survival after HTX in these patients compared with patients with an elevated PVR without former VAD implantation were inconclusive.3, 47, 48, 49, 50 Further therapeutic options for patients who are not eligible for regular HTX listing due to increased PVR cover heterotopic HTX or combined heart and lung transplantation but have a worse prognosis than standard orthotopic HTX.2, 25, 49
Altogether, we found an increased short‐term and midterm mortality after HTX along with a higher percentage of death due to transplant failure in patients with a moderately elevated pre‐transplant PVR. As survival after HTX may be affected by several risk factors and transplant failure after HTX is multifactorial, it is uncertain whether these findings can be applied to all patients who received HTX. Moreover, the therapeutic effects of applied pharmacological agents of vasodilation after HTX on survival in patients after HTX require further investigation in future studies.
4.4. Study limitations
Our findings were derived from a large observational retrospective single‐centre registry. Given the retrospective, non‐randomized study design, results should be interpreted with caution as the study design carries certain limitations and may be subject to unmeasured confounders. This retrospective study cannot proof or disproof a causal relationship between an elevated pre‐transplant PVR and an increased mortality in patients after HTX but merely indicates an association between the two. Despite its limitations, our analysis provides important and reliable information to the existing body of evidence. This large single‐centre study included a total of 561 patients with a complete 2 year follow‐up after HTX, which is comparable in participant numbers to multi‐centre studies. Furthermore, the used study design has the advantage of a standardized centre‐specific pre‐transplant, peri‐transplant, and post‐transplant course of treatment and follow‐up of patients after HTX. These positive aspects should be balanced against potential disadvantages.28, 29, 30, 31, 32, 33, 34, 35, 36
This analysis included patients receiving HTX at the Heidelberg Heart Center between 06/1989 and 12/2015. Because of the long study period, a possible era effect due to changes in medical care may have affected outcomes. As the initial standard immunosuppressive drug regimen was changed during the study period, a sensitivity analysis including a subgroup of patients with CsA and azathioprine was carried out to investigate a possible era effect and to test the robustness of the study results. This analysis showed similar results. A further change of medical treatment during the study period was the clinical introduction of ivabradine in 2006, which is used for heart rate reduction in patients after HTX. However, there was no significant difference in the use of ivabradine between both groups.33, 34
Finally, our results should be considered as hypothesis generating, especially in terms of post‐transplant survival as multiple factors may affect survival after HTX. Thus, to confirm our results, further large prospective multi‐centre trials are preferable to analyse the influence of an elevated pre‐transplant PVR on outcomes after HTX.28, 29, 30, 31, 32, 33, 34
4.5. Conclusions
Patients with a pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) had an increased 30 day mortality after HTX driven by a higher rate of death due to transplant failure. Multivariate analysis showed a PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) as an independent risk factor for impaired post‐transplant survival with a more than four‐fold increased risk of death within 30 days after HTX. Kaplan–Meier estimator additionally showed an inferior post‐transplant 2 year survival in patients with a PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units).
Furthermore, patients with a PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) had a higher incidence of AF within 30 days after HTX, whereas there was no significant difference between groups regarding the length of the initial hospital stay after HTX.
In summary, an elevated pre‐transplant PVR ≥ 300 dyn·s·cm−5 (≥3.75 Wood units) is associated with early post‐transplant AF and an inferior post‐transplant survival along with a higher percentage of death due to transplant failure.
Conflict of interest
None declared.
Funding
This work was supported by research grants from the Faculty of Medicine, University of Heidelberg (Medizinischen Fakultät Heidelberg, Universität Heidelberg; Physician Scientist Program Scholarship to R.R.), by the German Cardiac Society (Deutsche Gesellschaft für Kardiologie‐Herz und Kreislaufforschung; Research Scholarship to R.R.), and by the Ministry of Science, Research and the Arts Baden‐Württemberg (Sonderlinie Medizin to D.T.).
Acknowledgements
We thank Anna Daut, Viola Deneke, and Berthold Klein for their assistance and advice.
Rivinius, R. , Helmschrott, M. , Ruhparwar, A. , Schmack, B. , Darche, F. F. , Thomas, D. , Bruckner, T. , Doesch, A. O. , Katus, H. A. , and Ehlermann, P. (2020) Elevated pre‐transplant pulmonary vascular resistance is associated with early post‐transplant atrial fibrillation and mortality. ESC Heart Failure, 7: 176–187. 10.1002/ehf2.12549.
References
- 1. Stobierska‐Dzierzek B, Awad H, Michler RE. The evolving management of acute right‐sided heart failure in cardiac transplant recipients. J Am Coll Cardiol 2001; 38: 923–931. [DOI] [PubMed] [Google Scholar]
- 2. Vassileva A, Valsecchi O, Sebastiani R, Fontana A, Gamba A. Heterotopic heart transplantation for elevated pulmonary vascular resistance in the current era: long‐term clinical and hemodynamic outcomes. J Heart Lung Transplant 2013; 32: 934–936. [DOI] [PubMed] [Google Scholar]
- 3. Alba AC, Rao V, Ross HJ, Jensen AS, Sander K, Gustafsson F, Delgado DH. Impact of fixed pulmonary hypertension on post‐heart transplant outcomes in bridge‐to‐transplant patients. J Heart Lung Transplant 2010; 29: 1253–1258. [DOI] [PubMed] [Google Scholar]
- 4. Hosenpud JD, Bennett LE, Keck BM, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: seventeenth official report‐2000. J Heart Lung Transplant 2000; 19: 909–931. [DOI] [PubMed] [Google Scholar]
- 5. Griepp RB, Stinson EB, Dong E Jr, Clark DA, Shumway NE. Determinants of operative risk in human heart transplantation. Am J Surg 1971; 122: 192–197. [DOI] [PubMed] [Google Scholar]
- 6. Bozbaş H, Karaçağlar E, Ozkan M, Bozbaş SS, Eyüboğlu FÖ, Sade E, Altin C, Polat E, Sezgin A, Müderrisoğlu H. The prevalence and course of pulmonary hypertension and right ventricular dysfunction in patients undergoing orthotopic heart transplantation. Transplant Proc 2013; 45: 3538–3541. [DOI] [PubMed] [Google Scholar]
- 7. Gorlitzer M, Ankersmit J, Fiegl N, Meinhart J, Lanzenberger M, Unal K, Dunkler D, Kilo J, Wolner E, Grimm M, Grabenwoeger M. Is the transpulmonary pressure gradient a predictor for mortality after orthotopic cardiac transplantation? Transpl Int 2005; 18: 390–395. [DOI] [PubMed] [Google Scholar]
- 8. Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates—2006. J Heart Lung Transplant 2006; 25: 1024–1042. [DOI] [PubMed] [Google Scholar]
- 9. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger‐Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EA, Zuckermann A, International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases Council; International Society for Heart Lung Transplantation (ISHLT) Pediatric Transplantation Council; International Society for Heart Lung Transplantation (ISHLT) Heart Failure and Transplantation Council . The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10‐year update. J Heart Lung Transplant 2016; 35: 1–23. [DOI] [PubMed] [Google Scholar]
- 10. Addonizio LJ, Gersony WM, Robbins RC, Drusin RE, Smith CR, Reison DS, Reemtsma K, Rose EA. Elevated pulmonary vascular resistance and cardiac transplantation. Circulation 1987; 76: 52–55. [PubMed] [Google Scholar]
- 11. Kirklin JK, Naftel DC, Kirklin JW, Blackstone EH, White‐Williams C, Bourge RC. Pulmonary vascular resistance and the risk of heart transplantation. J Heart Transplant 1988; 7: 331–336. [PubMed] [Google Scholar]
- 12. Erickson KW, Costanzo‐Nordin MR, O'Sullivan EJ, Johnson MR, Zucker MJ, Pifarré R, Lawless CE, Robinson JA, Scanlon PJ. Influence of preoperative transpulmonary gradient on late mortality after orthotopic heart transplantation. J Heart Transplant 1990; 9: 526–537. [PubMed] [Google Scholar]
- 13. Bourge RC, Kirklin JK, Naftel DC, White C, Mason DA, Epstein AE. Analysis and predictors of pulmonary vascular resistance after cardiac transplantation. J Thorac Cardiovasc Surg 1991; 101: 432–445. [PubMed] [Google Scholar]
- 14. Costard‐Jäckle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol 1992; 19: 48–54. [DOI] [PubMed] [Google Scholar]
- 15. Murali S, Kormos RL, Uretsky BF, Schechter D, Reddy PS, Denys BG, Armitage JM, Hardesty RL, Griffith BP. Preoperative pulmonary hemodynamics and early mortality after orthotopic cardiac transplantation: the Pittsburgh experience. Am Heart J 1993; 126: 896–904. [DOI] [PubMed] [Google Scholar]
- 16. Chen JM, Levin HR, Michler RE, Prusmack CJ, Rose EA, Aaronson KD. Reevaluating the significance of pulmonary hypertension before cardiac transplantation: determination of optimal thresholds and quantification of the effect of reversibility on perioperative mortality. J Thorac Cardiovasc Surg 1997; 114: 627–634. [DOI] [PubMed] [Google Scholar]
- 17. Tenderich G, Koerner MM, Stuettgen B, Hornik L, Mirow N, Morshuis M, Mannebach H, Minami K, Koerfer R. Does preexisting elevated pulmonary vascular resistance (transpulmonary gradient >15 mm Hg or >5 wood) predict early and long‐term results after orthotopic heart transplantation? Transplant Proc 1998; 30: 1130–1131. [DOI] [PubMed] [Google Scholar]
- 18. Lindelöw B, Andersson B, Waagstein F, Bergh CH. High and low pulmonary vascular resistance in heart transplant candidates. A 5‐year follow‐up after heart transplantation shows continuous reduction in resistance and no difference in complication rate. Eur Heart J 1999; 20: 148–156. [DOI] [PubMed] [Google Scholar]
- 19. Delgado JF, Gómez‐Sánchez MA, Sáenz de la Calzada C, Sánchez V, Escribano P, Hernández‐Afonso J, Tello R, Gómez de la Cámara A, Rodríguez E, Rufilanchas JJ. Impact of mild pulmonary hypertension on mortality and pulmonary artery pressure profile after heart transplantation. J Heart Lung Transplant 2001; 20: 942–948. [DOI] [PubMed] [Google Scholar]
- 20. Klotz S, Deng MC, Hanafy D, Schmid C, Stypmann J, Schmidt C, Hammel D, Scheld HH. Reversible pulmonary hypertension in heart transplant candidates—pretransplant evaluation and outcome after orthotopic heart transplantation. Eur J Heart Fail 2003; 5: 645–653. [DOI] [PubMed] [Google Scholar]
- 21. Butler J, Stankewicz MA, Wu J, Chomsky DB, Howser RL, Khadim G, Davis SF, Pierson RN 3rd, Wilson JR. Pre‐transplant reversible pulmonary hypertension predicts higher risk for mortality after cardiac transplantation. J Heart Lung Transplant 2005; 24: 170–177. [DOI] [PubMed] [Google Scholar]
- 22. Chang PP, Longenecker JC, Wang NY, Baughman KL, Conte JV, Hare JM, Kasper EK. Mild vs severe pulmonary hypertension before heart transplantation: different effects on posttransplantation pulmonary hypertension and mortality. J Heart Lung Transplant 2005; 24: 998–1007. [DOI] [PubMed] [Google Scholar]
- 23. Klotz S, Wenzelburger F, Stypmann J, Welp H, Drees G, Schmid C, Scheld HH. Reversible pulmonary hypertension in heart transplant candidates: to transplant or not to transplant. Ann Thorac Surg 2006; 82: 1770–1773. [DOI] [PubMed] [Google Scholar]
- 24. Wever‐Pinzon O, Drakos SG, Kfoury AG, Nativi JN, Gilbert EM, Everitt M, Alharethi R, Brunisholz K, Bader FM, Li DY, Selzman CH, Stehlik J. Effect of reversible pulmonary hypertension on outcomes after heart transplantation. J Heart Lung Transplant 2007; 26: 319–323. [DOI] [PubMed] [Google Scholar]
- 25. Goland S, Czer LS, Kass RM, De Robertis MA, Mirocha J, Coleman B, Capelli C, Raissi S, Cheng W, Fontana G, Trento A. Pre‐existing pulmonary hypertension in patients with end‐stage heart failure: impact on clinical outcome and hemodynamic follow‐up after orthotopic heart transplantation. J Heart Lung Transplant 2007; 26: 312–318. [DOI] [PubMed] [Google Scholar]
- 26. Gude E, Simonsen S, Geiran OR, Fiane AE, Gullestad L, Arora S, Relbo A, Andreassen AK. Pulmonary hypertension in heart transplantation: discrepant prognostic impact of pre‐operative compared with 1‐year post‐operative right heart hemodynamics. J Heart Lung Transplant 2010; 29: 216–223. [DOI] [PubMed] [Google Scholar]
- 27. Vakil K, Duval S, Sharma A, Adabag S, Abidi KS, Taimeh Z, Colvin‐Adams M. Impact of pre‐transplant pulmonary hypertension on survival after heart transplantation: a UNOS registry analysis. Int J Cardiol 2014; 176: 595–599. [DOI] [PubMed] [Google Scholar]
- 28. Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Klein B, Erbel C, Gleissner CA, Akhavanpoor M, Frankenstein L, Darche FF, Thomas D, Ehlermann P, Bruckner T, Katus HA, Doesch AO. Analysis of malignancies in patients after heart transplantation with subsequent immunosuppressive therapy. Drug Des Devel Ther 2014; 9: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Erbel C, Gleissner CA, Akhavanpoor M, Frankenstein L, Darche FF, Schweizer PA, Thomas D, Ehlermann P, Bruckner T, Katus HA, Doesch AO. Long‐term use of amiodarone before heart transplantation reduces significantly early post‐transplant atrial fibrillation and is not associated with increased mortality after heart transplantation. Drug Des Devel Ther 2016; 10: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rivinius R, Helmschrott M, Ruhparwar A, Darche FF, Thomas D, Bruckner T, Katus HA, Doesch AO. Comparison of posttransplant outcomes in patients with no, acute, or chronic amiodarone use before heart transplantation. Drug Des Devel Ther 2017; 11: 1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rivinius R, Helmschrott M, Ruhparwar A, Rahm AK, Darche FF, Thomas D, Bruckner T, Ehlermann P, Katus HA, Doesch AO. Chronic digitalis therapy in patients before heart transplantation is an independent risk factor for increased post‐transplant mortality. Ther Clin Risk Manag 2017; 13: 1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rivinius R, Helmschrott M, Ruhparwar A, Erbel C, Gleissner CA, Darche FF, Thomas D, Bruckner T, Katus HA, Doesch AO. The influence of surgical technique on early posttransplant atrial fibrillation—comparison of biatrial, bicaval, and total orthotopic heart transplantation. Ther Clin Risk Manag 2017; 13: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rivinius R, Helmschrott M, Ruhparwar A, Rahm AK, Darche FF, Thomas D, Bruckner T, Ehlermann P, Katus HA, Doesch AO. Control of cardiac chronotropic function in patients after heart transplantation: effects of ivabradine and metoprolol succinate on resting heart rate in the denervated heart. Clin Res Cardiol 2018; 107: 138–147. [DOI] [PubMed] [Google Scholar]
- 34. Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Darche FF, Thomas D, Bruckner T, Katus HA, Ehlermann P, Doesch AO. Chronic obstructive pulmonary disease in patients after heart transplantation is associated with a prolonged hospital stay, early post‐transplant atrial fibrillation, and impaired post‐transplant survival. Clin Epidemiol 2018; 10: 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Helmschrott M, Rivinius R, Ruhparwar A, Schmack B, Erbel C, Gleissner CA, Akhavanpoor M, Frankenstein L, Ehlermann P, Bruckner T, Katus HA, Doesch AO. Advantageous effects of immunosuppression with tacrolimus in comparison with cyclosporine A regarding renal function in patients after heart transplantation. Drug Des Devel Ther 2015; 9: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Helmschrott M, Rivinius R, Bruckner T, Katus HA, Doesch AO. Renal function in heart transplant patients after switch to combined mammalian target of rapamycin inhibitor and calcineurin inhibitor therapy. Drug Des Devel Ther 2017; 11: 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anand V, Roy SS, Archer SL, Weir EK, Garg SK, Duval S, Thenappan T. Trends and outcomes of pulmonary arterial hypertension‐related hospitalizations in the United States: analysis of the nationwide inpatient sample database from 2001 through 2012. JAMA Cardiol 2016; 1: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 38. Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol 2018; 3: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fabregat‐Andrés Ó, Estornell‐Erill J, Ridocci‐Soriano F, García‐González P, Bochard‐Villanueva B, Cubillos‐Arango A, Espriella‐Juan Rde L, Fácila L, Morell S, Cortijo J. Prognostic value of pulmonary vascular resistance estimated by cardiac magnetic resonance in patients with chronic heart failure. Eur Heart J Cardiovasc Imaging 2014; 15: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 40. Gajarski RJ, Towbin JA, Bricker JT, Radovancevic B, Frazier OH, Price JK, Schowengerdt KO, Denfield SW. Intermediate follow‐up of pediatric heart transplant recipients with elevated pulmonary vascular resistance index. J Am Coll Cardiol 1994; 23: 1682–1687. [DOI] [PubMed] [Google Scholar]
- 41. Rajdev A, Garan H, Biviano A. Arrhythmias in pulmonary arterial hypertension. Prog Cardiovasc Dis 2012; 55: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hohnloser SH, Pajitnev D, Pogue J, Healey JS, Pfeffer MA, Yusuf S, Connolly SJ, Investigators ACTIVEW. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol 2007; 50: 2156–2161. [DOI] [PubMed] [Google Scholar]
- 43. Dasari TW, Pavlovic‐Surjancev B, Patel N, Williams AA, Ezidinma P, Rupani A, Sinacore JL, Heroux AL. Incidence, risk factors, and clinical outcomes of atrial fibrillation and atrial flutter after heart transplantation. Am J Cardiol 2010; 106: 737–741. [DOI] [PubMed] [Google Scholar]
- 44. Pavri BB, O'Nunain SS, Newell JB, Ruskin JN, William G. Prevalence and prognostic significance of atrial arrhythmias after orthotopic cardiac transplantation. J Am Coll Cardiol 1995; 25: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 45. Gasparova I, Kubatka P, Opatrilova R, Caprnda M, Filipova S, Rodrigo L, Malan L, Mozos I, Rabajdova M, Nosal V, Kobyliak N, Valentova V, Petrovic D, Adamek M, Kruzliak P. Perspectives and challenges of antioxidant therapy for atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol 2017; 390: 1–14. [DOI] [PubMed] [Google Scholar]
- 46. Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol 2007; 115: 135–143. [DOI] [PubMed] [Google Scholar]
- 47. Salzberg SP, Lachat ML, von Harbou K, Zünd G, Turina MI. Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur J Cardiothorac Surg 2005; 27: 222–225. [DOI] [PubMed] [Google Scholar]
- 48. Liden H, Haraldsson A, Ricksten SE, Kjellman U, Wiklund L. Does pretransplant left ventricular assist device therapy improve results after heart transplantation in patients with elevated pulmonary vascular resistance? Eur J Cardiothorac Surg 2009; 35: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 49. Beyersdorf F, Schlensak C, Berchtold‐Herz M, Trummer G. Regression of “fixed” pulmonary vascular resistance in heart transplant candidates after unloading with ventricular assist devices. J Thorac Cardiovasc Surg 2010; 140: 747–749. [DOI] [PubMed] [Google Scholar]
- 50. Tsukashita M, Takayama H, Takeda K, Han J, Colombo PC, Yuzefpolskaya M, Topkara VK, Garan AR, Mancini DM, Kurlansky PA, Naka Y. Effect of pulmonary vascular resistance before left ventricular assist device implantation on short‐ and long‐term post‐transplant survival. J Thorac Cardiovasc Surg 2015; 150: 1352–1361. [DOI] [PubMed] [Google Scholar]


