Abstract
Aims
In May 2016, a new version of the European Society of Cardiology (ESC) Guidelines for the management of heart failure (HF) was released. The aim of this study was to describe the management of HF with reduced ejection fraction after the publication of ESC Guidelines.
Methods and results
The Linx registry is a multicentre, observational, cross‐sectional study from 14 Catalan hospitals that enrolled 1056 patients with HF and reduced left ventricular ejection fraction (≤40%) from 1 February to 30 April 2017 in outpatient cardiology clinics. Results were compared between hospitals according to their level of complexity in our own registry and compared with previously published registries similar to ours. Sacubitril/valsartan was prescribed to 23.9% of patients in our population, as a consequence, use of angiotensin‐converting enzyme inhibitor and angiotensin receptor blockers in monotherapy decreased to 48.1% and 16.9%, respectively, and prescription of beta‐blockers (91.8%), mineralocorticoid receptor antagonists (72.7%), and ivabradine (21.4%) remained similar to previous registries. Target doses of beta‐blockers (25.4%), angiotensin‐converting enzyme inhibitors (24.9%), angiotensin receptor blockers (7.7%), sacubitril/valsartan (8.1%), and mineralocorticoid receptor antagonists (19.7%) were accomplished in a low proportion of patients. Our results also suggest that prescription and up‐titration of class I HF drugs were greater in hospitals with higher level of complexity.
Conclusions
The Linx registry shows an appropriate adherence to pharmacological recommendations from ESC HF Guidelines despite a low proportion of patients reached target doses. Almost one‐quarter of patients were under treatment with sacubitril/valsartan a few months after ESC HF Guidelines recommendations.
Keywords: Chronic heart failure, Guidelines, Heart failure, Management, Sacubitril/valsartan
1. Introduction
Heart failure (HF) is a global health issue with a prevalence between 1% and 2% in Western countries,1, 2 an estimated 26 million affected worldwide,3 and one of the leading sources of health care resource use and expenditure.4 Although in Western countries there is currently a trend towards a reduction in the incidence of HF with reduced ejection fraction [HFrEF; left ventricular ejection fraction (LVEF) ≤40%],5 it still represents almost one‐third of incident HF cases6 and has a mortality rate of 8.8% at 1 year.7
Pharmacological management of HFrEF aims to regulate the physiological compensatory response to low cardiac output, which in the long‐term prompts maladaptive cardiac remodelling finally leading to chronic HF.8 Blocking the adrenergic and the renin–angiotensin–aldosterone systems has been the classical target of HF drugs.9, 10, 11 In addition, drugs that reduce heart rate through inhibition of sinoatrial cardiac myocites, such as ivabradine,12 or increase plasma levels of natriuretic peptides through inhibition of neprilysin, such as sacubitril/valsartan,13 have shown clinical benefits in the last decade.
In May 2016, a new version of the European Society of Cardiology (ESC) Guidelines for the management of acute and chronic HF was released.14 The document included updated recommendations regarding the diagnosis, pharmacological and invasive treatment of these patients, such as introduction of sacubitril/valsartan as a class I therapy, or recommendations for the early initiation of key HF drugs.
To our knowledge, no major registries of HFrEF outpatients have been published after the publication of ESC 2016 Guidelines.14 The purpose of the present study was thus to (i) describe the characteristics of a large population HFrEF patients recruited after ESC 2016 Guidelines14 release, (ii) compare the pharmacological and invasive management of HFrEF patients by level of hospital complexity, and (iii) compare this management with those from previous registries.
2. Methods
2.1. Study design and population
The Linx registry is a multicentre, observational, cross‐sectional study from 14 Catalan hospitals (Figure 1 ) aimed at assessing the impact of the new ESC 2016 Guidelines14 in the management of HFrEF outpatients in the real‐world setting.
Figure 1.
Hospital geographical location. 1. Hospital Universitari de Bellvitge (L'Hospitalet de Llobregat), 2. Hospital Clinic (Barcelona), 3. Hospital Universitari Germans Trias i Pujol (Badalona), 4. Hospital de la Santa Creu i Sant Pau (Barcelona), 5. Hospital Universitari Vall d'Hebron (Barcelona), 6. Hospital Universitari Arnau de Vilanova (Lleida), 7. Hospital del Mar (Barcelona), 8. Corporació Sanitària Parc Taulí (Sabadell), 9. Hospital de Figueres (Figueres), 10. Hospital de Granollers (Granollers), 11. Hospital Moisès Broggi (Sant Joan Despí), 12. Hospital Sant Joan de Deu de Martorell (Martorell), 13. Hospital de Mataró (Mataró), 14. Hospital de Viladecans (Viladecans). 1–5 tertiary hospitals, 6–8 secondary hospitals, 9–14 primary hospitals.
For the Linx registry, patients were prospectively enrolled from the outpatient cardiology clinics of each participating hospital from 1 February to 30 April 2017. This included general cardiology clinics (mostly in primary hospitals) as well as specialized HF clinics (typically in tertiary hospitals). Inclusion criteria were a diagnosis of HF according ESC 2016 Guidelines14 and reduced LVEF (defined by LVEF ≤40% in the most recent echocardiography during follow‐up). Exclusion criteria were age under 18 years old, use of left ventricular assist devices, or heart transplantation before study entry. All of these patients were included in the present analysis.
Patients included in the Linx registry were managed according to the local HFrEF management protocols; at the time of inclusion, there were no regulatory restrictions to prescription of angiotensin receptor neprilysin inhibitor (ARNI). For the present analysis, the outpatient clinic visit in which patients were included in the study will be considered the ‘index' visit.
2.2. Data collection
Information on demographic characteristics, HF aetiology, key HF clinical variables (e.g. LVEF and New York Heart Association functional class), and relevant co‐morbidities was collected by local researchers using the local medical records. Information on vital signs and electrocardiographic parameters was obtained from the information generated in the most recent outpatient clinic visit before study entry. Data on blood sample tests [including levels of N‐terminal pro‐B type natriuretic peptide (NT‐proBNP)] and echocardiographic parameters were obtained from the most recent tests before study entry.
Data on pharmacological therapies [including beta‐blockers, angiotensin‐converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), ARNIs, and ivabradine, among others], their doses, and use of key HF devices [cardiac resynchronization therapy (CRT) and implantable cardioverter defibrillator (ICD)] were collected by local researchers at the end of the index visit.
2.3. Definition of hospital type
In order to compare the clinical characteristics and pharmacological management between hospitals according to their level of complexity, we classified recruiting centres in three categories: primary, secondary, and tertiary. Hospitals providing neither interventional cardiology nor cardiac surgery were classified as primary centres (six), hospitals providing interventional cardiology but not cardiac surgery were classified as secondary centres (three), and hospitals providing both were classified as tertiary centres (five). This classification has been widely used in previously published HF registries.15
2.4. Statistical analyses
Descriptive statistics were used to summarize the characteristics of the study participants, including therapies and drug doses. Categorical variables are presented as number and percentage, continuous variables with normal distribution are presented using mean and standard deviation, and non‐normal continuous variables are presented using median and interquartile range.
In the comparative analyses by level of hospital complexity, these characteristics were compared using chi‐squared tests for categorical variables, and ANOVA and non‐parametric tests for continuous variables, as appropriate.
The proportion of patients in whom target doses of each key HF pharmacotherapy were achieved (according to ESC 2016 Guidelines14) was also calculated, overall and by hospital type. As a graphical, intuitive summary, these proportions were plotted using a radial graph or ‘radar chart'.26 Although this graph has not been common in HF research, it allows accommodating in one single image multiple data (in the present analysis, multiple data on target dose use) in a more efficient way than traditional bar charts. It also allows calculating the area of the polygon, and the proportion over the total area, as summary measures. Specifically, we calculated the proportion over the total area for four polygons, as a percentage of the maximum theoretical target dose: overall (i.e. all hospitals), tertiary, secondary, and primary hospitals. More details on the calculations performed for the radial graph are presented in the Supporting Information, Data S1.
We also compared the overall proportions of users of each drug type and of HF devices in the Linx registry, with the results of the Spanish cohort of the ESC Heart Failure Long‐term Registry,16 a similar multicentre registry including 1526 Spanish HFrEF patients, published before the release of the ESC 2016 Guidelines. Proportions were compared again using the chi‐squared statistic.
Finally, we plotted the present results together with the results from similar, previously published landmark European HFrEF registries conducted since 2003, as means to provide historical context to the present findings.7, 17, 18, 19
All statistical analyses were centralized in one of the recruiting centres and were performed using Excel version 16 (Microsoft, Redmond, Washington, USA) and Stata software version 15.0 (StataCorp, College Station, Texas, USA). A two‐tailed P value <0.05 was considered statistically significant.
3. Results
3.1. Study participants
Between 1 February and 30 April 2017, 1056 HFrEF patients were prospectively enrolled in the registry; 604 patients (57.2%) were recruited from tertiary hospitals, 144 patients (13.6%) from secondary hospitals, and 308 patients (29.2%) from primary hospitals.
3.2. Demographic characteristics and clinical profile of the study participants
Table 1 summarizes the characteristics of 1056 HFrEF patients included in the registry. Overall, mean age was 66.5 years, and 78.9% of patients were men. Ischaemic cardiomyopathy was the most frequent aetiology of HF (42.3%), followed by idiopathic dilated cardiomyopathy (32.7%). Median LVEF was 30%, and only 14.5% of patients were asymptomatic, while more than half (58.4%) had mild HF symptoms (New York Heart Association II). Median NT‐proBNP was 1861 pg/mL, and 66.5% of patients had a previous admission for HF. Left bundle branch block was present in 33.6% of patients. With regard to co‐morbidities, 40% of patients had atrial fibrillation, 34.7% had chronic kidney disease, and 23.7% had chronic obstructive pulmonary disease.
Table 1.
Demographics and clinical profile of patients
| Overall (N = 1056) | Tertiary (N = 604) | Secondary (N = 144) | Primary (N = 308) | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 66.5 (12.3) | 64.3 (12.3) | 67.1 (12.1) | 70.4 (11.5) | <0.001 |
| Male sex | 815 (78.9) | 481 (80.8) | 98 (74.2) | 236 (77.1) | 0.162 |
| HF aetiology | <0.001 | ||||
| Ischaemic | 404 (42.3) | 232 (46.0) | 55 (38.2) | 117 (38.1) | |
| Valvular | 70 (7.3) | 33 (6.6) | 13 (9.0) | 24 (7.8) | |
| Hypertensive | 22 (2.3) | 9 (1.8) | 7 (4.9) | 6 (1.95) | |
| Alcoholic | 29 (3.0) | 18 (3.6) | 4 (2.8) | 7 (2.3) | |
| Other toxics | 18 (1.9) | 8 (1.6) | 10 (5.1) | 2 (0.6) | |
| Non‐compaction | 26 (2.7) | 20 (4.0) | 3 (2.1) | 3 (1.0) | |
| Mixed | 41 (4.3) | 27 (5.4) | 2 (1.4) | 12 (3.9) | |
| IDC | 312 (32.7) | 140 (27.8) | 44 (30.6) | 128 (41.7) | |
| Others | 33 (3.5) | 17 (3.4) | 8 (5.6) | 18 (2.6) | |
| LVEF (%) | 30 (25, 35) | 31 (25, 35) | 30 (25, 35) | 30 (25, 35) | 0.552 |
| NYHA class | <0.001 | ||||
| I | 153 (14.5) | 65 (10.8) | 32 (22.2) | 56 (18.2) | |
| II | 616 (58.4) | 341 (56.6) | 92 (63.9) | 183 (59.6) | |
| III | 265 (25.1) | 183 (30.4) | 19 (13.2) | 63 (20.5) | |
| IV | 20 (1.9) | 14 (2.3) | 1 (0.7) | 5 (1.6) | |
| Previous admission | 701 (66.5) | 396 (65.6) | 115 (79.9) | 190 (61.9) | 0.001 |
| Vital signs | |||||
| SBP (mmHg) | 117.6 (18.6) | 115.0 (18.2) | 120.9 (17.6) | 121.1 (19.0) | <0.001 |
| DBP (mmHg) | 70.9 (11) | 71.7 (10.0) | 69.8 (11.3) | 69.78 (12.5) | 0.020 |
| HR (b.p.m.) | 68.9 (12.6) | 67.7 (11.6) | 70.5 (14.8) | 70.4 (13.3) | 0.002 |
| Cardiovascular risk factors | |||||
| Current smoker | 136 (12.9) | 71 (11.8) | 24 (16.7) | 41 (13.4) | 0.275 |
| Previous smoker | 559 (53.7) | 337 (56.5) | 65 (45.8) | 157 (51.8) | 0.051 |
| Hypertension | 700 (66.4) | 389 (64.4) | 96 (66.7) | 215 (70.0) | 0.235 |
| Diabetes mellitus | 419 (39.7) | 231 (38.3) | 60 (41.7) | 128 (41.7) | 0.528 |
| Dyslipemia | 648 (61.5) | 371 (61.4) | 83 (57.6) | 194 (63.4) | 0.503 |
| Co‐morbidities | |||||
| OSAS | 132 (12.5) | 80 (13.3) | 19 (13.1) | 33 (10.8) | 0.551 |
| COPD | 250 (23.7) | 126 (20.9) | 40 (27.8) | 84 (27.5) | 0.041 |
| Stroke | 97 (9.2) | 66 (10.9) | 10 (6.9) | 21 (6.9) | 0.081 |
| Vasculopathy | 117 (11.1) | 70 (11.6) | 12 (8.3) | 35 (11.4) | 0.522 |
| CKD | 366 (34.7) | 211 (34.9) | 44 (30.8) | 111 (36.3) | 0.516 |
| AF | <0.001 | ||||
| No | 632 (60.0) | 379 (62.8) | 83 (57.6) | 170 (55.7) | |
| Paroxistic | 91 (8.6) | 43 (7.1) | 22 (15.3) | 26 (8.5) | |
| Persist/perman. | 144 (13.7) | 68 (11.3) | 31 (21.5) | 45 (14.8) | |
| Non‐defined | 186 (17.6) | 114 (18.9) | 8 (5.7) | 64 (21.0) | |
| Biomarkers | |||||
| Haemoglobin (g/dL) | 13.4 (1.9) | 13.4 (1.8) | 13.5 (2.3) | 13.3 (1.9) | 0.725 |
| Creatinine (mg/dL) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.5) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.4) | 0.619 |
| BNP (pg/mL) | 296 (200, 891) | 306 (204, 956) | 219 (136.9, 335) | 1782 (484, 3080) | 0.012 |
| NT‐proBNP (pg/mL) | 1861 (694, 4307) | 1418 (635, 4108) | 2957 (1182, 4794) | 2390 (1022.5, 4424.5) | 0.006 |
| Ferritin (ng/mL) | 154.5 (72, 284) | 143.5 (65, 280) | 145 (72, 328) | 180 (94, 294) | 0.268 |
| TSI (%) | 20.3 (15, 28) | 21 (15, 29) | 18.7 (14.5, 26) | 20 (15.6, 28) | 0.260 |
| ECG | |||||
| Rhythm | <0.001 | ||||
| Sinus | 715 (68.4) | 444 (74.0) | 93 (64.6) | 178 (58.9) | |
| AF | 227 (21.7) | 118 (19.7) | 31 (21.5) | 78 (25.8) | |
| Paced | 99 (9.5) | 34 (5.7) | 19 (13.2) | 46 (15.2) | |
| Atrial flutter | 5 (0.5) | 4 (0.7) | 1 (0.7) | 0 (0) | |
| LBBB | 353 (33.6) | 194 (32.1) | 48 (33.3) | 111 (36.5) | 0.425 |
| Echocardiography | |||||
| LVEDD (mm) | 63.1 (8.8) | 63.8 (9) | 62.8 (8.0) | 61.8 (8.6) | 0.005 |
| E/A | 1 (0.7, 1.8) | 1.1 (0.7, 2.0) | 0.9 (0.7, 1.4) | 0.8 (0.6, 1.5) | <0.001 |
| LA (mm) | 47.3 (8.5) | 47.1 (8.8) | 47.8 (8.6) | 47.4 (7.7) | 0.671 |
| MR mod/sev | 375 (35.9) | 223 (37.2) | 37 (25.9) | 115 (38.0) | 0.026 |
| PH | 267 (25.9) | 173 (29.1) | 27 (19.7) | 67 (22.3) | 0.019 |
AF, atrial fibrillation; BNP, brain natriuretic peptide; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; ECG, electrocardiogram; HF, heart failure; HR, heart rate; IDC, idiopathic dilated cardiomyopathy; LA, left atrium; LBBB, left bundle branch block; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NT‐proBNP, N‐terminal pro‐B type natriuretic peptide; NYHA, New York Heart Association; OSAS, obstructive sleep apnoea syndrome; PH, pulmonary hypertension; SBP, systolic blood pressure; TSI, transferrin saturation index.
In hospitals with higher level of complexity, patients were younger, and ischaemic cardiomyopathy was more frequent than in less complex centres. There were no differences between hospital types in terms of sex distribution or median LVEF. The burden of co‐morbidities was also similar across groups, except for atrial fibrillation and for chronic obstructive pulmonary disease, which were more frequent in hospitals with lower complexity. Median NT‐proBNP levels were higher in secondary hospitals, whereas the lowest levels were those of tertiary hospitals. Mean systolic blood pressure and mean heart rate were slightly lower in patients from tertiary hospitals compared with the other centres.
3.3. Pharmacological management and devices
Figure 2 shows the pharmacological and invasive management of our study population. Overall, beta‐blockers were used in 91.8% of patients, ACE‐I in 48.1%, ARB in 16.9%, sacubitril/valsartan in 23.9%, MRA in 72.7%, and ivabradine in 21.4% patients. CRT and ICD were used in 15.6% and 26.3% of patients, respectively.
Figure 2.
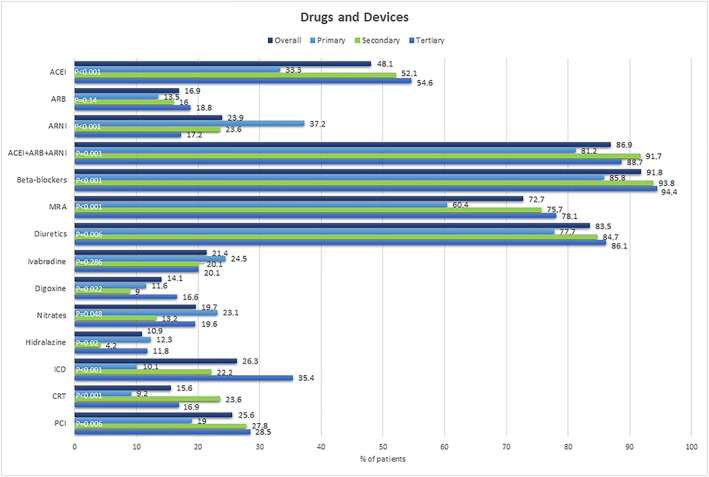
Drugs and devices. ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention.
In the comparative analyses by level of complexity, beta‐blockers were more frequently prescribed in tertiary hospitals, while renin–angiotensin inhibitors globally were more frequently prescribed in secondary centres. Primary hospitals had the highest proportion of patients under treatment with sacubitril/valsartan (37.2%), whereas there was a trend towards higher prescription of MRAs in hospitals with higher level of complexity (75.7% in secondary hospitals and 78.1% in tertiary hospitals).
There was a trend towards greater use of devices (ICD and CRT) in hospitals with higher complexity; and patients from tertiary and secondary hospitals had undergone percutaneous coronary revascularization more frequently.
Table 2 displays the combinations of drug prescription among patients. Overall, 62.7% of patients were under treatment with triple therapy (beta‐blockers + renin–angiotensin inhibitors + MRA). In line with the results showed in the previous paragraphs, there was also a trend towards higher proportion of patients under triple therapy in hospitals with higher level of complexity.
Table 2.
Combinations of drug prescription
| Overall (N = 1056) | Tertiary (N = 604) | Secondary (N = 144) | Primary (N = 308) | P value | |
|---|---|---|---|---|---|
| BB (monotherapy) | 60 (5.7%) | 26 (4.3%) | 2 (1.4%) | 32 (10.5%) | <0.001 |
| MRA (monotherapy) | 6 (0.6%) | 5 (0.8%) | 0 (0%) | 1 (0.3%) | 0.40 |
| iRA (monotherapy) | 24 (2.3%) | 5 (0.8%) | 3 (2.1%) | 16 (5.3%) | <0.001 |
| ACE‐I | 10 (1%) | 3 (0.5%) | 2 (1.4%) | 5 (1.6%) | 0.21 |
| ARB | 3 (0.3%) | 1 (0.2%) | 0 (0%) | 2 (0.7%) | 0.33 |
| ARNI | 9 (0.9%) | 1 (0.2%) | 1 (0.7%) | 7 (2.3%) | 0.004 |
| BB + iRA (bitherapy) | 190 (18.2%) | 94 (15.7%) | 29 (20.1%) | 67 (22.1%) | 0.048 |
| BB + ACE‐I | 109 (10.5%) | 58 (9.8%) | 18 (12.5%) | 33 (10.9%) | 0.612 |
| BB + ARB | 34 (3.2%) | 16 (2.7%) | 9 (6.3%) | 9 (3%) | 0.087 |
| BB + ARNI | 34 (3.2%) | 9 (1.5%) | 2 (1.4%) | 23 (7.6%) | <0.001 |
| BB + MRA (bitherapy) | 50 (4.8%) | 28 (4.7%) | 9 (6.3%) | 13 (4.3%) | 0.66 |
| iRA + MRA (bitherapy) | 46 (4.4%) | 20 (3.3%) | 5 (3.5%) | 21 (7%) | 0.035 |
| ACE‐I + MRA | 25 (2.4%) | 15 (2.5%) | 4 (2.8%) | 6 (2%) | 0.841 |
| ARB + MRA | 8 (0.8%) | 2 (0.3%) | 1 (0.7%) | 5 (1.7%) | 0.097 |
| ARNI + MRA | 11 (1.1%) | 2 (0.4%) | 0 (0%) | 9 (3%) | <0.001 |
| BB + iRA + MRA (triple therapy) | 653 (62.7%) | 415 (69.2%) | 95 (66%) | 143 (48%) | <0.001 |
| BB + ACE‐I + MRA | 328 (31.8%) | 226 (38.3%) | 51 (35.4%) | 51 (17.1%) | <0.001 |
| BB + ARB + MRA | 119 (11.4%) | 85 (14.1%) | 13 (9%) | 21 (7%) | 0.004 |
| BB + ARNI + MRA | 179 (17.2%) | 89 (14.8%) | 31 (21.5%) | 59 (20.1%) | 0.047 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blockers; iRA, renin–angiotensin inhibitors; MRA, mineralocorticoid receptor antagonist.
3.4. Specific drugs and drug doses
Table 3 describes the use of specific drugs within each family, and their mean doses, overall and by level of complexity. Overall, the most frequently prescribed drugs within each family were enalapril among ACEI (73.4%, mean dose 12 mg/day), bisoprolol among beta‐blockers (54.9%, mean dose 5.6 mg/day), losartan among ARBs (40.6%, mean dose 78 mg/day), and eplerenone among MRAs (65.8%, mean dose 29.7 mg/day). Mean dose of sacubitril/valsartan was 144.8 mg/day, and mean dose of ivabradine was 11 mg/day.
Table 3.
Drug prescription and doses (mg/day)
| Overall | Tertiary | Secondary | Primary | P value | |
|---|---|---|---|---|---|
| ACE‐I | |||||
| Enalapril (n, %) | 373 (73.4%) | 262 (79.6%) | 51 (65.4%) | 60 (59.4%) | <0.001 |
| Mean dose (SD) | 12 (9) | 12.2 (8.4) | 14.7 (11.4) | 8.7 (7.8) | — |
| Ramipril (n, %) | 118 (23.2%) | 58 (17.6%) | 23 (29.5%) | 37 (36.6%) | — |
| Mean dose (SD) | 4.5 (2.8) | 4.6 (2.8) | 5.3 (3.1) | 4.0 (2.6) | — |
| Lisinopril (n, %) | 9 (1.8%) | 6 (1.8%) | 1 (1.3%) | 2 (2%) | — |
| Mean dose (SD) | 13.1 (15.4) | 11.3 (14.3) | 5 (0) | 22.5 (24.7) | — |
| Captopril (n, %) | 1 (0.2%) | 0 (0%) | 0 (0%) | 1 (1%) | — |
| Mean dose (SD) | 25 (0) | — | — | 25 (0) | — |
| Perindopril (n, %) | 5 (1%) | 1 (0.3%) | 3 (3.9%) | 1 (1.0%) | — |
| Mean dose (SD) | 3.6 (2.6) | 8 (0) | 2 (0) | 4 (0) | — |
| Others (n, %) | 2 (0.4%) | 2 (0.6%) | 0 (0%) | 0 (0%) | — |
| ARB | |||||
| Valsartan (%) | 63 (37.1%) | 39 (35.8%) | 16 (66.7%) | 8 (21.6%) | 0.002 |
| Mean dose (SD) | 120.7 (85.1) | 115.6 (76.3) | 110.9 (83.6) | 320 (0) | — |
| Losartan (%) | 69 (40.6%) | 44 (40.4%) | 5 (20.8%) | 20 (54%) | — |
| Mean dose (SD) | 78 (46.6) | 80.4 (44.0) | — | 68.8 (57.9) | — |
| Candesartan (%) | 26 (15.3%) | 20 (18.4%) | 2 (8.3%) | 4 (10.8%) | — |
| Mean dose (SD) | 21.8 (16.9) | 22.2 (17.8) | — | 18 (2.8) | — |
| Olmesartan (%) | 6 (3.5%) | 1 (0.9%) | 1 (4.2%) | 4 (10.8%) | — |
| Mean dose (SD) | 40 (0) | — | — | 40 (0) | — |
| Irbesartan (%) | 5 (2.9%) | 5 (4.6%) | 0 (0%) | 0 (0%) | — |
| Mean dose (SD) | 150 (0) | 150 (0) | — | — | — |
| Telmisartan (%) | 1 (0.6%) | 0 (0%) | 0 (0%) | 1 (2.7%) | — |
| Mean dose (SD) | 80 (0) | — | — | 80 (0) | — |
| Beta‐blockers | |||||
| Bisoprolol (%) | 519 (54.9%) | 268 (48.6%) | 66 (48.3%) | 185 (71.4%) | <0.001 |
| Mean dose (SD) | 5.6 (3.3) | 6.4 (3.4) | 6.5 (3.8) | 4.1 (2.4) | — |
| Carvedilol (%) | 390 (41.2%) | 258 (46.8%) | 69 (50.7%) | 63 (24.3%) | — |
| Mean dose (SD) | 26.6 (18.4) | 26.2 (17.4) | 33.2 (21.3) | 20.8 (16.6) | — |
| Metoprolol (%) | 1 (0.1%) | 1 (0.2%) | 0 (0%) | 0 (0%) | — |
| Mean dose (SD) | 100 (0) | 100 (0) | — | — | — |
| Nevibolol (%) | 35 (3.7%) | 24 (4.4%) | 0 (0%) | 11 (4.3%) | — |
| Mean dose (SD) | 4.5 (2.5) | 4.7 (2.9) | — | 4 (1.5) | — |
| Others (%) | 1 (0.1%) | 0 (0%) | 1 (0.7%) | 0 (0%) | |
| MRA | |||||
| Spironolactone (%) | 262 (34.2%) | 161 (34.2%) | 31 (28.4%) | 70 (37.6%) | 0.275 |
| Mean dose (SD) | 28.2 (18.1) | 27.6 (18.7) | 27.8 (16.7) | 29.6 (17.3) | — |
| Eplerenone (%) | 504 (65.8%) | 310 (65.8%) | 78 (71.6%) | 116 (62.4%) | — |
| Mean dose (SD) | 29.7 (12.5) | 31.1 (13.6) | 29.9 (11.7) | 26.1 (8.7) | — |
| Ivabradine | |||||
| Mean dose (SD) | 11 (3.3) | 10.9 (3.6) | 11.2 (2.9) | 11.1 (2.9) | — |
| Sacubitril/valsartan | |||||
| Mean dose (SD) | 144.8 (94) | 160.5 (101) | 144.1 (94.4) | 130.4 (85.4) | — |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; n, number; SD, standard deviation.
The observations with regard to the most frequently used drug subtypes were also true across all levels of complexity, except for valsartan and carvedilol, which were the most frequently used ARB and beta‐blocker, respectively, in secondary hospitals.
3.5. Target doses of key heart failure drugs
The proportion of patients in whom target doses of each key HF pharmacotherapy were achieved (according to ESC 2016 Guidelines14) is displayed in Figure 3 , overall and by level of hospital complexity. Overall, target doses of ACEI and beta‐blockers were achieved in approximately 25% of patients, MRA in 20%, while for ARB and ARNI, target doses were achieved only in less than 10% of patients.
Figure 3.
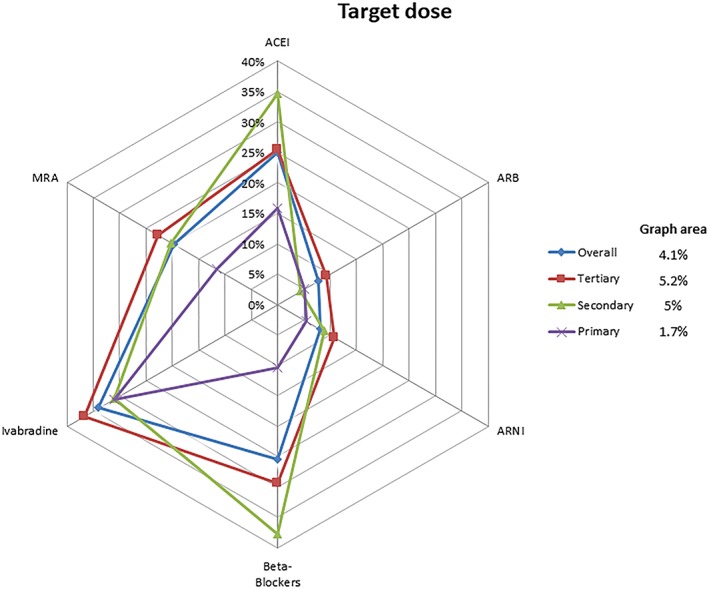
Target dose. ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist.
Although tertiary hospitals achieved target doses of ARBs, MRAs, ivabradine, and ARNI more frequently than the other centres, for beta‐blockers and ACEI, target doses were achieved more frequently in secondary hospitals. On the other hand, the lowest adherence to guideline‐recommended target doses was observed in primary centres (graph area 1.7%).
3.6. Comparison with prior registries
Table 4 compares the observations from our registry to a similar Spanish registry16 published before the release of the ESC 2016 Guidelines. In Linx, the use of ACEI, ARBs, and any renin–angiotensin inhibitors (ACE‐I, ARB, or ARNI) was slightly lower than in the 2015 registry (before commercialization of sacubitril/valsartan). There was also a statistically significant decrease in the use of digoxin after the ESC 2016 Guidelines.14 No other statistically significant differences were observed between the two studies.
Table 4.
Drugs prescription comparison with previous registries from Spain
| ESC Heart Failure Long‐term Registry17 (LVEF ≤ 40%) (N = 1526) | Linx registry (N = 1056) | P value | |
|---|---|---|---|
| ACE‐I (%) | 64.6 | 48.1 | <0.001 |
| ARB (%) | 29.1 | 16.9 | <0.001 |
| ARNI (%) | — | 23.9 | — |
| ACE‐I + ARB + ARNI (%) | 92.6 | 86.9 | <0.001 |
| Beta‐blockers (%) | 93.3 | 91.8 | 0.15 |
| MRA (%) | 74.5 | 72.7 | 0.31 |
| Diuretics (%) | 83.3 | 83.5 | 0.89 |
| Digoxin (%) | 22 | 14.1 | <0.001 |
| Ivabradine (%) | 19.7 | 21.4 | 0.29 |
| Nitrates (%) | 16.8 | 19.7 | 0.06 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; ESC, European Society of Cardiology; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist.
3.7. Temporal trends
The temporal trends in pharmacological and device management of outpatients with HFrEF in Europe from 2003 (SWEDE‐HEART registry17) to the present (Linx) are presented in Figure 4 , which summarizes the observations from key, comparable, European HFrEF observational studies. The proportion of patients treated with beta‐blockers and ACEI/ARBs remained stable over time (around 90%), until commercialization of sacubitril/valsartan, which, as evidenced in our registry, has been associated with a marked decrease in the use of ACEI. MRA use has steadily increased in the last decade, so has the use of ICD and CRT.
Figure 4.
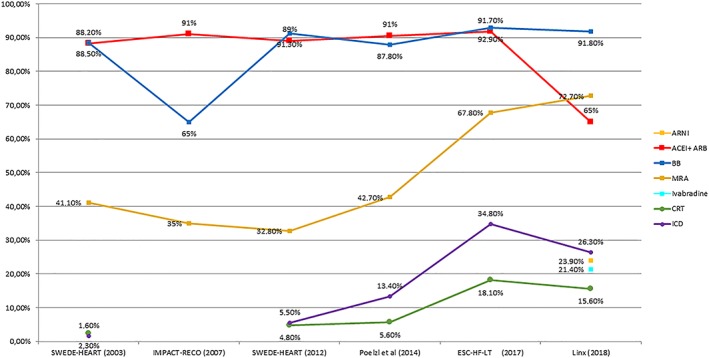
The temporal trends in pharmacological and device management of outpatients with heart failure with reduced ejection fraction in Europe. ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blockers; CRT, cardiac resynchronization therapy; ESC‐HF‐LT, European Society of Cardiology Heart Failure Long‐Term Registry; ICD, implantable cardioverter defibrillator; MRA, mineralocorticoid receptor antagonist.
4. Discussion
The Linx registry shows that after the release of the ESC 2016 Guidelines,14 use of beta‐blockers remained very high in HFrEF patients, while there was a marked decrease in the use of ACE‐I/ARBs in monotherapy, which was likely the consequence of a marked increase in the use of sacubitril/valsartan. When considering hospital complexity, we observed that beta‐blockers, MRAs, and devices were more frequently used in tertiary hospitals (where the population was younger and median NT‐proBNP levels were lower), while sacubitril/valsartan was more frequently used in primary hospitals. In all centres, achievement of the target drug doses recommended by the ESC 2016 Guidelines14 was low.
Our first main finding is the very high uptake of sacubitril/valsartan, only a few months after ESC 2016 Guidelines14 recommendation to replace ACE‐I for ARNI in ambulatory patients with HFrEF who remain symptomatic despite optimal medical treatment. The proportion reached 24% in our population and was even higher in primary hospitals. Given the recent publication of safety data showing that early initiation of ARNI might be feasible and tolerable,20 this proportion is likely to keep growing in the coming years. Further studies will provide valuable insights on this question.
Our results also suggest a trend towards a better adherence to ESC HF Guideline recommendations in hospitals with higher level of complexity. Whether these disparities are the consequence of differences in the clinical profiles of the patient populations from each type of hospital, or of other factors, cannot be concluded from the available data. Nevertheless, some differences in demographic and clinical characteristics were observed across centres—e.g. patients from tertiary hospitals were younger, which might explain a higher tolerance to beta‐blockers, and therefore the more frequent use of this drug group; and patients from secondary and primary hospitals were older and had more co‐morbidities, such as chronic obstructive pulmonary disease. Despite the fact that there are no previous studies comparing management of HFrEF outpatients by hospital type, some studies21, 22, 23 also suggest better outcomes in patients admitted for HF in higher volume hospitals, although in a recently published registry (GWTG‐HF)24 this trend was not confirmed.
We also observed a low proportion of patients meeting the recommended target doses of most class I HFrEF therapies. A potential explanation to this finding, as discussed in the ESC 2016 Guidelines,14 is the fact that some of the clinical trials in which those target doses were defined were conducted before the availability of modern therapies such as eplerenone or sacubitril/valsartan, among others. Also, the patient populations included in those studies tended to be young and to have few co‐morbidities; conversely, in our real‐life patient population, the prevalence of key co‐morbidities such as chronic kidney disease or chronic obstructive pulmonary disease was high. In addition, it is important to note that information on drug use, drug doses, and device use was recorded at study entry (i.e. not necessarily at end of titration). This means that many patients might have been treated with additional therapies, might have had some drug doses titrated, or might have been treated with specific HF devices later in time (i.e. during follow‐up in the cardiology clinic), as part of standard pharmacological titration and progressive therapeutic optimization. Therefore, the present observations regarding target doses and device use should be interpreted cautiously.
Importantly, our findings regarding the very low proportion of patients meeting target doses are consistent with those from prior registries.7, 17, 18, 19, 25 Indeed, achieving target doses of pharmacological therapies proven to reduce mortality remains as a major challenge for the management of patients with chronic HFrEF as recently described in CHAMP‐HF registry.25 Compared with clinical practice in the USA, we found no differences in achieving target doses of ACE‐I/ARB (20.6% vs. 17.5%) or beta‐blockers (25.4% vs. 27.6%), but we found fewer patients in target doses regarding ARNI (8.1% vs. 14%) and MRA (19.7% vs. 76.6%). The greater proportion of target doses of MRA might be partly explained by a much lower proportion of patients prescribed with MRA (72.7% vs. 33.1%) that might select a group of patients with better renal function.
4.1. Study strengths
To our knowledge, this is the first registry to evaluate the real‐world management of HFrEF in the outpatients setting in Europe since the publication of the ESC 2016 HF Guidelines. Moreover, because Linx is a multicentre, large registry, this maximized statistical power and the generalizability of the conclusions. In addition, because not only tertiary but also secondary and primary centres were included, we were able to compare hospitals by level of complexity, and this increased generalizability of our overall findings even further. Although secondary and primary centres are usually underrepresented in clinical trials and observational studies, they actually manage a large number of patients in routine clinical practice. Finally, the detailed information on drug dosage captured in Linx allowed evaluating adherence to guideline recommendations.
4.2. Study limitations
One of the major limitations of the registry comes from the cross‐sectional design, which limits causal inference based on the observed differences. Another limitation is the absence of information in the registry describing the reason for not prescribing or up‐titrating medication or devices. It is highly likely that formal contraindications as well as limited tolerance to specific drugs would explain most of these scenarios. Finally, although participating hospitals are spread around Catalonia, most patients were recruited from the metropolitan area of Barcelona, so patients from rural areas might have been underrepresented.
5. Conclusions
The Linx registry shows an appropriate adherence to pharmacological recommendations from ESC 2016 HF Guidelines despite that target doses of class I HF drugs were low in our population. These findings are consistent with the observations from prior observational, real‐life studies. On the other hand, almost one‐quarter of chronic HFrEF patients were under treatment with sacubitril/valsartan a few months after ESC 2016 HF Guidelines recommendation, prompting the reduction of ACE‐I/ARB use in this patient population. Use of sacubitril/valsartan was even greater in primary hospitals. Finally, use of beta‐blockers, MRA, and ivabradine remained similar to the prescription rates observed in previous Spanish HFrEF registries and was particularly high in tertiary centres.
Contributions
All authors contributed to patient inclusion and approved the final version of the manuscript. Miguel Cainzos‐Achirica performed statistical analysis and interpretation of results.
Conflict of interest
None declared.
Funding
The Linx registry was sponsored by the Catalan Society of Cardiology.
Supporting information
Data S1. Supplementary appendix.
de Frutos, F. , Mirabet, S. , Ortega‐Paz, L. , Buera, I. , Darnés, S. , Farré, N. , Andrés, B. , Adeliño, R. , Bascompte, R. , Pérez‐Rodón, J. , Aparicio, X. , Sutil‐Vega, M. , Soto, A. , Faraudo, M. , Cainzos‐Achirica, M. , and Manito, N. (2020) Management of Heart Failure with Reduced Ejection Fraction after ESC 2016 Heart Failure Guidelines: The Linx Registry. ESC Heart Failure, 7: 25–35. 10.1002/ehf2.12567.
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farré N, Vela E, Clèries M, Bustins M, Cainzos‐Achirica M, Enjuanes C, Moliner P, Ruiz S, Verdú‐Rotellar JM, Comín‐Colet J. Real world heart failure epidemiology and outcome: a population‐based analysis of 88,195 patients. PLoS ONE 2017; 12: e0172745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Failure 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 4. Farré N, Vela E, Clèries M. Medical resource use and expenditure in patients with chronic heart failure: a population‐based analysis of 88 195 patients. Eur J Heart Fail 2016; 18: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 5. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 6. Gurwitz JH, Magid DJ, Smith SH .Contemporary prevalence and correlates of incident heart failure with preserved ejection fraction. Am J Med 2013; 126: 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one‐year outcome in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 8. Lother A, Hein L. Pharmacology of heart failure: from basic science to novel therapies. Pharmacology & Therapeutics 2016; 166: 136–149. [DOI] [PubMed] [Google Scholar]
- 9. Yusuf S, Pitt B, Davis CE. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325: 293–302. [DOI] [PubMed] [Google Scholar]
- 10. Investigators CIBIS‐II and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999;353:9–13 [PubMed] [Google Scholar]
- 11. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 12. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L, SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomized placebo‐controlled study. Lancet 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJV, Packer M, Desai AS. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 15. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A, Heart Failure Association (HFA) of the European Society of Cardiology (ESC) . European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016; 18: 613–625. [DOI] [PubMed] [Google Scholar]
- 16. Crespo‐Leiro MG, Segovia‐Cubero J, González‐Costello J. Adherence to the ESC heart failure treatment guidelines in Spain: ESC heart failure long‐term registry. Rev Esp Cardiol (Engl Ed) 2015; 68: 785–793. [DOI] [PubMed] [Google Scholar]
- 17. Thorvaldsen T, Benson L, Dahlström U, Edner M, Lund LH. Use of evidence‐based therapy and survival in heart failure in Sweden 2003‐2012. Eur J Heart Fail 2016; 18: 503–511. [DOI] [PubMed] [Google Scholar]
- 18. De Groote P, Isnard R, Assyag P. Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur J Heart Fail 2007; 9: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 19. Poelzl G, Altenberger J, Pacher R. Dose matters! Optimisation of guideline adherence is associated with lower mortality in stable patients with chronic heart failure. Int J Cardiol 2014. [DOI] [PubMed] [Google Scholar]
- 20. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 21. Ross JS, Normand SLT, Wang Y, Ko DT, Chen J, Drye EE, Keenan PS, Lichtman JH, Bueno H, Schreiner GC, Krumholz HM. Hospital volume and 30‐day mortality for three common medical conditions. N Engl J Med 2010; 362: 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joynt KE, Orav EJ, Jha AK. The association between hospital volume and processes, outcomes, and costs of care for congestive heart failure. Ann Intern Med 2011. 18; 154: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McAllister FA, Youngson E, van Diepen S. Influence of hospital volume on outcomes for patients with heart failure: evidence from a Canadian national cohort study. Am Heart J 2018; 202: 148–150. [DOI] [PubMed] [Google Scholar]
- 24. Kumbhani DJ, Fonarow GC, Heidenreich PA, Schulte PJ, Lu D, Hernandez A, Yancy C, Bhatt DL. Association between hospital volume, processes of care, and outcomes in patients admitted with heart failure. Circulation 2018; 137: 1661–1670. [DOI] [PubMed] [Google Scholar]
- 25. Greene SJ, Butler J, Albert NM, DeVore A, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: The CHAMP‐HF Registry. J Am Coll Cardiol 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 26. Saary MJ. Radar plots: a useful way for presenting multivariate health care data. J Clin Epidemiol 2008; 60: 311–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary appendix.


