Abstract
Background
This study aimed to explore the correlation of lncRNA ANRIL/miR‐125a axis with disease risk, severity, and inflammatory cytokines of bronchial asthma.
Methods
Plasma samples from 90 patients with bronchial asthma at exacerbation (BA‐E), 90 with bronchial asthma at remission (BA‐R), and 90 controls (healthy subjects) were collected. The qPCR was used for lncRNA ANRIL and miR‐125a detection, and ELISA was adopted for pro‐inflammatory cytokines detection. Participants’ characteristics, laboratory tests, and the pulmonary ventilation function examinations were recorded.
Results
LncRNA ANRIL was negatively correlated with miR‐125a in BA‐E patients, BA‐R patients, and controls. LncRNA ANRIL/miR‐125a axis was upregulated in BA‐E patients compared with BA‐R patients and controls. ROC curve analyses illuminated that lncRNA ANRIL/miR‐125a axis was of good value in distinguishing BA‐E patients from BA‐R patients and controls. As to pulmonary ventilation functions, lncRNA ANRIL/miR‐125a axis was negatively associated with FEV1/FVC and FEV1%predicted in bronchial asthma patients, especially in BA‐E patients. Regarding inflammation, lncRNA ANRIL/miR‐125a axis was positively correlated with pro‐inflammatory cytokines in bronchial asthma patients, especially in BA‐E patients. In addition, lncRNA ANRIL/miR‐125a axis was positively correlated with exacerbation severity in BA‐E patients.
Conclusion
LncRNA ANRIL/miR‐125a is potentially indicative of disease exacerbation, exacerbation severity, and inflammation for bronchial asthma, while these findings are preliminary and need further confirmation.
Keywords: bronchial asthma, disease risk, exacerbation, inflammatory cytokines, LncRNA ANRIL/miR‐125a axis
1. INTRODUCTION
Bronchial asthma is a chronic inflammatory disorder characterized by reversible airway obstruction, airway inflammation, and bronchial hypersensitiveness, which is clinically manifested as recurrent episodes of wheezing, breathlessness, chest tightness, and coughing.1, 2 It is the most common chronic lung disease affecting around 300 million people worldwide.3 Many asthma patients are relieved by treatment with β2‐adrenergic receptor antagonist and inhaled corticosteroids or a leukotriene receptor antagonist, and however, the traditional anti‐inflammatory drugs have defects associated with high‐dose side effects, and novel therapeutic approaches such as antigen specific therapy and cytokine‐specific antagonists still require further improvement in safety and efficacy.3, 4 Moreover, there are still a large number of patients who succumb to more severe disease with more frequent symptoms and exacerbations.5 Therefore, exploring additional molecular targets would be of great benefit for general asthma patients including those with disease exacerbation.
Long non‐coding RNA (lncRNA) antisense non‐coding RNA in the INK4 locus (ANRIL) is transcribed from chromosome 9p21 in antisense orientation and is previously shown to regulate inflammatory responses in cardiovascular and inflammatory diseases.6, 7, 8 Besides, lncRNA ANRIL also facilitates the pathological progression in pulmonary diseases, and for instance, lncRNA ANRIL promotes cells proliferation and metastasis in non–small‐cell lung cancer (NSCLC).9, 10 MicroRNA‐125a (miR‐125a), as one of the target genes of lncRNA ANRIL, is downregulated in several inflammatory diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and Crohn's disease.11, 12, 13 In addition, decreased level of miR‐125a is associated with lung inflammation and acute alveolar hemorrhage in SLE patients by recruiting of neutrophils.12 Based on the existing evidence, lncRNA ANRIL/miR‐125a axis is reported to participate in oxidative damage during spinal cord injury, and it also facilitates carcinogenesis in several cancers.14, 15, 16, 17, 18 Considering that lncRNA ANRIL and miR‐125a are closely correlated with inflammatory diseases including lung inflammation, we speculated that lncRNA ANRIL/miR‐125a axis might participate in etiology of bronchial asthma as well. Nevertheless, limited study has been reported. Therefore, this study aimed to explore the correlation of lncRNA ANRIL/miR‐125a axis with disease risk, severity, and inflammatory cytokines of bronchial asthma.
2. MATERIALS AND METHODS
2.1. Participants
In this study, 90 patients with bronchial asthma at exacerbation (BA‐E), 90 patients with bronchial asthma at remission (BA‐R), and 90 controls were recruited from The Central Hospital of Wuhan between July 2017 and June 2018. The screening criteria for BA‐E patients were as follows: (a) diagnosed as bronchial asthma according to the Global Initiative for Asthma (GINA) guideline (2016);1 (b) presenting with an exacerbation or flare‐up, which was defined as an acute or sub‐acute worsening in symptoms and lung function from the patient's usual status; (c) age above 18 years; (d) no concurrent severe infections other than respiratory tract infections; and (e) had no malignant hematological diseases or tumors. The detailed diagnostic criteria for exacerbation severity were listed in Table S1. The screening criteria for BA‐R patients included the following: (a) diagnosed as bronchial asthma according to the GINA guideline (2016); (b) be in clinical remission status, which was defined as after treatment or without treatment, the symptoms and signs disappeared, and the pulmonary function recovered to the pre‐acute level and maintained for more than 3 months; (c) age above 18 years; (d) no concurrent severe infections other than respiratory tract infections; and (e) had no malignant hematological diseases or tumors. The recruited controls were healthy subjects who underwent health examination in the Hospital at the same period, and the inclusion criteria were as follows: (a) no history of bronchial asthma or other allergic diseases; (b) no treatment with corticosteroids, immunosuppressants, immunomodulators, or inflammatory transmitter antagonists within 4 weeks; (c) no infections within 4 weeks; and (d) no inflammatory diseases, malignant hematological diseases, or tumors.
2.2. Ethics statement
This study was approved by the Ethics Committee of the Hospital. All guardians of participants provided written informed consents prior to the recruitment.
2.3. Data and sample collection
After recruitment, participants’ characteristics, such as age, gender, and family history of asthma, were documented. Meanwhile, the laboratory tests (eg, immune globulin E (IgE)) and the pulmonary ventilation function examinations were recorded as well, which included forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), and the values of FEV1/FVC as well as FEV1%predicted were calculated. For the BA‐E patients, exacerbation severity was assessed on the day of hospital admission based on the degree of dyspnea, respiratory rate, pulse rate, oxygen saturation, and lung function. Besides, blood samples were collected from all participants using ethylenediaminetetraacetic acid (EDTA) tubes after eligibility was confirmed and then were immediately centrifuged at 1000 g for 15 minutes to isolate plasma which was stored at −80°C for following determinations.
2.4. Detection of lncRNA ANRIL and miR‐125a
Total RNA was extracted from plasma using QIAamp RNA Blood Mini Kit (Qiagen) with concentration of 5‐20 ng/μL, and then, nucleic acid concentration using ethanol precipitation method (75% ethanol) was performed, which raised the concentration of RNA samples to 200‐500 ng/μL. 0.8 μg RNA was reversely transcribed using iScript™ cDNA Synthesis Kit (Bio‐Rad), and stem‐loop reverse transcription was used for miRNA reverse transcription. Quantitative polymerase chain reaction (qPCR) was then conducted using TB Green™ Fast qPCR Mix (Takara), in which GAPDH was the internal reference for lncRNA ANRIL and U6 was the internal reference for miR‐125a. The Ct value of GAPDH was similar among BA‐E, BA‐R, and controls (P = .765). Primers were as follows: lncRNA ANRIL, forward (5'‐>3'): TGCTCTATCCGCCAATCAGG, reverse (5'‐>3'): GGGCCTCAGTGGCACATACC; miR‐125a forward (5'‐>3'): ACACTCCAGCTGGGTCCCTGAGACCCTTTAAC, reverse (5'‐>3'): TGTCGTGGAGTCGGCAATTC; GAPDH, forward (5'‐>3'): GAGTCCACTGGCGTCTTCAC, reverse (5'‐>3'): ATCTTGAGGCTGTTGTCATACTTCT; U6, forward (5'‐>3'): CTCGCTTCGGCAGCACATATACTA, reverse (5'‐>3'): ACGAATTTGCGTGTCATCCTTGC.
2.5. Measurement of inflammatory cytokines
The levels of tumor necrosis factor alpha (TNF‐α), interleukin‐1 beta (IL‐1β), and interleukin‐6 (IL‐6), and interleukin‐17 (IL‐17) in the plasma of participants were determined by the enzyme‐linked immunosorbent assay (ELISA) with the use of ELISA kits (Abcam) including Human TNF alpha ELISA Kit, Human IL‐1 beta ELISA Kit, Human IL‐6 ELISA Kit, and Human IL‐17 ELISA Kit. Standard curves were made by using standards provided in the kits, and the cytokine concentrations were appointed from the standard curves by use of linear regression analysis. All experiments were performed according to the protocols provided by the manufacturer.
2.6. Statistical analysis
SPSS 24.0 software (IBM Corp.) was used to perform data analysis, and GraphPad Prism 6.01 software (GraphPad Software Inc) was applied to plot graphs. Continuous variables were expressed as the mean and standard deviation (SD) or median (interquartile range), and the categorical variables were displayed as number (percentage). Comparisons among three groups were determined by chi‐square test or one‐way analysis of variance (ANOVA) or Kruskal‐Wallis test followed by Dunn's multiple comparisons test. Correlations between variables were analyzed by Spearman's rank correlation test. Receiver operating characteristic (ROC) curve analysis and the derived area under the curve (AUC) as well as 95% confidence interval (CI) were used to assess the diagnostic performance of variables. P value < .05 indicated a significant difference.
3. RESULTS
3.1. Baseline characteristics of participants
The mean age of 90 BA‐E patients was 36.4 ± 8.8 years, of which 44 (48.9%) cases were males and 46 (51.1%) cases were females (Table 1). The numbers of BA‐E patients with mild, moderate, and severe exacerbation severity were 23 (25.6%), 44 (48.9%), and 23 (25.6%), respectively. As for BA‐R patients, the mean age was 37.5 ± 9.3 years and 52 (57.8%) of them were males, while 38 (42.2%) of them were females. The mean age of controls was 35.2 ± 10.1 years, and 52 (57.8%) cases were males, while 38 (42.2%) cases were females. There was no difference in age (P = .260), gender (P = .384), or family history of asthma (P = .083) among BA‐E patients, BA‐R patients, and controls, while the IgE level (P < .001) was higher but FEV1/FVC (P < .001) and FEV1%predicted (P < .001) were lower in BA‐E patients and BA‐R patients compared with controls. In addition, the inflammatory cytokines including TNF‐α (P < .001), IL‐1β (P < .001), IL‐6 (P < .001), and IL‐17 (P < .001) were higher in BA‐E patients and BA‐R patients compared with controls. The detailed information on baseline characteristics among BA‐E patients, BA‐R patients, and controls was shown in Table 1.
Table 1.
Characteristics of participants
| Parameters | BA‐E patients (N = 90) | BA‐R patients (N = 90) | Controls (N = 90) | P value |
|---|---|---|---|---|
| Age, years, M ± SD | 36.4 ± 8.8 | 37.5 ± 9.3 | 35.2 ± 10.1 | .260 |
| Gender, No. (%) | ||||
| Male | 44 (48.9) | 52 (57.8) | 52 (57.8) | .384 |
| Female | 46 (51.1) | 38 (42.2) | 38 (42.2) | |
| Family history of asthma, No. (%) | 24 (26.7) | 19 (21.1) | 12 (13.3) | .083 |
| IgE, IU/mL, M ± SD | 265.8 ± 149.8 | 100.6 ± 64.0 | 33.8 ± 22.9 | <.001 |
| FEV1/FVC, %, M ± SD | 66.3 ± 6.6 | 80.2 ± 2.5 | 83.3 ± 2.6 | <.001 |
| FEV1%predicted, M ± SD | 76.0 ± 5.7 | 84.7 ± 4.9 | 100.5 ± 7.0 | <.001 |
| Exacerbation severity, No. (%) | ‐ | |||
| Mild | 23 (25.6) | ‐ | ‐ | |
| Moderate | 44 (48.9) | ‐ | ‐ | |
| Severe | 23 (25.6) | ‐ | ‐ | |
| TNF‐α, pg/mL, M ± SD | 57.8 ± 40.4 | 22.1 ± 13.7 | 13.1 ± 7.2 | <.001 |
| IL‐1β, pg/mL, M ± SD | 4.0 ± 2.9 | 1.5 ± 1.0 | 0.8 ± 0.5 | <.001 |
| IL‐6, pg/mL, M ± SD | 41.6 ± 34.3 | 14.0 ± 11.1 | 8.5 ± 6.3 | <.001 |
| IL‐17, pg/mL, M ± SD | 62.0 ± 59.0 | 23.8 ± 21.3 | 12.6 ± 11.1 | <.001 |
Comparisons were determined by the chi‐square test or one‐way analysis of variance (ANOVA). P value < .05 indicated a significant difference.
Abbreviations: BA‐E, bronchial asthma at exacerbation; BA‐R, bronchial asthma at remission; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IgE, immune globulin E; IL, interleukin; M ± SD, mean ± standard deviation; TNF‐α, tumor necrosis factor α.
3.2. The expression of lncRNA ANRIL/miR‐125a axis
LncRNA ANRIL/miR‐125a axis in BA‐E patients (9.048 [3.572‐13.875]), BA‐R patients (2.317 [0.762‐4.308]), and controls (0.981 [0.303‐2.423]) were shown, and lncRNA ANRIL/miR‐125a axis was higher in BA‐E patients compared with BA‐R patients (P < .001) and controls (P < .001), as well as in BA‐R patients compared with controls (P = .005) (Figure 1A). In addition, lncRNA ANRIL relative expression was 3.020 (2.296‐3.899) in BA‐E patients, 1.612 (0.807‐2.966) in BA‐R patients, and 0.984 (0.473‐1.814) in controls, and lncRNA ANRIL expression was higher in BA‐E patients compared with BA‐R patients (P < .001) and controls (P < .001), as well as in BA‐R patients compared with controls (P = .003) (Figure 1B). As for miR‐125a relative expression, it was 0.361 (0.246‐0.587) in BA‐E patients, 0.733 (0.560‐0.970) in BA‐R patients, and 0.976 (0.510‐2.310) in controls (P < .001), and miR‐125a expression was lower in BA‐E patients compared with BA‐R patients and controls (P < .001), but similar between BA‐R patients and controls (P = .350) (Figure 1C).
Figure 1.
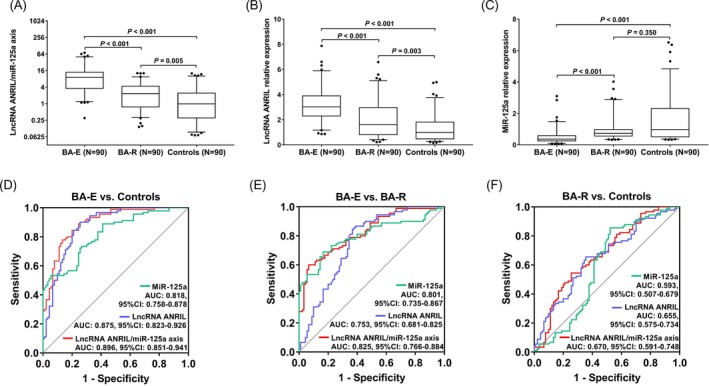
LncRNA ANRIL/miR‐125a axis, lncRNA ANRIL, and miR‐125a expression among BA‐E patients, BA‐R patients, and controls. LncRNA ANRIL/miR‐125a axis (A), lncRNA ANRIL (B), and miR‐125a (C) expression were compared among BA‐E patients, BA‐R patients, and controls. The values of LncRNA ANRIL/miR‐125a axis, lncRNA ANRIL, and miR‐125a in distinguishing BA‐E patients from controls (D), BA‐E patients from BA‐R patients (E), BA‐R patients from controls (F) were shown. The comparison among three groups was conducted by Kruskal‐Wallis test followed by Dunn's multiple comparisons test, and P values of comparison between two groups were shown. P < .05 was considered significant. The values of LncRNA ANRIL/miR‐125a axis, lncRNA ANRIL, and miR‐125a in differentiating BA‐E patients, BA‐R patients, and controls were assessed by ROC curve analyses. ANRIL, antisense non‐coding RNA in the INK4 locus; BA‐E, bronchial asthma at exacerbation; BA‐R, bronchial asthma at remission; LncRNA, long non‐coding RNA; miR‐125a, microRNA‐125a; ROC, receiver operating characteristic
Besides, the ability of lncRNA ANRIL/miR‐125a axis in distinguishing BA‐E patients, BA‐R patients, and controls was assessed by ROC curve analyses. LncRNA ANRIL/miR‐125a axis (AUC: 0.896, 95%CI: 0.851‐0.941), lncRNA ANRIL (AUC: 0.875, 95%CI: 0.823‐0.926), and miR‐125a (AUC: 0.818, 95%CI: 0.758‐0.878) were of good value in differentiating BA‐E patients from controls (Figure 1D). Also, lncRNA ANRIL/miR‐125a axis (AUC: 0.825, 95%CI: 0.766‐0.884), lncRNA ANRIL (AUC: 0.753, 95%CI: 0.681‐0.825), and miR‐125a (AUC: 0.801, 95%CI: 0.735‐0.867) were able to distinguish BA‐E patients from BA‐R patients (Figure 1E). In addition, lncRNA ANRIL/miR‐125a axis (AUC: 0.670, 95%CI: 0.591‐0.748), lncRNA ANRIL (AUC: 0.655, 95%CI: 0.575‐0.734), and miR‐125a (AUC: 0.593, 95%CI: 0.507‐0.679) could differentiate BA‐R patients from controls (Figure 1F). These indicated that lncRNA ANRIL/miR‐125a axis could differentiate BA‐E patients, BA‐R patients, and controls and was especially good at predicting the disease exacerbation.
3.3. Correlation between lncRNA ANRIL and miR‐125a in BA‐E patients, BA‐R patients, and controls
In BA‐E patients, lncRNA ANRIL expression was negatively correlated with miR‐125a expression (P < .001) (Figure 2A). As for in BA‐R patients, lncRNA ANRIL expression was also negatively associated with miR‐125a expression (P = .024) (Figure 2B). In addition, lncRNA ANRIL expression was negatively correlated with miR‐125a expression in controls as well (P = .002) (Figure 2C). These data indirectly suggested that miR‐125a was negatively correlated with lncRNA ANRIL, but the correlation was rather weak.
Figure 2.
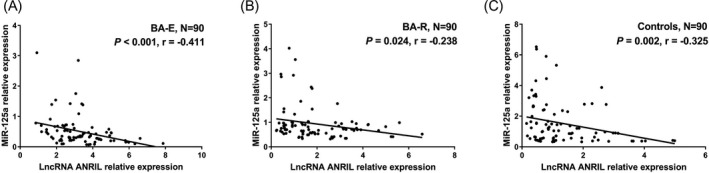
Negative correlation of lncRNA ANRIL with miR‐125a in BA‐E patients, BA‐R patients and controls. MiR‐125a expression was negatively correlated with lncRNA ANRIL in BA‐E patients (A), BA‐R patients (B), and controls (C). Correlations between variables were analyzed by Spearman's rank correlation test. P < .05 was considered significant. LncRNA, long non‐coding RNA; ANRIL, antisense non‐coding RNA in the INK4 locus; miR‐125a, microRNA‐125a; BA‐E, bronchial asthma at exacerbation; BA‐R, bronchial asthma at remission
3.4. Correlation of lncRNA ANRIL/miR‐125a axis with biochemical indexes and pulmonary ventilation functions in BA‐E patients, BA‐R patients, and controls
In BA‐E patients, lncRNA ANRIL/miR‐125a axis was negatively correlated with FEV1/FVC (P < .001) and FEV1%predicted (P < .001); lncRNA ANRIL was negatively associated with FEV1/FVC (P < .001) and FEV1%predicted (P = .003); and miR‐125a was positively correlated with FEV1/FVC (P < .001) and FEV1%predicted (P < .001) (Table 2). In BA‐R patients, lncRNA ANRIL/miR‐125a axis (P = .047) and lncRNA ANRIL (P = .025) were negatively correlated with FEV1/FVC, while miR‐125a was associated with reduced IgE level (P = .009). As in controls, miR‐125a was positively associated with FEV1/FVC (P = .036). These data indicated that lncRNA ANRIL/miR‐125a axis was correlated with advanced disease severity in bronchial asthma, especially in those with disease exacerbation.
Table 2.
Correlation of lncRNA ANRIL/miR‐125a axis, lncRNA ANRIL, and miR‐125a with biochemical indexes and pulmonary ventilation functions
| Items | lncRNA ANRIL/miR‐125a axis | lncRNA ANRIL | miR‐125a | |||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| BA‐E patients | ||||||
| IgE | .163 | .125 | .126 | .238 | −.120 | .259 |
| FEV1/FVC | −.621 | <.001 | −.395 | <.001 | .574 | <.001 |
| FEV1%predicted | −.519 | <.001 | −.308 | .003 | .548 | <.001 |
| BA‐R patients | ||||||
| IgE | .150 | .158 | .064 | .548 | −.272 | .009 |
| FEV1/FVC | −.210 | .047 | −.236 | .025 | .101 | .345 |
| FEV1%predicted | .027 | .800 | .019 | .860 | −.055 | .606 |
| Controls | ||||||
| IgE | −.067 | .532 | −.055 | .609 | .083 | .436 |
| FEV1/FVC | −.144 | .177 | .002 | .986 | .221 | .036 |
| FEV1%predicted | .148 | .165 | .096 | .367 | −.128 | .228 |
Correlation between variables was determined by Spearman's rank correlation test. P value < .05 indicated a significant difference.
Abbreviations: BA‐E, bronchial asthma at exacerbation; BA‐R, bronchial asthma at remission; IgE, immune globulin E; FEV1, Forced expiratory volume in 1 second; FVC, forced vital capacity.
3.5. Correlation of lncRNA ANRIL/miR‐125a axis with inflammatory cytokines in BA‐E patients, BA‐R patients, and controls
In BA‐E patients, lncRNA ANRIL/miR‐125a axis was positively correlated with TNF‐α (P < .001), IL‐1β (P < .001), IL‐6 (P = .028), and IL‐17 (P = .001); lncRNA ANRIL was positively associated with TNF‐α (P < .001), IL‐1β (P = .004), IL‐6 (P = .026), and IL‐17 (P < .001), while miR‐125a was negatively correlated with TNF‐α (P < .001), IL‐1β (P < .001), IL‐6 (P = .048), and IL‐17 (P = .009) (Table 3). In BA‐R patients, lncRNA ANRIL/miR‐125a axis was positively correlated with TNF‐α (P = .002), IL‐1β (P = .012), IL‐6 (P = .002), and IL‐17 (P < .001); lncRNA ANRIL was positively associated with TNF‐α (P = .004), IL‐1β (P = .011), IL‐6 (P = .011), and IL‐17 (P < .001), whereas miR‐125a was negatively correlated with TNF‐α (P = .039), IL‐6 (P = .023), and IL‐17 (P < .001). As for in controls, lncRNA ANRIL/miR‐125a axis was positively correlated with IL‐1β (P = .034), IL‐6 (P = .006), and IL‐17 (P = .011); lncRNA ANRIL was positively associated with IL‐6 (P = .012), while miR‐125a was negatively correlated with IL‐17 (P = .011). These implied that lncRNA ANRIL/miR‐125a axis was positively correlated with inflammatory cytokines in bronchial asthma, especially in patients with disease exacerbation.
Table 3.
Correlation of lncRNA ANRIL/miR‐125a axis, lncRNA ANRIL, and miR‐125a with inflammatory cytokines
| Items | lncRNA ANRIL/miR‐125a axis | lncRNA ANRIL | miR‐125a | |||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| BA‐E patients | ||||||
| TNF‐α | .419 | <.001 | .374 | <.001 | −.393 | <.001 |
| IL‐1β | .379 | <.001 | .298 | .004 | −.377 | <.001 |
| IL‐6 | .232 | .028 | .235 | .026 | −.209 | .048 |
| IL‐17 | .335 | .001 | .407 | <.001 | −.273 | .009 |
| BA‐R patients | ||||||
| TNF‐α | .319 | .002 | .300 | .004 | −.218 | .039 |
| IL‐1β | .263 | .012 | .268 | .011 | −.159 | .134 |
| IL‐6 | .322 | .002 | .268 | .011 | −.239 | .023 |
| IL‐17 | .455 | <.001 | .369 | <.001 | −.382 | <.001 |
| Controls | ||||||
| TNF‐α | .206 | .051 | .183 | .084 | −.156 | .143 |
| IL‐1β | .224 | .034 | .168 | .113 | −.172 | .105 |
| IL‐6 | .286 | .006 | .264 | .012 | −.205 | .052 |
| IL‐17 | .267 | .011 | .163 | .124 | −.268 | .011 |
Correlation between variables was determined by Spearman's rank correlation test. P value < .05 indicated a significant difference.
Abbreviations: BA‐E, bronchial asthma at exacerbation; BA‐R, bronchial asthma at remission; IL, interleukin; TNF‐α, tumor necrosis factor α.
3.6. Correlation of lncRNA ANRIL/miR‐125a axis with exacerbation severity in BA‐E patients
Among BA‐E patients, there were 23 cases, 44 cases, and 23 cases with mild, moderate, and severe exacerbation severity, respectively. LncRNA ANRIL/miR‐125a axis was correlated with increased exacerbation severity in BA‐E patients (P < .001) (Figure 3A). Regarding lncRNA ANRIL, positive correlation was also observed between lncRNA ANRIL with exacerbation severity in BA‐E patients (P < .001) (Figure 3B). However, miR‐125a was negatively associated with exacerbation severity in BA‐E patients (P < .001) (Figure 3C).
Figure 3.
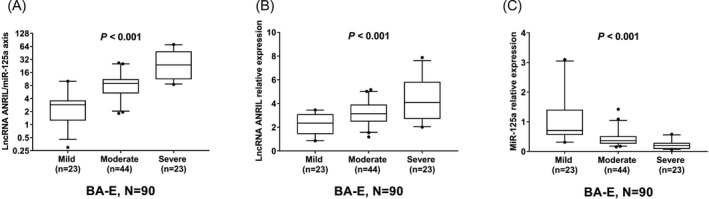
LncRNA ANRIL/miR‐125a axis in BA‐E patients with mild, moderate and severe exacerbation severity. LncRNA ANRIL/miR‐125a axis (A) and lncRNA ANRIL (B) expression were positively correlated with, while miR‐125a (C) expression was negatively correlated with exacerbation severity in BA‐E patients. The correlation of lncRNA ANRIL/miR‐125a axis, lncRNA ANRIL, and miR‐125a with exacerbation severity in BA‐E patients was assessed by Spearman's rank correlation test. P < .05 was considered significant. ANRIL, antisense non‐coding RNA in the INK4 locus; BA‐E, bronchial asthma at exacerbation; BA‐R, bronchial asthma at remission; LncRNA, long non‐coding RNA; miR‐125a, microRNA‐125a
4. DISCUSSION
Non‐coding RNAs are abundant RNA molecules with limited ability to translate into proteins, and the imbalances of non‐coding RNAs in human body have been implicated in pathology of a variety of human diseases.19 As a type of functionally important non‐coding RNA, long non‐coding RNAs (lncRNAs) are defined as non–protein‐coding transcripts with length longer than 200 nucleotides, which are recognized as key players in various biological processes including inflammation, angiogenesis, and oncogenesis.6, 7 The best well‐known mechanism of lncRNAs is their ability to sequester endogenous RNAs such as microRNAs (miRNAs), which are a small non‐coding RNA molecules around 22 nucleotides long, and regulate the downstream signaling pathways.20 And the clinical significance of lncRNA/miR axis has been increasingly investigated in extensive disease types.
LncRNA ANRIL is well known for its genetic implication in inflammatory diseases such as coronary artery disease and uric acid nephropathy.6, 8 And its role in pathogenesis of lung diseases is shown by its correlation with facilitated disease progression in NSCLC.9 In addition, miR‐125a low expression is previously reported to induce neutrophil recruitment and regulate lung inflammation in SLE.12 Known that miR‐125a is a target of lncRNA ANRIL,14, 15, 16, 17, 18 and both lncRNA ANRIL and miR‐125a regulate inflammatory responses including lung inflammation, we hypothesized that lncRNA ANRIL might interact with miR‐125a, and lncRNA ANRIL/miR‐125a axis might play critical roles in bronchial asthma as well. In our analysis, we discovered that lncRNA ANRIL/miR‐125a axis was of good predictive value for the risk of disease exacerbation in bronchial asthma as well as in distinguishing bronchial asthma patients from control. We also validated that lncRNA ANRIL was negatively correlated with miR‐125a in BA‐E patients, BA‐R patients, and controls. In addition, lncRNA ANRIL/miR‐125a axis was shown to be associated with poor pulmonary ventilation functions in bronchial asthma patients. This could be attributable to that: (a) LncRNA ANRIL/miR‐125a axis might regulate the downstream pathways, such as NF‐κB pathway and NLRP3 inflammasome activation that are associated with activation of inflammatory responses, thereby increased the disease risk of bronchial asthma.6, 7 It might also aggravate the immune response by raising the inflammatory cytokine levels and led to advanced disease severity in bronchial asthma patients. (b) LncRNA ANRIL/miR‐125a axis might cause damage to bronchial and alveolar cells by recruiting inflammatory cells, which might lead to lung inflammation and elevate the risk of disease exacerbation of bronchial asthma.
There have been several studies linking lncRNA ANRIL and miR‐125a to inflammatory cytokine levels, revealing the mechanism of lncRNA ANRIL and miR‐125a in regulating inflammatory responses in several inflammatory diseases. For instance, lncRNA ANRIL level is stimulated by TNF‐α, and it increases IL‐6 and IL‐8 levels in coronary artery disease,7 whereas miR‐125a is negatively correlated with C‐reactive protein, TNF‐α, and IL‐17 in patients with active Crohn's disease.11 Based on the previous evidence, lncRNA ANRIL and miR‐125a are both associated with inflammatory cytokine levels in inflammatory diseases. In addition, downregulated miR‐125a is previously shown to induce alveolar damage and hemorrhage in the lung in SLE patients.12 However, whether lncRNA ANRIL/miR‐125a axis affects inflammatory cytokine in inflammatory lung diseases, especially bronchial asthma, is unclear. To further determine the clinical significance of lncRNA ANRIL/miR‐125a axis in bronchial asthma, we measured its correlation with inflammatory cytokine levels among BA‐E patients, BA‐R patients, and controls. The analyses yielded that lncRNA ANRIL/miR‐125a axis were positively correlated with inflammatory cytokine levels in bronchial asthma patients and controls, especially in BA‐E patients. Here are the possible explanations: (a) Increased lncRNA ANRIL/miR‐125a axis might recruit immune and inflammation‐related cells such as neutrophils to the lung and facilitate the activation of inflammatory cascade and secretion of inflammatory cytokines.8, 11 Also, lncRNA ANRIL levels was shown to be elevated by TNF‐α, and subsequent release of other inflammatory cytokines such as IL‐6 was increased, and therefore, lncRNA ANRIL/miR‐125a axis was positively correlated with inflammatory cytokines in bronchial asthma patients.8, 11 (b) The correlation between lncRNA ANRIL/miR‐125a axis and inflammatory cytokines was especially apparent in BA‐E patients. This might be due to that there was an explosion of inflammatory response during disease exacerbation of bronchial asthma, which might cause the maladjustment of immune system. Under that circumstance, the dysregulation of lncRNA ANRIL/miR‐125a axis might be amplified, thereby lncRNA ANRIL/miR‐125a axis was more closely correlated with inflammatory cytokines in BA‐E patients.
The ROC curve analysis that evaluated the value of ANRIL/miR‐125a axis as well as inflammatory cytokines in distinguishing BA‐E, BA‐R, and controls was shown in Figure S1. ROC curve illustrated that ANRIL/miR‐125a axis was with diagnostic value non‐inferior to TNF‐α, IL‐1β, IL‐6, and IL‐17 in differentiating BA‐E patients from controls, BA‐E patients from BA‐R patients, and BA‐R patients from controls. Although there are existing inflammatory cytokines for asthma monitoring, the inflammatory cytokine level change is a result from inflammatory response, and their expression could be altered in various inflammatory diseases or affected by other pathological conditions other than asthma, which might lack specificity in asthma. However, ANRIL/miR‐125a axis is shown to regulate inflammatory response in genetic level and involves in pathogenesis of asthma in other ways such as damage to bronchial and alveolar cells.12, 21 Therefore, ANRIL/miR‐125a axis would be more beneficial to diagnose asthma exacerbation or to monitor the disease.
To our knowledge, this was the first study about the correlation of lncRNA ANRIL/miR‐125a axis with disease risk, severity, and inflammatory cytokines of bronchial asthma, while there remained several points to be improved. Firstly, the detailed mechanism of lncRNA ANRIL interacting with miR‐125 in bronchial asthma was not investigated, which needed further functional experiments. Secondly, study samples were recruited from a single center, which were not nationally representative. Lastly, the number of inflammatory cytokines used in this study was relatively small, and the correlation of lncRNA ANRIL/miR‐125a axis with inflammation in bronchial asthma patients might be further assessed by including more inflammatory markers.
In conclusion, lncRNA ANRIL/miR‐125a is potentially indicative of disease exacerbation, exacerbation severity, and inflammation for bronchial asthma, while these findings are preliminary and need further confirmation.
Supporting information
ACKNOWLEDGMENTS
This study supported by Clinical Observation of Specific Immunotherapy Combined with Inhalation of Budesonide/Formoterol Aerosol in the Treatment of Respiratory Allergic Diseases (No. WX17D06).
Ye S, Zhu S, Feng L. LncRNA ANRIL/miR‐125a axis exhibits potential as a biomarker for disease exacerbation, severity, and inflammation in bronchial asthma. J Clin Lab Anal. 2020;34:e23092 10.1002/jcla.23092
Shenglan Ye and Shan Zhu contributed equally to this work.
REFERENCES
- 1. Asthma GIf . Global Strategy for Asthma Management and. Prevention. 2016;http://www.ginasthma.org.
- 2. Detoraki A, Granata F, Staibano S, et al. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy. 2010;65(8):946‐958. [DOI] [PubMed] [Google Scholar]
- 3. Ryanna K, Stratigou V, Safinia N, et al. Regulatory T cells in bronchial asthma. Allergy. 2009;64(3):335‐347. [DOI] [PubMed] [Google Scholar]
- 4. Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. 2015;15(1):57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michalik M, Wojcik‐Pszczola K, Paw M, et al. Fibroblast‐to‐myofibroblast transition in bronchial asthma. Cell Mol Life Sci. 2018;75(21):3943‐3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu J, Wu H, Wang D, et al. LncRNA ANRIL promotes NLRP3 inflammasome activation in uric acid nephropathy through miR‐122‐5p/BRCC3 axis. Biochimie. 2019;157:102‐110. [DOI] [PubMed] [Google Scholar]
- 7. Zhou X, Han X, Wittfeldt A, et al. Long non‐coding RNA ANRIL regulates inflammatory responses as a novel component of NF‐kappaB pathway. RNA Biol. 2016;13(1):98‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang F, Su X, Liu C, et al. Prognostic Value of Plasma Long Noncoding RNA ANRIL for In‐Stent Restenosis. Med Sci Monit. 2017;23:4733‐4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nie FQ, Sun M, Yang JS, et al. Long noncoding RNA ANRIL promotes non‐small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther. 2015;14(1):268‐277. [DOI] [PubMed] [Google Scholar]
- 10. Lin L, Gu ZT, Chen WH, et al. Increased expression of the long non‐coding RNA ANRIL promotes lung cancer cell metastasis and correlates with poor prognosis. Diagn Pathol. 2015;10(1). 10.1186/s13000-015-0247-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun CM, Wu J, Zhang H, et al. Circulating miR‐125a but not miR‐125b is decreased in active disease status and negatively correlates with disease severity as well as inflammatory cytokines in patients with Crohn's disease. World J Gastroenterol. 2017;23(44):7888‐7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith S, Wu PW, Seo JJ, et al. IL‐16/miR‐125a axis controls neutrophil recruitment in pristane‐induced lung inflammation. JCI Insight. 2018;3(15):e120798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu HL, Nie ZQ, Lu Y, et al. Circulating miR‐125b but not miR‐125a correlates with acute exacerbations of chronic obstructive pulmonary disease and the expressions of inflammatory cytokines. Medicine (Baltimore). 2017;96(51):e9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li R, Yin F, Guo YY, et al. Knockdown of ANRIL aggravates H 2 O 2 ‐induced injury in PC‐12 cells by targeting microRNA‐125a. Biomed Pharmacother. 2017;92:952‐961. [DOI] [PubMed] [Google Scholar]
- 15. Li G, Zhu Y. Effect of lncRNA ANRIL knockdown on proliferation and cisplatin chemoresistance of osteosarcoma cells in vitro. Pathol Res Pract. 2019;215(5):931‐938. [DOI] [PubMed] [Google Scholar]
- 16. Hu X, Jiang H, Jiang X. Downregulation of lncRNA ANRIL inhibits proliferation, induces apoptosis, and enhances radiosensitivity in nasopharyngeal carcinoma cells through regulating miR‐125a. Cancer Biol Ther. 2017;18(5):331‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang LM, Ju HY, Wu YT, et al. Long non‐coding RNA ANRIL promotes tumorgenesis through regulation of FGFR1 expression by sponging miR‐125a‐3p in head and neck squamous cell carcinoma. Am J Cancer Res. 2018;8(11):2296‐2310. [PMC free article] [PubMed] [Google Scholar]
- 18. Chai L, Yuan Y, Chen C, et al. The role of long non‐coding RNA ANRIL in the carcinogenesis of oral cancer by targeting miR‐125a. Biomed Pharmacother. 2018;103:38‐45. [DOI] [PubMed] [Google Scholar]
- 19. Owen HC, Torrance HD, Jones TF, et al. Epigenetic regulatory pathways involving microRNAs may modulate the host immune response following major trauma. J Trauma Acute Care Surg. 2015;79(5):766‐772. [DOI] [PubMed] [Google Scholar]
- 20. Tian L, Hu X, He Y, et al. Construction of lncRNA‐miRNA‐mRNA networks reveals functional lncRNAs in abdominal aortic aneurysm. Exp Ther Med. 2018;16(5):3978‐3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin Y, Wu L, Ouyang Y, et al. MiR‐125a Is a critical modulator for neutrophil development. PLoS Genet. 2017;13(10):e1007027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


