Abstract
Heart failure (HF) is rapidly growing, conferring considerable mortality, morbidity, and costs. Dedicated HF clinics improve patient outcomes, and the development of a national HF clinics network aims at addressing this need at national level. Such a network should respect the existing health care infrastructures, and according to the capacities of hosting facilities, it can be organized into three levels. Establishing the continuous communication and interaction among the components of the network is crucial, while supportive actions that can enhance its efficiency include involvement of multidisciplinary health care professionals, use of structured HF‐specific documents, such as discharge notes, patient information leaflets, and patient booklets, and implementation of an HF‐specific electronic health care record and database platform.
Keywords: Heart failure, Heart failure clinics, Heart failure network, Multidisciplinary team, Discharge letter, Electronic health care record
1. The need for a heart failure network
Heart failure (HF) is the most rapidly growing cardiovascular condition globally and is associated with considerable mortality, morbidity, and hospitalization rates.1 Patients hospitalized for HF have a particularly adverse prognosis, with a high risk of mortality and rehospitalization and account for approximately 70% of the total expenditure related to the syndrome.2 As a result, HF is becoming a major challenge for the health care systems.3 Several community‐based studies and registries have consistently shown that evidence‐based therapies are underused in real‐life HF patients, while mortality and hospitalization rates are higher than those reported in randomized clinical trials.4
Dedicated HF clinics have been developed in many countries to systematically address the unmet needs for timely diagnosis, management, and clinical follow‐up of HF patients.5, 6 A wide variety of multidisciplinary strategies to manage HF patients have been examined including outpatient clinic‐based care, home‐visiting programmes, structure telephone support, telemonitoring, and patient education to enhance self‐care as compared with usual care.7 The optimal strategy according to patient status has not been proved, but involvement of a cardiologist in the care of HF patients improves clinical outcomes.8 A multi‐professional strategy for the management of HF patients has been shown to reduce all‐cause mortality and all‐cause and HF hospitalizations and improved adherence to life‐saving medications.9 Structured telephone support and non‐invasive telemonitoring were also found to reduce all‐cause mortality and HF‐related hospitalizations, whereas home‐visit programmes decreased primarily all‐cause and HF hospitalizations. Interestingly, follow‐up in a clinic with primary care supervision seems to be ineffective.7
2. Structure and functions of a national heart failure clinics network
The development of a national HF clinics network aims at three important aims: (i) the improvement of patient outcomes in terms of mortality, hospitalization, and quality of life, (ii) the reduction of HF‐related health care expenditure, and (iii) the improvement in the allocation of limited health care resources. The network is expected to provide expert consultation, assessment, and management to all patients with HF, including both HF with reduced and preserved left ventricular ejection fraction.
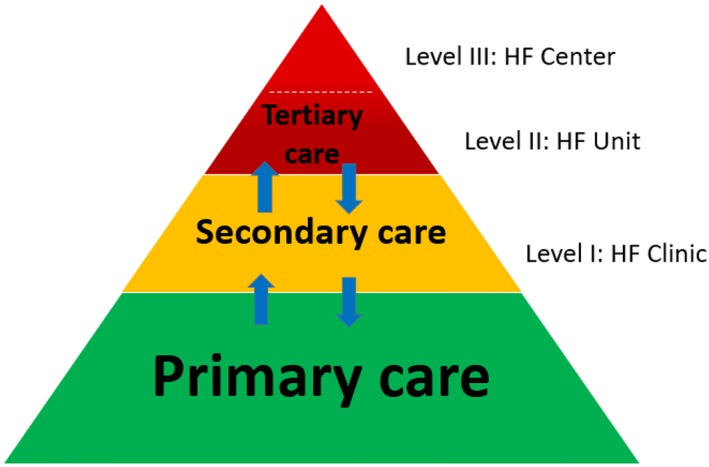
Establishing a national HF clinics network faces major challenges concerning the incorporation of existing clinical management processes and the integration of medical units providing different levels of care.5 In order for this network to be realistic, applicable, and functional, it has to respect the existing health care infrastructures, on which is expected to run. This aim would be better served if the HF network is organized into a three‐level structure according to capacities of the hosting health care facility.5 The main characteristics and requirements of this three‐level structure are summarized in Table 1. A similar graded structure of HF clinics is also proposed by the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) a means to ensure quality care for HF across Europe (M.F. Piepoli, personal communication).
Table 1.
Proposed organization levels and minimum requirements of medical units in the context of a national heart failure (HF) network
| Level | Unit name | Location | Personnel | Infrastructure | Diagnostic assessments | Therapeutic interventions |
|---|---|---|---|---|---|---|
| I | ‘HF clinic’ | Secondary regional/provincial hospitals | Cardiologists with HF training | Outpatient clinic, inpatient wards, general intensive care units (ICU), echocardiography lab, exercise testing lab | Clinical, electrocardiography, 6 min walk test, quality of life, natriuretic peptides, cardiac troponins and basic haematological and biochemical investigations, echocardiography, exercise stress testing | Clinical management, ward, and ICU hospitalization |
| II | ‘HF unit’ | Large secondary, tertiary, or university hospitals serving greater regions | Cardiologists, HF experts | The above plus cardiac catheterization lab, electrophysiology lab, computed tomography lab, cardiac care unit | The above plus cardiopulmonary exercise testing, transesophageal echo, cardiac catheterization, computed tomography imaging, basic electrophysiology | The above plus coronary artery interventions, device implantation, more advanced intensive care (venous–venous ultrafiltration) |
| III | ‘HF centre’ | Large tertiary or university hospitals serving urban areas | Cardiologists, HF experts | The above plus cardiac magnetic resonance imaging lab, nuclear cardiology lab, cardiac surgery | The above plus 3D echo, cardiac magnetic resonance imaging, nuclear cardiology imaging, advanced electrophysiology (navigation systems), endomyocardial biopsy | The above plus transcatheter valve implantation or repair, advanced electrophysiology interventions (VT ablation), cardiac surgery, mechanical circulatory support, assist device implantation, and/or cardiac transplantation |
The medical units serving at the first level of care will be termed ‘HF clinics’. These units reside at regional or provincial secondary hospitals through the country and consist of an outpatient clinic providing regular patient follow‐up linked to an inpatient cardiology department providing care in case of decompensation or acute de novo presentation (ward and general intensive care unit). HF clinics are expected to provide basic diagnostic and therapeutic services including clinical evaluation, electrocardiography, 6 min walk test, quality of life assessment, main haematological and biochemical investigations including natriuretic peptides and cardiac troponins, and echocardiographic imaging. In terms of personnel, HF clinics are populated by specialized cardiologists, ideally including at least one cardiologist with HF training and a nurse with experience in HF. HF clinic establishes affiliations with primary care units and physicians of the same region in order to ensure a seamless referral pathway of HF patients. HF clinics are further expected to contribute to national and international HF registries.
The medical units serving at the second level of care are termed ‘HF units’. These reside at larger secondary or tertiary hospitals covering the greater regions of the country with facilities for cardiac devices and more advanced cardiac procedures. In addition to the infrastructure and services provided by HF clinics, HF units are hosted in hospitals that provide more complex diagnostic and therapeutic procedures, including cardiopulmonary exercise testing, cardiac catheterization and electrophysiology (basic electrophysiology studies and device implantation), and computed tomography imaging, and are further equipped with a cardiac care unit and optionally with a cardiac magnetic resonance imaging lab or nuclear cardiology lab and cardiac surgery. HF units are populated by cardiologists with HF training and optionally with one HF expert (cardiologist specialized in HF) and an HF nurse, collaborating with other medical specialists such as nephrologist, pulmonologists, or cardiac rehabilitation specialists. Moreover, HF units are expected to contribute to HF research at least at a national level. HF units are further expected to contribute to HF education and training by running regional educational events.
Finally, the medical units at the third level of care are termed ‘HF centres’. These reside at large tertiary or university hospitals in main urban areas of the country. In addition to the infrastructure and services provided by hospitals hosting HF units, those hosting HF centres provide high‐end diagnostic and therapeutic procedures, including cardiac magnetic resonance, 3D echo imaging, nuclear cardiology, advanced electrophysiology, such as navigation systems and ventricular tachycardia ablation, endomyocardial biopsy and complex cardiac catheterization interventions such as transcatheter valve implantation or repair, mechanical circulatory support, and cardiac surgery offering ventricular assist device implantation and/or cardiac transplantation. The hosting hospitals provide wards and cardiac care units with dedicated HF beds and are populated by HF specialists with experience in the different areas of advanced HF management, experienced HF nurses, heart transplant/mechanical circulatory support specialists and technicians, and other affiliated medical specialists. HF centres further have a leading role in HF research at national and international level. Finally, they also hold a leading role in HF education and training.
In parallel to the aforementioned organizational structure, it is important to ensure the establishment of continuous communication, interaction, and networking among the different medical units that constitute the HF network. Important supportive actions that may enhance the efficiency of the network include the involvement of additional health care personnel besides the treating physicians, with a central role of HF nurses, the development and widespread use of structured documents such as an HF‐specific discharge note, an HF patient information leaflet, and an HF patient booklet as well as the development and implementation of a widely accessible HF‐specific electronic health care record and database platform, as described in detail below. Further support to this network should be provided by educational and training sessions originated from higher‐level to lower‐level structures in the context of the network. The application of widely accepted clinical protocols for diagnosis, treatment, and follow‐up as well as the training and accreditation of involved health care professionals in HF would enhance considerably efficiency. In addition, the performance of the units should be assessed regularly based on outcome measures or quality indicators such as mortality, readmissions and emergency room visits, patients' functional status, and health‐related quality of life (HR‐QoL). At a later stage of development, all medical units participating in the network will be required to apply for accreditation for the level of services that are expected to provide.
Heart failure is characterized by multisystem involvement, and therefore, the formation of a multidisciplinary team able to manage effectively the different aspects of HF health care is important, particularly at the higher‐level structures. Such a multidisciplinary HF team would facilitate referral of patients to specialists and allow joint consultation sessions with the participation of two or more specialists if this is deemed necessary. Besides HF specialists, cardiologists, and HF nurses, this team may consist of nephrologists, pulmonologists, psychiatrists, psychologists, physiotherapists, and other specialists. With the development of the HF network, the lower‐level structures will benefit from these multidisciplinary HF teams.
Exercise training and rehabilitation programmes constitute an indispensable aspect of HF care. As this network is designed to operate on the existing infrastructure, rehabilitation services will be provided only by certain units where such services are already available. A more extensive availability of cardiac rehabilitation remains an unmet need that should be addressed at a later stage of network development.
According to local or national needs, the network may also comprise highly specialized clinics, including clinics for genetic cardiomyopathies, cardiomyopathies associated with systemic conditions such as sarcoidosis, cardio‐oncology clinics, cardiac clinics for haemoglobinopathies, or clinics for adults with congenital heart disease.
3. Treatment and follow‐up protocols
Heart failure treatment and follow‐up strategies vary widely across hospitals and clinics even within the same region.10, 11, 12 There is therefore a need for implementation of structured follow‐up protocols. The optimal method of monitoring will depend on local organizational structures and resources as well as patient needs. Follow‐up may involve regular visits to community or hospital clinics, home visits or remote monitoring with or without implanted devices, or structured telephone support. More frequent monitoring may be required during periods of instability or titration of medication, as well as in older patients or those with important co‐morbidities. Particularly in recently discharged patients, optimal follow‐up decreases significantly readmission rates; the implementation of a 7 day follow‐up visits programme in a tertiary US hospital as a means of quality improvement resulted in a 30% decrease of HF 30 day readmission rate.13, 14
Regular monitoring may detect disease progression and/or complications or deterioration of symptoms that may require a change in management (e.g. the onset of atrial fibrillation or development of anaemia). Natriuretic peptides predict outcome in this patient population; a decrease in their levels during recovery from an episode of acute decompensation is associated with better prognosis.15 Natriuretic peptide‐guided therapy has been proposed as a method to monitor clinical status and tailor treatment; however, published studies have provided conflicting results, and this approach is not recommended by the current guidelines.16
Telemedicine provides the possibility of remote patient assessment. Several methods and technologies have been tested; results of clinical trials are conflicting, but meta‐analyses suggest some clinical benefit.17
The recent ESC HF guidelines provide specific recommendations regarding monitoring and follow‐up of the patient with HF, especially the elderly, encouraging physicians to
monitor frailty and seek and address reversible causes (cardiovascular and non‐cardiovascular) of deterioration in frailty score,
review medication and optimize doses of HF medication slowly and with frequent monitoring of clinical status,
reduce polypharmacy (number, doses, and complexity of regime),
consider stopping medication without an immediate effect on symptom relief or HR‐QoL (such as statin),
review the timing and dose of diuretic therapy to reduce risk of incontinence, and
consider the need to refer to specialist care of the elderly team and to general practitioner and social worker, for follow‐up and support for the patient and his or her family.16
The network may further provide consultation to patients with a diagnosis of HF who are being treated and followed outside the network, either on a regular basis or whenever this is deemed necessary. In other words, the care of patients may still remain with a treating physician outside the network, and the patients may be reviewed in an HF clinic within the network regularly or according to the needs.
4. Assessment of exercise capacity
Exercise intolerance is a main manifestation of HF directly related to the severity of the syndrome. Clinical variables and laboratory tests can be used to assess exercise intolerance. The simplest method is the New York Heart Association (NYHA) functional classification, which is based on patients' symptoms. Decreased maximal exercise capacity is associated with higher NYHA class and worse prognosis and quality of life. Unfortunately, symptom severity poorly correlates with left ventricular function and prognosis, and even patients with mild symptoms bare a significant risk of morbidity and mortality.16, 18 Furthermore, symptoms might reflect the severity of other non‐cardiovascular diseases and not HF per se. More comprehensive questionnaires such as the Kansas City Cardiomyopathy Questionnaire (KCCQ) that covers some very important domains besides symptoms (i.e. self‐efficacy, quality of life, and social limitation) may add incremental value to NYHA classification.19 The 6 min walk test is easy to implement, and it can give objective information about patient's exercise tolerance, especially in patients with severe symptoms, although it may not discriminate accurately HF severity among asymptomatic patients or those with mild symptoms.20 The best method to assess functional capacity in HF patients is cardiopulmonary exercise stress test, as it provides accurate measures and prognostic parameters and may discriminate between cardiac and non‐cardiac causes of exercise intolerance. However, the need for special equipment and experienced personnel preclude its widespread use.18, 21 In conclusion, NYHA classification and 6 min walk test should be implemented in all HF clinics, while the cardiopulmonary exercise test should be available in more advanced setting.
5. Assessment of the health‐related quality of life
Heart failure patients generally report worse HR‐QoL than the general population or even patients with other chronic conditions, such as diabetes mellitus, cancer, or Alzheimer's disease.22 The term HR‐QoL refers to the extent of a patient's general well‐being, which is usually affected by the health status. During the last years, the term disease‐specific QoL has been introduced and quantifies the limitation of a patient's physical, emotional, and social life that can be attributed to a specific disease or syndrome.
The assessment of HR‐QoL in HF patients includes HF‐specific self‐administered questionnaires, namely, the Minnesota Living with Heart Failure Questionnaire and the KCCQ.23, 24 These questionnaires are easily completed, and the results are summarized as functional, emotional, and overall components. Both of them are widely accepted and are proposed by the current European HF Guidelines, because they are excellent predictors of patients' morbidity and mortality.17 A shorter version of KCCQ is also available,25 while general quality of life questionnaires, such as the EQ‐5D26 may also be used.
Improving HR‐QoL represents a stand‐alone target in the management of HF patients. Many recent large clinical trials include improvement of QoL as an endpoint, moving towards a more patient‐centred approach. Thus, a combination of survival prolongation and QoL improvement, an outcome usually estimated by the quality‐adjusted life years, is increasingly used by clinical trials.
6. The role of nurses
A holistic approach by an interdisciplinary team in all health care settings, from primary to palliative, is needed for the effective management of HF.8, 16 Nurses may have an important role in such a comprehensive care. Prevention, early diagnosis of possible decompensation, and development of self‐care educational and follow‐up programmes are key domains that can be served by HF nurses, while they may act as care co‐ordinators and the liaison persons, who connect patients and their family with the health care system.27, 28 A recent meta‐analysis has shown that one of the determinants of a successful HF management programme is whether HF nurses were included in the programme.29 In addition, when post‐discharge follow‐up programmes were led by nurses, a decrease in readmission rates was achieved.28 As the educational background of nurses involved in HF programmes is pivotal, the HFA of the ESC has developed a curriculum for HF nurses.30, 31
7. Networking with primary and community health care
While the acute phase of HF usually requires admission to hospital for diagnostic interventions and treatment, the great majority of patients live with chronic HF, and as the number of these patients increases exponentially worldwide, out‐of‐hospital community and primary care settings should be involved in their care. Primary care services vary among countries and even within each country.
Prevention of HF and rapid referral to a cardiologist for diagnosis of HF in patients with symptoms are two aspects of the disease to which primary care may contribute. Furthermore, there are multiple areas in HF management in which primary care could play an important role32, 33; education and support of stable, low‐risk patients and caregivers, promotion of self‐care, counselling on lifestyle modifications, adherence to treatment and correct use of medications, and co‐ordination of care for co‐morbidities. The 2016 ESC HF guidelines suggest that referral to primary care for long‐term follow‐up may be considered for stable HF patients who are on optimal therapy to monitor for effectiveness of treatment, disease progression, and patient adherence (class IIb recommendation).16 Rigorous follow‐up at periods of clinical instability, that is, transition phase after hospital discharge, and early identification of clinical worsening remain challenging areas in which primary care could offer great help. End‐of‐life, palliative care, and home‐care for frail patients unable to move are also sensitive aspects of care.34
Although primary care physicians and nurses play a key role in HF care,16 the current status of HF management in primary care remains suboptimal.34, 35 Collaborative models of care where the primary care physician may share the care with a cardiologist have been shown to improve patient outcomes compared with a primary physician only.36 Technology may play a leading role in this regard, as telemedicine platforms have been designed to link teams of experts within an academic hub with primary care clinicians to improve care provision and enhance primary physicians' learning by providing mentoring and feedback from specialists.37 Therefore, designing and establishing highly collaborative HF networks that co‐ordinate all groups involved in HF care provision at various levels may improve HF patient outcomes and ensure that limited resources are effectively deployed.
Establishing proper communication among physicians, and providing a clear patient pathway when required, is a crucial need that an HF network should address and actually one of the key reasons in building a network. Figure 1 provides an idea of patient flow through the proposed three‐level structure. In this context, establishing criteria for patient referral is important, and this is an issue that this network will address in the near future.
Figure 1.
A proposed scheme for the design of a heart failure care network.
8. Training and accreditation in heart failure
The complexity of HF syndrome dictates the need of training and accreditation of physicians through a curriculum widely accepted across Europe.2 HF subspecialty should be acknowledged at a national level and offered as an additional option of further training to cardiologists that express special interest. The HFA of the ESC has provided a framework that can be used as a blueprint and has defined the required knowledge, the necessary skills, and the professionalism that should be attained.38
The proposed programme of training in HF consists of two 12 month periods. The first period is dedicated to diagnosis and investigation of HF, treatment of the underlying aetiology, diagnosis and treatment of co‐morbidities, medical treatment of HF, lifestyle changes, device therapy for HF, monitoring of HF patients, acute HF, and functioning as part of multidisciplinary team. In the second period, HF specialist trainee can choose between special modules and obtain further expertise in advanced imaging (echocardiography and cardiac magnetic resonance), device therapy, or heart transplantation and mechanical circulation support. Finally, specific assessment methods of trainee's knowledge, skills, competence, and professionalism are mandatory and universally used: logbook for all procedures, direct observation of procedural skills, assessment of behaviours in outpatient and inpatient management, assessment of ability to function in and lead a multidisciplinary team, overall management of complex cases, participation in continuing medical education, and assessment of advanced knowledge by online HFA education programme with multiple choice questions. Subspecialty HF accreditation programmes have been developed in US by American Board of Internal Medicine39 and UK,40 and a hands‐on HF certification course is also provided by HFA.41
9. Heart failure‐specific tools
9.1. Heart failure discharge note
Patients discharged with an HF diagnosis have a markedly high readmission rate, reaching 30% at 3 months.42 In addition, readmission after index hospitalization carries a significantly higher risk of mortality compared with index hospitalization.43 Undoubtedly, the major issue with the most widely used discharge notes is the lack of information on post‐discharge care transition and outpatient follow‐up.44, 45 In contrast, comprehensive discharge notes can significantly reduce readmission rates.45, 46, 47 Discharge notes should constitute a link between the patient, the hospital physicians, the primary care and community physicians, and any other health care professional involved in patient's care. This will ensure awareness of patient's medical issues and assist in clinical decision making with the ultimate goal of improving patient outcomes and health care efficiency.47
A discharge note should contain information on diagnosis and cause of HF; clinical status at admission (NYHA class); echocardiographic findings and natriuretic peptide levels confirming diagnosis; electrocardiogram mentioning QRS duration, rhythm, presence of atrioventricular, bundle branch, or fascicular block; laboratory tests results (urea, creatinine, haemoglobin, BNP or NT‐proBNP, and discharge electrolytes); patient weight at discharge; blood pressure and heart rate at discharge; medications and dosing during hospitalization and discharge; titration instructions and reasons for use of smaller target doses or document reason for no initiation; instructions concerning blood pressure, heart rate, and body weight targets; arrhythmic risk stratification; programming for implantation of a cardiac rhythm management device; encouragement for daily monitoring of body weight; smoking cessation and referral to specialized centres; targeted dietary instructions; administration and monitoring of anticoagulation therapy, on indication, as well as cautions for co‐administration with certain medications (antibiotics) and annual flu vaccination. The components of an HF discharge note are outlined in Table 2.
Table 2.
Components of a structured heart failure‐specific discharge note
| • Demographics and administrative data |
| • Diagnosis and cause of heart failure |
| • Clinical status at admission (NYHA class) |
| • Echocardiographic findings and natriuretic peptide levels confirming diagnosis |
| • Copy of an electrocardiogram mentioning QRS duration, rhythm, presence of atrioventricular or bundle branch, or fascicular block |
| • Laboratory tests results (urea, creatinine, haemoglobin, BNP or NT‐proBNP, and discharge electrolytes) |
| • Patient weight at discharge (‘dry weight’) |
| • Blood pressure and heart rate at discharge |
| • Medications and dosing during hospitalization and following discharge. If no beta‐blockers, MRAs, ACEIs, or, alternatively, ARBs are administered record and document reason |
| • Titration instructions and reasons for use of smaller target doses |
| • Instructions concerning blood pressure, heart rate, and body weight targets |
| • Arrhythmic risk stratification and, on indications, programming for implantation of a cardiac rhythm management device (defibrillator, biventricular pacemaker) |
| • Encouragement for daily monitoring of body weight and, in case of abrupt increase—more than 2 kg in 3 days, contact with treating physician |
| • Encouragement for smoking cessation and referral to specialized centres |
| • Targeted dietary instructions |
| • Instructions for administration and monitoring of anticoagulation therapy, on indications, as well as cautions for co‐administration with certain medications (antibiotics) |
| • Instructions for annual flu vaccination |
| • Instructions for reassessment at a dedicated heart failure outpatient clinic following a laboratory workup (which should be detailed) |
| • Names of treating physicians, with attached copies of their instructions |
| • Patient information regarding contact details for Heart Failure Outpatient Clinic, both of the discharging hospital and hospitals near patient's residence |
9.2. Heart failure patient booklet and information leaflet
The recent European HF Guidelines suggest that a ‘seamless’ system of health care for patients leaving hospital and returning to the community is key to success.16 In this context, an HF patient booklet is a means to improve medical management, access to care during episodes of decompensation, follow‐up after discharge, patient education, and treatment adherence. Such a booklet should offer an integrated and comprehensive view of administrative and health‐related information as outlined in Table 3. The booklet may be delivered in a hard copy paper format, while a printed electrocardiogram and copies of clinic notes may be parts of it, and data can be updated at follow‐up consultations. Used as a hand‐held tool during patient review, the booklet may rapidly inform the health care professional about the key features of the HF patient, which are required to optimize care.
Table 3.
Components of a heart failure patient booklet
| • Personal data, family and social history, contact details |
| • Past medical history, co‐morbidities, surgeries and other procedures, allergies and adverse drug reactions, blood transfusions |
| • HF aetiology, disease course, phenotype, device therapy, hospitalizations for decompensation |
| • A review of medications and dosing, including possible side effects, need for changes and up‐titration goals |
| • A clinical assessment of vital signs, cardiac rhythm, functional capacity, 6 min walk test, fluid status, nutritional and cognitive status |
| • Reports of imaging (echocardiography, chest X‐ray, SPECT, coronary angiography, cardiac ΜRΙ), cardiorespiratory stress test with VO2 max, electrophysiology study and ablation, myocardial biopsy, genetic testing |
| • Laboratory test results (serum urea, creatinine, electrolytes, haemoglobin, ferritin, TSH, NT proBNP) |
| • Vaccinations |
| • Consultation of other specialists |
| • Free space for special notes |
In addition to an HF patient booklet, a patient information leaflet, containing all important pieces of information that the patient should know about HF, is useful. Written in simple language, this leaflet would provide a reliable source of information to address the needs of the broad spectrum of HF patients, including not only those with poor access to sources of knowledge, such as elderly people, but also those that are exposed to a wealth of information that may sometimes be unreliable and misleading. The proposed components of this leaflet are summarized in Table 4, while a complete example of such a leaflet can be found in Supporting Information, Data S1.
Table 4.
Components of a heart failure patient information leaflet
| • What is heart failure? |
| • What should I do to live better? |
| ○ Medications |
| ○ Diet—Salt |
| ○ Exercise |
| ○ Lifestyle |
| ○ Vaccinations |
| • How should I adjust my activities? |
| ○ Driving |
| ○ Travel |
| ○ Sex |
| • How do I deal with emotions and feelings? |
| • How should I assess and manage my condition? |
| • What kind of services are provided by the heart failure clinics, and why should I attend one? |
| • Where can I find more information (websites)? |
Continuous patient education remains an important aim of this network. Besides the HF patient booklet and the informational leaflet, important information for patient guidance is also included in the HF‐specific discharge note, as previously mentioned. In addition to provided knowledge, the patient leaflet encourages the patients to seek further knowledge in suggested Internet resources, such as the webpage hosting the Greek translation of the ‘Heart Failure Matters’ project. HF classes for patients and other educational events are further encouraged and supported by the network, but they depend upon local potentials. Besides these modalities, patient education remains a continuous process that should ideally occur during every contact with the physician.
9.3. Heart failure e‐platform
The management of HF is characterized by lifelong care, with multiple outpatient clinic visits, frequent hospital admissions, and multidisciplinary care with visits to other medical specialists. This creates a bulk of medical information with huge medical records that are not able to handle in paper. As a result, HF management imposes the use of electronic medical records. In addition, an indispensable part of an HF network is research activities. Actually, HF networks are ideal for generation of real‐world evidence that can be used for the identification of unmet health care needs, the design of public health policies, or the documented advocacy for health system reforms as well as for participation in clinical research projects.48 Exploitation of HF patient data for research requires the development of a well‐organized medical database that cannot be performed without a proper electronic platform. An electronic platform that would combine the aforementioned functions would be ideal for the needs of an HF network. The features of such a platform include a well‐structured and functional medical record for outpatient and inpatient contacts, the widest possible accessibility from inside and outside the medical units, which can be provided only by a web‐based platform, the possibility of multiple simultaneous users, the extraction of anonymized data for research use, the possibility of customization for special clinical or research purposes, and a high‐level data safety with the use of secure data servers and proper encryption algorithms in accordance with the international standards. Additional features could render the system more attractive for clinicians, such as the generation of automated documents like prescription or an appointment support system. In addition, the e‐platform supports the development of fully customizable dedicated data entry forms that can be used to host surveys and registries. The features and structure of an HF‐specific electronic platform are summarized in Table 5.
Table 5.
Main features of an electronic platform for heart failure networks
| e‐platform features |
| • Structured HF‐specific electronic medical record |
| • Extractable anonymized datasets for research exploitation |
| • Wide accessibility form inside and outside medical units—web‐based |
| • Multiple simultaneous users |
| • Customization for clinical or research purposes |
| • Automatic document generation (e.g. discharge note and prescriptions) |
| • Appointment support system |
| • Established data safety protocols |
| • Secured data server complying with international standards |
| Medical record structure |
| • Basic medical record |
| • Demographics |
| • Medical history |
| • Visits |
| ‐ Outpatient visit |
| ‐ Inpatient visit |
| ‐ Special clinic visit (e.g. cardio‐oncology) |
| Visit structure |
| • Clinical data |
| • Laboratory investigations |
| • Drug therapy |
| • In‐hospital interventions (for inpatients visit) |
| • Medical instructions/prescription |
| ‐ Medications |
| ‐ Investigations |
| ‐ Referrals |
| ‐ Next appointment data and time |
Conflicts of interest
None declared.
Supporting information
Data S1. Example of a heart failure patient information leaflet.
The Task force of the Hellenic Heart Failure Clinics Network (2020) How to develop a national heart failure clinics network: a consensus document of the Hellenic Heart Failure Association. ESC Heart Failure, 7: 15–24. 10.1002/ehf2.12558.
References
- 1. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlstrom U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A. Heart Failure Association of the European Society of C. European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016; 18: 613–625. [DOI] [PubMed] [Google Scholar]
- 2. Filippatos G, Angermann CE, Cleland JGF, Lam CSP, Dahlström U, Dickstein K, Ertl G, Hassanein M, Hart KW, Lindsell CJ, Perrone SV, Guerin T, Ghadanfar M, Schweizer A, Obergfell A, Collins SP. Global Differences in Characteristics, Precipitants, and Initial Management of Patients Presenting with Acute Heart Failure [published online ahead of print, 2020. Jan 8]. JAMA Cardiol. 2020. 10.1001/jamacardio.2019.5108 [DOI] [PMC free article] [PubMed]
- 3. Farmakis D, Parissis J, Karavidas A, Karvounis C, Triposkiadis F, Filippatos G, Lekakis J. Collaborators. In‐hospital management of acute heart failure: practical recommendations and future perspectives. Int J Cardiol 2015; 201: 231–236. [DOI] [PubMed] [Google Scholar]
- 4. Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Metra M, Zwinderman AH. Determinants and clinical outcome of uptitration of ACE‐inhibitors and beta‐blockers in patients with heart failure: a prospective European study. Eur Heart J 2017; 38: 1883–1890. [DOI] [PubMed] [Google Scholar]
- 5. Anguita Sanchez M, Lambert Rodriguez JL, Bover Freire R, Comin Colet J, Crespo Leiro MG, Gonzalez Vilchez F, Manito Lorite N, Segovia Cubero J, Ruiz Mateas F, Elola Somoza FJ, Iniguez RA. Classification and quality standards of heart failure units: scientific consensus of the Spanish Society of Cardiology. Rev Esp Cardiol 2016; 69: 940–950. [DOI] [PubMed] [Google Scholar]
- 6. Hauptman PJ, Rich MW, Heidenreich PA. Chin J, Cummings N, Dunlap ME, Edwards ML, Gregory D, O'connor CM, Pezzella SM, Philbin E; Heart Failure Society of America. The heart failure clinic: a consensus statement of the Heart Failure Society of America. J Card Fail 2008; 14: 801–815. [DOI] [PubMed] [Google Scholar]
- 7. McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810–819. [DOI] [PubMed] [Google Scholar]
- 8. McDonagh TA. Blue L, Clark AL, Dahlstrom U, Ekman I, Lainscak M, McDonald K, Ryder M, Stromberg A, Jaarsma T, European Society of Cardiology Heart Failure Association Committee on Patient C. European Society of Cardiology Heart Failure Association Standards for delivering heart failure care. Eur J Heart Fail 2011; 13: 235–241. [DOI] [PubMed] [Google Scholar]
- 9. Gandhi S, Mosleh W, Sharma UC, Demers C, Farkouh ME, Schwalm JD. Multidisciplinary heart failure clinics are associated with lower heart failure hospitalization and mortality: systematic review and meta‐analysis. Can J Cardiol 2017; 33: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 10. Maggioni AP, Anker SD, Dahlstrom U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L. Heart Failure Association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology Guidelines? Evidence from 12,440 patients of the esc heart failure long‐term registry. Eur J Heart Fail 2013; 15: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 11. Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L. Heart Failure Association of the European Society of C. EURObservational Research Programme: regional differences and 1‐year follow‐up results of the heart failure pilot survey (ESC‐HF pilot). Eur J Heart Fail 2013; 15: 808–817. [DOI] [PubMed] [Google Scholar]
- 12. Komajda M, Follath F, Swedberg K, Cleland J, Aguilar JC, Cohen‐Solal A, Dietz R, Gavazzi A, Van Gilst WH, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, Widimsky J, Freemantle N, Eastaugh J, Mason J. Study group on diagnosis of the working group on heart failure of the European Society of C. The EuroHeart Failure Survey Programme—a survey on the quality of care among patients with heart failure in Europe. Part 2: Treatment. Eur Heart J 2003; 24: 464–474. [DOI] [PubMed] [Google Scholar]
- 13. Ryan J, Andrews R, Barry MB, Kang S, Iskandar A, Mehla P, Ganeshan R. Preventability of 30‐day readmissions for heart failure patients before and after a quality improvement initiative. Am J Med Qual 2014; 29: 220–226. [DOI] [PubMed] [Google Scholar]
- 14. Ryan J, Kang S, Dolacky S, Ingrassia J, Ganeshan R. Change in readmissions and follow‐up visits as part of a heart failure readmission quality improvement initiative. Am J Med 2013; 126: 989–994. [DOI] [PubMed] [Google Scholar]
- 15. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes‐Genis A, Mueller T, Richards M. Januzzi JL Jr; Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019; 21: 715–731. [DOI] [PubMed] [Google Scholar]
- 16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 17. Giamouzis G, Mastrogiannis D, Koutrakis K, Karayannis G, Parisis C, Rountas C, Adreanides E, Dafoulas GE, Stafylas PC, Skoularigis J, Giacomelli S, Olivari Z, Triposkiadis F. Telemonitoring in chronic heart failure: a systematic review. Cardiol Res Pract 2012; 2012: 410820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Heart 2007; 93: 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawwa N, Vest AR, Kumar R, Lahoud R, Young JB, Wu Y, Gorodeski EZ, Cho L. Comparison between the Kansas City Cardiomyopathy Questionnaire and New York Heart Association in assessing functional capacity and clinical outcomes. J Card Fail 2017; 23: 280–285. [DOI] [PubMed] [Google Scholar]
- 20. Yap J, Lim FY, Gao F, Teo LL, Lam CS, Yeo KK. Correlation of the New York Heart Association Classification and the 6‐minute walk distance: a systematic review. Clin Cardiol 2015; 38: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keteyian SJ, Patel M, Kraus WE, Brawner CA, McConnell TR, Pina IL, Leifer ES, Fleg JL, Blackburn G, Fonarow GC, Chase PJ, Piner L, Vest M, O'Connor CM, Ehrman JK, Walsh MN, Ewald G, Bensimhon D, Russell SD, Investigators H‐A. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol 2016; 67: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Comin‐Colet J, Anguita M, Formiga F, Almenar L, Crespo‐Leiro MG, Manzano L, Muniz J, Chaves J, de Frutos T, Enjuanes C. researchers V‐Ims. Health‐related quality of life of patients with chronic systolic heart failure in Spain: results of the VIDA‐IC study. Rev Esp Cardiol 2016; 69: 256–271. [DOI] [PubMed] [Google Scholar]
- 23. Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure Questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J 1992; 124: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 24. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 25. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015; 8: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Filippatos G, Maggioni AP, Lam CSP, Pieske‐Kraigher E, Butler J, Spertus J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Bamber L, Gheorghiade M, Pieske B. Patient‐reported outcomes in the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (SOCRATES‐PRESERVED) study. Eur J Heart Fail 2017; 19: 782–791. [DOI] [PubMed] [Google Scholar]
- 27. Shepperd S, Parkes J, McClaren J, Phillips C. Discharge planning from hospital to home. Cochrane Database Syst Rev 2013: CD000313. [DOI] [PubMed]
- 28. Lambrinou E, Kalogirou F, Lamnisos D, Sourtzi P. Effectiveness of heart failure management programmes with nurse‐led discharge planning in reducing re‐admissions: a systematic review and meta‐analysis. Int J Nurs Stud 2012; 49: 610–624. [DOI] [PubMed] [Google Scholar]
- 29. Oyanguren J, Latorre Garcia PM, Torcal Laguna J, Lekuona Goya I, Rubio Martin S, Maull Lafuente E, Grandes G. Effectiveness and factors determining the success of management programs for patients with heart failure: a systematic review and meta‐analysis. Rev Esp Cardiol 2016; 69: 900–914. [DOI] [PubMed] [Google Scholar]
- 30. Jaarsma T, Stromberg A, De Geest S, Fridlund B, Heikkila J, Martensson J, Moons P. Scholte op Reimer W, Smith K, Stewart S, Thompson DR. Heart failure management programmes in Europe. Eur J Cardiovasc Nurs 2006; 5: 197–205. [DOI] [PubMed] [Google Scholar]
- 31. Riley JP, Astin F, Crespo‐Leiro MG, Deaton CM, Kienhorst J, Lambrinou E, McDonagh TA, Rushton CA, Stromberg A, Filippatos G, Anker SD. Heart Failure Association of the European Society of Cardiology heart failure nurse curriculum. Eur J Heart Fail 2016; 18: 736–743. [DOI] [PubMed] [Google Scholar]
- 32. Schou M, Gustafsson F, Videbaek L, Tuxen C, Keller N, Handberg J, Sejr Knudsen A, Espersen G, Markenvard J, Egstrup K, Ulriksen H, Hildebrandt PR. NorthStar Investigators amoTDHFCN. Extended heart failure clinic follow‐up in low‐risk patients: a randomized clinical trial (NorthStar). Eur Heart J 2013; 34: 432–442. [DOI] [PubMed] [Google Scholar]
- 33. Luttik ML, Jaarsma T, van Geel PP, Brons M, Hillege HL, Hoes AW, de Jong R, Linssen G, Lok DJ, Berge M, van Veldhuisen DJ. Long‐term follow‐up in optimally treated and stable heart failure patients: primary care vs. heart failure clinic. Results of the COACH‐2 study. Eur J Heart Fail 2014; 16: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 34. Cardiac Care Network. Strategy for community management of heart failure in Ontario . http://www.ccn.on.ca/ccn_public/uploadfiles/files/Strategy_for_Community_Mgmt_in_HF_in_ON.pdf (February 2014).
- 35. Smeets M, Van Roy S, Aertgeerts B, Vermandere M, Vaes B. Improving care for heart failure patients in primary care, GPs' perceptions: a qualitative evidence synthesis. BMJ Open 2016; 6: e013459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Driscoll A, Meagher S, Kennedy R, Hay M, Banerji J, Campbell D, Cox N, Gascard D, Hare D, Page K, Nadurata V, Sanders R, Patsamanis H. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord 2016; 16: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Project ECHO, authors. https://echo.unm.edu/
- 38. McDonagh TA, Gardner RS, Lainscak M, Nielsen OW, Parissis J, Filippatos G, Anker SD. Heart failure association of the European society of cardiology specialist heart failure curriculum. Eur J Heart Fail 2014; 16: 151–162. [DOI] [PubMed] [Google Scholar]
- 39. The American Board of Internal Medicine. Advanced heart failure and trans‐plant cardiology policies . http://www.abim.org/pdf/publications/certification-in-advanced-heart-failure-transplant-cardiology.pdf Assessed 30/12/2019.
- 40. Cardiology SAC Curriculum for Cardiovascular Medicine. BCS Website , 2010. http://www.bcs.com/documents/2010_Cardiovascular_Medicine_Curriculum.pdf (01 November 2013).
- 41. https://www.escardio.org/Education/Postgraduate-Programmes/Courses-Heart-Failure 16 July 2019.
- 42. Farmakis D, Parissis J, Lekakis J, Filippatos G. Acute heart failure: epidemiology, risk factors, and prevention. Rev Esp Cardiol 2015; 68: 245–248. [DOI] [PubMed] [Google Scholar]
- 43. Fernandez‐Gasso L, Hernando‐Arizaleta L, Palomar‐Rodriguez JA, Abellan‐Perez MV, Pascual‐Figal DA. Trends, causes and timing of 30‐day readmissions after hospitalization for heart failure: 11‐year population‐based analysis with linked data. Int J Cardiol 2017; 248: 246–251. [DOI] [PubMed] [Google Scholar]
- 44. Horwitz LI, Jenq GY, Brewster UC, Chen C, Kanade S, Van Ness PH, Araujo KL, Ziaeian B, Moriarty JP, Fogerty RL, Krumholz HM. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med 2013; 8: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Al‐Damluji MS, Dzara K, Hodshon B, Punnanithinont N, Krumholz HM, Chaudhry SI, Horwitz LI. Hospital variation in quality of discharge summaries for patients hospitalized with heart failure exacerbation. Circ Cardiovasc Qual Outcomes 2015; 8: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Farmakis D, Filippatos G, Parissis J, Karavidas A, Sideris A, Trikas A, Triposkiadis F, Karvounis C, Adamopoulos S. Structured discharge instructions for hospitalized heart failure patients to improve guideline implementation and patient outcomes. Int J Cardiol 2016; 220: 143–145. [DOI] [PubMed] [Google Scholar]
- 47. Walz SE, Smith M, Cox E, Sattin J, Kind AJ. Pending laboratory tests and the hospital discharge summary in patients discharged to sub‐acute care. J Gen Intern Med 2011; 26: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parissis J, Farmakis D, Triposkiadis F. Heart failure registries: how far can we go? Eur J Heart Fail 2016; 18: 626–628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Example of a heart failure patient information leaflet.


