Abstract
Aims
The aim of this study is to investigate the prognostic impact of ischaemic heart disease (IHD) in heart failure (HF) and its association to age, sex, left ventricular ejection fraction (EF), and HF duration, and furthermore, to evaluate if the impact of IHD has changed over time, in light of improved therapy.
Methods and results
We studied 30 946 patients with non‐valvular HF, by accessing the Swedish Heart Failure Registry, from years 2000 to 2012. The mortality in 17 778 patients with clinical IHD was compared with 13 168 patients without IHD (non‐IHD). There was a significantly worse outcome in IHD, with the crude mortality of 41.1% and the event rate per 100 person‐years [95% confidence interval (CI)] of 14.8 (14.4–15.1), compared with 28.2% and 9.7 (9.4–10.0) in non‐IHD. After multivariable adjustment, the hazard ratio (HR) (95% CI) for mortality, IHD vs. non‐IHD, was 1.16 (1.11–1.22; P < 0.0001). Subgroup analyses showed significantly increased mortality in IHD, in all age subgroups, in all subgroups with EF < 50%, in both men and women, and regardless of heart failure duration more or less than 6 months. Analyses for the combination of age and EF showed the highest HR for time to death in the youngest with the lowest EF, HR (95% CI) 2.05 (1.59–2.64) for patients <60 years of age with EF < 30%. Although a numerical reduction of the HR for mortality was seen over time, the risk for mortality in IHD, compared with the non‐IHD group, was greater throughout the study period.
Conclusions
In non‐valvular heart failure, IHD was associated with significantly increased mortality, compared with non‐IHD, in groups of EF below 50%, in all age groups, and regardless of sex or HF duration. The risk increase associated with EF reduction diminished with increasing age. The mortality in IHD, compared with non‐IHD, remained significantly higher throughout the 13 year study period.
Keywords: Mortality/survival, Heart failure, Chronic ischaemic heart disease, Prognosis, Aetiology, Risk factors
1. Introduction
Ischaemic heart disease (IHD) is a well‐known major cause of heart failure (HF), and the risk of in hospital death is increased when ischaemia is the precipitating factor of acute HF.1 Studies of chronic HF have associated IHD with increased mortality in patients with HF with reduced ejection fraction (EF < 40%, HFrEF),2, 3 and in a contemporary HF cohort, implantable cardioverter defibrillators reduced all‐cause mortality in patients with ischaemic aetiology, as opposed to non‐ischaemic HFrEF.4 In HF with preserved EF (EF ≥ 50%, HFpEF), there is conflicting evidence of the prognostic importance of IHD,2, 3, 5 and HF with mid‐range EF (EF 40–49%, HFmrEF) is less explored in this regard, even though often described as an intermediate group with a higher prevalence of cardiovascular disease but otherwise with co‐morbidities and all‐cause mortality resembling that of HFpEF.6, 7
In the past decades, we have witnessed a gradual decline in the incidence of, and death by, myocardial infarction8, 9, 10 likely due to more effective pharmacotherapies such as dual antiplatelet therapies,11 anticoagulants,12, 13 lipid‐lowering treatment,14 possibly more effective invasive techniques,15 and stent development,16 as well as widespread use of early revascularization strategies in acute myocardial infarction.
It is unclear whether the improved treatment in IHD has improved outcome also in patients with IHD and HF.
In the present study, we aimed to perform a comprehensive assessment over time of the outcome in non‐valvular HF, with and without clinical ischaemic heart disease, and the possible interaction between ischaemic heart disease, age, sex, left ventricular EF, and HF duration.
2. Methods
2.1. The Swedish Heart Failure Registry
The Swedish Heart Failure Registry (SwedeHF) has been described elsewhere.17 In brief, the inclusion criterion for entry in the registry was an established diagnosis of HF based on clinical assessment. The protocol, registration form, and annual report are available at ‘http://www.swedehf.se'.
Approximately 80 variables were recorded at discharge from the hospital or outpatient visit at cardiology, internal medicine, and primary care clinics and entered into a web‐based database managed by the Uppsala Clinical Research Center (http://www.ucr.uu.se/en). Additional data regarding co‐morbidities and mortality were obtained from the Swedish National Patient Register and the Cause‐Specific Death Register. Registration of discharge diagnoses coded in accordance with the International Classification of Diseases system and cause of death is mandatory for all patients in Sweden since 1987 and 1961, respectively (http://www.socialstyrelsen.se). Establishment of the registry and analysis of the data were approved by a multisite ethics committee. The registry and this study are in accordance with the 1964 Declaration of Helsinki and its later amendments.
2.2. Inclusion and exclusion criteria
All patients registered in the SwedeHF, from 11 May 2000 to 31 December 2012, were eligible for inclusion (n = 51 060). Index date was defined as the date of the outpatient visit or the date for discharge during index hospitalization, resulting in 1180 (2.3%) individuals being excluded because of death during hospitalization. IHD was defined as either reported IHD, presence of angina or previous myocardial infarction, or performed percutaneous coronary intervention or coronary artery bypass graft surgery. Patients with missing data for prevalent IHD [n = 672 (1.3%)], or missing data for EF [n = 7667 (15.0%)], were excluded. EF is registered in the database in categories: <30, 30–39, 40–49, and ≥50%. In SwedeHF, valvular heart disease was registered if it was assessed to be clinically significant; however, because we did not have data on the grade or the mechanism, patients with valvular diseases and history of valve intervention were excluded [n = 10 595 (20.8%)]. Thus, the final sample consisted of 30 946 (60.6%) individuals available for analysis: 17 778 were categorized as HF with IHD and 13 168 as HF without IHD (non‐IHD).
2.3. Baseline characteristics
Baseline data included clinical characteristics, medical history, co‐morbidities, heart rate, blood pressure, laboratory findings, and medical and device treatment. Additional baseline data on co‐morbidities were obtained from the Swedish National Patient Register (http://www.socialstyrelsen.se) based on the International Statistical Classification of Diseases and Related Health Problems, 10th Revision.
2.4. Outcome measure
The outcome was all‐cause mortality occurring after the index date until the end of follow‐up (31 December 2012). This measure was studied for all IHD vs. non‐IHD patients, as well as for subgroups stratified for sex, age group (<60, 60 to <70, 70 to <80, and ≥80 years), EF group (<30, 30 to <40, 40 to <50, and ≥50%), duration of HF (<6 and ≥6 months), and calendar period (2000–2004, 2005–2006, 2007–2008, 2009–2010, and 2011–2012, with the first 4 years merged because of low numbers).
2.5. Statistical analysis
For baseline characteristics, continuous variables are presented as mean ± standard deviation or median and inter‐quartile range (IQR), where appropriate, while categorical variables are presented as frequencies and percentages. Comparing the differences in characteristics among groups, we used Fisher's exact test (lowest one‐sided P‐value multiplied by 2) for dichotomous variables, the Mantel–Haenszel χ 2 test for ordered categorical variables, χ 2 test for non‐ordered categorical variables, and the Mann–Whitney U test for continuous variables. Crude event rates for all‐cause mortality were calculated as the number of events per 100 person‐years, using Poisson‐based 95% confidence intervals (CIs). Studying time trends over calendar periods, the estimated mortality rates were standardized for the distribution of age and sex, for the first calendar period, 2000–2004. For these analyses, we chose to adjust only for time‐updated age, sex, EF group, and HF duration, for minimizing the impact of missing data. Descriptively, unadjusted cumulative incidence of all‐cause mortality for IHD and non‐IHD was estimated using the Kaplan–Meier technique. Time to all‐cause mortality was assessed using a Cox proportional hazards model. The effects of subgroups were evaluated by studying the interaction between the IHD group and the subgrouping variable of interest. From these models, HRs with 95% CIs are presented along with the associated P‐values. Adjustments for background variables were carried out in a stepwise manner: in the first model, we adjusted for age and sex; in the second, also for HF duration more or less than 6 months and EF group; and in the last, for 22 additional co‐morbidities or variables of presumed importance.
For interaction analysis, we adjusted for age (time‐updated age in the time‐trend analyses), sex (unless a subgroup variable), EF group (unless a subgroup variable), HF duration (unless subgroup variables), index period, follow‐up specialty, smoking, hypertension, atrial fibrillation/flutter, diabetes, lung disease, peripheral artery disease, stroke/transient ischaemic attack, malignant cancer within the past 3 years, estimated glomerular filtration rate, haemoglobin, systolic blood pressure, New York Heart Association functional class, angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs), beta‐blockers, mineralocorticoid receptor antagonists, diuretics, digoxin, statins, oral anticoagulants, and device therapy. For categorical variables, missing values were treated as a single, unknown category. For continuous variables, missing values [2399 (7.8%)] were not imputed and therefore excluded from the fully adjusted model.
The proportional hazards assumption was assessed for violation by introducing an interaction term between the IHD group and log (time). This procedure showed that the proportional hazards assumption was satisfied.
For all tests, statistical significance was set to P ˂ 0.05 (two tailed). Analyses were performed, and artworks were created using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Baseline characteristics
The IHD group differed significantly from the non‐IHD group, in that they were older, more often male, and more frequently had HF duration of ≥6 months. We found no significant interaction with regard to EF. The prevalence of cancer within 3 years, liver disease, and sleep apnoea was similar. All other co‐morbidities differed significantly; the prevalence of alcoholism and atrial fibrillation was higher in the non IHD group, while all other co‐morbidities were more frequent in the IHD group. Patients with IHD were significantly more often treated with beta‐blockers and nitrates but less often with mineralocorticoid receptor antagonists. The groups were similar regarding the use of ACEIs, ARBs, and diuretics (Table 1).
Table 1.
Baseline data by aetiology of heart failure in all individuals
| Variable | Total (n = 30 946) | IHD (n = 17 778) | Non‐IHD (n = 13 168) | P‐value |
|---|---|---|---|---|
| Age (years) | 72.6 (12.2) | 74.6 (10.4) | 69.8 (13.7) | <0.0001 |
| Age group | ||||
| <60 years | 4367 (14.1%) | 1596 (9.0%) | 2771 (21.0%) | <0.0001 |
| 60 to <70 years | 6633 (21.4%) | 3567 (20.1%) | 3066 (23.3%) | |
| 70 to <80 years | 9698 (31.3%) | 6062 (34.1%) | 3636 (27.6%) | |
| ≥80 years | 10 248 (33.1%) | 6553 (36.9%) | 3695 (28.1%) | |
| Sex | ||||
| Male | 20 081 (64.9%) | 12 023 (67.6%) | 8058 (61.2%) | <0.0001 |
| Female | 10 865 (35.1%) | 5755 (32.4%) | 5110 (38.8%) | |
| Index period | ||||
| 2000–2004 | 1928 (6.2%) | 1248 (7.0%) | 680 (5.2%) | <0.0001 |
| 2005–2006 | 4206 (13.6%) | 2570 (14.5%) | 1636 (12.4%) | |
| 2007–2008 | 8148 (26.3%) | 4895 (27.5%) | 3253 (24.7%) | |
| 2009–2010 | 8456 (27.3%) | 4722 (26.6%) | 3734 (28.4%) | |
| 2011–2012 | 8208 (26.5%) | 4343 (24.4%) | 3865 (29.4%) | |
| Planned follow‐up level | ||||
| Primary care/other | 10 733 (37.0%) | 6486 (39.1%) | 4247 (34.3%) | <0.0001 |
| Specialty care | 18 259 (63.0%) | 10 106 (60.9%) | 8153 (65.8%) | |
| Duration of HF | ||||
| <6 months | 16 313 (53.1%) | 8154 (46.2%) | 8159 (62.4%) | <0.0001 |
| ≥6 months | 14 416 (46.9%) | 9505 (53.8%) | 4911 (37.6%) | |
| Smoking | ||||
| Never | 10 047 (41.1%) | 5375 (38.4%) | 4672 (44.7%) | <0.0001 |
| Previous | 10 851 (44.4%) | 6752 (48.2%) | 4099 (39.2%) | |
| Current | 3549 (14.5%) | 1869 (13.4%) | 1680 (16.1%) | |
| Medical history per SwedeHF or patient register | ||||
| Hypertension | 18 762 (60.6%) | 11 466 (64.5%) | 7296 (55.4%) | <0.0001 |
| Atrial fibrillation/flutter | 16 020 (51.8%) | 8446 (47.5%) | 7574 (57.5%) | <0.0001 |
| Diabetes | 8667 (28.0%) | 6052 (34.0%) | 2615 (19.9%) | <0.0001 |
| Lung disease | 8841 (28.6%) | 5380 (30.3%) | 3461 (26.3%) | <0.0001 |
| Medical history according to SwedeHF | ||||
| Ischaemic heart disease | 14 849 (49.5%) | 14 849 (85.0%) | 0 (0.0%) | <0.0001 |
| Dilated cardiomyopathy | 3909 (13.0%) | 1282 (7.4%) | 2627 (20.4%) | <0.0001 |
| Co‐morbidities according to patient register | ||||
| Myocardial infarction | 12 753 (41.2%) | 12 753 (71.7%) | 0 (0.0%) | <0.0001 |
| PCI/CABG | 8660 (28.0%) | 8660 (48.7%) | 0 (0.0%) | <0.0001 |
| Angina | 9701 (31.3%) | 9701 (54.6%) | 0 (0.0%) | <0.0001 |
| Peripheral artery disease | 2851 (9.2%) | 2253 (12.7%) | 598 (4.5%) | <0.0001 |
| Stroke/TIA | 4997 (16.1%) | 3372 (19.0%) | 1625 (12.3%) | <0.0001 |
| Anaemia | 4067 (13.1%) | 2720 (15.3%) | 1347 (10.2%) | <0.0001 |
| Renal failure | 3095 (10.0%) | 2188 (12.3%) | 907 (6.9%) | <0.0001 |
| Malignant cancer within the past 3 years | 4059 (13.1%) | 2381 (13.4%) | 1678 (12.7%) | 0.097 |
| Dialysis | 188 (0.6%) | 136 (0.8%) | 52 (0.4%) | <0.0001 |
| Liver disease | 494 (1.6%) | 263 (1.5%) | 231 (1.8%) | 0.063 |
| Sleep apnoea | 1125 (3.6%) | 650 (3.7%) | 475 (3.6%) | 0.85 |
| Alcoholism | 1280 (4.1%) | 595 (3.3%) | 685 (5.2%) | <0.0001 |
| Gout | 1317 (4.3%) | 896 (5.0%) | 421 (3.2%) | <0.0001 |
| Laboratory and physical examination | ||||
| Latest ECG, sinus rhythm | 17 259 (56.5%) | 10 789 (61.7%) | 6470 (49.6%) | <0.0001 |
| Latest ECG, LBBB | 4708 (18.3%) | 2757 (18.9%) | 1951 (17.5%) | 0.0025 |
| EF group | ||||
| <30% | 9075 (29.3%) | 4867 (27.4%) | 4208 (32.0%) | 0.096 |
| 30–39% | 8720 (28.2%) | 5514 (31.0%) | 3206 (24.3%) | |
| 40–49% | 6728 (21.7%) | 4056 (22.8%) | 2672 (20.3%) | |
| ≥50% | 6423 (20.8%) | 3341 (18.8%) | 3082 (23.4%) | |
| NT‐proBNP | 4558 (6586) n = 9877 | 5018 (7184) n = 5134 | 4060 (5831) n = 4743 | <0.0001 |
| Haemoglobin (g/L) | 133.7 (17.2) n = 30 945 | 131.5 (16.7) n = 17 778 | 136.6 (17.4) n = 13 167 | <0.0001 |
| Estimated glomerular filtration rate | 68.4 (33.6) n = 28 724 | 63.2 (31.0) n = 16 579 | 75.6 (35.7) n = 12 145 | <0.0001 |
| Systolic blood pressure (mmHg) | 128.4 (21.3) n = 30 550 | 128.1 (21.1) n = 17 530 | 128.8 (21.6) n = 13 020 | 0.015 |
| Diastolic blood pressure (mmHg) | 74.1 (12.3) n = 30 515 | 72.7 (11.8) n = 17 516 | 75.9 (12.6) n = 12 999 | <0.0001 |
| BMI (kg/cm2) | 27.2 (5.5) n = 14 214 | 27.0 (5.2) n = 8218 | 27.4 (6.0) n = 5996 | 0.010 |
| NYHA | ||||
| I | 2910 (12.7%) | 1458 (11.4%) | 1452 (14.5%) | <0.0001 |
| II | 11 101 (48.6%) | 5990 (46.7%) | 5111 (51.1%) | |
| III | 8079 (35.4%) | 4881 (38.0%) | 3198 (32.0%) | |
| IV | 748 (3.3%) | 508 (4.0%) | 240 (2.4%) | |
| Medical treatment at discharge or revisit | ||||
| ACEIs/ARBs | 26 912 (87.2%) | 15 409 (86.9%) | 11 503 (87.6%) | 0.076 |
| Beta‐blockers | 26 983 (87.6%) | 15 740 (88.9%) | 11 243 (85.8%) | <0.0001 |
| Diuretics | 23 909 (77.6%) | 13 768 (77.8%) | 10 141 (77.4%) | 0.47 |
| MRAs | 8639 (28.1%) | 4875 (27.6%) | 3764 (28.8%) | 0.027 |
| Digoxin | 5091 (16.5%) | 2289 (12.9%) | 2802 (21.4%) | <0.0001 |
| Statins | 14 681 (47.7%) | 11 583 (65.4%) | 3098 (23.7%) | <0.0001 |
| Nitrates | 5106 (16.6%) | 4691 (26.6%) | 415 (3.2%) | <0.0001 |
| Oral anticoagulants | 11 323 (36.8%) | 5511 (31.2%) | 5812 (44.4%) | <0.0001 |
| Device therapy | ||||
| ICD without CRT | 644 (2.1%) | 481 (2.7%) | 163 (1.2%) | <0.0001 |
| CRT without ICD | 343 (1.1%) | 220 (1.3%) | 123 (0.9%) | |
| CRT with ICD | 339 (1.1%) | 245 (1.4%) | 94 (0.7%) | |
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; BMI, body mass index; CABG, coronary artery bypass graft surgery; CRT, cardiac resynchronization therapy; ECG, electrocardiogram; EF, ejection fraction; HF, heart failure; ICD, implantable cardioverter defibrillator; IHD, ischaemic heart disease; LBBB, left bundle branch block; MRAs, mineralocorticoid receptor antagonists; non‐IHD, non‐ischaemic heart disease; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SwedeHF, Swedish Heart Failure Registry; TIA, transient ischaemic attack.
For categorical variables, n (%) is presented. For continuous variables, mean (standard deviation)/median (min; max)/n is presented. For comparison between groups, Fisher's exact test (lowest one‐sided P‐value multiplied by 2) was used for dichotomous variables and the Mantel–Haenszel χ 2 test for ordered categorical variables and χ 2 for non‐ordered categorical variables and the Mann–Whitney U test for continuous variables.
3.2. All‐cause mortality in ischaemic heart disease vs. non‐ischaemic heart disease
Median follow‐up time was 2.4 years (IQR: 1.0–4.2) in IHD and 2.6 years (IQR: 1.1–4.3) in non‐IHD. The crude mortality was higher in the IHD group (n = 7315, 41.1%) than in the non‐IHD group (n = 3711, 28.2%), with an event rate per 100 person‐years (95% CI) of 14.8 (14.4–15.1) in IHD vs. 9.7 (9.4–10.0) in non‐IHD (Table 2). The probability of survival in patients with IHD was lower than those without IHD (Figure 1).
Table 2.
Deaths, mortality event rate, and follow‐up time for IHD vs. non‐IHD
| Deaths (%) | Mortality event rate per 100 person‐years | Median follow‐up time, years (IQR) | ||||
|---|---|---|---|---|---|---|
| IHD | Non‐IHD | IHD | Non‐IHD | IHD | Non‐IHD | |
| All individuals | 41.1 | 28.2 | 14.8 (14.4–15.1) | 9.7 (9.4–10.0) | 2.4 (1.0–4.2) | 2.6 (1.1–4.3) |
| Sex | ||||||
| Male | 40.3 | 26.8 | 14.3 (13.9–14.8) | 9.0 (8.6–9.3) | 2.4 (1.0–4.2) | 2.7 (1.1–4.4) |
| Female | 43.0 | 30.4 | 15.7 (15.1–16.3) | 10.9 (10.3–11.4) | 2.3 (0.9–4.1) | 2.4 (1.0–4.2) |
| Age | ||||||
| <60 years | 16.4 | 10.0 | 4.5 (4.0–5.1) | 2.8 (2.5–3.2) | 3.4 (1.7–5.2) | 3.3 (1.5–5.1) |
| 60 to <70 years | 26.3 | 17.6 | 8.2 (7.7–8.8) | 5.5 (5.1–6.0) | 2.9 (1.3–4.8) | 2.9 (1.3–4.7) |
| 70 to <80 years | 38.5 | 28.9 | 13.0 (12.5–13.6) | 9.9 (9.3–10.5) | 2.7 (1.1–4.4) | 2.6 (1.1–4.2) |
| ≥80 years | 57.7 | 49.9 | 26.3 (25.4–27.1) | 22.4 (21.4–23.5) | 1.7 (0.7–3.3) | 1.7 (0.7–3.4) |
| EF | ||||||
| <30% | 46.7 | 23.5 | 17.9 (17.2–18.7) | 7.7 (7.2–8.2) | 2.1 (0.8–3.9) | 2.7 (1.2–4.6) |
| 30–39% | 38.6 | 24.5 | 13.5 (12.9–14.0) | 8.2 (7.6–8.8) | 2.4 (1.0–4.3) | 2.6 (1.1–4.4) |
| 40–49% | 36.9 | 28.8 | 12.4 (11.8–13.0) | 10.2 (9.5–10.9) | 2.7 (1.1–4.5) | 2.5 (1.1–4.1) |
| ≥50% | 42.4 | 37.8 | 15.9 (15.1–16.7) | 14.0 (13.2–14.8) | 2.2 (0.9–4.0) | 2.3 (0.9–4.0) |
| HF duration | ||||||
| <6 months | 31.9 | 22.7 | 11.2 (10.7–11.6) | 7.8 (7.5–8.2) | 2.5 (1.1–4.3) | 2.6 (1.1–4.3) |
| ≥6 months | 49.3 | 37.6 | 18.0 (17.5–18.5) | 12.7 (12.1–13.2) | 2.3 (0.9–4.2) | 2.6 (1.1–4.5) |
EF, ejection fraction; HF, heart failure; IHD, ischaemic heart disease; IQR, inter‐quartile range; non‐IHD, non‐ischaemic heart disease.
Figure 1.
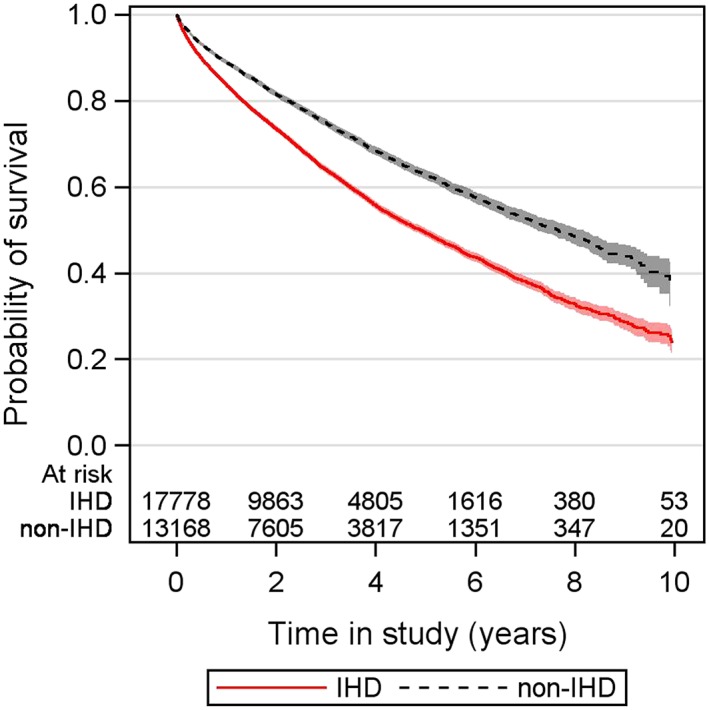
Probability of survival in all individuals by aetiology. IHD, ischaemic heart disease; non‐IHD, non‐ischaemic heart disease.
3.3. Interaction between baseline characteristics and mortality in ischaemic heart disease vs. non‐ischaemic heart disease
A Cox proportional hazards regression model was used to evaluate the effect of IHD vs. non‐IHD on all individuals and by sex, age groups, EF groups, and HF duration more or less than 6 months (Table 3). After adjustment for age and sex (unless subgroup variable), the HR for mortality, IHD vs. non‐IHD, was significantly increased in ‘all individuals', all age groups, all groups of EF < 50%, and both groups of HF duration. Further adjustments of additional variables did not change these results. After multivariable adjustment, the estimated overall effect of IHD on mortality compared with non‐IHD was HR (95% CI) 1.16 (1.11–1.22; P < 0.0001).
Table 3.
Adjusted Cox proportional hazards models for time to death: IHD vs. non‐IHD for selected subgroups
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| All individuals | 1.23 (1.18–1.28) | <0.0001* | 1.18 (1.13–1.23) | <0.0001* | 1.16 (1.11–1.22) | <0.0001* |
| Sex | ||||||
| Male | 1.25 (1.18–1.31) | 0.40** | 1.19 (1.13–1.26) | 0.45** | 1.16 (1.10–1.23) | 0.83** |
| Female | 1.20 (1.13–1.28) | 1.16 (1.09–1.23) | 1.15 (1.08–1.24) | |||
| Age (group) | ||||||
| <60 years | 1.58 (1.34–1.88) | <0.0001** | 1.62 (1.36–1.92) | <0.0001** | 1.56 (1.30–1.87) | <0.0001** |
| 60 to <70 years | 1.48 (1.33–1.65) | 1.43 (1.28–1.59) | 1.42 (1.27–1.59) | |||
| 70 to <80 years | 1.29 (1.20–1.39) | 1.24 (1.15–1.33) | 1.18 (1.09–1.28) | |||
| ≥80 years | 1.16 (1.10–1.23) | 1.11 (1.05–1.17) | 1.10 (1.04–1.17) | |||
| EF (group) | ||||||
| <30% | 1.55 (1.44–1.67) | <0.0001** | 1.43 (1.32–1.54) | <0.0001** | 1.39 (1.28–1.51) | <0.0001** |
| 30–39% | 1.30 (1.20–1.41) | 1.24 (1.14–1.35) | 1.20 (1.10–1.31) | |||
| 40–49% | 1.09 (1.00–1.19) | 1.06 (0.98–1.16) | 1.12 (1.02–1.23) | |||
| ≥50% | 1.03 (0.96–1.12) | 1.00 (0.92–1.08) | 0.96 (0.88–1.04) | |||
| HF duration | ||||||
| <6 months | 1.16 (1.09–1.23) | 0.71** | 1.19 (1.12–1.26) | 0.75** | 1.18 (1.11–1.26) | 0.37** |
| ≥6 months | 1.18 (1.11–1.24) | 1.17 (1.11–1.24) | 1.14 (1.07–1.21) | |||
CI, confidence interval; EF, ejection fraction; HF, heart failure; HR, hazard ratio; IHD, ischaemic heart disease; IQR, inter‐quartile range; non‐IHD, non‐ischaemic heart disease.
Model 1: adjusted for age and sex (unless subgroup variable). Model 2: additionally adjusted for EF (group) and HF duration (unless subgroup variables). Model 3: additionally adjusted for index period, smoking, hypertension, atrial fibrillation, diabetes, lung disease, creatinine clearance, haemoglobin, systolic blood pressure, New York Heart Association class, angiotensin‐converting enzyme inhibitors/angiotensin‐converting enzyme inhibitors, beta‐blockers, mineralocorticoid receptor antagonists, diuretics, digoxin, statins, oral anticoagulants, peripheral artery disease, stroke/transient ischaemic attack, cancer, follow‐up specialty, and device therapy. For categorical variables, missing values were treated as a single, unknown category. For continuous variables, missing values were not imputed and therefore excluded from Model 3. Missing data in the analysis of HF duration, Model 3: 2595 (8.4%). Missing data in the analysis of other variables, Model 3: 2399 (7.8%).
P‐value.
P‐value for interaction.
The categories of sex and HF duration showed no significant interactions with the IHD group.
Subgroup analyses for age showed significantly higher mortality in IHD than in non‐IHD, in all age groups. The difference remained significant after multivariable adjustment, with a pattern of decreasing difference with increasing age, HR (95% CI) ranging between 1.56 (1.30–1.87) for age <60 years and 1.10 (1.04–1.17) for age ≥80 years, P < 0.0001, for interaction.
Subgroup analyses for EF showed higher crude mortality rates in IHD for all EF groups in the stratification, with a more than two‐fold mortality rate per 100 person‐years (95% CI) in EF < 30%, 17.9 (17.2–18.7) in IHD vs. 7.7 (7.2–8.2) in non‐IHD (Table 2). After multivariable adjustment, the mortality in IHD compared with non‐IHD remained significantly increased in groups with systolic dysfunction, with a decreasing difference with increasing EF: HR (95% CI) 1.39 (1.28–1.51) for EF < 30% and 1.12 (1.02–1.23) for EF 40–49%. For EF ≥ 50%, the adjusted mortality outcome was similar in the two groups (Table 3).
Analyses of IHD vs. non‐IHD for the combination of EF and age showed the highest HR for time to death in the youngest age group with EF < 30%, HR (95% CI) 2.05 (1.59–2.64). For patients with EF ≥ 50%, the effect of IHD vs. non‐IHD for time to death did not significantly differ for any of the age groups (Figure 2 ).
Figure 2.
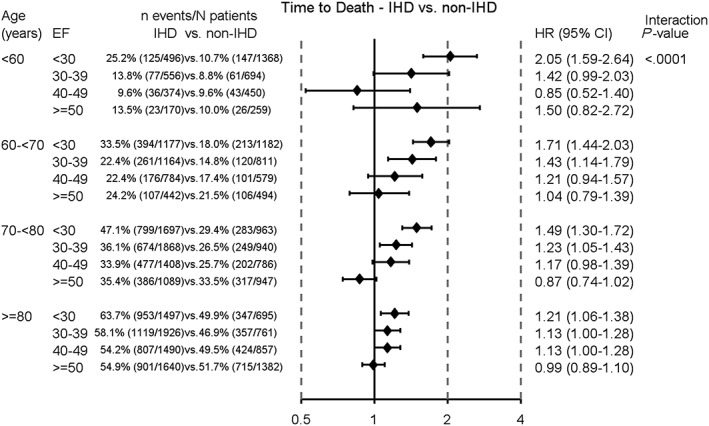
Cox proportional hazards for analysis of time to death. Ischaemic heart disease (IHD) vs. non‐ischaemic heart disease (non‐IHD) for subgroups of age and ejection fraction (EF) groups. Adjusted for index period, smoking, hypertension, atrial fibrillation, diabetes, lung disease, estimated glomerular filtration rate, haemoglobin, systolic blood pressure, New York Heart Association functional class, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, beta‐blockers, mineralocorticoid receptor antagonists, diuretics, digoxin, statins, oral anticoagulants, peripheral artery disease, stroke/transient ischaemic attack, malignant cancer within the past 3 years, follow‐up specialty, and device therapy. CI, confidence interval; HR, hazard ratio.
3.4. Trend in mortality in ischaemic heart disease vs. non‐ischaemic heart disease during the study period
Ischaemic heart disease was associated with a significantly higher all‐cause mortality compared with the non‐IHD group, during the entire study period. The HR for mortality, IHD vs. non‐IHD, after adjusting for time‐updated age, sex, EF group, and HF duration, did not significantly decrease over time: HR (95% CI) 1.40 (1.11–1.78) for the period 2000–2004 and 1.15 (CI: 1.07–1.23) for the period 2011–2012, P = 0.28, for interaction between IHD group and calendar periods (Figure 3).
Figure 3.
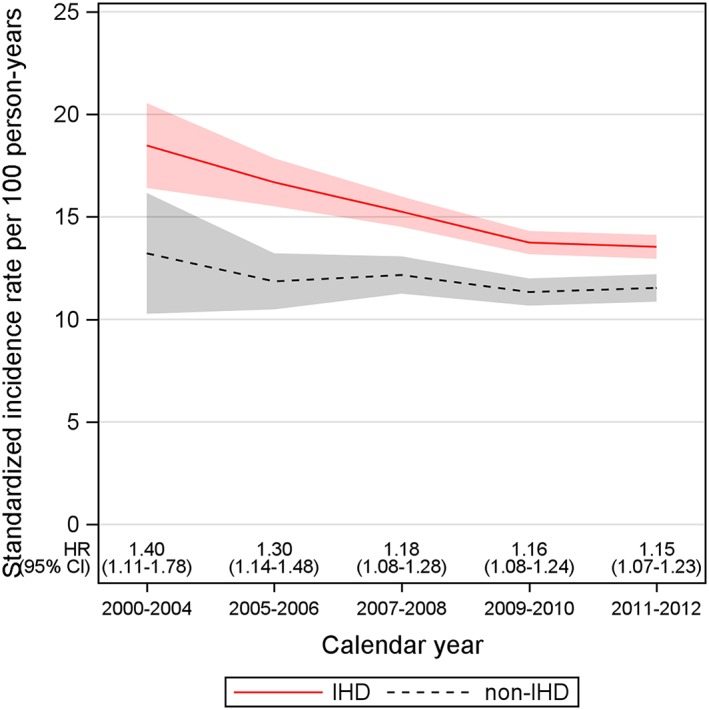
Standardized incidence rates for mortality over calendar periods and hazard ratio (HR) [95% confidence interval (CI)] for mortality [ischaemic heart disease (IHD) vs. non‐ischaemic heart disease (non‐IHD)] adjusted for time‐updated age, sex, ejection fraction group, and heart failure duration. For interaction between IHD group and calendar periods, P = 0.28.
4. Discussion
Our study, with its large sample size from the SwedeHF, demonstrated that the all‐cause mortality was higher in patients with HF and clinical IHD than in patients with HF without IHD. Moreover, this prognostic difference remained significant throughout the 13 year study period.
The cohort was treated well with ACEIs/ARBs and beta‐blockers (87 and 89% in IHD vs. 88 and 86% in non‐IHD). The use of implantable cardioverter defibrillator and cardiac resynchronization therapy was low in both groups (4.1 and 2.6% in IHD vs. 2.0 and 1.6% in non‐IHD, including combined devices).
Our results are consistent with previous studies,2, 18 showing that in HF, IHD is associated with a worse outcome than non‐IHD. After multivariable adjustment, the mortality in IHD was increased in all age categories, in both men and women, and for both short and long duration of HF. With regard to EF, however, IHD was associated with a higher all‐cause mortality only in groups of EF < 50%, and subgroup analyses showed gradually decreasing HR for mortality with increasing EF, within each age group. The pattern was not as clear in the youngest, but the low number of events limits the interpretation. Overall, the impact of IHD diminished with increasing age, likely reflecting the increasing importance of other diseases. In patients older than 80 years of age, we found no difference in mortality in those with EF ≥ 30%.
We speculate that the worse prognosis in IHD is inherent in the double burden of disease. As one of the pathogenic causes of HF, the presence of IHD may not only be causally responsible for myocardial damage and adverse cardiac remodelling but also related to or involved in other pathological mechanisms, such as progressive inflammatory and circulatory compromising conditions and unstable electrical substrates capable of initiating and sustaining arrhythmias.19, 20 If the underlying pathological mechanisms are ongoing, the increased risk of mortality will likely remain. Indeed, it is known that patients with prior myocardial infarction and multi‐vessel coronary disease are still at an elevated risk for recurrent coronary events despite revascularization.21, 22, 23 Coronary artery disease already manifested in younger patients seems to be more aggressive, which may explain the higher risk compared with non‐IHD. In our study, IHD was associated with a more than 50% increased risk for mortality in patients below the age of 60 years. In IHD, EF reduction often indicates more severe myocardial damage, and those with reduced EF appear to be more vulnerable with a higher risk of mortality. It is notable that patients with EF < 30% without IHD had the lowest crude mean mortality event rate of all groups, while patients with EF < 30% and IHD had the highest. In non‐IHD, the higher mortality rates in groups of preserved EF, or less reduced EF, are likely caused by the varying aetiologies of HF and existing co‐morbidities.
In line with another SwedeHF study by Vedin et al.,18 we demonstrated that IHD was not associated with increased mortality in patients with EF ≥ 50%. However, in contrast to our study, patients with valvular heart disease were included and comprised approximately 23% in HFrEF, 25% in HFmrEF, and 33% in HFpEF. Valvular heart disease (in SwedeHF also including atrial and ventricular septal defects) was registered if considered to be of haemodynamic significance. However, no description of factors of importance for left ventricle load and prognosis, such as the severity, location, or type of failure of the afflicted valve was made. We also believe that the mechanical effects of either significantly obstructive or regurgitant valvular disease were likely to be intrinsically progressive irrespective of either HF or IHD, making interpretation of the results difficult, and we therefore excluded all patients with noted valvular heart disease.
In studies of Swedish patients from years 1995 to 2014, the mortality was found to decrease in ST‐elevation myocardial infarctions until 2007–2008,9 and in non‐ST‐elevation myocardial infarctions until 2009–2010,10 after which the mortality levelled out and remained rather unchanged. The proposed main reasons for the reductions were in‐hospital coronary interventions and improved medication. As shown in our study, the difference in mortality between IHD patients and non‐IHD patients was lowest in the groups of preserved or slightly reduced EF. Despite increased early reperfusion therapy, likely reducing the extent of myocardial damage and increasing the proportion of patients with minor cardiac dysfunction, the reduction in HR for mortality, in IHD vs non‐IHD, over time was non‐significant.
Our study has limitations. As with other registry studies, causality cannot be inferred. The analyses depend on the data provided, including missing data and possible misclassifications. Categorization of IHD was based on the diagnoses registered in the SwedeHF and the Swedish National Patient Register; hence, underestimation of subclinical coronary heart disease is possible. Reversely, 1148 of the 17 778 IHD patients (6.5%) were categorized on the basis of angina without the requirement of objective imaging techniques, and it cannot be ruled out that the symptoms were due to HF or even non‐cardiac causes. When excluding patients with valvular disease, we may have inadvertently excluded patients with less severe valvular dysfunction. Mild‐to‐moderate mitral regurgitation is not believed to cause HF but is associated with IHD, reduced EF, and worse outcome,24, 25 and the exclusion might therefore have influenced the prognosis, possibly reducing the true mortality especially in the IHD group and in low EF subgroups.
To conclude, in this large Swedish cohort study of non‐valvular HF in years 2000 to 2012, we observed that in patients with IHD, compared with non IHD, the mortality was increased in all age subgroups, in all subgroups with EF below 50%, and regardless of sex or HF duration. The increased risk diminished with increasing age and EF. Despite significant improvements in the treatment of coronary artery disease, the mortality was significantly higher in IHD than in non‐IHD, throughout the 13 year study period, implicating that IHD remains a significant prognostic risk factor in HF.
Conflict of interest
M.F. reports unrelated modest consulting fees from Novartis, Pfizer, Boehringer Ingelheim, Vifor Pharma, and AstraZeneca. U.D. reports unrelated research funding/honoraria from AstraZeneca and Novartis. No other conflict of interest or relationship with the industry declared.
Funding
This work was supported by the Swedish Heart‐Lung Foundation (20170453 to M.F.) and the regional ALF agreement between the Region Västra Götaland and University of Gothenburg (ALFGBG‐721961 to M.F.). The Swedish Heart Failure Registry is funded by the Swedish National Board of Health and Welfare, the Swedish Association of Local Authorities and Regions, and the Swedish Society of Cardiology.
Acknowledgements
J.S., H.S., E.B., M.F., and U.D. designed the study. The statistical analyses were performed by A.P. J.S. wrote the draft. All authors interpreted the data, critically revised the manuscript, and approved the submitted version.
Silverdal, J. , Sjöland, H. , Bollano, E. , Pivodic, A. , Dahlström, U. , and Fu, M. (2020) Prognostic impact over time of ischaemic heart disease vs. non‐ischaemic heart disease in heart failure. ESC Heart Failure, 7: 264–273. 10.1002/ehf2.12568.
References
- 1. Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart failure 2016; 4: 464–472. [DOI] [PubMed] [Google Scholar]
- 2. Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation 2009; 119: 3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rusinaru D, Houpe D, Szymanski C, Levy F, Marechaux S, Tribouilloy C. Coronary artery disease and 10‐year outcome after hospital admission for heart failure with preserved and with reduced ejection fraction. Eur J Heart Fail 2014; 16: 967–976. [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 5. Badar AA, Perez‐Moreno AC, Hawkins NM, Jhund PS, Brunton AP, Anand IS, McKelvie RS, Komajda M, Zile MR, Carson PE, Gardner RS, Petrie MC, McMurray JJ. Clinical characteristics and outcomes of patients with coronary artery disease and angina: analysis of the irbesartan in patients with heart failure and preserved systolic function trial. Circ Heart Fail 2015; 8: 717–724. [DOI] [PubMed] [Google Scholar]
- 6. Lam CSP, Gamble GD, Ling LH, Sim D, Leong KTG, Yeo PSD, Ong HY, Jaufeerally F, Ng TP, Cameron VA, Poppe K, Lund M, Devlin G, Troughton R, Richards AM, Doughty RN. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi‐ethnic cohort study. Eur Heart J 2018; 39: 1770–1780. [DOI] [PubMed] [Google Scholar]
- 7. Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid‐range (borderline) ejection fraction: clinical implications and future directions. JACC Heart Failure 2017; 5: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The National Board of Health and Welfare (Socialstyrelsen) . Myocardial infarctions in Sweden 1990. –2013. 2014. [Google Scholar]
- 9. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, James S, Kellerth T, Lindahl B, Ravn‐Fischer A, Rydberg E, Yndigegn T, Jernberg T. Improved outcomes in patients with ST‐elevation myocardial infarction during the last 20 years are related to implementation of evidence‐based treatments: experiences from the SWEDEHEART registry 1995‐2014. Eur Heart J 2017; 38: 3056–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, James S, Kellerth T, Lindahl B, Ravn‐Fischer A, Rydberg E, Yndigegn T, Jernberg T. Relations between implementation of new treatments and improved outcomes in patients with non‐ST‐elevation myocardial infarction during the last 20 years: experiences from SWEDEHEART registry 1995 to 2014. Eur Heart J 2018; 39: 3766–3776. [DOI] [PubMed] [Google Scholar]
- 11. Sabatine MS, Cannon CP, Gibson CM, Lopez‐Sendon JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill KA, Skene AM, McCabe CH, Braunwald E. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation. N Engl J Med 2005; 352: 1179–1189. [DOI] [PubMed] [Google Scholar]
- 12. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). G Ital Cardiol (Rome) 2016; 17: 831–872. [DOI] [PubMed] [Google Scholar]
- 13. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130: e344–e426. [DOI] [PubMed] [Google Scholar]
- 14. Stenestrand U, Wallentin L. Early statin treatment following acute myocardial infarction and 1‐year survival. JAMA 2001; 285: 430–436. [DOI] [PubMed] [Google Scholar]
- 15. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361: 13–20. [DOI] [PubMed] [Google Scholar]
- 16. Feinberg J, Nielsen EE, Gluud C, Jakobsen JC. Cochrane Corner: drug‐eluting stents versus bare‐metal stents for acute coronary syndrome. Heart. 2018; 104: 1895–1895. [DOI] [PubMed] [Google Scholar]
- 17. Jonsson A, Edner M, Alehagen U, Dahlstrom U. Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail 2010; 12: 25–31. [DOI] [PubMed] [Google Scholar]
- 18. Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, Braun OO, Savarese G, Dahlstrom U, Lund LH. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail 2017; 10: pii:e003875. [DOI] [PubMed] [Google Scholar]
- 19. Priori SG, Blomstrom‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Rev Esp Cardiol (Engl Ed) 2016; 69: 176. [DOI] [PubMed] [Google Scholar]
- 20. Ghuran AV, Camm AJ. Ischaemic heart disease presenting as arrhythmias. Br Med Bull 2001; 59: 193–210. [DOI] [PubMed] [Google Scholar]
- 21. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Rother J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010; 304: 1350–1357. [DOI] [PubMed] [Google Scholar]
- 22. Fox KA, Carruthers KF, Dunbar DR, Graham C, Manning JR, De Haedt H, Buysschaert I, Lambrechts D, Van de Herf F. Underestimated and under‐recognized: the late consequences of acute coronary syndrome (GRACE UK–Belgian Study). Eur Heart J 2010; 31: 2755–2764. [DOI] [PubMed] [Google Scholar]
- 23. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post‐myocardial infarction patients: nationwide real world data demonstrate the importance of a long‐term perspective. Eur Heart J 2015; 36: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 24. Lamas GA, Mitchell GF, Flaker GC, Smith SC Jr, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators Circulation 1997; 96: 827–833. [DOI] [PubMed] [Google Scholar]
- 25. Bursi F, Enriquez‐Sarano M, Nkomo VT, Jacobsen SJ, Weston SA, Meverden RA, Roger VL. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005; 111: 295–301. [DOI] [PubMed] [Google Scholar]


