Abstract
Aim
To illustrate the pre‐hospital management arsenals and protocols in different EMS units, and to estimate the perceived difficulty of diagnosing suspected acute heart failure (AHF) compared with other common pre‐hospital conditions.
Methods and results
A multinational survey included 104 emergency medical service (EMS) regions from 18 countries. Diagnostic and therapeutic arsenals related to AHF management were reported for each type of EMS unit. The prevalence and contents of management protocols for common medical conditions treated pre‐hospitally was collected. The perceived difficulty of diagnosing AHF and other medical conditions by emergency medical dispatchers and EMS personnel was interrogated.
Ultrasound devices and point‐of‐care testing were available in advanced life support and helicopter EMS units in fewer than 25% of EMS regions. AHF protocols were present in 80.8% of regions. Protocols for ST‐elevation myocardial infarction, chest pain, and dyspnoea were present in 95.2, 80.8, and 76.0% of EMS regions, respectively. Protocolized diagnostic actions for AHF management included 12‐lead electrocardiogram (92.1% of regions), ultrasound examination (16.0%), and point‐of‐care testings for troponin and BNP (6.0 and 3.5%). Therapeutic actions included supplementary oxygen (93.2%), non‐invasive ventilation (80.7%), intravenous furosemide, opiates, nitroglycerine (69.0, 68.6, and 57.0%), and intubation 71.5%. Diagnosing suspected AHF was considered easy to moderate by EMS personnel and moderate to difficult by emergency medical dispatchers (without significant differences between de novo and decompensated heart failure). In both settings, diagnosis of suspected AHF was considered easier than pulmonary embolism and more difficult than ST‐elevation myocardial infarction, asthma, and stroke.
Conclusions
The prevalence of AHF protocols is rather high but the contents seem to vary. Difficulty of diagnosing suspected AHF seems to be moderate compared with other pre‐hospital conditions.
Keywords: Acute heart failure, Pre‐hospital, Emergency care, Dispatching centre, Emergency medical services
Introduction
Acute heart failure (AHF) is a common medical condition encountered in the emergency departments (ED) and pre‐hospital settings.1, 2 From 11–53% of AHF patients arrive to the ED by ambulance.3, 4, 5 While the prognosis of AHF patients' remains poor,6, 7, 8, 9 the importance of early phase and pre‐hospital management by emergency medical services (EMS) has been recently underlined.10, 11, 12, 13
Traditionally, EMS units are categorized by their resources for diagnosis and care and the level of personnel. Typically, advanced life support (ALS) units have a physician, nurse, or paramedic aboard (depending on the country) with readiness for intravenous (IV) line insertion and IV‐medication administration. Most helicopter EMS (HEMS) units also correspond to this category. In contrast, basic life support (BLS) units are usually resourced and staffed for less critical situations.
The routine AHF management includes 12‐lead electrocardiogram (ECG) recording, vital sign monitoring, option for treatment with supplementary oxygen, non‐invasive ventilation (NIV), and administration of IV diuretics and vasodilators.11, 12 However, the administration of pre‐hospital medication seems scarce.3, 4, 13, 14 Moreover, earlier studies suggest that it might be difficult for EMS personnel to differentiate AHF from other underlying causes of dyspnoea14, 15, 16, 17 especially when the diagnosis is based only on patient's medical history and clinical signs and symptoms.18, 19
Illustrative data on the possibilities of EMS to treat and diagnose AHF in the pre‐hospital setting in accordance with the guidelines are scarce. Bearing in mind these gaps in current knowledge, the present study was designed to investigate the possibilities to diagnose and treat AHF in the pre‐hospital setting. We also assessed the prevalence of specific management protocols for dyspnoeic conditions and the perceived difficulty of diagnosing suspected AHF and other critical conditions encountered in EMS.
Methods
The EMS‐AHF study was based on a multinational survey. The surveys were sent to persons in charge of an EMS region. These regional EMS leaders were contacted by key national emergency physicians who agreed to participate in the present study. Data were collected between November 2017 and February 2018 from 104 EMS regions in 18 countries. Fifteen of these countries were European (Belgium, Czech Republic, Denmark, Estonia, Finland, France, Germany, Italy, Lithuania, Monaco, Norway, Poland, Slovenia, Spain, and Switzerland). In addition, Canada, Singapore, and the United States were included.
The collected data included information about the different types of EMS units and the diagnostic and therapeutic arsenals on board; BLS, ALS, and HEMS units were assessed separately. The availability of diagnostic tools was classified into three categories: 0 = ‘not on board', 1 = ‘in some ambulances', and 2 = ‘in all ambulances'. Likewise, availability of the treatment options was classified as 0 = ‘not available', 1 = ‘permission needed', and 2 = ‘permanent standing order'. In addition, data on the prevalence of specific pre‐hospital management protocols for AHF, dyspnoea, chest pain, and ST‐elevation myocardial infarction (STEMI) were collected. The data included information about diagnostic and therapeutic actions, which were again classified as 0 = ‘not on board', 1 = ‘permission needed', and 2 = ‘permanent standing order'. For statistical analyses, we combined Categories 1 and 2 and compared with Category 0. Finally, the perceived difficulty of diagnosing suspected AHF (distinguishing between de novo AHF and acute‐decompensated heart failure [ADHF]) and other common conditions encountered in EMS (stroke, acute coronary syndrome in general, STEMI, asthma attack, pulmonary embolism, and sepsis) were estimated and graded according to a 5‐grade scale: 1 = ‘very easy', 2 = ‘easy', 3 = ‘moderate', 4 = ‘difficult', and 5 = ‘very difficult'. Separated scores grading difficulty of diagnosing suspected AHF by emergency medical dispatchers (EMD) and by EMS personnel were collected.
The categorical variables are presented as absolute values and percentages and compared by Fisher's exact test. Perceived difficulty is presented as a quantitative variable ranging from 1 (minimal difficulty) to 5 (maximal difficulty), reported as mean and standard deviation, and compared by Student's t‐test. Statistical analysis was performed using SPPS Version 25 (IBM Corp., Armonk, NY, USA).
Results
The survey covered more than 20% of the respective country's population in 11 out of 18 countries (Singapore 100.0%, Monaco 97.5%, Spain 79.0%, Estonia 70.5%, Finland 66.2%, Switzerland 39.5%, Lithuania 29.4%, Norway 29.4%, Belgium 27.4%, Denmark 22.7%, and France 21.1%). The population coverage ranged from 2.2–13.2% in Canada, Czech Republic, Germany, Italy, Poland, Slovenia, and United states.
The capability of EMS personnel to run diagnostic tests (Figure 1 ) or to provide therapeutic treatments (Figure 2 ) potentially needed for managing AHF patient largely varied according with the type of EMS unit. The diagnostic and therapeutic arsenals were more commonly available in ALS and HEMS units than in BLS units. However, the availability of point‐of‐care testing (POCT) and ultrasound, even in the ALS and HEMS units, was quite low (less than 25% EMS regions had these). With respect to therapeutic arsenal, apart from supplementary oxygen, AHF treatments were available in roughly one‐third, or less, of BLS units, whereas majority of ALS and HEMS units had these treatment options. Main AHF medications (diuretics and nitroglycerine) were available in approximately half of ALS units as permanent standing order.
Figure 1.
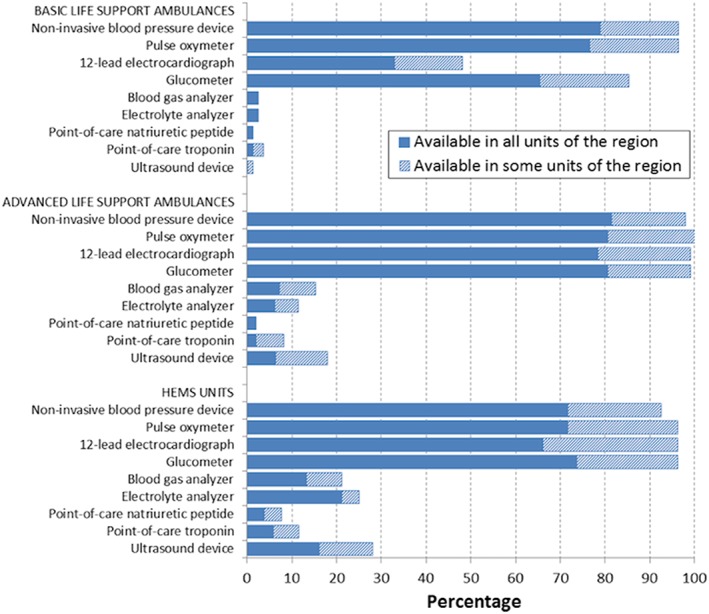
Availability of diagnostic tools in different type of emergency medical service units. HEMS, helicopter emergency medical services.
Figure 2.
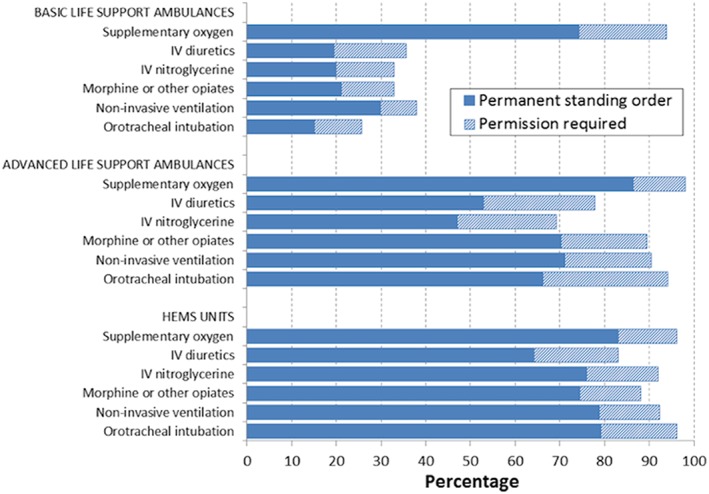
Possibility of administration of common therapeutic treatments for acute heart failure in different emergency medicine service units including those with permanent standing order and those requiring permission. HEMS, helicopter emergency medical services; IV, intravenous.
A specific protocol for pre‐hospital AHF management was present in 84 regions (80.8%). Prevalence of protocols for chest pain (84 regions, 80.8%, P = 1.00) and dyspnoea (79 regions, 76.0%, P = 0.50) was similar to AHF protocols. Whereas, the prevalence of STEMI protocols was significantly higher than AHF protocols (99 regions, 95.2%, P < 0.001). The contents of the different management protocols are shown in Table 1. With respect to diagnostic actions, 12‐lead ECG was included in almost all AHF protocols, whereas POCTs for BNP and troponin were included only in few protocols and ultrasound in 14–16% of protocols. In general, AHF management most commonly—in over 90% of AHF protocols—involved acquisition of 12‐lead ECG, insertion of an IV line, and administration of supplementary oxygen. With respect to therapeutic actions, after supplementary oxygen, NIV was the most common action (present in the AHF protocols of 80.7% EMS regions), followed by intubation (71.5%), IV diuretics (69%), IV opiates (68.6%), and IV nitroglycerine (57.0%). Compared with the non‐specific dyspnoea protocols, AHF protocols more frequently included IV diuretic and nitroglycerine administration; compared with chest pain, IV diuretics and NIV were more frequently recommended. Compared with STEMI protocols, AHF protocols less frequently included ECG and IV opiate administration, and more frequently IV diuretic and NIV use (Table 1).
Table 1.
Protocolized actions contained in the acute heart failure management protocol and in the other three additional pre‐hospital protocols surveyed
| AHF | Dyspnoea | Chest pain | STEMI | |
|---|---|---|---|---|
| N = 84 | N = 79 | N = 84 | N = 99 | |
|
Diagnostic actions Total % (% permission request needed/% permanent standing order) | ||||
| Take a 12‐lead ECG | 92.1 (20.5/71.6) *** | 84.5 (20.2/64.3) | 98.8 (21.8/77.0) | 99.0 (19.0/80.0) |
| Run a POC‐testing for troponin | 6.0 (2.4/3.6) | 7.2 (3.6/3.6) | 8.2 (3.5/4.7) | 8.2 (1.0/7.2) |
| Run a POC‐testing for BNP/NT‐proBNP | 3.5 (3.5/0.0) | 3.6 (3.6/0.0) | 3.5 (3.5/0.0) | 2.1 (0.0/2.1) |
| Do ultrasound | 16.0 (5.7/10.3) | 15.5 (6.0/9.5) | 14.9 (5.7/9.2) | 15.0 (4.0/11.0) |
|
Therapeutic actions Total % (% permission request needed/% permanent standing order) | ||||
| Insert an IV line | 94.3 (21.6/72.7) | 90.4 (21.4/69.0) | 95.4 (21.8/73.6) | 98.0 (20.2/77.8) |
| Provide supplementary oxygen | 93.2 (21.6/71.6) | 92.8 (23.8/69.0) | 86.2 (17.2/69.0) | 87.0 (24.0/63.0) |
| Provide IV diuretics | 69.0(29.9/39.1)* , ** , *** | 49.4 (24.1/25.3) | 32.6 (14.0/18.6) | 36.4 (17.2/19.2) |
| Provide morphine or another opiate | 68.6 (22.1/46.5)*** | 54.9 (19.5/35.4) | 80.0 (25.9/54.1) | 89.8 (29.6/60.2) |
| Provide IV nitroglycerine | 57.0 (31.4/25.6)* | 31.4 (16.9/14.5) | 50.6 (18.8/31.8) | 60.2 (24.5/35.7) |
| Provide non‐invasive ventilation | 80.7(25.0/55.7)** , *** | 82.2 (28.6/53.6) | 45.4 (16.3/29.1) | 50.5 (22.8/27.7) |
| Perform intubation | 71.5 (29.5/42.0) | 77.3 (33.3/44.0) | 64.3 (24.1/40.2) | 65.0 (27.0/38.0) |
AHF, acute heart failure; BNP, brain natriuretic peptide; ECG, electrocardiogram; IV, intravenous; NT‐proBNP, N terminal pro brain natriuretic peptide; POC, point of care; STEMI, ST‐elevation myocardial infarction.
P < 0.05 in comparison with the dyspnea protocol.
P < 0.05 in comparison with the chest pain protocol.
P < 0.05 in comparison with the STEMI protocol.
Surveys about the perceived difficulty of diagnosing suspected AHF by EMS personnel and by EMD were provided by 101 (97.1%) and 96 (92.3%) participants, respectively. They did not report significant differences between diagnostic difficulty of de novo AHF and ADHF in either scenario. De novo AHF and ADHF were both graded to be easy to moderate to suspect by EMS personnel and moderate to difficult by EMD (Figure 3 ). For EMD, both types of AHF were significantly more difficult to suspect than stroke and acute asthma and easier than pulmonary embolism. On the other hand, for EMS personnel, AHF (de novo and ADHF) was significantly more difficult to suspect than STEMI, stroke, and acute asthma and easier than pulmonary embolism (Figure 3 ). For all the conditions assessed, suspicion by EMD was always considered to be significantly more difficult than by EMS personnel.
Figure 3.
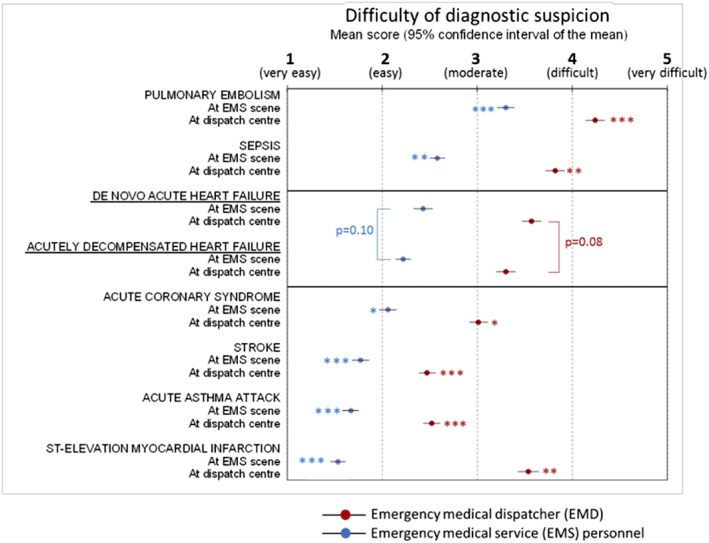
Perceived difficulty of diagnosing suspected acute heart failure (differentiated by de novo and decompensated) by emergency centre dispatchers (blue) and by emergency medical service personnel at scene (red). Comparisons were established between pairs in the same setting. * P < 0.05 compared with de novo acute heart failure. ** P < 0.05 compared with acutely decompensated heart failure.
Discussion
The EMS‐AHF study describes for the first time the pre‐hospital management of AHF from a multinational perspective. Our study provides three main findings. First, though only minority of EMS units carry diagnostic tools that can help in AHF diagnosis, the majority of units have the possibility to provide recommended AHF treatments. Second, AHF management protocols are common in the pre‐hospital setting. Yet, the contents of these protocols vary between EMS regions. Third, diagnosing suspected AHF is perceived to be easy to moderate at scene but moderate to difficult at dispatching centres, with no significant difference reported between ADHF and de novo AHF.
The differential diagnosis between AHF and other medical conditions causing dyspnoea is difficult without the use of diagnostic tools20 and may lead to misdiagnosis and inappropriate, even harmful, treatment of dyspnoeic patients.1 Our survey showed that the prevalence of diagnostic tools is low in the EMS units, even in ALS and HEMS units. Thus, there is room for improvement in the availability of diagnostic tools, such as POCTs for BNP and troponin and ultrasound devices in the EMS units' arsenals.
The availability of therapeutic arsenals varies between different types of EMS units, as could be expected from their roles in the EMS organization. IV diuretics and vasodilators such as nitroglycerine (the mainstay of AHF treatment)21 are on board in the majority of ALS and HEMS units, whereas only a minority of BLS units can provide these treatments. Very recent data suggest that early IV diuretic administration may improve mortality in AHF.9, 10, 11, 12 Considering these two findings, it is important that EMD provides the most appropriate EMS unit for a patient with suspected AHF. However, the reported proportion of AHF patients calling to dispatching centres varies from 11% in Finland 3 to 53% of cases in Spain,4 and less than one‐third of AHF patients are managed by an ALS unit.3, 4 The SEMICA study (Emergency Medical Response Systems for Patients with Acute Heart Failure) explored factors associated with EMS unit provision by EMD and found out that ALS unit assignment was well related to the severity of AHF.4, 22
In our survey, most of the EMS regions had a specific management protocol for AHF. The prevalence of AHF protocols was similar to that of chest pain and dyspnoea protocols. Some earlier studies show that delays in AHF management may increase mortality and morbidity.19, 23 The use of specific management protocols in EMS might reduce the delays in the initiation of pre‐hospital management. The prevalence of AHF management protocols can be considered rather high as the early diagnosis and treatment of AHF have been emphasized somewhat recently.11, 12, 13 As expected, management protocols for STEMI were even more prevalent because of stronger evidence and recommendation in guidelines.24 The importance of time to treatment in STEMI is unambiguous, as the condition evolves more abruptly compared with AHF. In contrast, the prognostic significance of time to treatment in AHF still remains controversial.10, 25 More importantly, AHF manifests in several clinical phenotypes, which vary in their acuity and severity.26 Specific causes of AHF like acute coronary syndrome, hypertensive emergency, or arrhythmias justify cause‐specific pre‐hospital management as well. Interestingly, chest pain protocols were no more common than AHF protocols, and dyspnoea protocols were reported only in 76% of EMS regions.
The contents of the management protocols vary between the EMS regions. The prevalence of IV opiates was high in the AHF management protocols, which is somewhat alarming because opiates are not a routine medication for all AHF patients and is recommended to be used with caution.21, 27 Moreover, IV opiates were included in AHF management protocols more often than IV nitroglycerine, which in contrast is one of the mainstay medications well available in EMS units. A vast majority of AHF protocols included respiratory support, which is an essential part of AHF management, and timely NIV may reduce the need for intubation in pulmonary oedema.28
The diagnosis of both de novo AHF and ADHF is perceived to be easy to moderate. It was found to be significantly more difficult in the dispatching centres. In comparison, STEMI was considered the easiest pre‐hospital condition to diagnose among all the conditions surveyed, probably because of the rather unequivocal diagnostic ECG criteria based on ST changes and the high availability of 12‐lead ECG. In the case of AHF, the POCT for BNP could play a similar role.
Further studies should be done to evaluate the accuracy of the pre‐hospital AHF diagnosis.
Limitations
There are some limitations to be acknowledged. First, the national representativeness of the surveys in different countries was variable. Still, we feel that our survey gives an adequate overview of the current status. Second, the diagnostic difficulty in the pre‐hospital setting was based on subjective views of the EMS regional leaders. However, the interview of individual staff members was beyond the scope of the present study.
Conclusions
The prevalence of AHF protocols is rather high and, AHF‐treatment options, especially respiratory support, are readily available in ALS and HEMS units. The pre‐hospital diagnosis of critical medical conditions is perceived to be significantly more difficult in dispatching centre compared with the EMS at scene. The diagnosis of both ADHF and de novo AHF is reported to be easy, even though the prevalence of diagnostic tools in EMS units is scarce. Future studies are warranted to investigate the accuracy of the EMS diagnosis and the cause behind the perceived diagnostic easiness of AHF.
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was supported by Department of Emergency Medicine, Helsinki University Hospital, University of Helsinki, Helsingin ja Uudenmaan sairaanhoitopiiri to P.H.
Acknowledgements
The members of EMS‐AHF study group:
Belgium: Florence Dupriez (Hôpital de Jolimont, La Louvière, Belgium), Said Idrissi (UZ Ghent, University of Ghent, Belgium), Robert Leach (Hôpital Saint‐Jean, Brussels, Belgium), Pierre Mols (CHU Saint‐Pierre, Brussels, Belgium), Frédéric Thys (Grand Hopital de Charleroi, Charleroi, Belgique), Marc Vannuffelen (Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium), Olivier Vermylen (CHU Brugmann, Brussels, Belgium), Canada: Justin Ezekowitz (University of Alberta, Alberta, Canada), Dale Weiss (Emergency Medical Service, Alberta Health Services, Alberta, Canada), Czech Republic: Roman Gregor (Emergency Medical Services of Moravian‐Silesian Region, Czech Republic), Denmark: Nanna Kruse (Department of Anesthesiology, North Zealand Hospital, Hilleroed, Denmark), Leif Rognås (Prehospital Critical Care Service, Aarhus University Hospital, Aarhus, Denmark), Estonia: Ago Kõrgvee (Department of Anaesthesiology and Intensive Care, Tartu University Hospital, Tartu, Estonia), Finland: Sanna Hoppu (Emergency Medical Service, Tampere University Hospital, Tampere, Finland), Timo Iirola (Emergency Medical Services, Turku University Hospital and University of Turku, Turku, Finland), Jouni Kurola (Prehospital Emergency Care, Kuopio University Hospital, University of Eastern Finland), Vesa Lund (Emergency Department, Satakunta Central Hospital, Pori, Finland), Matti Martikainen (Emergency Medical Services and Emergency Department, Oulu University Hospital, Oulu, Finland), Pekka Mäkelä (Emergency Medical Services, Tampere University Hospital, Tampere, Finland), Kari Törrönen (Emergency Medical Services, North Karelia Hospital District, Joensuu, Finland), Susanna Wilen (Emergency Department, North Karelia Central Hospita, Joensuu, Finland), France: Emilie Bot (Rennes, France), François Braun (Metz, France), Tahar Chouihed (Nancy, France), Pierre‐Géraud Claret (Nîmes, France), Thibaut Desmettre (Besançon, France), Mohammed Dyani (Auxerre, France), Patrick Goldstein (Lille, France), Said Laribi (Emergency Medicine Department, University Hospital of Tours, Tours, France), Cherif Mansour (Chateauroux, France), Isabelle Meyer (Bourges, France), Olivier Mimoz (Poitiers, France), Emmanuel Montassier (Nantes, France), Agnes Ricard‐Hibon (Pontoise, France), Paul‐Georges Reuter (Garches, France), Karim Tazarourte (Lyon, France), Germany: Martin Möckel (Department of Emergency Medicine, Campus Charité Mitte and Campus Virchow‐Klinikum, Charite Hospital, Berlin, Germany), Italy: Salvatore Di Somma (Emergency Department, San Andrea Hospital, University Sapienza, Rome, Italy), Marco Metra (Institute of Cardiology, University of Brescia, Brescia, Italy), Lithuania: Jelena Čelutkienė (Institute of Clinical Medicine, Faculty of Medicine, Vilnius University), Vanda Pumputienė (Vilnius Emergency Station), Monaco: Yann‐Erick Claessens, Norway: Mårten Sandberg (Air Ambulance Department, Oslo University Hospital, Oslo), Poland: Marek Banaszewski (Institute of Cardiology, Intensive Cardiac Therapy Clinic, Warsaw, Poland), Jerzy Rekosz (Medical Deputy Director of the Ambulance Service in Warsov), Singapore: Lim Swee Han (Department of Emergency Medicine, Singapore General Hospital), Slovenia: Mitja Lainscak (Division of Cardiology, General Hospital Murska Sobota, Slovenia), Spain: Javier Martin (Emergency Department, Hospital Clínico San Carlos, Madrid, Spain), and U.S.A: Sean P. Collins (Department of Emergency Medicine at Vanderbilt University, Nashville, Tennessee), Robert B. Dunne (Wayne State University,Detroit, Michigan), Jeffrey M. Goodloe (The University of Oklahoma School of Community Medicine, Tulsa, Oklahoma), David Guss (Department of Emergency Medicine, UC San Diego Health System, San Diego, USA), Andrew J. Harrell (University of New Mexico, Albuquerque, New Mexico), Melissa Hazlitt (Department of Emergency Medicine, Yale University School of Medicine, New Haven, CT, USA), Rick Hong (Cooper University Health Care, Camden, New Jersey), Joshua M. Knapp (PennState Health, Hershey, Pennsylvania), Jeffrey S. Lubin (PennState Health, Hershey, Pennsylvania), Jeffrey H. Luk (Case Western Reserve University, Cleveland, Ohio), James MacNeal (MercyHealth, Janesville, Wisconsin), Jared McKinney (Department of emergency medicine, Vanderbilt University Medical Center, Nashville, Tennessee and Nashville Fire Department, Nashville, Tennessee), Mary P. Mercer (University of California ‐ San Francisco, San Francisco, California), Roberto C. Portela (East Carolina University, Greeneville, North Carolina), Kim Pruett (University of New Mexico, Albuquerque, New Mexico), Paul Rosenberg (Southwestern Medical Center at Dallas, Dallas, Texas), Doug Swanson (Carolinas Health Care, Charlotte, North Carolina), Stacy N. Weisberg, MD, MPH (UMass Memorial Medical Center, Worcester, Massachusetts), Allen Yee (Virginia Commonwealth University, Richmond, Virginia).
Harjola, P. , Miró, Ò. , Martín‐Sánchez, F. J. , Escalada, X. , Freund, Y. , Penaloza, A. , Christ, M. , Cone, D. C. , Laribi, S. , Kuisma, M. , Tarvasmäki, T. , Harjola, V.‐P. , and on behalf of the EMS‐AHF Study Group (2020) Pre‐hospital management protocols and perceived difficulty in diagnosing acute heart failure. ESC Heart Failure, 7: 289–296. 10.1002/ehf2.12524.
References
- 1. Mosesso VN, Dunford J, Blackwell T, Griswell JK. Prehospital therapy for acute congestive heart failure: state of the art. Prehosp Emerg Care 2003; 7: 13–23. [DOI] [PubMed] [Google Scholar]
- 2. Hubble MW, Richards ME, Jarvis R, Millikan T, Young D. Effectiveness of prehospital continuous positive airway pressure in the management of acute pulmonary edema. Prehosp Emerg Care 2006; 10: 430–439. [DOI] [PubMed] [Google Scholar]
- 3. Harjola P, Boyd J, Tarvasmaki T, Mattila J, Koski R, Kuisma M, Harjola VP. The impact of emergency medical services in acute heart failure. Int J Cardiol 2017; 232: 222–226. [DOI] [PubMed] [Google Scholar]
- 4. Miro O, Llorens P, Escalada X, Herrero P, Jacob J, Gil V, Xipell C, Sanchez C, Aguilo S, Martin‐Sanchez FJ, Grupo de Investigacion ICA‐SEMES. Prehospital emergency care of patients with acute heart failure in Spain: the SEMICA study (Emergency Medical Response Systems for Patients with Acute Heart Failure). Emergencias 2017; 29: 223–230. [PubMed] [Google Scholar]
- 5. Ezekowitz JA, Podder M, Hernandez AF, Armstrong PW, Starling RC, O'Connor CM, Califf RM. Arrival by ambulance in acute heart failure: insights into the mode of presentation from Acute Studies of Nesiritide in Decompensated Heart Failure (ASCEND‐HF). BMJ Open 2016; 6: e010201–e012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tavazzi L, Senni M, Metra M, Gorini M, Cacciatore G, Chinaglia A, Di Lenarda A, Mortara A, Oliva F, Maggioni AP. IN‐HF (Italian Network on Heart Failure) outcome investigators. Multicenter prospective observational study on acute and chronic heart failure: one‐year follow‐up results of IN‐HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail 2013; 6: 473–481. [DOI] [PubMed] [Google Scholar]
- 7. Miro O, Javaloyes P, Gil V, Jacob J, Herrero‐Puente P, Martin‐Sanchez FJ, Salvo E, Alonso H, Juan Gomez MA, Parissis J, Llorens P, en nombre del grupo de investigacion ICA‐SEMES . Mortality after an episode of acute heart failure in a cohort of patients with intermediate ventricular function: Global analysis and relationship with admission department. Med Clin (Barc) 2018; 151: 223–230. [DOI] [PubMed] [Google Scholar]
- 8. Chivite D, Formiga F, Corbella X, Conde‐Martel A, Aramburu O, Carrera M, Davila MF, Perez‐Silvestre J, Manzano L, Montero‐Perez‐Barquero M, RICA Investigators . Basal functional status predicts one‐year mortality after a heart failure hospitalization in elderly patients—the RICA prospective study. Int J Cardiol 2018; 254: 182–188. [DOI] [PubMed] [Google Scholar]
- 9. Harikrishnan S, Sanjay G, Agarwal A, Kumar NP, Kumar KK, Bahuleyan CG, Vijayaraghavan G, Viswanathan S, Sreedharan M, Biju R, Rajalekshmi N, Nair T, Suresh K, Jeemon P. One‐year mortality outcomes and hospital readmissions of patients admitted with acute heart failure: data from the Trivandrum Heart Failure Registry in Kerala, India. Am Heart J 2017; 189: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, Okumura T, Kida K, Mizuno A, Oishi S, Inuzuka Y, Akiyama E, Matsukawa R, Kato K, Suzuki S, Naruke T, Yoshioka K, Miyoshi T, Baba Y, Yamamoto M, Murai K, Mizutani K, Yoshida K, Kitai T. Time‐to‐furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017; 69: 3042–3051. [DOI] [PubMed] [Google Scholar]
- 11. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray J, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail 2015; 17: 544–558. [DOI] [PubMed] [Google Scholar]
- 12. Mebazaa A, Tolppanen H, Mueller C, Lassus J, DiSomma S, Baksyte G, Cecconi M, Choi DJ, Cohen Solal A, Christ M, Masip J, Arrigo M, Nouira S, Ojji D, Peacock F, Richards M, Sato N, Sliwa K, Spinar J, Thiele H, Yilmaz MB, Januzzi J. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med 2016; 42: 147–163. [DOI] [PubMed] [Google Scholar]
- 13. Miro O, Hazlitt M, Escalada X, Llorens P, Gil V, Martin‐Sanchez FJ, Harjola P, Rico V, Herrero‐Puente P, Jacob J, Cone DC, Mockel M, Christ M, Freund Y, di Somma S, Laribi S, Mebazaa A, Harjola VP, ICA‐SEMES Research Group . Effects of the intensity of prehospital treatment on short‐term outcomes in patients with acute heart failure: the SEMICA‐2 study. Clin Res Cardiol 2018; 107: 347–361. [DOI] [PubMed] [Google Scholar]
- 14. Eckstein M, Suyehara D. Ability of paramedics to treat patients with congestive heart failure via standing field treatment protocols. Am J Emerg Med 2002; 20: 23–25. [DOI] [PubMed] [Google Scholar]
- 15. Hoffman JR, Reynolds S. Comparison of nitroglycerin, morphine and furosemide in treatment of presumed pre‐hospital pulmonary edema. Chest 1987; 92: 586–593. [DOI] [PubMed] [Google Scholar]
- 16. Pan A, Stiell IG, Dionne R, Maloney J. Prehospital use of furosemide for the treatment of heart failure. Emerg Med J 2015; 32: 36–43. [DOI] [PubMed] [Google Scholar]
- 17. Jaronik J, Mikkelson P, Fales W, Overton DT. Evaluation of prehospital use of furosemide in patients with respiratory distress. Prehosp Emerg Care 2006; 10: 194–197. [DOI] [PubMed] [Google Scholar]
- 18. Prosen G, Klemen P, Strnad M, Grmec S. Combination of lung ultrasound (a comet‐tail sign) and N‐terminal pro‐brain natriuretic peptide in differentiating acute heart failure from chronic obstructive pulmonary disease and asthma as cause of acute dyspnea in prehospital emergency setting. Crit Care 2011; 15: R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maisel AS, Peacock WF, McMullin N, Jessie R, Fonarow GC, Wynne J, Mills RM. Timing of immunoreactive B‐type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol 2008; 52: 534–540. [DOI] [PubMed] [Google Scholar]
- 20. Teboul A, Gaffinel A, Meune C, Greffet A, Sauval P, Carli P. Management of acute dyspnoea: use and feasibility of brain natriuretic peptide (BNP) assay in the prehospital setting. Resuscitation 2004; 61: 91–96. [DOI] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. Authors/task force members, document reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 22. Harjola P, Harjola VP. Can we do more for patients with acute heart failure before arrival at the hospital? Emergencias 2017; 29: 221–222. [PubMed] [Google Scholar]
- 23. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail 2009; 15: 256–264. [DOI] [PubMed] [Google Scholar]
- 24. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P, ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 25. Park JJ, Kim SH, Oh IY, Choi DJ, Park HA, Cho HJ, Lee HY, Cho JY, Kim KH, Son JW, Yoo BS, Oh J, Kang SM, Baek SH, Lee GY, Choi JO, Jeon ES, Lee SE, Kim JJ, Lee JH, Cho MC, Jang SY, Chae SC, Oh BH. The effect of door‐to‐diuretic time on clinical outcomes in patients with acute heart failure. JACC Heart Fail 2018; 6: 286–294. [DOI] [PubMed] [Google Scholar]
- 26. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez‐Fernandez S, Miani D, Filippatos G, Maggioni AP, Heart Failure Long‐Term Registry Investigators ESC. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 27. Miro O, Gil V, Martin‐Sanchez FJ, Herrero‐Puente P, Jacob J, Mebazaa A, Harjola VP, Rios J, Hollander JE, Peacock WF, Llorens P, ICA‐SEMES Research Group(*) . Morphine use in the ED and outcomes of patients with acute heart failure: a propensity score‐matching analysis based on the EAHFE registry. Chest 2017; 152: 821–832. [DOI] [PubMed] [Google Scholar]
- 28. Berbenetz N, Wang Y, Brown J, Godfrey C, Ahmad M, Vital FMR, Lambiase P, Banerjee A, Bakhai A, Chong M. Non‐invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev 2019;4:CD005351. [DOI] [PMC free article] [PubMed] [Google Scholar]


