Abstract
Background
Galectin‐3 is an inflammatory marker that is raised in myocardial fibrosis and inflammation. Recent studies have explored its role in predicting atrial fibrillation (AF) outcomes. The aim of this systematic review and meta‐analysis is to examine the association between serum concentration of galectin‐3 and AF.
Methods
PubMed, EMBASE, and the Cochrane Database were searched. A total of 280 studies were identified, of which 28 studies involving 10 830 patients were included in our meta‐analysis.
Results
Galectin‐3 is present at higher concentrations in patients with AF than those in sinus rhythm (mean difference [MD] = −0.68 ng/mL, 95% CI: −0.92, −0.44, Z = 5.61, P < .00001). Galectin‐3 levels were significantly higher in the persistent AF than in the paroxysmal AF group (MD = −0.94 ng/mL, 95% CI: −1.85, −0.03, Z = 2.04, P = .04). Higher galectin‐3 levels were associated with a 45% increase in the odds of developing AF (odds ratio [OR] = 1.45, 95% CI: 1.15, 1.83, Z = 3.11, P = .002) and risk of AF recurrence (hazard ratio [HR] =1.17, 95% CI: 1.06, 1.29, Z = 3.12, P = .002).
Conclusions
Our meta‐analysis found that galectin‐3 is significantly higher in patients with persistent AF than in those with paroxysmal AF, and can predict both AF development and recurrence after treatment.
Keywords: atrial fibrillation, galectin‐3, meta‐analysis, recurrence
1. INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia observed in clinical practice with a rising prevalence in part due to an aging population. By 2020, AF is expected to affect 10‐15 million patients in the United States alone.1 Patients with AF have increased risks for developing complications such as heart failure, stroke, and premature death. The pathophysiology of AF is complex and is thought to involve pro‐inflammatory responses, leading to structural remodeling and in turn tissue fibrosis and electrophysiological remodeling. The end result is a pro‐arrhythmic substrate for arrhythmogenesis. As with other disorders, blood markers have been used for risk stratification purposes.2, 3, 4, 5, 6, 7 More recently, galectin‐3, which is raised in the context of myocardial fibrosis, inflammation, and immune response activation, has emerged as a promising biomarker for risk stratification.8 A recent meta‐analysis has demonstrated that galectin‐3 provides incremental prognostic value that extends beyond that of traditional risk factors in the context of heart failure.9 However, the evidence on AF has been controversial with some studies reporting prognostic values while others have demonstrated little utility. In this study, therefore, we conducted a systematic review and meta‐analysis of published studies to evaluate the prognostic value of galectin‐3 in the context of AF.
2. MATERIALS AND METHODS
2.1. Search strategy
This systematic review and meta‐analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) statement. We searched studies that examined association between serum concentration of galectin‐3 and atrial fibrillation (AF). Two independent reviewers (MG and AC) systematically and independently searched the electronic databases of PubMed, EMBASE, and the Cochrane Database to identify relevant studies from their inception through June 24, 2018. The search terms used were as follows: (galectin 3 or gal 3) and (atrial fibrillation or AF). There were no restrictions with date of publication or language. The search details of different databases were recorded in Table S1. Excluded studies encompassed duplicate studies or ineligible for our study selection criteria. The disagreement was resolved by discussion with a senior reviewer (TL).
2.2. Selection criteria
The following inclusion criteria were applied: (a) The study design was a observational study (included prospective cohort, retrospective cohort, and case‐control); (b) there were measured serum concentration of galectin‐3 at least about two groups in one study; (c) compared groups were AF group and sinus rhythm group, or paroxysmal AF group and persistent AF group, or recurrence AF group and without recurrence AF group; and d) the hazard ratios (HRs)/odds ratio (OR) and the corresponding 95% confidence intervals (CI) or mean ± standard deviation (SD) were reported for galectin‐3. If the reported data of galectin‐3 in some studies can translate to means ± SD by calculation, we also included. Regarding multiple articles originating from the same cohort and reporting the same event, only those with the largest sample and the longest follow‐up duration were included.
2.3. Data extraction
Two blinded reviewers (MG and AC) independently extracted the relevant data from each eligible study using a standard data extraction form and cross‐checked. The following data were extracted: first author's last name, publication year, location, study design, number of participants, male ratio, mean age, duration of follow‐up, study population, and measurement methods of galectin‐3. Any disagreement was resolved by consensus with a senior reviewer (TL). If there was no sinus group and the two groups were different types of atrial fibrillation, we defined paroxysmal AF group as the control group.
2.4. Quality assessment
To limit heterogeneity secondary to differences among study designs, the methodological quality of included articles was evaluated by two blinded reviewers (MG and AC) applying the Newcastle‐Ottawa Score (NOS) checklist. We graded the quality as good (≥7 stars), fair (4‐6 stars), and poor (<4 stars).
2.5. Statistical analysis
The demographic characteristics of included patients are provided as mean ± SD, or median (interquartile range, IQR), or a percentage, as appropriate. All data of galectin‐3 were pooled analysis by means ± SD or HR or OR. The primary outcome was the serum concentration of galectin‐3 for different groups. Pooled effect sizes were presented as the mean ± SD for each study. Since the related data were occasionally absent, we utilized raw data to calculate mean ± SD. We use the method of translation median and IQR to mean ± SD by Wan et al10 and Luo et al11 In brief, q1 is the first quartile, m is the median, q3 is the third quartile, n is the sample size, and therefore, mean ≈ (0.7 + 0.39/n)(q1 + q3)/2+(0.3‐0.39/n)m.11 When Q ≤ 50, SD ≈ (q3‐q1)/η(n), n = 4Q + 1, we use the numerical values of η(n) were given by Wan et al10; When Q > 50, we used the formula that SD ≈ (q3‐q1)/1.35.12
Continuous data were expressed as mean difference (MD) and 95% CI, pooled analysis by inverse variance. Statistical heterogeneity across studies was assessed by chi‐square test and quantified with the use of the I2 statistic. An I2 >50% was indicative of at least moderate heterogeneity, and we used random effect model to analyze this result. To assess the effect of individual studies on the estimated relative risk, we also performed a sensitivity analysis by recalculating the pooled relative risk after omitting one study at a time and checking the consistency of the overall effect estimate. Furthermore, publication bias was evaluated by inspecting the funnel plot for each outcome. Statistical significance was defined as a 2‐tailed P‐value of .05. All statistical analyses were performed with the Review Manager, version 5.3 (RevMan; The Cochrane Collaboration).
3. RESULTS
A flow diagram of the search procedure is illustrated in Figure 1. A total of 280 studies were identified from PubMed, EMBASE, and Cochrane Library by the initial search. Of these, 57 duplicate citations and 223 ineligible studies were excluded for the following reasons: That the study was an experimental or animal study, review article, or outcome of the study was not related to AF or galectin‐3. Among the 38 full‐text articles assessed for eligibility, ten were excluded for the following reasons: One study lacked a control group;13 one study population was heart failure;14 four studies lacked available data for further analysis;15, 16, 17, 18 and four reported duplicate data from studies that later published as full text.19, 20, 21, 22 Finally, 28 studies involving 10 830 patients were included in our meta‐analysis,23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 with their baseline characteristics shown in Tables 1 and 2.
Figure 1.
Flow diagram of study selection process
Table 1.
Characteristics of included studies for meta‐analysis of association of galectin‐3 and AF
| First author | Country | Design | Study population | Number of patients | Follow‐up | Measurement methods of galectin‐3 | Quality score |
|---|---|---|---|---|---|---|---|
| Szadkowska 2013 | Poland | PC | First acute MI treated with pPCI | 145 | Until discharge | VIDAS Galectin‐3 kit | 6 |
| Clementy 2014 | France | Case‐control | Symptomatic AF | 187 | NA | VIDAS Galectin‐3 kit | 6 |
| Gurses 2014 | Turkey | PC | Lone AF underwent cryoballoon‐based PVI | 100 | 12 mo | ELISA | 6 |
| Ho 2014 | United States | PC | AF and SR | 3306 | 10 y | ELISA | 7 |
| Lee 2014 | China | PC | AF | 96 | 18 mo | ELISA | 6 |
| Sonmez 2014 | Turkey | Case‐control | AF and SR | 85 | NA | ELISA | 7 |
| Yalcin 2014 | Turkey | Case‐control | Lone AF and SR | 256 | NA | ELISA | 6 |
| Gurses 2015a | Turkey | Case‐control | AF and SR | 151 | NA | ELISA | 8 |
| Gurses 2015b | Turkey | PC | Persistent AF | 65 | 3 mo | ELISA | 6 |
| Kornej 2015 | Germany | PC | AF underwent catheter ablation | 119 | 6 mo | ELISA | 6 |
| Wu 2015 | China | PC | Persistent AF and SR | 96 | 17 mo | Milliplex MAP Kits | 9 |
| A 2016 | Russian | Case‐control | Metabolic syndrome with AF and SR | 100 | NA | ELISA | 5 |
| Alexandre 2016 | France | PC | SR underwent CABG with/without AVR | 137 | 27 d | ELISA | 9 |
| Chen 2016 | Australia | Case‐control | New onset AF and chronic AF (control) | 131 | NA | ELISA | 7 |
| Clementy 2016 | France | PC | Symptomatic AF | 160 | 12 mo | VIDAS Galectin‐3 kit | 7 |
| Ionin 2016 | Russian Federation. | PC | Metabolic syndrome with AF and SR | 230 | NA | ELISA | 5 |
| Mohanty 2016 | United States | PC | AF underwent catheter ablation | 145 | 15 mo | NA | 6 |
| Takemoto 2016 | United States | PC | AF underwent radiofrequency ablation | 55 | 12 mo | ELISA | 8 |
| Begg 2017a | UK | PC | Persistent AF and SR | 119 | 383 d | ELISA | 8 |
| Begg 2017b | UK | Case‐control | Paroxysmal AF underwent catheter ablation and SR | 129 | NA | ELISA | 8 |
| Berger 2017 | Netherlands | PC | AF underwent thoracoscopic surgical ablation | 98 | 20.7 mo | ELISA | 6 |
| Dzeshka 2017 | Belarus | Case‐control | Paroxysmal AF and SR | 76 | NA | ELISA | 5 |
| Fashanu 2017 | United States | PC | SR | 4257 | 15.7 y | Chemiluminescent microparticle immunoassay | 6 |
| Hernandez‐romero 2017 | Spain | PC | Undergoing CABG without AF | 100 | Until discharge | ELISA | 6 |
| Pavlovic 2017 | Serbia | PC | NSTEMI with AF and SR | 54 | 461 d | ELISA | 8 |
| Begg 2018 | UK | PC | AF underwent radiofrequency ablation | 92 | 1 y | ELISA | 7 |
| Kang 2018 | China | Case‐control | AF underwent radiofrequency ablation and SR | 30 | NA | ELISA | 8 |
| Tang 2018 | China | PC | AF | 113 | NA | ELISA | 7 |
Abbreviations: AVR, aortic valve replacement; CABG, coronary artery bypass graft; ELISA, enzyme‐linked immunosorbent assay; PC, prospective cohort; pPCI, Primary percutaneous coronary intervention; PVI, pulmonary vein isolation.
Table 2.
Characteristics of included patients in the meta‐analysis
| First author | Age (years) | Male (%) | Hypertension (%) | Diabetes (%) | LAD (mm) | LVEF (%) |
|---|---|---|---|---|---|---|
| Szadkowska 2013 | 61.8 ± 10.4 | 76.3 | 77.4 | 24 | NR | 54.8 ± 9.5 |
| Clementy 2014 | 62 ± 10 | 68 | 50 | 18 | 42 ± 7 | 54 ± 11 |
| Gurses 2014 | 56.95 ± 11.36 | 43.8 | 0 | 0 | 39.1 ± 4.7 | NR |
| Ho 2014 | 58.6 ± 9.2 | 47 | NR | 14.5 | NR | NR |
| Lee 2014 | NR | NR | NR | NR | NR | NR |
| Sonmez 2014 | 70 ± 10 | 37 | 63.2 | 24.2 | NR | 53.3 ± 12.8 |
| Yalcin 2014 | NR | NR | 0 | 0 | 37.1 ± 4.4 | NR |
| Gurses 2015a | 58.1 ± 10.2 | 47.1 | 0 | 0 | NR | 65.9 ± 3.3 |
| Gurses 2015b | 56.09 ± 8.03 | 46.2 | NR | NR | NR | NR |
| Kornej 2015 | 61.5 ± 8.6 | 57.5 | NR | NR | NR | NR |
| Wu 2015 | 47.6 ± 9.4 | 94.8 | 0 | 0 | 37.6 ± 4.7 | 63.2 ± 4.9 |
| A 2016 | NR | NR | NR | NR | NR | NR |
| Alexandre 2016 | 67.2 ± 10.7 | 86.7 | 78.1 | 38.7 | NR | 60.5 ± 9.9 |
| Chen 2016 | 70.3 ± 11.8 | 59 | 52.5 | 26 | NR | NR |
| Clementy 2016 | 61 ± 10 | 71 | 49 | 17 | 42 ± 8 | 54 ± 11 |
| Ionin 2016 | 50 ± 22.4 | NR | NR | NR | NR | NR |
| Mohanty 2016 | NR | 69 | NR | NR | NR | NR |
| Takemoto 2016 | 62.7 ± 1.1 | 82 | NR | NR | 44.3 ± 1.1 | 59.2 ± 0.8 |
| Begg 2017a | 62.8 ± 10.0 | 68.8 | 52.5 | 13.2 | 42.8 ± 6.1 | 55.7 ± 12.4 |
| Begg 2017b | 57.8 ± 11.4 | 69.4 | 40.2 | 11.8 | 40.1 ± 6.7 | 58.5 ± 9.2 |
| Berger 2017 | 59.8 ± 8.6 | 76 | 57 | 7 | NR | 50.2 ± 10.3 |
| Dzeshka 2017 | 62.16 | 57.5 | NR | NR | NR | NR |
| Fashanu 2017 | 62.7 ± 5.7 | 41.6 | NR | 15.5 | NR | NR |
| Hernandez‐romero 2017 | 65.1 ± 9.5 | 77 | 70 | 47 | 40.71 ± 5.80 | NR |
| Pavlovic 2017 | 68.1 ± 10.9 | 60.6 | 91.6 | 29.8 | NR | 55.12 ± 8.9 |
| Begg 2018 | 58.23 ± 15.47 | 69.9 | 33.5 | 10.15 | NR | NR |
| Kang 2018 | 62.45 ± 5.14 | NR | NR | NR | 38.8 ± 3.61 | 63.25 ± 2.49 |
| Tang 2018 | 66.7 ± 9.4 | 50.6 | 54.1 | 22.25 | 38.6 ± 4.9 | 42.2 ± 9.0 |
Abbreviations: LAD, left atrium diameter; LVEF, left ventricular ejection fraction; NR, not reported.
Fourteen studies compared serum concentrations of galectin‐3 between the sinus rhythm group and AF group.26, 28, 29, 31, 32, 33, 37, 40, 42, 44, 46, 47, 49, 50 Our meta‐analysis shows that the AF group had higher concentrations of galectin‐3 than the sinus rhythm (SR) group (mean difference [MD] = −0.68 ng/mL, 95% CI: −0.92, −0.44, Z = 5.61, P < .00001) (Figure 2A). Furthermore, we showed that higher galectin‐3 levels were associated with a 45% increase in the odds of developing AF (odds ratio [OR] = 1.45, 95% CI: 1.15, 1.83, Z = 3.11, P = .002) (Figure 2B). Six studies compared galectin‐3 levels between paroxysmal AF and persistent AF patients.24, 35, 37, 41, 48, 50 The pooled analysis showed that galectin‐3 levels were significantly higher in the persistent AF group (MD = −0.94 ng/mL, 95% CI: −1.85, −0.03, Z = 2.04, P = .04) (Figure 2C).
Figure 2.
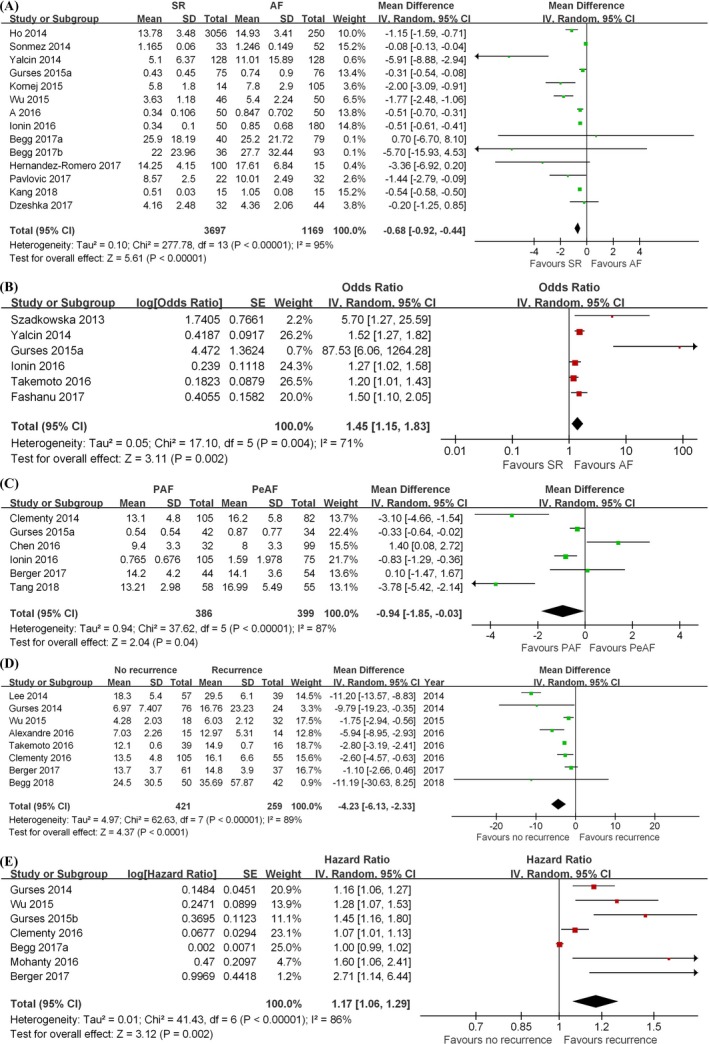
Forest plots of meta‐analysis. A, Serum concentrations of galectin‐3 between the SR group and AF group. B, The odds of concentrations of galectin‐3 developing AF. C, Serum concentrations of galectin‐3 between PAF group and PeAF group. D, Serum concentrations of galectin‐3 between AF recurrence group and sinus rhythm restoration group. E, The hazard ratio of concentrations of galectin‐3 in AF recurrence. SR, sinus rhythm; AF, atrial fibrillation; CI, confidence interval; M‐H, Mantel‐Haenszel; SD, standard deviation; IV, Inverse Variance
Several published studies also examined the value of galectin‐3 in predicting patients who will have AF recurrence after different treatments for SR restoration. Our meta‐analysis shows that patients with no recurrence had significantly lower galectin‐3 levels than those with disease recurrence (MD = −4.23 ng/mL, 95% CI: −6.13, −2.33, Z = 4.37, P < .0001) (Figure 2D). Furthermore, higher galectin‐3 levels were associated with higher risk of AF recurrence (hazard ratio [HR] = 1.17, 95% CI: 1.06, 1.29, Z = 3.12, P = .002) (Figure 2E).
Funnel plot results suggested that publication bias may be present (Figures S1‐S5).
4. DISCUSSION
AF is the most frequently cardiac arrhythmia observed in clinical practice, with an increasing prevalence due to an aging population and the rising burden of comorbid cardiovascular diseases.51 It is important at the public health level because of its predisposition to stroke, heart failure, dementia, premature mortality, and disability.52 In this condition, there is an ongoing cardiomyopathic process of the atrial myocardium,53, 54 involving a number of cellular and molecular mechanisms revolving around inflammation.55, 56 One of the consequences is fibrosis, characterized by increased turnover of the extracellular matrix, producing conduction abnormalities that provide the necessary substrate for arrhythmogenesis.57, 58 A number of blood biomarkers3, 5, 6, 7, 59, 60, 61, 62 and electrocardiographic predictors54, 63, 64 have been found in association with AF onset, development, and recurrence. Of these, galectin‐3 plays a key role in acute and chronic pro‐inflammatory responses and mediates activation of quiescent fibroblasts and synthesis of collagen.65 Previous studies have reported the prognostic value of galectin‐3 in cardiovascular pathologies such as acute coronary syndrome,66 heart failure,67, 68 and in the general population.16, 69 Recently, a meta‐analysis examined its prognostic value in the context of heart failure and in the general population,9, 18 but whether it aids risk stratification in AF remains controversial. Several trends have emerged from this meta‐analysis regarding the correlation between galectin‐3 concentrations and AF. Firstly, galectin‐3 levels are higher in AF patients than those in SR and increased levels are associated with higher odds of AF development. Secondly, galectin‐3 levels did significantly differ between AF subtypes. Thirdly, galectin‐3 levels are higher in patients with AF recurrence than those with no recurrence after SR restoration.
Galectin‐3 was originally discovered in 1982 as Mac‐2,70 cloned in 1991, and subsequently recognized as a β‐galactoside‐binding lectin.71 It has diverse biological functions such as regulation of cell adhesion,72 immunity,73 inflammation,74 and fibrosis.75 Its pathological role in the heart, specifically heart failure, has been discussed in detail by the excellent review here.76 It is upregulated in response to increased stressors such as angiotensin II and pressure overload. It is thought to play a critical role in the transition from compensatory remodeling to decompensation, as originally shown in an animal model.77 AF can induce tissue injury, leading to increased synthesis and subsequent release of galectin‐3 by macrophages.77 Galectin‐3 can itself mediate macrophage activation through both classical and alternative pathways,78 as well as induce adverse structural and electrophysiological remodeling in the atria.42 The latter effect may be independent of heart failure, since galectin‐3 is raised in AF patients without structural heart disease.32 The following mechanisms underlying galectin‐3‐mediated atrial dysfunction have been identified. The extracellular pentameric domain of galectin‐3 can interact with pro‐fibrotic signals, such as transforming growth factor‐β/SMAD, which can initiate fibrosis.39 Furthermore, galectin‐3 can form complexes, which can cross‐link glycosylated ligands to form a lattice.79 This lattice could potentially trap transforming growth factor‐β receptors to amplify the pro‐fibrotic signaling pathways in the atria. It should be noted that AF may further induce galectin‐3 release from macrophages, producing a vicious cycle that can perpetuate AF progression.8
There are several strengths of this meta‐analysis. Firstly, this study adhered to PRISMA guidelines, which ensured the quality of the systematic evaluation and minimization of bias. Secondly, a large sample size of 10 830 patients from a total of 28 studies was included, meaning that we are confident. Finally, galectin‐3 levels were determined using enzyme‐linked immunosorbent assay (ELISA) in 21 of the studies, and therefore. we can be confident that the values provided are comparable. However, some limitations must be noted. Firstly, only 14 of the 28 included studies had quality scores of 7 or above, suggesting quality of the remaining 14 studies requires cautious interpretation. Secondly, attempts were made to identify the origin of the high heterogeneity. There are several reasons as to why this may be the case, for example, differing characteristics of the study groups, such as acute myocardial infarction, metabolic syndrome, or after coronary artery bypass grafting surgery or ablation procedures; possible variable contributions from confounders such as heart failure and other comorbid conditions; different follow‐up periods; and different study designs.
5. CONCLUSIONS
Our meta‐analysis found that galectin‐3 is significantly higher in patients with persistent AF than in those with paroxysmal AF and can predict both AF development and recurrence after treatment.
Supporting information
ACKNOWLEDGMENTS
This work was supported by grants (81970270, 81570298 to TL, 81270259 to QS.W.) from the National Natural Science Foundation of China, Tianjin Natural Science Foundation (16JCZDJC34900 to TL), and Shanghai Science and Technology Development Funds (17411954600 to QS.W.).
Gong M, Cheung A, Wang Q‐S, et al. Galectin‐3 and risk of atrial fibrillation: A systematic review and meta‐analysis. J Clin Lab Anal. 2020;34:e23104 10.1002/jcla.23104
Contributor Information
Gary Tse, Email: gary.tse@doctors.org.uk.
Tong Liu, Email: liutongdoc@126.com.
REFERENCES
- 1. Anumonwo JM, Kalifa J. Risk factors and genetics of atrial fibrillation. Heart Fail Clin. 2016;12:157‐166. [DOI] [PubMed] [Google Scholar]
- 2. Lee YT, Gong M, Chau A, et al. Pentraxin‐3 as a marker of sepsis severity and predictor of mortality outcomes: a systematic review and meta‐analysis. J Infect. 2018;76(1):1‐10 [DOI] [PubMed] [Google Scholar]
- 3. Cheung A, Gong M, Bellanti R, et al. Cancer antigen‐125 and risk of atrial fibrillation: a systematic review and meta‐analysis. Heart Asia. 2018;10:e010970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foris V, Kovacs G, Tscherner M, Olschewski A, Olschewski H. Biomarkers in pulmonary hypertension: what do we know? Chest. 2013;144:274‐283. [DOI] [PubMed] [Google Scholar]
- 5. Weymann A, Ali‐Hasan‐Al‐Saegh S, Sabashnikov A, et al. Prediction of new‐onset and recurrent atrial fibrillation by complete blood count tests: a comprehensive systematic review with meta‐analysis. Med Sci Monit Basic Res. 2017;23:179‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qi W, Zhang N, Korantzopoulos P, et al. Serum glycated hemoglobin level as a predictor of atrial fibrillation: a systematic review with meta‐analysis and meta‐regression. PLoS ONE. 2017;12:e0170955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weymann A, Ali‐Hasan‐Al‐Saegh S, Popov AF, et al. Haematological indices as predictors of atrial fibrillation following isolated coronary artery bypass grafting, valvular surgery, or combined procedures: a systematic review with meta‐analysis. Kardiol Pol. 2018;76:107‐118. [DOI] [PubMed] [Google Scholar]
- 8. Lippi G, Cervellin G, Sanchis‐Gomar F. Galectin‐3 in atrial fibrillation: Simple bystander, player or both? Clin Biochem. 2015;48:818‐822. [DOI] [PubMed] [Google Scholar]
- 9. Imran TF, Shin HJ, Mathenge N, et al. Meta‐analysis of the usefulness of plasma galectin‐3 to predict the risk of mortality in patients with heart failure and in the general population. Am J Cardiol. 2017;119:57‐64. [DOI] [PubMed] [Google Scholar]
- 10. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Stat Methods Med Res. 2018;27:1785‐1805. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley Online Library; 2008.
- 13. Selcoki Y, Aydin HI, Celik TH, et al. Galectin‐3: a biochemical marker to detect paroxysmal atrial fibrillation? Clin Invest Med. 2016;39:27528. [PubMed] [Google Scholar]
- 14. Lashkul D, Syvolap VD. Biomarkers of cardiovascular stress in assessment the left atrial remodeling in patients with ischemic chronic heart failure with atrial fibrillation. Europace. 2015;17:iii253. [Google Scholar]
- 15. Benhenda N, Clementy N, Bernard‐Brunet A, Babuty D. Left atrial mechanics and galactic 3 assessment in patients undergoing atrial fibrillation ablation. Heart Rhythm. 2017;14:S457. [Google Scholar]
- 16. van der Velde AR, Meijers WC, Ho JE, et al. Serial galectin‐3 and future cardiovascular disease in the general population. Heart. 2016;102:1134‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hussein AA, DeFilippi C, Bartz T, et al. Association of two biomarkers of pathways involved in cardiac structural remodeling with the risk of incident atrial fibrillation: The cardiovascular health study. Heart Rhythm. 2014;11:S535. [Google Scholar]
- 18. Shaposhnikova Y, Ilchenko I, Bobronnikova L. Assessment of combination of elevated Gal‐3 and NT‐proBNP levels as an independent predictors of new‐onset AF in patients with acute coronary syndrome. Eur Heart J. 2016;5:373‐374. [Google Scholar]
- 19. Wu XY, Bai R, Wen SN, et al. Plasma galectin‐3 elevated in patients with lone persistent and paroxysmal atrial fibrillation and associated with left atrial enlargement. J Am Coll Cardiol. 2014;63:A459‐A459. [Google Scholar]
- 20. Ionin VA, Soboleva AV, Listopad OV, et al. Galectin 3 and aldosterone in patients with atrial fibrillation and metabolic syndrome. Russ J Cardiol. 2015;120:79‐83. [Google Scholar]
- 21. Berger WR, Jagu B, Van Den Berg NWE, et al. Galectin‐3 in the human atria and serum of patients with atrial fibrillation does not reflect progression of the fibrotic substrate. Eur Heart J. 2016;37:885. [Google Scholar]
- 22. Wu XY, Bai R, Wen SN, et al. Plasma galectin‐3 levels and recurrent arrhythmia after successful ablation of lone atrial fibrillation. J Am Coll Cardiol. 2014;63:A464‐A464. [Google Scholar]
- 23. Szadkowska I, Wlazel RN, Migala M, et al. The association between galectin‐3 and clinical parameters in patients with first acute myocardial infarction treated with primary percutaneous coronary angioplasty. Cardiol J. 2013;20:577‐582. [DOI] [PubMed] [Google Scholar]
- 24. Clementy N, Piver E, Benhenda N, et al. Galectin‐3 in patients undergoing ablation of atrial fibrillation. IJC Metab Endocr. 2014;5:56‐60. [Google Scholar]
- 25. Gurses KM, Yalcin MU, Kocyigit D, et al. Serum galectin‐3 level predicts late recurrence following cryoballoon‐based pulmonary vein isolation in lone atrial fibrillation patients. Circulation. 2014;130:A15237. [Google Scholar]
- 26. Ho JE, Yin X, Levy D, et al. Galectin 3 and incident atrial fibrillation in the community. Am Heart J. 2014;167(5):729‐34.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee JK, Lin CL. A novel biomarker‐based approach for rhythm outcomes after catheter ablation of atrial fibrillation. Heart Rhythm. 2014;11:S510. [Google Scholar]
- 28. Sonmez O, Ertem FU, Vatankulu MA, et al. Novel fibro‐inflammation markers in assessing left atrial remodeling in non‐valvular atrial fibrillation. Med Sci Monit. 2014;20:463‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yalcin MU, Gurses KM, Kocyigit D, et al. Serum galectin‐3 levels are elevated in patients with lone atrial fibrillation. Anadolu Kardiyoloji Dergisi. 2014;14:58. [Google Scholar]
- 30. Gurses K, Yalcin M, Kocyigit D, et al. Serum galectin‐3 level predicts recurrence following successful electrical cardioversion in persistent atrial fibrillation patients. J Am Coll Cardiol. 2015;65:A307. [Google Scholar]
- 31. Kornej J, Schmidl J, Ueberham L, et al. Galectin‐3 in patients with atrial fibrillation undergoing radiofrequency catheter ablation. PLoS ONE. 2015;10:e0123574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu XY, Li SN, Wen SN, et al. Plasma galectin‐3 predicts clinical outcomes after catheter ablation in persistent atrial fibrillation patients without structural heart disease. Europace. 2015;17:1541‐1547. [DOI] [PubMed] [Google Scholar]
- 33. Va A, Zaslavskaya EL, Soboleva AV, et al. Galectin‐3 in patients with paroxysmal and persistent atrial fibrillation and metabolic syndrome. Kardiologiia. 2016;56:41‐45. [DOI] [PubMed] [Google Scholar]
- 34. Alexandre J, Saloux E, Chequel M, et al. Preoperative plasma aldosterone and the risk of atrial fibrillation after coronary artery bypass surgery: a prospective cohort study. J Hypertens. 2016;34:2449‐2457. [DOI] [PubMed] [Google Scholar]
- 35. Chen D, Procter N, Goh V, et al. New onset atrial fibrillation is associated with elevated galectin‐3 levels. Int J Cardiol. 2016;223:48‐49. [DOI] [PubMed] [Google Scholar]
- 36. Clementy N, Benhenda N, Piver E, et al. Serum Galectin‐3 Levels Predict Recurrences after Ablation of Atrial Fibrillation. Sci Rep. 2016;6:34357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ionin VA, Zaslavskaya EL, Soboleva AV, et al. Can we predict the effect of antiarrhythmic pharmacotherapy in atrial fibrillation and metabolic syndrome patients: Focus on galectin‐3. Eur Heart J. 2016;37:881. [Google Scholar]
- 38. Mohanty S, Mohanty P, Metz T, et al. Galectin‐3 predicts long‐term procedure outcome in atrial fibrillation patients undergoing catheter ablation. Eur Heart J. 2016;37:883‐883. [Google Scholar]
- 39. Takemoto Y, Ramirez RJ, Yokokawa M, et al. Galectin‐3 regulates atrial fibrillation remodeling and predicts catheter ablation outcomes. JACC Basic Transl Sci. 2016;1:143‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Begg GA, Karim R, Oesterlein T, et al. Intra‐cardiac and peripheral levels of biochemical markers of fibrosis in patients undergoing catheter ablation for atrial fibrillation. Europace. 2017;19:1944‐1950. [DOI] [PubMed] [Google Scholar]
- 41. Berger WR, Jagu B, van den Berg NWE, et al. The change in circulating galectin‐3 predicts absence of atrial fibrillation after thoracoscopic surgical ablation. Europace. 2018;20(5):764‐771. [DOI] [PubMed] [Google Scholar]
- 42. Hernandez‐Romero D, Vilchez JA, Lahoz A, et al. Galectin‐3 as a marker of interstitial atrial remodelling involved in atrial fibrillation. Sci Rep. 2017;7:40378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fashanu OE, Norby FL, Aguilar D, et al. Galectin‐3 and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2017;192:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pavlovic M, Apostolovic S, Stokanovic D, et al. The association between galectin‐3 and hs‐CRP and the clinical outcome after Non‐ST‐elevation myocardial infarction with preexisting atrial fibrillation. Sci Rep. 2017;7:15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Begg GA, Karim R, Oesterlein T, et al. Left atrial voltage, circulating biomarkers of fibrosis, and atrial fibrillation ablation. A prospective cohort study. PLoS ONE. 2018;13:e0189936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dzeshka MS, Appadoo K, Shantsila E, Snezhitskiy VA, Lip GYH. P1720 Increased evidence of left ventricular myocardial fibrosis in patients with paroxysmal atrial fibrillation and sinus node dysfunction. Eur Heart J. 2017;38:379. [Google Scholar]
- 47. Kang Q, Li X, Yang M, Fernando T, Wan Z. Galectin‐3 in patients with coronary heart disease and atrial fibrillation. Clin Chim Acta. 2018;478:166‐170. [DOI] [PubMed] [Google Scholar]
- 48. Tang Z, Zeng L, Lin Y, et al. Circulating galectin‐3 is associated with left atrial appendage remodelling and thrombus formation in patients with atrial fibrillation. Heart Lung Circ. 2019;28:923‐931. [DOI] [PubMed] [Google Scholar]
- 49. Begg GA, Lip GY, Plein S, Tayebjee MH. Circulating biomarkers of fibrosis and cardioversion of atrial fibrillation: a prospective, controlled cohort study. Clin Biochem. 2017;50:11‐15. [DOI] [PubMed] [Google Scholar]
- 50. Gurses KM, Yalcin MU, Kocyigit D, et al. Effects of persistent atrial fibrillation on serum galectin‐3 levels. Am J Cardiol. 2015;115:647‐651. [DOI] [PubMed] [Google Scholar]
- 51. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583‐596. [DOI] [PubMed] [Google Scholar]
- 52. Mongkhon P, Naser AY, Fanning L, et al. Oral anticoagulants and risk of dementia: A systematic review and meta‐analysis of observational studies and randomized controlled trials. Neurosci Biobehav Rev. 2018;96:1‐9. [DOI] [PubMed] [Google Scholar]
- 53. Tse G, Wong CW, Gong M, et al. Predictive value of inter‐atrial block for new onset or recurrent atrial fibrillation: a systematic review and meta‐analysis. Int J Cardiol. 2018;250:152‐156. [DOI] [PubMed] [Google Scholar]
- 54. Tse G, Liu T, Baranchuk A. Authors' reply: low prevalence of inter‐atrial block in the general population from China: a possible reason for its low rates of atrial fibrillation. Int J Cardiol. 2018;260:112. [DOI] [PubMed] [Google Scholar]
- 55. Korantzopoulos P, Letsas K, Fragakis N, Tse G, Liu T. Oxidative stress and atrial fibrillation: an update. Free Radic Res. 2018;52:1199‐1209. [DOI] [PubMed] [Google Scholar]
- 56. Korantzopoulos P, Letsas KP, Tse G, et al. Inflammation and atrial fibrillation: a comprehensive review. J Arrhythm. 2018;34:394‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tse G, Lai ET, Yeo JM, Tse V, Wong SH. Mechanisms of electrical activation and conduction in the gastrointestinal system: lessons from cardiac electrophysiology. Front Physiol. 2016;7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tse G, Lai ET, Lee AP, Yan BP, Wong SH. Electrophysiological mechanisms of gastrointestinal arrhythmogenesis: lessons from the heart. Front Physiol. 2016;7:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shao Q, Korantzopoulos P, Letsas KP, et al. Red blood cell distribution width as a predictor of atrial fibrillation. J Clin Lab Anal. 2018;32:e22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weymann A, Popov AF, Sabashnikov A, et al. Baseline and postoperative levels of C‐reactive protein and interleukins as inflammatory predictors of atrial fibrillation following cardiac surgery: a systematic review and meta‐analysis. Kardiol Pol. 2018;76:440‐451. [DOI] [PubMed] [Google Scholar]
- 61. Zhang N, Fan C, Gong M, et al. Leucocyte telomere length and paroxysmal atrial fibrillation: a prospective cohort study and systematic review with meta‐analysis. J Clin Lab Anal. 2018;32:e22599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang N, Tse G, Liang X, Li G, Liu T. Telomere length: a newly marker for predicting atrial fibrillation? Int J Cardiol. 2017;239:21. [DOI] [PubMed] [Google Scholar]
- 63. Alexander B, Haseeb S, van Rooy H, et al. Reduced P‐wave voltage in lead i is associated with development of atrial fibrillation in patients with coronary artery disease. J Atr Fibrillation. 2017;10:1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang N, Gong M, Tse G, et al. Prolonged corrected QT interval in predicting atrial fibrillation: a systematic review and meta‐analysis. Pacing Clin Electrophysiol. 2018;41:321‐327. [DOI] [PubMed] [Google Scholar]
- 65. Vergaro G, Del Franco A, Giannoni A, et al. Galectin‐3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int J Cardiol. 2015;184:96‐100. [DOI] [PubMed] [Google Scholar]
- 66. Agnello L, Bivona G, Lo Sasso B, et al. Galectin‐3 in acute coronary syndrome. Clin Biochem. 2017;50:797‐803. [DOI] [PubMed] [Google Scholar]
- 67. Amin HZ, Amin LZ, Wijaya IP. Galectin‐3: a novel biomarker for the prognosis of heart failure. Clujul Med. 2017;90:129‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gehlken C, Suthahar N, Meijers WC, de Boer RA. Galectin‐3 in heart failure: an update of the last 3 years. Heart Fail Clin. 2018;14:75‐92. [DOI] [PubMed] [Google Scholar]
- 69. de Boer RA, van Veldhuisen DJ, Gansevoort RT, et al. The fibrosis marker galectin‐3 and outcome in the general population. J Intern Med. 2012;272:55‐64. [DOI] [PubMed] [Google Scholar]
- 70. Ho MK, Springer TA. Mac‐2, a novel 32,000 Mr mouse macrophage subpopulation‐specific antigen defined by monoclonal antibodies. J Immunol. 1982;128:1221‐1228. [PubMed] [Google Scholar]
- 71. Raz A, Carmi P, Raz T, et al. Molecular cloning and chromosomal mapping of a human galactoside‐binding protein. Cancer Res. 1991;51:2173‐2178. [PubMed] [Google Scholar]
- 72. Yang EH, Rode J, Howlader MA, et al. Galectin‐3 alters the lateral mobility and clustering of beta1‐integrin receptors. PLoS ONE. 2017;12:e0184378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Park AM, Hagiwara S, Hsu DK, Liu FT, Yoshie O. Galectin‐3 plays an important role in innate immunity to gastric infection by Helicobacter pylori . Infect Immun. 2016;84:1184‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gittens BR, Bodkin JV, Nourshargh S, Perretti M, Cooper D. Galectin‐3: a positive regulator of leukocyte recruitment in the inflamed microcirculation. J Immunol. 2017;198:4458‐4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lok DJ, Van Der Meer P, de la Porte PW, et al. Prognostic value of galectin‐3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL‐HF study. Clin Res Cardiol. 2010;99:323‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Boer RA, van der Velde AR, Mueller C, et al. Galectin‐3: a modifiable risk factor in heart failure. Cardiovasc Drugs Ther. 2014;28:237‐246. [DOI] [PubMed] [Google Scholar]
- 77. Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin‐3 marks activated macrophages in failure‐prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121‐3128. [DOI] [PubMed] [Google Scholar]
- 78. MacKinnon AC, Farnworth SL, Hodkinson PS, et al. Regulation of alternative macrophage activation by galectin‐3. J Immunol. 2008;180:2650‐2658. [DOI] [PubMed] [Google Scholar]
- 79. Nabi IR, Shankar J, Dennis JW. The galectin lattice at a glance. J Cell Sci. 2015;128:2213‐2219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


