Introduction
Gastroesophageal reflux disease (GERD) affects one in five Americans and is one of the most common diagnoses managed in the outpatient gastroenterology clinic.1, 2 According to the Montreal Consensus, GERD is defined as the presence of troublesome symptoms and/or complications that develop due to retrograde reflux of gastric contents in the esophagus. 2 Historically, GERD management has relied on empirical treatment approaches, and proven unsuccessful. Traditionally, GERD is diagnosed based on presence of symptoms and managed reflexively with acid suppressive medication, particularly proton pump inhibitors (PPIs). However, up to 40% of patients treated with PPIs may have incomplete or no symptom response to therapy.4 Since various symptoms (e.g., heartburn, regurgitation, chest pain, globus, dysphagia, throat symptoms, cough, belching) can be attributed to GERD, and a multitude of mechanisms can result in gastro-esophageal reflux physiology, it is no wonder that individuals with GERD respond differently to a single-pronged treatment approach.
Recent advancements in esophageal physiology and psychology have led to the development of sophisticated diagnostic tools to evaluate GERD. Clinical sites across the world have adopted these diagnostic tools, such as pH monitoring and high-resolution manometry systems, to evaluate esophageal disorders. At the same time, therapeutic options for GERD beyond PPI therapy and laparoscopic fundoplication have expanded. Today treatment options for GERD span from behavioral interventions, various pharmacologic applications, and minimally invasive endoluminal and laparoscopic techniques. These state-of-the-art concepts, diagnostic tools, and treatment options offer tremendous opportunity for personalizing the management of GERD. Therefore, this paper will review a personalized approach for the evaluation and management of GERD.
Theory of Personalization in GERD
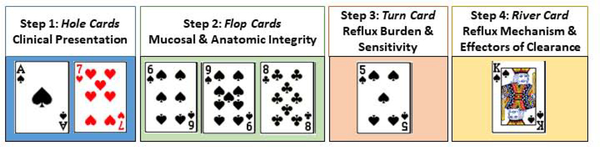
Patients with GERD symptoms can be classified based on multiple factors including clinical history (predominant symptom and response to PPI), endoscopic findings (gastroesophageal flap valve, hiatal hernia, esophagitis), ambulatory reflux testing (acid exposure, reflux events), and esophageal function (reflux clearance, alternate etiologies of symptoms). Different permutations of these factors can yield more than 50 classifications, or phenotypes, of GERD. This concept is akin to a game of poker where a particular combination of cards (factors) may lead to different hands of cards (phenotypes of GERD) (Figure 1). In poker, betting utilizes probability theory to ascertain the odds of the particular poker hand. Similarly, in a phenotype approach to personalizing the management of GERD, the treatment decision is based on the predicted outcome for the particular phenotype of GERD.
Figure 1. The combination of factors in phenotyping GERD are akin to the combination of cards in a game of poker.
Phenotypes of GERD, or a combination of cards, are revealed in a step-wise fashion. At any step a provider, or player, may decide to stop further evaluation and treat given a very high odds of outcome for a certain phenotype.
Four Steps of Phenotyping in a Personalized Approach for GERD
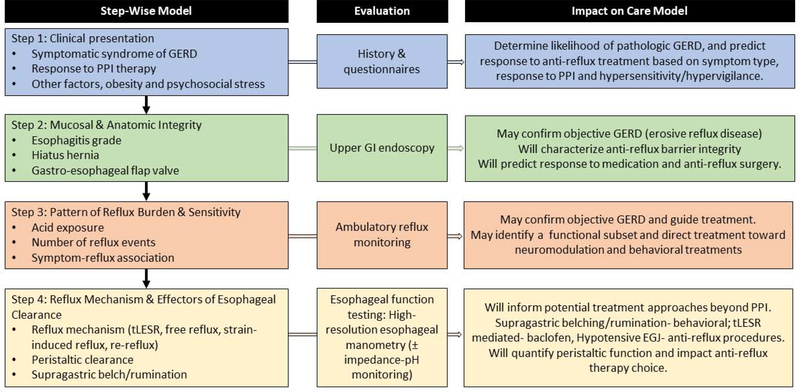
The proposed personalized approach for GERD employs a step-wise method that optimizes phenotypic yield and outcome while minimizing invasiveness, risk and cost [Figure 2]. Continuing with the poker analogy, in Texas hold’em cards are revealed to players in a step-wise method, and bets are placed at each step. At any step a player may decide to go all in (bet everything) given a very high odds of winning, or alternatively fold given a very high odds of losing. Similarly, throughout the step-wise method for phenotyping GERD a clinician may at any point elect to halt further testing (akin to folding or going all in) and proceed with management if the information available identifies a distinct GERD phenotype.
Figure 2.
Four steps of phenotyping in GERD to define important biomarkers and clinical predictors of treatment and disease outcomes.
Step 1: Clinical presentation to assess symptom and response to PPI (Hole Cards)
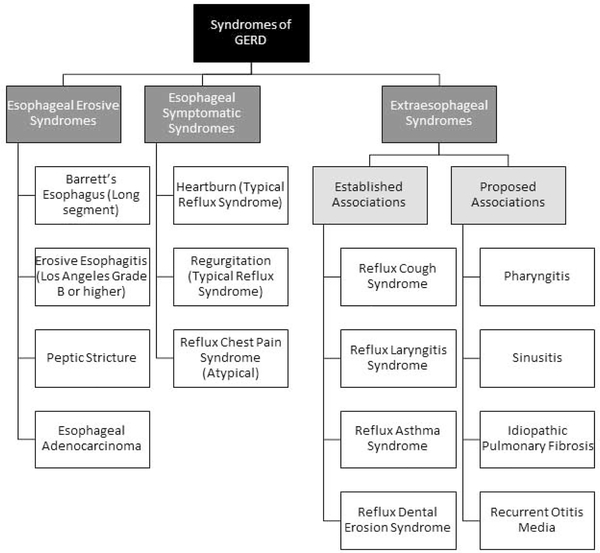
A good history is crucial in step 1 of the evaluation of GERD. This is typically focused on the symptom presentation, the response to a PPI trial and also other key factors that may implicate and modulate reflux severity, such as obesity and underlying comorbidities like scleroderma or sleep apnea. Additionally, an underlying assessment of visceral anxiety and hypervigilance may be important clues in developing the phenotypes and treatment strategies as these factors will modulate response across all GERD phenotypes. Typically, GERD is initially diagnosed based on symptom presentation and further classified based on response to anti-reflux treatment. Clinically diagnosed symptomatic syndromes of GERD include typical reflux syndromes in the setting of heartburn and/or regurgitation, or reflux chest pain syndrome. 2 Further, clinically diagnosed GERD may be due extra-esophageal syndromes such as reflux cough, reflux laryngitis, or reflux asthma. 2 [Figure 3] The therapeutic gain of PPI therapy, the mainstay treatment for clinically suspected GERD, over placebo in treating GERD syndromes varies. 3,4 When esophagitis is not present, therapeutic efficacy is greatest for heartburn symptoms, less so for regurgitation or chest pain, and least for extra-esophageal syndromes. 5 Variation in outcomes to PPI therapy is likely a function of pathophysiologic mechanism. For instance, acidification of the esophagus can provoke heartburn symptoms, and therefore, acid suppression is an effective treatment option for acid-mediated heartburn. 6 Regurgitation, on the other hand, is a function of gastro-esophageal reflux volume rather than acidic nature of refluxate, and thus less responsive to acid suppression. Extra-esophageal symptoms are the least responsive to PPI therapy for various reasons. In up to 60% of cases, extra-esophageal symptoms will be caused by non-reflux conditions such as environmental exposures, sinopulmonary conditions, and voice use. 7 Studies also suggest that higher pH levels can impart noxious stimuli and injury in the hypopharynx compared to the distal esophagus. 8 For these reasons, the response to PPI therapy for extra-esophageal symptoms is close to that of placebo. 9, 10 Therefore, clinical assessment of esophageal and/or extra-esophageal symptoms, and response to PPI therapy is an important initial component in GERD phenotyping. This is combined with the underlying patient characteristics that may be related to the development of heartburn, such as recent weight gain and new medications which reduce lower esophageal sphincter (LES) pressure.
Figure 3.
Syndromes of GERD (Adapted from the Montreal Classification) classifies GERD as an esophageal syndrome or extra-esophageal syndrome. Esophageal syndromes may be those associated with esophageal injury or based on symptom presentation. (From Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900–20; with permission.)
Step 2: Upper GI endoscopy to assess mucosal & anatomic integrity (Flop Cards)
Information from step 1 helps to guide the need for upper GI endoscopy (step 2). Upper GI endoscopy is the next step for patients with typical reflux symptoms without adequate response to PPI therapy, atypical reflux symptoms, as well as warning signs or symptoms such as dysphagia, GI bleeding, weight loss, or iron deficiency anemia. 11 First, endoscopic assessment of the esophagus will determine presence and severity of erosive reflux disease. The presence of Los Angeles C or D esophagitis, long-segment Barrett’s esophagus, and/or peptic stricture provides objective confirmation of pathologic GERD and signifies a higher likelihood of response to antireflux therapy. 12 While the Lyon consensus considers Los Angeles B inconclusive for GERD, the finding of Los Angeles B esophagitis along with typical symptoms has a high likelihood of underlying reflux unless alternative factors are noted, such as eosinophilic esophagitis, or a dermatologic or non-peptic esophagitis pattern.
Next, endoscopic assessment of the integrity of the antireflux barrier will impact the likelihood for medical management to succeed. The anti-reflux barrier is a high-pressure zone comprised of the LES attached to the crural diaphragm via the phreno-esophageal ligament which forms a tight gastro-esophageal flap valve to prevent pathologic gastroesophageal reflux. 13 Disruption to the anti-reflux barrier may lead to increased reflux burden and acid exposure. Axial separation between the crural diaphragm and LES results in a hiatal hernia. Hiatal hernia, as well as reduced tonicity of the intrinsic LES will reduce integrity of the gastro-esophageal flap valve mechanism. Therefore, endoscopic assessment of the anti-reflux barrier via characterization of hernia, if present, measurement of length of separation between the diaphragmatic pinch (crural diaphragm) and the proximal extent of the gastric folds (lower esophageal sphincter), and grading of the gastro-esophageal flap valve are important steps in phenotyping GERD. 13
Endoscopy also enables evaluation of alternate sources of esophageal symptoms such as eosinophilic esophagitis, mechanical obstruction, other non-peptic esophagitis or peptic ulcer disease.
Step 3: Reflux monitoring to characterize pattern of reflux burden and reflux sensitivity (Turn Card)
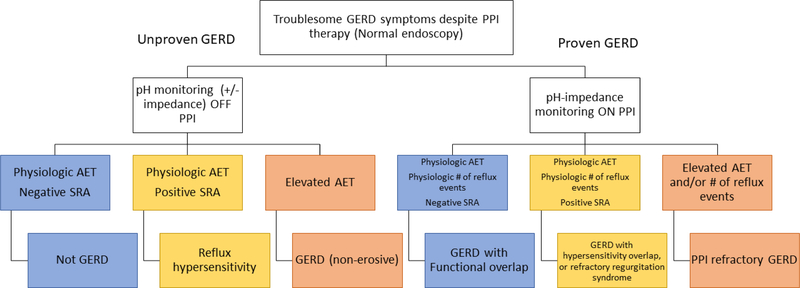
When erosive reflux disease is not present, the next step is to characterize the reflux burden with reflux monitoring to assess for acid exposure, reflux events, and association between symptom perception and reflux events. 14 Ambulatory reflux monitoring is available as 24 hour transnasal catheter recording with or without impedance monitoring, and wireless pH monitoring with prolonged monitoring capabilities. When the pre-test likelihood of GERD is low, defined by the Lyon Consensus as lack of erosive reflux disease, ambulatory reflux monitoring is performed off acid suppression in order to establish presence of GERD at baseline. 15 On the other hand, if the pre-test likelihood of GERD is high, defined by objective evidence of reflux by way of erosive reflux disease on endsocopy or previous positive reflux monitoring, ambulatory reflux monitoring is performed on acid suppression to evaluate for refractory GERD [Figure 4]. 16
Figure 4. Reflux monitoring for PPI non-response for patients with non-erosive GERD symptoms.
For patients with unproven GERD ambulatory reflux monitoring off PPI is recommended to assess for presence or absence of pathologic acid exposure time (AET) and symptom reflux association (SRA) in order to phenotype GERD. In setting of previously proven GERD ambulatory reflux monitoring with pH impedance on PPI is recommended to identify whether GERD with a functional or hypersensitivity overlap is present, whether a regurgitation syndrome is present, or whether PPI refractory GERD is present.
According to the recent Lyon Consensus for GERD, esophageal acid exposure time greater than 6.0% is consistent with acidic gastro-esophageal reflux disease, acid exposure time less than 4.0% is physiologic, and acid exposure times between 4.0 to 6.0% are inconclusive and require further testing. Also, per the Lyon Consensus, more than 80 reflux events is consistent with elevated reflux burden, below 40 reflux events is physiologic, and between 40 to 80 is inconclusive. 16 These values are basically levels of confidence, or in other words the amount of money you would wager, that the patient has underlying GERD.
Furthermore, reflux monitoring evaluates the association between reflux events and symptom perception. A positive symptom index (SI) (50% or more of a symptom are associated with a reflux event) and a positive symptom association probability (SAP) (>95%) are consistent with positive symptom-reflux association. When acid exposure and reflux events are within normal limits, a symptom-reflux association indicates esophageal hypersensitivity. 16
Esophageal hypersensitivity is symptom perception of heartburn/chest pain under non-pathologic reflux settings, and is a function of allodynia and hyperalgesia due to central and peripheral sensitization. As such, treatments to modulate neural perception such as behavioral interventions or low-dose antidepressants are therapies for esophageal hypersensitivity. 6 In contrast, patients with a positive symptom association for the perception of regurgitation on PPI therapy may have significant non-acid reflux burden and this is different than reflux hypersensitivity as it is most commonly due to overt defects in the antireflux barrier.
Therefore, for patients with unproven GERD reflux monitoring can classify reflux burden as non-erosive reflux disease with acid burden, reflux hypersensitivity, regurgitation of acid or non-acid refluxate, or absence of GERD.
Step 4: Esophageal function testing to identify reflux mechanism, characterize peristaltic clearance, and evaluate for alternate etiologies (River card)
Esophageal function testing with esophageal manometry with or without impedance-pH monitoring can provide adjunctive qualitative assessments in GERD. Esophageal manometry is another method to evaluate the anti-reflux barrier, and to additionally characterize the gastro-esophageal reflux mechanism. For instance, esophageal manometry can identify transient LES relaxations (TLESRs), prolonged relaxations in the LES associated with inhibition of the crural diaphragm that occur in response to gastric distention in the absence of a swallow. 17, 18 TLESRs are the primary mechanism of initiating reflux in the context of an intact anti-reflux barrier, and can be pharmacologically inhibited with GABA agonists.19–22 When the anti-reflux barrier is disrupted, esophageal manometry may identify pathologic reflux as a function of strain-induced reflux, free-reflux, or re-reflux of contents from a non-reducible hiatal hernia.23 In these cases pharmacologic treatment options are limited and may indicate the need for mechanical restoration of the crural diaphragm.
Manometric properties can also be assessed following a meal to evaluate for behavioral conditions such as rumination or supragastric belching. 24, 25 The sensitivity and specificity of high-resolution impedance manometry for diagnosis of rumination are 80% and 100%, and when the pre-test suspicion for rumination is high post-prandial testing on manometry can identify rumination in up to 20% of cases. 26, 27,17 Supragastric belching is identifiable on pH-impedance monitoring and high-resolution impedance manometry. 28
Further, esophageal manometry provides valuable assessment of peristaltic function and esophageal clearance properties, and helps to predict response to treatment and risk of post-treatment symptoms. For instance, a reduced distal contractile integral (less than 450mmHg-s-cm) in the majority of swallows will indicate a hypomotile esophageal motor condition, and an insufficient distal contractile integral following a multiple rapid swallow will indicated reduced peristaltic reserve. 29, 30 Hypomotile patterns have an increased risk of post-fundoplication dysphagia, and in these cases tailored fundoplication techniques or alternative anti-reflux procedures should be considered.
Therapeutic Strategies Personalized to GERD Phenotype
GERD is not one in the same, and as such, all treatments available to manage GERD are not appropriate across all patients. Therapeutic strategies should be personalized to the GERD phenotype. Below are examples of different scenarios of GERD patients. Through a step-wise phenotype approach, a personalized therapeutic strategy for the distinct GERD phenotype is established. Following this chapter, the next chapters will describe in detail therapeutic options for GERD options such as acid suppression, endoluminal interventions, and surgery.
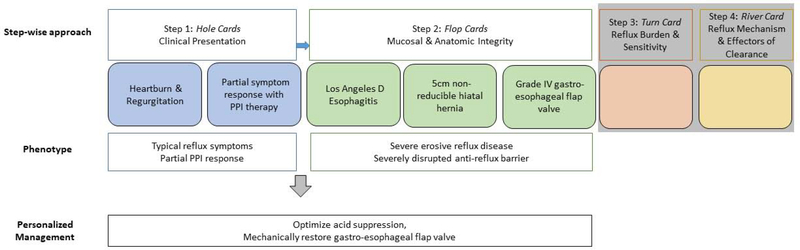
Example 1 (Figure 5A): A 65 year-old man is referred to clinic for:
Heartburn and regurgitation that responds partially to PPI therapy (hole cards)
- Undergoes upper GI endoscopy which reveals a 5cm non-reducible hiatal hernia with grade IV gastroesophageal flap valve and LA D esophagitis (flop cards)
- Thus, this patient’s phenotype is: typical reflux symptoms with partial PPI response, erosive reflux disease and a severely disrupted anti-reflux barrier. At this point you don’t need further diagnostic evaluation with reflux monitoring or esophageal function testing. In other words you can go all in without the turn or river card. Management should focus on optimizing acid suppression and mechanical restoration of the gastro-esophageal flap valve.
Figure 5. Step-wise phenotype approach for three patient examples (A, B, C).
Example 5A: A 65 year old man is referred for heartburn and regurgitation partially responsive to PPI (hole cards) and undergoes upper GI endoscopy which reveals a 5cm non-reducible hiatal hernia with grade IV gastroesophageal flap valve and LA D esophagitis (flop cards). This patient’s phenotype is clear at this point (typical reflux symptoms with partial PPI response, erosive reflux disease and a severely disrupted anti-reflux barrier), and thus phenotype guided management can proceed without further evaluation. Management in this case will hinge on optimizing acid suppression and mechanical restoration of the gastro-esophageal flap valve.
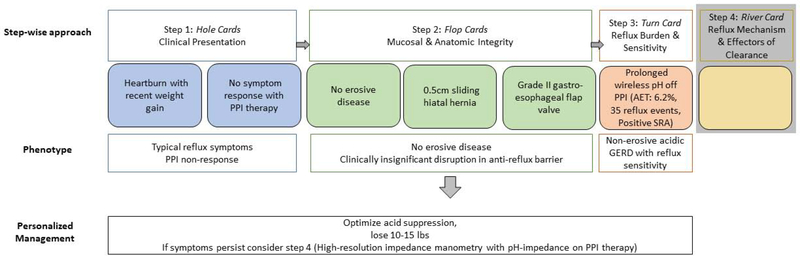
Example 5B: A 65-year old man with recent weight gain, heartburn, and no response to PPI therapy (hole cards) undergoes upper GI endoscopy which reveals normal esophageal mucosa, a 0.5cm sliding hiatal hernia and grade II gastroesophageal flap valve (flop cards). The GERD phenotype is not clear at this point, and the next step is ambulatory reflux monitoring. Since the patient has a low pre-test probability of GERD, the patient undergoes prolonged wireless pH monitoring off PPI therapy. The acid exposure time (AET) is elevated. Thus, this patient’s phenotype is: heartburn symptoms with recent weight gain, PPI non-responder with non-erosive acidic reflux disease. At this point you can attempt management without further diagnostic evaluation focused on weight loss and optimizing acid suppression. However, if symptoms persist further evaluation with esophageal manometry and impedance-pH monitoring on PPI (river card) would be warranted.
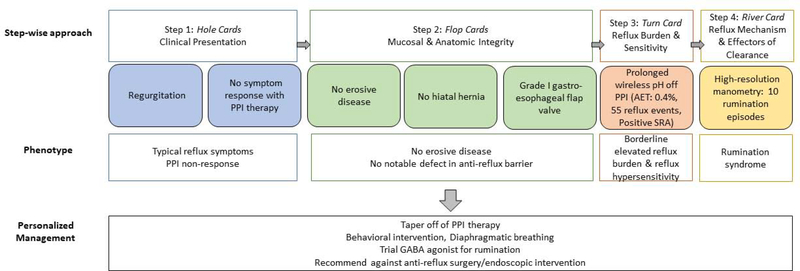
Example 5C: A 65-year old man is referred to clinic for regurgitation and no response to PPI therapy (hole cards) and undergoes upper GI endoscopy which reveals normal esophageal mucosa, no hiatal hernia, and grade I gastroesophageal flap valve (flop cards). The GERD phenotype is unclear and so the next step is ambulatory reflux monitoring, which demonstrates an inconclusive number of reflux events, normal acid exposure, and positive symptom-reflux association for regurgitation (SI 100%, SAP 99%). (turn card) Given inconclusive findings and suspicion for rumination the next step is esophageal physiologic testing with manometry and pH impedance on PPI therapy which uncovers rumination syndrome. At this point the phenotype is clear (PPI non-responder with rumination) and management will focus on behavioral intervention, diaphragmatic breathing and trial of GABA agonist. In this case you will refrain from referring for anti-reflux surgery.
Example 2 (Figure 5B): A 65-year old man is referred to clinic for:
Heartburn without response to PPI therapy and recent weight gain (hole cards)
Undergoes upper GI endoscopy which reveals normal esophageal mucosa, a 0.5cm sliding hiatal hernia and grade II gastroesophageal flap valve (flop cards)
- The next step is ambulatory reflux monitoring. Since the patient has a low pre-test probability of GERD, the patient undergoes prolonged wireless pH monitoring off PPI therapy. The acid exposure is 6.2%, 35 reflux events per day, and there is a positive symptom reflux association (SI 75% and SAP 98%) for 25 symptoms of heartburn.
- Thus, this patient’s phenotype is: heartburn symptoms with recent weight gain, PPI non-responder with non-erosive reflux disease likely acid predominant with component of reflux sensitivity. At this point you can attempt management without further diagnostic evaluation (bet high without river card), focused on weight loss and optimizing acid suppression. However, if the wager is whether or not to proceed with anitreflux surgery, obtaining high-resolution manometry (river card) at this point is extremely important as this may help guide decisions into the type of antireflux intervention used to treat the underlying GERD. Since the patient’s symptoms persist the patient undergoes esophageal manometry and impedance-pH monitoring on PPI (river card). In this case testing reveals frequent tLESRs with gastro-esophageal reflux events, and controlled acid exposure on PPI. Adjunctive management in this case could include the addition of tLESR inhibition.
Example 3 (Figure 5C): A 65-year old man is referred to clinic for:
Regurgitation and no response to PPI therapy (hole cards)
Undergoes upper GI endoscopy which reveals normal esophageal mucosa, no hiatal hernia, and grade I gastroesophageal flap valve (flop cards)
- The next step is ambulatory reflux monitoring, which demonstrates 55 reflux events, normal acid exposure, and positive symptom-reflux association for regurgitation (SI 100%, SAP 99%). (turn card)
- Thus, the phenotype at this point is PPI non-responder with regurgitation and borderline elevated reflux burden. You can add on a mucosal protective agent, but since you suspect rumination and given borderline testing you pursue additional testing.
- High-resolution esophageal manometry and impedance-pH monitoring on PPI are performed and reveal 10 rumination episodes during the 60 minute post-prandial testing period. (river card)
- Thus this patient is a PPI non-responder with rumination, and management will focus on behavioral intervention, diaphragmatic breathing and trial of GABA agonist. 31 In this case you will refrain from referring for anti-reflux surgery.
Summary
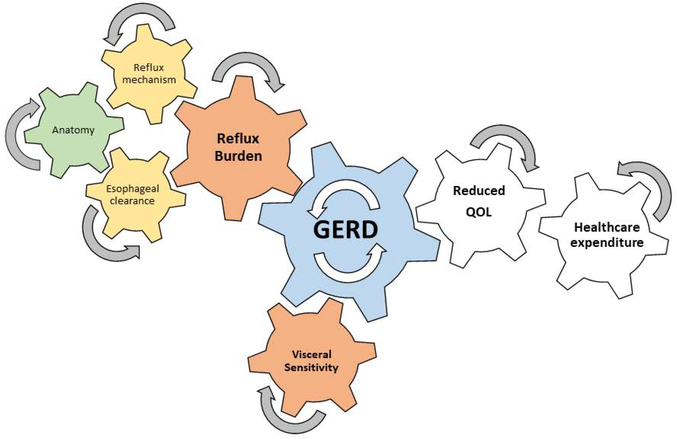
In summary, GERD is prevalent disorder and patients will present with heterogeneous phenotypes which require personalized treatment strategies. Personalization of treatment for GERD requires an understanding of the patient’s GERD phenotype. The framework to phenotype GERD hinges on a step-wise Bayesian evaluation method which minimizes risk and cost while maximizes phenotypic yield (Figure 2). The step-wise evaluation begins with characterization of symptom profile and response to PPI therapy. In cases of inadequate symptom response, the next step is endoscopic evaluation of mucosal and anatomic integrity. In the absence of erosive reflux disease and a severe disruption, the third step is ambulatory reflux monitoring, off PPI therapy in the case of unproven GERD, to characterize reflux burden and evaluate for esophageal hypersensitivity. At this juncture if the GERD phenotype remains unclear the next step is to perform esophageal function testing with high resolution impedance manometry with or without pH impedance testing to evaluate for mechanism of gastro-esophageal reflux event, peristaltic clearance from esophagus, and alternate sources of symptoms. Esophageal function testing is also a part of the pre-operative evaluation for anti-reflux surgery. Therapeutic strategies should be focused on the GERD phenotype, and begin with the least invasive and safest treatment options. Additionally, factors focused on visceral anxiety and hypervigilance need to be addressed as these features can effect symptom severity and healthcare utilization. These treat-to-mechanism phenotype approaches to GERD are critical to reducing the tremendous health care burden of GERD that is generated from a combination of these important anatomic, physiologic and psychologic biomarkers (Figure 6).
Figure 6.
Conceptual model of the interaction of the components of phenotyping in GERD that lead to reduced quality of life and increased healthcare utilization.
Synopsis.
Patients with gastroesophageal reflux disease (GERD) present with heterogeneous symptoms, response to treatment and physiologic profiles, requiring distinct and personalized management. This chapter provides a step-wise framework to phenotype GERD beginning with (1) characterization of symptom profile and response to acid suppression, (2) endoscopic evaluation of mucosal and anatomic integrity, (3) ambulatory reflux monitoring to characterize reflux burden and sensitivity, and (4) esophageal physiologic testing to assess gastro-esophageal reflux mechanism, effectors of reflux clearance and evaluate for alternate etiologies.
Key Points.
GERD is a prevalent disorder associated with tremendous quality of life impairment and healthcare expenditure
Patients with GERD present with heterogeneous phenotypes and require personalized diagnostic and treatment strategies
- A step-wise framework to phenotype GERD aims to optimize phenotypic yield while minimizing risk and cost
- Step 1 is to characterize symptom profile and response to PPI therapy
- Step 2 is indicated when symptom response is inadequate, patient presentation is atypical, and/or warning signs/symptoms are present. Step 2 is endoscopic evaluation for mucosal and anatomic integrity, and to assess for alternative diagnoses.
- Step 3 is indicated in the absence of erosive reflux disease or severe disruption of the anti-reflux barrier. Step 3 is ambulatory reflux monitoring, typically off acid suppression, to characterize reflux burden and evaluate for reflux hypersensitivity
- Step 4 is indicated if the GERD phenotype remains unclear. Step 4 is esophageal function testing with high-resolution impedance manometry with or without pH impedance testing to assess for reflux mechanism, peristaltic clearance, and alternate sources of symptoms
Acknowledgments
Research Support: Dr. Yadlapati is supported by the ACG Research Institute – Junior Faculty Development Award (Yadlapati). Dr. Yadlapati & Dr. Pandolfino are supported by the NIH R01DK092217-04A1 (Pandolfino)
Abbreviations
- GERD
Gastroesophageal reflux disease
- PPI
proton pump inhibitor
- LES
lower esophageal sphincter
- TLESRs
transient LES relaxations
- SI
symptom index
- SAP
symptom association probability
- AET
acid exposure time
- SRA
symptom reflux association
Footnotes
Disclosures: Dr. Pandolfino is a consultant for Crospon, Ironwood, Torax, Astra Zeneca, Takeda, Impleo, Medtronic, and Diversatek
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peery AF, Crockett SD, Barritt AS, et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015;149:1731–1741 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900–20; quiz 1943. [DOI] [PubMed] [Google Scholar]
- 3.de Bortoli N, Martinucci I, Savarino E, et al. Proton pump inhibitor responders who are not confirmed as GERD patients with impedance and pH monitoring: who are they? Neurogastroenterol Motil 2014;26:28–35. [DOI] [PubMed] [Google Scholar]
- 4.Bytzer P, Jones R, Vakil N, et al. Limited ability of the proton-pump inhibitor test to identify patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2012;10:1360–6. [DOI] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Boeckxstaens G. Failure of reflux inhibitors in clinical trials: bad drugs or wrong patients? Gut 2012;61:1501–9. [DOI] [PubMed] [Google Scholar]
- 6.Aziz Q, Fass R, Gyawali CP, et al. Functional Esophageal Disorders. Gastroenterology 2016. [DOI] [PubMed] [Google Scholar]
- 7.de Bortoli N, Nacci A, Savarino E, et al. How many cases of laryngopharyngeal reflux suspected by laryngoscopy are gastroesophageal reflux disease-related? World J Gastroenterol 2012;18:4363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang D, Ang TL, Teo EK, et al. Is impedance pH monitoring superior to the conventional 24-h pH meter in the evaluation of patients with laryngorespiratory symptoms suspected to be due to gastroesophageal reflux disease? J Dig Dis 2011;12:341–8. [DOI] [PubMed] [Google Scholar]
- 9.Lechien JR, Saussez S, Schindler A, et al. Clinical outcomes of laryngopharyngeal reflux treatment: A systematic review and meta-analysis. Laryngoscope 2019;129:1174–1187. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Wang H, Liu K. Meta-analysis of the efficacy of proton pump inhibitors for the symptoms of laryngopharyngeal reflux. Braz J Med Biol Res 2016;49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329. [DOI] [PubMed] [Google Scholar]
- 12.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill LD, Kozarek RA. The gastroesophageal flap valve. J Clin Gastroenterol 1999;28:194–7. [DOI] [PubMed] [Google Scholar]
- 14.Cheng FK, Albert DM, Maydonovitch CL, et al. Categorization of patients with reflux symptoms referred for pH and impedance testing while off therapy. Clin Gastroenterol Hepatol 2015;13:867–73. [DOI] [PubMed] [Google Scholar]
- 15.Park EY, Choi MG, Baeg M, et al. The value of early wireless esophageal pH monitoring in diagnosing functional heartburn in refractory gastroesophageal reflux disease. Dig Dis Sci 2013;58:2933–9. [DOI] [PubMed] [Google Scholar]
- 16.Roman S, Gyawali CP, Savarino E, et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil 2017;29:1–15. [DOI] [PubMed] [Google Scholar]
- 17.Kessing BF, Bredenoord AJ, Smout AJ. Objective manometric criteria for the rumination syndrome. Am J Gastroenterol 2014;109:52–9. [DOI] [PubMed] [Google Scholar]
- 18.Roman S, Holloway R, Keller J, et al. Validation of criteria for the definition of transient lower esophageal sphincter relaxations using high-resolution manometry. Neurogastroenterol Motil 2016. [DOI] [PubMed] [Google Scholar]
- 19.Ren LH, Chen WX, Qian LJ, et al. Addition of prokinetics to PPI therapy in gastroesophageal reflux disease: a meta-analysis. World J Gastroenterol 2014;20:2412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sifrim D, Castell D, Dent J, et al. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 2004;53:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roman S, Holloway R, Keller J, et al. Validation of criteria for the definition of transient lower esophageal sphincter relaxations using high-resolution manometry. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 22.Hillman L, Yadlapati R, Thuluvath AJ, et al. A review of medical therapy for proton pump inhibitor nonresponsive gastroesophageal reflux disease. Dis Esophagus 2017;30:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadlapati R, DeLay K. Proton Pump Inhibitor-Refractory Gastroesophageal Reflux Disease. Med Clin North Am 2019;103:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bravi I, Woodland P, Gill RS, et al. Increased prandial air swallowing and postprandial gas-liquid reflux among patients refractory to proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2013;11:784–9. [DOI] [PubMed] [Google Scholar]
- 25.Herregods TV, Troelstra M, Weijenborg PW, et al. Patients with refractory reflux symptoms often do not have GERD. Neurogastroenterol Motil 2015;27:1267–73. [DOI] [PubMed] [Google Scholar]
- 26.Rommel N, Tack J, Arts J, et al. Rumination or belching-regurgitation? Differential diagnosis using oesophageal impedance-manometry. Neurogastroenterol Motil 2010;22:e97–104. [DOI] [PubMed] [Google Scholar]
- 27.Yadlapati R, Tye M, Roman S, et al. Postprandial High-Resolution Impedance Manometry Identifies Mechanisms of Nonresponse to Proton Pump Inhibitors. Clin Gastroenterol Hepatol 2018;16:211–218 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessing BF, Bredenoord AJ, Velosa M, et al. Supragastric belches are the main determinants of troublesome belching symptoms in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2012;35:1073–9. [DOI] [PubMed] [Google Scholar]
- 29.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fornari F, Bravi I, Penagini R, et al. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil 2009;21:718–e41. [DOI] [PubMed] [Google Scholar]
- 31.Pauwels A, Broers C, Van Houtte B, et al. A Randomized Double-Blind, Placebo-Controlled, Cross-Over Study Using Baclofen in the Treatment of Rumination Syndrome. Am J Gastroenterol 2018;113:97–104. [DOI] [PubMed] [Google Scholar]