Abstract
The head−neck system of birds is a highly complex structure that performs a variety of demanding and competing tasks. Morphofunctional adaptations to feeding specializations have previously been identified in the head and neck, but performance is also influenced by other factors such as its phylogenetic history. In order to minimize the effects of this factor, we here analyzed the anatomy of three closely related vultures that distinctly differ in feeding strategy. Vultures, as obligate scavengers, have occupied a special ecological niche by exclusively feeding on carrion. However, competition among sympatric vultures led to ecological differences such as preference of certain types of food from a carcass. Via comparative dissections we systematically described the craniocervical anatomy in the Griffon vulture (Gyps fulvus), the Cinereous vulture (Aegypius monachus) and the Hooded vulture (Necrosyrtes monachus) that exploit the same food resources in different ways. Our results revealed differences in the number of cervical vertebrae, in the morphology of the atlas−axis complex as well as in the neck musculature despite overall similarities in the musculoskeletal system. Gulpers, rippers and scrappers adopt specific postures while feeding from a carcass, but the cervical vertebral column is indispensable to position the head during all kinds of behavior. The great range of demands may explain the conservation of the overall muscle topography of the neck across the studied taxa.
Keywords: birds, craniocervical anatomy, ecology, muscular adaptations, scavengers
Vultures, as obligate scavengers, have occupied a special ecological niche by exclusively feeding on carrion. Competition among sympatric taxa led to preference of certain types of food from a carcass. Via comparative dissections we systematically described the anatomy of the head−neck system in three sympatric species of vultures representing gulpers, rippers and scrappers. The overall muscle topography is conserved, but we found differences in the musculoskeletal system that may reflect functional adaptations to the specific feeding habits.
Introduction
Among extant vertebrates, vultures are the only obligate scavengers meaning that they strictly rely on carrion for their survival and reproduction (Ruxton & Houston, 2004; Dermody et al. 2011). They have evolved a highly specialized anatomy with a range of particular adaptations. In terms of feeding specializations, this generally includes a very sharp beak, a relatively bald head (and neck), very strong stomach acids for digestion of rotting and diseased meat, and feet adapted to locomotion on the ground rather than grasping as it is the case in other birds of prey (Fisher, 1946; Houston & Copsey, 1994; Hertel et al. 2015; Bright et al. 2016). Despite these commonalities, the 23 different species of vultures (Mundy et al. 1992; Thiollay, 1994) display very different phenotypes, which is thought to be linked to ecological differences. Competition among sympatric vultures led to differences in their feeding strategy, such as preference of certain types of food from a carcass (Kruuk, 1967; Hertel, 1994; O'Neal Campbell, 2016). Based on behavioral data, three feeding groups have been previously identified (Kruuk, 1967; Houston, 1988). ‘Gulpers’ feed primarily on the softer viscera, and include most species of griffons (Gyps sp.) as well as a few other species such as the American black vulture (Coragyps atratus). In contrast, ‘rippers’ feed primarily on the tough skin and hide of a carcass: they include large vultures such as the Cinereous vulture (Aegypius monachus) and the Red‐headed vulture (Sarcogyps calvus) or the King vulture (Sarcoramphus papa). ‘Scrappers’ (or ‘peckers’) feed primarily on small scraps on and around the carcass. This group involves the Egyptian vulture (Neophron percnopterus) and the Hooded vulture (Necrosyrtes monachus). These differences in feeding ecology have been related to differences in size and shape of their beak, skull and mandible (Hertel, 1994), and are linked to differences in head movements. However, movements of the head are executed around several or even all joints of the cervical vertebral column (Berthoz et al. 1992) and, to date, we lack knowledge on the detailed muscular anatomy of the neck in vultures.
The avian neck comprises a large number of bones and articulations in the cervical vertebral column that make it an anatomically highly complex structure. Additionally, there is a wide range of variation in the length of the neck and the number of cervical vertebrae (10–26 in extant birds; Böhmer et al. 2019). Approximately 200 muscle slips are active during positioning of the head−neck system (Zweers et al. 1987). Some muscles are rather long, spanning (almost) the entire length of the neck, whereas other muscles are short attaching between successive vertebrae within the cervical vertebral column. Previous studies investigating qualitative differences in the neck musculature revealed that the muscles most directly involved in vital actions such as the feeding process show marked adaptations (Boas, 1929; Zusi, 1962). A general observation was that these neck muscles are hypertrophied and have greater attachment surfaces and leverage that are active during prevalent movements of the head−neck system (e.g. well‐developed dorsal neck muscles that help to stabilize the neck during prey striking in the Black Skimmer; Zusi, 1962). We would, thus, expect that adaptations in the neck musculature correspond to specialized feeding habits in birds. The aim of the present study is to test the hypothesis that the specific feeding habits in vultures are reflected in the cervical musculoskeletal system. In order to reveal the functional adaptations in the avian neck, we investigate the anatomy of the neck in three sympatric species of vultures representing gulpers, rippers and scrappers.
Materials and methods
Study specimens
The musculoskeletal system of the neck in three accipitrid species representing each scavenging group was analyzed via comparative dissections: Gyps fulvus (N = 2) as ‘gulper’; A. monachus (N = 2) as ‘ripper’; and N. monachus (N = 1) as ‘scrapper’ (Table 1). The carcasses were received from the animal care center of the Goupil Association. The specimens were adult animals, did not display any pathologies, and died a natural death. Before dissection we obtained the number of cervical vertebrae for each species by using X‐ray imaging (Philips Diagnost C generator, 4 kV, 5 mA, 25 mS). Next, the head and neck were scanned in situ with surrounding soft‐tissue using micro‐computer tomography (µCT; RX Solutions EasyTom Micro, 80 kV). The 3D models of the vertebrae were reconstructed from the µCT scans using the software Avizo (Version 6.3, Visualization Science Group) and the software MeshLab (ISTI‐CNR, Pisa).
Table 1.
Studied taxa
| Taxon | Common name | Mean body mass (kg) | Ecological category | #CV |
|---|---|---|---|---|
| Gyps fulvus | Griffon vulture | 7.4 | Gulper | 15 |
| Aegypius monachus | Cinereous vulture | 9.3 | Ripper | 13 |
| Necrosyrtes monachus | Hooded vulture | 2.0 | Scrapper | 13 |
All three vultures belong to the family Accipitridae. The mean body mass is taken from Wilman et al. (2014), and the assignment to the respective ecological category is based on Kruuk (1967), Houston (1988) and Hertel (1994) (refer to Materials and methods for a more detailed description).
#CV, number of cervical vertebrae.
There are potential differences in cervical count in the literature that depend on the criteria for assigning the cervicothoracic transition. Birds have ribs that are completely fused to the cervical vertebrae (cervical ribs). Following Böhmer et al. (2019), we identified the cervical count as the number of all vertebrae cranial to the first thoracic vertebra, which is defined as having articular facets for freely movable ribs (Romer, 1976). By contrast, Baumel et al. (1993) consider the first thoracic vertebra as the cranial‐most vertebra with a complete rib that articulates with the sternum. Therefore, the caudal cervical vertebrae may bear movable ribs, not reaching the sternum. However, the latter definition makes it more complicated to include fossil specimens in future analyses because the preservation may not allow identifying if the rib reached the sternum or not.
Dissection and documentation
After removing feathers, skin and fascia, each muscle of the neck was identified and systematically dissected by carefully detaching it from both the origin and insertion (Table 2). The origin of a neck muscle is its caudal attachment point, which does not move during contraction. The insertion of a neck muscle is its cranial attachment point, which moves during contraction. All muscles are present in both the left and right sides of the neck (bilateral organization). The vertebra of muscle attachment was identified by palpation and using the previously obtained X‐ray images. Muscle attachment sites were noted as they bear significantly on the biomechanical roles of the muscles. We also assessed the presence and the ability of osteological correlates to identify muscle attachment and insertion sites, which has implications for the ability to reconstruct soft‐tissue in extinct taxa. Fiber architecture was recorded as well and photographs were taken at each stage of the dissection. These images were used as reference for illustrations of the muscle topography.
Table 2.
Summary of head and neck muscles
| Muscle | Synonym | Origin | Insertion | Main function |
|---|---|---|---|---|
| Craniocervical system | ||||
| M. complexus | M. cucullaris, caput part | Dorsolateral on cranial CV | Dorsal on occiput (skull) | Dorsolateral flexor |
| M. splenius capitis (pars medialis and pars lateralis) | M. rectus capitis posticus major | Dorsal on Atlas‐axis complex (first two CV) | Dorsal on occiput (skull) | Dorsal flexor and rotator |
| M. rectus capitis ventralis | M. flexor capitis inferior; M. rectus capitis anticus lateralis | Ventral on cranial CV | Ventral on occiput (skull) | Ventrolateral flexor |
| M. rectus capitis dorsalis | M. trachelomastoideus; M. rectus capitis superior | Lateral on cranial CV | Ventral on occiput (skull) | Ventral flexor |
| M. rectus capitis lateralis | M. rectus capitis anticus minor; M. rotator capiti | Ventral on cranial CV | Lateral on occiput (skull) | Lateral flexor and rotator |
| Dorsal system | ||||
| M. biventer cervicis | – | Dorsal on last CV and first TV | Dorsal on occiput (skull) | Dorsal flexor |
| M. longus colli dorsalis, pars cranialis | M. longus colli posticus; M. splenius colli; M. longus colli posticus, pars anterior | Lateral on cranial CV | Dorsal on axis (second cervical vertebra) | Dorsolateral flexor |
| M. longus colli dorsalis, pars caudalis | M. spinalis cervicis, M. spinalis, M. longus colli posticus, pars posterior | Dorsal on last CV and first TV | Dorsal on intermediate CV | Dorsolateral flexor |
| M. longus colli dorsalis, pars profunda | M. longus colli posticus; Mm. dorsales pygmaei; M. profundus colli posticus | Dorsolateral on intermediate CV | Dorsal on intermediate CV | Dorsal flexor |
| Mm. ascendentes cervicales | M. obliquus colli; M. cervicalis ascendens | Lateral on intermediate and caudal CV | Dorsolateral on intermediate and caudal CV | Dorsal flexor and rotator |
| Ventral system | ||||
| M. longus colli ventralis | M. longus colli; M. longus colli anticus | Ventral on cranial TV | Ventrolateral on all CV (except the first two CV) | Ventrolateral flexor |
| Intervertebral system | ||||
| Mm. interspinales | − | Dorsal on cranial CV | Dorsal on cranial CV | Dorsal flexor |
| Mm. intercristales | Mm. obliquotransversales | Dorsolateral on intermediate and caudal CV | Dorsolateral on intermediate and caudal CV | Dorsal flexor |
| Mm. intertransversarii (including Mm. inclusi) | Mm. intertransversales; M. colli lateralis | Lateral on all CV (except the first two CV) | Lateral on all CV (except the first CV) | Lateral flexor |
The origin, insertion and main function of each muscle are given in a general manner in order to summarize the musculoskeletal arrangement. Detailed descriptions including interspecific differences are provided in the main text. Anatomical nomenclature and description follows primarily Boas (1929) and Baumel et al. (1993). Synonyms of muscles are given as well (refer to the main text for references).
CV, cervical vertebra; M., Musculus; TV, thoracic vertebra.
The function of a muscle can generally be inferred to some extent from the study of structure. We deduced the main function of each muscle using elementary mechanical principles, and complemented our observations with previous descriptions and physiological studies. Following Zweers et al. (1987) and Davies & Green (1994), the muscular system of the cervical vertebral column in birds can be distinguished into four main units based on their topology and major function: (1) the craniocervical system; (ii) the dorsal system; (iii) the ventral system; and (iv) the intervertebral system.
Anatomical terminology followed primarily Boas (1929) and Baumel et al. (1993). Osteological structures are described with English equivalents of the Latin terms. The Latin terminology is retained for muscles and ligaments. Synonyms of the analyzed cervical muscles are provided in the text and summarized in Table 2 in order to facilitate comparisons with previous works on the avian neck.
Results
We did not observe any distinct intraspecific differences. In the following, we present the results principally referring to the Griffon vulture (G. fulvus) and indicating differences to the Cinereous vulture (A. monachus), where present. Our analysis revealed that the Hooded vulture (N. monachus) is very similar in its musculoskeletal anatomy to A. monachus, but differences are presented as well.
Osteology
The number of cervical vertebrae is 15 in G. fulvus, and 13 in A. monachus and N. monachus (Table 1). Apart from some differences in size, the general morphology of the cervical vertebrae is similar in all three vultures except for the first and second cervical vertebra. Correspondingly to the previously observed differences in skull shape (Hertel, 1994), the atlas−axis complex is significantly different between G. fulvus and A. monachus (Fig. 1). The morphology of the first two cervical vertebrae of N. monachus globally resembles that of A. monachus.
Figure 1.
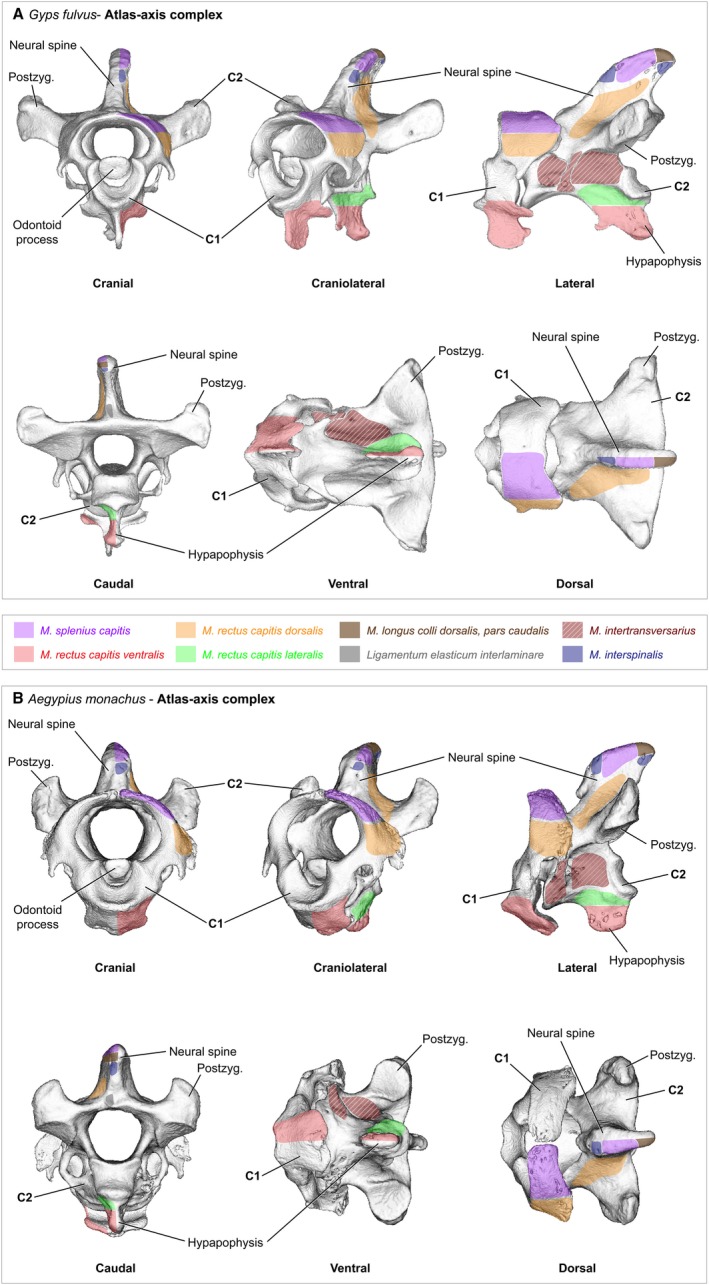
Cervical vertebrae – atlas−axis complex. The first and second cervical vertebra of (A) Gyps fulvus, (B) Aegypius monachus. The two vertebrae were scanned in situ with surrounding soft‐tissue. The 3D models were reconstructed from the micro‐computer tomography (μCT) scans using the software Avizo (Version 6.3, Visualization Science Group) and the software MeshLab (ISTI‐CNR, Pisa). The areas of origin and insertion of the neck muscles are shown only for the left side of the bones. The muscles are labeled in color and the bone structures are labeled in black
The long and rather narrow skull of G. fulvus ends in a ball‐shaped occipital condyle that perfectly fits into the socket‐shaped depression of the atlas (cervical vertebra 1, CV1; Figs 1A and 15A). The first two cervical vertebrae are rather long and narrow. CV1 bears a distinct ventral process as well as laterocaudally projecting transverse processes (Fig. 1A). In lateral view, the neural spine of CV2 is relatively slender. The distal tip of the ventral process of CV2 points caudally in lateral and ventral view (Fig. 1A). The short and very broad skull of A. monachus has a rounded but rather rectangular shape that articulates with the atlas (Figs 1B and 15B). The first two cervical vertebrae are relatively short and broad. In contrast to G. fulvus, the CV1 of A. monachus does not bear a distinct ventral process, but is rather smooth (Fig. 1B). In lateral view, the neural spine of CV2 is very broad. The ventral process of CV2 projects ventrally in lateral view (Fig. 1B).
Figure 15.
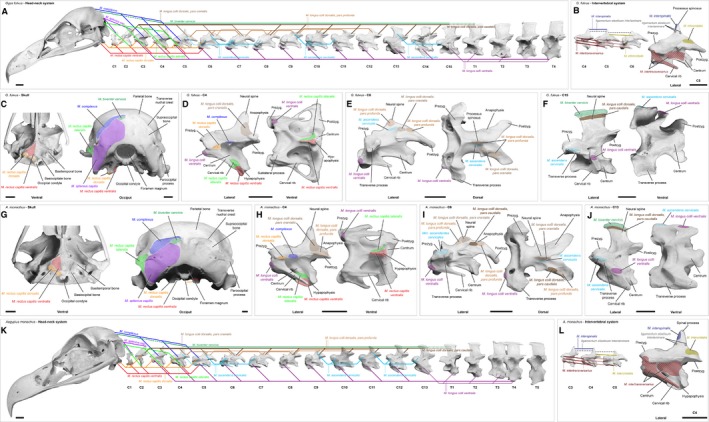
Musculoskeletal system of the head and neck in vultures. Diagram summarizing the musculature of the head−neck system of (A−F) Gyps fulvus and (G−L) Aegypius monachus. The muscles are shown as solid lines, whereas tendons are given as dashed lines. The pointed lines indicate the aponeurosis of the notarium. Note that the M. longus colli dorsalis, pars profunda and the smaller intervertebral muscles are displayed in a simplified manner with only some representative muscle slips. Bottom: detailed view of the skull and representative cervical vertebrae showing the areas of origin and insertion of the neck muscles. The muscles are labeled in color, the ligament in gray (dashed lines) and the bone structures are labeled in black. The color coding indicates the different muscles and corresponds to the colors used in all previous figures
Muscular anatomy
The craniocervical group includes muscles that insert around the foramen magnum on the occipital region of the skull. They position the head relative to the cranial region of the neck. The muscles of the dorsal group are relatively long, and move the head and neck in the dorsal direction. The ventral group is composed of one large, multi‐slip muscle (Musculus longus colli ventralis) that flexes the neck in the ventral direction. The intervertebral group includes a series of mono‐articular muscles that serve to stabilize the cervical vertebral column and that control motion in dorsal and lateral directions.
Craniocervical system
The craniocervical system forms the connection between the head and the cranial part of the neck. Five muscles contribute to the stabilization and motion of the head relative to the neck.
Musculus complexus
Synonymy: M. cucullaris, caput part (Fisher & Goodman, 1955).
This relatively broad muscle lies superficially on the dorsal side of the neck and inserts on the skull (Fig. 2) in all three vultures. The fibers run parallel to the muscle’s long axis. The area of insertion on the occiput is more pronounced in A. monachus compared with G. fulvus (Fig. 15) and N. monachus. In G. fulvus, the right and left M. complexus lie relatively closely to each other and overlie completely the cranial part of the M. biventer cervicis (Fig. 2A). In contrast, the two muscle parts are slightly more separated from each other by connective tissue in A. monachus (Fig. 2B) and N. monachus. The M. complexus appears to have a more inclined position in A. monachus. The muscle originates with a muscle slip at CV3, CV4 and CV5 (Fig. 2C and 2) in all three vultures.
Figure 2.
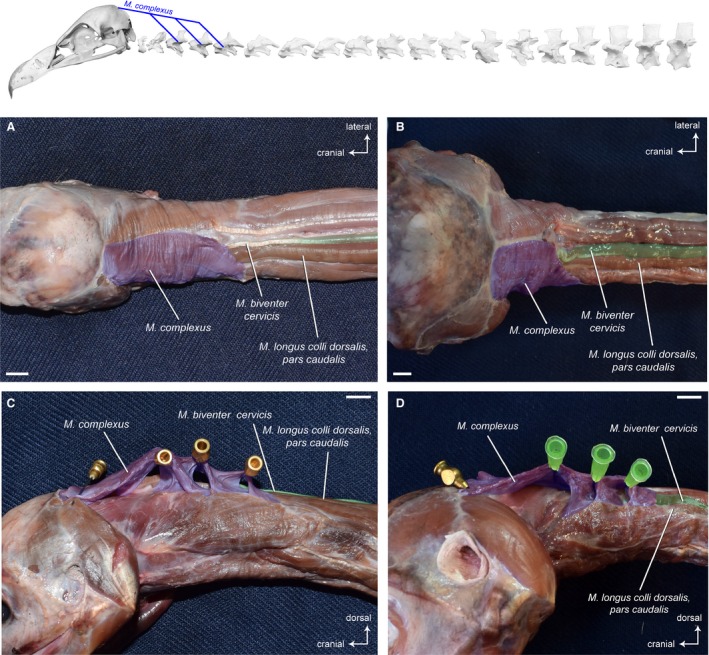
Craniocervical system – dorsal, superficial muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology. The M. complexus in dorsal view (A) Gyps fulvus, (B) Aegypius monachus, and lateroventral view (C) Gyps fulvus, (D) Aegypius monachus. In both vultures, the muscle originates with a muscle slip at cervical vertebrae 3, 4 and 5. and inserts into the supraoccipital bone at the transverse nuchal crest of the skull. Scale bar: 1 cm
Osteological correlates for the origin of the M. complexus are not developed. On the skull, the distinct depression at the transverse nuchal crest is an osteological correlate marking the insertion of the muscle.
Origin: Transverse processes of CV3−CV5.
Insertion: Supraoccipital bone at the transverse nuchal crest of the skull (dorsal to the insertion of the M. biventer cervicis).
Function: The M. complexus raises the head and the cranial region of the neck with respect to the rest of the vertebral column. During unilateral contraction it also contributes to flexion of the head in the lateral direction. Furthermore, the results of an electromyographic (EMG) study indicate that the muscle functions to cause roll of the head (Snively et al. 2014). The M. complexus is also called ‘hatching’ muscle because it plays an important role in the hatching process. Around the time of hatching, the muscle undergoes transient hypertrophy. It forces the egg tooth against the shell and, thus, facilitates the breaking of the shell (Keibel, 1914; Smail, 1964).
Musculus splenius capitis (pars medialis and pars lateralis).
Synonymy: M. rectus capitis posticus major (Shufeldt, 1890; Kaupp, 1918).
This rather short, triangular‐shaped muscle that lies deep to the M. complexus and M. biventer cervicis connects the skull with the first two cervical vertebrae (Fig. 3) in all three vultures. The fiber arrangement in the muscle is bipennate. In all three vultures, a medial and a lateral part are easily distinguishable. Near the origin, the right and left M. splenius capitis are closely connected along the sagittal plane.
Figure 3.
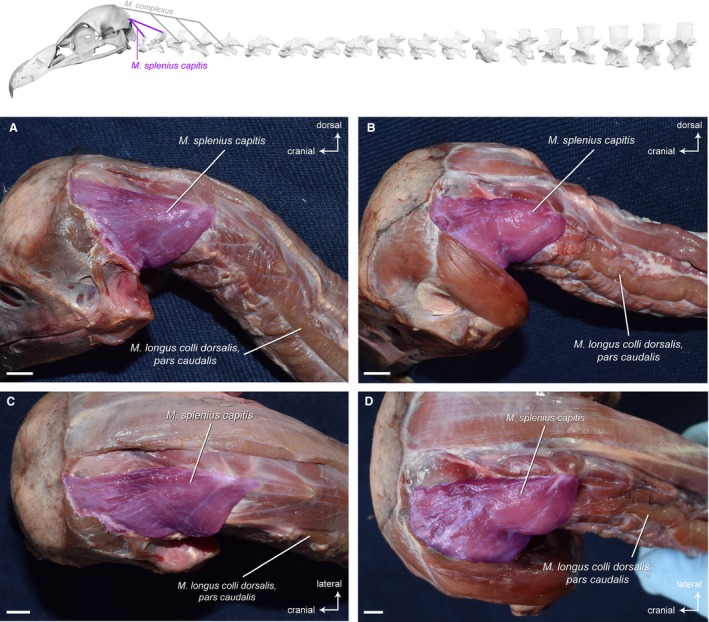
Craniocervical system – dorsal deep muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscle is highlighted in color). The M. splenius capitis (pars medialis and pars lateralis) in dorsolateral view (A) Gyps fulvus, (B) Aegypius monachus, and dorsal view (C) Gyps fulvus, (D) Aegypius monachus. In both vultures, the muscle originates from the neural spines of the cervical vertebrae 1 and 2, and inserts into the supraoccipital bone ventral to the transverse nuchal crest of the skull. Scale bar: 1 cm
Osteological correlates for the origin of the M. splenius capitis, such as a marked depression on the neural spines of the vertebrae, are not developed. On the skull, there are scars marking the ventrolateral extent of the muscle’s insertion site.
Origin: Neural spines of CV1 and CV2.
Insertion: Supraoccipital bone ventral to the transverse nuchal crest of the skull (ventral to the insertion of the M. biventer cervicis).
Function: The M. splenius capitis raises the head and provides lateral stability for the head. It also contributes to tilting of the head.
Musculus rectus capitis ventralis
Synonymy: M. flexor capitis inferior (Shufeldt, 1890; Kaupp, 1918); M. rectus capitis anticus lateralis (Kuroda, 1962).
This relatively long, triangular‐shaped muscle covers the cranial, ventral side of the neck (Fig. 4). It originates from the ventral processes of the first six cervical vertebrae in G. fulvus (Fig. 15A), and of the first five cervical vertebrae in A. monachus (Fig. 15K) and N. monachus. The insertion of the M. rectus capitis ventralis is on the entire ventral surface of the basitemporal plate. In all three vultures, the medial part of the muscle inserts fleshy on the base of the skull, whereas the lateral part inserts tendinously on the basitemporal plate (Fig. 4). The tendon of the lateral part of the M. rectus capitis ventralis is well developed in all three vultures, but appears slightly stronger in A. monachus. The right and left M. rectus capitis ventralis are fused at the sagittal plane and lie between the branches of the carotid artery. At the origin of the muscle, there is some degree of fusion with the M. longus colli ventralis at CV6 and CV5, respectively (the most caudal extent of the muscle). Although the muscle forms a compact muscle mass, several muscle slips each originating from a cervical vertebra are partially identifiable (Fig. 4). The fibers in the unipennate muscle diverge from their origins in a fanlike configuration towards the skull.
Figure 4.
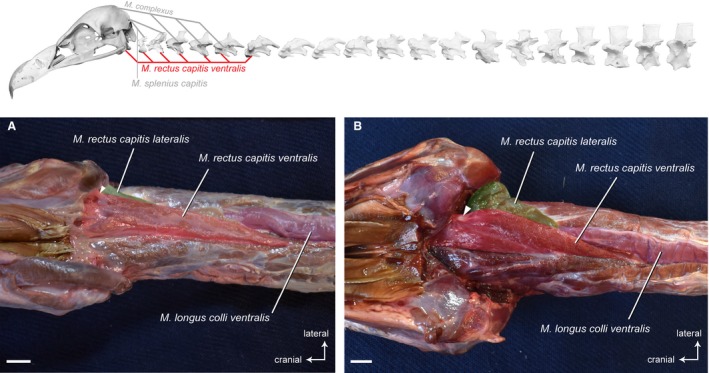
Craniocervical system – ventral muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscle is highlighted in color). The M. rectus capitis ventralis in ventral view (A) Gyps fulvus, (B) Aegypius monachus. The muscle originates from the ventral processes of the cervical vertebrae 1−6 in G. fulvus, and of the cervical vertebrae 1−5 in A. monachus. It inserts into the basioccipital bone of the skull in both vultures. The white rectangle indicates the tendon of the lateral part of the M. rectus capitis ventralis. It inserts laterally into the basitemporal plate. Scale bar: 1 cm
Osteological correlates for the origin of the M. rectus capitis ventralis are present in the form of the ventral processes (hypapophyses) on the cervical vertebrae. On the skull, the basitemporal plate is the osteological correlate indicating the insertion of the muscle.
Origin: Hypapophyses and sublateral processes of CV1−CV6 in G. fulvus; of CV1−CV5 in A. monachus and N. monachus (together with the M. rectus capitis lateralis).
Insertion: Basitemporal bone of the skull (medial and ventral to the insertion of the M. rectus capitis dorsalis).
Function: The M. rectus capitis ventralis flexes the head and the cranial region of the neck in the ventral direction. During unilateral contraction, it also contributes to turning the head in the lateral direction.
Musculus rectus capitis dorsalis
Synonymy: M. trachelomastoideus (Shufeldt, 1890; Kaupp, 1918; Fujioka, 1963); M. rectus capitis superior (Boas, 1929; Fisher & Goodman, 1955; Zusi & Storer, 1969).
The M. rectus capitis dorsalis lies ventrally to the M. complexus on the lateral side of the neck. It comprises several muscle slips that originate dorsolaterally from the first six cervical vertebrae in G. fulvus, and from the first five cervical vertebrae in A. monachus and N. monachus (Fig. 5). The slips of the unipennate muscle merge into a stout muscle belly that inserts mainly tendinously into the skull in all three vultures. After removing the M. rectus capitis ventralis, the insertion area is clearly visible. There is one very well‐developed tendon that inserts into a prominent knob of the basitemporal plate, and another well‐developed tendon that inserts into the basioccipital bone near the occipital condyle.
Figure 5.
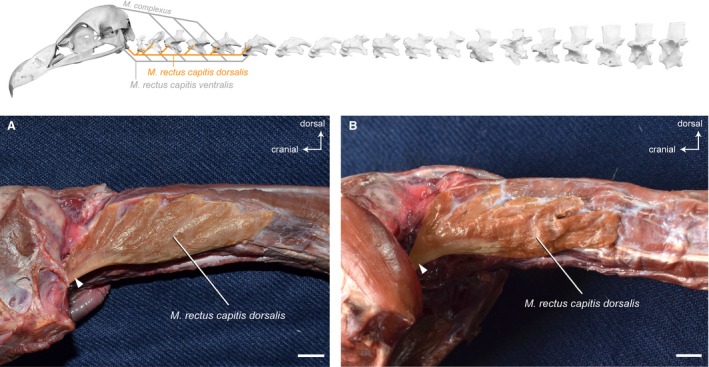
Craniocervical system – ventrolateral muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscle is highlighted in color). The M. rectus capitis dorsalis in lateral view (A) Gyps fulvus, (B) Aegypius monachus. The muscle originates dorsolaterally from the cervical vertebrae 1−6 in G. fulvus, and of the cervical vertebrae 1−5 in A. monachus. It inserts with two tendons into the basitemporal plate and the basioccipital bone of the skull in both vultures. The white rectangle indicates the tendon that inserts into a prominent knob of the basitemporal plate. Scale bar: 1 cm
There are no distinct osteological correlates marking the origin of the M. rectus capitis dorsalis. On the skull, the scars on the basioccipital and basitemporal bone mark the caudal extent of the muscle’s insertion site.
Origin: Lateral and on the transverse processes of CV1−CV6 in G. fulvus; of CV1−CV5 in A. monachus; of CV1−CV5 in N. monachus.
Insertion: Basioccipital and basitemporal bone of the skull (lateral and dorsal to the insertion of the M. rectus capitis ventralis).
Function: The M. rectus capitis dorsalis flexes the head and the cranial region of the neck in the ventral direction.
Musculus rectus capitis lateralis
Synonymy: M. rectus capitis anticus minor (Shufeldt, 1890; Kaupp, 1918); M. rotator capitis (Kuroda, 1962).
This strap‐like, parallel‐fibered muscle lies between the M. rectus capitis dorsalis and the M. rectus capitis ventralis on the ventrolateral side of the neck. After removing the M. rectus capitis ventralis, the area of origin is clearly visible. It originates ventrolaterally from the ventral processes of the first four postatlantal vertebrae in G. fulvus, and from the first three postatlantal vertebrae in A. monachus (Fig. 6) and N. monachus. The M. rectus capitis lateralis inserts fleshy into the supraoccipital bone extending on the paroccipital process (Fig. 15).
Figure 6.
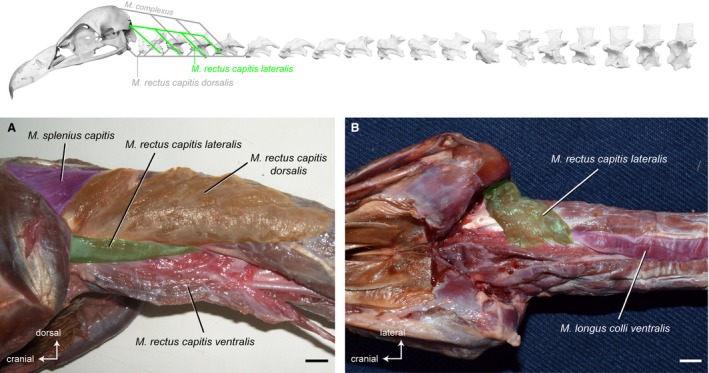
Craniocervical system – ventrolateral muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscle is highlighted in color). The M. rectus capitis lateralis in ventrolateral view (A) Gyps fulvus. The M. rectus capitis ventralis is deflected medially. The M. rectus capitis lateralis in ventral view (B) Aegypius monachus. The M. rectus capitis ventralis has been removed to reveal the origin of the M. rectus capitis lateralis. The muscle originates ventrolaterally from the cervical vertebrae 2−5 in G. fulvus, and of the cervical vertebrae 2−4 in A. monachus. It inserts fleshy into the supraoccipital bone and the paroccipital process of the skull in both vultures. Scale bar: 1 cm
There are no distinct osteological correlates marking the origin of the M. rectus capitis lateralis. On the skull, the paroccipital process marks the lateral extent of the muscle’s insertion site.
Origin: Lateral on the hypapophyses of CV2−CV5 in G. fulvus; of CV2−CV4 in A. monachus and N. monachus.
Insertion: Supraoccipital bone and paroccipital process (lateral from M. splenius capitis).
Function: The M. rectus capitis lateralis raises the head and provides lateral stability for the head. It turns the head and the cranial region of the neck in lateral direction. The muscle also rotates the head about its axis.
Dorsal system
The dorsal system includes multi‐slip muscles that pass the entire length of the neck as well as intervertebral muscles that pass only one or two segments but are interconnected to each other. They position the neck relative to the trunk and move the neck in the dorsal direction.
Musculus biventer cervicis
Synonymy: Cranially covered by the M. complexus, the M. biventer cervicis is a superficial, parallel‐fibered muscle. It extends the entire length of the neck lateral to the sagittal line. The muscle consists of a cranial and a caudal muscle belly that are connected via a relatively long tendon (Fig. 7A and 7). The M. biventer cervicis is rather slender, but relatively broader in A. monachus and N. monachus as compared with G. fulvus (Fig. 7). It is the only muscle of the cervical vertebral column that connects the notarium (formed by the fusion of the first thoracic vertebrae, TV) with the cranium (Baumel et al. 1993). In contrast to G. fulvus in which the muscle inserts tendinously onto the skull (Fig. 7C), the insertion of the M. biventer cervicis in A. monachus (Fig. 7D) and N. monachus is fleshy. It is also larger in A. monachus, which may highlight the relative importance of this muscle in this taxon. Correspondingly, the area of insertion on the occiput is more pronounced in A. monachus compared with G. fulvus (Fig. 15). The proportional lengths of the cranial and caudal part and the tendon in between are more or less equivalent in all three vultures. On average, the cranial part comprises about 22% of the total length of the M. biventer cervicis, the tendon about 15% and the caudal part about 63% (Table 3).
Figure 7.
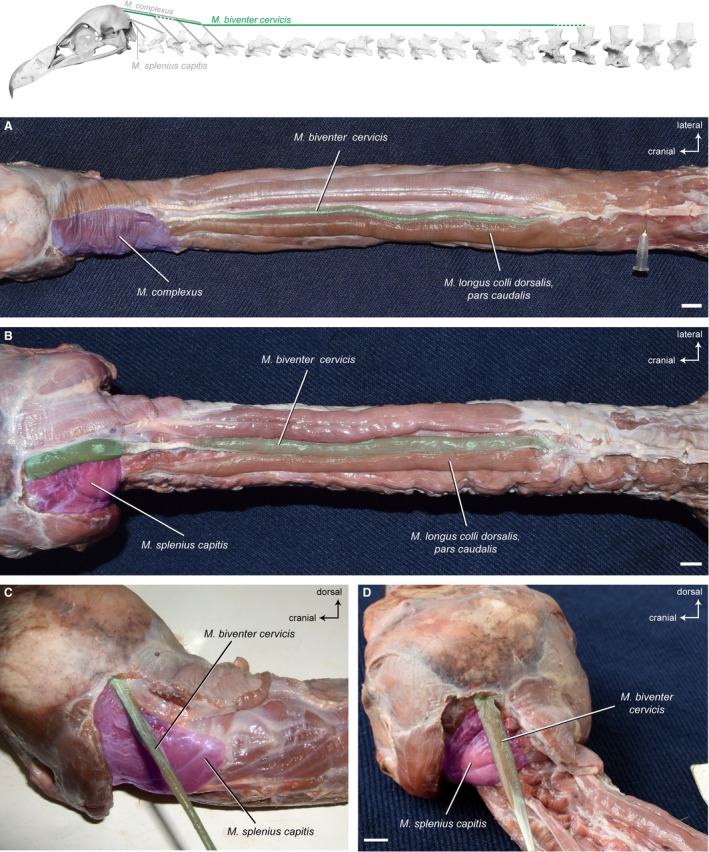
Dorsal system – superficial muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscle is highlighted in color). The M. biventer cervicis in dorsal view (A) Gyps fulvus, (B) Aegypius monachus. The muscle originates dorsally from the aponeurosis of the notarium (neural spine of the last cervical vertebra and first thoracic vertebra) in both vultures and inserts onto the occiput (ventral to the M. complexus). A cranial and a caudal fleshy part are connected to each other by a tendon. The M. biventer cervicis, pars cranialis in dorsolateral view (C) G. fulvus, (D) A. monachus. It inserts tendinously onto the skull in G. fulvus, whereas the insertion in A. monachus is fleshy. The M. complexus has been removed to reveal the area of insertion of the M. biventer cervicis. Scale bar: 1 cm
Table 3.
Muscle−tendon proportions
| Taxon | Pars cranialis | Tendon | Pars caudalis | |||
|---|---|---|---|---|---|---|
| Gyps fulvus #1 | 6.9 cm | 24% | 3.6 cm | 13% | 17.8 cm | 63% |
| Gyps fulvus #2 | 5.2 cm | 22% | 3.5 cm | 15% | 15.0 cm | 63% |
| Aegypius monachus #1 | 3.7 cm | 24% | 2.4 cm | 15% | 9.7 cm | 61% |
| Aegypius monachus #2 | 3.6 cm | 17% | 3.1 cm | 15% | 14.0 cm | 68% |
| Necrosyrtes monachus | 3.0 cm | 21% | 2.8 cm | 19% | 8.7 cm | 60% |
Proportional lengths of the cranial and caudal part of the M. biventer cervicis and the tendon in between the muscle bellies in all three vulture species.
There are no distinct osteological correlates marking the origin of the M. biventer cervicis because it originates from fibrous tissue of the notarium. On the skull, the depression at the transverse nuchal crest (ventromedial to the insertion of the M. complexus) marks the muscle’s insertion.
Origin: Aponeurosis (plate‐like fibrous tissue) of the notarium (in particular on neural spine of last CV and TV1); dorsal to origin of M. longus colli dorsalis, pars caudalis.
Insertion: Transverse nuchal crest (on the skull).
Function: The M. biventer cervicis flexes the head and neck in the dorsal direction. It straightens the cranial region of the neck. The muscle raises the cranial region of the neck on the intermediate region and the intermediate region of the neck on the caudal region.
Musculus longus colli dorsalis
Ventral to the M. biventer cervicis, the M. longus colli dorsalis covers the entire dorsal length of the neck (Fig. 8A). Generally, it forms the major part of the dorsal muscular system and can be divided into three parts based on topological differences: a cranial part (pars cranialis); a caudal part (pars caudalis); and a profound part (pars profunda). Shufeldt (1890) calls the muscle M. longus colli posticus and does not give separate names for the different parts.
Figure 8.
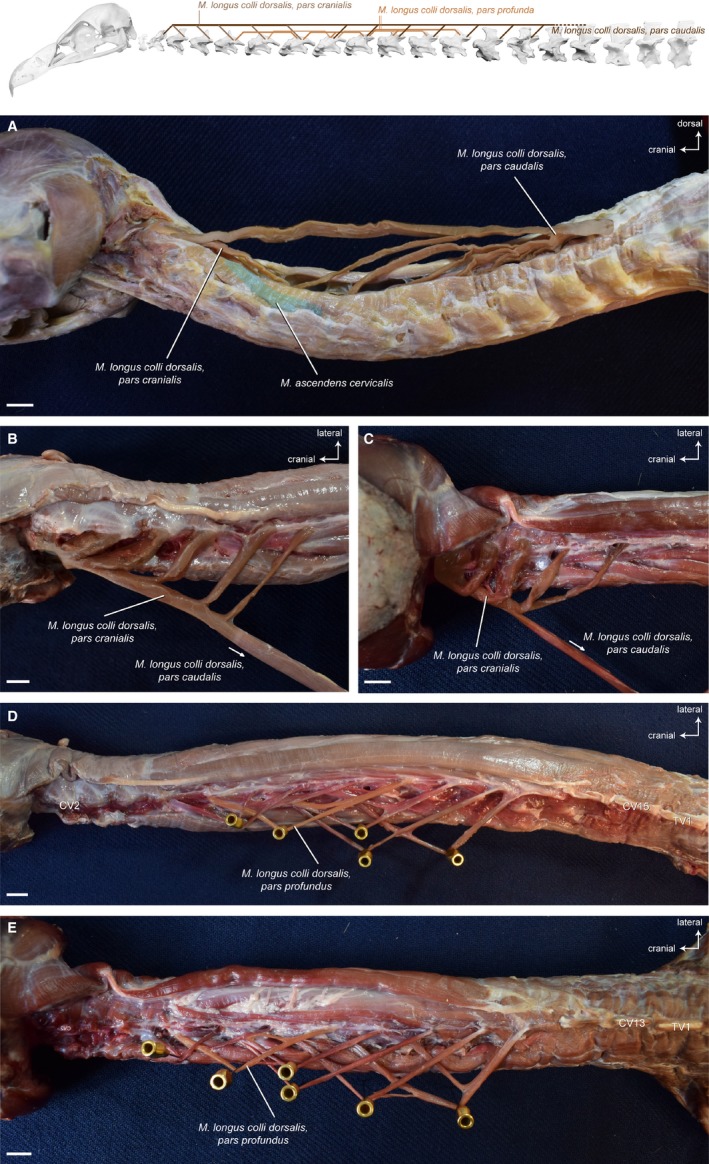
Dorsal system – deep muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscle is highlighted in color). The M. longus colli dorsalis consists of a cranial, a caudal and a profound part. The M. longus colli dorsalis, pars caudalis in lateral view (A) Aegypius monachus (only one muscle slip of the Mm. ascendentes cervicales is illustrated in color). The muscle originates from the aponeurosis of the notarium (neural spine of the last cervical vertebra and first thoracic vertebra) and inserts onto seven caudal vertebrae in both vultures (CV8−CV14 in Gyps fulvus, CV6−CV12 in A. monachus). The longest muscle slip inserts onto the second cervical vertebra, where the M. longus colli dorsalis, pars caudalis fuses with the M. longus colli dorsalis, pars cranialis. The M. longus colli dorsalis, pars cranialis in dorsal view (B) Gyps fulvus, (C) A. monachus. The cranial part of the M. longus colli dorsalis originates from five cervical vertebrae (CV3−CV7) in G. fulvus and from four cervical vertebrae (CV3−CV6) in A. monachus. The muscle slips converge to insert tendinously into the second cervical vertebra in both vultures. The M. longus colli dorsalis, pars profunda in dorsal view (D) G. fulvus, (E) A. monachus. The muscle consists of a series of partially overlapping muscle slips connecting two−three cervical vertebrae in the intermediate region of the neck (CV5−CV13 in G. fulvus, CV4−CV11 in A. monachus). Only one muscle slip of the M. longus colli dorsalis, pars profunda is illustrated in color. Scale bar: 1 cm
Musculus longus colli dorsalis, pars cranialis
Synonymy: M. longus colli posticus (Shufeldt, 1890; Burk, 1893; Kaupp, 1918); M. splenius colli (Boas, 1929; Fisher & Goodman, 1955; Zusi & Storer, 1969); M. longus colli posticus, pars anterior (Kuroda, 1962).
Overall, the shape of this unipennate muscle is similar in all three vultures. The M. longus colli dorsalis, pars cranialis originates with a separate stout muscle slip from several cranial cervical vertebrae (Fig. 8B and 8). There are five muscle slips in G. fulvus, and four muscle slips in A. monachus and N. monachus. The muscle inserts into CV2, and its insertion is fused together with the most cranial insertion of the M. longus colli dorsalis, pars caudalis (Fig. 8B and 8). In A. monachus and N. monachus, the most caudal muscle slip of the M. longus colli dorsalis, pars cranialis originates at the cervical vertebra into which the second most cranial muscle slip of the M. longus colli dorsalis, pars caudalis inserts (Fig. 15). This is in contrast to G. fulvus in which the second most cranial muscle slip of the M. longus colli dorsalis, pars caudalis inserts into one cervical vertebra after the most caudal muscle slip of the M. longus colli dorsalis, pars cranialis.
There are no distinct osteological correlates marking the origin and insertion of the M. longus colli dorsalis, pars cranialis.
Origin: Lateral on neural spines and anapophyses of CV3−CV7 in G. fulvus; of CV3−CV6 in A. monachus and N. monachus.
Insertion: Lateral on neural spine of CV2.
Function: The M. longus colli dorsalis, pars cranialis straightens and laterally bends the cranial region of the neck. It also raises the cranial region of the neck on the intermediate region. An EMG study showed that the muscle is active during both protraction and retraction of the head (Snively et al. 2014).
Musculus longus colli dorsalis, pars caudalis
Synonymy: M. longus colli posticus (Shufeldt, 1890); M. spinalis cervicis (Boas, 1929; Fisher & Goodman, 1955; Zusi, 1962; Zusi & Storer, 1969); M. spinalis (Burton, 1974); M. longus colli posticus, pars posterior (Kuroda, 1962).
The overall shape of this muscle is similar in all three vultures. The M. longus colli dorsalis, pars caudalis originates from the base of the neck and gives rise to a number of relatively strong muscle slips that insert each into one of several caudal cervical vertebrae (Fig. 8A). The muscle slips run superficially along the muscle bellies of the Mm. ascendentes. There are eight muscle slips in G. fulvus, A. monachus and N. monachus. The strongest, dorsal‐most muscle slip passes over the entire length of the neck and inserts tendinously into the CV2 (Tendo axialis) converging with the origin of the M. longus colli dorsalis, pars cranialis (Fig. 15).
There are no distinct osteological correlates marking the origin of the M. longus colli dorsalis, pars caudalis because it originates from fibrous tissue of the notarium. On the cervical vertebrae, the accessory process (anapophysis) arising at the dorsal side between neural spine and postzygapophysis is an osteological correlate marking the insertion of the muscle.
Origin: Aponeurosis (plate‐like fibrous tissue) of the notarium (neural spine of last CV and TV1); ventral to origin of M. biventer cervicis.
Insertion: Anapophysis and neural spine of CV2 and anapophyses of CV8−CV14 in G. fulvus; of CV6−CV12 in A. monachus and N. monachus.
Function: The M. longus colli dorsalis, pars caudalis flexes the neck in the dorsal direction. It raises the cranial region of the neck on the intermediate region and the intermediate region of the neck on the caudal region. This muscle straightens the caudal region of the neck and also bends the cranial region of the neck laterally.
Musculus longus colli dorsalis, pars profunda
Synonymy: M. longus colli posticus (Shufeldt, 1890); Mm. dorsales pygmaei (Boas, 1929; Fisher & Goodman, 1955; Zusi, 1962; Burton, 1971); M. profundus colli posticus (Kuroda, 1962).
This parallel‐fibered muscle is confined to the intermediate region of the neck, and consists of a series of rather thin, partially overlapping muscle slips each connecting two−three cervical vertebrae. Some of the muscle bellies subdivide into two or three slips. Overall, the appearance of the M. longus colli dorsalis, pars profunda is similar in all three vultures, although slight variations in the exact pattern of slips occur (Fig. 8D and 8). The muscle is tendinous at its origin and rather fleshy at its insertion.
There are no distinct osteological correlates marking the origin and insertion of the M. longus colli dorsalis, pars profunda.
Origin: Lateral on neural spines of CV7−CV13 in G. fulvus; of CV7−CV11 in A. monachus; of CV9−CV11 in N. monachus.
Insertion: Dorsal on postzygapophyses of CV5−CV8 in G. fulvus; of CV4−CV7 in A. monachus; of CV5−CV9 in N. monachus.
Function: The M. longus colli dorsalis, pars profunda flexes the intermediate region of the neck in the dorsal direction.
Mm . ascendentes cervicales
Synonymy: M. obliquus colli (Shufeldt, 1890; Kaupp, 1918); M. cervicalis ascendens (Zusi, 1985).
This dorsolateral muscle unit consists of a number of serially arranged, obliquely‐lying muscle slips (Fig. 9A). The unipennate, fusiform muscle bellies overlap each other and lie along almost the entire length of the neck (until the M. complexus). Each muscle slip originates laterally from the transverse processes of generally two cervical vertebrae and inserts cranially onto the neural spine of one vertebra (Fig. 9B and 9). The Mm. ascendentes cervicales are relatively regular in their appearance in all three vultures. At the base of the neck, the muscles transition into the Mm. ascendentes thoracis that attach to the thoracic vertebrae.
Figure 9.
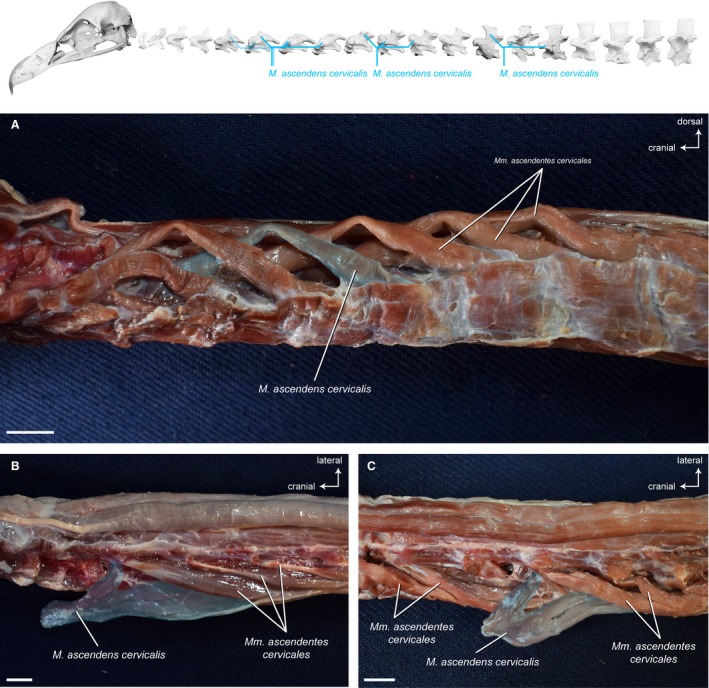
Dorsolateral system – deep muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology. The Mm. ascendentes cervicales in dorsolateral view (A) A. monachus. A series of obliquely‐lying muscle slips originate each from the transverse processes of generally two cervical vertebrae and insert onto the neural spine of one vertebra. The Mm. ascendentes cervicales in dorsal view (B) G. fulvus, (C) A. monachus. One muscle belly has been deflected laterally from its insertion on the neural spine. Scale bar: 1 cm
There are no distinct osteological correlates marking the origin and insertion of the Mm. ascendentes cervicales.
Origin: Lateral on transverse process of CV6−CV15 in G. fulvus; of CV5−CV13 in A. monachus and N. monachus.
Insertion: Lateral on neural spine of CV5−CV13 in G. fulvus; of CV4−CV11 in A. monachus and N. monachus.
Function: Contraction of the Mm. ascendentes cervicales as a group dorsally flexes the cervical vertebral column. One M. ascendens flexes one vertebra on the other (intervertebral flexion) in the dorsal direction. Unilateral contraction of the muscle causes slight torsion of the neck.
Ventral system
Acting in opposition to the M. longus colli dorsalis, there is one comparatively large, multi‐slip muscle that controls motion of the neck in the ventral direction. Some authors divide the M. longus colli ventralis into a cranial part (M. flexor colli brevis) and caudal part (M. longus colli ventralis), and sometimes even a profound part (M. flexor colli profundus). Here, we considered it as one muscle because no clear separation could be identified in the three vultures.
Musculus longus colli ventralis
Synonymy: M. longus colli (Shufeldt, 1890; Fisher & Goodman, 1955); M. longus colli anticus (Kuroda, 1962).
This unipennate muscle spans almost the entire ventral side of the neck from the cranial thoracic vertebrae until CV3 (Fig. 10). At the origin, a caudal part can be distinguished from the remaining muscle, but it fuses at its insertion with the cranial part. The left and right M. longus colli ventralis lie close to each other along a shallow sagittal channel in the caudal and intermediate region of the neck. Only in the cranial region of the neck, the left and right M. longus colli ventralis are more separate from each other and encompass the left and right M. rectus capitis ventralis. The M. longus colli ventralis originates fleshy from the ventral process of the fourth thoracic vertebra in all three vultures. It inserts more or less tendinously into all cervical vertebrae until CV3. The tendons are more developed in G. fulvus in comparison to A. monachus (Fig. 10) and N. monachus.
Figure 10.
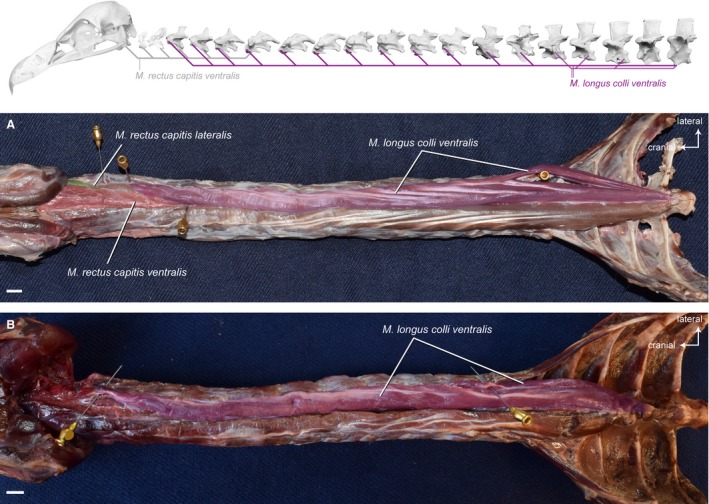
Ventral system. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscle is highlighted in color). The M. longus colli ventralis in ventral view (A) Gyps fulvus and (B) Aegypius monachus (the M. rectus capitis ventralis and the M. rectus capitis lateralis have been removed in A. monachus). The muscle originates from the ventral processes of the first four thoracic vertebrae. It inserts ventrolaterally into the last cervical vertebra (CV15 in G. fulvus, CV13 in A. monachus). Scale bar: 1 cm
There are no unambiguous osteological correlates marking the origin and insertion of the M. longus colli ventralis.
Origin: Lateral on hypapophyses of TV1−TV3 and ventrolateral on hypapophysis of TV4.
Insertion: Ventrolateral on the transverse processes of CV3−CV15 in G. fulvus; CV3−CV13 in A. monachus and N. monachus.
Function: The M. longus colli ventralis flexes the neck in the ventral direction. It lowers the cranial region of the neck on the intermediate region and the intermediate region of the neck on the caudal region. This muscle straightens the intermediate region of the neck and also contributes to lateral bending of the neck.
Intervertebral system
There are three major groups of intervertebral muscles: Mm. interspinales; Mm. intercristales; and Mm. intertransversarii. These are mono‐articular muscles that connect two successive vertebrae and that contribute to stabilization of the neck.
Mm. interspinales
Synonymy: For Zusi (1985), the Mm. intercristales include the Mm. interspinales and Mm. splenii accessorii of other authors.
The Mm. interspinales are small, midline muscles between the neural spines of two successive cervical vertebrae in the cranial region. There are three muscles in the cranial neck region of G. fulvus, but only two muscles in A. monachus and N. monachus. The Mm. interspinales are a continuation of the Mm. intercristales that are developed starting from CV5 in G. fulvus, and from CV4 in A. monachus (Fig. 11) and N. monachus. In contrast to the Mm. intercristales in which the bilateral muscle slips are clearly separated because they originate and insert laterally into the neural spines, the right and left parts of the Mm. interspinales are very close to each other that they appear as one muscle belly connecting the neural spines of two adjacent cervical vertebrae. The fibers run parallel to the muscle’s long axis.
Figure 11.
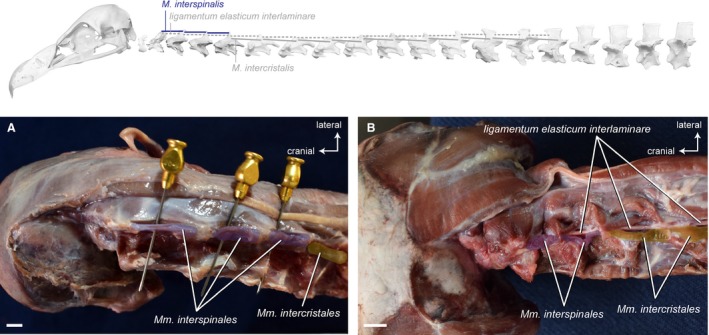
Intervertebral system – cranial muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscles are highlighted in color). The Mm. interspinales in dorsal view (A) Gyps fulvus and (B) Aegypius monachus. The muscles originate cranially from the neural spines of three cervical vertebrae (CV3−CV5) in G. fulvus and two cervical vertebrae (CV3−CV4) in A. monachus. They insert caudally into the neural spines of the respective precedent cervical vertebrae. Scale bar: 1 cm
There are no unambiguous osteological correlates marking the origin and insertion of the Mm. interspinales.
Origin: Cranial on the neural spines of CV3−CV5 in G. fulvus; of CV3−CV4 in A. monachus and N. monachus.
Insertion: Caudal on the neural spines of CV2−CV4 in G. fulvus; of CV2−CV3 in A. monachus and N. monachus.
Function: The Mm. interspinales are responsible for intervertebral flexion in the dorsal direction. They straighten the cranial region of the neck and contribute to the fine control.
Mm . intercristales
Synonymy: Mm. obliquotransversales (Shufeldt, 1890).
This segmental group of profound muscles lies parallel to the ligamentum elasticum interlaminare (discontinuous ligaments connecting the neural spines of successive cervical vertebrae) and directly on the dorsal side of the bone connecting two successive cervical vertebrae (Fig. 12). The parallel‐fibered muscle slips of the Mm. intercristales are rather flat and strap‐like. They originate from the craniodorsal portion of the vertebral arch and insert into the caudodorsal portion of the vertebral arch and laterally into the base of the neural spine.
Figure 12.
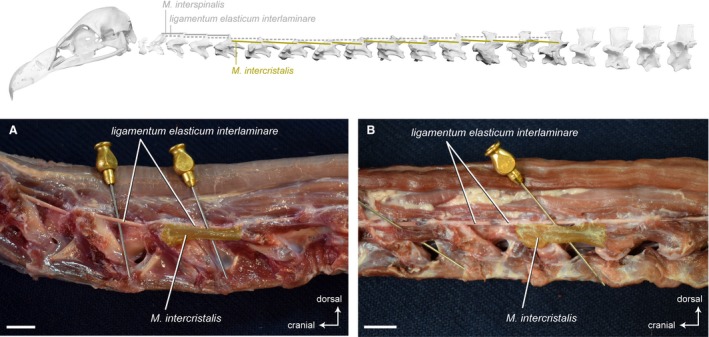
Intervertebral system – dorsolateral muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscles are highlighted in color). The Mm. intercristales in dorsolateral view (A) Gyps fulvus and (B) Aegypius monachus. Only one muscle belly is colored. The muscle belly just cranial to the colored one has been removed to reveal the midline interspinous ligament. The M. intercristalis originates dorsolaterally from one vertebra and inserts dorsolaterally onto the precedent vertebra. The Mm. intercristales are present between all cervical vertebrae starting from CV5 in G. fulvus and from CV4 in A. monachus. Scale bar: 1 cm
Osteological correlates for the origin and insertion of the M. intercristales are present in the form of transverso‐oblique crests on the cervical vertebrae.
Origin: Dorsolateral on the vertebral arches (transverso‐oblique crest) of CV6−CV15 in G. fulvus; of CV5−CV13 in A. monachus and N. monachus.
Insertion: Dorsolateral on the vertebral arches (transverso‐oblique crest) of CV5−CV14 in G. fulvus; of CV4−CV12 in A. monachus and N. monachus.
Function: The Mm. intercristales are responsible for intervertebral flexion in the dorsal direction. They straighten the cranial and the caudal region of the neck and contribute to the fine control of the neck.
Mm. intertransversarii (including Mm. inclusi)
The Mm. intertransversarii and the deeper Mm. inclusi cover the entire lateral and lateroventral half of the neck. The muscles are very intimately associated with each other (Jenni, 1981; Zusi & Bentz, 1984) and, thus, rarely treated as separate muscles. For instance, Shufeldt (1890) refers to the Mm. inclusi as the medial part of the Mm. intertransversarii. Boas (1929) names them separately as Mm. inclusi, but explains that they are a special part of the Mm. intertransversarii. The Mm. inclusi are usually considered derivatives of the Mm. intertransversarii (Baumel et al. 1993). It was not possible to distinguish between the two muscle complexes in the three vultures and, consequently, we discuss them as one group.
Synonymy: Mm. intertransversales (Shufeldt, 1890); M. colli lateralis (Kuroda, 1962).
This muscle complex consists of a series of intervertebral, multipennate muscles that cover the lateral side of the cervical vertebrae spanning the entire length of the neck (Fig. 13). These rectangular muscles form the principal lateral musculature of the neck in birds. Dorsally, they overlie the origins of the Mm. ascendentes cervicales and Mm. intercristales. Ventrally, they are in contact with the M. longus colli ventralis. In all three vultures, the Mm. intertransversales contain some tendinous fibers.
Figure 13.
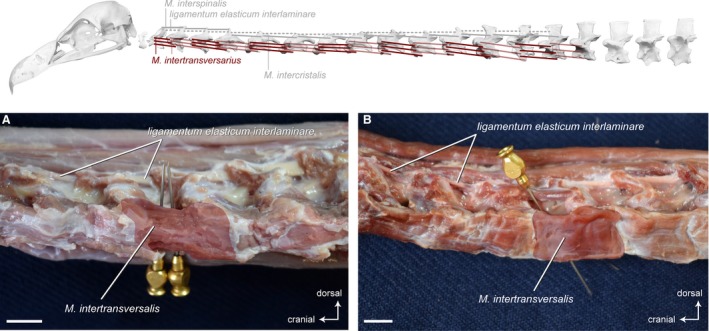
Intervertebral system – lateral muscles. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the relevant muscles are highlighted in color). The Mm. intertransversarii in lateral view (A) Gyps fulvus and (B) Aegypius monachus. Only one muscle belly is colored. The other intervertebral muscles have largely been removed to reveal the midline interspinous ligament. The M. intertransversarius originates laterally on the cranial part of one vertebra and inserts laterally onto the caudal part of the precedent vertebra. The Mm. intertransversarii are present between all cervical vertebrae starting from CV2 in both vultures. Scale bar: 1 cm
There are no distinct osteological correlates marking the origin and insertion of the Mm. intertransversarii.
Origin: Lateral on the transverse processes and ribs of CV3 to TV1.
Insertion: Lateral on the transverse processes and cervical ribs of CV2−CV15 in G. fulvus; to CV13 in A. monachus and N. monachus. The insertion of each muscle lies caudal to the origin of its precedent muscle.
Function: Unilateral contraction of the Mm. intertransversarii as a group laterally flexes the cervical vertebral column. The muscles are involved in the fine control of the head and neck, and they also contribute to intervertebral stability of the neck. The Mm. intertransversarii flex the intermediate region of the neck upward and raise it on the caudal region. They also straighten the caudal segment.
Ligaments
In between the right and left muscle slips of the Mm. intercristales, unpaired, elastic ligaments connect the neural spines of successive cervical vertebrae. Generally, the discontinuous ligamentum elasticum interlaminare (Baumel et al. 1993) is present from the second to the last cervical vertebra (Boas, 1929). There is no such ligament neither between the skull and CV1 nor between the CV1 and CV2 (Boas, 1929). This is the case for the three species of vultures. The ligamentum elasticum interlaminare is present between all cervical vertebrae except between the skull and the first two cervical vertebrae. There is variation in the thickness of the ligament. In G. fulvus, the midline interspinous ligaments are well developed between CV2 until CV5, relatively thin between CV5 until CV9, and strongly developed between CV10 and CV15 (Fig. 14A−D). In A. monachus and N. monachus, the thickness of the ligament is intermediate between CV2 until CV6, relatively thin between CV6 until CV10, and strongly developed between CV10 and CV13 (Fig. 14E−H).
Figure 14.
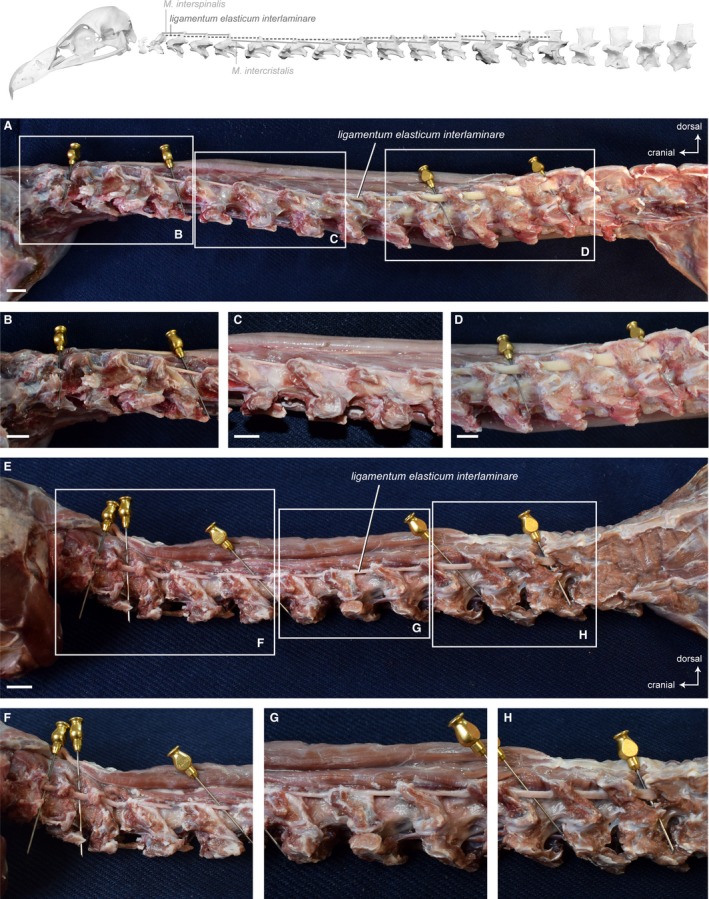
Ligaments. Top: schematic diagram (based on Gyps fulvus) visualizing the muscle topology (the ligament is represented as dashed lines). The ligamentum elasticum interlaminare along the entire neck in lateral view (A) Gyps fulvus and (E) Aegypius monachus. Note the different thickness/strength of the interspinal tendons. Detailed view of the tendon in (B, F) the caudal region, (C, G) the intermediate region, (D, H) the cranial region of the neck. Scale bar: 1 cm
Osteological correlates marking the origin and insertion of the ligamentum elasticum interlaminare are present in the form of more or less pronounced concavities on the cranial and caudal side of the base of the neural spines.
Function: The primary function of the ligaments is to absorb gravitational forces that act on the neck. It helps to keep the neck in an erect neutral position.
Discussion
We found differences in the anatomy of the neck in the three analyzed species of vultures. In the following, we will interpret the relationship between the musculoskeletal system of the neck and the respective feeding strategy. The discussion will mainly focus on the comparison between G. fulvus and A. monachus because N. monachus is relatively similar to the latter.
Craniocervical junction
The craniocervical junction − defined as the occiput, atlas (CV1) and axis (CV2) − represents the complex transitional zone between the skull and the vertebral column (Goel & Cacciola, 2011). It consists of two anatomically and functionally unique joints (Occiput−CV1 and CV1−CV2) that combine stability and motion, making the craniocervical junction the most mobile region in the cervical vertebral column. The degree of head mobility is directly correlated with the curvature of the condyle. The morphology of the structure in G. fulvus indicates free motion in all directions, whereas it suggests some degree of constraint in A. monachus. The craniocervical junction allows for not only the movement between the head and neck, but also provides attachment areas for the craniocervical musculature. In contrast to G. fulvus, the morphology of the occiput, atlas and axis of A. monachus indicate emphasized traction exerted by the craniocervical muscles because the attachment areas are very deep (occiput; Fig. 15G) and large (neural spine and hypapophysis on CV2; Fig. 1B). Overall, the comparative morphology of the craniocervical junction in the analyzed vultures implies different functional adaptations that correspond well to the previously identified of the skull, beak and mandible (Hertel, 1994).
Comparative anatomy of the neck
Muscles
Our comparative analysis revealed that G. fulvus, A. monachus and N. monachus overall share a similar cervical myology. All muscles in the neck are present, and the interspecific differences include mainly variations in the cervical position of the attachment points of certain muscles. These differences correspond partly to the differing number of cervical vertebrae; for instance, the most caudal insertion of the M. longus colli ventralis is on CV15 in G. fulvus, and on CV13 in A. monachus and N. monachus. However, other muscles differ in their arrangement by only one cervical vertebra, which may have functional implications; for example, the M. rectus capitis dorsalis originates on six cervical vertebrae in G. fulvus, but on five cervical vertebrae in A. monachus and N. monachus. The same applies for the M. rectus capitis ventralis that originates on six cervical vertebrae in G. fulvus and five cervical vertebrae in A. monachus and N. monachus (Fig. 4). The higher number of muscle attachment sites indicates that relatively more cranial cervical vertebrae in the short‐necked vultures (A. monachus and N. monachus) can be moved by these ventral flexors of the craniocervical system. It is also interesting to note that the ventral processes (hypapophyses) onto which the M. rectus capitis ventralis attaches are broader in A. monachus as compared with G. fulvus (Fig. 15D and H). This may emphasize the strong traction exerted by this muscle. Equivalently, the insertion areas on the skull for the dorsal flexors (M. complexus, M. biventer cervicis, M. splenius capitis) are deeper in A. monachus than in G. fulvus (Fig. 15C and G), indicating the high mechanical stress exerted by the action of this muscle. In particular, the M. complexus and the M. splenius capitis that contribute to considerable and quick movements of the head (Den Boer, 1953) are well developed in A. monachus. The structure of the M. biventer cervicis (cranial muscle belly, tendon, caudal muscle belly) must be seen as a way to transfer the force over a greater distance than a bundle of parallel fibers could do (Den Boer, 1953). The proportions between muscle bellies and tendon are similar in all three analyzed vultures, but the cranial muscle belly is more prominently developed in A. monachus (Fig. 7D).
Ligaments
According to Boas (1929), the midline interspinous ligaments are more or less well developed near the skull. They become thinner in the intermediate region of the neck and are strongest in the caudal cervical region (Boas, 1929). Similarly, the ligamentum elasticum interlaminare is regionalized into three sections in all three vultures. It is not surprising that our comparative analysis did not reveal specific functional adaptations in the structure of these ligaments because their primary function is to passively stabilize the head and neck. They keep the neck of the birds in the S‐shape, without or with the reduced action of the muscles (Kaupp, 1918; Boas, 1929). We would not expect that this demand is different between the analyzed vultures.
Bennett & Alexander (1987) concluded that the ligaments are not sufficient to support the extended head and neck without the aid of muscles, but they help reducing the muscular energy expenditure in a head‐down posture (Bennett & Alexander, 1987). This is in contrast to the ligamentum nuchae of mammals that is a more effective bracing device (Bennett & Alexander, 1987; Nickel et al. 2003; Tsuihiji, 2004). In palaeognath birds such as the ostrich or the rhea, there is additionally the ligamentum elasticum interspinale that crosses multiple intervertebral joints and probably evolved from the ligamentum elasticum interlaminare (Boas, 1929; Tsuihiji, 2004). Functionally, it is similar to the ligamentum nuchae in mammals (Dimery et al. 1985). It has been shown that ventral mobility in the neck of the ostrich is largely constrained by the ligamentum elasticum interspinale (Cobley et al. 2013).
Ecomorphological implications of foraging variability
The three species of vultures analyzed in this study can be clearly distinguished according to their feeding strategy (Kruuk, 1967; Houston, 1988). This ecological separation is reflected in morphological differences of the skull, beak and mandible (Hertel, 1994). It is less evident in the qualitative muscular anatomy of the neck (this study).
Gulpers (represented in this study by G. fulvus) who penetrate the corpse to pull out soft insides and fleshy parts usually use one or no foot for tearing and holding the dead body (Kruuk, 1967; Houston, 1988). This suggests that they strongly rely on their neck to bring their head in position and to fix the dead body during manipulation. It would, thus, have been expected that gulpers have a muscular system that facilitates pulling when holding the neck in an inflected form. This would primarily concern the main ventral flexor muscle (M. longus colli ventralis). It is indeed more strongly developed and more tendinous in G. fulvus than in A. monachus (Fig. 10). Furthermore, the caudal part of the muscle (M. longus colli ventralis, pars caudalis) is more distinctly developed in G. fulvus, suggesting more fine control when moving the caudal and intermediate vertebrae, respectively. It would also have been expected that the craniocervical muscles in gulpers allow for rotating the head and cranial part of the neck to reach into the carcass. This would concern the main rotator muscles (M. splenius capitis and M. rectus capitis lateralis). However, they are not distinctly different between G. fulvus and A. monachus (Figs 3 and 6).
Rippers (represented in this study by A. monachus) who tear strips and pieces off bones regularly use their feet to assist in tearing food from a carcass (Kruuk, 1967; Houston, 1988). This suggests that they predominantly adopt a straight and vertical neck posture for tearing. Consequently, we would expect that rippers have a muscular system that facilitates pulling when holding the neck straightly bend down to the ground. The pulling back of the entire neck is primarily effected by the main dorsal flexor muscles (M. biventer cervicis and M. longus colli dorsalis). They are indeed more strongly developed in A. monachus; in particular the M. biventer cervicis and the M. longus colli dorsalis pars profunda (Figs 7 and 8). We would also expect that the craniocervical muscles in rippers allow for ventral flexion of the head and the cranial part of the neck to reach to the food item that is held with their feet. This would concern the main ventral flexor muscles of the head−neck system (M. rectus capitis ventralis and M. rectus capitis dorsalis). Both muscles are relatively longer in A. monachus because the most caudal origins of M. rectus capitis ventralis and M. rectus capitis dorsalis are from CV5 (as compared with G. fulvus that has two more cervical vertebrae; Figs 5 and 8).
Scrappers (represented in this study by N. monachus) usually feed with ‘chicken‐pecks’ around the main carcass rather than actually from it (Lippens, 1936; Petrides, 1959; Kruuk, 1967). Pecking is a fundamental motor repertoire in avian foraging (Bermejo et al. 1989) and, thus, we would not expect distinct specializations in the muscular system of the neck in N. monachus. Nevertheless, it is a specialist in terms of foraging behavior because vultures rely on carrion for survival and reproduction. This may explain in part why the neck anatomy between N. monachus and A. monachus is not distinctly different. As compared with rippers, scrappers have to reach food items at the level of their feet as well. Thus, the craniocervical muscles in scrappers should allow for ventral flexion as in rippers. Indeed, the main ventral flexor muscles of the head−neck system in N. monachus closely resemble those of A. monachus. Although N. monachus has a thin, slender bill adapted to pecking rather than to pulling or tearing (Kruuk, 1967), it is able to tear pieces from carcasses. This concerns primarily the main dorsal flexor muscles. The M. biventer cervicis is indeed similarily developed in N. monachus as in A. monachus. In contrast, the M. longus colli dorsalis pars profunda is shorter by one cervical vertebra in N. monachus as compared with A. monachus. Both taxa have the same number of cervical vertebrae. The similarity in cervical count may also contribute to the similarities in the macroscopic neck musculature between N. monachus and A. monachus. There may be microscopic differences between the taxa that are not within the scope of this study.
Conclusion
Investigating the anatomy of closely related species that differ in their behavior allows for evaluating functional and possibly adaptive variation in the muscular system. The present study provided a detailed and systematic description of the anatomy of the neck in three species of vultures that exploit the same food resources in different ways. Gulpers, rippers and scrappers adopt specific postures while feeding from a carcass that may impose a selective influence on the musculoskeletal system. We found evidence for the relationship between the musculoskeletal system of the neck and feeding strategy in the three analyzed species of vultures. Yet, the strength of the functional signals is different as discussed above. The cervical vertebral column is indispensable to position the head during all kinds of behavior (Boas, 1929; Zusi, 1962; Heidweiller et al. 1992). Consequently, the anatomy of the neck may appear not extremely specialized as a result of the great range of demands. The comparison of the craniocervical muscular anatomy in G. fulvus (representing gulpers), A. monachus (representing rippers) and N. monachus (representing scrappers) revealed similarities in the overall conformation and structure of the bones and muscles. Nevertheless, we found distinct morphological differences that reflect their functional adaptations.
To conclude, our data are crucial for interpretation of the results of studies comparing the functional adaptations in the neck of birds. Furthermore, they will serve as a foundation for future analyses of avian neck biomechanics.
Conflict of interest
The authors declare no conflict of interests.
Author contributions
A.A. and C.B. conceived and designed the study. C.B. and J.P. collected data and conducted the analyses. C.B., J.P., A.A. and O.D. interpreted the data. C.B. designed the figures. J.P. participated in data visualization. C.B. wrote the manuscript. A.A. and O.D. provided critical feedback. All authors commented on the manuscript and contributed to its final version. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the Simon Potier (Montpellier) and the Goupil Association for providing bird specimens for dissection. The authors express their gratitude to Christine Lefèvre (Muséum National d’Histoire Naturelle Paris) who kindly permitted access to the museum collection under her care, Xavier de La Bernardie (SUBATECH, Unite Mixte de Recherche CNRS‐IN2P3/IMT‐Atlantique/Univ.de Nantes) for providing the CT scans, Pauline Provini (Muséum National d’Histoire Naturelle Paris) for fruitful discussion and helpful advice for segmentation of the CT data. The authors thank all members and collaborators of the research consortium AVINECK for stimulating discussion. The authors thank Stephan Lautenschlager and an anonymous reviewer for their valuable suggestions and helpful comments. This study was financially supported by the Agence National de la Recherche (ANR): Project ID #ANR‐16‐CE33‐0025 (AVINECK), Project coordinator: A. Abourachid.
References
- Baumel JJ, King AS, Breazile JE, et al. (1993) Handbook of avian anatomy: nomina anatomica avium. Cambridge: Nuttall Ornithological Club. [Google Scholar]
- Bennett MB, Alexander RM (1987) Properties and function of extensible ligaments in the necks of turkeys (Meleagris gallopavo) and other birds. J Zool 212, 275–281. [Google Scholar]
- Bermejo R, Allan RW, Houben AD, et al. (1989) Prehension in the pigeon. I. Descriptive analysis. Exp Brain Res 75, 569–576. [DOI] [PubMed] [Google Scholar]
- Berthoz A, Graf W, Vidal PP (1992) The Head‐Neck Sensory Motor System, p. 748 Oxford: Oxford University Press. [Google Scholar]
- Boas JEV (1929) Biologisch‐anatomische Studien über den Hals der Vögel. Kongelige Danske Videnskabernes Selskabs Skrifter 9, 101–222. [Google Scholar]
- Den Boer PJ (1953) On the correlation between the cervical muscles and the structure of the skull in Phasianus colchicus L. and Perdix perdix L. II. Koninklijke Nederlandse Akademie van Wetenschappen 56, 455–473. [Google Scholar]
- Böhmer C, Plateau O, Cornette R, et al. (2019) Correlated evolution of neck length and leg length in birds. R Soc Open Sci 6, 181 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright JA, Marugan‐Lobon J, Cobb SN, et al. (2016) The shapes of bird beaks are highly controlled by nondietary factors. Proc Natl Acad Sci 113, 5352–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk CM (1893) The myology of the pigeon (Columba livia). A study of the muscular system of the pigeon. Philadelphia: Biodiversity Heritage Library. [Google Scholar]
- Burton PJK (1971) Comparative anatomy of head and neck in the Spoon‐billed sandpiper, Eurynorhynchus pygmeus and its allies. J Zool 163, 145–163. [Google Scholar]
- Burton PJK (1974) Anatomy of head and neck in the Huia (Heteralocha acutirostris) with comparative notes on other Callaeidae. Bull Br Mus (Natural History) Zool 27, 1–48. [Google Scholar]
- Cobley MJ, Rayfield EJ, Barrett PM (2013) Inter‐vertebral flexibility of the ostrich neck: implications for estimating sauropod neck flexibility. PLoS ONE 8, e72187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MNO, Green PR (1994) Perception and motor control in birds. An ecological approach, p. 364 Berlin: Springer. [Google Scholar]
- Dermody BJ, Tanner CJ, Jackson AL (2011) The evolutionary pathway to obligate scavenging in Gyps vultures. PLoS ONE 6, e24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimery NJ, Alexander RM, Deyst K, et al. (1985) Mechanics of the ligamentum nuchae of some artiodactyls. J Zool 206, 341–351. [Google Scholar]
- Fisher HI (1946) Adaptations and comparative anatomy of the locomotor apparatus of New World vultures. Am Midl Nat 35, 545–727. [Google Scholar]
- Fisher HI, Goodman DC (1955) The myology of the Whooping crane, Grus americana . Ill Biol Monogr 24, 1–127. [Google Scholar]
- Fujioka T (1963) Comparative and topographical anatomy of the fowl. IV. Origins and insertions of muscles of the head and neck in the fowl. Part 1. Muscles of the head. Nihon Juigaku Zasshi (Japanese Journal of Veteerinary Science) 25, 207–226. [DOI] [PubMed] [Google Scholar]
- Goel A, Cacciola F (2011) The craniovertebral junction: Diagnosis, pathology, surgical techniques, p. 592 Stuttgart: Thieme. [Google Scholar]
- Heidweiller J, Van der Leeuw AHJ, Zweers GA (1992) Cervical kinematics during drinking in developing chickens. J Exp Zool 262, 135–153. [DOI] [PubMed] [Google Scholar]
- Hertel F (1994) Diversity in body size and feeding morphology within past and present vulture assemblages. Ecology 75, 1074–1084. [Google Scholar]
- Hertel F, Maldonado JE, Sustaita D (2015) Wing and hindlimb myology of vultures and raptors (Accipitriformes) in relation to locomotion and foraging. Acta Zool 96, 283–295. [Google Scholar]
- Houston DC (1988) Competition for food between Neotropical vultures in forest. Ibis 130, 402–417. [Google Scholar]
- Houston DC, Copsey JA (1994) Bone digestion and intestinal morphology of the bearded vulture. J Raptor Res 28, 73–78. [Google Scholar]
- Jenni L (1981) Das Skelettmuskelsystem des Halses von Buntspecht und Mittelspecht Dendrocopos major und medius . J Ornithol 122, 37–63. [Google Scholar]
- Kaupp BF (1918) The anatomy of the domestic fowl. Philadelphia: W. B. Saunders. [Google Scholar]
- Keibel F (1914) Über die Veränderung des M. complexus der Vögel zur Zeit des Ausschlüpfens. Zeitschrift für Morphologie und Anthropologie 18, 73–84. [Google Scholar]
- Kruuk H (1967) Competition for food between vultures in East Africa. Ardea 55, 172–193. [Google Scholar]
- Kuroda N (1962) On the cervical muscles of birds. J Yamashina Inst Ornithol 12, 189–211. [Google Scholar]
- Lippens L (1936) Les vautours de la Plaine de la Semliki. Cercle Zoologique Congolais 13, 96–100. [Google Scholar]
- Mundy P, Butchart D, Ledger J, et al. (1992) The vultures of Africa. St Louis: Academic Press. [Google Scholar]
- Nickel R, Schummer A, Seiferle E (2003) Lehrbuch der Anatomie der Haustiere I: Bewegungsapparat. Stuttgart: Parey. [Google Scholar]
- O'Neal Campbell M (2016) Vultures. Their evolution, ecology and conservation. New York: CRC Press. [Google Scholar]
- Petrides GA (1959) Competition for food between five species of East African vultures. Auk 76, 104–106. [Google Scholar]
- Romer AS (1976) Osteology of the Reptiles. Chicago: The University of Chicago Press. [Google Scholar]
- Ruxton GD, Houston DC (2004) Obligate vertebrate scavengers must be large soaring fliers. J Theor Biol 228, 431–436. [DOI] [PubMed] [Google Scholar]
- Shufeldt RW (1890) The myology of the raven (Corvus corax sinuatus). London: MacMillan. [Google Scholar]
- Smail JR (1964) A possible role of the Musculus complexus in pipping the chicken egg. Am Midl Nat 72, 499–506. [Google Scholar]
- Snively E, Russell AP, Powell GL, et al. (2014) The role of the neck in the feeding behavior of the Tyrannosauridae: inference based on kinematics and muscle function of extant avians. J Zool 292, 290–303. [Google Scholar]
- Thiollay JM (1994) Family Accipitridae (Hawks and Eagles) In: Handbook of the Birds of the World (eds del Hoyo J, Elliot A, Sargatal J.), pp. 52–205. Barcelona: Lynx Edición. [Google Scholar]
- Tsuihiji T (2004) The ligament system in the neck of Rhea americana and its implications for the birfurcated neural spines of sauropod dinosaurs. J Vertebr Paleontol 24, 165–172. [Google Scholar]
- Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W (2014) EltonTraits 1.0: Species‐level foraging attributes of the world's birds and mammals. Ecology 95(7), 2027. [Google Scholar]
- Zusi RL (1962) Structural adaptations of the head and neck in the Black Skimmer. Publications of the Nuttall Ornithological Club 3, 1–101. [Google Scholar]
- Zusi RL (1985) Muscles of the neck, trunk and tail in the Noisy Scrub‐bird, Atrichornis clamosus, and Superb Lyrebird, Menura novaehollandiae (Passeriformes: Atrichornithidae and Menuridae). Rec Aust Mus 37, 229–242. [Google Scholar]
- Zusi RL, Bentz GD (1984) Myology of the Purple‐throated Carib (Eulampis jugularis) and other hummingbirds (Aves: Trochilidae). Smithson Contrib Zool 385, 1–70. [Google Scholar]
- Zusi RL, Storer RW (1969) Osteology and myology of the head and neck of the Pied‐billed grebes (Podilymbus). Misc Publ Mus Zool Univ Mich 139, 1–49. [Google Scholar]
- Zweers GA, Vanden Berge JC, Koppendraier R (1987) Avian cranio‐cervical systems. Part I: Anatomy of the cervical column in the chicken (Gallus gallus L.). Acta Morphol Neerl Scand 25, 131–155. [PubMed] [Google Scholar]


