Abstract
In mammals, the accessory olfactory or vomeronasal system exhibits a wide variety of anatomical arrangements. In caviomorph rodents, the accessory olfactory bulb (AOB) exhibits a dichotomic conformation, in which two subdomains, the anterior (aAOB) and the posterior (pAOB), can be readily distinguished. Interestingly, different species of this group exhibit bias of different sign between the AOB subdomains (aAOB larger than pAOB or vice versa). Such species‐specific biases have been related with contrasting differences in the habitat of the different species (e.g. arid vs. humid environments). Aiming to deepen these observations, we performed a morphometric comparison of the AOB subdomains between two sister species of octodontid rodents, Octodon lunatus and Octodon degus. These species are interesting for comparative purposes, as they inhabit similar landscapes but exhibit contrasting social habits. Previous reports have shown that O. degus, a highly social species, exhibits a greatly asymmetric AOB, in which the aAOB has twice the size of the pAOB and features more and larger glomeruli in its glomerular layer (GL). We found that the same as in O. degus, the far less social O. lunatus also exhibits a bias, albeit less pronounced, to a larger aAOB. In both species, this bias was also evident for the mitral/tufted cells number. But unlike in O. degus, in O. lunatus this bias was not present at the GL. In comparison with O. degus, in O. lunatus the aAOB GL was significantly reduced in volume, while the pAOB GL displayed a similar volume. We conclude that these sister species exhibit a very sharp difference in the anatomical conformation of the AOB, namely, the relative size of the GL of the aAOB subdomain, which is larger in O. degus than in O. lunatus. We discuss these results in the context of the differences in the lifestyle of these species, highlighting the differences in social behaviour as a possible factor driving to distinct AOB morphometries.
Keywords: accessory olfactory bulb, glomerular layer, octodontid rodents, social behaviour, vomeronasal system
We performed a detailed morphometric comparison of the subdomains of the accessory olfactory bulb (AOB) between two sister species of South American stricognath (octodontid) rodents, Octodon lunatus and Octodon degus. The comparison is of interest because these species live in similar habitats, but exhibit contrasting social behaviours. We found a very sharp morphometric difference between these species, namely, the relative size of the glomerular layer of the anterior AOB subdomain, which was larger in O. degus. than in O. lunatus. We suggest that the differences in social behaviours may drive to different AOB morphometries even between closely related species.
Introduction
Among the various olfactory sensory modalities present in mammals, the accessory or vomeronasal olfactory system (VNS) plays a key role in the sensing of pheromone‐like semiochemicals, and in the orchestration of a wide variety of neuroendocrine and behavioural responses (Tirindelli et al. 2009). The sensory surface of the VNS is the vomeronasal organ (VNO), a tubular structure located bilaterally at the base of the nasal septum. In rodents, the VNO neuroepithelium contains two spatially segregated populations of chemosensitive neurons, each co‐expressing either V1R receptors and Gαi2 protein or V2R receptors and Gαo protein (Jia & Halpern, 1996; Halpern et al. 1998). V1R receptors feature enhanced chemical affinity for small volatile molecules (Inamura et al. 1999; Inamura & Kashiwayanagi, 2000; Leinders‐Zufall et al. 2000; Sugai et al. 2006), while V2R receptors display greater affinity for large insoluble molecules (Leinders‐Zufall et al. 2004, 2009; Kimoto et al. 2005). In turn, V1R and V2R neurons project separately towards different subdomains of the accessory olfactory bulb (AOB). Gαi2‐expressing axons end in glomeruli located in the rostral, or anterior, half of the AOB (aAOB), while Gαo‐axons end in glomeruli of the caudal, or posterior, half of the AOB (pAOB; Jia & Halpern, 1996; Halpern et al. 1998). From the AOB, secondary projection neurons (mitral and tufted cells) of each subdomain send segregated although partially overlapped projections to multiple forebrain regions associated with neuroendocrine regulation, including the medial amygdala, posteromedial cortical amygdala, the bed nucleus of the stria terminalis and the bed nucleus of the accessory olfactory tract (Scalia & Winans, 1975; von Campenhausen & Mori, 2000; Martinez‐Marcos, 2009).
In addition to rodents, the dichotomous segregation of vomeronasal pathways has been described in opossums (Halpern et al. 1995) and rabbits (Villamayor et al. 2018) also, and was initially thought to represent a common feature to all mammals. However, subsequent reports showed that Primates and Laurasiatherians do not show this dichotomous segregation, such that either they possess the V1R pathway only (such as in goats, marmosets, shrews, domestic carnivores, elephants and hippopotami) or no vomeronasal system at all (such as in apes, some bats and aquatic species; Meisami & Bhatnagar, 1998; Takigami et al. 2000, 2004; Ngwenya et al. 2011; Zhao et al. 2011; Kondoh et al. 2017). Moreover, the lesser Tenrec (Echinops telfairi), a basal Afrotheria, has a dichotomous but not strictly segregated VNS (Suárez et al. 2009b), while the hyrax (Procavia capensis), another basal Afrotheria, possesses the V1R pathway only (Suárez et al. 2011a). Furthermore, not all rodents have a dichotomous VNS; the California ground squirrel (Otospermophilus beecheyi), a sciurid rodent, has the V1R pathway only (Suárez et al. 2011a). Thus, the dichotomous organization of the VNS displays a remarkable variability within and between taxa, suggesting that this trait may be more closely related to the species’ lifestyles rather than their phylogenetic backgrounds.
These observations have raised the question about the biological rationale behind (the main factors explaining) the existence, conservation or disappearance of these segregated pathways. Evidence from neuronal recording and neural activity markers expression experiments, obtained mostly in rodents, suggest that the V1R pathway mediates male−female attraction, while the V2R pathway seems more related with male−male aggression (Dudley & Moss, 1999; Inamura et al. 1999; Kumar et al. 1999; Matsuoka et al. 1999; Inamura & Kashiwayanagi, 2000; Peele et al. 2003; Sugai et al. 2006; Chamero et al. 2011). In addition, comparative observations across mammals have shown that independently of its phylogenetic position, species with visible sexual dimorphisms show a deterioration of the V2R pathway (Suárez et al. 2011a), a result that is consistent with a sexually related functional segregation of the AOB pathways.
South American hystricognath rodents constitute an interesting model group to study the question posed above. Several recent studies have shown that these wild rodents feature a dichotomous, but asymmetric, organization of the VNS, in which one of the subdomains of the AOB is larger than the other. More interestingly, both aAOB bias and pAOB bias have been described in different species of this group, in association with conspicuous differences in environmental traits. In Octodon degus, a semi‐arid inhabitant, the aAOB has twice the overall volume than the pAOB, and contains more and larger glomeruli (Suárez & Mpodozis, 2009a), while in the semi‐aquatic capybara (Hydrochoerus hydrochaeris), the pAOB has a larger volume and contains more glomeruli than the aAOB (Suárez et al. 2011b).
Given that social interactions in mammals rely to a great extent on pheromones, it could be expected that in addition to sexual and environmental factors, the degree of sociality would also be an important lifestyle trait driving the VNS to different anatomical conformations in different species. Here, we performed a morphometric study of the subdomains of the AOB in Octodon lunatus and compared the resulting measurements with that of its sister species, O. degus. Octodon degus is a diurnal, open field inhabitant, which exhibits an elaborate sociality, including communal nesting, cooperative burrowing, cooperative surveillance and alarm calls display (Ebensperger & Bozinovic, 2000; Ebensperger et al. 2004, 2006; Jesseau et al. 2009, Fulk, 1976; Yáñez, 1976; Cecchi et al. 2003). Octodon lunatus is a mainly nocturnal shrub inhabitant that is far less social than O. degus (Sobrero et al. 2014). In the field, O. lunatus form small groups consisting of two–four adult individuals, which occasionally share resting areas during daytime. Engagement in cooperative behaviours has not been described in this species. We found significant differences between these species, in particular regarding the morphometric bias of the glomerular layer (GL) between AOB subdomains. We conclude that differences in sociality may led to morphometric AOB differences, even between closely related species.
Materials and methods
Five adult specimens of O. lunatus and six adult specimens of O. degus were used in these experiments. These animals were captured in the wild and maintained in an animal facility at the University of Chile. Octodon lunatus were captured in Los Molles (Region de Valparaiso, Chile) and O. degus were captured in both Rinconada de Maipú (Region Metropolitana, Chile) and Parque Nacional Bosque Fray Jorge (Region de Coquimbo, Chile). All procedures were performed under guidelines established by the Universidad de Chile Bioethical Committee (120731‐DB‐FSH‐UCH) and adhered to Chilean laws [permits 1‐130 154.2010 (7989), 1‐109.2011 (6749), 1‐90.2011 (4731) and 1‐95‐2012 (4486) by the Servicio Agrícola y Ganadero and 013/2011 by the Corporación Nacional Forestal].
Brain extraction
The animals were deeply anaesthetized using a dose of ketamine/xylazine (2.4 mL kg−1 and 1.2 mL kg−1, respectively) and transcardially perfused with 0.9% saline serum, followed by 4% paraformaldehyde in 0.1 m phosphate‐buffered saline (PBS) solution. The brains were removed, weighed and postfixed for several days. The olfactory bulbs (OBs) were removed and placed in a 30% sucrose in 0.01 m PBS solution for cryoprotection, until they sank. Then, 40‐μm sagittal OB sections were obtained using a freezing‐sliding microtome (Leitz Wetzlar 1400, Leitz, Germany). The sections were collected in two series of 40‐μm intervals and maintained in a solution of 0.01 m PBS + 0.01% sodium azide. One series was mounted onto gelatin‐coated slides, dehydrated and stained using Cresyl violet solution (Fluka; 61123, 0.2% solution, pH 4.3) for stereological quantification.
Immunohistochemistry
The second series of OB sections was free‐floating incubated with 3% H2O2 + 10% methanol for 10 min, followed by 10 mm sodium citrate solution (pH 6.0), for 30 min at 80 °C. Posteriorly, the sections were incubated in a 5% normal goat serum (NGS) + 0.05% Triton X‐100 in 0.01 m PBS (PBST) for 1 h. The slices were incubated with anti‐Gαi2 antibody (1 : 500; cat no. sc‐13534, Santa Cruz Biotechnology) with 3% NGS in PBST for 16 h at 5 °C. Subsequently, the sections were rinsed and incubated in biotinylated goat anti‐mouse secondary antibody (1 : 500; cat no. BA‐9200, Vector Laboratories) for 2 h. In the final steps, the biotinylated sections were incubated with the avidin‐biotin complex (ABC Elite Kit; Vector Laboratories) for 1 h, and reacted using 0.25 mg mL−1 of 3.3‐diaminobenzidine (cat no. D‐5905, Sigma‐Aldrich) and 0.001% H2O2 for 3 min.
Stereological measures
Measurements of AOB volumes and counting of mitral/tufted (M/T) cells were estimated in Cresyl violet‐stained serial sections. Stereological measures were made using a StereoInvestigator 8.0 software (MBF Bioscience, Williston, Vt., USA), a CCD video camera (Microfare, Optometrics®) and a bright‐field microscope (Eclipse E400, Nikon). AOB samples of each subject were examined every two sections, covering its whole lateral−medial extension. We measured the overall volume and GL volume of both aAOB and pAOB subdomains. For the overall AOB subdomains measurements, the vomeronasal nerve layer (VNL), the GL, the external plexiform layer (EPL) and the mitral/tufted cells layer (M/TL) were considered. The indentation splitting the AOB layers was used to distinguish the aAOB from the pAOB subdomain, as in previous reports (Suárez & Mpodozis, 2009a; Suárez et al. 2011b). The volume measurements were performed using the Cavalieri stereological estimator (CE). In all samples, we used a 30‐μm square grid covering the whole AOB area, matched to a 40 × lens. For CE, the Gundersen coefficients of error (m = 1) obtained ranged between 0.009 and 0.018 for O. lunatus, and 0.003 and 0.012 for O. degus. For comparative purposes, we divided the estimated volume of a given structure by brain volume, taking account for any allometric effects. Brain volume was calculated by dividing the brain mass by fixed brain tissue density (1.036 g mm−3; Iwaniuk & Wylie, 2007; Vega‐Zuniga et al. 2013). Thus, volumes are expressed as relative to the brain volume. Cell counts of AOB M/T cells were performed in both species, considering only that restricted to M/TL. The anatomical indentation splitting the M/TL was considered to separate both anterior M/TL and posterior M/TL. The M/T cells number and the M/TL volume of both AOB portions were obtained using an optical fractionator (OF) procedure, matched to a × 100 oil immersion lens. A 100‐μm sampling grid was used, and all 50 × 50 μm frames were counted using a dissector height of 15 μm and 7.5 μm guard zones. For OF, the Gundersen coefficients of error (m = 1) obtained ranged between 0.03 and 0.07 for O. lunatus, and 0.03 and 0.06 for O. degus. From these measurements, the density of M/T cells, expressed as cells mm−3, of each AOB portion was calculated.
Statistical analyses
Non‐parametric statistics were performed to analyse the data, as they did not show a normal distribution; the Mann–Whitney U‐test was chosen to compare between O. lunatus and O. degus AOB measures, and the Wilcoxon‐matched pairs test was used to compare between aAOB and pAOB measures within each of the species. All the statistical analyses were performed using Statistica 6.0 (StatSoft, Tulsa, OK, USA). P < 0.05 or P ≤ 0.01 values are related with significant differences in both statistical tests.
Figure preparation
All the images were obtained using a bright‐field microscope and a SPOT camera (Spot Advanced, Diagnostic Instrument). Microphotographs were processed using Adobe Photoshop CS4 (Adobe Systems, San Jose, CA, USA). All the graphs were plotted using SigmaPlot (Systat Software).
Results
Anatomical characterization of the Octodon lunatus AOB
In O. lunatus, the AOB appears as a prominent structure located in the caudal/dorsal region of the main olfactory bulb, with the vomeronasal nerve innervating it from its lateral aspect (as shown by Suárez et al. 2011b; Figs 1a and 2a). The cross‐sectional area of the AOB varies across its medial−lateral axis, reaching a maximum value at the middle zone (Fig. 2b). Both the anterior and posterior AOB portions are clearly distinguishable by a glial indentation splitting all layers. At both subdomains, all AOB layers previously reported in other rodent species appear well defined and readily recognizable: VNL, GL, EPL, M/TL, lateral olfactory tract (lot) and granular cells layer (GrL; Fig. 1a). The glomeruli’s profiles in both AOB portions are numerous, poorly defined and exhibit different sizes. The soma of M/T cells was mainly located in M/TL, but some scant cells were also observed scattered in the EPL.
Figure 1.
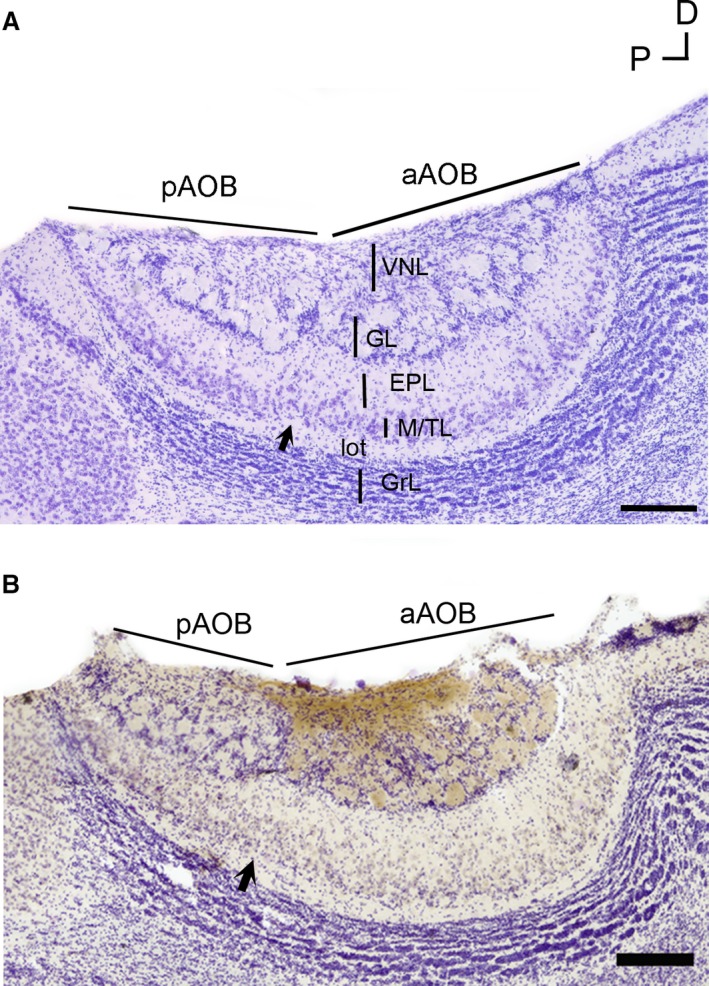
Accessory olfactory bulb (AOB) anatomy of Octodon lunatus. (a) Microphotograph of a Nissl‐stained section through the medial portion of the AOB. All layers of the AOB appear readily identifiable. Note the glial indentation splitting the anterior (aAOB) and posterior (pAOB) subdomains (black arrow). (b) Anti‐Gαi2‐immunostained, Nissl‐counterstained section. The stain (brown) is exclusively restricted to the aAOB portion. EPL, external plexiform layer; GL, glomerular layer; GrL, granular cells layer; lot, lateral olfactory tract; M/TL, mitral/tufted cells layer; VNL, vomeronasal nerve layer. Scale bar: 250 μm.
Figure 2.
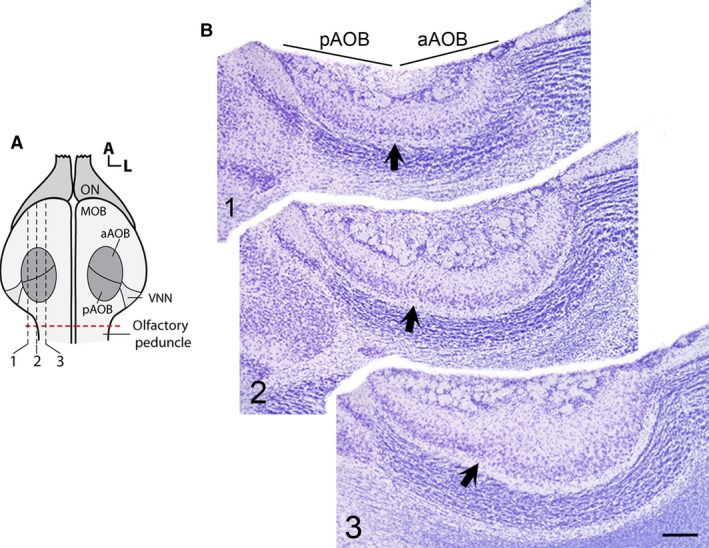
Sagittal series of accessory olfactory bulb (AOB) profiles in Octodon lunatus. (a) Schema (dorsal view) of the olfactory bulb illustrating the relative position of the AOB subdomains. The red dotted line in (a) indicates the position of the cut made to separate the olfactory bulbs (OBs) from the brain. Dashed lines and numbers indicate the approximate level of each of the sections depicted in (b). (b) Microphotographs of the corresponding series of sagittal sections, stained with Nissl. The glial indentation dividing the AOB subdomains is appreciable at all levels (dark arrows). Note the variation of the cross‐sectional area of the AOB. MOB, main olfactory bulb; ON, olfactory nerve; VNN, vomeronasal nerve. Other abbreviations as in Fig. 1. Scale bar: 250 μm.
The presence of an anatomical split delimiting AOB subdomains coincides with a segregated expression of Gαi2, a protein located exclusively in V1R neurons (Fig. 1b). We observed the Gαi2 + immunostaining in both VNL and GL restricted to the aAOB. We did not perform a study of Gαo expression (V2R neurons marker) in this species; however, a conserved, fully segregated pattern of expression, similar to that previously reported in O. degus, can be expected to be found in O. lunatus (Suárez & Mpodozis, 2009a).
AOB morphometry of Octodon lunatus
We performed a series of morphometric measurements on the AOB of O. lunatus. Specifically, we measured the overall volume of each subdomain, the volume of the GL in each subdomain, and the number of M/T cells at M/TL associated to each subdomain (for more details, see Materials and methods). We found that the overall aAOB volume was significantly larger than the pAOB volume (aAOB: 0.689 ± 0.04 mm3; pAOB: 0.507 ± 0.03 mm3; Z = 2.02, P = 0.04, n = 5; Fig. 3a). Concordantly, the number of M/T cells counted in the aAOB M/TL was double the number of cells counted in the pAOB M/TL (aAOB: 10 341 ± 1022; pAOB: 5227 ± 297; Z = 2.02, P = 0.04, n = 5; Fig. 3b). There was also not a significant difference in M/T cells density between both AOB subdomains (aAOB: 183 751 ± 82 176 cells mm−3; pAOB: 184 253 ± 82 400 cells mm−3; Z = 0.13, P = 0.8, n = 5). In contrast, the volume of the GL did not show a significant difference between both AOB subdomains (aAOB: 0.069 ± 0.008 mm3; pAOB: 0.064 ± 0.007 mm3; Z = 1.21, P = 0.2, n = 5; Fig. 3c). Thus, both the overall volume of the AOB subdomains and the number of M/TL cells showed a significant bias towards the aAOB, while for the GL no noticeable bias was found.
Figure 3.
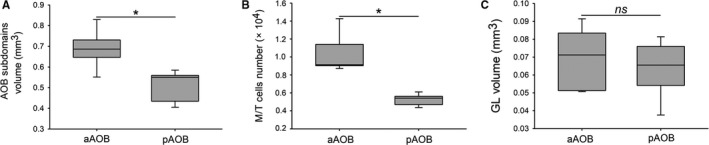
Octodon lunatus exhibits an emphasized anterior accessory olfactory bulb (aAOB), with exception of the glomerular layer (GL). Box plot graphs showing the measures for overall volume (a), mitral/tufted (M/T) cells number (b) and GL volume (c), corresponding to aAOB and posterior (pAOB). The graphs show the two central percentiles and the median, with error bars. *P < 0.05, significant differences obtained running Wilcoxon‐matched pair tests. ns, not significant.
AOB morphometric comparison between Octodon lunatus and Octodon degus
In agreement with previous reports (Suárez & Mpodozis, 2009a), we found that the AOB of O. degus shows a marked bias to a larger aAOB, regarding its overall volume (aAOB: 0.616 ± 0.06 mm3; pAOB: 0.363 ± 0.03 mm3; Z = 2.2, P = 0.03, n = 6; Fig. 4a), number of neurons in the M/TL (aAOB: 14 219 ± 2522; pAOB: 6883 ± 1030; Z = 2.02, P = 0.04, n = 6; Fig. 4b) and GL volume (aAOB: 0.1 ± 0.01 mm3; pAOB: 0.061 ± 0.005 mm3; Z = 2.2, P = 0.03, n = 6; Fig. 4c). There was not a significant difference in M/T cells density between both AOB subdomains (aAOB: 110 145 ± 9112 cells mm−3; pAOB: 99 125 ± 11 543 cells mm−3; Z = 0.67, P = 0.6, n = 5). Based on these data, we performed a series of inter‐species comparisons, as described below.
Figure 4.
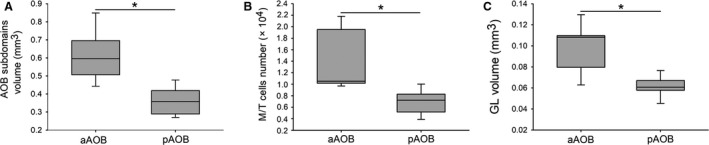
Octodon degus exhibits a full‐bias to a larger anterior accessory olfactory bulb (aAOB). Box plot graphs showing the measures for overall volume (a), mitral/tufted (M/T) cells number (b) and glomerular layer (GL) volume (c), corresponding to aAOB and posterior (pAOB). The graphs show the two central percentiles and the median, with error bars. *P < 0.05, significant differences obtained running Wilcoxon‐matched pair tests. ns, not significant.
First, we compared the morphometric measurement obtained for each subdomain in each of the species. For the aAOB we did not observe significant inter‐species differences, in the overall volume (U = 8, Z = 1.19, P = 0.2, n deg = 6, n lun = 5; Fig. 5a) or in the number of M/T cells (U = 5, Z = 1.46, P = 0.14, n deg = 5, n lun = 5; Fig. 5b). However, the GL volume of aAOB was significantly smaller in O. lunatus than in O. degus (U = 1, Z = 2.46, P = 0.01, n deg = 6, n lun = 5; Fig. 5c). For the pAOB we did not find significant inter‐species differences for any morphometric measurements: overall volume (U = 14, Z = 0.09, P = 0.9, n deg = 6, n lun = 5; Fig. 5a), number of M/T cells (U = 6, Z = 1.25, P = 0.21, n deg = 5, n lun = 5; Fig. 5b) and GL volume (U = 4, Z = 1.92, P = 0.06, n deg = 6, n lun = 5; Fig. 5c).
Figure 5.
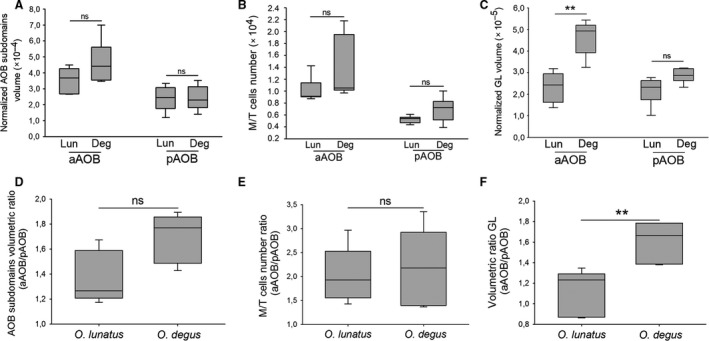
(a−c) Octodon lunatus and O. degus exhibit similar accessory olfactory bulb (AOB) measures, with the exception of the anterior (aAOB) glomerular layer (GL). Box plot graphs showing the measures for normalized overall volume (a), mitral/tufted (M/T) cells number (b), and normalized GL volume (c), corresponding to aAOB and posterior (pAOB). The graphs show the two central percentiles and the median, with error bars. ** P ≤ 0.01, significant difference obtained running Mann−Whitney U‐tests. ns, not significant. (d−f) Octodon lunatus and O. degus exhibit similar AOB bias extent, with the exception of the GL. aAOB/pAOB ratio for overall volume (d), M/T cells number (e) and GL volume (f). Box plot graphs showing the two central percentiles and median, with error bars. **P ≤ 0.01, significant differences obtained running Mann−Whitney U‐tests. ns, not significant.
Moreover, we performed a comparison of the ratio aAOB/pAOB for each of the morphometric measurements performed on each species. We did not find significant differences between these species in the ratio of overall volume of the subdomains (aAOB/pAOB: U = 4, Z = −1.92, P = 0.06, n deg = 6, n lun = 5; Fig. 5d) or in the ratio of the number of M/T cells per subdomain (aAOB/pAOB: U = 5, Z = 1.46, P = 0.1, n deg = 6, n lun = 5; Fig. 5e). However, for the GL, O. lunatus exhibited a significantly smaller volumetric ratio than O. degus (aAOB/pAOB: U = 0, Z = −2.64, P = 0.008, n deg = 6, nlun = 5; Fig. 5f).
Discussion
Our morphometric measurements show that O. lunatus has an overall volumetric bias favouring the aAOB subdomain. Interestingly, such bias is of the same sign, albeit less pronounced, than the AOB bias exhibited by its sister species, O. degus. Just as in O. degus, in O. lunatus we found that this bias was readily evident at the M/TL. But, unlike in O. degus, in O. lunatus this bias was not present at the GL. In comparison with O. degus, in O. lunatus the aAOB GL was significantly reduced in volume, while the pAOB GL displayed a similar volume. Thus, we conclude that between these sister species there is a very specific difference in the anatomical conformation of the AOB, namely, the relative size of the GL of the aAOB subdomain, which is larger in O. degus than in O. lunatus. In what follows we discuss these results in the context of differences in the life mode in these species.
Octodon lunatus vs Octodon degus AOB differences: A matter of physical environmental factors?
It has been suggested that salient cues of the species habitat could be a key underlying factor explaining the asymmetries observed in the relative size of anterior and posterior portions of the AOB in caviomorph rodents. A bias to a more prominent aAOB has been reported in O. degus, which lives in a semi‐arid habitat, and performs scent‐marking behaviours (such as dustbathing) that likely involve the usage of volatile pheromones (Fulk, 1976; Ebensperger & Caiozzi, 2002). This scent‐marking mode implies the preferential use of the V1R pathway, which would explain the greater development of the aAOB observed in this species (Suárez & Mpodozis, 2009a). In clear contrast, a bias to a more prominent pAOB has been reported in the capybara (H. hydrochaeris), a semi‐aquatic wetland inhabitant. The capybara engages in scent‐marking behaviours using oily, non‐volatile molecules secreted by two kinds of scent glands: anal glands and the morrillo gland (located at the top of the snout; MacDonald et al. 1984; Herrera & MacDonald, 1994). Such scent‐marking mode would be associated with the preferential usage of the V2R pathway and, hence, with an anatomical bias favouring the pAOB (Suárez et al. 2011b).
Unlike O. degus, which have a preference to live in open areas, or savannas, of the mediterranean region of central Chile, O. lunatus is a shrub inhabitant, more frequently observed in the hills of the coastal range of this same region (Glanz & Meserve, 1982; Sobrero et al. 2014). Both species come to coexist, however, inhabiting different patches in xeric or sclerophic landscapes affected by the same climatic regime. Even when at a microscale level the habitat of O. lunatus may be slightly more humid than that of O. degus, this difference in humidity is far from comparable to the one that exists between the wetlands of the capybaras and the savanna of the degus. In agreement, and unlike the capybara, O. lunatus lack specialized glands producing non‐volatile pheromones, and do not present an anatomical bias towards the pAOB. Thus, a preferred usage of non‐volatile cues in this species is very unlikely. Furthermore, the overall volume of the pAOB, as well as the volume of the pAOB GL appears to be similar in these species, suggesting no differences between them in the usage of non‐volatile pheromones. Thus, the anatomical differences in the conformation of the AOB reported here are unlikely to be due to environmental factors. Instead, they point to the existence of idiosyncratic species‐specific differences in VNS‐dependent aspects of behaviour.
AOB differences and pheromonal sensing
It is widely accepted that the size and degree of specialization of a sensory structure correlate with the relevance that the corresponding sensory modality has for the realization of the ‘umwelt’ (organism/environment relationship) of a given organism (Jerison, 1973). In the case of olfaction, the main olfactory bulb (MOB) of nocturnal, olfaction‐dependent species tends to be larger than the OB of more visually dependent diurnal species, a trait observed in mammals (Barton et al. 1995). In a similar way, we might expect that the volume of the GL of a given AOB subdomain, which reflects the number and size of the glomeruli it contains, very likely correlates with the frequency and diversity of the pheromone‐dependent behaviours mediated by such subdomains.
As we stated above, the most striking differences between O. degus and O. lunatus concern the volume of the GL in the aAOB, which is markedly reduced in the latter species. Previous studies have shown that in O. degus the aAOB GL features more and larger glomeruli than the pAOB GL, indicating an enhanced sensory activity of the aAOB over the pAOB in this species (Suárez & Mpodozis, 2009a). We were unable to perform equivalent measurements in O. lunatus, due to difficulties in the stereological quantification of glomeruli. However, our qualitative observations indicate that unlike O. degus, in O. lunatus the aAOB GL exhibit glomerular profiles of similar size to those located at the pAOB. Altogether, these observations indicate that the aAOB subdomain is less active in O. lunatus than in O. degus, while the pAOB subdomain seems to be equally active in both species. Given that the aAOB activity is mainly driven by volatile pheromones (Inamura et al. 1999; Inamura & Kashiwayanagi, 2000; Leinders‐Zufall et al. 2000; Sugai et al. 2006), we conclude that the sensing of volatile pheromones is less frequent, less intense and/or less relevant in O. lunatus than in O. degus.
In addition, a remarkable aspect of our results is that while in O. lunatus the GL show no significant bias between AOB subdomains, the M/T cells, whose dendrites also contribute to the glomeruli, show a definite bias in number towards the aAOB. This bias is similar in magnitude and direction to the one found in O. degus. We think that this apparent incongruence might be explained by the pattern of connections of M/T cells inside the AOB. AOB M/T cells differ from MOB M/T cells in that the former are not restricted to innervate only one glomerulus, but the latter are (Del Punta et al. 2002). Instead, AOB M/T cells perform synaptic contacts with 6–10 glomeruli sharing innervation from vomeronasal nerve (VNN) neurons expressing a common vomeronasal receptor (Del Punta et al. 2002; Hovis et al. 2012). This property might allow AOB M/T cells to stay post‐ and pre‐synaptically active, hence alive, even if some of the glomeruli they innervate become less active or even obliterated. A more detailed study focused on how the bias in the M/T cells number arises during ontogeny may help to clarify this latter point.
AOB bias and social environment
Social interactions in mammals rely to a great extent upon semiochemical interchanges (for review, see Liberles, 2014). This observation led us to hypothesize that species exhibiting different degrees of sociality would have differences in the specialization of the VNS. Interestingly, even though O. degus and O. lunatus are closely related species, they exhibit marked differences in their social behaviours. On the one hand, O. degus exhibits a very rich social system. Individuals of this species live in groups composed of a dominant male and two–five females, plus their offspring. Members of a group share the same burrow system, including a communal nest site, and exhibit a variety of cooperative behaviours, such as communal play, construction and maintenance of burrows, anti‐predatory surveillance and alarm calls emission (Ebensperger & Bozinovic, 2000; Ebensperger et al. 2004, 2006; Jesseau et al. 2009). In contrast, O. lunatus individuals do not share burrows nor do they form communal groups or engage in cooperative behaviours. However, males and females exhibit a partial overlap in their household domains, reflecting that social bonds, up to a certain degree, also take place in this species (Sobrero et al. 2014). Such contrasting social behaviours very likely imply differences in the frequency and diversity of the pheromonal interactions that take place during the lifespan of individuals of each species. In O. degus, the conformation of the social ensemble likely involves a high frequency and a variety of interactions in which pheromonal cues, along with olfactory cues, are rehearsed. In O. lunatus, in which social interactions seem to be less relevant, a comparatively low frequency of pheromonal interactions can be expected.
Can AOB plasticity be driven by social behaviour?
In this context, it is relevant to note that the neural components of the vertebrate olfactory system exhibit a remarkable activity‐dependent neuronal plasticity. More specifically, it has been repeatedly shown that glomeruli maturation, size and number, and even the overall size of the MOB, are dependent on olfactory activity at early and advanced stages of ontogeny, in animals as distant as fishes, rodents and humans. (Sullivan et al. 1989; Zou et al. 2004; Li et al. 2005; Kerr & Belluscio, 2006; Valle‐Leija et al. 2012; Cheetham et al. 2016; Negoias et al. 2017; Scheib et al. 2019) In the case of the VNS, recent studies in mice reported that the pool (in terms of different chemosensitive types) of VN neurons is subject to considerable changes in composition after prolonged exposition to different combinations of semiochemicals (Xu et al. 2016). In mice, the AOB glomeruli underwent an increment in the size of the VNN axonal arborization and a delay in its coalescence after prolonged exposition to pheromones during early postnatal development (Hovis et al. 2012).
There is also evidence suggesting that social activity could be a driving factor of the plasticity of the AOB components. In mice, male−male social interactions led to changes in the excitatory/inhibitory synaptic balance between mitral and granular AOB cells (Cansler et al. 2017). In the solitary tree shrews, Tupaia glis, the total AOB volume becomes significantly smaller in individuals raised in captivity than in wild animals, likely as a consequence of the disruption of normal social interactions during captivity (Frahm et al. 1984). In hamsters, the size of synaptic contact zones and the somal area of M/T cells in the AOB become larger in animals raised in social conditions compared with those raised in isolation (Matsuoka et al. 1994, 1998). Our own preliminary observations indicated that degus raised in captivity features at adulthood a less marked bias between AOB subdomains, especially at the GL (data not shown).
In summary, our results, together with the previous evidence, lead us to conjecture that: (i) in octodontid rodents, the orchestration of social behaviours involves active volatile pheromonal signalling; and (ii) the frequency and relevance of the social interactions enacted in the life mode of these species may lead the AOB to reach different structural outcomes in different species, both in ontogeny and phylogeny, in what appears to be a ‘Baldwinian’ case of canalization of phenotypic plasticity (Baldwin, 1896; Pigliucci et al. 2006). It is worth noting that this latter process seems to act specifically over certain AOB components, namely the GL, leaving others, the M/T cells, unaffected.
Altogether, the evidence suggests that the structure of the VNS system can be shaped, in a very specific and direct manner, by the behavioural demands entailed in the lifestyle of different species. The mechanisms underlying this process deserve to be studied more. In particular, the comparative study of the ontogeny of the VNS in different species may serve to validate or discard these conjectures.
Author contributions
PFA conceived and performed the experiments, analysed the results and co‐wrote the manuscript. SED performed the experiments and analysed the results. RS provided the materials and analysed the results. JM conceived the experiments, supervised the project, and wrote the manuscript.
Acknowledgements
The authors gratefully acknowledge Elisa Sentis and Solano Henriquez for their technical assistance. Thanks to Dr Camilo Libedinsky for his comments on the manuscript. This study was supported by FONDECYT 1170027 (JM), MECESUP Doctoral Fellowship UCH0713 (PFA) and FONDECYT 3150306 (RS). The authors declare no competing financial interests.
Data availability statement
The data and images that support the findings of this study are available from the corresponding authors upon reasonable request.
References
- Baldwin JM (1896) A new factor in evolution. Am Nat 30, 536–553. [Google Scholar]
- Barton RA, Purvis A, Harvey PH (1995) Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Philos Trans R Soc Lond B Biol Sci 348, 381–392. [DOI] [PubMed] [Google Scholar]
- von Campenhausen H, Mori K (2000) Convergence of segregated pheromonal pathways from the accessory olfactory bulb to the cortex in the mouse. Eur J Neurosci 12, 33–46. [DOI] [PubMed] [Google Scholar]
- Cansler HL, Maksimova MA, Meeks JP (2017) Experience‐dependent plasticity in accessory olfactory bulb interneurons following male‐male social interaction. J Neurosci 37, 7240–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi C, Penna M, Vásquez RA. (2003) Alarm calls in the social caviomorph rodent Octodon degus. Paper presented at the XXVIII International Ethological Conference, Florianópolis, Brasil. Revista de Etología (Suplemento).
- Chamero P, Katsoulidou V, Hendrix P, et al. (2011) G protein Gαo is essential for vomeronasal function and aggressive behavior in mice. Proc Natl Acad Sci USA 108, 12 898–12 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham CEJ, Park U, Belluscio L (2016) Rapid and continuous activity‐dependent plasticity of olfactory sensory input. Nat Comm 7, 10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley CA, Moss RL (1999) Activation of an anatomically distinct subpopulation of accessory olfactory bulb neurons by chemosensory stimulation. Neuroscience 91, 1549–1556. [DOI] [PubMed] [Google Scholar]
- Ebensperger LA, Bozinovic F (2000) Communal burrowing in the hystricognath rodent, Octodon degus: A benefit of sociality? Behav Ecol Sociobiol 47, 365–369. [Google Scholar]
- Ebensperger LA, Caiozzi A (2002) Male degus, Octodon degus, modify their dustbathing behavior in response to social familiarity of previous dustbathing marks. Rev Chil Hist Nat 75, 157–163. [Google Scholar]
- Ebensperger LA, Hurtado MJ, Soto‐Gamboa M, et al. (2004) Communal nesting and kinship in degus (Octodon degus). Naturwissenschaften 91, 391–395. [DOI] [PubMed] [Google Scholar]
- Ebensperger LA, Hurtado MJ, Ramos‐Jiliberto R (2006) Vigilance and collective detection of predators in degus (Octodon degus). Ethology 112, 879–887. [Google Scholar]
- Frahm HD, Stephan H, Baron G (1984) Comparison of accessory olfactory bulb volumes in the common tree shrew (Tupaia glis). Acta Anat 119, 129–135. [DOI] [PubMed] [Google Scholar]
- Fulk G (1976) Notes on the activity, reproduction, and social behavior of Octodon degus . J Mammal 57, 495–505. [Google Scholar]
- Glanz WE, Meserve PL (1982) An ecological comparison of the small mammal communities in California and Chile In: Dynamics and Management of Mediterranean‐type Ecosystems. (edsConrad CE, Oechel WC), pp. 220–226. Berkeley, CA: United States Department of Agriculture and Forest Service. Gen Tech Rep PSW‐58, 1–637. [Google Scholar]
- Halpern M, Shapiro LS, Jia C (1995) Differential localization of G proteins in the opossum vomeronasal system. Brain Res 677, 157–161. [DOI] [PubMed] [Google Scholar]
- Halpern M, Jia C, Shapiro LS (1998) Segregated pathways in the vomeronasal system. Microsc Res Tech 41, 519–529. [DOI] [PubMed] [Google Scholar]
- Herrera E, MacDonald D (1994) Social significance of scent marking in capybaras. J Mammal 75, 410–415. [Google Scholar]
- Hovis KR, Ramnath R, Dahlen JE, et al. (2012) Activity regulates functional connectivity from the vomeronasal organ to the accessory olfactory bulb. J Neurosci 32, 7907–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura K, Kashiwayanagi M (2000) Inward current responses to urinary substances in rat vomeronasal sensory neurons. Eur J Neurosci 12, 3529–3536. [DOI] [PubMed] [Google Scholar]
- Inamura K, Matsumoto Y, Kashiwayanagi M, et al. (1999) Laminar distribution of pheromone‐receptive neurons in rat vomeronasal epithelium. J Physiol 517, 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniuk AN, Wylie DR (2007) Neural specialization for hovering in hummingbirds: Hypertrophy of the pretectal nucleus lentiformis mesencephali. J Comp Neurol 500, 211–221. [DOI] [PubMed] [Google Scholar]
- Jerison H (1973) Evolution of the Brain and Intelligence. New York: Academic Press. [Google Scholar]
- Jesseau SA, Holmes WG, Lee TM (2009) Communal nesting and discriminative nursing by captive degus, Octodon degus . Anim Behav 78, 1183–1188. [Google Scholar]
- Jia C, Halpern M (1996) Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Gαi2 and Goα) and segregated projections to the accessory olfactory bulb. Brain Res 719, 117–128. [DOI] [PubMed] [Google Scholar]
- Kerr MA, Belluscio L (2006) Olfactory experience accelerates glomerular refinement in the mammalian olfactory bulb. Nat Neurosci 9, 484–486. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, et al. (2005) Sex‐specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature 437, 898–901. [DOI] [PubMed] [Google Scholar]
- Kondoh D, Watanabe K, Nishihara K, et al. (2017) Histological properties of main and accessory olfactory bulbs in the common hippopotamus. Brain Behav Evol 90, 224–231. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dudley CA, Moss RL (1999) Functional dichotomy within the vomeronasal system: distinct zones of neuronal activity in the accessory olfactory bulb correlate with sex‐specific behaviors. J Neurosci 19, RC32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders‐Zufall T, Lane AP, Puche AC, et al. (2000) Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature 405, 792–796. [DOI] [PubMed] [Google Scholar]
- Leinders‐Zufall T, Brennan P, Widmayer P, et al. (2004) MHC class I peptides as chemosensory signals in the vomeronasal organ. Science 306, 1033–1037. [DOI] [PubMed] [Google Scholar]
- Leinders‐Zufall T, Ishii T, Mombaerts P, et al. (2009) Structural requirements for the activation of vomeronasal sensory neurons by MHC peptides. Nat Neurosci 12, 1551–1558. [DOI] [PubMed] [Google Scholar]
- Li J, Mack JA, Souren M, et al. (2005) Early development of functional spatial maps in the zebrafish olfactory bulb. J Neurosci 25, 5784–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles SD (2014) Mammalian pheromones. Annu Rev Physiol 76, 151–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D, Krantz K, Aplin R (1984) Behavioural, anatomical and chemical aspects of scent marking amongst capybaras, Hydrochoeris hydrochaeris (Rodentia: Caviomorpha). J Zool 202, 341–360. [Google Scholar]
- Martinez‐Marcos A (2009) On the organization of olfactory and vomeronasal cortices. Prog Neurogibol 87, 21–30. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Mori Y, Hoshino K, et al. (1994) Social environment affects synaptic structure in the glomerulus of the accessory olfactory bulb of the hamster. Neurosci Res 19, 187–193. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Mori Y, Ichikawa M (1998) Morphological changes of synapses induced by urinary stimulation in the hamster accessory olfactory bulb. Synapse 28, 160–166. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Yokosuka M, Mori Y, et al. (1999) Specific expression pattern of Fos in the accessory olfactory bulb of male mice after exposure to soiled bedding of females. Neurosci Res 35, 189–195. [DOI] [PubMed] [Google Scholar]
- Meisami E, Bhatnagar KP (1998) Structure and diversity in mammalian accessory olfactory bulb. Microsc Res Tech 43, 476–499. [DOI] [PubMed] [Google Scholar]
- Negoias S, Pietsch K, Hummel T (2017) Changes in olfactory bulb volume following lateralized olfactory training. Brain Imaging Behav 11, 998–1005. [DOI] [PubMed] [Google Scholar]
- Ngwenya A, Patzke N, Ihunwo AO, et al. (2011) Organisation and chemical neuroanatomy of the african elephant (Loxodonta africana) olfactory bulb. Brain Struct Func 216, 403–416. [DOI] [PubMed] [Google Scholar]
- Peele R, Salazar I, Mimmack M, et al. (2003) Low molecular weight constituents of male mouse urine mediate the pregnancy block effect and convey information about the identity of the mating male. Eur J Neurosci 18, 622–628. [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Murren CJ, Schlichting CD (2006) Phenotypic plasticity and evolution by genetic assimilation. J Exp Biol 209, 2362–2367. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Puche A, Adams NC, et al. (2002) A divergent pattern of sensory axonal projections is rendered convergent by second‐order neurons in the accessory olfactory bulb. Neuron 35, 1057–1066. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS (1975) The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol 161, 31–55. [DOI] [PubMed] [Google Scholar]
- Scheib JJ, Pozzuto JM, Byrd‐Jacobs CA (2019) Reversible deafferentation of the zebrafish olfactory bulb with wax plug insertion. J Neurosci Methods 311, 47–56. [DOI] [PubMed] [Google Scholar]
- Sobrero R, Prieto ÁL, Ebensperger LA (2014) Activity, overlap of range areas, and sharing of resting locations in the moon‐toothed degu, Octodon lunatus . J Mammal 95, 91–98. [Google Scholar]
- Suárez R, Mpodozis J (2009a) Heterogeneities of size and sexual dimorphism between the subdomains of the lateral‐innervated accessory olfactory bulb (AOB) of Octodon degus (Rodentia: Hystricognathi). Behav Brain Res 198, 306–312. [DOI] [PubMed] [Google Scholar]
- Suárez R, Villalón A, Künzle H, et al. (2009b) Transposition and intermingling of Gαi2 and Gαo afferences into single vomeronasal glomeruli in the Madagascan lesser tenrec Echinops telfairi . PLoS ONE 4, e8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez R, Fernández‐Aburto P, Manger PR, et al. (2011a) Deterioration of the Gαo vomeronasal pathway in sexually dimorphic mammals. PLoS ONE 6, e26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez R, Santibáñez R, Parra D, et al. (2011b) Shared and differential traits in the accessory olfactory bulb of caviomorph rodents with particular reference to the semiaquatic capybara. J Anat 218, 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai T, Yoshimura H, Kato N, et al. (2006) Component‐dependent urine responses in the rat accessory olfactory bulb. NeuroReport 17, 1663–1667. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M (1989) Norepinephrine and learning‐induced plasticity in infant rat olfactory system. J Neurosci 9, 3998–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigami S, Mori Y, Ichikawa M (2000) Projection pattern of vomeronasal neurons to the accessory olfactory bulb in goats. Chem Senses 25, 387–393. [DOI] [PubMed] [Google Scholar]
- Takigami S, Mori Y, Tanioka Y, et al. (2004) Morphological evidence for two types of mammalian vomeronasal system. Chem Senses 29, 301–310. [DOI] [PubMed] [Google Scholar]
- Tirindelli R, Dibattista M, Pifferi S, et al. (2009) From pheromones to behavior. Physiol Rev 89, 921–956. [DOI] [PubMed] [Google Scholar]
- Valle‐Leija P, Blanco‐Hernández E, Drucker‐Colín R, et al. (2012) Supernumerary formation of olfactory glomeruli induced by chronic odorant exposure: A constructivist expression of neural plasticity. PLoS ONE 7, e35358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega‐Zuniga T, Medina FS, Fredes F, et al. (2013) Does nocturnality drive binocular vision? Octodontine rodents as a case study. PLoS ONE 8, e84199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamayor PR, Cifuentes JM, Fdz‐de‐Troconiz P, et al. (2018) Morphological and immunohistochemical study of the rabbit vomeronasal organ. J Anat 233, 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PS, Lee D, Holy TE (2016) Experience‐dependent plasticity drives individual differences in pheromone‐sensing neurons. Neuron 91, 878–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez J (1976) Eco‐etología de Octodon degus . Bachelors thesis, Universidad de Chile, Santiago, Chile.
- Zhao H, Xu D, Zhang S, et al. (2011) Widespread losses of vomeronasal signal transduction in bats. Mol Biol Evol 28, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou DJ, Feinstein P, Rivers AL, et al. (2004) Postnatal refinement of peripheral olfactory projections. Science 304, 1976–1979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and images that support the findings of this study are available from the corresponding authors upon reasonable request.


