Abstract
The array of end organ innervations of the vagus nerve, coupled with increased basic science evidence, has led to vagus nerve stimulation (VNS) being explored as a management option in a number of clinical disorders, such as heart failure, migraine and inflammatory bowel disease. Both invasive (surgically implanted) and non‐invasive (transcutaneous) techniques of VNS exist. Transcutaneous VNS (tVNS) delivery systems rely on the cutaneous distribution of vagal afferents, either at the external ear (auricular branch of the vagus nerve) or at the neck (cervical branch of the vagus nerve), thus obviating the need for surgical implantation of a VNS delivery device and facilitating further investigations across a wide range of uses. The concept of electrically stimulating the auricular branch of the vagus nerve (ABVN), which provides somatosensory innervation to several aspects of the external ear, is relatively more recent compared with cervical VNS; thus, there is a relative paucity of literature surrounding its operation and functionality. Despite the increasing body of research exploring the therapeutic uses of auricular transcutaneous VNS (tVNS), a comprehensive review of the cutaneous, intracranial and central distribution of ABVN fibres has not been conducted to date. A review of the literature exploring the neuroanatomical basis of this neuromodulatory therapy is therefore timely. Our review article explores the neuroanatomy of the ABVN with reference to (1) clinical surveys examining Arnold’s reflex, (2) cadaveric studies, (3) fMRI studies, (4) electrophysiological studies, (5) acupuncture studies, (6) retrograde tracing studies and (7) studies measuring changes in autonomic (cardiovascular) parameters in response to auricular tVNS. We also provide an overview of the fibre composition of the ABVN and the effects of auricular tVNS on the central nervous system. Cadaveric studies, of which a limited number exist in the literature, would be the ‘gold‐standard’ approach to studying the cutaneous map of the ABVN; thus, there is a need for more such studies to be conducted. Functional magnetic resonance imaging (fMRI) represents a useful surrogate modality for discerning the auricular sites most likely innervated by the ABVN and the most promising locations for auricular tVNS. However, given the heterogeneity in the results of such investigations and the various limitations of using fMRI, the current literature lacks a clear consensus on the auricular sites that are most densely innervated by the ABVN and whether the brain regions secondarily activated by electrical auricular tVNS depend on specific parameters. At present, it is reasonable to surmise that the concha and inner tragus are suitable locations for vagal modulation. Given the therapeutic potential of auricular tVNS, there remains a need for the cutaneous map of the ABVN to be further refined and the effects of various stimulation parameters and stimulation sites to be determined.
Keywords: Acupuncture therapy, Autonomic nervous system, Cadaver, Cardiovascular system, Cranial nerves, Electrical stimulation, Magnetic resonance imaging, Neuroanatomy, Pain, Vagus nerve, Vagus nerve stimulation
A schematic diagram of the approximate intracranial relationships between the facial, glossopharyngeal and vagus nerves.
Background
The autonomic nervous system (ANS) via its sympathetic and parasympathetic divisions influences the function of numerous organs, glands and involuntary muscles throughout the body. A major component of the parasympathetic nervous system is the vagus nerve, the 10th and longest of the cranial nerves, which serves an important bidirectional conduit between the body and brain, largely serving to maintain homeostasis. Derived from the Latin word meaning ‘wandering’, the vagus nerve courses from the brainstem to the proximal two‐thirds of the colon, innervating multiple thoracic and abdominal viscera en route. It is a mixed nerve composed of 20% efferent fibres and 80% afferent fibres (Bonaz et al. 2018).
Imbalances in the activity of the constituent parts of the ANS have been linked with several clinical disorders (Farmer et al. 2016): heart failure (De Ferrari et al. 2011), inflammatory bowel disease (Ghia et al. 2006) and chronic pain syndromes (Farmer et al. 2014). In general, the reported imbalance involves relatively higher sympathetic activity associated with a paucity of parasympathetic activity (Farmer et al. 2016). In conjunction with deep slow‐paced breathing, tVNS has been proposed as novel non‐pharmacological analgesic intervention for pain management (Botha et al. 2014; Farmer & Aziz, 2015). In healthy subjects, we have previously demonstrated that central sensitisation can be prevented by physiological stimulation of the vagus nerve, using deep slow‐paced breathing, in a validated model of oesophageal pain hypersensitivity (Botha et al. 2015). Therefore, efforts to therapeutically rebalance this equilibrium with techniques such as vagus nerve stimulation (VNS), which putatively increases the activity of the vagus nerve, are of interest across a range of clinical disciplines.
Both invasive (surgically implanted) and non‐invasive (transcutaneous) techniques of VNS exist. Invasive VNS (iVNS) involves the surgical implantation of a programmable pulse generator device in the chest wall and the placement of electrodes around the left (typically) cervical vagus nerve. As it currently stands, iVNS has several potential risks. There are reports of bradycardia and asystole occurring during intraoperative lead testing, as a result of unintentional direct stimulation of the cardiac branches of the vagus nerve, occurring in approximately 1 in 1000 implantations (Asconape et al. 1999). In the direct postoperative period, implantation can result in a peri‐incisional haematoma, dyspnoea and localised infection around the wound site (Fahy, 2010). Up to two‐thirds of patients suffer from transient dysphonia and some patients can experience paraesthesia and pain (Watkins et al. 1995; Malow et al. 2000; Santos, 2003). Further information on iVNS, which is outside the scope of this article, is available elsewhere (Farmer et al. 2016).
Novel non‐invasive (or transcutaneous) VNS delivery systems rely on the cutaneous distribution of vagal fibres, either at the external ear (auricular branch of the vagus nerve) or at the neck (cervical branch of the vagus nerve), thus obviating the need for surgical implantation and facilitating further investigations across a wide range of uses (Ben‐Menachem et al. 2015). Functional magnetic resonance imaging (fMRI) of the brain has demonstrated that tVNS can stimulate brain areas consistent with the contemporaneously accepted understanding of central vagal projections (Frangos et al. 2015). Several devices can deliver tVNS. For example, the NEMOS® (CerboMed, Erlangen, Germany) stimulates the concha of the outer ear and is CE‐marked (European Conformity) for the European market for the management of epilepsy. The electrode is connected to a stimulating box, and the stimulation intensity can be adjusted by the patient, caregiver or treating physician (increased at steps of 0.1 mA until the perception threshold of the electrical stimulation is reached; the stimulation frequency is predefined at 25 Hz, the pulse width of 250 µs). The hand‐held stimulator gammaCore (Electrocore LLC, Basking Ridge, NJ, USA) is used in transcutaneous stimulation of the cervical branch of the vagus nerve and is approved by the Food and Drug Administration for the management of episodic cluster headache (Mwamburi et al. 2017). The device produces a pulsatile waveform (1‐ms pulses comprising 5‐Hz sine waves repeated at 25 Hz). The recommended duration of stimulation is 2 min and can be administered several times (up to 12 times) a day (Holle‐Lee & Gaul, 2016). Recognised side‐effects of tVNS can include local skin irritation from electrode placement, headache and nasopharyngitis (Redgrave et al. 2018). A common side‐effect of cervical tVNS is painless, mild facial twitching (Holle‐Lee & Gaul, 2016).
Although Eastern medicine has utilised the analgesic effects of auricular acupuncture for thousands of years (Asher et al. 2010; He et al. 2012; Usichenko et al. 2017), the concept of electrically stimulating the auricle is a more recent one (Ventureyra, 2000). The feasibility of auricular tVNS was first demonstrated using recordings of vagus somato‐sensory evoked potentials from the scalp (Fallgatter et al. 2003) and since then has been proposed as an effective therapeutic strategy in the management of several clinical disorders including epilepsy (Rong et al. 2014), depression (Kong et al. 2018), migraine (Straube et al. 2015) and tinnitus (Hyvarinen et al. 2015). The basis of auricular tVNS is the cutaneous representation of the auricular branch of the vagus nerve (ABVN), also termed Arnold’s nerve, which provides somatosensory innervation to the external ear. The latter name of the nerve is an eponym for the German anatomist Friedrich Arnold (1803–1890), who was the first to document that irritation of the posterior wall of the external acoustic meatus could induce a cough reflex in humans (Lekakis, 2003). The ABVN is sometimes referred to as Alderman's nerve; a reference to the centuries‐old Aldermen of the City of London and their practice of using rosewater bowls at ceremonial banquets, where attendees were encouraged to place a napkin moistened with rosewater behind their ears in the belief that this would aid digestion (Murray et al. 2016). Studies have shown that the ear–cough reflex occurs in between 1.7–4.2% of individuals (Bloustine et al. 1976; Gupta et al. 1986; Tekdemir et al. 1998). The ABVN is additionally credited as the afferent pathway to other somewhat unusual somatovisceral reflexes, including (1) the gastro‐auricular phenomenon, (2) the pulmono‐auricular phenomenon, (3) the auriculo‐genital reflex and (4) the auriculo‐uterine reflex (Engel, 1979; Gupta et al. 1986).
The ABVN continues to be an area of pathophysiological interest across a number of clinical disciplines. As research into the use of auricular tVNS is rapidly growing, a review exploring the anatomical basis of this neuromodulatory technique is timely. To date, there has been no review of the literature investigating the anatomical basis of the ABVN, with reference to its cutaneous, intracranial and central distribution. Our overall aim was to address the aforementioned gap in the literature.
Anatomy of the external ear
The construction of devices for auricular tVNS relies on a precise knowledge of the anatomy and cutaneous innervation of the external ear. Fig. 1 outlines a schematic diagram of the external ear and the current consensus on the cutaneous map of the nerve fibres innervating the lateral auricle, although this dermatome map should be interpreted with caution. Indeed, a precise cutaneous map of the external ear is not practical for several reasons: (1) the cutaneous distribution of a particular nerve root can vary considerably, (2) some nerve fibres cross‐communicate with other nerve fibres along their intracranial course and (3) the boundaries between particular dermatomes are not necessarily distinct and often overlap. The challenges of producing a precise cutaneous map of the auricle and the inter‐study variability in the distribution of ABVN fibres are explored herein.
Figure 1.
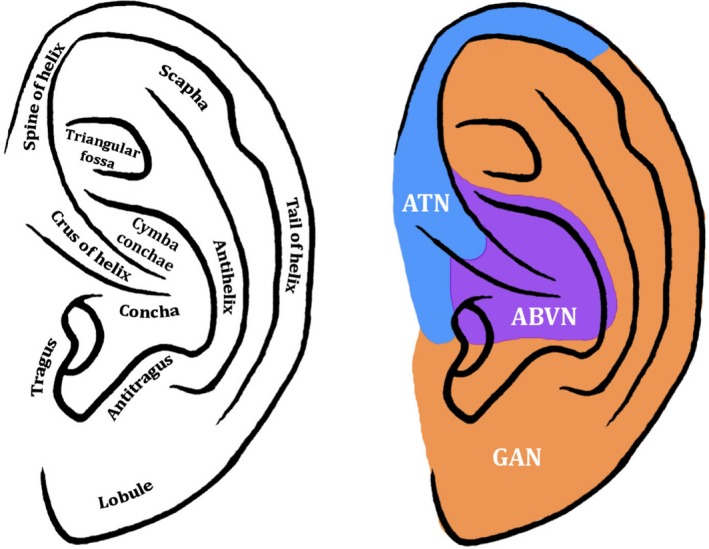
Diagram of the external ear and its hypothesised cutaneous innervation. Permissions: No permissions required
Most of the commercially available devices used for auricular tVNS target the concha of the external ear (Bermejo et al. 2017; Redgrave et al. 2018), whose innervation is complicated by multiple neural communications of partly somatogenic and branchiogenic origin: the ABVN, the auriculotemporal nerve – a sensory branch of the posterior division of the mandibular division of the trigeminal nerve (Schmalfuss et al. 2002), the facial nerve, the greater auricular nerve and the lesser occipital nerve. The latter two nerves are superficial branches of the cervical plexus, contributed by fibres from the C2 and C3 spinal nerves (Ginsberg & Eicher, 2000).
Evolutionary and embryological development of the ABVN
The auricle (visible part of the ear that resides outside of the head) is formed by contributions from the first two pharyngeal (or branchial) arches – the mandibular and hyoid – whereas the external auditory meatus (which extends from the deepest part of the concha to the tympanic membrane) is formed from the first pharyngeal groove. The pharyngeal arches arise in the 4th week of embryonic development and act as precursors for head and neck structures. The depressions between the pharyngeal arches are known as pharyngeal grooves and are composed of ectoderm, itself a precursor to skin. The surface depressions between the pharyngeal arches on the endodermal surface will form the pharyngeal pouches. The ABVN is the remnant of a more extensive embryonic nerve which once supplied the first pharyngeal arch (Gupta et al. 1986) and is thought to be derived from nerves supplying the lateral line organs (a system of sense organs used to detect movement, vibration and pressure gradient in surrounding water) in aquatic vertebrates, such as fish (Hoagland, 1933; Engel, 1979). A comprehensive review exploring the embryologic development of the external ear is provided elsewhere (Wright, 1997).
Classical studies exploring the cutaneous distribution of the ABVN
It was Friedrich Arnold who, after discovering the ear–cough reflex, conducted a dissection which confirmed that the ABVN served as the afferent pathway of this unusual reflex (Arnold, 1831). However, it was not until Sherrington (1857–1952) applied his technique of ‘remaining aesthesia' that a more refined sensory map of the ABVN and other cranial and cervico‐brachial nerve roots supplying the outer ear materialised (Sherrington, 1898). After severing the trigeminus and the highest three cervical nerves in the vertebral canal of two macaques, Sherrington noted sensitive skin in ‘practically the whole of the concha, the antitragus, part of the tragus and part of the antihelix; also part of the fossa of the antihelix'. He concluded with ‘little doubt’ that the sensitive skin was supplied by the un‐severed vagus nerve, which was otherwise impossible to section, as this rendered the animal lame. Findings ‘extremely like those of Sherrington on the macaque’ were later reported in a human study conducted by Temple Fay (1895–1963) following a subtotal avulsion of the posterior root of the trigeminus (section of the outer and lower two‐thirds of the posterior root) and intracranial section of the posterior root of the vagus in a patient with intractable pain in the ear and throat due to a metastatic tongue carcinoma (Fay, 1927). Fay reported that sectioning of the trigeminal nerve caused analgesia ‘that was complete over the tragus, anterior wall of the internal auditory canal [and] over the cheek in the third divisional area’. Sectioning of the trigeminal nerve followed by the vagus root caused the cutaneous area of anaesthesia to enlarge ‘from the base of the tragus and anterior wall of the internal auditory canal to include the posterior wall, the concha and, to a slight degree for pain only, the antihelix and antitragus’.
Contemporary studies exploring the cutaneous distribution of the ABVN
Clinical surveys
Several clinical surveys have suggested the external acoustic meatus (EAM) as a region innervated by the ABVN and therefore a potential site for auricular tVNS to be effectively delivered. In one such study (Gupta et al. 1986), 21 of 500 (4.2%) randomly selected outpatients had a clinically positive Arnold's ear–cough reflex when the posterior and inferior quadrants of the EAM were palpated, and the reflex could also be elicited by palpating the anterior quadrant of the EAM in 10 (2%) patients. Similarly, Tekdemir et al. (1998) demonstrated Arnold's ear–cough reflex in 11 of 514 (2.1%) patients after palpating the postero‐inferior aspect of the EAM and one (0.2%) patient after palpating the antero‐inferior aspect of the EAM. Although the frequencies are different, these crude clinical surveys suggest that it is anatomically possible for the ABVN to be distributed to the anterior and posterior walls of the EAM, with the posterior wall likely to have a denser innervation of ABVN fibres compared with the anterior wall.
Cadaveric studies
A list of cadaveric studies that have examined the cutaneous and intracranial course of the ABVN is outlined in Table 1. In an attempt to discern the cutaneous and intracranial course of the ABVN, Tekdemir et al. (1998) demonstrated that ABVN fibres innervated the EAM; however, those authors were unable to determine the precise sensory map of these fibres, as the terminal branches of the ABVN were ‘extremely fine’ and may have been destroyed during dissection. In a Japanese study (Kiyokawa et al. 2014), the nerve distribution around the EAM and the auricle, as well as the intracranial relationships between the ABVN, facial and glossopharyngeal nerves, were determined. That study revealed the antero‐superior aspect of the EAM to be innervated by the auriculotemporal nerve, and the inferior wall by the greater auricular nerve. In comparison, the postero‐superior wall of the acoustic meatus was innervated by two types of nerve branches, both of which originated from the superior ganglion of the vagus nerve. The outcomes of such cadaveric studies provide further evidence supporting the suggestion that the EAM, and particularly its posterior aspect, may be a suitable location to deliver auricular tVNS.
Table 1.
A summary of cadaveric studies that have assessed the intracranial and/or cutaneous map of the auricular branch of the vagus nerve (ABVN).
| Reference | Number of cadavers (sex if available) | Mean age of cadavers (range if available) | Number of ears | Summary of salient findings in relation to the ABVN |
|---|---|---|---|---|
| (Ueno et al. 1993) | 2m, 1f |
71m (70–72) 65f (65) |
5 (3m, 2f) |
|
| (Tekdemir et al. 1998) | 18m | 57 (49–71) | 16 |
|
| (Peuker & Filler, 2002) | 7 (m and f) | N/A (68–84) | 14 |
|
| (Kiyokawa et al. 2014) |
7m, 4f |
79.6 (N/A) | 18 |
|
| (Watanabe et al. 2016) | 3 | N/A | 6 |
|
Permissions: No permissions are required.
ABVN, Auricular branch of the vagus nerve; f, female; M, male.
In addition to the EAM, there is cadaveric evidence that the inner tragus and skin surrounding the cymba concha may be innervated by the ABVN. One dissection study – arguably the most cited piece of work in the field of auricular tVNS – performed on 7 German cadavers (14 ears) systematically reported the sensory map of the lateral auricle (Peuker & Filler, 2002). Nevertheless, there are several apparent inconsistencies in this study which limit the interpretability of its findings (Burger & Verkuil, 2018). Importantly, there are contradictions between the outcomes described in the main text and those listed in the manuscript’s table regarding the innervation of the antihelix, the tragus and the cavity of the concha. According to the original table in the manuscript, Peuker & Filler (2002) report the tragus to be innervated by the ABVN in 45% of the exposed auricles. However, in the main text, the authors state that the tragus is innervated either by the greater auricular nerve (45% of all exposed auricles) or by the auriculotemporal nerve (9%), or by both of these nerves (46%). The main text does not mention that the tragus is innervated by the ABVN, and this is inconsistent with their table (Burger & Verkuil, 2018).
Contemporary cadaveric studies have detailed the intracranial course of the ABVN and its inter‐neural connections with the glossopharyngeal, trigeminal and facial nerves (Tekdemir et al. 1998; Kiyokawa et al. 2014; Watanabe et al. 2016). After arising from the superior jugular ganglion, the ABVN is soon accompanied by fibres from the inferior ganglion of the glossopharyngeal nerve. The ABVN then passes outwards behind the bulb of the internal jugular vein, and ascends through the mastoid canaliculus on the lateral surface of the jugular fossa (Proctor, 1989) or runs in a groove on the inferior aspect of the temporal bone (Guild, 1953). In the mastoid, it courses over the fallopian canal approximately 4–5 mm above the stylomastoid foramen (Tekdemir et al. 1998). There it divides into two main branches. The first branch provides sensory innervation to the dura mater of the posterior cranial fossa and has a connection with the chorda tympani, a branch of the facial nerve (Watanabe et al. 2016). The second branch divides into two smaller nerves, one connecting to the posterior auricular nerve – a branch of the facial nerve – and the second innervating the postero‐inferior skin of the external auditory canal and the adjacent tympanic membrane (Watanabe et al. 2016). The second branch also has an inferior division connecting to the posterior auricular branch of the facial nerve after exiting the stylomastoid foramen (Watanabe et al. 2016). A schematic diagram of the relationships between the various cranial nerves is given in Fig. 2. For a detailed description of the relationship between the facial nerve and ABVN we direct the reader to the review by Diamond et al. (2010).
Figure 2.
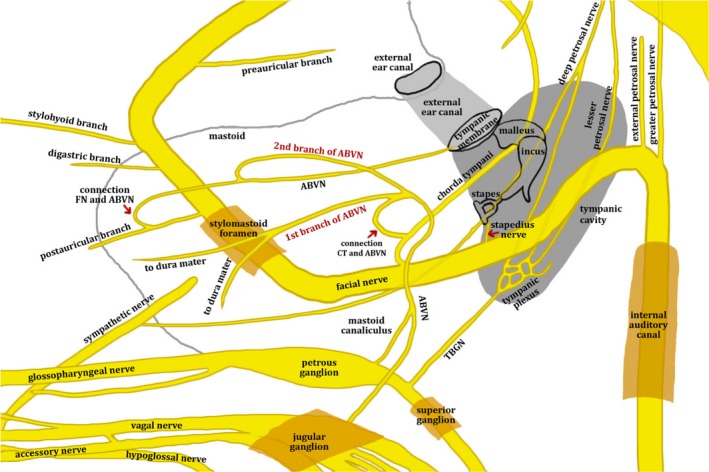
A schematic diagram of the approximate relationships between the facial, glossopharyngeal and vagus nerves. The ABVN originates from the petrous ganglion of the glossopharyngeal nerve and jugular ganglion of the vagus nerve and ascends through the mastoid canaliculus. In the mastoid, it crosses the fallopian canal 3–4 mm above the stylomastoid foramen and divides into two branches. The first branch of the ABVN has a connection with the chorda tympani and supplies the sensory part of the posterior cranial fossa dura mater, while the second branch supplies the posterior skin of the external auditory meatus and adjacent tympanic membrane. The second branch has an inferior division connecting to the posterior auricular branch of the facial nerve after exiting the stylomastoid foramen. ABVN, auricular branch of the vagus nerve; CT, chorda tympani nerve; FN, facial nerve; TBGN, tympanic branch of the glossopharyngeal nerve. Permissions: No permissions required.
To summarise, there is considerable heterogeneity in the outcomes of cadaveric studies investigating the cutaneous distribution of the ABVN. Outcomes from cadaveric studies make it tempting to speculate that the EAM, and in particular the posterior aspect, is supplied by the ABVN; this would be reasonable as Arnold’s cough reflex has been consistently evoked in clinical studies by touching the inferoposterior wall of the ear canal (Gupta et al. 1986; Tekdemir et al. 1998). Nevertheless, fMRI evidence (see below) does not suggest that the posterior wall of the ear canal is a suitable site for ABVN stimulation, and these studies generally accept the inner tragus (Dietrich et al. 2008; Kraus et al. 2013; Yakunina et al. 2017; Badran et al. 2018b) and cymba concha (Frangos et al. 2015; Yakunina et al. 2017; Wang et al. 2018) as active sites for vagal modulation, as supported by the cadaveric study conducted by Peuker & Filler (2002).
Functional magnetic resonance imaging (fMRI)
Utilising fMRI, it may be possible to compare the brain structures activated by auricular tVNS with iVNS, making it a useful surrogate modality for discerning the cutaneous map of the ABVN. A summary of fMRI studies that have been conducted to date and the auricular sites reported to engage the classical vagal centres is given in Table 2. fMRI studies of iVNS (Bohning et al. 2001; Lomarev et al. 2002; Narayanan et al. 2002; Liu et al. 2003; Nahas et al. 2007) and auricular tVNS (see Table 2) have reported activity within the same afferent vagal projection sites. The cell bodies of (human) afferent vagal nerve fibres are housed in the inferior vagal ganglion, thence projecting centrally to the brainstem where their processes terminate primarily in the nucleus of the solitary tract (Berthoud & Neuhuber, 2000; Knowles & Aziz, 2009).
Table 2.
A summary of functional magnetic resonance imaging (fMRI) studies that have investigated the brain regions secondarily activated by auricular transcutaneous vagal nerve stimulation.
| Reference | n | Subjects | Location of auricular stimulation | Location of sham stimulation | Parameters | Key findings | Effect on the autonomic nervous system (cardiovascular parameters) | One‐line summary |
|---|---|---|---|---|---|---|---|---|
| (Kraus et al. 2007) | 22 | Healthy controls | Left external acoustic meatus on the inner side of the tragus | Earlobe |
20 μs 8 Hz 30 s On 120 s Off |
Tragus vs. Control:
|
|
|
| (Dietrich et al. 2008) | 4 | Healthy controls | Inner wall of the left tragus | No (compared with baseline) |
250 μs 25 Hz 50 s On 100 s Off |
Tragus vs. baseline:
|
|
|
| (Kraus et al. 2013) | 16 | Healthy controls | Inner wall of the left tragus vs. posterior side of the left ear canal | Earlobe |
20 μs 8 Hz 30 s On 60 s Off |
Tragus vs. Control
Posterior side of the left ear canal vs. sham
Anterior side of the left ear canal vs. posterior side
Anterior versus posterior stimulation
Brain stem regions
|
|
|
| (Frangos et al. 2015) | 12 | Healthy controls | L cymba concha | Earlobe |
250 μs 25 Hz 7 min On 11 min Off |
Cymba concha vs. control
|
|
|
| (Yakunina et al. 2017) | 37 | Healthy controls |
The left inner tragus, left inferoposterior wall of the ear canal, left cymba concha |
Earlobe |
500 μs 25 Hz 6 min On 90 s Off |
|
|
|
| (Badran et al. 2018b) | 17 | Healthy controls | Left tragus | Earlobe |
500 μs 25 Hz 60 s On 60 s Off |
Tragus vs. control
|
|
|
The number of subjects, location of stimulation, site of sham stimulation, parameters of stimulation and pertinent findings are listed for each study.
Permissions: No permissions are required.
The nucleus of the solitary tract receives approximately 95% of projections from the vagus nerve (Magdaleno‐Madrigal et al. 2002; Lulic et al. 2009) and in turn projects to several other forebrain, limbic and brainstem sites, including the spinal trigeminal nucleus, parabrachial area, dorsal raphe, periaqueductal gray, thalamus, amygdala, insula, nucleus accumbens, bed nucleus of the stria terminalis, and hypothalamus (Sawchenko, 1983; Ruggiero et al. 2000; Knowles & Aziz, 2009). Importantly, the nucleus of the solitary tract projects to the locus coeruleus, the major noradrenergic brain structure in the brain that is thought to mediate several of the therapeutic effects underlying VNS (Fornai et al. 2011; Yakunina et al. 2017). Both the nucleus of the solitary tract and locus coeruleus are the assumed key targets of auricular tVNS (Keute et al. 2018b) and activation of these brainstem structures can be considered (indirect) proof of vagal activity.
The outcomes of fMRI studies presented in Table 2 demonstrate considerable heterogeneity in the brain regions secondarily activated by auricular tVNS, which may be a result of differences in sex distribution, frequencies of stimulation and locations of outer‐ear stimulation: the inner wall of the tragus (Kraus et al. 2007; Dietrich et al. 2008; Kraus et al. 2013; Yakunina et al. 2017; Badran et al. 2018b), the posterior aspect of the ear canal (Kraus et al. 2013) and the cymba concha (Frangos et al. 2015; Yakunina et al. 2017; Wang et al. 2018). Indeed, there is evidence that different sites on the external ear can activate vagal centres to different degrees (Yakunina et al. 2017). In one study (Yakunina et al. 2017), stimulation at the posterior aspect of the ear canal failed sufficiently to activate the locus coeruleus and nucleus of the solitary tract, which indicates that the vagal pathway was not sufficiently activated at this location. On the other hand, the same study showed that stimulation at the inner wall of the tragus and cymba concha successfully activated the vagal afferent pathway, with the greatest activation seen at the cymba concha, suggesting that this is the most promising site for auricular tVNS (Yakunina et al. 2017). In another study (Kraus et al. 2013), a head‐to‐head assessment of BOLD‐signal changes secondary to tVNS at the anterior vs. posterior wall of the auditory canal, revealed BOLD‐signal decreases in the locus coeruleus and nucleus of the solitary tract following stimulation at the anterior wall of the auditory canal, whereas stimulation at the posterior wall resulted in non‐specific changes of the BOLD‐signal within the solitary tract. The fMRI findings that the posterior wall of the ear canal is an unlikely site of ABVN innervation is interesting, as Arnold’s cough reflex has consistently been evoked in clinical surveys following physical stimulation at the inferoposterior wall of the ear canal (Gupta et al. 1986; Tekdemir et al. 1998). It is therefore likely that the cutaneous distribution of the ABVN exhibits significant heterogeneity, which is supported by the findings from dissection studies (see section on Cadaveric studies).
The nucleus of the solitary tract is indirectly and directly connected to a network of brain regions, including the amygdala and nucleus accumbens, which are postulated to influence the pathogenesis of depression (Mayberg, 1997; Mayberg et al. 1999; Dagyte et al. 2011). Thus, there may be a basis for the use of tVNS in managing depressive symptoms (Liu et al. 2016). fMRI can be used to investigate the relationship between changes in resting‐state functional connectivity (rsFC) secondary to tVNS. rsFC measures the temporal dependency of neuronal activation patterns between anatomically separated brain regions during rest (Biswal et al. 1995), which allows a study of the functional correlation of one brain region with other brain regions in terms of networks (Fang et al. 2016). Studies on patients with depression have demonstrated aberrant rsFC between the amygdala – the brain region implicated in emotional processing, fear and motivation – and regions in the frontal cortex, and that this is related to overall depression severity (Tang et al. 2013; Cullen et al. 2014; Pannekoek et al. 2014). In one study (n = 49, patients with mild/moderate depression), tVNS applied at the auricular concha area of both ears simultaneously (30 min, carried out twice a day, at least 5 days per week, for a 4‐week duration) compared with sham stimulation (superior scapha) resulted in increased rsFC between the right amygdala and left dorsolateral prefrontal cortex (Liu et al. 2016).
The ‘gold‐standard’ sham stimulation site in fMRI studies
A sham stimulation site – the ear lobe in the majority of published reports – ensures that the pattern of brain changes observed by fMRI are not caused by the conscious perception of touch, pressure, vibration or pain that may accompany auricular tVNS. A gold‐standard sham location should meet the following two (non‐exhaustive) criteria: (1) the site should not be innervated by ABVN fibres and (2) both the tVNS and sham operations should be clinically indistinguishable to the patient.
The earlobe has been widely used as a sham as it is thought to be relatively free of vagal afferents (Peuker & Filler, 2002; Yakunina et al. 2017) and innervated by the greater auricular nerve – a branch of the superficial cervical plexus. However, stimulation of the earlobe is not physiologically inert and has been shown to produce similar fMRI patterns to ABVN stimulation. In one tVNS study, several regions of the limbic system were deactivated by both sham stimulation (applied at the earlobe) and tVNS (applied at the inner surface of the tragus, inferoposterior wall of the external acoustic meatus and cymba concha): namely, the hippocampus, the posterior cingulate gyrus, parahippocampal gyrus and amygdala (Yakunina et al. 2017). Low‐intensity stimulation of the earlobe can be used as part of cranial electrotherapy stimulation, an FDA‐approved therapeutic strategy for the management of insomnia, depression and anxiety (Feusner et al. 2012). fMRI analyses of the neurophysiological changes induced by cranial electrotherapy stimulation have revealed negative BOLD changes in several brain regions, including the precuneus, posterior cingulate gyrus, precentral and postcentral gyri, and occipital cortex (Feusner et al. 2012). Several of these brain regions have also been affected in the published tVNS fMRI studies (see Table 2).
Limitations and future potential of fMRI
fMRI is a useful surrogate modality for determining the sites most likely innervated by the ABVN, granted that its use has several limitations. First, it is impossible to demonstrate conclusively that the observed fMRI patterns are a direct cause of stimulation of the ABVN, as definitive proof would require direct nerve recordings and systematic cadaveric studies. Secondly, researchers should appreciate that stimulation of the earlobe is not physiologically inert and can produce very similar fMRI patterns to ABVN stimulation, although there is no substantial published evidence to date that the locus coeruleus and nucleus of the solitary tract (central vagal relay centres) are activated by electrical stimulation of the earlobe (Frangos et al. 2015; Yakunina et al. 2017). Therefore, there remains a need to research the ‘gold‐standard’ sham site to ensure that accurate conclusions can be drawn from the effects found. Thirdly, the challenges of employing fMRI are compounded by swallowing artefacts, subject motion, heart rate and breathing, which can make it challenging to image small brainstem structures, such as the locus coeruleus, measuring (approximately) 1 mm at the widest in the axial plane (Keren et al. 2009). Therefore, researchers using fMRI in auricular tVNS studies should remember to use advanced fMRI techniques that can permit a closer study of these small brainstem regions. Lastly, fMRI studies have employed a range of stimulation techniques with heterogeneous parameters (e.g. frequency, on–off cycle and current intensity), stimulation durations and control sites. Therefore, a clear consensus on the precise auricular sites that allow access to the vagus nerve and whether the brain regions that are secondarily activated depend on specific parameters has yet to be determined.
There is a paucity of high‐quality head‐to‐head fMRI trials of tVNS applied at the three most likely sites of ABVN innervation: tragus vs. posterior aspect of the ear canal vs. cymba concha. Such investigations would help to discern the region most densely innervated by the ABVN and the most promising sites for tVNS. It would be worthwhile if future head‐to‐head fMRI studies also studied peripheral biomarkers, such as autonomic variables (e.g. heart rate and blood pressure) and behavioural changes, as these are some of the variables through which tVNS can exert its therapeutic benefits in clinical practice. Hitherto, functional connectivity studies conducted on patients with mild/moderate depression have identified the cymba concha as a suitable location to modulate brain connectivity, although there is still insufficient evidence that this is due to vagal activity, and future studies may want to consider electrical stimulation at other auricular sites.
Interestingly, no fMRI study to date has described an ear–cough reflex following electrical auricular tVNS, which highlights the rarity of this phenomenon. There is some evidence that the ear–cough reflex is associated with hypersensitivity of vagal nerve afferents (sensory neuropathy of the vagus nerve), which may be responsible for idiopathic or refractory cough (Ryan et al. 2014). Therefore, since fMRI studies have used healthy participants, it is unlikely that the ear–cough phenomenon would be observed at a rate similar to the estimated population prevalence of 1.7–4.2% (Bloustine et al. 1976; Gupta et al. 1986; Tekdemir et al. 1998).
Retrograde tracing studies
fMRI studies have demonstrated activation of the nucleus of the solitary tract and locus coeruleus (the central vagal relay centres that are assumed to be the key targets of auricular tVNS), following electrical auricular tVNS at specific auricular points. However, this pattern of fMRI changes may not necessarily be truly unique to vagus activation, and it is possible that these effects are mediated by the two other nerves supplying the external ear, namely, the greater auricular and auriculotemporal nerves. Retrograde tracing studies (necessarily conducted in animal models) can help to clarify the central terminations of the ABVN, greater auricular and auriculotemporal temporal nerve afferent fibres.
Central projections of the greater auricular nerve in animals have been examined only in rabbit (n = 18) by means of transganglionic transport of horseradish peroxidase (HRP) (Liu & Hu, 1988). HRP applied to the cut central end of the greater auricular nerve was detected ipsilaterally in the dorsal root ganglion cells (C2–C3) and the superior cervical ganglion cells. The transganglionically labelled fibres were also noted in the dorsal column of the upper four segments of the spinal cord and were strongly stained in laminae I–V at the C2 level. The afferent greater auricular nerve projections also had intense staining in the cranial nerve nuclei in the medulla, accounting for caudal subnuclei of the spinal trigeminal nerve, the solitary nucleus, and also medial and lateral cuneate nuclei (Liu & Hu, 1988).
Studies on central termination of the auriculotemporal have been conducted in rodent models (Jacquin et al. 1982; Takemura et al. 1987). HRP‐labelled primary afferent fibres of the auriculotemporal nerve were confined to the ipsilateral brainstem. The nerve terminals projected to the caudal medulla; specifically staining the dorsolateral border of the mandibular division of the trigeminal principal nucleus, cuneate nucleus and also paratrigeminal nucleus (Pa5). Travelling further caudally until the 3rd cervical level, a termination of the auriculotemporal nerve was also found in the dorsolateral spinal dorsal horn complex at laminae I–V, but most intensely in III–IV (Takemura et al. 1987).
Retrograde tracing studies conducted on afferent projections from the external ear demonstrate some degree of overlap in the central nervous system (Fig. 3). All of the auricular nerve tracing studies (greater auricular nerve, auriculotemporal nerve and ABVN) ipsilaterally labelled laminae III and IV of the dorsal horn (Nomura & Mizuno, 1983; Takemura et al. 1987; Liu & Hu, 1988). Interestingly, this was not the case when vagal tracing was performed directly from the vagal trunk. A summary of the auricular vagal nerve studies and the cervical vagal studies is presented in Table 3.
Figure 3.
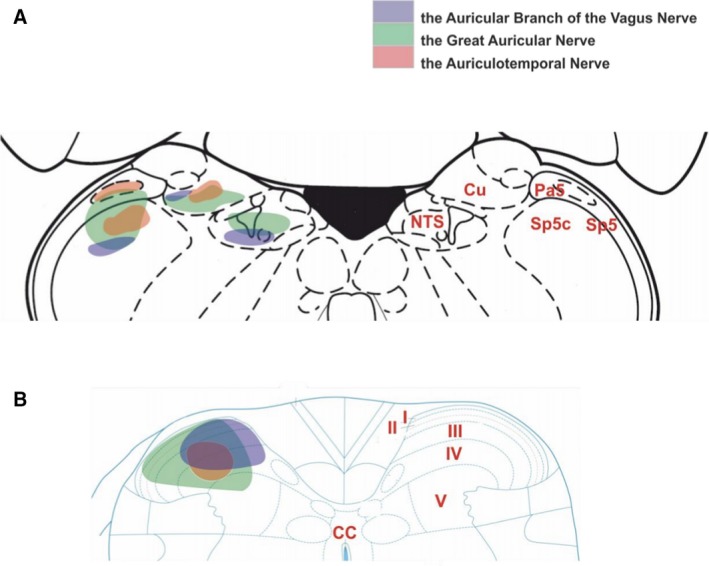
A schematic diagram of external auricle afferent projections to the brainstem and upper cervical spinal cord. (A) In the brainstem, the greater auricular nerve (green) projects into the trigeminal tract, cuneate nucleus and a little in the nucleus of the solitary tract. The auriculotemporal nerve (red) terminates in the trigeminal tract, caudal trigeminal nucleus and cuneate nucleus. The ABVN (blue) is projected into the nucleus of the solitary tract, cuneate nucleus, and caudal trigeminal nucleus. (B) Projection of the greater auricular nerve into upper cervical cord has wide coverage from laminae I to laminae V, and smaller coverage by the auriculotemporal nerve concentrated in the laminae III–IV. The ABVN afferents terminate in the laminae I‐IV. The level central nervous axis was omitted for clarity. Permissions: We wish to thank Prof. Jim Deuchars (University of Leeds) and Dr Mohd Kaisan Bin Mahadi for providing this figure. No formal permissions required.
Table 3.
A comparison between neuronal tracing studies of the nerves supplying the lateral auricle (the ABVN, the greater auricular nerve and the auriculotemporal nerve) and the cervical vagal trunk.
| Study | Reference | Location of injection | Tracer | Species | NTS | Vagal | Trigeminal | Cervical | AP | Cu | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | D | Comm | M | D | I | V | DVN | Pa5 | SP5n | SP5 | C1 | C2 | C3 | |||||||
| Non‐isolated ABVN | (Chien et al. 1996) | Middle and caudal auricle | Horseradish peroxidase | Dogs | + | + | + | + | + | + | + | + | + | |||||||
| (He et al. 2013) | Auricular concha | Cholera toxin B | Rats | + | + | + | + | + | ||||||||||||
| Isolated ABVN | (Nomura & Mizuno, 1984) | ABVN | Horseradish peroxidase | Cats | + | + | + | + | + | + | + | + | + | + | ||||||
| Vagal nerve | (Kalia & Sullivan, 1982) | Cervical vagal trunk | Horseradish peroxidase | Rats | + | + | + | + | + | + | + | + | + | + | + | |||||
| (Nomura & Mizuno, 1983) | Cervical vagal trunk | Horseradish peroxidase | Cats | + | + | + | + | + | + | + | + | + | + | |||||||
| Isolated greater auricular nerve | (Liu & Hu, 1988) | Greater auricular nerve root | Horseradish peroxidase | Rabbit | + | + | + | + | + | + | + | |||||||||
| Isolated auriculotemporal nerve | (Takemura et al. 1987) | Auriculotemporal nerve branch | Horseradish peroxidase | Rats | + | + | + | + | + | + | + | |||||||||
Permissions: We wish to thank Professor Jim Deuchars (University of Leeds) and Dr Mohd Kaisan Bin Mahadi for providing this table. No formal permissions required.
L, Lateral; DL, dorsolateral; Comm, commissural; M, medial; D, dorsal; I, intermediate; V, ventral; DVN, dorsal vagal nucleus; Pa5, paratrigeminal nucleus; SP5n, spinal trigeminal nucleus; SP5, spinal trigeminal tract; AP, area postrema; Cu, cuneate nucleus.
Vagus somato‐sensory evoked potentials (VSEPs)
The feasibility of auricular tVNS was first demonstrated using recordings of scalp potentials induced from stimulation of the inner tragus which were thought to originate from vagal nuclei, hence the term ‘vagus somato‐sensory evoked potentials’ (VSEPs) (Fallgatter et al. 2003). Provided the reported signals arise from vagal nuclei, they may be used to compare the scalp‐recorded VSEPs of auricular tVNS with iVNS, providing (in theory) a surrogate method for determining the cutaneous map of the ABVN. Table 4 outlines the result of electrophysiological studies that have investigated scalp potentials following auricular tVNS. Researchers have consistently demonstrated three reproducible peaks (P1, N1 and P2) from C4–F4 recordings after stimulation of various regions around the tragus. This pattern of peaks has also been shown with left cervical iVNS (Usami et al. 2013).
Table 4.
A summary of electrophysiological studies that have investigated vagus somatosensory evoked potentials (VSEPs) – a measure of the activity of vagus brain stem nuclei – secondary to auricular tVNS.
| Reference | n | Subjects | Location of auricular stimulation | Location of sham stimulation | Parameters | Findings |
|---|---|---|---|---|---|---|
| (Fallgatter et al. 2003) | 5 | Healthy subjects | Inner side of the tragus at the outer ventral edge of the external acoustic meatus |
In a single subject only: lobulus auriculae, the scapha, the crus antihelicis superior and the top of the helix |
8 mA |
|
| (Fallgatter et al. 2005) | 65 | 22 young healthy participants vs. 43 healthy elderly participants | Inner side of the tragus at the outer ventral edge of the internal auditory meatus | None | Stimulus intensity was 8 mA. Stimuli were electrical square impulses of 0.1‐ms duration. The interstimulus interval was 2 s |
components (N1, P1 and N2) were significantly longer in the elderly as compared to the younger participants, while the amplitudes showed less clear‐cut differences between groups |
| (Polak et al. 2007) | 10 | Patients diagnosed with Alzheimer’s disease (7/10) and mild cognitive impairment (3/10) | Inner side of the tragus at the outer ventral edge of the external acoustic meatus | None | Stimulus intensity was 8 mA, the sequence of stimuli was randomised. Stimuli were electrical square impulses of 0.1 msec duration. The interstimulus interval was 2 s |
|
| (Polak et al. 2009) | 20 | Young healthy participants | Inner side of the tragus at the outer ventral edge of the internal auditory meatus | None | Stimuli were electrical square impulses of 0.1‐ms duration with interstimulus interval of 2 s and stimulus intensity of 5, 8, and 10 mA in randomized sequence |
|
| (Leutzow et al. 2013) | 14 | Patient scheduled for elective extracranial surgery under total intravenous anaesthesia. Patients required administration of a muscle‐relaxing agent | Inner side of the right tragus and to the concha of the auricle | None | Electrical square impulses with 0.1 ms with a frequency of 0.5 Hz and an intensity of 8 mA |
|
| (Nonis et al. 2017) | 12 | Healthy volunteers | Medial region of the tragus close to the entry of the acoustic meatus | None |
Mean stimulation intensity 8 mA. 50 stimulations delivered at a frequency of 2 Hz and a pulse duration of 500 ms |
Stimulation evoked two reproducible peaks (P1, N1) in nine of 12 subjects |
The number of subjects, location of stimulation, site of sham stimulation, parameters of stimulation and pertinent findings are listed for each study.
Permissions: No permissions are required.
Usami et al. (2013) recorded short‐latency component VSEPs during left cervical iVNS of 25 patients with drug‐resistant epilepsy and found the response to consist of four peaks (P1, N1, P2 and N2) at the scalp level. The early complexes P1–N1 of responses to direct stimulation of the vagus nerve did not disappear after the administration of a neuromuscular blocking agent (which would preclude myogenic scalp potentials). However, the later complex P2–N2 disappeared after the administration of a neuromuscular blocking agent, and thus this is a muscular component.
Leutzow et al. (2013) conducted a study investigating the artefact‐free (using neuromuscular blocking agents) VSEPs elicited by auricular tVNS to the inner side of the tragus of the auricle. The scalp responses to the auricular stimulation were recorded before and after induction of general anaesthetic in 14 patients, during non‐depolarising muscle relaxing agent, cis‐atracurium (C‐AR) and after recovery from C‐AR under anaesthesia. Typical response curves with P1, N1 and P2 peaks were reported in all patients before and after general anaesthesia induction, which disappeared during the neuromuscular block. The authors postulated that the scalp responses reported in previous VSEP studies were a result of the activation of trigeminal or facial nerves, given the partially overlapping innervation of the auricle. The authors suggested a possible role for the occipitofrontal muscle (innervated by branches of the facial nerve) and the parietotemporal muscle (innervated by the trigeminal nerve) for the myogenic scalp potentials.
Despite the weighty evidence against the study of scalp potentials originating from vagal nuclei (VSEPs), there is little denying that VSEP studies have made important contributions to the tVNS field. A review ignoring such studies would be both incomplete, given it was a study of VSEPs that initially provided researchers with the impetus to explore this landscape (Fallgatter et al. 2003). Nevertheless, all available evidence seems to suggest that any recorded evoked potentials after auricular tVNS may be a result of facial or trigeminal nerve stimulation, and not stimulation of the vagus. Therefore, at present, there is no reason to refer to these signals as VSEPs, as the vagus likely has little to do with them. The VSEP field ought to remain an active research frontier. Indeed, postmortem studies have demonstrated that vagal nuclei are selectively vulnerable to the early pathophysiological processes of Alzheimer’s (Parvizi et al. 2001) and Parkinson’s diseases (Braak et al. 2003; Rietdijk et al. 2017), thus a method to assess the function of vagal nuclei, potentially by employing tVNS, may be used to detect these neurodegenerative processes in their primitive stages and permit earlier therapeutic interventions (Hagen et al. 2015).
Acupuncture studies
Eastern medicine has utilised the analgesic effects of auricular acupuncture for thousands of years (Asher et al. 2010; He et al. 2012; Usichenko et al. 2017) and numerous human studies have shown that vagal tone can be elicited by auricular acupuncture (He et al. 2012). Usichenko et al. (2017) extracted data concerning the auricular acupuncture points, used for the treatment of patients with acute and chronic pain in randomised controlled trials, from a previously published meta‐analysis (Asher et al. 2010). The three most frequently used auricular acupuncture points were: ‘Shenmen’ (TF4) (12/17 studies), located at the apex of the triangular fossa of the auricle, ‘Lung’ (CO14) (7/17 studies), located at the cavum concha, and thalamus (5/17 studies), located close to the antitragus (see Fig. 4). All 17 RCTs investigating the therapeutic effect of auricular acupuncture targeted the ABVN either exclusively or in a region of shared innervation by the ABVN and greater auricular nerve, as previously reported by Peuker & Filler (2002), and that group therefore concluded that the analgesic effects of auricular acupuncture were mediated by stimulation of the ABVN.
Figure 4.
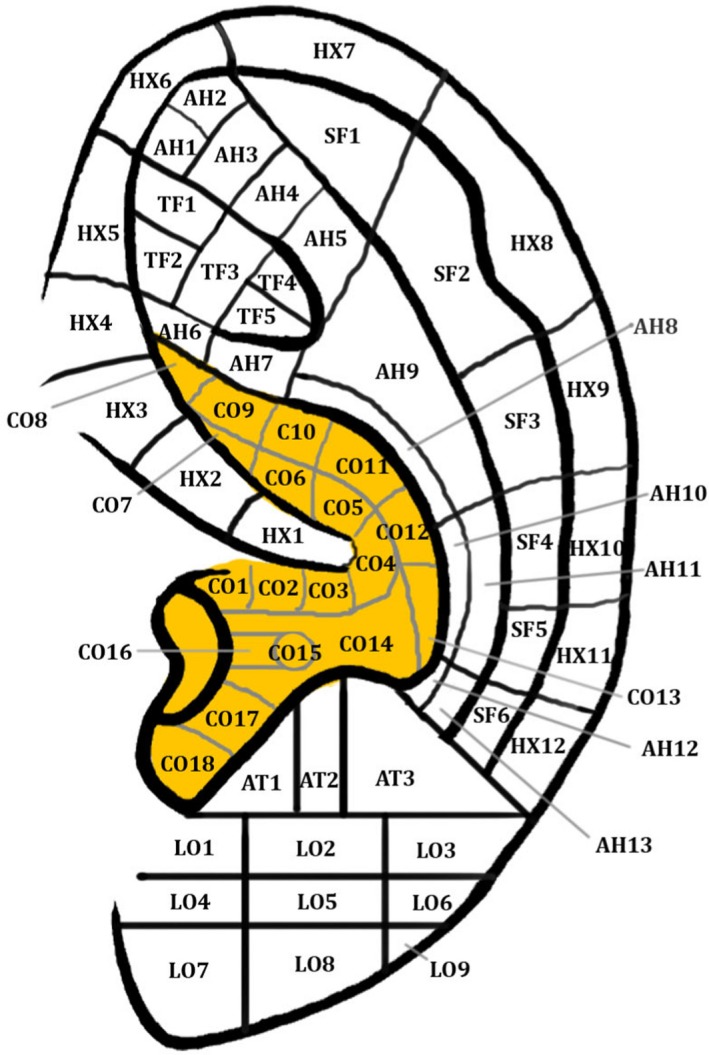
Auricular acupoints. Permissions: No permissions required.
Moreover, studies have examined the association between auricular acupoint ‘Heart’ (CO15), located in the cavum concha, and cardiovascular regulation (He et al. 2012). In 14 healthy volunteers, a significant decrease in heart rate and increase in heart rate variability has been observed after manual ear acupressure at auricular acupoint CO15, which is located in the cavum concha (Gao et al. 2012). Moreover, a study by Huang & Liang (1992) of auricular acupuncture at auricular acupoint CO15 in a group of 30 vascular hypertensive patients revealed a marked immediate short‐term and long‐term depressor effect and marked effects on angiotensin II in grade III hypertension. Interestingly, the same study reported that acupuncture at auricular acupoint ‘Stomach’ (located at the crus helix) produced no depressor effect on vascular hypertension, which fits with the cutaneous map of the ABVN described by Peuker & Filler (2002), in which the crus helix was innervated in 20% of cases by the ABVN (according to the table) and 0% (according to the manuscript). An acupuncture study (n = 12) reported that a single needle insertion in the left inferior hemi‐concha of the ear resulted in a statistically significant increase in the high frequency component (an indirect measure of parasympathetic outflow) of heart rate variability both during and post‐stimulation compared with pre‐stimulation (Haker et al. 2000) without affecting the heart rate. A more complete analysis of heart rate variability is described later in this review article.
Autonomic nervous system – the cardiovascular system
Sympathoexcitation is a key feature of many cardiovascular diseases, such as heart failure, hypertension and obstructive sleep apnoea (Charkoudian & Rabbitts, 2009; Malpas, 2010). Device‐based autonomic modulation by simultaneously decreasing sympathetic and increasing parasympathetic nervous system activity has been hypothesised to improve outcomes and quality for patients with a broad range of cardiovascular diseases (Schwartz et al. 2015). Table 5 outlines the human and animal studies that have investigated the effects of auricular tVNS applied at different locations of the auricle on various cardiovascular parameters.
Table 5.
The effect of auricular tVNS on various cardiovascular parameters.
| Reference | Subjects | n | Sampling group | Location of auricular stimulation | Control group | Parameters | Findings |
|---|---|---|---|---|---|---|---|
| Animal studies | |||||||
| Yu et al. (2013) | Dogs | 16 | AF | Right tragus | N/A |
20 Hz, 1‐ms square waves LLTS was defined as a voltage of stimulation setting at 80% below the threshold that slowed the sinus rate or AV conduction (threshold mean: 9.8 ± 2.6 V) |
|
| Wang et al. (2014) | Dogs | 30 | MI | Bilateral tragus stimulation | Sham surgery without tragus stimulation |
20 Hz, 1 ms square waves LLTS was defined as a voltage of stimulation setting at 80% below the threshold that slowed the sinus rate. Actual electrical stimulation value 16–24 V (range) |
|
| Chen et al. (2015a) | Dogs | 15 | AF | Right tragus | Sham surgery for RAP and no tragus stimulation |
20 Hz, 1‐ms square waves LLTS was defined as a voltage of stimulation setting at 80% below the threshold that slowed the sinus rate (mean threshold value/ actual electrical stimulation range unreported) |
|
| Chen et al. (2015b) | Dogs | 32 | AF | Left tragus | Sham surgery for RAP and no tragus stimulation |
20 Hz, 1 ms square waves LLTS was defined as a voltage of stimulation setting at 80% below the threshold that slowed the sinus rate (mean threshold value/ actual electrical stimulation range unreported) |
|
| Zhou et al. (2016) | Dogs | 16 | Tachycardia | Right tragus | Sham surgery without stimulation |
20 Hz, 2‐millisecond pulse width LLTS was defined as a voltage of stimulation setting at 80% below the threshold that slowed the sinus rate (mean threshold value/ actual electrical stimulation range unreported) |
|
| Human studies | |||||||
| Zamotrinsky et al. (1997) | Humans | 20 | Preoperative coronary artery disease patients | Bilateral cymba concha | Patients with a similar degree of coronary artery disease not treated with auricular tVNS | 0.2–1.5 mA, 1.5 ms, 3 Hz |
|
| Zamotrinsky et al. (2001) | Humans | 38 | Preoperative coronary artery disease patients | Bilateral cymba concha | Untreated patients with coronary artery disease | 0.2–1.5 mA, 1.5 ms, 3 Hz |
Relative to baseline, patients treated with auricular tVNS had:
|
| Popov et al. (2013) | Humans | 48 | Coronary artery disease patients | Bilateral cymba concha | Untreated patients with coronary artery disease | 0.05–0.15 mA |
Relative to baseline, patients treated with auricular tVNS had:
|
| Clancy et al. (2014) | Humans | 48 | Healthy | Right tragus | Healthy patients who did not undergo auricular tVNS | 200 ms, 30 Hz |
Relative to baseline, patients treated with auricular tVNS had:
|
| Stavrakis et al. (2015) | Humans | 40 | Paroxysmal atrial fibrillation | Right tragus | Patients with atrial fibrillation who did not undergo auricular tVNS | 1 ms, 20 Hz |
Relative to baseline, experimental group participants:
|
| De Couck et al. (2017) | Humans | 60 | Healthy | Right or left concha | Patients not treated with auricular tVNS | 0.7 mA, 250 μs, 25 Hz |
|
Permissions: No permissions are required.
AF, Atrial fibrillation; AV, atrioventricular; HF, high frequency component of heart rate variability; LF, low frequency; LLTS, low level electrical transcutaneous tragal stimulation; RAP, rapid atrial pacing.
Some studies noted in Table 5 refer to the autonomic variables heart rate variability (HRV), high frequency (HF) and low frequency (LF) components. HRV refers to the beat‐to‐beat alterations in heart rate (determined from the R‐R intervals of an ECG), and several lines of evidence suggest that HRV is a marker, albeit non‐specific, of autonomic tone (De Couck et al. 2017). Power spectral analysis of the R‐R intervals produces two components which are hypothesised to reflect sympathetic activity (HF component) and parasympathetic/vagus nerve activity (LF component) (Malfatto et al. 2001; Chapleau & Sabharwal, 2011).
Several studies have reported that electrical stimulation of the inner tragus, most probably due to ABVN stimulation, which is hypothesised to activate parasympathetic afferents, can modulate heart rate and HRV (Clancy et al. 2014; Badran et al. 2018c). Badran et al. (2018c) showed that (only) two parameters (500 µs 10 Hz and 500 µs 25 Hz) significantly decreased heart rate during active stimulation of the inner wall of the tragus compared with sham stimulation at the earlobe. Clancy et al. (2014) investigated the effects of auricular tVNS (applied to the inner and outer aspects of the tragus) on cardiovascular autonomic function in 48 healthy participants. The group found that auricular tVNS (200‐μs pulses at 30 Hz, 10–50 mA, slightly below the level at which subjects could feel the electrical stimulus) significantly decreased the LF : HF ratio of HRV, indicative of a (potential) increase in parasympathetic activity. Micrographic recordings revealed a significant decrease in frequency and incidence of muscle sympathetic nerve activity, hence stimulation of the tragus significantly reduced the incidence of firing of single sympathetic fibres. Interestingly, although the group showed a decrease in the LF : HF ratio during auricular tVNS, they did not report a statistically significant increase in the HF component (an indirect measure of parasympathetic outflow) or a statistically significant decrease in the LF component (an indirect measure of sympathetic outflow) of HRV. A study investigating the short (10 min) and prolonged (1 h) effects of auricular tVNS (right vs. left tragus) on HRV in healthy subjects (n = 60) showed no consistent changes in HRV, greater effects on HRV using left auricular tVNS or more consistent changes in women compared with men (De Couck et al. 2017).
Studies investigating the therapeutic potential of tVNS in sympathetic nervous system‐driven disorders, such as pain and angina, have failed to demonstrate any effect of electrical auricular tVNS on cardiovascular parameters. In one study, auricular tVNS (supplied at the exhalation phase of respiration) in female patients (n = 15) with chronic pelvic pain reported no statistically significant change in heart rate or any HRV indices (HF, LF and LF : HF ratios) (Napadow et al. 2012). Busch et al. (2013) reported that auricular tVNS applied at the left inner tragus reduced the sensitivity of mechanically evoked pain and had an inhibitory effect on the temporal summation of noxious tonic heat in healthy volunteers. However, with regard to autonomic variables, the researchers did not show any a statistically significant change in heart rate (other HRV indices were not measured). Another study investigating the effects of electrical auricular tVNS (located on the internal surfaces of the auricle) in male patients (n = 48) with angina demonstrated that auricular tVNS did not cause any statistically significant changes in the LF : HF ratio (Popov et al. 2013). However, the patients had a significantly reduced number of ventricular extrasystoles (at both time points) compared with baseline.
Several observations could explain the inconsistent effects of auricular tVNS on HRV parameters. Different groups have used different stimulation sites, e.g. the concha (De Couck et al. 2014) and tragus (Clancy et al. 2014), and studies have also employed different stimulation parameters, which could activate vagal afferents to different degrees. Moreover, auricular tVNS operates via a complex indirect system of brain regions and nuclei (see section on fMRI studies), hence the effects of auricular tVNS on HRV may be minimal (De Couck et al. 2017). The complex nature of LF power, its poor relationship to sympathetic nerve activation, and the non‐linear interactions between sympathetic and parasympathetic activity that are confounded by the mechanical effects of respiration and heart rate mean that the LF: HF sympatho‐vagal balance hypothesis is inaccurate (Billman, 2013; Shaffer & Ginsberg, 2017). Therefore, it is challenging to determine the cutaneous map of the ABVN using the current evidence base investigating the autonomic effects of auricular tVNS.
Hitherto, it has been unclear whether the autonomic effects of auricular tVNS are the result of the activation of the hypothesised afferent central targets of the vagus system (ear → brain → vagus nerve → organ) or whether there is direct activation of the efferent vagal projections from the ear to body organs (ear → vagus → heart) (Murray et al. 2016; Fig. 5). Afferent ABVN fibres are thought to modulate neuronal activity in the nucleus of the solitary tract, dorsal vagal nucleus, nucleus ambiguous, caudal ventrolateral medulla and rostroventral medulla (Fig. 6). An investigation of the role of afferent and efferent vagal fibres in mediating the therapeutic effect of tVNS would benefit from in vivo microelectrode recording of nerve dissections in animal models, or in animal models where the ABVN is lesioned.
Figure 5.
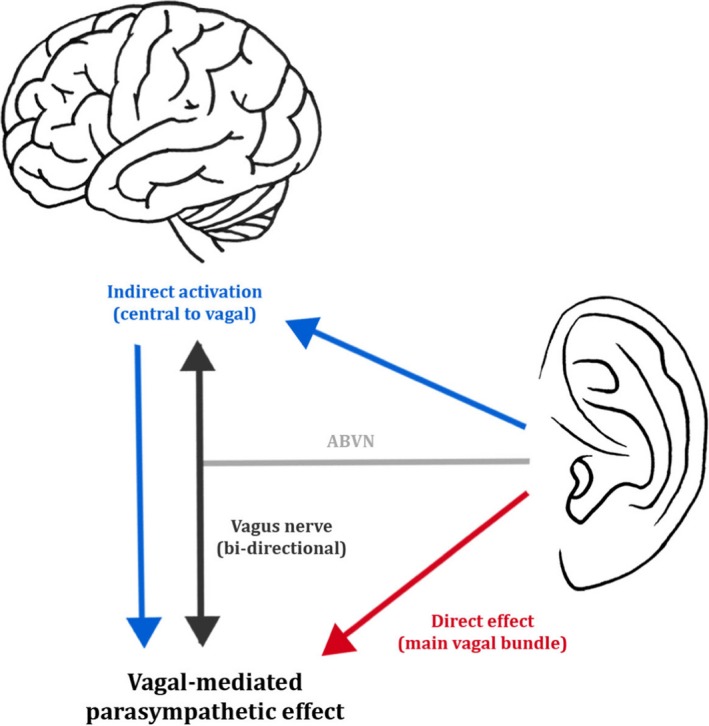
Two possible mechanisms for parasympathetic activation via the auricular branch of the vagus nerve (ABVN). The blue arrows represent the activation of vagal afferents and stimulation of the main vagal bundle via the central nervous system. The red arrow represents the direct activation of vagal efferents and stimulation of the main vagal bundle. Permissions: No permissions required.
Figure 6.
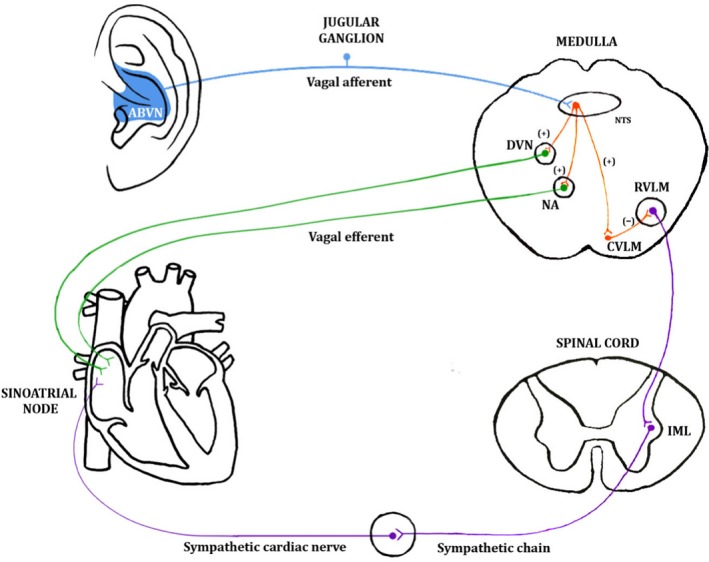
A schematic diagram of one of the hypothesised pathways through which stimulation of the auricular branch of the vagus nerve (ABVN) can influence the cardiovascular system Stimulation of the ABVN increases input to the nucleus of the solitary tract (NTS) in the medulla and influences the activity of NTS neurones projecting to the cardioinhibitory vagal efferent neurones of the dorsal vagal nucleus (DVN) and nucleus ambiguous (NA). These vagal efferent neurones propagate the vagal tone to the sinoatrial node (SA). Stimulation of the ABVN may also excite NTS neurons, sending excitatory projections to the caudal ventrolateral medulla (CVLM). The CVLM inhibits the rostroventrolateral medulla (RVLM), which is the primary source of excitatory drive to sympathetic preganglionic neurons in the intermediolateral cell column (IML) of the spinal cord. This inhibition would decrease sympathetic activity. Permissions: No permissions required.
ABVN fibre composition
Thick afferent Aβ axons have been consistently shown to mediate the therapeutic effects of cervical iVNS (Evans et al. 2004). Group C nerve fibres (C‐fibres) are not thought to be involved in mediating the physiological effects of VNS. In a study investigating the therapeutic effect of iVNS in stimulation‐induced seizures in rats, capsaicin‐induced destruction of peripheral C‐fibre populations did not alter the therapeutic mechanism of iVNS for seizure suppression in rats (Krahl et al. 2001). To our knowledge, only one study to date has determined the number of myelinated axons in the human ABVN (Safi et al. 2016). In that study, researchers investigated the numbers and caliber of myelinated axons in 18 segments of the ABVN (9 left, 9 right) in 16 German cadavers. The human ABVN was shown to contain thick myelinated axons ≥ 7 mm belonging to the Aβ class, which were reportedly five to six times less numerous than those found in the cervical vagus nerve. The study (Safi et al. 2016) revealed that the number of Aβ myelinated axons represent, on average, about one‐fifth of the total myelinated axon count and can vary widely between individuals, which may help to explain why auricular tVNS may not be effective in some patients, or indeed why the ear–cough reflex is elicited in so few people (Gupta et al. 1986; Tekdemir et al. 1998) and has not been reported in any of the fMRI studies published to date. These seminal findings may represent the anatomical basis for the clinical effectiveness of auricular tVNS.
Effects of auricular tVNS on the central nervous system (CNS)
The therapeutic mechanism of auricular tVNS and iVNS is thought to be mediated by concentration shifts of the neurotransmitters noradrenaline, γ‐aminobutyric acid (GABA) and acetylcholine (ACh) in the CNS (Van Leusden et al. 2015), which induce neuroplastic changes in the cerebral cortex. The majority of studies investigating the therapeutic mechanisms underlying VNS have studied the locus coeruleus: the major noradrenergic brain structure (Krahl et al. 1998; Van Bockstaele et al. 1999; Groves et al. 2005; Fornai et al. 2011). The first study (Capone et al. 2015) to assess the GABA‐mediated mechanism of tVNS employed transcranial magnetic stimulation and demonstrated significantly reduced cortical excitability in 10 healthy participants following 1 h of tVNS at the inner side of the left tragus (relative to sham stimulation at the left ear lobe) as measured by increased short‐interval intra‐cortical inhibition (SICI). SICI is a transmagnetic stimulation protocol which is a surrogate marker of GABAA activity within the motor cortex, and an increase in SICI suggests increased GABA neurotransmitter concentration (Stagg et al. 2011). Recently, Keute et al. (2018a) demonstrated, using 16 healthy young human volunteers, that auricular tVNS applied to the cymba concha of the left ear induced a GABAergic neuromodulation during automatic inhibition, as measured by the behavioural marker negative compatibility effect (NCE), which is robustly negatively correlated to GABA concentration (Boy et al. 2010). Interestingly, the group reported that auricular tVNS produced an increased NCE, in contradiction to the decrease in NCE that they had hypothesised. The group postulated that GABA activity may not have increased globally by auricular tVNS but was rather differentially modified in different brain regions, as has been previously reported (Greenhouse et al. 2016).
Conclusion
Our review article has explored the neuroanatomy of the ABVN with reference to (1) clinical surveys examining Arnold’s reflex, (2) cadaveric studies, (3) fMRI studies, (4) electrophysiological studies, (5) acupuncture studies, (6) retrograde tracing studies and (7) studies measuring changes in autonomic (cardiovascular) parameters in response to auricular tVNS. We have also provided an overview of the composition of the ABVN and the effects of auricular tVNS on the central nervous system. Cadaveric studies, of which a limited number exist in the literature, would be the ‘gold‐standard’ approach to studying the cutaneous map of the ABVN and thus there is a need for more such studies to be conducted by individuals with advanced training in microdissection of the head and neck, as the ABVN diameter is only of 1 µm resolution. Teams should also consider age, ethnicity, trauma and prior health history in their selection of cadavers (Badran et al. 2018a). fMRI may (in theory) offer a useful surrogate modality for determining the auricular locations that best activate central vagal centres (when compared with the fMRI patterns observed with invasive vagal nerve stimulation), accepting that its use has several limitations. Importantly, fMRI studies cannot discern whether the observed fMRI patterns are a direct result of activation of the ABVN as opposed to the greater auricular and auriculotemporal nerves. Importantly, stimulation of the earlobe – an auricular site innervated by the greater auricular nerve and a popular auricular location for sham electrical stimulation in fMRI studies – is not physiologically inert and there is therefore a need to explore alternative sham stimulation sites. A clear consensus on the auricular sites that are most densely innervated by the ABVN and whether the brain regions secondarily activated by electrical auricular tVNS depend on specific parameters has yet to be achieved. At present, the outcomes of fMRI studies published to date make it reasonable to surmise that the concha and inner tragus are suitable locations for vagal modulation. Given the therapeutic potential of auricular tVNS (Rong et al. 2014; Hyvarinen et al. 2015; Straube et al. 2015; Kong et al. 2018), there remains a need for the cutaneous map of the ABVN to be further refined and the effects of various stimulation parameters and stimulation sites to be determined.
Conflict of interest
There are no known conflicts of interest associated with the publication of this work. No author received financial support.
Acknowledgements
We are indebted to Dr Mohd Kaisan Bin Mahadi and Prof. Jim Deuchars (University of Leeds, School of Biomedical Sciences) for providing us with Fig. 3 and Table 3. We also thank Ms Reya Srivastava (Barts and The London School of Medicine and Dentistry) for expertly drawing the figures contained in this article.
Dr. Adam D. Farmer, FRCP PhD and Prof. Qasim Aziz, FRCP PhD are joint senior authors.
References
- Arnold F (1831) Der Kopftheil des vegatativen Nervensystems beim Menschen. Heidelberg: Karl Groos. [Google Scholar]
- Asconape JJ, Moore DD, Zipes DP, et al. (1999) Bradycardia and asystole with the use of vagus nerve stimulation for the treatment of epilepsy: a rare complication of intraoperative device testing. Epilepsia 40, 1452–1454. [DOI] [PubMed] [Google Scholar]
- Asher GN, Jonas DE, Coeytaux RR, et al. (2010) Auriculotherapy for pain management: a systematic review and meta‐analysis of randomized controlled trials. J Altern Complement Med 16, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran BW, Glusman CE, Badran AW, et al. (2017) The physiological and neurobiological effects of transcutaneous auricular vagus nerve stimulation (taVNS). Brain Stimul 10, 378. [Google Scholar]
- Badran BW, Brown JC, Dowdle LT, et al. (2018a) Tragus or cymba conchae? Investigating the anatomical foundation of transcutaneous auricular vagus nerve stimulation (taVNS). Brain Stimul 11, 947–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran BW, Dowdle LT, Mithoefer OJ, et al. (2018b) Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul 11, 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran BW, Mithoefer OJ, Summer CE, et al. (2018c) Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter‐specific effects on heart rate. Brain Stimul 11, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Menachem E, Revesz D, Simon BJ, et al. (2015) Surgically implanted and non‐invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol 22, 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo P, López M, Larraya I, Chamorro J, Cobo JL, Ordóñez S, Vega JA (2017). Innervation of the human cavum conchae and auditory canal: anatomical basis for transcutaneous auricular nerve stimulation. Biomed Res Int 2017, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL (2000) Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85, 1–17. [DOI] [PubMed] [Google Scholar]
- Billman GE (2013) The LF/HF ratio does not accurately measure cardiac sympatho‐vagal balance. Front Physiol 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, et al. (1995) Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Bloustine S, Langston L, Miller T (1976) Ear‐cough (Arnold's) reflex. Ann Otol Rhinol Laryngol 85, 406–407. [DOI] [PubMed] [Google Scholar]
- van Bockstaele EJ, Peoples J, Telegan P (1999) Efferent projections of the nucleus of the solitary tract to peri‐locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol 412, 410–428. [DOI] [PubMed] [Google Scholar]
- van Leusden JWR, Sellaro R, Colzato LS (2015) Transcutaneous vagal nerve stimulation (tVNS): a new neuromodulation tool in healthy humans? Front Psychol 6, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Lomarev MP, Denslow S, et al. (2001) Feasibility of vagus nerve stimulation‐synchronized blood oxygenation level‐dependent functional MRI. Invest Radiol 36, 470–479. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Bazin T, Pellissier S (2018) The vagus nerve at the interface of the microbiota‐gut‐brain axis. Front Neurosci 12, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha C, Farmer AD, Nilsson M, et al. (2014) Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut 64, 611–617. [DOI] [PubMed] [Google Scholar]
- Botha C, Farmer AD, Nilsson M, et al. (2015) Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut 64, 611–617. [DOI] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, et al. (2010) Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol 20, 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, et al. (2003) Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 110, 517–536. [DOI] [PubMed] [Google Scholar]
- Burger AM, Verkuil B (2018) Transcutaneous nerve stimulation via the tragus: are we really stimulating the vagus nerve? Brain Stimul 11, 945–946. [DOI] [PubMed] [Google Scholar]
- Busch V, Zeman F, Heckel A, et al. (2013) The effect of transcutaneous vagus nerve stimulation on pain perception–an experimental study. Brain Stimul 6, 202–209. [DOI] [PubMed] [Google Scholar]
- Capone F, Assenza G, di Pino G, et al. (2015) The effect of transcutaneous vagus nerve stimulation on cortical excitability. J Neural Transm 122, 679–685. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Sabharwal R (2011) Methods of assessing vagus nerve activity and reflexes. Heart Fail Rev 16, 109–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Rabbitts JA (2009) Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc 84, 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Yu L, Liu Q, et al. (2015a) Low level tragus nerve stimulation is a non‐invasive approach for anti‐atrial fibrillation via preventing the loss of connexins. Int J Cardiol 179, 144–145. [DOI] [PubMed] [Google Scholar]
- Chen M, Zhou X, Liu Q, et al. (2015b) Left‐sided noninvasive vagus nerve stimulation suppresses atrial fibrillation by upregulating atrial gap junctions in canines. J Cardiovasc Pharmacol 66, 593–599. [DOI] [PubMed] [Google Scholar]
- Chien CH, Shieh JY, Ling EA, et al. (1996) The composition and central projections of the internal auricular nerves of the dog. J Anat 189, 349–362. [PMC free article] [PubMed] [Google Scholar]
- Clancy JA, Mary DA, Witte KK, et al. (2014) Non‐invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 7, 871–877. [DOI] [PubMed] [Google Scholar]
- de Couck M, Nijs J, Gidron Y (2014) You may need a nerve to treat pain: the neurobiological rationale for vagal nerve activation in pain management. Clin J Pain 30, 1099–1105. [DOI] [PubMed] [Google Scholar]
- de Couck M, Cserjesi R, Caers R, et al. (2017) Effects of short and prolonged transcutaneous vagus nerve stimulation on heart rate variability in healthy subjects. Auton Neurosci 203, 88–96. [DOI] [PubMed] [Google Scholar]
- de Ferrari GM, Crijns HJ, Borggrefe M, et al. (2011) Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 32, 847–855. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes‐Dougan B, et al. (2014) Abnormal amygdala resting‐state functional connectivity in adolescent depression. JAMA Psychiatry 71, 1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagyte G, den Boer JA, Trentani A (2011) The cholinergic system and depression. Behav Brain Res 221, 574–582. [DOI] [PubMed] [Google Scholar]
- Diamond M, Wartmann CT, Tubbs RS, et al. (2010) Peripheral facial nerve communications and their clinical implications. Clin Anat 24, 10–18. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Smith J, Scherzinger C, et al. (2008) A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed Tech (Berl) 53, 104–111. [DOI] [PubMed] [Google Scholar]
- Engel D (1979) The gastroauricular phenomenon and related vagus reflexes. Arch Psychiatr Nervenkr 1970, 271–277. [DOI] [PubMed] [Google Scholar]
- Evans MS, Verma‐Ahuja S, Naritoku DK, et al. (2004) Intraoperative human vagus nerve compound action potentials. Acta Neurol Scand 110, 232–238. [DOI] [PubMed] [Google Scholar]
- Fahy BG (2010) Intraoperative and perioperative complications with a vagus nerve stimulation device. J Clin Anesth 22, 213–222. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Neuhauser B, Herrmann MJ, et al. (2003) Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 110, 1437–43. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Ehlis AC, Ringel TM, et al. (2005) Age effect on far field potentials from the brain stem after transcutaneous vagus nerve stimulation. Int J Psychophysiol 56, 37–43. [DOI] [PubMed] [Google Scholar]
- Fang J, Rong P, Hong Y, et al. (2016) Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry 79, 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer AD, Aziz Q 2015. Vagally mediated analgesia: breath‐holding during exhalation as a simple manipulation to reduce pain Perception‐Reyes Del Paso et al. Pain medicine 2015. Pain Med 16, 2417. [DOI] [PubMed] [Google Scholar]
- Farmer AD, Coen SJ, Kano M, et al. (2014) Psychophysiological responses to visceral and somatic pain in functional chest pain identify clinically relevant pain clusters. Neurogastroenterol Motil 26, 139–148. [DOI] [PubMed] [Google Scholar]
- Farmer AD, Albu‐Soda A, Aziz Q (2016) Vagus nerve stimulation in clinical practice. Br J Hosp Med (Lond) 77, 645–651. [DOI] [PubMed] [Google Scholar]
- Fay T (1927) Observations and results from intracranial section of the glossopharyngeus and vagus nerves in man. J Neurol Psychopathol 8, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Madsen S, Moody TD, et al. (2012) Effects of cranial electrotherapy stimulation on resting state brain activity. Brain Behav 2, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Ruffoli R, Giorgi FS, et al. (2011) The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. Eur J Neurosci 33, 2169–2178. [DOI] [PubMed] [Google Scholar]
- Frangos E, Ellrich J, Komisaruk BR (2015) Non‐invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul 8, 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XY, Wang L, Gaischek I, et al. (2012) Brain‐modulated effects of auricular acupressure on the regulation of autonomic function in healthy volunteers. Evid Based Complement Alternat Med 2012, 714391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, Kumar‐Ondiveeran H, et al. (2006) The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131, 1122–1130. [DOI] [PubMed] [Google Scholar]
- Ginsberg LE, Eicher SA (2000) Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol 21, 568–571. [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Noah S, Maddock RJ, et al. (2016) Individual differences in GABA content are reliable but are not uniform across the human cortex. NeuroImage 139, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves DA, Bowman EM, Brown VJ (2005) Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett 379, 174–179. [DOI] [PubMed] [Google Scholar]
- Guild SR (1953) The glomus jugulare, a nonchromaffin paraganglion, in man. Ann Otol Rhinol Laryngol 62, 1045–1071. [DOI] [PubMed] [Google Scholar]
- Gupta D, Verma S, Vishwakarma SK (1986) Anatomic basis of Arnold's ear‐cough reflex. Surg Radiol Anat 8, 217–220. [DOI] [PubMed] [Google Scholar]
- Hagen K, Ehlis AC, Haeussinger FB, et al. (2015) The relation of SMI and the VSEP in a risk sample for neurodegenerative disorders. J Neural Transm (Vienna) 122, 1167–1174. [DOI] [PubMed] [Google Scholar]
- Haker E, Egekvist H, Bjerring P (2000) Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects. J Auton Nerv Syst 79, 52–59. [DOI] [PubMed] [Google Scholar]
- He W, Wang X, Shi H, et al. (2012) Auricular acupuncture and vagal regulation. Evid Based Complement Alternat Med 2012, 786839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Jing XH, Zhu B, et al. (2013) The auriculo‐vagal afferent pathway and its role in seizure suppression in rats. BMC Neurosci 14, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland H (1933) Electrical responses from the lateral‐line nerves of catfish. I. J Gen Physiol 16, 695–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holle‐Lee D, Gaul C (2016) Noninvasive vagus nerve stimulation in the management of cluster headache: clinical evidence and practical experience. Ther Adv Neurol Disord 9, 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Liang S (1992) Acupuncture at otoacupoint heart for treatment of vascular hypertension. J Tradit Chin Med 12, 133–136. [PubMed] [Google Scholar]
- Hyvarinen P, Yrttiaho S, Lehtimaki J, et al. (2015) Transcutaneous vagus nerve stimulation modulates tinnitus‐related beta‐ and gamma‐band activity. Ear Hear 36, e76–e85. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Semba K, Rhoades RW, et al. (1982) Trigeminal primary afferents project bilaterally to dorsal horn and ipsilaterally to cerebellum, reticular formation, and cuneate, solitary, supratrigeminal and vagal nuclei. Brain Res 246, 285–291. [DOI] [PubMed] [Google Scholar]
- Kalia M, Sullivan JM (1982) Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 211, 248–265. [DOI] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, et al. (2009) In‐Vivo Mapping of the Human Locus Coeruleus. NeuroImage 47, 1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keute M, Ruhnau P, Heinze H‐J, et al. (2018a) Behavioral and electrophysiological evidence for GABAergic modulation through transcutaneous vagus nerve stimulation. Clin Neurophysiol 129, 1789–1795. [DOI] [PubMed] [Google Scholar]
- Keute M, Ruhnau P, Zaehle T (2018b) Reply to ‘Reconsidering sham in transcutaneous vagus nerve stimulation studies’. Clin Neurophysiol 129, 2503–2504. [DOI] [PubMed] [Google Scholar]
- Kiyokawa J, Yamaguchi K, Okada R, et al. (2014) Origin, course and distribution of the nerves to the posterosuperior wall of the external acoustic meatus. Anat Sci Int 89, 238–245. [DOI] [PubMed] [Google Scholar]
- Knowles CH, Aziz Q (2009) Basic and clinical aspects of gastrointestinal pain. Pain 141, 191–209. [DOI] [PubMed] [Google Scholar]
- Kong J, Fang J, Park J, et al. (2018) Treating depression with transcutaneous auricular vagus nerve stimulation: State of the Art and future perspectives. Front Psychiatry 9: doi: 10.3389/fpsyt.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahl SE, Clark KB, Smith DC, et al. (1998) Locus coeruleus lesions suppress the seizure‐attenuating effects of vagus nerve stimulation. Epilepsia 39, 709–714. [DOI] [PubMed] [Google Scholar]
- Krahl SE, Senanayake SS, Handforth A (2001) Destruction of peripheral C‐fibers does not alter subsequent vagus nerve stimulation‐induced seizure suppression in rats. Epilepsia 42, 586–589. [DOI] [PubMed] [Google Scholar]
- Kraus T, Hosl K, Kiess O, et al. (2007) BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 114, 1485–1493. [DOI] [PubMed] [Google Scholar]
- Kraus T, Kiess O, Hosl K, et al. (2013) CNS BOLD fMRI effects of sham‐controlled transcutaneous electrical nerve stimulation in the left outer auditory canal – a pilot study. Brain Stimul 6, 798–804. [DOI] [PubMed] [Google Scholar]
- Lekakis GK (2003) Philipp Friedrich Arnold, Ludvig Levin Jacobson and their contribution to head and neck anatomy. J Laryngol Otol 117, 28–31. [DOI] [PubMed] [Google Scholar]
- Leutzow B, Lange J, Gibb A, et al. (2013) Vagal sensory evoked potentials disappear under the neuromuscular block ‐ an experimental study. Brain Stimul 6, 812–816. [DOI] [PubMed] [Google Scholar]
- Liu D, Hu Y (1988) The central projections of the great auricular nerve primary afferent fibers – an HRP transganglionic tracing method. Brain Res 445, 205–210. [DOI] [PubMed] [Google Scholar]
- Liu WC, Mosier K, Kalnin AJ, et al. (2003) BOLD fMRI activation induced by vagus nerve stimulation in seizure patients. J Neurol Neurosurg Psychiatry 74, 811–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Fang J, Wang Z, et al. (2016) Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J Affect Disord 205, 319–326. [DOI] [PubMed] [Google Scholar]
- Lomarev M, Denslow S, Nahas Z, et al. (2002) Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res 36, 219–227. [DOI] [PubMed] [Google Scholar]
- Lulic D, Ahmadian A, Baaj AA, et al. (2009) Vagus nerve stimulation. Neurosurg Focus 27, E5. [DOI] [PubMed] [Google Scholar]
- Magdaleno‐Madrigal VM, Valdes‐Cruz A, Martinez‐Vargas D, et al. (2002) Effect of electrical stimulation of the nucleus of the solitary tract on the development of electrical amygdaloid kindling in the cat. Epilepsia 43, 964–969. [DOI] [PubMed] [Google Scholar]
- Malfatto G, Branzi G, Gritti S, et al. (2001) Different baseline sympathovagal balance and cardiac autonomic responsiveness in ischemic and non‐ischemic congestive heart failure. Eur J Heart Fail 3, 197–202. [DOI] [PubMed] [Google Scholar]
- Malow BA, Edwards J, Marzec M, et al. (2000) Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology 55, 1450–1454. [DOI] [PubMed] [Google Scholar]
- Malpas SC (2010) Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90, 513–557. [DOI] [PubMed] [Google Scholar]
- Mayberg HS (1997) Limbic‐cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9, 471–481. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, et al. (1999) Reciprocal limbic‐cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 156, 675–682. [DOI] [PubMed] [Google Scholar]
- Murray AR, Atkinson L, Mahadi MK, et al. (2016) The strange case of the ear and the heart: The auricular vagus nerve and its influence on cardiac control. Auton Neurosci 199, 48–53. [DOI] [PubMed] [Google Scholar]
- Mwamburi M, Liebler EJ, Tenaglia AT (2017) Review of non‐invasive vagus nerve stimulation (gammaCore): efficacy, safety, potential impact on comorbidities, and economic burden for episodic and chronic cluster headache. Am J Manag Care 23, S317–s325. [PubMed] [Google Scholar]
- Nahas Z, Teneback C, Chae JH, et al. (2007) Serial vagus nerve stimulation functional MRI in treatment‐resistant depression. Neuropsychopharmacology 32, 1649–1660. [DOI] [PubMed] [Google Scholar]
- Napadow V, Edwards RR, Cahalan CM, et al. (2012) Evoked pain analgesia in chronic pelvic pain patients using respiratory‐gated auricular vagal afferent nerve stimulation. Pain Med 13, 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan JT, Watts R, Haddad N, et al. (2002) Cerebral activation during vagus nerve stimulation: a functional MR study. Epilepsia 43, 1509–1514. [DOI] [PubMed] [Google Scholar]
- Nomura S, Mizuno N (1983) Central distribution of efferent and afferent components of the cervical branches of the vagus nerve. A HRP study in the cat. Anat Embryol (Berl) 166, 1–18. [DOI] [PubMed] [Google Scholar]
- Nomura S, Mizuno N (1984) Central distribution of primary afferent fibers in the Arnold's nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain Res 292, 199–205. [DOI] [PubMed] [Google Scholar]
- Nonis R, D'Ostilio K, Schoenen J, et al. (2017) Evidence of activation of vagal afferents by non‐invasive vagus nerve stimulation: An electrophysiological study in healthy volunteers. Cephalalgia 37, 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek JN, van der Werff SJ, Meens PH, et al. (2014) Aberrant resting‐state functional connectivity in limbic and salience networks in treatment‐naive clinically depressed adolescents. J Child Psychol Psychiatry 55, 1317–1327. [DOI] [PubMed] [Google Scholar]
- Parvizi J, van Hoesen GW, Damasio A (2001) The selective vulnerability of brainstem nuclei to Alzheimer's disease. Ann Neurol 49, 53–66. [DOI] [PubMed] [Google Scholar]
- Peuker ET, Filler TJ (2002) The nerve supply of the human auricle. Clin Anat 15, 35–37. [DOI] [PubMed] [Google Scholar]
- Polak T, Ehlis AC, Langer JB, et al. (2007) Non‐invasive measurement of vagus activity in the brainstem – a methodological progress towards earlier diagnosis of dementias? J Neural Transm (Vienna) 114, 613–619. [DOI] [PubMed] [Google Scholar]
- Polak T, Markulin F, Ehlis AC, et al. (2009) Far field potentials from brain stem after transcutaneous vagus nerve stimulation: optimization of stimulation and recording parameters. J Neural Transm (Vienna) 116, 1237–1242. [DOI] [PubMed] [Google Scholar]
- Popov SV, Afanasiev SA, Kurlov IO, Pisklova AV 2013. Drug‐free correction of the tone of the autonomic nervous system in the management of cardiac arrhythmia in coronary artery disease. Int J BioMed, 3, 74‐77. [Google Scholar]
- Proctor B (1989) Surgical anatomy of the ear and temporal bone. New York: Thieme Medical Publishers. [Google Scholar]
- Redgrave J, Day D, Leung H, et al. (2018) Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain Stimul 11, 1225–1238. [DOI] [PubMed] [Google Scholar]
- Rietdijk CD, Perez‐Pardo P, Garssen J, et al. (2017) Exploring Braak’s Hypothesis of Parkinson’s disease. Front Neurol 8, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P, Liu A, Zhang J, et al. (2014) An alternative therapy for drug‐resistant epilepsy: transcutaneous auricular vagus nerve stimulation. Chin Med J (Engl) 127, 300–304. [PubMed] [Google Scholar]
- Ruggiero DA, Underwood MD, Mann JJ, et al. (2000) The human nucleus of the solitary tract: visceral pathways revealed with an ‘in vitro’ postmortem tracing method. J Auton Nerv Syst 79, 181–190. [DOI] [PubMed] [Google Scholar]
- Ryan NM, Gibson PG, Birring SS (2014) Arnold's nerve cough reflex: evidence for chronic cough as a sensory vagal neuropathy. J Thorac Dis 6, S748–S752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi S, Ellrich J, Neuhuber W (2016) Myelinated axons in the auricular branch of the human vagus nerve. Anat Rec 299, 1184–1191. [DOI] [PubMed] [Google Scholar]
- Santos PM (2003) Evaluation of laryngeal function after implantation of the vagus nerve stimulation device. Otolaryngol Head Neck Surg 129, 269–273. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE (1983) Central connections of the sensory and motor nuclei of the vagus nerve. J Auton Nerv Syst 9, 13–26. [DOI] [PubMed] [Google Scholar]
- Schmalfuss IM, Tart RP, Mukherji S, et al. (2002) Perineural tumor spread along the auriculotemporal nerve. AJNR Am J Neuroradiol 23, 303–311. [PMC free article] [PubMed] [Google Scholar]
- Schwartz PJ, la Rovere MT, de Ferrari GM, et al. (2015) Autonomic modulation for the management of patients with chronic heart failure. Circ Heart Fail 8, 619–628. [DOI] [PubMed] [Google Scholar]
- Shaffer F, Ginsberg JP (2017) An overview of heart rate variability metrics and norms. Front Public Health 5, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS (1898) Experiments in examination of the peripheral distribution of the fibres of the posterior roots of some spinal nerves. Philos Trans R Soc Lond B Biol Sci 190, 45–186. [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, et al. (2011) Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol 589, 5845–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrakis S, Humphrey MB, Scherlag BJ, et al. (2015) Low‐level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 65, 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube A, Ellrich J, Eren O, et al. (2015) Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t‐VNS): a randomized, monocentric clinical trial. J Headache Pain 16: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura M, Sugimoto T, Sakai A (1987) Topographic organization of central terminal region of different sensory branches of the rat mandibular nerve. Exp Neurol 96, 540–557. [DOI] [PubMed] [Google Scholar]
- Tang Y, Kong L, Wu F, et al. (2013) Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment‐naive patients with major depressive disorder: a resting‐state functional magnetic resonance imaging study. Psychol Med 43, 1921–1927. [DOI] [PubMed] [Google Scholar]
- Tekdemir I, Aslan A, Elhan A (1998) A clinico‐anatomic study of the auricular branch of the vagus nerve and Arnold's ear‐cough reflex. Surg Radiol Anat 20, 253–257. [PubMed] [Google Scholar]
- Ueno N, Sudo H, Hattori Y, et al. (1993) Innervation of the external ear in humans and the musk shrew. Nihon Jibiinkoka Gakkai Kaiho 96, 212–218. [DOI] [PubMed] [Google Scholar]
- Usami K, Kawai K, Sonoo M, et al. (2013) Scalp‐recorded evoked potentials as a marker for afferent nerve impulse in clinical vagus nerve stimulation. Brain Stimul 6, 615–623. [DOI] [PubMed] [Google Scholar]
- Usichenko T, Hacker H, Lotze M (2017) Transcutaneous auricular vagal nerve stimulation (taVNS) might be a mechanism behind the analgesic effects of auricular acupuncture. Brain Stimul 10, 1042–1044. [DOI] [PubMed] [Google Scholar]
- Ventureyra EC (2000) Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv Syst 16, 101–102. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu L, Wang S, et al. (2014) Chronic intermittent low‐level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ Heart Fail 7, 1014–1021. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fang J, Liu J, et al. (2018) Frequency‐dependent functional connectivity of the nucleus accumbens during continuous transcutaneous vagus nerve stimulation in major depressive disorder. J Psychiatr Res 102, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Tubbs RS, Satoh S, et al. (2016) Isolated deep ear canal pain: possible role of auricular branch of vagus nerve – case illustrations with cadaveric correlation. World Neurosurg 96, 293–301. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Goehler LE, Relton JK, et al. (1995) Blockade of interleukin‐1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune‐brain communication. Neurosci Lett 183, 27–31. [DOI] [PubMed] [Google Scholar]
- Wright CG (1997) Development of the human external ear. J Am Acad Audiol 8, 379–382. [PubMed] [Google Scholar]
- Yakunina N, Kim SS, Nam EC (2017) Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation 20, 290–300. [DOI] [PubMed] [Google Scholar]
- Yu L, Scherlag BJ, Li S, et al. (2013) Low‐level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm 10, 428–435. [DOI] [PubMed] [Google Scholar]
- Zamotrinsky A, Afanasiev S, Karpov RS, et al. (1997) Effects of electrostimulation of the vagus afferent endings in patients with coronary artery disease. Coron Artery Dis 8, 551–557. [PubMed] [Google Scholar]
- Zamotrinsky AV, Kondratiev B, de Jong JW (2001) Vagal neurostimulation in patients with coronary artery disease. Auton Neurosci 88, 109–116. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhou L, Wang S, et al. (2016) The use of noninvasive vagal nerve stimulation to inhibit sympathetically induced sinus node acceleration: a potential therapeutic approach for inappropriate sinus tachycardia. J Cardiovasc Electrophysiol 27, 217–223. [DOI] [PubMed] [Google Scholar]


