Abstract
Background
Trauma‐induced coagulopathy (TIC) is a disorder of the blood clotting process that occurs soon after trauma injury. A diagnosis of TIC on admission is associated with increased mortality rates, increased burdens of transfusion, greater risks of complications and longer stays in critical care. Current diagnostic testing follows local hospital processes and normally involves conventional coagulation tests including prothrombin time ratio/international normalized ratio (PTr/INR), activated partial prothrombin time and full blood count. In some centres, thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are standard tests, but in the UK they are more commonly used in research settings.
Objectives
The objective was to determine the diagnostic accuracy of thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for TIC in adult trauma patients with bleeding, using a reference standard of prothrombin time ratio and/or the international normalized ratio.
Search methods
We ran the search on 4 March 2013. Searches ran from 1970 to current. We searched The Cochrane Library, MEDLINE (OvidSP), EMBASE Classic and EMBASE, eleven other databases, the web, and clinical trials registers. The Cochrane Injuries Group's specialised register was not searched for this review as it does not contain diagnostic test accuracy studies. We also screened reference lists, conducted forward citation searches and contacted authors.
Selection criteria
We included all cross‐sectional studies investigating the diagnostic test accuracy of TEG and ROTEM in patients with clinically suspected TIC, as well as case‐control studies. Participants were adult trauma patients in both military and civilian settings. TIC was defined as a PTr/INR reading of 1.2 or greater, or 1.5 or greater.
Data collection and analysis
We piloted and performed all review stages in duplicate, including quality assessment using the QUADAS‐2 tool, adhering to guidance in the Cochrane Handbook for Diagnostic Test Accuracy Reviews. We analysed sensitivity and specificity of included studies narratively as there were insufficient studies to perform a meta‐analysis.
Main results
Three studies were included in the final analysis. All three studies used ROTEM as the test of global haemostatic function, and none of the studies used TEG. Tissue factor‐activated assay EXTEM clot amplitude (CA) was the focus of the accuracy measurements in blood samples taken near to the point of admission. These CAs were not taken at a uniform time after the start of the coagulopathic trace; the time varied from five minutes, to ten minutes and fifteen minutes. The three included studies were conducted in the UK, France and Afghanistan in both civilian and military trauma settings. In two studies, median Injury Severity Scores were 12, inter‐quartile range (IQR) 4 to 24; and 22, IQR 12 to 34; and in one study the median New Injury Severity Score was 34, IQR 17 to 43.
There were insufficient included studies examining each of the three ROTEM CAs at 5, 10 and 15 minutes to make meta‐analysis and investigation of heterogeneity valid. The results of the included studies are thus reported narratively and illustrated by a forest plot and results plotted on the receiver operating characteristic (ROC) plane.
For CA5 the accuracy results were sensitivity 70% (95% CI 47% to 87%) and specificity 86% (95% CI 82% to 90%) for one study, and sensitivity 96% (95% CI 88% to 100%) and specificity 58% (95% CI 44% to 72%) for the other.
For CA10 the accuracy results were sensitivity 100% (95% CI 94% to 100%) and specificity 70% (95% CI 56% to 82%).
For CA15 the accuracy results were sensitivity 88% (95% CI 69% to 97%) and specificity 100% (95% CI 94% to 100%).
No uninterpretable ROTEM study results were mentioned in any of the included studies.
Risk of bias and concerns around applicability of findings was low across all studies for the patient and flow and timing domains. However, risk of bias and concerns around applicability of findings for the index test domain was either high or unclear, and the risk of bias for the reference standard domain was high. This raised concerns around the interpretation of the sensitivity and specificity results of the included studies, which may be misleading.
Authors' conclusions
We found no evidence on the accuracy of TEG and very little evidence on the accuracy of ROTEM. The value of accuracy estimates are considerably undermined by the small number of included studies, and concerns about risk of bias relating to the index test and the reference standard. We recognise that the reference standards of PT and INR are imperfect, but in the absence of embedded clinical consensus these are judged to be the best reflection of current clinical practice. We are unable to offer advice on the use of global measures of haemostatic function for trauma based on the evidence on test accuracy identified in this systematic review. This evidence strongly suggests that at present these tests should only be used for research. We consider more thoroughly what this research could be in the Discussion section.
Keywords: Adult, Humans, Blood Coagulation Disorders, Blood Coagulation Disorders/diagnosis, Blood Coagulation Disorders/etiology, Cross‐Sectional Studies, Hemorrhage, Hemorrhage/blood, Hemorrhage/etiology, Thrombelastography, Thrombelastography/methods, Thrombelastography/standards, Wounds and Injuries, Wounds and Injuries/blood, Wounds and Injuries/complications
Plain language summary
TEG and ROTEM for diagnosing trauma‑induced coagulopathy (disorder of the clotting system) in adult trauma patients with bleeding
What is 'trauma‐induced coagulopathy'?
Trauma‐induced coagulopathy (TIC) is a disorder of the blood clotting process that can occur soon after trauma injury that can lead to the patient bleeding to death. A diagnosis of TIC on admission to hospital is associated with increases in death rates, blood transfusions, risks of complications and length of stay in hospital.
How is TIC diagnosed?
Current testing for TIC normally involves coagulation tests on the patient's blood.
What are thromboelastography (TEG) and rotational thromboelastometry (ROTEM)?
Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are tests which involve a group of assessments that can be used to diagnose TIC. In some centres TEG and ROTEM are used routinely to test patients' blood, but in the UK their use is usually restricted to experimental and research settings.
The purpose of this research
The purpose of this research was to determine how good the TEG and ROTEM assessments are at diagnosing TIC in adult trauma patients who are bleeding. The accuracy of TEG and ROTEM was compared against another test that is currently used (the reference standard), which was the prothrombin time/international normalized ratio (PTr/INR).
What we discovered
We identified 3 studies (with 300, 90 and 40 participants; 430 in total) that compared the diagnostic test accuracy of TEG or ROTEM for identifying TIC in bleeding adult trauma patients within the emergency setting against PTr/INR. We recognise that the reference standards of PT and INR are imperfect, but in the absence of embedded clinical consensus these are judged to be the best reflection of current clinical practice. Readers should note that the assessment of test accuracy was not the single purpose of any of these 3 included studies.
None of the 3 studies investigated the accuracy of the TEG assessment; they all investigated the ROTEM assessment. The 3 studies provided very little evidence on the accuracy of ROTEM, and provided results for only one potential indicator of TIC (clot amplitude (CA) at 5, 10 and 15 minutes (CA5, CA10 and CA15)), although other indicators could have been used.
The overall reliability of the estimates of accuracy for CA was undermined by the low number of studies (2 for CA5 measurements and 1 each for CA10 and CA15 measurements), as well as concerns that the studies might be subject to bias concerning aspects of the ROTEM test and the PTr/INR test being used as the reference standard.
There was not enough research available on the test accuracy of TEG or ROTEM for the researchers to determine whether these assessments provide a good test for diagnosing TIC in bleeding adult trauma patients.
This evidence strongly suggests that at the moment these tests should only be used for research. The review emphasises that it is not enough to define the index test solely in terms of the device (TEG and ROTEM). Both ROTEM and TEG offer a number of measures: time to initiate clotting; time of clot formation; alpha angle; clot amplitude; maximum strength of clot; time to maximum clot strength; time to lysis of different degrees. These are illustrated in Figure 7. In addition, the protocol for initiating clotting also needs to be specified e.g. INTEM, EXTEM or FIBTEM in the case of ROTEM. Greater clarity is needed on which of these measures is most reliable and which is most relevant for particular clinical tasks; there may be more than one. Finally, different test evaluations may help in assessing these various aspects of the tests. Evaluations of predictive studies may shed light on the link between test result and patient outcome, and provide insight into the best treatment strategies for this condition and patient group. The authors of this review are currently conducting a review of such predictive studies, and this is registered on the International Prospective Register of Systematic Reviews (PROSPERO).
Summary of findings
Summary of findings'. 'Summary of findings table.
| What is the test accuracy of thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma‑induced coagulopathy (TIC) in adult trauma patients with bleeding? | |||||
| Patients | Adult trauma patients with bleeding at risk of TIC | ||||
| Prior testing | None | ||||
| Setting | Hospital; civilian or military | ||||
| Index tests | Tests of global haemostatic function especially thromboelastography (TEG) and rotational thromboelastometry (ROTEM). Any device output measure considered |
||||
| Reference standard |
|
||||
| Study design | Cross‐sectional or case‐control test accuracy studies; all included studies were cross‐sectional | ||||
| Test |
No of participants (no. of studies) |
Accuracy (sensitivity (95% CI)) |
Accuracy (specificity (95% CI)) |
Risk of bias | Implications |
| ROTEM EXTEM Clot amplitude 5 minutes (CA5) | 409 (2) |
Davenport 2011a: 70% (47,87) Woolley 2012: 96% (88,100) |
Davenport 2011a: 86% (82,90) Woolley 2012: 58% (44,72) |
High | Accuracy estimates potentially misleading |
| ROTEM EXTEM Clot amplitude 10 minutes (CA10) | 109 (1) | 100% (94,100) | 70% (56,82) | High | Accuracy estimates potentially misleading |
| ROTEM EXTEM Clot amplitude 15 minutes (CA15) | 88 (1) | 88% (69,97) | 100% (94,100) | High | Accuracy estimates potentially misleading |
Concerns about risk of bias arose from consideration of the index test and the reference standard
Background
Target condition being diagnosed
Trauma‐induced coagulopathy (TIC) can be defined as an impairment of blood clotting that occurs soon after injury (Frith 2010). A diagnosis of TIC on admission to hospital carries a mortality rate amongst patients of up to 50%, and is often associated with increased burdens of transfusion, greater risks of organ injury and septic complications, and longer stays in critical care (Brohi 2003; MacLeod 2003; Maegele 2007). Worldwide, trauma is the leading cause of mortality and disability in adults under the age of 36 years (Hess 2009), and in the UK 40% of all trauma deaths are as a result of haemorrhage (Frith 2010), whilst shock and coagulopathy upon admission have both been independently associated with both massive transfusion and increased mortality (Spinella 2009). Equally in the combat setting, bleeding is the largest cause of death on the battlefield (Holcomb 2007).
Various terms such as TIC, ‘acute traumatic coagulopathy' (ATC) and ‘acute coagulopathy of trauma shock’ are used to describe these early coagulation changes. None of these terms have taken particular precedence and all are widespread within the trauma literature. For the purposes of this review we will use the term TIC to describe the hypocoagulable changes that occur within the first 24 hours following injury due to a variety of different and highly interlinked causes, i.e. hypoperfusion, ongoing bleeding and consumption of clotting factors, haemodilution, acidosis, hypothermia and ATC.
In the absence of embedded clinical consensus, the coagulopathic range we use is based on prothrombin time ratio (PTr)/international normalized ratio (INR). Two different coagulopathic ranges are commonly used within the research and clinical literature: a PTr/INR count of 1.2 or above; and a PTr/INR count of 1.5 or above is considered coagulopathic (further detail is given in the section on Reference standards). We will be including both these ranges within our review. This decision was reached through review of the literature and discussion with the report authors, including experts in haematology and trauma medicine.
The aetiology of coagulopathy associated with trauma is not fully understood. In non‐trauma situations, blood clots form through a chain of actions; first, platelets form a sticky clump on the blood vessel wall at the site of injury. This clot is weak, but soon a cascade of clotting proteins generates fibrin, a protein that meshes the platelets and some red blood cells together to produce a far stronger clot. This process is called coagulation, but it can become disordered; this happens in around a quarter of trauma patients. The underlying reasons for this disruption are still unknown, but the combination of tissue damage and shock are contributory factors, as is the presence of hypoperfusion through severe blood loss (Barts & The London 2011).
Early recognition of the nature of the clotting defect has been acknowledged as increasingly important to guide replacement of clotting factors alongside blood volume maintenance and red cell replacement. There are, however, no validated methods to guide therapy effectively. This leads to both over‐transfusion and under‐transfusion, reduction in efficacy, increased wastage and exposure to risk. These issues can be exacerbated in disasters where timely availability of blood and component therapy is vital but severely resource constrained.
Clinical pathway
Standard blood tests are performed as soon as possible on every patient with bleeding who arrives at the hospital emergency department (see diagnostic pathway in Figure 1). There is no hierarchy of tests performed at admission, but rather a group of tests are used ‐ i.e. activated partial thromboplastin time (APTT), PTr/INR and full blood count (FBC). The choice of these tests is highly variable and follows local hospital practice. In some centres, especially across Europe, thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are standard tests. In the UK, the use of TEG and ROTEM is increasing, but has ‐ up until now ‐ been mainly used in research settings.
1.

Clinical pathway for emergency department identification of trauma‐induced coagulopathy
Current tests
Traditional measures of clotting (such as platelet count, bleeding time, prothrombin time (PT and APTT) have some limitations in the context of managing trauma. Amongst these,
platelet count provides data about how many platelets are present, but gives no information about how they function;
bleeding time measured through the application of a cuff also assesses platelet function, but is impractical in the bleeding patient and is thus rarely used;
fibrinogen tests measure the functional ability of the available fibrinogen, but this test therefore measures only one part of the coagulation system and does not give an overall indication of haemostatic potential; and
PT and APTT only provide a measure of time before initial thrombin generation, they are performed on platelet‐poor plasma, were designed to evaluate clotting factor deficiencies (not acquired coagulopathy), and are known to be poor predictors of bleeding in these circumstances (Dzik 2004).
In addition, evidence has suggested that APTT and PT are not able to provide an indication of when a patient is in a hypercoagulable state (Park 2009).
Despite these weaknesses, in practical terms PT remains the current standard of practice, although it measures a late change in haemostasis and is not a sensitive measure (Brohi 2013).
Index tests
Newer global haemostatic function technologies such as TEG and ROTEM enable ‘point of care’ measurement, using whole blood samples, of the initiation and progress of coagulation as well as final clot strength and lysis and the dynamics of clot formation. For the purposes of this study, TEG and ROTEM are envisaged as a replacement test for traditional coagulation tests. Both tests are currently used in routine clinical practice as both a diagnostic tool and to guide treatment.
TEG (trademark of Haemonetics Corporation, USA: www.haemonetics.com) and ROTEM (trademark of TEM International GmbH: www.rotem.de) work by measuring shear elastic modulus during clot formation and subsequent fibrinolysis. In both tests the whole blood sample is placed in a sample cup or ‘cuvette’ into which a cylindrical pin is immersed, leaving a small gap between the bottom of the pin and the base of the cuvette. The subsequent movement of the blood (designed to emulate sluggish circulation) is where the main difference lies between the two methods. When the sample blood begins to clot (i.e. fibrin begins to form, measured as clotting time or ‘time to clot’), the movement of the pin becomes restricted with increasing firmness and this kinetic is transferred to the machinery of the TEG or ROTEM unit.
The next stage of the coagulation process is platelet aggregation, where platelets build in the blood vessel walls at the site of injury. Fibrin binds to the platelets, which then form a stronger clot, measured by both TEG and ROTEM in shear elasticity units as ‘clot stability’. Eventually lysis – or clot break down – is measured, and a graphic is produced that represents haemostatic performance at all these stages: clotting time, clot formation, clot stability and lysis (see detailed description in Appendix 1).
Whilst both TEG and ROTEM measure clotting time, clot formation, clot strengthening, amplitude of clot, maximum strength of clot, and clot lysis, they use slightly different terms or lettering to designate these features. These differences are detailed in Table 2.
1. Normal reference values for ROTEM and TEG.
| TEG | ROTEM | |
| Clotting time (period to 2 mm amplitude) | R (reaction time) N (whole blood) 4 to 8 min N (Cit, kaolin) 3 to 8 min |
CT (clotting time) N (Cit, INTEM) 137 to 246 s N (Cit, EXTEM) 42 to 74 s |
| Clot kinetics (period from 2 to 20 mm amplitude) |
K (kinetics) N (WB) 1 to 4 min N (Cit, kaolin) 1 to 3 min |
CFT (clot formation time) N (Cit, INTEM) 40 to 100 s N (Cit, EXTEM) 46 to 148 s |
| Alpha angle (clot strengthening) | α (slope between r and k) N (WB) 47° to 74° N (Cit, kaolin) 55° to 78° |
α (slope of tangent at 2 mm amplitude) N (Cit, INTEM) 71° to 82° N (Cit, EXTEM) 63° to 81° |
| Amplitude (at set time) | A or CA | A or CA |
| Maximum strength | MA (maximum amplitude) N (WB) 55 to 73 mm N (Cit, kaolin) 51 to 69 mm |
MCF (maximum clot firmness) N (Cit, INTEM) 52 to 72 mm N (Cit, EXTEM) 49 to 71 mm N (Cit, FIBTEM) 9 to 25 mm |
| Lysis (at fixed time) | CL30, CL60 | LY30, LY60 |
TEG: N = normal values for kaolin‐activated TEG in native whole blood (WB) or citrated and recalcified blood samples (Cit) ROTEM: N = normal values for contact (partial thromboplastin phospholipids, INTEM), tissue factor (EXTEM) and tissue factor plus platelet inhibitor cytochalasin D (FIBTEM) activated citrated and recalcified blood samples Reference values depend on reference population, blood sampling technique, other preanalytical factors, and coagulation activator (Ganter 2008) min: minute(s)
Rationale
This systematic review forms part of the evidence for a wider NIHR‐funded research programme ('Traumatic Coagulopathy & Massive Transfusion ‐ Improving Outcomes & Saving Blood' RP‐PG‐0407‐10036), which aims to improve outcomes for severely injured bleeding trauma patients. This programme is designed around the principle that early identification of patients who present with a TIC and effective, directed therapy will lead to improved outcomes, reduced complications and rationalised transfusions. In addition, these initiatives will result in reduced costs to the National Health Service (NHS), and a reduced logistical burden to military and humanitarian organisations (such as the Red Cross) within austere combat environments. These tests, however, require proper evaluation. Test accuracy studies have been conducted amongst evaluations thus far and should be systematically reviewed.
To complement this review we are also conducting a systematic review of prognosis studies linking measures from TEG/ROTEM with patient outcome (Hunt 2014).
Objectives
The objective was to determine the diagnostic accuracy of thromboelastography and rotational thromboelastometry for TIC in adult trauma patients with bleeding, using a reference standard of Prothrombin Time ratio and/or the International Normalized Ratio.
Methods
Criteria for considering studies for this review
Types of studies
We included all cross‐sectional studies investigating the diagnostic test accuracy of TEG or ROTEM in patients with clinically suspected TIC. We would have included case‐control studies due to the small number of cross‐sectional studies retrieved, but we found none.
Participants
We included all studies involving adult trauma patients with clinically suspected TIC in both military and civilian settings.
Index tests
This review focused on two global tests of haemostatic function; TEG (thromboelastography ‐ trademark of the Haemonetics Corporation, USA) and ROTEM (rotational thromboelastometry ‐ trademark of TEM International GmbH). Thresholds are indicated in Table 2.
Target conditions
The target condition was TIC defined by standard clotting times of prothrombin time ratio (PTr) and international normalized ratio (INR).
Reference standards
In the absence of embedded clinical consensus, we used a coagulopathic range based on PTr/INR; the lower limit of the coagulopathic range being a PTr/INR reading of 1.2 or greater (Frith 2010), and the higher limit of the coagulopathic range being a PTr/INR reading of 1.5 or greater (Stainsby 2006). There is no upper threshold: anyone with a PTr/INR count of above 1.2, or above 1.5, is considered coagulopathic. These figures were reached through discussion by the report authors, including experts in haematology and trauma medicine.
PTr differs from INR, although the final numbers may be the same. The PTr calculated varies according to local thresholds and separate batches of different manufacturers' reagent involved in conducting the prothrombin time test. In an effort to standardise this measurement, the INR is calculated as the ratio of a patient’s PTr compared to a mean normal PTr (calculated by determining the mean of 30 or more patients who are representative of the local hospital population), computed to the power of the International Sensitivity Index (ISI), which is itself calculated by the manufacturer, to give an indication of how each batch of tissue factor corresponds to an international reference. The equation for calculation is shown in Figure 2.
2.
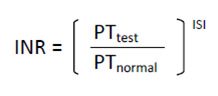
INR equation
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date or publication status. We used a sensitive search strategy to identify literature relating to the index tests for this review. This strategy was not limited by language but was limited by date to 1970 to current and to ‘human only’ populations. The search strategy was modified to increase sensitivity after the protocol had been published. This amendment is recorded in Appendix 2.
Electronic searches
We searched the following bibliographic sources:
The Cochrane Library (all databases; 4 March 2013);
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (1946 to 4 March 2013);
Embase Classic and Embase (OvidSP) (1947 to 4 March 2013);
PsycINFO (OvidSP) (1806 to February Week 4 2013);
CINAHL (EBSCO Host) (1981 to 4 March 2013);
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to March 2013);
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to March 2013);
Prospero (2011 to March 2013);
LILACS (4 March 2013);
BIOSIS (1969 to 4 March 2013);
British Nursing Index (Proquest) (1994 to 4 March 2013);
HMIC (4 March 2013);
Transfusion Evidence Library (1980 to 4 March 2013);
Centre for Reviews and Dissemination (CRD) (4 March 2013).
The Cochrane Injuries Group Specialised Register was not searched for this review as it does not contain diagnostic test accuracy studies.
We searched the following trials registers:
Current Controlled Trials (http://www.controlled‐trials.com/) (accessed 4 March 2013);
Clinical Trials.Gov (www.clinicaltrials.gov) (accessed 4 March 2013);
The World Health Organization (WHO) International Trials Registry Platform (http://www.who.int/ictrp/en/) (accessed 4 March 2013).
We searched the following websites (15/03/2012):
Aggressive Research Intelligence Facility (ARIF) (http://tinyurl.com/3u9tevp);
C‐EBLM (ww.ifcc.org);
Cochrane Diagnostic Test Accuracy Working Group (http://srdta.cochrane.org/);
MEDION database (http://www.mediondatabase.nl);
Haemonetics Corporation (http://www.haemonetics.com/en.aspx);
TEM Innovations GmbH (http://www.rotem.de/site/index.php).
Searching other resources
We conducted citation chasing on all studies included for full text screening. Where necessary we attempted to contact authors for any additional or supporting information.
For further details on the search, including the search strategy, please see Appendix 2.
Data collection and analysis
Selection of studies
Searches and deduplication were performed by the information specialist (CC) before transferring the results to HH, CH and ZZ for screening. All sources were managed using Review Manager software version 5.2 (RevMan 2012). The inclusion criteria were based on the Criteria for considering studies for this review. Three authors (HH, CH and ZZ) made decisions independently on the inclusion/exclusion of studies, using piloted criteria. Any disagreements were resolved with reference to a fourth experienced author, although in the event this was not necessary.
Data extraction and management
The reviewer extracted the following data (where available) into a bespoke data extraction table.
Author, year of study, year of publication, journal reference.
Study design and timing of data collection (prospective/retrospective).
Study population and participant characteristics (age, sex, setting – e.g. hospital, region, country, other details given).
-
Trauma type:
blunt/penetrating;
traumatic brain injury (TBI)/no TBI;
site of injury.
-
Trauma severity as measured by:
Injury Severity Score (ISS);
New ISS (NISS); and
Trauma ISS (TRISS).
Length of time from injury to admission.
Percentage receiving massive transfusion (defined as ≥ 10 units packed red blood cells in 24 hours, or the replacement of an equivalent amount of blood to an entire circulating blood volume of the patient within 24 hours (Doran 2010)).
Mean and interquartile range (IQR) number of units of blood and blood components (fresh frozen plasma, platelets and cryoprecipitate) transfused.
Temperature (% hypothermic at 33 degrees or below), systolic blood pressure (% shocked), and base deficit (% with hypoperfusion) on admission.
Duration of bleed at point of testing.
Reference test used (PTr/INR) and any other measures taken (of, for example, PT, APTT, fibrinogen level, platelet count, fibrinogen degradation products).
Index test used (TEG/ROTEM) and version of device.
Any details about device reliability.
When tests were carried out in treatment phase (i.e. pre/post transfusion, timings).
Data from the 2 x 2 table will be extracted where presented, i.e. true positives, false positives, true negatives and false negatives.
QUADAS‐2 items (see Table 3).
2. Quality appraisal (using QUADAS‐2).
| QUADAS‐2 quality appraisal | Included studies | ||
| Davenport 2011a | Rugeri 2007 | Woolley 2012 | |
| Summary patient domain | |||
| Was a consecutive or random sample of patients enrolled? | Yes Near consecutive, bar exclusions |
Yes Clear statement that patients were consecutive. p 290 col 1 line 10 |
Unclear Lack of clarity regarding the proportion of T1 or T2 casualties over the study period for the whole population, and also how the sub‐population that contributed to the accuracy study were chosen |
| Was case control study design avoided? | Yes | Yes Could be confusion about determination of normal values for ROTEM from healthy volunteers |
Yes Could be confusion about the role of the samples taken from the 50 uninjured control subjects |
| Did the study avoid inappropriate exclusions? | Yes Several exclusion criteria, these all seem reasonable |
Yes Minimal exclusions; 2 out of 90 |
Yes Exclusions not mentioned |
| Risk of bias overall? | No | No | |
| Is there a concern that the included patients do not match the review question? | Concern: low | Concern: low | Concern: low |
| Index test domain | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear The relative objectivity of both the ROTEM measure and the reference standard was judged to reduce the importance of this issue |
Yes Clear statement that clinicians were not informed of ROTEM results p 291 col 1 line 3 |
Unclear No clear statement. The relative objectivity of both the ROTEM measure and the reference standard was judged to reduce the importance of this issue |
| If a threshold was used was it pre‐specified? | No Clear statement that choice of ROTEM measure and threshold were chosen because "there was good separation of normal and ATC curves at this time point" p 2655 col 3 para 6 |
No Thresholds were derived from a normal range in healthy subjects measured in the study. This is less of a problem than failure to pre‐specify the exact ROTEM measure. This choice seems to have been largely made on the basis of performance |
Unclear Some pre‐statement for preference for measure as CA5 and CA10. Reasons why this was chosen over other possible early values such as clotting time, clot formation time or alpha angle were not provided. The threshold for CA5 and CA10 was not pre‐specified, but appeared to be derived from independently measured normal values (Table 1 p 595) |
| Risk of bias overall: could the conduct or interpretation of the index test have introduced bias? | Yes The failure to pre‐specify was considered to represent a major threat to validity |
Yes Although blind interpretation is a positive feature that was not present in other studies, the failure to pre‐specify was considered to represent a major threat to validity |
Unclear As for the other included studies there is a risk of bias associated with failure to pre‐specify measure and threshold, but this was thought to be less than in the other two included studies, and was hence marked Risk: unclear rather than Risk: yes |
| Is there a concern that the index test, its conduct or interpretation differ from the review question? | Concern: unclear There is clarity about the test apparatus. There is less clarity about whether the exact measure and threshold are those that would be used in standard practice, both because these were not pre‐specified and because standard practice is not established |
Concern: high There is clarity about the test apparatus. There is less clarity about whether the exact measure and threshold are those that would be used in standard practice, both because these were not pre‐specified and because standard practice is not established |
Concern: unclear There is clarity about the test apparatus. There is less clarity about whether the exact measure and threshold are those that would be used in standard practice, both because these were not pre‐specified and because standard practice is not established |
| Reference standard domain | |||
| Is the reference standard likely to correctly classify the target condition? | No There are concerns that prolonged prothrombin time may not capture all cases of coagulopathy, even though it is the most established of the measures of coagulopathy |
No There are concerns that prolonged prothrombin time may not capture all cases of coagulopathy, even though it is the most established of the measures of coagulopathy |
No There are concerns that prolonged prothrombin time may not capture all cases of coagulopathy, even though it is the most established of the measures of coagulopathy |
| Were the reference standard test results interpreted without knowledge of the results of the index test? | Unclear No clear statement. The relative objectivity of both the ROTEM measure and the reference standard was judged to reduce the importance of this issue |
Yes Clear statement that clinicians were not informed of ROTEM results p 291 col 1 line 3 |
Unclear No clear statement. The relative objectivity of both the ROTEM measure and the reference standard was judged to reduce the importance of this issue |
| Risk of bias overall? | Yes Reassurance about this possibility could have been provided if discrepant samples had been examined. This was not done |
Yes Reassurance about his possibility could have been provided if discrepant samples had been examined. This was not done |
Yes Reassurance about his possibility could have been provided if discrepant samples had been examined. This was not done |
| Is there a concern that the target condition as defined by the reference standard does not match the review question? | Concern: low The reference standard matches the review question. There are however noted concerns about the risk of bias arising from imperfection in the reference standard, particularly where careful analysis of the discrepant samples (especially false positives) was not carried out |
Concern: low The reference standard matches the review question. There are however noted concerns about the risk of bias arising from imperfection in the reference standard, particularly where careful analysis of the discrepant samples (especially false positives) was not carried out |
Concern: low The reference standard matches the review question. There are however noted concerns about the risk of bias arising from imperfection in the reference standard, particularly where careful analysis of the discrepant samples (especially false positives) was not carried out |
| Flow and timing domain | |||
| Was there an appropriate interval between index test and reference standard? | Yes Clear evidence that ROTEM and PT done on same sample. |
Yes Reasonable evidence that ROTEM and PT done on same sample |
Yes Reasonable evidence that ROTEM and PT done on same sample |
| Did all patients receive a reference standard? | Yes | Yes | Yes |
| Did all patients receive the same reference standard? | Yes | Yes | Yes |
| Were all patients included in the analysis? | Yes | Yes | Yes |
| Risk of bias overall? | No | No | No |
| Other issues | ‐ | Multiple samples seem to have been taken from each patient, but seems reasonably clear that only the samples at admission (H0) were used in the analysis | Lack of clarity about whether some samples came from the same patient |
Abbreviations
CA5: clot amplitude at 5 minutes CA10: clot amplitude at 10 minutes col: column H0: hospital/ emergency room admission p: page para: paragraph PT: prothrombin time T1: triaged casualty group 1 ‐ immediate or urgent clinical problems requiring full trauma team activation (as T2 below). T2: triaged casualty group 2 ‐ immediate or urgent clinical problems requiring full trauma team activation (as T1 above).
We recorded variability between operators and assay conditions where available, although this was often not reported in primary studies. Particular care was required in recording many of these items (particularly index test and reference standard) due lack of standardisation. Two authors (HH and CH) piloted the extraction form using two primary diagnostic studies, with a third author (NC) in place to resolve disagreements. The data extraction form was accompanied by a briefing document explaining how it should be used. Data were extracted by one author (HH) and checked by a second (CH), with a third author (NC) providing moderation as required.
Assessment of methodological quality
We carried out quality assessment using a checklist approach to assess the quality of primary studies, on the QUADAS‐2 instrument and in line with advice given in Reitsma 2009. Independently, we scored each item as ‘yes’, ‘no’ or ‘unclear’ as recommended by the Cochrane Handbook for Diagnostic Test Accuracy Reviews (Deeks 2010). A categorisation of 'unclear' is generally considered to be a marker of poor quality, so we took care to account for the possibility that failing to report an item was reasonable given the circumstances in which the study was conducted. Results are presented in the Methodological quality of included studies with further detail in Table 3.
Statistical analysis and data synthesis
We used Review Manager software (version 5.2) to conduct our analysis. We analysed the accuracy of TEG and ROTEM compared to the reference standard as detailed in the review protocol (Hunt 2013), with the intention to consider values greater than 1.2 and greater than 1.5 separately ‐ although insufficient data were available to render this necessary. Updates may be able to incorporate this discrimination if sufficient data are available. Our a priori commitment to exploring the TEG and ROTEM test type as a potential source of heterogeneity was unnecessary, as all of the studies included used ROTEM as their index test. However, regardless of results it should be noted that ROTEM and TEG are not interchangeable. Whilst the underlying mechanism of measuring shear elastic modulus is similar, the tests use different clotting activators, different methodology and require different treatment algorithms (Hagemo 2013b; Sankarankutty 2012). Therefore, TEG and ROTEM would not have been formally compared.
Results are test accuracy data which form the components of the 2 x 2 table, sensitivity and specificity and their 95% confidence interval (CI). These results have been tabulated and are presented according to the different ROTEM sub‐measures used in Data table 1, Data table 2 and Data table 3. These data are also presented graphically (in forest plots Figure 3, and summary receiver operating characteristic (ROC) plots Figure 4). A narrative analysis was conducted with conclusions based on patterns of results. Quantitative meta‐analysis was not appropriate as there were too few studies to estimate the parameters of meta‐analytic models.
1. Test.

ROTEM CA5.
2. Test.

ROTEM CA10.
3. Test.

ROTEM CA15.
3.
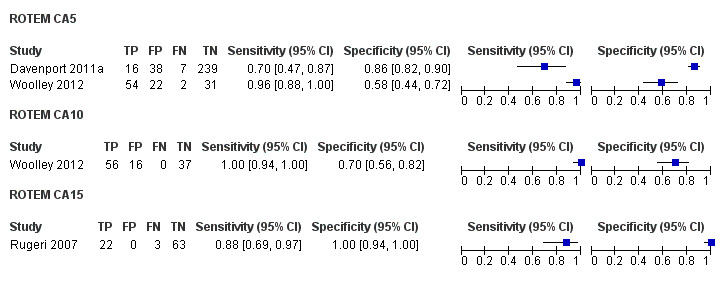
Forest plot of tests: ROTEM CA5, ROTEM CA10, ROTEM CA15
4.
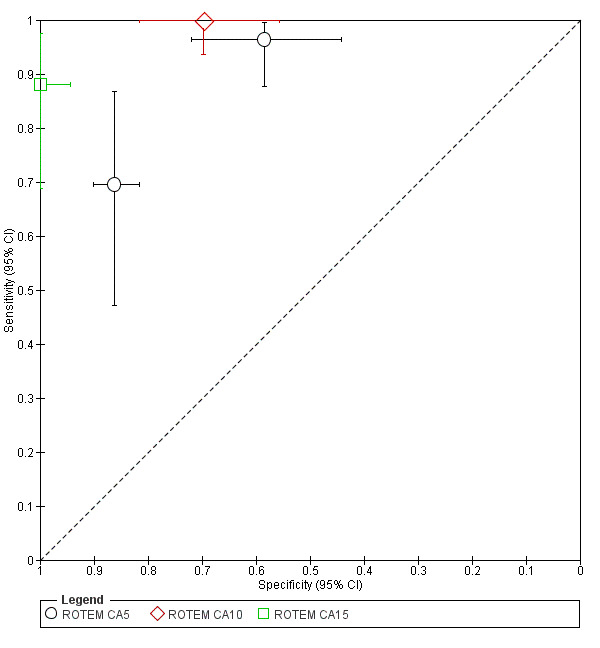
Summary ROC Plot of tests: ROTEM CA5, ROTEM CA10, ROTEM CA15
Investigations of heterogeneity
There was an insufficient number of studies included in the review for us to conduct formal investigations of heterogeneity. The approach specified in the protocol is recorded in Appendix 3, and will be used, should the review be updated in the future.
Sensitivity analyses
As no meta‐analysis was conducted, no sensitivity analysis was required. Should the review be updated in future, we will follow guidance in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Macaskill 2010).
Assessment of reporting bias
We did not assess reporting bias because its impact in test accuracy is unclear and the tools for investigating it are in the early stages of development.
Results
Results of the search
We screened 9013 citations and examined 91 full text articles in detail to reveal three included studies Rugeri 2007, Woolley 2012 and Davenport 2011a. The characteristics of the included studies are tabulated in Table 4 and Table 5. The PRISMA study flow diagram is shown in Figure 5.
3. Characteristics of studies ‐ study details.
|
Author, year of publication [year of study] |
Study design and timing of data collection (prospective/ retrospective) | Study population and participant characteristics (age, sex, setting – e.g. hospital, region, country, other details given) | Exclusion criteria |
Trauma type: blunt/ penetrating, traumatic brain injury (TBI)/no TBI [% receiving massive transfusion] |
Trauma severity * |
SBP (% shocked) on admission [When tests were carried out in treatment phase] |
|
Davenport, 2011 [2007‐2009] |
Multiple measurements at one point of time in each of a group of trauma patients. Data were collected prospectively (i.e. after study had been designed) | Adult trauma patients who met local criteria for full trauma team activation. Between 0800 and 2000 when study personnel present. N = 300. Age: median 33 IQR 23‐48. Sex: M 246 (82%). Setting: Level 1 trauma centre (more details given). Region & country: UK, urban | Arrival in ED > 2 h after injury; > 2000 ml of iv fluid before ED; transfer from another hospital; burns covering > 5% body area | 62 (21%) penetrating injuries. No information on TBI [11 (4%) received >10 packed red cell units] |
ISS median 12 IQR 4‐24. ISS > 15 126 (42%) | 53 (18%) < 100 SBP at admission [on admission] |
|
Rugeri, 2007 [2004] |
Multiple measurements at several points in time in each of a group of trauma patients. Blood tests at admission were the main focus of the results | Consecutive trauma patients July‐October 2004. N = 90; 2 excluded because on oral anticoagulants. Age: mean 34 SD 16 years. Sex: M 68 (77%). Setting: Teaching hospital. Region & country: France, Lyon (urban) |
On oral anticoagulants | Not stated [Not stated] |
ISS median 22 IQR 12‐34. [ISS derived from the Abbreviated Injury Score] | Not stated [on admission‐ assumed] |
|
Woolley, 2012 [2009] |
Multiple measurements at several points in time in each of a group of trauma patients | Seriously injured patients presenting to Role 3 Field Hospital at Camp Bastion 21 May 2009 to 3 July 2009. N = 48 (108 samples). Age: mean 24 IQR 21‐26. Sex: male 48 (100%): Setting: Military field hospital. Region & country: Camp Bastion, Afghanistan. Test accuracy vs conventional coagulation undertaken on 40 samples from 30 patients. No details of characteristics of these patients | None stated | Whole population: 48% improvised explosive devices; 29% ballistic injuries; 4% burns; 4% road traffic accidents. No information on TBI. No information on accuracy population [Not stated; although median of 10 packed red blood cells suggests > 50%] |
Whole population: NISS median 34 IQR 17‐43 (range 5‐75). No information on accuracy population | Whole population: mean BP 92 mmHg (SD 24; range 40‐152). No information on accuracy population [not stated] |
Abbreviations
BP: blood pressure ED: emergency department IQR: inter‐quartile range ISS: Injury Severity Score iv: intravascular M: male NISS: New Injury Severity Score SD: standard deviation TBI: traumatic brain injury
4. Characteristics of studies ‐ test details.
|
Author, year of publication [year of study] |
Reference test used (PTr/INR) | Any other measures taken (e.g. PT, APTT, fibrinogen level, platelet count, fibrinogen degradation products) | Index test used (TEG/ROTEM) and version of device | Measure used and threshold | Origin of threshold | Other available index test measures | % disease prevalence (PTr > specified range) |
|
Davenport, 2011 [2007‐2009] |
Laboratory PT ratio of > 1.2 | Platelet and fibrinogen measured but not used to define reference standard. Need for massive transfusion also considered as marker of coagulopathy and sensitivity of TEG to predict massive transfusion calculated | ROTEM delta (Pentapharm GmbH, Munich, Germany). STARTEM (recalcitrant) and EXTEM (tissue factor derived from rabbit brain) protocols employed | EXTEM CA5 (clot amplitude at 5 minutes) ≤ 35 mm | Based on maximum separation between normal and acute traumatic coagulopathy patients in study. Also noted to be 1 SD below normal range | Clotting time (s); clot formation time (s); alpha angle (degrees); maximum clot firmness (mm) | 8% |
|
Rugeri, 2007 [2004] |
PT ratio of > 1.5 of control PT. MDA II instrument used to measure all coagulation measures | APTT > 1.5 of control; fibrinogen < 1 g/L (Fibriquick/Clauss technique); platelets < 50 x109/L (SE‐9500) ‐ all used as alternative definitions of coagulopathy | ROTEM (model not specified). INTEM, EXTEM and FIBTEM screening tests | EXTEM CA15 (clot amplitude at 15 minutes) = 32 mm | Unclear, but appears to be based on results of correlation between ROTEM results and standard measures of coagulation | Clotting time (s); clot formation time (s); alpha angle (degrees); maximum clot firmness (mm); CA10 and CA15 | 28% |
|
Woolley, 2012 [2009] |
"Standard lab testing in the hospital lab". PT > 1.5 times normal (corresponding to PT > 18 s) | Main focus of evaluation was examining accuracy relative to ROTEM EXTEM MCF < 40 mm | ROTEM (TEM International GmBH, Munich, Germany). STARTEM (calcium), EXTEM (tissue factor) and FIBTEM (platelet inhibitor, cytochalasin D) screening tests | EXTEM CA5 and CA10 below reference range. For Camp Bastion CA5 32‐71 mm CA10 40‐72 mm (derived from 50 uninjured volunteers). Manufacturer CA10 43‐65 mm | Reference range. For Camp Bastion results based on 50 uninjured volunteers ‐ members of the Emergency Blood Donor Panel | Clotting time (s); clot formation time (s); alpha angle (degrees); maximum clot firmness (mm) | 51% |
Abbreviations
APTT: activated partial thromboplastin time CA5: clot amplitude at 5 minutes CA10: clot amplitude at 10 minutes CA15: clot amplitude at 15 minutes EXTEM: tissue factor activated citrated and recalcified blood sample FIBTEM: tissue factor plus platelet inhibitor cytochalasin D activated citrated and recalcified blood samples INR: International Normalized Ratio PT: prothrombin time PTr: pro‐thrombin time ratio ROTEM: rotational thromboelastometry SD: standard deviation STARTEM: a liquid system reagent for recalcifying citrated blood or plasma TEG: thromboelastography
5.
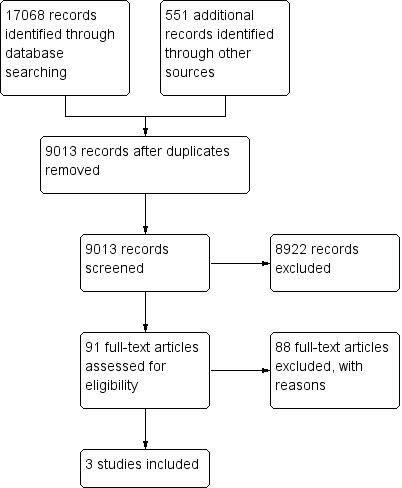
Study flow diagram
All three included studies used ROTEM as the test of global haemostatic function, and none used TEG. Whilst there may be a difference of one or two versions between ROTEM models, fundamentally the technology and tests are the same. Of the many measurements that can be made by ROTEM, EXTEM (tissue factor) clot amplitude (CA) was the focus of the accuracy measurements in blood samples taken near to the point of admission. However, these CAs were not measured at a uniform time after the start of the coagulopathic trace. The time varied from five minutes (A5 or CA5; Davenport 2011a; Woolley 2012), ten minutes (A10 or CA10; Woolley 2012) and fifteen minutes (A15 or CA15; Rugeri 2007). Concerning the thresholds for the CA measurements, the two studies using EXTEM CA5 used a threshold of 35 mm or below (Davenport 2011a), and below a reference range of 32 mm to 71 mm (Woolley 2012); the study using CA10 used a threshold of below a reference range of 40 mm to 72 mm (Woolley 2012); and the study using EXTEM CA15 used a threshold of less than 32 mm (Rugeri 2007).
In accordance with the review inclusion criteria, we made accuracy measures in all the included studies relative to a reference standard of PTr. However in two cases the PTr value was greater than 1.5 (Rugeri 2007; Woolley 2012), and in one case greater than 1.2 (Davenport 2011a). We also examined accuracy relative to other reference standards defining TIC in the included studies, but none of the alternatives were used consistently. The need for massive transfusion (systolic blood pressure less than 90 mmHg, poor response to initial fluid infusion and suspicion of ongoing haemorrhage) was considered as a marker for coagulopathy by Davenport 2011a. Rugeri 2007 also used an APTT value of more than 1.5 of control, fibrinogen less than 1 g/L (Fibriquick/Clauss technique), and platelets less than 50 x 109 L‐1 (SE‐9500 ‐ Sysmex, Kobe, Japan) as alternative definitions of coagulopathy. Finally, Woolley 2012 examined accuracy relative to ROTEM EXTEM Maximum Clot Firmness (MCF) below 40 mm, and this was, in fact, the main focus of their study.
The three included studies were conducted in the UK (Davenport 2011a), France (Rugeri 2007) and Afghanistan (Woolley 2012), in both civilian and military trauma settings. The trauma appeared moderate to severe in all the included studies, and was most severe in the military setting where there were injuries from improvised explosive devices and ballistics (Woolley 2012), and least severe in the UK NHS (Davenport 2011a). As a corollary, the frequency of TIC (PTr above the defined range) was 8% and 28% in the civilian settings (Davenport 2011a; Rugeri 2007, respectively), and 51% in the military setting (Woolley 2012). The studies varied in size from 300 participants to 90 and 48. The number of patients contributing to the accuracy estimates was complicated, particularly in the study by Woolley, where there were multiple samples from individual patients (Woolley 2012). This also complicates what can be inferred about whether the blood samples were taken close to the point of admission in all cases (see above).
All studies employed standard test accuracy designs where index tests and reference standards were applied to naturally occurring groups of patients presenting to trauma centres. There were no diagnostic case‐control studies. However it is worthy of note that in no case was the assessment of accuracy the single objective of the included studies and indeed may not have been the main objective. The studies were a mixture of attempts to identify a normal range in healthy patients (and to compare values in trauma patients) (Rugeri 2007; Woolley 2012), correlations of usual coagulation test results with ROTEM values (Rugeri 2007), and exploration of the threshold for ROTEM results (Davenport 2011a; Rugeri 2007; Woolley 2012).
All three studies declared support from external sources. The Davenport 2011a study received equipment and materials from Pentapharm GMbH (manufacturers of ROTEM ‐ Munich, Germany), and two study authors received unrestricted equipment and materials grants from the manufacturers. Rugeri 2007 stated that they were grateful to BIODIS (Signes, France) for their support of the study, but declared no conflicts of interest. Woolley 2012 declared that the work was funded by and formed part of the Human Dimension and Medical Sciences Domain Research programme within the UK Ministry of Defence (MoD) Defence Science and Technology Laboratory (DSTL) Programme Office.
Methodological quality of included studies
This is summarised in the QUADAS‐2 tables with further information provided in Table 3. A 'Risk of bias and applicability concerns' graph is presented in Figure 6.
6.
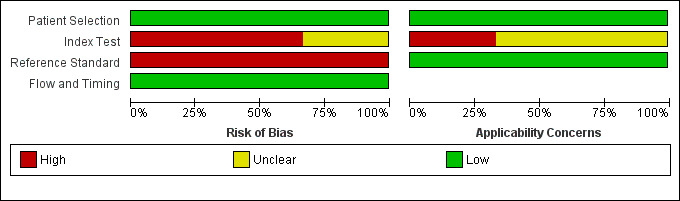
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
Concerning risk of bias, we felt the risk to be low across the included studies for the patient and flow and timing domains. There was some concern regarding timing domains arising from multiple samples being taken from the same patient in the Woolley 2012 study, but this was not felt to be a major problem.
The risk of bias for the index test domain for the trials was either high or unclear, and arose, principally, from failure to prespecify the threshold. Davenport 2011a based their threshold on maximum separation between normal and acute traumatic coagulopathy patients. The origin of the threshold from the study by Rugeri 2007 appears to have been based on results of correlation between ROTEM results and standard measures of coagulation. Woolley 2012 created a reference range for Camp Bastion, with results based on 50 uninjured volunteers (existing members of the Emergency Blood Donor Panel). In summary, only Woolley 2012 seems to have generated a threshold external to the participants to generate the accuracy estimations, and even this could be criticised, as it relies on test values lying outside a normal range.
The risk of bias for the reference standard domain was also high. This principally rose from concern that PTr is not a completely robust measure to define coagulopathy, even though, pragmatically, it is the most consistently used definition. Our view was that it could only be relied on if there was some examination of discrepant samples, particularly to explore the possibility that some 'true' cases of TIC had normal PT, which, anecdotally, is claimed to occur. There were no such discrepant analyses.
Concerning applicability, we have no concerns as far as the patient population is concerned, though we have concerns about the index test domain. Although CA as a ROTEM measure is clearly relevant to the review question, the concern arose because it was unclear why this measure had been chosen over other measures. Furthermore, there is a need to wait for at least 10 and 15 minutes to obtain a result for CA10 and CA15, which might limit their usefulness relative to measures that can be obtained earlier in the ROTEM trace. There was a complete absence of information for the other commonly used device, TEG.
Findings
See Characteristics of included studies; Characteristics of excluded studies; Table 4; Table 5.
There were insufficient included studies examining each of the three ROTEM CAs at 5, 10 and 15 minutes to make the meta‐analysis and investigation of heterogeneity valid. The results of the included studies are thus reported narratively and illustrated by a forest plot (Figure 3), with results plotted on the ROC plane (Figure 4).
For CA5 the accuracy results were sensitivity 70% (95% CI 47,87) and specificity 86% (95% CI 82, 90) for one study (Davenport 2011a), and sensitivity 96% (95% CI 88,100) and specificity 58% (95% CI 44,72) for the other (Woolley 2012).
For CA10 the accuracy results were sensitivity 100% (95% CI 94,100) and specificity 70% (95% CI 56,82) (Woolley 2012).
For CA15 the accuracy results were sensitivity 88% (95% CI 69,97) and specificity 100% (95% CI 94,100) (Rugeri 2007).
None of the included studies mentioned uninterpretable ROTEM study results.
Discussion
Summary of main results
There is no evidence for the accuracy of TEG and very little evidence for the accuracy of ROTEM. The latter is limited to information on the accuracy of CA at 5, 10 and 15 minutes, as opposed to the many other features of the ROTEM trace that might be used. Furthermore, the value of the accuracy estimates for CA are considerably undermined by the number of studies (two for CA5 and one each for CA10 and CA15) and concerns about risk of bias arising from considerations about the index test and the reference standard.
Strengths and weaknesses of the review
The review was conducted using a pre‐specified protocol designed by a large multi‐disciplinary team with expertise in the condition, the test and the evaluation methodology. There were no departures from this protocol, bar failure to use meta‐analysis because of an insufficient number of included studies. We conducted a very comprehensive search that was screened in triplicate. We were able to obtain additional information from many study investigators because of the strong links many of the review team have with researchers in the field. This gave us insight that studies rarely, however, address accuracy alone and are frequently undertaken as one small component of wider evaluations, opening the risk of overlooking accuracy results. Those review authors who were members of the investigating teams of any included studies were not involved in the quality appraisal of their own study.
Publication bias is an ever present threat that, despite the comprehensive search undertaken for this review, is difficult to guard against completely. The nature of publication bias in test accuracy studies is still not completely clear. Also the very limited number of included studies, and the inability to conclude definitively from them, restricts the potential importance of possible publication bias in this review.
The main limitation of the review is the very limited number of included studies and the possibility of bias in these. Together these conspire to leave the accuracy of global measures of haemostatic function virtually unknown at present. This raises the question of whether the absence of this information is important and, if so, how evidence on it should be obtained in future. It also prompts questions concerning whether other types of evaluation of TEG and ROTEM, beyond accuracy, should be examined in parallel or in preference to accuracy data. Both of these issues are considered in further detail in recommendations for research below.
Applicability of findings to the review question
Although the three included studies match the review question, they only cover a fraction of the issues needing to be addressed to fully examine the accuracy of global measures of haemostatic function. There are no evaluations of TEG and the evaluations of ROTEM are restricted to measures of CA at different points in time.
Authors' conclusions
Implications for practice.
We found no evidence on the accuracy of thromboelastography (TEG) and very little evidence on the accuracy of rotational thromboelastometry (ROTEM). The value of accuracy estimates are considerably undermined by the small number of included studies, and concerns about risk of bias relating to the index test and the reference standard. We are therefore unable to offer advice on the use of global measures of haemostatic function for trauma based on the evidence on test accuracy identified in this systematic review. This evidence strongly suggests that at present these tests should only be used for research.
Implications for research.
Based on the findings outlined above, the evidence strongly suggests that currently these tests should only be used for research. We consider below what this research could be.
Which test?
The review emphasises that it is insufficient to define the index test solely in terms of the device.
Both ROTEM and TEG offer a number of measures: time to initiate clotting; time of clot formation; alpha angle; clot amplitude; maximum strength of clot; time to maximum clot strength; time to lysis of different degrees. These are illustrated in Figure 7. Furthermore the protocol for initiating clotting also needs to be specified e.g. INTEM, EXTEM or FIBTEM in the case of ROTEM. Greater clarity is needed on which of the measures is most robust and which is most relevant for specific clinical tasks; there may be more than one. In the context of trauma, a measure that is available early in the trace would seem to be the most valuable. It may be, however, that there is a trade‐off, where timeliness is achieved at the expense of accuracy. This does not appear to have been investigated, although it may be that this has been established in applications beyond trauma. Even if this is the case there may still be a need to repeat the exercise in trauma patients.
7.
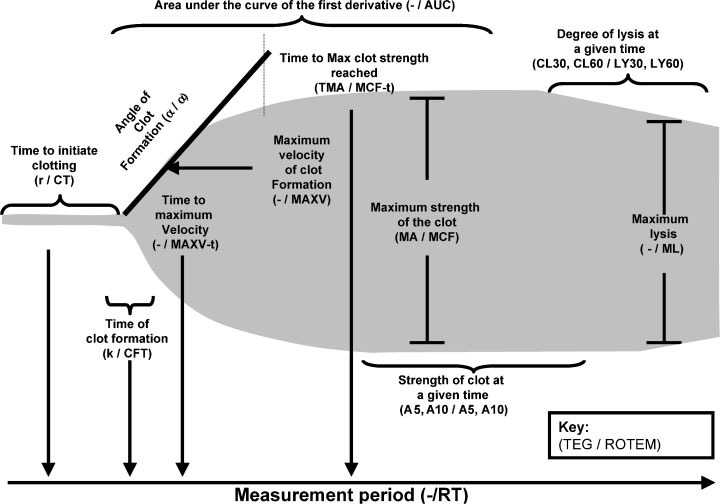
Viscoelastic haemostatic assays terminology and parameters
α, alpha angle; AUC, area under the curve; CFT, clot formation time; CL (t), clot lysis (at time t); CT, clot time; k, rate of clot formation; LY (t), lysis (at time t); MA, maximum amplitude; MAXV, maximum velocity; MAXV‐t, time to maximum velocity; MCF, maximum clot firmness; MCF‐t, time to maximum clot firmness; ML, maximum lysis; r, time to clot initiation; ROTEM, Rotational Thromboelastogram; RT, reaction time; TEG, Thromboelastograph; TMA, time to maximum amplitude; '‐'; no equivalent parameter.
Reproduced by kind permission of Dr Roger Luddington, Addenbrooke's Hospital, UK
What test threshold?
Given the lack of clarity about the specific test measures most likely to be helpful in diagnosing coagulopathy, it is not surprising that the thresholds that define when the disease is present and absent still appear to be unclear. Once the precise measures have been identified, however, it is important that these thresholds are derived from a data‐set independent of that used to measure the accuracy. This principle did not seem to be widely appreciated in the included studies where the same data‐sets were used. Furthermore, the reference ranges employed in the included studies, although they provided a useful starting point, may not be sufficient to define thresholds, as they assume a complete separation between non‐disease and disease, which is unusual.
Is test accuracy important?
Given the uncertainty about the reference standard it may be reasonable to question the value of accuracy of data in evaluating global measures of haemostatic function. However, even with the concerns about pro‐thrombin time ratio (PTr) either greater than 1.2 or greater than 1.5 as a reference standard, further steps could have been taken in the accuracy studies we included to improve their value.
Investigators could have examined discrepant samples, particularly 'false positives' to consider whether by using all available clinical data there was evidence that ROTEM measures gave a better indication of the presence of coagulopathy than the current reference standard. We encountered studies that did this for individual cases, but not in the context of an accuracy study.
Investigators could have looked at PTr alongside other markers of coagulopathy, creating a composite reference standard definition consisting for instance of high PTr or low fibrinogen levels or low platelets.
Investigators could have used quasi‐clinical definitions of coagulopathy incorporating features such as uncontrollable bleeding, need for transfusion or massive transfusion.
Investigators in the included accuracy studies indicated that they had considered such approaches, but they appear to have lacked confidence to apply them consistently and in combination.
In time it may be reasonable to use complete data from a coagulation trace to validate and assess the accuracy of single early trace measures, however, this requires authoritative demonstration of the accuracy of the complete trace data, which the current evidence base does not provide. Given all of these considerations, further research on test accuracy is justified.
Irrespective of the nature of the reference standard in future accuracy studies, attention must be paid to minimising bias through good conduct and reporting adhering to the STARD criteria (Bossuyt 2003).
Are other test evaluations needed?
There is a case that the objections to an imperfect reference standard may not be completely overcome, or that better accuracy studies will leave aspects of evaluation uncovered.
There are certainly other test designs that might help to understand the value of a new test, particularly one that appears to offer advantages over current reference standards. This is the case for global measures of haemostatic function, because it is clear that they offer the ability to examine the whole coagulation process rather than just specific components of it.
The link between test result and outcome is potentially very informative. Such prediction or prognosis studies are particularly achievable in this scenario because patients succumb to coagulopathy or survive it over a short time interval. In addition, the current impact of alternative treatments may not be pronounced because optimal treatment strategies are not clearly identified. It is important to review prognosis studies and a protocol for one has just been registered on PROSPERO by this review group. Depending on its results, further studies of prognosis may be helpful.
Ultimately evaluation may require interventional studies in which the effect on patient outcomes is compared in patients using TEG/ROTEM informed management with those using normal practice. Randomised trials of this type have been undertaken for the use of TEG/ROTEM in cardiac surgery and liver transplantation, and these have been the subject of a Cochrane Review (Afshari 2011b). It should be noted that the Afshari 2011b review included eight trials of routine cardiac surgery with only one trial of liver transplantation, although the review title does not make this explicit. Although the results from studies in routine cardiac surgery are not generalisable to the use of TEG/ROTEM in trauma, they do illustrate the feasibility of interventional studies. At least one controlled clinical trial appears to have been conducted (Messenger 2011). However, the appropriateness of such trials where there appears to be lack of clarity about the specific TEG and ROTEM measures to use, and how results in particular ranges from these measures should influence management, is debatable.
Feedback
Comments submitted by Matthew D Neal, MD, 28 February 2015
Summary
The recent Cochrane review by Hunt and colleagues draws the conclusion that viscoelastic testing such as TEG and ROTEM in trauma should, at present, be limited to research purposes and not clinical practice (1). Although the review of the literature is robust, the included studies are all fundamentally flawed, and, as such, the review and conclusions are equally limited. The three main studies included by Hunt all focus on a comparison of ROTEM to conventional coagulation testing (CCT), including the prothrombin time (PT) and international normalized ratio (INR). It is well documented, as Hunt and colleagues mention in their discussion, that the use of CCT as a standard for the measurement of trauma‐induced coagulopathy (TIC) has never been rigorously and prospectively validated. In fact, correlation with clinical and hemostatic history is necessary for accurate interpretation of CCT, and measurement of PT and INR alone miss major components of coagulation, including the intrinsic pathway, platelet function, and the presence of inhibitors (2).
Furthermore, hyperfibrinolysis is a major component of TIC and has been shown to directly correlate with mortality in trauma patients (3,4). Treatment with antifibrinolytic therapy has demonstrated reduced mortality in bleeding trauma patients, as demonstrated by a prior Cochrane review (5). TEG and ROTEM provide a quantitative assessment of fibrinolysis which is missed by using only PT and INR. A thorough assessment of TIC should include clinical evidence of impaired hemostasis and potentially can be monitored through transfusion requirements. Using these endpoints, multiple authors have demonstrated that viscoelastic measurements are superior to CCT in predicting the risk of bleeding and need for transfusion (6,7).
In summary, including only studies using comparison of ROTEM to PT and INR without any assessment of clinical bleeding inserts a major form of selection bias into the referenced Cochrane review by Hunt and colleagues (1). Caution should be exercised in concluding that TEG and ROTEM should be limited to research ‐ what is lacking in the literature is a robust, prospective randomized study to compare TEG/ROTEM to PT/INR in the context of clinically important markers of coagulopathy. Numerous studies comparing TEG/ROTEM to relevant clinical assessments of TIC, as referenced above, provide clinical equipoise for such an analysis as well as the ongoing use of viscoelastic testing in clinical practice until such a study is completed.
References:
1. Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, Zhelev Z, Hyde C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev. 2015 Feb 16;2:CD010438. [Epub ahead of print]
2. Kamal AH, Tefferi A, Pruthi RK, How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007 2007 Jul;82(7):864‐73)
3. Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg 2012; 73:365‐370; discussion 370
4. Chapman MP, Moore EE, Ramos CR, et al. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg 2013; 75:961‐967; discussion 967
5. Roberts I, Shakur H, Ker K, Coats T; CRASH‐2 Trial collaborators. Antifibrinolytic therapy for acute traumatic injury. Cochrane Database Syst Rev. 2012;12:CD004896. Review.
6. McCully SP, Fabricant LJ, Kunio NR, Groat TL, Watson KM, Differding JA, Deloughery TG, Schreiber MA. The International Normalized Ratio overestimates coagulopathy in stable trauma and surgical patients. J Trauma Acute Care Surg. 2013 Dec;75(6):947‐53
7. Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, Adams PR, McCarthy JJ, Cotton BA. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012 Sep;256(3):476‐86
Reply
Our thanks to the commentator for his interest and observations on our recently‐published systematic review 'Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma‐induced coagulopathy (TIC) in adult trauma patients with bleeding', and we welcome the opportunity to engage with those who have an interest in this area. We address each main point in turn.
The commentator disputes the acceptability of using PT and INR as reference standards. We have sympathy with this assertion and address the issue at some length within the review, both in terms of recognition within the Objectives and Background sections and suggestions for future research in the Discussion section. Within the Discussion section, we explicitly address the issues raised by the commentator in the section headed ’Is test accuracy important?’ (p.16‐17). In particular, we advance the potential for authors creating a composite reference standard definition comprising, for instance, high PTr or low fibrinogen levels, and suggest the potential for using quasi‐clinical definitions of coagulopathy.
The commentator appropriately raises the potential importance of hyperfibrinolysis. However we note that hyperfibrinolysis is a specific component of TIC which is usually considered separately, and this is the approach we took. This is largely because the optimal indicators of TEG/ROTEM suggesting hyperfibrinolysis are probably not the same as those suggesting hypocoagulability (although they are clearly inter‐connected); the most appropriate reference standard in an accuracy study would be different too. Evidence suggests that whereas marked hyperfibrinolysis is readily identifiable by ROTEM, mild or moderate hyperfibrinolysis is not (Raza et al., 2013); thus its value is not self‐evident as is suggested. Further, the benefit from treatment with tranexamic acid mentioned as being demonstrated by CRASH‐2 did not require specific diagnosis of hyperfibrinolysis by TEG/ROTEM.
The commentator cites two studies, claiming they “demonstrate that viscoelastic measurements are superior to CCT in predicting the risk of bleeding and the need for transfusion”. We examined this claim. McCully et al. (2013) examines measures of TEG and CCT before and after FFP transfusions in 106 stable trauma and surgical patients. The majority of patients appear to be surgical with only 36/106 identified as being from the “Trauma/critical care” service. Massive transfusion was an exclusion criterion. Our main concern is thus whether the circumstances in which TEG is being used are equivalent to those of interest in our review. Further risk of bleeding and need for transfusion do not seem to have been addressed by the study.
Holcomb et al. (2012) is a study of 1974 consecutive trauma patients in which admission rapid TEG (r‐TEG) and CCTs (PT; aPTT; INR; platelets; and fibrinogen) were correlated against each other and outcome measures such as 0‐6h transfusion of RBC, plasma, platelets; substantial bleeding; massive transfusion; and mortality. With the exception of using r‐TEG rather than TEG, the study is typical of one which we would include in our review of prognosis studies proposed in our discussion section. However this study usefully illustrates the difficulty of interpreting such studies, particularly in isolation. Thus in Holcomb et al. for the outcome predicting massive transfusion, the r‐TEG measure alpha‐angle <56o is the strongest predictor with an odds ratio (OR) of 8.99 [this means that massive transfusion is nearly 9 times more likely where alpha‐angle <56o, than in patients where it is 56o or above], and so consistent with the claim that r‐TEG are stronger predictors than CCTs (where the OR are lower). However the 95% CI around the OR of 8.99 are 2.9 to 28.3, showing that there is considerable uncertainty. As a corollary the risk associated with INR>1.5 is 3.4, so the difference in risk between the r‐TEG value and the CCT value could be explained by chance. To reinforce this issue in the outcome predicting substantial bleeding, the strongest predictor is this time a CCT measure, INR>1.5 OR 3.4 (95% CI 1.7 to 7.0) but again the difference between this and the strongest r‐TEG predictor, alpha‐angle <56o, OR 2.7 could be explained by chance. A further important issue is that it is likely that both CCT and r‐TEG measures were being used to guide transfusion during the study so the risks measured include a treatment effect. Given these issues we think the claim by the commentator, and indeed the conclusion by the original authors that “Admissions CCTs can be replaced with r‐TEG.” is overstated.
Finally the commentator contests our suggestion that TEG and ROTEM as used in adult trauma patients with bleeding should only be used for research. However, the commentator acknowledges that a robust prospective randomized study is required to compare TEG/ROTEM to PT/INR, so he is agreeing with us; as such trials are unlikely if TEG/ROTEM is in widespread use in day‐to‐day trauma care practice. We also draw attention in our review to the fact that such trials are in progress and the help that similar trials have provided in clarifying the use of TEG/ROTEM in routine cardiac surgery. However, we also note that such trials are unlikely to be successful where TEG/ROTEM measures which indicate treatment decisions are not specified, and the nature of these treatments decisions is vague.
References:
Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Annals of Surgery 2012;256(3),476‐86.
McCully SP, Fabricant LJ, Kunio NR, Groat TL, Watson KM, Differding JA et al. The International Normalized Ratio overestimates coagulopathy in stable trauma and surgical patients. Journal of Trauma and Acute Care Surgery 2013;75(6),947‐53.
Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C et al. The incidence and magnitude of fibrinolytic activation in trauma patients. Journal of Thrombosis and Haemostasis 2013;11:307‐14.
Contributors
Harriet Hunt, Simon Stanworth, Nicola Curry, Tom Woolley, Chris Cooper, Obioha Ukoumunne, Zhivko Zhelev and Chris Hyde
Comments submitted by Shannon Kilkelly, Vanderbilt University Medical Center, 1 May 2015
Summary
We read the recent Cochrane review (1) concerning the use of thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for the evaluation of trauma induced coagulopathy (TIC) with interest. Given that TIC is a multifactorial disease, the ideal test for its detection should be one that incorporates all vital elements of coagulation as well as thrombolysis. Clinicians have recognized for years that the standard laboratory tests (prothrombin time [PT], international normalized ratio [INR], and partial thromboplastin time [aPTT]) correlate poorly with observed hemostasis during trauma surgery (2). The stated objective of the review was to “determine the accuracy” of TEG and ROTEM for TIC, using the reference standards PT or INR. Because TEG and ROTEM, which mimic venous clotting, are global approaches to the assessment of coagulation incorporating platelet and clotting factor function as well as the elements of thrombolysis, is not surprising that a single test of a factor pathway (PT or INR) doesn’t correlate well with a test of comprehensive hemostasis. It is well known that the mortality of trauma patients is significantly higher when fibrinolysis is present (4). Both TEG and ROTEM are very sensitive to fibrinolysis, whereas the PT and INR are not. Therefore, we believe that PT or INR are not ideal comparators for TEG and ROTEM in patients with TIC.
Essential to the care of critically injured patients is the prediction of which patients will require transfusion therapy, and which blood components they will require. In this regard, recent work by Holcomb et al demonstrated the superiority of rTEG to conventional coagulation tests in the prediction of transfusion requirements for all four major blood products (3). Furthermore, recent work by McCully et al (5) suggests that using INR as a measure of TIC may actually be harmful in that INR overestimates TIC when compared to TEG and clotting factor activity. This overestimation led directly to the transfusion of additional units of FFP, increasing these patients’ risk of a transfusion related complication (6), as well as their cost of care.
A common criticism regarding the utility of TEG or ROTEM in the evaluation of TIC is not test accuracy or validity, but rather the time involved in getting results. Trauma patients can require a large volume of blood products over a very short period of time. Because both TEG and ROTEM can often require greater than 30 minutes for test completion, the clinical picture may drastically change during this time period, rendering the results unhelpful. The exception is the diagnosis of fibrinolysis, which usually does not improve with blood component therapy. However, advances in TEG technology have led to the development of the Rapid Thromboelastograph (rTEG), which adds tissue factor to the standard kaolin to activate the clotting process. Cotton et al (7) demonstrated that with the addition of a bedside remote graphical display of the evolving tracing, key elements of the rTEG were available to guide resuscitative efforts within 5 minutes, and that a near complete tracing was observable in 15 minutes. It is on the basis of these observations that we submit that TEG technology does in fact have a role in the evaluation and management of the exsanguinating trauma patient.
In summary, we believe that PT and INR are poor reference standards for the evaluation of TEG or ROTEM in trauma patients, because they clearly test different aspects of clotting function.
Shannon Kilkelly, Lawrence Lottenberg, Mark J Rice
References 1. Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, Zhelev Z, Hyde C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma‐induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev. 2015 Feb 16;2
2. Kashuk et al. Primary fibrinolysis is integral in the pathogenesis of acute coagulopathy of trauma. Annuals of Surgery 2010:252:434‐42
3. Holcomb et al. Admission Rapid Thromboelastography Can Replace Conventional Coagulation Tests in the Emergency Department. Annals of Surgery 2012 256:476‐84
4. Cotton et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. Journal of Trauma and Acute Care Surgery 2012 Aug;73(2):365‐70
5. McCully et al. The International Normalized Ratio Overestimates Coagulopathy in Stable Trauma and Surgical Patients. Journal of Trauma and Acute Care Surgery. 2014 76(2) 264‐71
6. Watson GA, Sperry JL, RosengartMR, et al. Inflammation and Host Response to Injury Investigators. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67(2):221‐227
7. Cotton et al. Rapid thrombelastography delivers real‐time results that predict transfusion within 1 hour of admission. Journal of Trauma 2011 Aug;71(2):407‐14
Reply
We thank the correspondents for their comments on our systematic review Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma‐induced coagulopathy (TIC) in adult trauma patients with bleeding. As indicated in our response to the previous feedback on this review (Feedback 1), we have sympathy with the assertion that PT and INR are not ideal reference standards. We address the issue of imperfect reference standards at some length within the review, both in terms of recognition within the Objectives and Background sections and suggestions for future research in the Discussion section. Within the Discussion section we address these issues in detail, particularly advancing potential for researchers to create a composite reference standard as well as the possible utility of quasi‐clinical definitions of coagulopathy. In light of the feedback received, we recognise that this information could be made explicit in the more frequently‐accessed Abstract and Plain Language Summary sections. We have therefore updated these sections to recognise the limitations of reference standards, and thank the commentators for their contribution.
Contributors
Harriet Hunt, Simon Stanworth, Nicola Curry, Tom Woolley, Chris Cooper, Obioha Ukoumunne, Zhivko Zhelev and Chris Hyde
What's new
| Date | Event | Description |
|---|---|---|
| 5 June 2015 | Feedback has been incorporated | The authors have responded to Feedback 2. |
History
Protocol first published: Issue 3, 2013 Review first published: Issue 2, 2015
| Date | Event | Description |
|---|---|---|
| 21 April 2015 | Feedback has been incorporated | The authors have responded to Feedback 1. |
Acknowledgements
Sincere thanks to Dr Pablo Perel for his contributions to this review. We are grateful to Dr Roger Luddington for the use of his diagram of the thromboelastic trace, and to all contacted authors who contributed information and copies of full papers. Thanks also to Jenny Lowe for providing administrative support.
This project was supported by the National Institute for Health Research, UK, through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. TEG and ROTEM equivalent methods
Clotting time
In TEG, clotting time is measured as R (reaction time), N (whole blood; normal values for kaolin activated TEG in whole blood) and N (Cit, kaolin; normal values for kaolin activated TEG in citrated and recalcified blood). In ROTEM, clotting time is measured as CT (clotting time), N (Cit, INTEM; normal values for contact) and N (Cit, EXTEM; normal values for tissue factor).
Clot formation
Clot formation is the time from the start of clot formation until the clot firmness (or strength) reaches an arbitrary pre‐defined value (in both ROTEM and TEG this is 20 mm firmness). Clot formation is measured in TEG as K (kinetics) and ‐ as above ‐ N (whole blood) and N (Cit, kaolin). In ROTEM, CFT (clot formation time) is measured as before as N (Cit, INTEM) and N (Cit, EXTEM).
Alpha angle
This denotes the angle of the curve during initial clot formation and is a measure of the rapidity of fibrin polymerisation. The alpha angle in TEG is defined as the slope between R and K and in ROTEM is the slope of tangent at 2 mm amplitude. Again, both tests give the alpha angle as N (whole blood) and N (Cit, kaolin) for TEG and N (Cit, INTEM) and N (Cit, EXTEM) for ROTEM.
Amplitude of clot/clot amplitude
Amplitude of clot ('CA' or ‘A’) is given at set times in both tests.
Maximum clot firmness
The maximum strength of the clot is measured in TEG as maximum amplitude (MA) and in ROTEM as maximum clot firmness (MCF), and both tests give this measurement both as N (whole blood) and N (Cit, kaolin) for TEG and N (Cit, INTEM) and N (Cit, EXTEM) for ROTEM ‐ although ROTEM also reports tissue factor plus platelet inhibitor cytochalasin D (Cit, FIBTEM).
Clot lysis
Both tests give readings for clot lysis (CL in TEG, e.g. CL30, CL60; and LY in ROTEM, e.g. LY30, LY60).
Appendix 2. Search strategies
Literature searching
A line in the search strategy was altered between publication of the protocol and the searches being run. A truncation marker was moved to increase the sensitivity of the search, in order to account for thromboelastometry if it was expressed as thrombelasto‐metry/‐graphy.
Previous line: (thromboelastom$ or thrombelastom$ or (thromb$ adj2 elastom$) or (rotational adj2 thrombelast) or ROTEM or tem international).mp.
New line: (thromboelasto$ or thrombelasto$ or (thromb$ adj2 elastom$) or (rotational adj2 thrombelast) or ROTEM or tem international).mp.
All strategies were checked by CC and HH.
The Cochrane Library
Host: http://www.thecochranelibrary.com/view/0/index.html
Data Parameters: CDSR Issue 2 of 12 (Feb 2013); CENTRAL Issue 1 of 12 January 2013; DARE Issue 1 of 4 Jan 2013; Methods Issue 1 of 4, Jan 2013; HTA Issue 1 of 4 Jan 2013; NHS EEDS Issue 1 of 4 Jan 2013
Strategy:
#1 MeSH descriptor: [Thrombelastography] explode all trees 141
#2 (Thrombelastogra* or Thromboelastogra* or (thromb* near/3 elastogra*) or TEG or haemoscope or haemonetics) 351
#3 (thromboelasto* or thrombelasto* or (thromb* near/3 elastom*) or (rotational near/3 thrombelast) or ROTEM or "tem international") 273
#4 #1 or #2 or #3 from 1970 368
Hits: 368 (CDSR: 17; DARE: 3; CENTRAL: 339; Methods: 1; HTA: 3; NHS EEDS: 5)
MEDLINE (Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R))
Strategy:
| # | Searches | Results |
| 1 | (Thrombelastogra$ or Thromboelastogra$ or (thromb$ adj2 elastogra$) or TEG or haemoscope or haemonetics).mp. | 4711 |
| 2 | Thrombelastography/ | 3190 |
| 3 | (thromboelasto$ or thrombelasto$ or (thromb$ adj2 elastom$) or (rotational adj2 thrombelast) or ROTEM or "tem international").mp. | 4174 |
| 4 | 1 or 2 or 3 | 4832 |
| 5 | exp animals/ not humans.sh. | 3,765,894 |
| 6 | 4 not 5 | 4247 |
| 7 | limit 6 to yr="1970 ‐Current" | 3336 |
Embase (OvidSP)
Data Parameters: Embase 1980 to 2013 Week 09, Embase 1974 to 1979, Embase Classic 1947 to 1973
Strategy:
| # | Searches | Results |
| 1 | (Thrombelastogra$ or Thromboelastogra$ or (thromb$ adj2 elastogra$) or TEG or haemoscope or haemonetics).mp. | 7712 |
| 2 | thromboelastography/ | 5167 |
| 3 | (thromboelasto$ or thrombelasto$ or (thromb$ adj2 elastom$) or (rotational adj2 thrombelast) or ROTEM or "tem international").mp. | 6740 |
| 4 | 1 or 2 or 3 | 8264 |
| 5 | exp animal/ not human/ | 4,754,994 |
| 6 | 4 not 5 | 7323 |
| 7 | limit 6 to yr="1970 ‐Current" | 5913 |
Transfusion Evidence Library
Host: http://www.transfusionguidelines.org.uk/Index.aspx?Publication=SRI&Section=24&pageid=7559
Data Parameters: 1980‐Present
Strategy:
(Thrombelastogra* or Thromboelastogra* or (thromb* and elastogra*) or TEG or haemoscope or haemonetics) [in Search All Text] OR (thromboelasto* or thrombelasto* or (thromb* and elastom*) or (rotational and thrombelast) or ROTEM or tem international) [in Search All Text]
Hits: 24
British Nursing Index (Proquest) (1994 ‐ current)
Strategy:
S1 all((Thrombelastogra* OR Thromboelastogra* OR (thromb* NEAR/2 elastogra*) OR TEG OR haemoscope OR haemonetics))
S2 (thromboelasto* OR thrombelasto* OR (thromb* NEAR/2 elastom*) OR (rotational NEAR/2 thrombelast) OR ROTEM OR tem international)
S3 S1 or S2
Hits: 8
BIOSIS (ISI) (1969‐present)
Strategy:
| # 1 | 2785 | Topic=((Thrombelastogra* or Thromboelastogra* or (thromb* NEAR/2 elastogra*) or TEG or haemoscope or haemonetics)) Databases=BCI Timespan=1970‐2013 |
| # 2 | 2153 | Topic=((thromboelasto* or thrombelasto* or (thromb* NEAR/2 elastom*) or (rotational NEAR/2 thrombelast) or ROTEM or "tem international")) Databases=BCI Timespan=1970‐2013 |
| # 3 | 3060 | #2 OR #1 Databases=BCI Timespan=1970‐2013 |
Centre for Reviews and Dissemination (http://www.crd.york.ac.uk/crdweb/SearchPage.asp)
Strategy:
1. (Thrombelastogra* or Thromboelastogra* or (thromb* NEAR2 elastogra*) or TEG or haemoscope or haemonetics)
2. (thromboelasto* or thrombelasto* or (thromb* NEAR2 elastom*) or (rotational N2 thrombelast) or ROTEM or tem international)
3. 1 or 2
Hits: 13
CINAHL (EBSCO Host) (1981 to present)
Strategy:
S1 TI ( (Thrombelastogra* or Thromboelastogra* or (thromb* N2 elastogra*) or TEG or haemoscope or haemonetics) ) OR AB ( (Thrombelastogra* or Thromboelastogra* or (thromb* N2 elastogra*) or TEG or haemoscope or haemonetics) )
S2 TI ( (thromboelasto* or thrombelasto* or (thromb* N2 elastom*) or (rotational N2 thrombelast) or ROTEM or tem international) ) OR AB ( (thromboelasto* or thrombelasto* or (thromb* N2 elastom*) or (rotational N2 thrombelast) or ROTEM or tem international) )
S1 OR S2
Hits: 263
HMIC (OvidSP) (1979 to January 2013)
Strategy:
| # | Searches | Results |
| 1 | (Thrombelastogra$ or Thromboelastogra$ or (thromb$ adj2 elastogra$) or TEG or haemoscope or haemonetics).mp. | 1 |
| 2 | (thromboelasto$ or thrombelasto$ or (thromb$ adj2 elastom$) or (rotational adj2 thrombelast) or ROTEM or "tem international").mp. | 0 |
| 3 | 1 or 2 | 1 |
| 4 | limit 3 to yr="1970 ‐Current" | 1 |
PsycINFO (OvidSP) (1806 to February Week 4 2013)
Strategy:
| # | Searches | Results |
| 1 | (Thrombelastogra$ or Thromboelastogra$ or (thromb$ adj2 elastogra$) or TEG or haemoscope or haemonetics).mp. | 21 |
| 2 | (thromboelasto$ or thrombelasto$ or (thromb$ adj2 elastom$) or (rotational adj2 thrombelast) or ROTEM or "tem international").mp. | 11 |
| 3 | 1 or 2 | 25 |
| 4 | limit 3 to yr="1970 ‐Current" | 22 |
Hits: 22
ISI WOS: Conference Proceedings Citation Index ‐ Science (CPCI‐S) (1990‐present); Conference Proceedings Citation Index ‐ Social Science & Humanities (CPCI‐SSH) (1990‐present); Science Citation Index Expanded (SCI‐EXPANDED) (1970‐present); Social Sciences Citation Index (SSCI) (1970‐present).
Strategy:
| # 1 | 3715 | Topic=((Thrombelastogra* or Thromboelastogra* or (thromb* NEAR/2 elastogra*) or TEG or haemoscope or haemonetics)) Databases=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=1970‐01‐01 ‐ 2013‐03‐04 |
| # 2 | 2570 | Topic=((thromboelasto* or thrombelasto* or (thromb* NEAR/2 elastom*) or (rotational NEAR/2 thrombelast) or ROTEM or "tem international")) Databases=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=1970‐01‐01 ‐ 2013‐03‐04 |
| # 3 | 4052 | #2 OR #1 Databases=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=1970‐01‐01 ‐ 2013‐03‐04 |
Hits: 4052
Prospero (http://www.crd.york.ac.uk/prospero/)
Strategy:
(Thrombelastogra* or Thromboelastogra* or (thromb* NEAR2 elastogra*) or TEG or haemoscope or haemonetics)
(thromboelasto* or thrombelasto* or (thromb* NEAR2 elastom*) or (rotational N2 thrombelast) or ROTEM or tem international)
Hits: 0
LILACS (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F)
Strategy:
(Thrombelastogra* or Thromboelastogra* or TEG or thromboelasto* or thrombelasto* or ROTEM)
Hits: 25
Trials registries
Current Controlled Trials (http://www.controlled‐trials.com/)
Strategy: (TEG or ROTEM)
Clinical Trials.Gov (http://clinicaltrials.gov/ct2/home)
Strategy: (TEG or ROTEM)
WHO International Trials Registry Platform (http://www.who.int/ictrp/en/)
Strategy: (TEG or ROTEM)
Websearching
Aggressive Research Intelligence Facility (ARIF) via http://tinyurl.com/3u9tevp
C‐EBLM
Diagnostic Test Accuracy Working Group (Cochrane) via http://srdta.cochrane.org/
Google
MEDION database via http://www.mediondatabase.nl/
Haemonetics Corporation http://www.haemonetics.com/en.aspx
TEM Innovations GmbH http://www.rotem.de/site/index.php
Fowards Citation Chasing
| Citation | N |
| Functional definition and characterization of acute traumatic coagulopathy. Critical Care Medicine, 39, 2652‐2658. | 44 |
| Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. Journal of Thrombosis and Haemostasis, 5, 289‐295. | 173 |
| Early determination of hypocoagulopathy based on interim ROTEM values for clot strength. British Journal of Surgery, 97, 21‐21. | 1 |
| total | 218 |
| Duplicates removed | 156 |
| Unique records to screen | 62 |
Appendix 3. Methods from protocol [Art. No.: CD010438]
Methods
Criteria for considering studies for this review
Types of studies
Cross‐sectional studies investigating the diagnostic test accuracy of TEG or ROTEM in patients with clinically suspected TIC will be eligible. We will expand the inclusion criteria to include case control studies if the number of sources retrieved is insufficient for a valid systematic review and possible meta‐analysis. Pragmatically we have set this level at less than 100 patients in total in the included studies.
Participants
All studies including adult trauma patients with clinically suspected TIC will be eligible. Studies in both military and civilian settings will be included.
Index tests
Two global tests of haemostatic function will be used, TEG (thromboelastography ‐ whose name is a trademark of the Haemoscope Corporation, USA http://www.haemoscope.com/) and ROTEM (rotational thromboelastometry ‐ trademark of TEM International GmbH http://www.rotem.de/site/index.php). Normal thresholds are indicated in Table 2.
Target conditions
The target condition will be TIC defined by standard clotting times of PTr and INR.
Reference standards
In the absence of embedded clinical consensus, the coagulopathic range we will be using is based on pro‐thrombin time ratio (PTr)/International Normalized Ratio (INR), with the lower limit of the range a PTr/INR reading of 1.2 or greater Frith 2010), and the upper limit of 1.5 or greater (Stainsby 2006). There is no upper limit to the range – anyone with a PTr/INR count of above 1.2, or above 1.5, is considered coagulopathic. These figures were reached through discussion by the report authors, including experts in haematology and trauma medicine.
PTr differs from INR, although the final numbers may be the same. The PTr calculated varies depending on local thresholds and separate batches of different manufacturer’s reagent involved in conducting the prothrombin time (PT) test. In an effort to standardise this measurement, the INR is calculated as the ratio of a patient’s prothrombin time compared to a mean normal PT (calculated by determining the mean of 30 or more patients who are representative of the local hospital population), computed to the power of the International Sensitivity Index (ISI), which is itself calculated by the manufacturer to give an indication of how each batch of tissue factor corresponds to an international reference. The equation for calculation is in Figure 1.
Search methods for identification of studies
A sensitive search strategy will be used to identify literature relating to the index test for this review. This strategy will not be limited by language but will be limited by date to 1990‐current and to human only populations. The test technology has been established since 1948, but the date limit has been set to 1990 in order to maximise study quality and capture the more recent versions of the technology in current use.
Electronic searches
The following bibliographic resources will be searched: British Nursing Index, Biosis, Centre for Reviews and Dissemination databases, CINAHL, The Cochrane Library, Conference Proceedings Citation Index‐ Science (CPCI‐S), Conference Proceedings Citation Index‐ Social Science & Humanities (CPCI‐SSH), EMBASE, HMIC, MEDLINE in Process, MEDLINE, PsycINFO, Science Citation Index Expanded (SCI‐EXPANDED), Social Sciences Citation Index (SSCI), LILACS and the Transfusion Evidence Library.
The following trials registers will be hand‐searched: Current Controlled Trials, Clinical Trials.Gov and the WHO International Trials Registry Platform via http://www.who.int/ictrp/en/
The following websites will be searched:
Aggressive Research Intelligence Facility (ARIF) via http://tinyurl.com/3u9tevp
C‐EBLM
Diagnostic Test Accuracy Working Group (Cochrane) via http://srdta.cochrane.org/
Google
MEDION database via http://www.mediondatabase.nl/
Haemonetics Corporation http://www.haemoscope.com/
TEM Innovations GmbH http://www.rotem.de/site/index.php
Searching other resources
Citation chasing will be conducted on all studies included on full text. Attempts will be made to conduct authors for any additional or supporting information. For further details on the search, including the strategy, please see Appendix 2.
Data collection and analysis
Selection of studies
All sources will be managed using Review Manager 5 software. The inclusion criteria will be based on the “Criteria for considering the studies for this review” detailed above. Decisions on inclusion/exclusion of studies will be made independently by two reviewers (HH and CH) using piloted criteria. Disagreements will be resolved with reference to a third experienced reviewer (SS and PP). The systematic review of diagnostic test accuracy of coagulation tests will target prospective cohort studies measuring accuracy relative to a reference standard and rigorous evaluation of time taken to obtain coagulation results.
Data extraction and management
We will extract the following data (where available) into a bespoke data extraction table.
Author, year of study, year of publication, journal reference.
Study design and timing of data collection (prospective/retrospective).
Study population and participant characteristics (age, sex, setting – e.g. hospital, region, country, other details given).
Trauma type and severity (Injury Severity Score – ISS).
Patient history.
Pre‐admission treatment, especially blood transfusion and/or additives.
Blood temperature and duration of bleed at point of testing.
Reference test used (PTr/INR) and any other measures taken (e.g. PT, APTT, Fibrinogen level, platelet count, fibrinogen degradation products).
Index test used (TEG/ROTEM) and version of device.
Any details about device reliability.
When tests were carried out in treatment phase (i.e. pre/post transfusion, timings).
Data from the 2 x 2 table will be extracted where presented, i.e. true positives, false positives, true negatives and false negatives.
QUADAS‐2 items (see Table 3).
Where available, variability between operators and assay conditions will be recorded. Particular care is likely to be required on many of these items (index test and reference standard) because of lack of standardisation. The abstraction form will be piloted by two authors (HH and CH) using two primary diagnostic studies. A third author (NC) will resolve disagreements. The form will be accompanied by a briefing document explaining how it should be used. Data will be abstracted by one reviewer (HH) and checked by a second (CH), with a third author (NC) providing moderation as required.
Assessment of methodological quality
Quality assessment will be carried out using a checklist approach to assess the quality of primary studies based on the QUADAS‐2 instrument (see Table 3) in line with advice given in Chapter 9, ‘Assessing Methodological Quality’ in the Cochrane Handbook for Diagnostic Test Accuracy Reviews (Reitsma 2009). We will independently score each item as ‘Yes’, ‘No’ or ‘Unclear’, and will omit three reporting items from the QUADAS‐2 list, addressing the description of the index test, reference standard and selection criteria, as recommended by the Cochrane Handbook for Diagnostic Test Accuracy (Deeks 2010). A categorisation of 'unclear' will generally be considered a marker of poor quality, so care will be taken to account for the possibility that failing to report an item was reasonable given the circumstances in which the study was conducted. Results will be presented narratively in the text, and in an appropriate graphic representation of quality assessment (such as a table).
Statistical analysis and data synthesis
We will consider the accuracy of TEG and ROTEM compared to the reference standard as detailed above. Results will be the components of the 2 x 2 table, sensitivity and specificity and their 95% confidence intervals (CI). These will be tabulated and presented graphically (forest plots and receiver operating characteristic (ROC) space). The initial approach to analysis is likely to be qualitative, with conclusions based on patterns of results. Quantitative meta‐analysis may be appropriate where the quantity and nature of the included studies permit. If meta‐analysis is possible, the approach will be to calculate a summary receiver operating characteristic (SROC) curve using a hierarchical SROC (HSROC) model. Use of a bivariate model will also be considered depending on the data (Reitsma 2009), but a priori uncertainty about thresholds and the likelihood of implicit thresholds suggests the HSROC model may be slightly preferable in the first instance. A summary of results table will be generated. If feasible and appropriate, translation of any summary results into natural frequencies and other metrics such as predictive values will be considered to facilitate improved understanding to readers.
The number of uninterpretable results will be tabulated and commented on. Analysis and presentation of results will be carried out in line with advice in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Macaskill 2010).
We will carefully scrutinise all the included studies for any further investigation of discrepant results between the index test and reference standards (False Positives – FP ‐ and False Negatives ‐ FN), ideally based on independent clinical review of all available findings with the purpose of considering whether it was global haemostatic function or traditional measures of clotting which was giving the better indication of true disease state. Any results will tabulated and summarised narratively.
Investigations of heterogeneity
With respect to test accuracy results, we will assume that important heterogeneity beyond that accounted for by chance will be present and will need to be investigated. Our initial approach will be to perform sequential sub‐group analyses using the analytical framework detailed below. We will consider whether using co‐variates in the HSROC model will add to any insights gained from these sub‐group analyses.
The provisional framework for investigating heterogeneity will include the following.
Type of global measure of haemostatic function (TEG/ROTEM).
Time blood sample taken relative to trauma (< 1 h/> 1 h).
Nature of reference standard (INR/PTr of 1.2 ≥; INR/PTr of 1.5 ≥).
Prevalence of acute traumatic coagulopathy (ATC; excluding case‐control studies if these are included).
Participant type especially severity of trauma and mechanism of injury (blunt/penetrating).
Setting (military or civilian).
Whether trauma associated with massive transfusion (yes or mixed/no).
Case‐control study design (if these are included).
Other aspects of study quality, particularly blinding of index test and reference standard.
There are no specific plans for the investigation of heterogeneity of the data concerning uninterpretable results or further investigation of discrepant results.
Sensitivity analyses
In the unlikely event that heterogeneity is not present and the effect of important covariates has not already been analysed, we will investigate the robustness of any summary estimates of test accuracy to the aspects of study quality indicated in the framework for investigating heterogeneity above.
Assessment of reporting bias
We will not be assessing reporting bias because its impact in test accuracy is unclear and the tools for investigating it are in the early stages of development.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 ROTEM CA5 | 2 | 409 |
| 2 ROTEM CA10 | 1 | 109 |
| 3 ROTEM CA15 | 1 | 88 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Davenport 2011a.
| Study characteristics | |||
| Patient sampling | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Patient characteristics and setting | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Index tests | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Target condition and reference standard(s) | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Flow and timing | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were exclusions accounted for? | Yes | ||
| Low | |||
Rugeri 2007.
| Study characteristics | |||
| Patient sampling | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Patient characteristics and setting | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Index tests | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Target condition and reference standard(s) | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Flow and timing | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Comparative | |||
| Notes | Published study reported Negative Predictive Value (NPV) = 99, but calculated in this report using RevMan software NPV = 95 [figure used in analysis]. Study author could not explain the difference and raw data was no longer available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were exclusions accounted for? | Yes | ||
| Low | |||
Woolley 2012.
| Study characteristics | |||
| Patient sampling | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Patient characteristics and setting | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Index tests | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Target condition and reference standard(s) | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Flow and timing | See study characteristics in Table 4 and Table 5 and quality appraisal details in Table 3 | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were exclusions accounted for? | Yes | ||
| Low | |||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afshari 2011a | Not a primary study |
| Afshari 2011b | Not a primary study |
| Anonymous 2007 | Unobtainable |
| Anonymous 2008 | Unobtainable |
| Avikainen 1977 | Not a DTA study |
| Blackbourne 2012 | Not a primary study |
| Cap 2011 | Outcome not in line with inclusion criteria |
| Carroll 2009 | Outcome not in line with inclusion criteria |
| Chandler 2010 | Intervention not in line with inclusion criteria |
| Cheng 2009 | Outcome not in line with inclusion criteria |
| Cotton 2011 | Not a DTA study |
| Cotton 2012a | Condition not in line with inclusion criteria |
| Cotton 2012b | Not DTA study |
| Craft 2008 | Not a DTA study |
| Curry 2011 | Not a DTA study |
| Davenport 2009 | Not a DTA study |
| Davenport 2011b | Not a DTA study |
| David 2011 | Not a primary study |
| Differding 2011 | Outcome not in line with inclusion criteria |
| Ettinger 1970 | Population not in line with inclusion criteria |
| Floccard 2012 | Intervention not in line with inclusion criteria |
| Franschman 2012 | Intervention not in line with inclusion criteria |
| Frink 2009 | Not a primary study |
| Hagemo 2013a | Not a primary study |
| Hagemo 2013b | Reference standard not in line with inclusion criteria |
| Jambor 2009 | Not primary study |
| Jeger 2009 | Not a DTA study |
| Jeger 2010 | Not a primary study |
| Jeger 2011a | Abstract ‐ author contacted |
| Jeger 2011b | Duplicate of Jeger 2011a |
| Jeger 2012 | Not a DTA study (FT of Jeger 2011a) |
| Kashuk 2010 | Abstract ‐ author contacted |
| Kashuk 2012 | Not DTA study (FT of Kashuk 2010) |
| Kaufmann 1995 | Abstract ‐ author contacted |
| McCann 2011 | Not a DTA study |
| Messenger 2011 | Not a DTA study |
| Nystrup 2011 | Not a DTA study |
| Ostrowski 2011 | Not a DTA study |
| Ostrowski 2012a | Not a DTA study |
| Ostrowski 2012b | Not a DTA study |
| Park 2008 | Not a DTA study |
| Park 2009 | Outcome not in line with inclusion criteria |
| Pezold 2012 | Outcome not in line with inclusion criteria |
| Plotkin 2008 | Outcome not in line with inclusion criteria |
| Raza 2011a | Outcome not in line with inclusion criteria |
| Raza 2011b | Outcome not in line with inclusion criteria |
| Raza 2013 | Outcome not in line with inclusion criteria |
| Rizoli 2011 | Outcome not in line with inclusion criteria |
| Rourke 2012 | Outcome not in line with inclusion criteria |
| Schochl 2005 | Unobtainable |
| Schochl 2007 | Abstract of Schochl 2009 ‐ not a DTA study |
| Schochl 2009 | Not a DTA study |
| Schochl 2011a | Not a DTA study |
| Schochl 2011b | Not a DTA study |
| Schochl 2011c | Not a DTA study |
| Schochl 2012a | Not a DTA study |
| Schochl 2012b | Not a DTA study |
| Schreiber 2005 | Not a DTA study |
| Schreiber 2009 | Not a DTA study |
| Shah 2012 | Not a DTA study |
| Sharma 2010 | Population not in line with inclusion criteria |
| Sixta 2013 | Not a DTA study |
| Solomon 2011 | Not a DTA study |
| Spoors 2011 | Not a DTA study |
| Tanaka 2012 | Not a DTA study |
| Tapia 2013 | Not a DTA study |
| Theusinger 2011 | Not a DTA study |
| Theusinger 2013 | Not a DTA study |
| Watters 2010 | Outcome not in line with inclusion criteria |
| Weiss 2011 | Not a DTA study |
| Windelov 2011 | Not a DTA study |
| Wohlauer 2012 | Not a DTA study |
| Woolley 2010 | Not a DTA study |
Abbreviation
FT: full text
Differences between protocol and review
Searches ran from 1970 onwards, whereas in the published protocol (Hunt 2013) searches are listed as running from 1990 onwards. This date was amended in response to feedback from protocol reviewer comments.
The complete protocol methods are available in Appendix 3. The entire protocol is available in the Cochrane Library (Hunt 2013).
Contributions of authors
Writing the first draft of the review ‐ Harriet Hunt, Chris Hyde
Methodological advice ‐ Chris Hyde
Content advice ‐ all authors
Editing review ‐ all authors
Sources of support
Internal sources
-
Dutch Cochrane Centre, Netherlands.
Training
-
Cochrane Diagnostic Test Accuracy Working Group, UK.
Technical support
External sources
National Institute for Health Research: Programme Grant for Applied Research (RP‐PG‐0407‐10036)., UK.
-
NIHR CLAHRC, UK.
We acknowledge support from the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health in England.
Declarations of interest
Harriet Hunt: none known. Simon Stanworth: a member of the research team for included study Davenport 2011a, and excluded study Davenport 2009, but not involved in screening, quality assessment or analysis of those studies within this review. Nicola Curry: a member of the research team for excluded study Rourke 2012 but not involved in screening of that study within this review. Tom Woolley: a member of the research team for included study Woolley 2012, but not involved in screening, quality assessment or analysis of that study within this review. Chris Cooper: none known Obioha Ukoumunne: none known Zhivko Zhelev: none known Chris Hyde: CH was an employee of NHS Blood and Transplant when this review was commenced. This arrangement ended in 2009.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Davenport 2011a {published and unpublished data}
- Davenport R, Manson J, De'Ath H, Platton S, Coates A, Allard S, Hart D, et al. Functional definition and characterization of acute traumatic coagulopathy. Critical Care Medicine 2011;39(12):2652‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rugeri 2007 {published data only (unpublished sought but not used)}
- Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. Journal of Thrombosis and Haemostasis 2007;5(2):289‐95. [DOI] [PubMed] [Google Scholar]
Woolley 2012 {published and unpublished data}
- Woolley T, Midwinter M, Spencer P, Watts S, Doran C, Kirkman E. Utility of interim ROTEM values of clot strength, A5 and A10, in predicting final assessment of coagulation status in severely injured battle patients. Injury 2013;44:593‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Afshari 2011a {published data only}
- Afshari A. Evidence based evaluation of immuno‐coagulatory interventions in critical care. Danish Medical Bulletin 2011;58:B4316. [PubMed] [Google Scholar]
Afshari 2011b {published data only}
- Afshari A, Wikkelso A, Brok J, Moller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007871.pub2] [DOI] [PubMed] [Google Scholar]
Anonymous 2007 {published data only}
- Anonymous. Abstracts of Papers to be Presented at the Annual Scientific Meeting of the Trauma Association of Canada, May 10 ‐12, 2007, Ottowa, Ontario, Canada. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2007;62(3):787‐804. [Google Scholar]
Anonymous 2008 {published data only}
- Anonymous. Congress of the German Interdisciplinary Association of Intensive Care and Emergency Medicine 3–6 December 2008 CCH Congress Centre, Hamburg [Kongress der Deutschen Interdisziplinären Vereinigung für Intensivmedizin und Notfallmedizin 3–6 Dezember 2008 CCH Congress Center, Hamburg]. Intensivmedizin und Notfallmedizin. 2008; Vol. 45 supplement:1‐100.
Avikainen 1977 {published data only}
- Avikainen V. Coagulation disorders in severely and critically injured patients. Annales Chirurgiae et Gynaecologiae 1977;66:269‐77. [PubMed] [Google Scholar]
Blackbourne 2012 {published data only}
- Blackbourne LH, Baer DG, Eastridge BJ, Renz EM, Chung KK, DuBose J, et al. Military medical revolution: deployed hospital and en route care. Journal of Trauma and Acute Care Surgery 2012;73:S378‐87. [DOI] [PubMed] [Google Scholar]
Cap 2011 {published data only}
- Cap AP, Spinella PC. Severity of head injury is associated with increased risk of coagulopathy in combat casualties. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2011;71:S78‐81. [DOI] [PubMed] [Google Scholar]
Carroll 2009 {published data only}
- Carroll R C, Craft RM, Langdon RJ, Clanton CR, Snider CC, Wellons DD, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Translational Research 2009;154:34‐9. [DOI] [PubMed] [Google Scholar]
Chandler 2010 {published data only}
- Chandler WL, Ferrell C, Trimble S, Moody S. Development of a rapid emergency hemorrhage panel. Transfusion 2010;50:2547‐52. [DOI] [PubMed] [Google Scholar]
Cheng 2009 {published data only}
- Cheng L, Yao X. Significance of thrombelastogram in the study on coagulation disorder of patients with severe traumatic brain injury. [Chinese]. Chinese Journal of Contemporary Neurology and Neurosurgery 2009;9:270‐4. [Google Scholar]
Cotton 2011 {published data only}
- Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, et al. Rapid thrombelastography delivers real‐time results that predict transfusion within 1 hour of admission. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2011;71:407‐14. [DOI] [PubMed] [Google Scholar]
Cotton 2012a {published data only}
- Cotton BA, Minei KM, Radwan ZA, Matijevic N, Pivalizza E, Podbielski J, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. Journal of Trauma and Acute Care Surgery 2012;72:1470‐7. [DOI] [PubMed] [Google Scholar]
Cotton 2012b {published data only}
- Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schoechl H, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. Journal of Trauma and Acute Care Surgery 2012;73:365‐70. [DOI] [PubMed] [Google Scholar]
Craft 2008 {published data only}
- Craft R, Carroll R, Langdon R, Clanton C, Snider C, Lawson C, et al. Point‐of‐care thrombelastography assays for coagulopathy in trauma patients. Transfusion 2008;48:81A. [Google Scholar]
Curry 2011 {published data only}
- Curry NS, Rourke C, Khan S, Beavis J, Kingsland S, Brohi K, et al. Fibrinogen replacement in traumatic bleeding. Journal of Thrombosis and Haemostasis 2011;9:938. [Google Scholar]
Davenport 2009 {published data only}
- Davenport R, Coates A, Allard S, Pasi J, MacCallum P, Stanworth S, et al. Trauma haemorrhage: evolving role of ROTEM for predicting transfusion requirements. Shock 2009;32:15. [Google Scholar]
Davenport 2011b {published data only}
- Davenport R, Rourke C, Manson J, De'Ath H, Platton S, Coates A, et al. Activated protein C is a principle mediator of acute traumatic coagulopathy. Journal of Thrombosis and Haemostasis 2011;9:51‐2. [Google Scholar]
David 2011 {published data only}
- David J‐S, Marchal V, Levrat A, Inaba K. Which is the most effective strategy: early detection of coagulopathy with thromboelastometry or use of hemostatic factors or both?. Critical Care (London, England) 2011;15:433; author reply 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Differding 2011 {published data only}
- Differding JA, Underwood SJ, Van PY, Khaki RA, Spoerke NJ, Schreiber MA. First Place Residents' Competition: trauma induces a hypercoagulable state that is resistant to hypothermia as measured by thrombelastogram. American Journal of Surgery 2011;201:585‐9. [DOI] [PubMed] [Google Scholar]
Ettinger 1970 {published data only}
- Ettinger MG. Coagulation abnormalities in subarachnoid hemorrhage. Stroke; a journal of cerebral circulation 1970;1:139‐42. [DOI] [PubMed] [Google Scholar]
Floccard 2012 {published data only}
- Floccard B, Rugeri L, Faure A, Saint Denis M, Boyle EM, Peguet O, et al. Early coagulopathy in trauma patients: an on‐scene and hospital admission study. Injury 2012;43:26‐32. [DOI] [PubMed] [Google Scholar]
Franschman 2012 {published data only}
- Franschman G, Boer C, Andriessen TM, Naalt J, Horn J, Haitsma I, et al. Multicenter evaluation of the course of coagulopathy in patients with isolated traumatic brain injury: relation to CT characteristics and outcome. Journal of Neurotrauma 2012;29:128‐36. [DOI] [PubMed] [Google Scholar]
Frink 2009 {published data only}
- Frink M, Zeckey C, Mommsen P, Haasper C, Krettek C, Hildebrand F. Polytrauma management ‐ a single centre experience. Injury 2009;40:S5‐S11. [DOI] [PubMed] [Google Scholar]
Hagemo 2013a {published data only}
- Hagemo JS. Prehospital detection of traumatic coagulopathy. Transfusion 2013;53:48S‐51S. [DOI] [PubMed] [Google Scholar]
Hagemo 2013b {published data only}
- Hagemo JS, Naess PA, Johansson P, Windeløv NA, Cohen MJ, Røislien J, et al. Evaluation of TEG and RoTEM inter‐changeability in trauma patients. Injury 2013;44:600‐5. [DOI] [PubMed] [Google Scholar]
Jambor 2009 {published data only}
- Jambor C, Heindl B, Spannagl M, Rolfes C, Dinges G K, Frietsch T. Blood transfusion haemostaseological management in polytrauma ‐ status of patient‐oriented diagnostic methods. Anasthesiologie Intensivmedizin Notfallmedizin Schmerztherapie 2009;44:200‐9. [DOI] [PubMed] [Google Scholar]
Jeger 2009 {published data only}
- Jeger V, Zimmermann H, Exadaktylos AK. Can RapidTEG accelerate the search for coagulopathies in the patient with multiple injuries?. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2009;66:1253‐7. [DOI] [PubMed] [Google Scholar]
Jeger 2010 {published data only}
- Jeger V, Zimmermann H, Exadaktylos AK. Thrombelastography in trauma, the closer the better. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2010;68:508. [DOI] [PubMed] [Google Scholar]
Jeger 2011a {published data only}
- Jeger V, Willi S, Liu T, Omert L, Orr A, Popovsky MA, et al. A comparison of point of care thrombelastography (TEG) versus conventional coagulation tests (CCT) in the emergency department management of trauma. British Journal of Surgery 2011;98:15. [Google Scholar]
Jeger 2011b {published data only}
- Jeger V, Willi S, Liu T, Omert L, Orr A, Popovsky MA, et al. Comparison of point‐of‐care thrombelastography versus conventional coagulation tests in the emergency department management of trauma. Critical Care 2011;15:S154. [Google Scholar]
Jeger 2012 {published data only}
- Jeger V, Willi S, Liu T, Yeh DD, Moya M, Zimmermann H, et al. The rapid TEG alpha‐angle may be a sensitive predictor of transfusion in moderately injured blunt trauma patients. ScientificWorldJournal 2012;2012:821794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kashuk 2010 {published data only}
- Kashuk J, Moore E, Wohlauer M, Johnson J, Pezold M, Lawrence J, et al. Initial experiences with point of care (POC) rapid thrombelastography (TEG) for management of life threatening postinjury coagulopathy (PIC). Shock 2010;33:27‐8. [DOI] [PubMed] [Google Scholar]
Kashuk 2012 {published data only}
- Kashuk JL, Moore EE, Wohlauer M, Johnson JL, Pezold M, Lawrence J, et al. Initial experiences with point‐of‐care rapid thrombelastography for management of life‐threatening postinjury coagulopathy. Transfusion 2012;52:23‐33. [DOI] [PubMed] [Google Scholar]
Kaufmann 1995 {published data only}
- Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thromboelastography in assessment of trauma patient coagulation. Journal of Trauma 1995;39:170. [DOI] [PubMed] [Google Scholar]
McCann 2011 {published data only}
- McCann K, Walsh M, Castellino F, Yount R, Thomas S, Evans E, et al. Expanding role of platelet mapping in traumatic brain injury. Journal of Neurotrauma 2011;28 (6):A51. [Google Scholar]
Messenger 2011 {published data only}
- Messenger BM, Craft RM, Carroll RC, Daley BJ, Enderson B, Snider CG. TEG‐guided massive transfusion in trauma patients. Anesthesia and Analgesia. 2011; Vol. 112 Suppl 5:S‐90.
Nystrup 2011 {published data only}
- Nystrup KB, Windelov NA, Thomsen AB, Johansson PI. Reduced clot strength upon admission, evaluated by thrombelastography (TEG), in trauma patients is independently associated with increased 30‐day mortality. Scandinavian Journal of Trauma Resuscitation & Emergency Medicine 2011;19. [DOI: 10.1186/1757-7241-19-52.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostrowski 2011 {published data only}
- Ostrowski SR, Sorensen AM, Larsen CF, Johansson PI. Thrombelastography and biomarker profiles in acute coagulopathy of trauma: a prospective study. Scandinavian Journal of Trauma, Resuscitation & Emergency Medicine 2011;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostrowski 2012a {published data only}
- Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. Journal of Trauma and Acute Care Surgery 2012;73:60‐6. [DOI] [PubMed] [Google Scholar]
Ostrowski 2012b {published data only}
- Ostrowski SR, Sorensen AM, Windelov NA, Perner A, Welling KL, Wanscher M, et al. High levels of soluble VEGF receptor 1 early after trauma are associated with shock, sympathoadrenal activation, glycocalyx degradation and inflammation in severely injured patients: a prospective study. Scandinavian Journal of Trauma Resuscitation & Emergency Medicine 2012;20:27. [DOI: 10.1186/1757-7241-20-27.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2008 {published data only}
- Park MS, Salinas J, Wade CE, Wang JF, Martini WJ, Pusateri AE, et al. Combining early coagulation and inflammatory status improves prediction of mortality in burned and nonburned trauma patients. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2008;64:S188‐94. [DOI] [PubMed] [Google Scholar]
Park 2009 {published data only}
- Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, et al. Thromboelastography as a better Indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. The Journal of Trauma 2009;67(2):266‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pezold 2012 {published data only}
- Pezold M, Moore EE, Wohlauer M, Sauaia A, Gonzalez E, Banerjee A, et al. Viscoelastic clot strength predicts coagulation‐related mortality within 15 minutes. Surgery 2012;151:48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plotkin 2008 {published data only}
- Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2008;64:S64‐8. [DOI] [PubMed] [Google Scholar]
Raza 2011a {published data only}
- Raza I, Davenport R, Brohi K, Rourke C, Platton S, Khan S, et al. Dilutional coagulopathy following blood transfusion in trauma. Journal of Thrombosis and Haemostasis 2011;9:692‐3. [Google Scholar]
Raza 2011b {published data only}
- Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, et al. Occult activation of fibrinolysis in trauma. Journal of Thrombosis and Haemostasis 2011;9:848. [Google Scholar]
Raza 2013 {published data only}
- Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. Journal of Thrombosis and Haemostasis 2013;11:307‐14. [DOI] [PubMed] [Google Scholar]
Rizoli 2011 {published data only}
- Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, et al. Clotting factor deficiency in early trauma‐associated coagulopathy. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2011;71:S427‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rourke 2012 {published data only}
- Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. Journal of Thrombosis and Haemostasis 2012;10:1342‐51. [DOI] [PubMed] [Google Scholar]
Schochl 2005 {published data only}
- Schochl H. Intraoperative coagulation management in polytrauma. [German]. Journal fur Anasthesie und Intensivbehandlung 2005;12:119‐22. [Google Scholar]
Schochl 2007 {published data only}
- Schochl H, Frietsch T, Drews P, Jambor C. 21st Annual Meeting of the Eastern Association for the Surgery of Trauma, Jacksonville, FL, USA, January 15‐19, 2008. The Journal of TRAUMA ‐ Injury, Infection & Critical Care. 2007; Vol. 63:1426‐40.
Schochl 2009 {published data only}
- Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2009;67:125‐31. [DOI] [PubMed] [Google Scholar]
Schochl 2011a {published data only}
- Schochl H, Solomon C, Redl H, Bahrami S. Rotational thromboelastometry in patients with severe traumatic brain injury: possible risk stratification?. Shock 2011;36:31. [Google Scholar]
Schochl 2011b {published data only}
- Schochl H, Solomon C, Redl H, Bahrami S. Rotational thromboelastometric (rotem (r)) test results in the emergency room predicts massive transfusion in major trauma patients. Shock 2011;36:30. [Google Scholar]
Schochl 2011c {published data only}
- Schochl H, Cotton B, Inaba K, Nienaber U, Fischer H, Voelckel W, et al. FIBTEM provides early prediction of massive transfusion in trauma. Critical Care (London, England) 2011;15:R265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schochl 2012a {published data only}
- Schochl H, Solomon C, Voelckel W. Goal‐directed coagulation management in trauma‐related bleeding. Shock 2012;38:32‐3. [Google Scholar]
Schochl 2012b {published data only}
- Schochl H, Solomon C, Voelckel W, Redl H, Bahrami S. Hyperfibrinolysis in trauma. Shock 2012;38:7‐8. [Google Scholar]
Schreiber 2005 {published data only}
- Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullins RJ, Timberlake GA, et al. Hypercoagulability is most prevalent early after injury and in female patients. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2005;58:475‐81. [DOI] [PubMed] [Google Scholar]
Schreiber 2009 {published data only}
- Schreiber M, Park M, Kashuk J, Park M. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time: discussion. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2009;67:275‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2012 {published data only}
- Shah B, Subramanium A, Agrawal D, Mukhopadhyay A. Thromboelastography‐viscoelastic haemostatic assay (VHA) as an indicator of overt disseminated intravascular coagulation in brain injury patients within 24 hours after injury. Brain Injury 2012;26(4‐5):586‐7. [Google Scholar]
Sharma 2010 {published data only}
- Sharma P, Saxena R. A novel thromboelastographic score to identify overt disseminated intravascular coagulation resulting in a hypocoagulable state. American Journal of Clinical Pathology 2010;134:97‐102. [DOI] [PubMed] [Google Scholar]
Sixta 2013 {published data only}
- Sixta SL, Schreiber M, Cohen M, Wade CE, Holcomb JB, Cotton BA. Coagulopathy after head injury: uncommon by conventional coagulation testing and associated with platelet‐fibrinogen dysfunction. Journal of Surgical Research 2013;179(2):272‐3. [Google Scholar]
Solomon 2011 {published data only}
- Solomon C, Traintinger S, Ziegler B, Hanke A, Rahe‐Meyer N, Voelckel W, et al. Platelet function following trauma. A multiple electrode aggregometry study. Thrombosis and Haemostasis 2011;106:322‐30. [DOI] [PubMed] [Google Scholar]
Spoors 2011 {published data only}
- Spoors C, Rourke C, Manson J, De'Ath H, Raza I, Khan S, et al. Rotem indices of platelet function in acute traumatic coagulopathy. British Journal of Surgery. 2011; Vol. 98 (S2):8:O24.
Tanaka 2012 {published data only}
- Tanaka KA, Bolliger D, Vadlamudi R, Nimmo A. Rotational thromboelastometry (ROTEM)‐based coagulation management in cardiac surgery and major trauma. Journal of Cardiothoracic and Vascular Anesthesia 2012;26:1083‐93. [DOI] [PubMed] [Google Scholar]
Tapia 2013 {published data only}
- Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall M J Jr, et al. TEG‐guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. Journal of Trauma and Acute Care Surgery 2013;74:378‐86. [DOI] [PubMed] [Google Scholar]
Theusinger 2011 {published data only}
- Theusinger OM, Wanner GA, Emmert MY, Billeter A, Eismon J, Seifert B, et al. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM (R)) Is associated with higher mortality in patients with severe trauma. Anesthesia and Analgesia 2011;113:1003‐12. [DOI] [PubMed] [Google Scholar]
Theusinger 2013 {published data only}
- Theusinger OM, Levy JH. Point of care devices for assessing bleeding and coagulation in the trauma patient. Anesthesiology Clinics 2013;31:55‐65. [DOI] [PubMed] [Google Scholar]
Watters 2010 {published data only}
- Watters JM, Sambasivan CN, Zink K, Kremenevskiy I, Englehart MS, Underwood SJ, et al. Splenectomy leads to a persistent hypercoagulable state after trauma. American Journal of Surgery 2010;199:646‐51. [DOI] [PubMed] [Google Scholar]
Weiss 2011 {published data only}
- Weiss G, Lison S, Glaser M, Herberger S, Johanning K, Strasser T, et al. Observational study of fibrinogen concentrate in massive hemorrhage: evaluation of a multicenter register. Blood Coagulation & Fibrinolysis 2011;22:727‐34. [DOI] [PubMed] [Google Scholar]
Windelov 2011 {published data only}
- Windelov NA, Welling KL, Ostrowski SR, Johansson PI. The prognostic value of thrombelastography in identifying neurosurgical patients with worse prognosis. Blood Coagulation and Fibrinolysis 2011;22:416‐9. [DOI] [PubMed] [Google Scholar]
Wohlauer 2012 {published data only}
- Wohlauer MV, Thomas SG, Moore EE, Walsh M, Musunuru H, Davis PK, et al. Platelet dysfunction as an early marker for traumatic brain induced coagulopathy in the absence of platelet inhibitors. Journal of the American College of Surgeons 2012;1:S60. [Google Scholar]
Woolley 2010 {published data only}
- Woolley T, Midwinter M, Spencer P, Doran C, Kirkman E. Early determination of hypocoagulopathy based on interim ROTEM values for clot strength. British Journal of Surgery 2010;97:21. [Google Scholar]
Additional references
Barts & The London 2011
- Barts, The London School of Medicine, Dentistry. The science of the bleeding obvious! Trauma surgery. Summer Science Exhibition 2011, The Royal Society 5‐10 July 2011.
Bossuyt 2003
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CE, Glasziou PE, Irwig LE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Annals of Internal Medicine 2003;138(1):W1‐12. [DOI] [PubMed] [Google Scholar]
Brohi 2003
- Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2003;54(6):1127‐30. [DOI] [PubMed] [Google Scholar]
Brohi 2013
- Brohi, K. Query concerning 2003 paper [personal communication]. email to: H Hunt 14 September 2013.
Deeks 2010
- Deeks JJ, Bossuyt PM, Gatsonis M, Constantine A [editors]. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. The Cochrane Collaboration, 2010, issue Version 1.0.
Doran 2010
- Doran CM, Woolley T, Midwinter MJ. Surgeon General’s Operational Policy Letter 10/07 – Management of massive haemorrhage on operations. Defence Medical Services Department 05/01/02, dated June 14, 2007 quoted in Feasibility of using rotational thromboelastometry to assess coagulation status of combat casualties in a deployed setting. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2010; Vol. 69 Suppl 1 July:40‐8. [DOI] [PubMed]
Dzik 2004
- Dzik WH. Predicting hemorrhage using preoperative coagulation screening assays. Current Hematology Reports 2004;3(5):324‐30. [PubMed] [Google Scholar]
Frith 2010
- Frith D, Goslings JC, Gaardner C, Maegele M, Cohen MJ, Allard S, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. Journal of Thrombosis and Haemostasis 2010;8:1919‐25. [DOI] [PubMed] [Google Scholar]
Ganter 2008
- Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point of care coagulation devices. International Anaesthesia Research Society 2008;106(5):1366‐75. [DOI] [PubMed] [Google Scholar]
Hess 2009
- Hess JR, Lindell AL, Stansbury LG, Dutton RP, Scalea TM. The prevalence of abnormal results of conventional coagulation tests on admission to a trauma center. Transfusion 2009;49(1):34‐9. [DOI] [PubMed] [Google Scholar]
Holcomb 2007
- Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen‐Gerdes L, et al. Causes of death in U.S. Special Operations forces in the global war on terrorism. Annals of Surgery 2007;245(6):968‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 2013
- Hunt H, Hyde C, Stanworth S, Curry N, Perel P, Woolley T, et al. Thromboelastography (TEG) and thromboelastometry (ROTEM) for trauma‐induced coagulopathy in adult trauma patients with bleeding. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010438] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 2014
- Hunt H, Hyde CJ, Stanworth S, Curry N, Perel P, Woolley T, et al. A systematic review of studies indicating the additional prognostic value of tests of global haemostasis (TEG/ROTEM) in the immediate care of adult trauma patients. PROSPERO‐registered protocol 7 April 2014; Vol. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014009060.
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. 1.0. The Cochrane Collaboration, 2010. [Google Scholar]
MacLeod 2003
- MacLeod JB, Lynn M, McKenney MG, Dohn SM, Murthda M. Early coagulopathy predicts mortality in trauma. The Journal of TRAUMA ‐ Injury, Infection & Critical Care 2003;55(1):39‐44. [DOI] [PubMed] [Google Scholar]
Maegele 2007
- Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury 2007;38(3):298‐304. [DOI] [PubMed] [Google Scholar]
Reitsma 2009
- Reitsma JB, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ. Chapter 9: Assessing methodological quality. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors),Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version1.0.0. The Cochrane Collaboration, 2009. Available from: http://srdta.cochrane.org/.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.2. Copenhagen: The Cochrane Collaboration, 2012.
Sankarankutty 2012
- Sankarankutty A, Nascimento B, Luz LT, Rizoli S. TEG and ROTEM in trauma: similar test but different results. World Journal of Emergency Surgery 2012;Suppl 1:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinella 2009
- Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Reviews 2009;23:231‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stainsby 2006
- Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. Guidelines on the management of massive blood loss. British Journal of Haematology 2006;135:634‐41. [DOI] [PubMed] [Google Scholar]


