Abstract
Sinonasal mucosal melanoma (SNMM) is a rare tumor, comprising less than 10% of sinonasal malignancies. SNMM most frequently occurs in the nasal cavity (70%) and maxillary sinus (14%), typically as black patches. Overall, SNMM harbors a very poor prognosis; 5-year survival is less than 30%. Nasal cavity tumors confer a better prognosis than sinus melanoma. The primary management for SNMM is surgery, when feasible, followed by adjuvant radiotherapy. Recent studies suggest that immunotherapy may confer survival benefit to patients with advanced disease. The multidisciplinary team approach has been shown to optimize treatment, reduce costs, and minimize adverse events, while maximizing the chances for cure.
Keywords: mucosal melanoma, head and neck, multidisciplinary
Introduction
Sinonasal mucosal melanoma (SNMM) is a rare tumor, comprising about 1% of all melanomas and about 4–8% of sinonasal malignancies.1,2 In recent years, SNMM has been considered and managed as a separate disease from cutaneous melanoma, including its staging system and treatment modalities.3
SNMM most commonly affects the nasal cavity (70%) and maxillary sinus (14%). Patients with nasal cavity origin have been shown to have a better prognosis than those with paranasal sinus tumors.4 The reported 5-year survival rate of SNMM is less than 30%.3,5
Treatment of early SNMM has traditionally been surgical, followed by adjuvant radiotherapy.3 A role for biologic treatment, as well as immunotherapy, has emerged over the last decade. However, SNMM is characterized by high variability in tumor characteristics, poor prognosis, complexity of treatment, and a vast range of new therapeutic targets. Hence, a single physician cannot manage all the aspects of treatment with state-of-the-art approaches. Rather, a multidisciplinary team (MDT) approach is mandatory for planning the best treatment modality and follow the disease course.
In this review paper, we present the latest updates on SNMM treatment, with an emphasis on the MDT approach.
The Multidisciplinary Team Approach
It can be challenging to manage patients who have been diagnosed with malignant nasal tumors, undergone tumor debulking in a low-volume service, and later been referred for further treatment at a tertiary cancer center. Although infrequent, such management may reduce the chance of cure and confer unjustified detriment to the patient. According to the guidelines of the National Comprehensive Cancer Network and the European Society for Medical Oncology, MDTs are equal-level structures that include clinicians who play various roles, and have different areas of specialization and degrees of expertise. An MDT thus enables the creation of a network in which the patient is at the center of the decision-making process.6–8 At presentation, the focus is to determine the stage of the disease (early, intermediate, or locally-advanced) and the role of surgery in its management. The objective is to make a correct diagnosis and provide patients with the best possible treatment.9
The head and neck cancer MDT includes physicians and paramedical staff from multiple disciplines.10 This tumor board is directed toward attaining a comprehensive evaluation of cancer patients from different points of view.11 Tumor board discussions incorporate information based on the fields of expertise, experience, and knowledge of all the participating specialists, including surgery, medical oncology, radiation oncology, radiology, nuclear medicine, and pathology.12 The entire team is thus able to arrive at a wide-ranging assessment of a patient’s case, at one time, rather than over the course of several days and a number of separate visits with specialists.9,12
As regards melanomas, in comparison to conventional care, the MDT approach has been shown to reduce healthcare costs by optimizing treatments and reducing treatment-associated adverse events.13 In the best settings, an MDT workup will ensure accurate assessment, evidence-based decision-making, and the most advantageous treatment planning and delivery of care.14
The Role of the Multidisciplinary Team Workup
Studies in non-small-cell lung cancer and breast cancer showed that MDTs led to changing treatment decisions in half of the patients, and eventually improved the survival of those individuals.15,16
A number of studies have reported outcomes from the MDT approach in head and neck cancers. A prospective study by Wheless et al17 evaluated the tumor characteristics and treatment plans for 120 patients who were presented to MDT meetings of the head and neck tumor board in a tertiary academic hospital, 84 with malignant and 36 with benign tumors. The multidisciplinary head and neck tumor board conferences were attended by multiple (3 to 5) head and neck surgeons, a medical oncologist, and a radiation oncologist. When needed, representatives of other specialties were also present (eg, neurosurgery, plastic surgery, pathology, radiology; dental, oral and maxillofacial surgery, and social work). Compared to pre-conference decisions, the board’s results showed changes in tumor diagnoses, disease stage, or treatment plans in about 27% of the studied cases. Changes in treatment were significantly more common in cases of malignancy, occurring for 24% of patients versus 6% of those with benign tumors. Such changes were largely due to escalations in management related to multimodality care. Approximately 7% of these patients required a further diagnostic work-up before definitive treatment planning could advance.17
Another study that examined the role of the MDT approach in treatment decision making in head and neck cancers analyzed the retrospective data of 781 head and neck cancer patients.9 Staging or restaging by imaging, pathology, or immunohistochemical or molecular analyses was deemed necessary for 49% of the patients.9 Immediately following MDT evaluation, diagnoses were changed in 3% of the patients without any further need for additional diagnostic investigations. Treatment plans were modified for 10% of the patients. Notably, restaging was required more for those with rare, rather than common cancer types (60% vs 43%).9
Compared to patients who are managed by one physician at a time, MDT-managed cancer patients have better survival outcomes18 and shorter wait times before obtaining consultations with other experts or securing treatment.19 Moreover, they benefit from a more vigorous treatment decision making process because they have rapid access to several expert opinions.20
One large-scale British study compared the outcomes of head and neck cancer patients with and without an MDT approach during two time periods: 1996–1997 (556 patients) and 1999–2000 (727 patients). Patients assessed by an MDT exhibited improved survival (1997: P=0.1; 2000: hazard ratio 0.7, P=0.02). This suggests a correlation between patient care in a multidisciplinary clinic and patient survival.18
Ganti et al analyzed SNMM patients in a large national database to better understand treatment modalities applied and approaches taken with these individuals.3 In a univariate analysis, the study found that, due to the aggressiveness of the disease, a significant predictor of survival was the time between an SNMM diagnosis and initiation of treatment. These data emphasize the importance of fast, multidisciplinary discussion, staging, and treatment decision-making to improve survival.3
Taken together, the accumulating data on the treatment of head and neck cancer, especially the less common entities, indicate that the MDT approach is required for initial management. This allows for better definition of patient stage and adjusting the treatment plan, accordingly.9
The Authors’ Perspective on Implementing the Multidisciplinary Approach
At our tertiary academic head and neck center, we conduct two MDT meetings weekly to discuss head and neck cancer and skull base tumors. The head and neck tumor board is comprised of head and neck surgeons, a medical oncologist, a radiation oncologist, a radiologist, a nuclear imaging specialist, a reconstructive surgeon, and a maxillofacial surgeon. The skull base tumor board is complemented by a neurosurgical team. The aim of these meetings is, first and foremost, to determine the best treatments for patients. In addition, they have educational value.
The role of each member of the MDT is defined and known, and the opinions of the surgeons and oncologists have equal weight regarding the best course of treatment. All verdicts are made by a joint decision.
The Molecular Profile of SNMM
Considerable variation exists in the genetic alterations within the subtypes of melanoma, such as cutaneous, mucosal, and uveal.21 The majority of skin melanomas show an increased number of BRAF mutations, particularly BRAFV600E. On the other hand, a greater number of KIT gene alterations in SNMM and a complete lack of BRAFV600E alterations have been demonstrated.22–24
According to various scientific studies, the somatic oncogenic mutations that characterize SNMM are as follows: KIT, 0–40%; NRAS, 10–50%; and BRAF, 0–10%.25,26 In all tumors obtained from 17 mucosal melanoma patients, fluorescent in-situ hybridization assays showed amplification of the Ras-responsive element binding protein 1 (RREB1) gene in chromosome 6p25. The loss of proto-oncogene MYB, and the cell cycle regulator, cyclin D1 (CCND1) was reported in 76% and 15% of patients with mucosal tumors, respectively.25,27 The absence of the tumor suppressor genes PTEN and p16 was observed in SNMM patients as well, though no alterations were seen in PIK3CA, which is commonly associated with the loss of PTEN.28 Inhibition of expression or complete loss of PTEN was observed in about 40% of the patients, which compares with inhibition of p16 in 50%.25 Furthermore, the overexpression of pAkt and pERK due to alterations of multiple proteins in this signaling cascade resulted in the combined activation of PI3K/Akt and RAS-MAPK signaling pathways.24,25 High levels of pAkt1, which are frequently seen in SNMM patients, can serve as individual prognostic markers.29 Moreover, in non-metastatic BRAFV600E//Cdkn2aNull mouse, overexpression of pAkt1 was shown to be responsible for lung and brain metastasis, which gives further insight into molecular mechanisms driving melanoma progression.30 The identification and characterization of these mutations are essential as they provide potential targeting opportunities for systemic therapy. Inhibition of the effectors in the aforementioned signaling pathways will aid in the development of effective forms of therapy tailored for SNMM patients.
From an epigenetic standpoint, SNMM is known to have a specific pattern of chromosomal alterations, which are absent in cutaneous and uveal melanoma.31 In tumors obtained from SNMM patients, 100%, 93%, and 57% gains have been reported in chromosome arms 1q, 6p, and 8q, respectively. Furthermore, ploidy analysis showed significant clear and high copy gains in 75% of triploid and tetraploid tumors.31,32 Another aspect of SNMM is the high frequency of telomerase reverse transcriptase promoter mutations, which has been observed in 8% of SNMM patients. This results in higher transcriptional activity and an increased number of driver mutations.33 The current clinical significance of these alterations is unknown. However, they can facilitate the characterization of tumors and may eventually serve as therapeutic targets.
The identification of the mitochondrial expression profile in mucosal melanomas paved the way for the development of new mitochondrial biomarkers which could be utilized as potential targets for treating this disease.34 The overexpression mitochondrial biomarkers like antimitochondrial fission protein 1 (FIS1) and mitofusin‐2 (MFN2) has been observed in sinonasal melanoma patients. The former resulted in higher rates of vascular invasion in mucosal melanomas and the latter showed higher rates of distant metastasis, and both are responsible for reducing overall survival rates.35,36
Treatment Paradigms for SNMM
Surgery
The preferred method of treatment for SNMM is surgery, followed by radiation therapy. However, its patchy and multicentric pattern of growth makes complete surgical resection challenging and often impossible. Thus, other treatment interventions, such as systemic therapies, are required. The rarity of SNMM, together with its high metastatic potential, makes it hard to find efficient systemic therapies to combat this form of melanoma.
Surgical resection with negative margins is the primary treatment modality for SNMM. Surgery can be performed using an open or endoscopic approach. Proponents of endoscopic resection claim it has lower complication rates, though the tumor is resected in a piecemeal manner and not en bloc. Several studies have examined the effect of the surgical approach on patient outcomes.3,37,38 Surgery, whether open or endoscopic, has been shown not to affect overall survival. Whereas post-surgical morbidity appears comparable following endoscopic resection and open resections,37,38 quality of life has been shown to be better after endoscopic resection,39–41 Although level one evidence has not demonstrated the superiority of endoscopic resection over open approaches, endoscopic surgery currently replaces open surgery in over 80% of SNMM cases.42
Regarding surgical margins, the aim of surgery should be complete resection with clear margins. An analysis of a large database of 1874 patients with SNMM found that complete surgical resection with negative margins significantly improves patient prognosis.3 Several other studies showed associations of positive surgical margins with a higher rate of distant metastases, decreased survival measures, and a significantly higher risk of death compared to patients with negative surgical margins.43–45 Thus, surgeons should consider the complex anatomy of the sinonasal cavity, the proximity of the tumor to vital structures, and the pattern of locally-advanced disease (eg orbital invasion, dural invasion, or brain invasion). It is unclear whether radical surgery, which often comes at the cost of significant cosmetic and functional impairments, is justified. Surgical excision as a single-modality therapy should be reserved for patients with small tumors, localized disease, and negative marigns.46
SNMM does not tend to spread to lymph nodes and throughout nerves.3,46,47 Reported rates of regional lymph node metastasis in SNMM are 8–11%.3,48,49 Patients who present with positive lymph nodes require therapeutic neck dissection. Those with regional spread do not have worse outcomes than those without regional spread.3,48 Due to the low rate of regional spread and the lack of effect on survival, elective neck dissection is not recommended.48
Chemotherapy
The role of chemotherapy is minor compared to the biological and immunological systemic therapies. For example, dacarbazine-based chemotherapy was previously the preferred option for treating patients with metastatic mucosal melanoma.50 However, the lack of association of chemotherapy alone with improved overall survival eventually led to its discontinuation as the standard of care.51
Currently, chemotherapy is used as an adjuvant systemic therapy in combination with other immunotherapeutic and biological drugs after surgical resection in mucosal melanoma.52 Chemotherapeutic drugs, such as dacarbazine, are being used in conjunction with MEK, PD-1, and CTLA-4 inhibitors (see below), as well as with biochemotherapeutic drugs, like interleukin-2 and interferon alfa-2b.53 Most of these combinatorial treatments have yielded disappointing results, with no significant impact on overall survival. Due to the rarity of mucosal melanoma and the stringency with which the benefit of the treatment is determined, chemotherapy is unlikely to be further developed as a monotherapeutic regimen.54
In a Phase II biochemotherapy (BCT) trial, patients were treated with cisplatin, vinblastine, and dacarbazine (CVD) as monotherapies or in conjunction with interleukin-2 and interferon alfa-2b (BCT).55,56 Though BCT showed a higher response rate than CVD, 19.5% vs 13.8% (P=0.140), patients experienced a toxicity of grade 3 or higher. Despite promising results in phase II trials, overall survival rates did not improve in Phase III trials.57 High-dose interferon, which is often used as an adjuvant therapy, or BCT, consisting of CVD, interleukin-2, and granulocyte colony-stimulating factor, were administered to high-risk patients with melanoma in an intergroup phase III trial (S0008). Among the patients receiving BCT, relapse-free survival, but not overall survival improved.58 Hence, BCT provides an alternate approach to treatment. Its primary advantage is the short duration of the treatment, whereas its main caveat is high toxicity. Comparing adjuvant therapies after the resection of mucosal melanoma, temozolomide-based chemotherapy demonstrated better relapse-free survival than did high-dose interferon. A combination of temozolomide with cisplatin is a superior systemic option and may result in better survival rates.59
Biological Therapy
Aberrations in the KIT gene are highly prevalent in SNMM. This provides an opportunity for systemic therapy by using selective KIT inhibitors. In a phase II trial, the tyrosine kinase inhibitor, imatinib mesylate, showed significant effects in patients with the K642E KIT gene mutation.26 The use of imatinib resulted in control of the disease by 77%, as well as a 54% tumor response rate against the development of an advanced stage of the disease with c-KIT mutations.60 Another tyrosine kinase inhibitor, dasatanib, showed promising results in clinical trials that investigated the most common L576P KIT gene mutation in mucosal melanomas.61 The selective inhibition of various KIT alterations provides an attractive opportunity for developing adjuvant therapies for mucosal melanoma.
Although it’s not as common in SNMM as in other forms of melanoma, the overexpression of the Ras-MAPK signaling pathway in SNMM is another example in which systemic therapy can be implemented. MEK162 is one such allosteric MEK1 and MEK2 inhibitor that has shown promising results in phase III trials conducted in patients with NRAS mutated malignant melanoma. This drug was also shown to be effective against melanomas with BRAF mutations.62 In phase III trials of BRAF-altered metastatic melanoma, vemurafinib, a BRAF kinase inhibitor, showed greater efficacy and tolerability, and also a 20% increase in the 6-month survival rate, compared to the chemotherapeutic dacarbazine.63,64 The effects of the MEK inhibitor, binimetinib (MEK162), were compared to those of dacarbazine in a randomized phase III trial consisting of 400 patients harboring the NRAS mutation in cutaneous melanoma. The administration of binimetinib, before or after one round of immunotherapy, showed better overall response, progression-free survival, and disease control than dacarbazine.62,65 Similar results were seen in another randomized phase III trial in which binimetinib was used in combination with the BRAF inhibitor encorafenib. Compared to vemurafinib monotherapy, this combination showed greater efficacy and tolerability in patients with malignant melanomas.66
Immunotherapy
In a randomized phase III trial, the use of the cytotoxic T-lymphocyte-associated antigen 4 blocker (CTLA4), ipilimumab, showed a significant increase in median survival (10.1 months), compared to the administration of the glycoprotein 100 (gp100) peptide vaccine (6.4 months).67 In another phase III study, 502 patients with untreated metastatic melanoma were treated with a 1:1 combination of ipilimumab and dacarbazine, where outcomes were compared to dacarbazine monotherapy. The combination treatment showed a statistically significant increase in overall survival of 11.2 months, compared to 9.1 months obtained from dacarbazine monotherapy.68 Hence, multiple prospective and retrospective studies support the use of ipilimumab against this disease.69
The checkpoint inhibitor nivolumab operates by inhibiting interactions of ligands PD-L1 and PD-L2 with its receptor, programmed death-1 receptor (PD-1), thereby blocking T-cell activation. In a phase III trial of patients with ipilimumab-refractory metastatic melanoma, Nivolumab showed a higher overall survival rate (72.9%) than dacarbazine (42.1%).70 Furthermore, in a randomized phase III trial (CheckMate 037), patients who progressed after ipilimumab monotherapy or the combination of ipilimumab and BRAF inhibitor, reported a higher response to nivolumab compared to standard chemotherapy.71 Nivolumab in combination with ipilimumab showed a higher overall response rate (37%) than nivolumab (23%) or ipilimumab (8%) monotherapies, respectively.72 This indicates that the abovementioned combination therapy is more efficient than the systemic therapies that are currently available for mucosal melanoma patients. In addition to nivolumab, other checkpoint inhibitors, such as pembrolizumab, have shown more improvement in progression-free survival, toxicity, and overall survival than ipilimumab, or the investigator-choice chemotherapy drug in the KEYNOTE trials 006 and 002.73,74 Other anti-PD-L1 antibody monotherapies, such as durvalumab and atezolizumab, have also been tested without much success.75
A multivariate analysis of patients with SNMM showed better survival following immunotherapy among patients with metastatic disease (HR: 0.14, 95% CI: 0.04–0.49), but not among those without metastatic disease. This concurs with the more frequent utilization of systemic therapies for advanced rather than localized disease. In that cohort, chemotherapy remained a nonsignificant predictor of survival.3
Treatment of SNMM is challenging due to the complexity and rarity of the disease, as well as the aggressiveness of tumors. Currently, early detection and surgical excision is considered the primary method of treatment. Recent clinical trials demonstrate the effectiveness of systemic therapies in increasing survival rates, thus encouraging the performance of large-scale clinical trials. Furthermore, poor prognosis should provide the impetus to investigate the role of new systemic neoadjuvant combination therapies in the treatment of mucosal melanoma. Table 1 summarizes the ongoing clinical trials for head and neck mucosal melanoma.
Table 1.
Ongoing Clinical Trials for Head and Neck Mucosal Melanoma
| Number and Name of the Study | Phase | Biologics/Drugs | Target | Number of Participants |
|---|---|---|---|---|
|
NCT02506153: High-Dose Recombinant Interferon Alfa-2B, Ipilimumab, or Pembrolizumab in Treating Patients With Stage III–IV High Risk Melanoma That Has Been Removed by Surgery |
III |
Biological: Ipilimumab Biological: Pembrolizumab Other: Quality-of-life assessment Biological: Recombinant Interferon Alfa-2b |
CTLA-4 PD-1 |
1378 |
|
NCT03313206: Neoadjuvant Treatment Associated With Maintenance Therapy by Anti-PD1 Immunotherapy in Patients With Resectable Head and Neck Mucosal Melanoma (IMMUQ) |
II |
Drug: Pembrolizumab Procedure: Surgery Radiation: IMRT |
PD-1 |
50 |
|
NCT02748564: Aldesleukin and Pembrolizumab in Treating Patients With Stage III–IV Melanoma |
II |
Biological: Aldesleukin Other: Laboratory biomarker analysis Biological: Pembrolizumab |
PD-1 |
65 |
| NCT03758729: Phase II Study of Nivolumab in Combination With Radiation Therapy as Definitive Treatment for Patients With Locally Advanced, Unresectable Head and Neck Mucosal Melanoma | II | Drug: Nivolumab Radiation: Radiation therapy |
PD-1 | 26 |
Abbreviation: IMRT, intensity-modulated radiation therapy.
Radiation Therapy
Radiation therapy (RT) can be administered to patients with SNMM as a definitive or adjuvant treatment following surgery. Samstein et al reported a retrospective analysis of 78 patients with localized, non-metastatic SNMM who were surgically treated.76 The rate of negative surgical margins was 38%. Eighty-two percent of the patients received RT, 68% as adjuvant treatment and 32% as definitive treatment, due to gross residual disease. Intensity-modulated RT was administered to 45 patients (70%) with the remainder receiving 3D conformal RT. Five-year local recurrence-free survival was higher among patients who received RT than among those who did not (59% vs 35%). The local recurrence rate was 33% among patients who received adjuvant RT, compared to 71% among those who did not. Importantly, the overall survival and disease-specific survival of the two groups was similar.76
Other studies also showed an association of adjuvant RT with reduced locoregional occurrence, but not with overall survival.3,77 According to a recent publication,78 this may be due to low SNMM recurrence of about 20% during the first five years following the commencement of treatment, alongside a high risk of systemic disease. The latter is evidenced by the occurrence of distant metastases in up to 80% of patients within the same period.
Particle beam therapy has also been used to facilitate the delivery of high doses to the residual tumor while minimizing exposure to the surrounding normal tissues.79–81 The reported results of treatment with carbon ion radiotherapy in head and neck mucosal melanoma were 84% for 5-year local control and 27% for 5-year overall survival with acceptable toxicity.81 In patients treated with proton beam therapy, 3-year and 5-year overall survival were 58% and 51% respectively,79,82 Five-year disease-free survival was 38%.82
The role of RT as a primary treatment modality for SNMM remains unclear.83,84 Data in the literature show mainly retrospective outcomes of patients who had definitive RT for unresectable or advanced tumors. Various analyses revealed that definitive RT does not improve survival or disease control of patients with SNMM.1,44,85
Outcomes and Risk Assessment
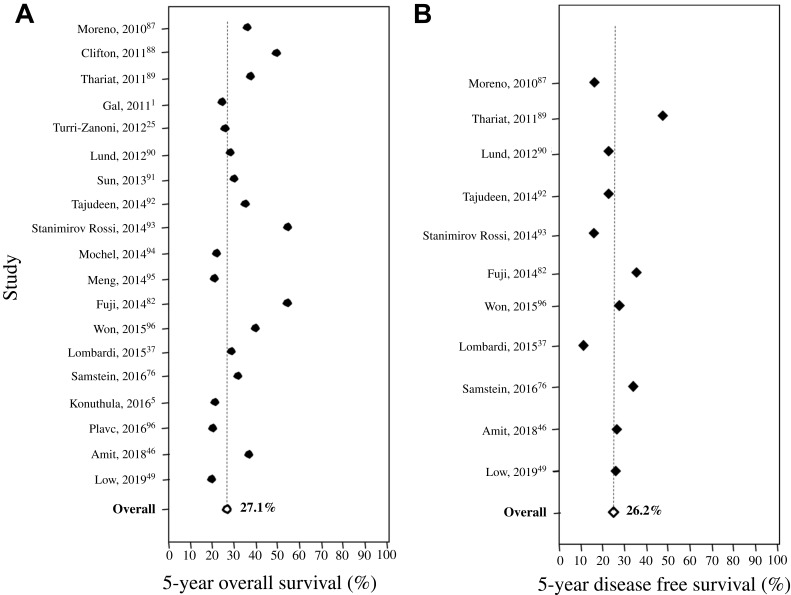
The prognosis of patients with SNMM remains poor. The reported 5-year overall survival is in the range of 20–60%, while the mean is 27% (Figure 1). Mean, five-year disease-free survival is about 26% (Figure 1). Advanced age is associated with decreased survival.3,5,49 Tumor characteristics that have been associated with worse survival include T4 disease and distant metastasis.3,5,49,76,86 Disease of nasal origin confers better survival than disease of sinus origin.5,49,76,86
Figure 1.
Survival rates of patients with sinonasal mucosal melanoma. (A) Five-year overall survival. (B) Five-year disease-free survival.
An analysis of 1874 patients with SNMM in the National Cancer Database sought to find correlations between treatment modalities and survival measures.3 Surgical resection with negative margins was shown to improve survival (HR: 0.44; 95% CI: 0.30–0.65). This concurs with previous studies.5 Although its effect on survival is yet to be fully realized, immunotherapy was shown to improve the survival rate in the subset of patients with SNMM and distant metastases (HR: 0.14; 95% CI: 0.04–0.49). Surgical treatment, radiotherapy, and chemotherapy were not found to be significant predictors of survival.3 A retrospective study of 198 patients with SNMM, conducted by Amit et al, revealed a recurrence rate of 48%. The most common cause of treatment failure was distant metastasis in 69 (35%), followed by local [36 (18%)] and regional [22 (11%)] recurrence.86 The most common sites for distant metastases were the lungs (20%), followed by the liver (13%), bones (8%), and the brain (5%). Factors with adverse prognostic effects were tumor thickness >4 mm for disease-free survival, positive margins for local recurrence, and bone invasion for distant metastases.86
Similarly, Samstein el al76 reported 66% distant failure, 23% local failure, and 13% regional lymph node recurrence. A subset of patients (n=30) underwent diagnostic genomic testing for mutations of the KIT, BRAF, NRAS, and GNAQ genes. Overall, 30% of them had at least one mutation. Analysis of outcomes in these patients did not reveal a significant prognostication between the presence of mutations and patient outcomes.76
Conclusions
SNMM is a rare disease that harbors a poor prognosis. Distant metastasis is the most common cause of treatment failure. The variability of the disease and the emergence of new treatment modalities necessitate an MDT approach for the optimal management of treatment choice, treatment side effects, and follow-up.
Surgery remains the mainstay of treatment in patients with SNMM. Adjuvant RT seems to improve local control, without a substantial effect on overall survival. Chemotherapy does not play a significant role as a primary treatment, while immunotherapy may confer survival benefit to patients with metastatic disease.
Acknowledgment
Cindy Cohen is thanked for her editorial assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gal TJ, Silver N, Huang B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope. 2011;121(9):2026–2033. doi: 10.1002/lary.21925 [DOI] [PubMed] [Google Scholar]
- 2.Gilain L, Houette A, Montalban A, Mom T, Saroul N. Mucosal melanoma of the nasal cavity and paranasal sinuses. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(6):365–369. doi: 10.1016/j.anorl.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 3.Ganti A, Raman A, Shay A, et al. Treatment modalities in sinonasal mucosal melanoma: a national cancer database analysis. Laryngoscope. 2019. doi: 10.1002/lary.27995 [DOI] [PubMed] [Google Scholar]
- 4.Jethanamest D, Vila PM, Sikora AG, Morris LGT. Predictors of survival in mucosal melanoma of the head and neck. Ann Surg Oncol. 2011;18(10):2748–2756. doi: 10.1245/s10434-011-1685-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konuthula N, Khan MN, Parasher A, et al. The presentation and outcomes of mucosal melanoma in 695 patients. Int Forum Allergy Rhinol. 2017;7(1):99–105. doi: 10.1002/alr.21831 [DOI] [PubMed] [Google Scholar]
- 6.Head and neck cancers. Available from: http://www.nccn.org. Accessed February13, 2020.
- 7.Licitra L, Bossi P, Locati LD. A multidisciplinary approach to squamous cell carcinomas of the head and neck: what is new? Curr Opin Oncol. 2006;18(3):253–257. doi: 10.1097/01.cco.0000219254.53091.35 [DOI] [PubMed] [Google Scholar]
- 8.Chan ATC, Gregoire V, Lefebvre J-L, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol off J Eur Soc Med Oncol. 2012;23 Suppl 7:vii83–5. doi: 10.1093/annonc/mds266 [DOI] [PubMed] [Google Scholar]
- 9.Bergamini C, Locati L, Bossi P, et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54–57. doi: 10.1016/j.oraloncology.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Shah JP, Gil Z. Current concepts in management of oral cancer–surgery. Oral Oncol. 2009;45(4–5):394–401. doi: 10.1016/j.oraloncology.2008.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489–501. doi: 10.4065/83.4.489 [DOI] [PubMed] [Google Scholar]
- 12.Mazzaferro V, Majno P. Principles for the best multidisciplinary meetings. Lancet Oncol. 2011;12(4):323–325. doi: 10.1016/S1470-2045(11)70010-9 [DOI] [PubMed] [Google Scholar]
- 13.Lewis CM, Hessel AC, Roberts DB, et al. Prereferral head and neck cancer treatment: compliance with national comprehensive cancer network treatment guidelines. Arch Otolaryngol Head Neck Surg. 2010;136(12):1205–1211. doi: 10.1001/archoto.2010.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol. 2006;7(11):935–943. doi: 10.1016/S1470-2045(06)70940-8 [DOI] [PubMed] [Google Scholar]
- 15.Forrest LM, McMillan DC, McArdle CS, Dunlop DJ. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;93(9):977–978. doi: 10.1038/sj.bjc.6602825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JH, Vines E, Bertsch H, et al. The impact of a multidisciplinary breast cancer center on recommendations for patient management: the University of Pennsylvania experience. Cancer. 2001;91(7):1231–1237. doi: [DOI] [PubMed] [Google Scholar]
- 17.Wheless SA, McKinney KA, Zanation AM. A prospective study of the clinical impact of a multidisciplinary head and neck tumor board. Otolaryngol Head Neck Surg. 2010;143(5):650–654. doi: 10.1016/j.otohns.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birchall M, Bailey D, King P. Effect of process standards on survival of patients with head and neck cancer in the south and west of England. Br J Cancer. 2004;91(8):1477–1481. doi: 10.1038/sj.bjc.6602118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics. Do they work? Cancer. 1997;79(12):2380–2384. doi: 10.1002/(ISSN)1097-0142 [DOI] [PubMed] [Google Scholar]
- 20.Sidhom MA, Poulsen MG. Multidisciplinary care in oncology: medicolegal implications of group decisions. Lancet Oncol. 2006;7(11):951–954. doi: 10.1016/S1470-2045(06)70942-1 [DOI] [PubMed] [Google Scholar]
- 21.Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol. 2007;56(5):828–834. doi: 10.1016/J.JAAD.2006.06.017 [DOI] [PubMed] [Google Scholar]
- 22.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–4346. doi: 10.1200/JCO.2006.06.2984 [DOI] [PubMed] [Google Scholar]
- 23.Torres-Cabala CA, Wang W-L, Trent J, et al. Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral-lentiginous/mucosal type. Mod Pathol. 2009;22(11):1446–1456. doi: 10.1038/modpathol.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zebary A, Jangard M, Omholt K, Ragnarsson-Olding B, Hansson J. KIT, NRAS and BRAF mutations in sinonasal mucosal melanoma: a study of 56 cases. Br J Cancer. 2013;109(3):559–564. doi: 10.1038/bjc.2013.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turri-Zanoni M, Medicina D, Lombardi D, et al. Sinonasal mucosal melanoma: molecular profile and therapeutic implications from a series of 32 cases. Head Neck. 2013;35(8):1066–1077. doi: 10.1002/hed.23079 [DOI] [PubMed] [Google Scholar]
- 26.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327. doi: 10.1001/jama.2011.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastian BC. Understanding the progression of melanocytic neoplasia using genomic analysis: from fields to cancer. Oncogene. 2003;22(20):3081–3086. doi: 10.1038/sj.onc.1206463 [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22(20):3113–3122. doi: 10.1038/sj.onc.1206451 [DOI] [PubMed] [Google Scholar]
- 29.Soares C, Melo de Lima Morais T, Carlos R, et al. Phosphorylated Akt1 expression is associated with poor prognosis in cutaneous, oral and sinonasal melanomas. Oncotarget. 2018;9(99):37291–37304. doi: 10.18632/oncotarget.26458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho JH, Robinson JP, Arave RA, et al. AKT1 activation promotes development of melanoma metastases. Cell Rep. 2015;13(5):898–905. doi: 10.1016/j.celrep.2015.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dijk M, Sprenger S, Rombout P, et al. Distinct chromosomal aberrations in sinonasal mucosal melanoma as detected by comparative genomic hybridization. Genes Chromosom Cancer. 2003;36(2):151–158. doi: 10.1002/gcc.10156 [DOI] [PubMed] [Google Scholar]
- 32.Woodman SE, Davies MA. Targeting KIT in melanoma: a paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol. 2010;80(5):568–574. doi: 10.1016/J.BCP.2010.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jangard M, Zebary A, Ragnarsson-Olding B, Hansson J. TERT promoter mutations in sinonasal malignant melanoma: a study of 49 cases. Melanoma Res. 2015;25(3):185–188. doi: 10.1097/CMR.0000000000000148 [DOI] [PubMed] [Google Scholar]
- 34.Su DM, Zhang Q, Wang X, et al. Two types of human malignant melanoma cell lines revealed by expression patterns of mitochondrial and survival-apoptosis genes: implications for malignant melanoma therapy. Mol Cancer Ther. 2009;8(5):1292–1304. doi: 10.1158/1535-7163.MCT-08-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soares CD, de Lima Morais TM, Mariano FV, et al. Expression of mitochondrial dynamics markers during melanoma progression: comparative study of head and neck cutaneous and mucosal melanomas. J Oral Pathol Med off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2019;48(5):373–381. doi: 10.1111/jop.12855 [DOI] [PubMed] [Google Scholar]
- 36.Soares CD, de Lima Morais TM, Carlos R, et al. Prognostic importance of mitochondrial markers in mucosal and cutaneous head and neck melanomas. Hum Pathol. 2019;85:279–289. doi: 10.1016/j.humpath.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 37.Lombardi D, Bottazzoli M, Turri-Zanoni M, et al. Sinonasal mucosal melanoma: a 12-year experience of 58 cases. Head Neck. 2016;38 Suppl 1:E1737–45. doi: 10.1002/hed.24309 [DOI] [PubMed] [Google Scholar]
- 38.Swegal W, Koyfman S, Scharpf J, et al. Endoscopic and open surgical approaches to locally advanced sinonasal melanoma: comparing the therapeutic benefits. JAMA Otolaryngol Head Neck Surg. 2014;140(9):840–845. doi: 10.1001/jamaoto.2014.1321 [DOI] [PubMed] [Google Scholar]
- 39.Amit M, Abergel A, Fliss DM, Gil Z. The clinical importance of quality-of-life scores in patients with skull base tumors: a meta-analysis and review of the literature. Curr Oncol Rep. 2012;14(2):175–181. doi: 10.1007/s11912-012-0222-3 [DOI] [PubMed] [Google Scholar]
- 40.Abergel A, Cavel O, Margalit N, Fliss DM, Gil Z. Comparison of quality of life after transnasal endoscopic vs open skull base tumor resection. Arch Otolaryngol Head Neck Surg. 2012;138(2):142–147. doi: 10.1001/archoto.2011.1146 [DOI] [PubMed] [Google Scholar]
- 41.Cavel O, Abergel A, Margalit N, Fliss DM, Gil Z. Quality of life following endoscopic resection of skull base tumors. J Neurol Surg B Skull Base. 2012;73(2):112–116. doi: 10.1055/s-0032-1301392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gil Z, Fliss DM. Contemporary management of head and neck cancers. Isr Med Assoc J. 2009;11(5):296–300. [PubMed] [Google Scholar]
- 43.Tajudeen BA, Vorasubin N, Sanaiha Y, Palma-Diaz MF, Suh JD, Wang MB. Sinonasal mucosal melanoma: 20-year experience at a tertiary referral center. Int Forum Allergy Rhinol. 2014;4(7):592–597. doi: 10.1002/alr.21324 [DOI] [PubMed] [Google Scholar]
- 44.Lee SP, Shimizu KT, Tran LM, Juillard G, Calcaterra TC. Mucosal melanoma of the head and neck: the impact of local control on survival. Laryngoscope. 1994;104(2):121–126. doi: 10.1288/00005537-199402000-00001 [DOI] [PubMed] [Google Scholar]
- 45.Penel N, Mallet Y, Mirabel X, Van JT, Lefebvre J-L. Primary mucosal melanoma of head and neck: prognostic value of clear margins. Laryngoscope. 2006;116(6):993–995. doi: 10.1097/01.mlg.0000217236.06585.a9 [DOI] [PubMed] [Google Scholar]
- 46.Amit M, Na’ara S, Hanna EY. Contemporary treatment approaches to sinonasal mucosal melanoma. Curr Oncol Rep. 2018;20(2):10. doi: 10.1007/s11912-018-0660-7 [DOI] [PubMed] [Google Scholar]
- 47.Gil Z, Carlson DL, Gupta A, et al. Patterns and incidence of neural invasion in patients with cancers of the paranasal sinuses. Arch Otolaryngol Head Neck Surg. 2009;135(2):173–179. doi: 10.1001/archoto.2008.525 [DOI] [PubMed] [Google Scholar]
- 48.Amit M, Tam S, Abdelmeguid AS, et al. Approaches to regional lymph node metastasis in patients with head and neck mucosal melanoma. Cancer. 2018;124(3):514–520. doi: 10.1002/cncr.31083 [DOI] [PubMed] [Google Scholar]
- 49.Low CM, Price DL, Moore EJ, et al. Nodal and distant metastases in sinonasal mucosal melanoma: a population-based analysis. Laryngoscope. 2019. doi: 10.1002/lary.28065 [DOI] [PubMed] [Google Scholar]
- 50.Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9):2745. doi: 10.1200/JCO.1999.17.9.2745 [DOI] [PubMed] [Google Scholar]
- 51.Tyrrell H, Payne M. Combatting mucosal melanoma: recent advances and future perspectives. Melanoma Manag. 2018;5(3):MMT11. doi: 10.2217/mmt-2018-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amit M, Tam S, Abdelmeguid AS, et al. Role of adjuvant treatment in sinonasal mucosal melanoma. J Neurol Surg B Skull Base. 2017;78(6):512–518. doi: 10.1055/s-0037-1604350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spencer KR, Mehnert JM. Mucosal Melanoma: Epidemiology, Biology and Treatment. Cham: Springer; 2016. 295–320. doi: 10.1007/978-3-319-22539-5_13 [DOI] [PubMed] [Google Scholar]
- 54.Cheng Y-F, Lai -C-C, Ho C-Y, Shu C-H, Lin C-Z. Toward a Better Understanding of Sinonasal Mucosal Melanoma: Clinical Review of 23 Cases. Vol. 70 2007. doi: 10.1016/S1726-4901(09)70296-5 [DOI] [PubMed] [Google Scholar]
- 55.Legha S, Ring S, Eton O, Bedikian A, Plager C, Papadopoulos N. Development and results of biochemotherapy in metastatic melanoma: the university of texas MD anderson cancer center experience. Cancer J Sci Am. 1977;3:S9–S15. [PubMed] [Google Scholar]
- 56.Keilholz U, Goey SH, Punt CJ, et al. Interferon alfa-2a and interleukin-2 with or without cisplatin in metastatic melanoma: a randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Clin Oncol. 1997;15(7):2579–2588. doi: 10.1200/JCO.1997.15.7.2579 [DOI] [PubMed] [Google Scholar]
- 57.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26(35):5748–5754. doi: 10.1200/JCO.2008.17.5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flaherty LE, Othus M, Atkins MB, et al. Southwest Oncology Group S0008: a phase III trial of high-dose interferon Alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma–an intergroup study of cancer and leukemia Group B, Children’s Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. J Clin Oncol. 2014;32(33):3771–3778. doi: 10.1200/JCO.2013.53.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lian B, Si L, Cui C, et al. Phase II randomized trial comparing high-dose IFN- 2b with temozolomide plus cisplatin as systemic adjuvant therapy for resected mucosal melanoma. Clin Cancer Res. 2013;19(16):4488–4498. doi: 10.1158/1078-0432.CCR-13-0739 [DOI] [PubMed] [Google Scholar]
- 60.Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified kit arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31(26):3182–3190. doi: 10.1200/JCO.2012.47.7836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodman SE, Trent JC, Stemke-Hale K, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009;8(8):2079–2085. doi: 10.1158/1535-7163.MCT-09-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label Phase 2 study. Lancet Oncol. 2013;14(3):249–256. doi: 10.1016/S1470-2045(13)70024-X [DOI] [PubMed] [Google Scholar]
- 63.Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAFV600 mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 2014;15(4):436–444. doi: 10.1016/S1470-2045(14)70051-8 [DOI] [PubMed] [Google Scholar]
- 64.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flaherty K, Arenberger P, Ascierto PA, et al. NEMO: a Phase 3 trial of binimetinib (MEK162) versus dacarbazine in patients with untreated or progressed after first-line immunotherapy unresectable or metastatic NRAS -mutant cutaneous melanoma. J Clin Oncol. 2014;32(15_suppl):TPS9102–TPS9102. doi: 10.1200/jco.2014.32.15_suppl.tps9102 [DOI] [Google Scholar]
- 66.Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS -mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(4):435–445. doi: 10.1016/S1470-2045(17)30180-8 [DOI] [PubMed] [Google Scholar]
- 67.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 69.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from Phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 71.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 72.D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35(2):226–235. doi: 10.1200/JCO.2016.67.9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 74.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Redman JM, Gibney GT, Atkins MB. Advances in immunotherapy for melanoma. BMC Med. 2016;14(1):20. doi: 10.1186/s12916-016-0571-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samstein RM, Carvajal RD, Postow MA, et al. Localized sinonasal mucosal melanoma: outcomes and associations with stage, radiotherapy, and positron emission tomography response. Head Neck. 2016;38(9):1310–1317. doi: 10.1002/hed.24435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li W, Yu Y, Wang H, Yan A, Jiang X. Evaluation of the prognostic impact of postoperative adjuvant radiotherapy on head and neck mucosal melanoma: a meta-analysis. BMC Cancer. 2015;15:758. doi: 10.1186/s12885-015-1750-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crippen MM, Kilic S, Eloy JA. Updates in the management of sinonasal mucosal melanoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26(1):52–57. doi: 10.1097/MOO.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 79.Zenda S, Kawashima M, Nishio T, et al. Proton beam therapy as a nonsurgical approach to mucosal melanoma of the head and neck: a pilot study. Int J Radiat Oncol Biol Phys. 2011;81(1):135–139. doi: 10.1016/j.ijrobp.2010.04.071 [DOI] [PubMed] [Google Scholar]
- 80.Yanagi T, Mizoe J-E, Hasegawa A, et al. Mucosal malignant melanoma of the head and neck treated by carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74(1):15–20. doi: 10.1016/j.ijrobp.2008.07.056 [DOI] [PubMed] [Google Scholar]
- 81.Mizoe J-E, Hasegawa A, Jingu K, et al. Results of carbon ion radiotherapy for head and neck cancer. Radiother Oncol. 2012;103(1):32–37. doi: 10.1016/j.radonc.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 82.Fuji H, Yoshikawa S, Kasami M, et al. High-dose proton beam therapy for sinonasal mucosal malignant melanoma. Radiat Oncol. 2014;9:162. doi: 10.1186/1748-717X-9-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Douglas CM, Malik T, Swindell R, Lorrigan P, Slevin NJ, Homer JJ. Mucosal melanoma of the head and neck: radiotherapy or surgery? J Otolaryngol Head Neck Surg. 2010;39(4):385–392. [PubMed] [Google Scholar]
- 84.Krengli M, Jereczek-Fossa BA, Kaanders JHAM, Masini L, Beldi D, Orecchia R. What is the role of radiotherapy in the treatment of mucosal melanoma of the head and neck? Crit Rev Oncol Hematol. 2008;65(2):121–128. doi: 10.1016/j.critrevonc.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 85.Bachar G, Loh KS, O’Sullivan B, et al. Mucosal melanomas of the head and neck: experience of the Princess Margaret Hospital. Head Neck. 2008;30(10):1325–1331. doi: 10.1002/hed.20878 [DOI] [PubMed] [Google Scholar]
- 86.Amit M, Tam S, Abdelmeguid AS, et al. Patterns of treatment failure in patients with sinonasal mucosal melanoma. Ann Surg Oncol. 2018;25(6):1723–1729. doi: 10.1245/s10434-018-6465-y [DOI] [PubMed] [Google Scholar]
- 87.Moreno MA, Roberts DB, Kupferman ME, et al. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson Cancer Center. Cancer. 2010;116(9):NA–NA. doi: 10.1002/cncr.24976 [DOI] [PubMed] [Google Scholar]
- 88.Clifton N, Harrison L, Bradley PJ, Jones NS. Malignant melanoma of nasal cavity and paranasal sinuses: report of 24 patients and literature review. J Laryngol Otol. 2011;125(5):479–485. doi: 10.1017/S0022215110002720 [DOI] [PubMed] [Google Scholar]
- 89.Thariat J, Bolle S, Demizu Y, et al. New techniques in radiation therapy for head and neck cancer. Anticancer Drugs. 2011;22(7):596–606. doi: 10.1097/CAD.0b013e328340fd2b [DOI] [PubMed] [Google Scholar]
- 90.Lund VJ, Chisholm EJ, Howard DJ, Wei WI. Sinonasal malignant melanoma: an analysis of 115 cases assessing outcomes of surgery, postoperative radiotherapy and endoscopic resection. Rhinology. 2012;50(2):203–210. doi: 10.4193/Rhino11.267 [DOI] [PubMed] [Google Scholar]
- 91.Sun C-Z, Li Q-L, Hu Z-D, Jiang Y-E, Song M, Yang A-K. Treatment and prognosis in sinonasal mucosal melanoma: a retrospective analysis of 65 patients from a single cancer center. Head Neck. 2014;36(5):675–681. doi: 10.1002/hed.23355 [DOI] [PubMed] [Google Scholar]
- 92.Clair JM, Arshi A, Tajudeen B, Abemayor E, St John M. Epidemiology and treatment of lacrimal gland tumors. JAMA Otolaryngol Neck Surg. 2014;140(12):1110. doi: 10.1001/jamaoto.2014.2846 [DOI] [PubMed] [Google Scholar]
- 93.Stanimirov Rossi O, Vital D, Soyka MB, Roth TN, Huber GF, Holzmann D. Multilocular sinonasal malignant melanoma: a poor prognostic subgroup? Eur Arch Oto Rhino Laryngol. 2015;272(1):123–129. doi: 10.1007/s00405-014-3098-z [DOI] [PubMed] [Google Scholar]
- 94.Mochel MC, Duncan LM, Piris A, Kraft S. Primary mucosal melanoma of the sinonasal tract: a clinicopathologic and immunohistochemical study of thirty-two cases. Head Neck Pathol. 2015;9(2):236–243. doi: 10.1007/s12105-014-0570-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meng X-J, Ao H-F, Huang W-T, et al. Impact of different surgical and postoperative adjuvant treatment modalities on survival of sinonasal malignant melanoma. BMC Cancer. 2014;14(1):608. doi: 10.1186/1471-2407-14-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim DW, Barcena E, Mehta UN, et al. Prolonged survival of a patient with metastatic leptomeningeal melanoma treated with BRAF inhibition-based therapy: a case report. BMC Cancer. 2015;15(1):400. doi: 10.1186/s12885-015-1391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Plavc G, But-Hadžić J, Aničin A, Lanišnik B, Didanović V, Strojan P. Mucosal melanoma of the head and neck: a population-based study from Slovenia, 1985–2013. Radiat Oncol. 2016;11(1):137. doi: 10.1186/s13014-016-0712-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Head and neck cancers. Available from: http://www.nccn.org. Accessed February13, 2020.