Abstract
Purpose
To compare toric intraocular lens (IOL) outcome accuracy after using an online toric calculator that accounted for posterior corneal astigmatism versus a traditional calculator that only accounted for anterior corneal astigmatism.
Patients and Methods
This was a single-arm, non-masked, non-randomized prospective study in a single private practice in Norfolk, Virginia, USA, evaluating clinical outcomes of toric IOL implantation based on a calculator that considered posterior corneal astigmatism (PCA) and effective lens position (ELP). Of interest was the distribution of the residual refraction (sphere and cylinder) at 40–70 days postoperative. Residual refractive cylinder (RRC) was compared to the back-calculated theoretical results using a legacy calculator that did not consider PCA. Distance visual acuity (best-corrected and uncorrected) and the manifest refraction were also measured, along with preoperative and postoperative keratometry.
Results
Forty-six eyes of 34 subjects were available for analysis. All eyes had a spherical equivalent refraction within 0.5D of intended. Uncorrected visual acuity was 20/25 or better in 86% of eyes targeted for emmetropia. Residual cylinder was 0.50D or less in 96% of eyes, with a maximum of 0.75D measured. The difference between residual cylinder and the expected cylinder from calculations was significantly lower for the calculator that included consideration of PCA and ELP relative to the one that did not.
Conclusion
Use of a toric IOL calculator that includes consideration of posterior corneal astigmatism is recommended to optimize clinical outcomes.
Keywords: posterior corneal astigmatism, cylinder, residual refraction, toric calculator
Plain Language Summary
When surgeons perform cataract surgery, they replace the cloudy natural lens with an intraocular lens, a miniature lens that sits inside the eye. They can choose the power of that lens, based on measuring various features of the eye, particularly how long the eye is and how curved the front of the eye (the cornea) is. Patients may then find less need for glasses to see clearly after surgery, at least for distance vision. The degree of success is often related to the reliability of the measurements and the accuracy of the calculator being used.
Toric intraocular lenses correct astigmatism – they are used when the cornea has a different curvature in two directions (e.g. the cornea is shaped like a football, rather than a baseball). Surgeons must calculate how much astigmatism to correct, and this can be complicated. Results in the past have been good, but there was room for improvement. A new calculator includes consideration of the front and back surface of the cornea. This study was designed to compare results with such a new calculator to theoretical results if the new calculator had not been used.
Results show that there are significant benefits to using the new calculator, with patients having less astigmatism after surgery, which leads to better distance vision. This new calculator appears to provide a better model of the optical properties of the eye. It is recommended that surgeons use such a model when planning to correct astigmatism at the time of cataract surgery.
Introduction
Approximately a third of patients presenting for cataract surgery have significant corneal astigmatism, which would benefit from treatment at the time of surgery.1 The most effective treatment in most cases is a toric intraocular lens.2 Early efforts to correct measured (anterior) corneal astigmatism met with good, but not excellent success, with 50–75% of eyes generally exhibiting 0.50D or less of refractive astigmatism post-surgery.3 Savini et al4 concluded that posterior corneal astigmatism (PCA) had the greatest impact on residual astigmatism when compared to the effects of surgically induced astigmatism (SIA), lens misalignment and effective lens position. Reitblat et al5 noted that considering PCA when performing toric intraocular lens (IOL) calculations was likely to significantly increase the percentage of patients with residual astigmatism within 0.50D. Additional work by Koch6 and Goggin7 around the same time also demonstrated the likely beneficial effects of considering PCA.
Several methods are utilized to account for PCA including using nomograms/formulas to adjust the measured anterior corneal astigmatism, or directly measuring PCA. Adjustments are typically based on a calculated adjustment coefficient,7,8 though using the ratio of anterior-to-posterior corneal power is also possible.9 Published results with these methods have shown improvement in residual astigmatism correction after toric lens implantation, resulting in an average of about 0.2 D less residual astigmatism when compared to the use of the unadjusted anterior corneal astigmatism.7–9 The majority of available calculators for toric intraocular lens cylinder power are based on an adjustment of anterior corneal power.
Directly measuring PCA is also possible with certain diagnostic devices. Some published results have shown higher percentages of eyes with 0.50D or less of refractive astigmatism postoperatively with this approach,10 but it is not in common use. Variability has been noted in the measurement of PCA.4
Historically, toric intraocular lens calculators did not consider the effects of PCA. One such calculator was the original AcrySof®/Alcon toric calculator. Results with this calculator were reported by Savini et al,11 with a mean residual refractive astigmatism using the calculator varying from 0.40 to 0.95 DC. One of the suggestions from their work was to consider PCA to improve outcomes with the calculator. Applying PCA adjustments to toric IOL calculators has been implemented, with good published results.12 A commonly used toric calculator that considers PCA using a proprietary algorithm is the Barrett toric calculator. This calculator also includes consideration of effective lens position (ELP) for appropriately adjusting IOL cylinder power to the corneal plane. While perhaps less important than the effects of PCA, consideration of ELP has been demonstrated to affect toric IOL calculations.13
Results with the Barrett toric calculator show less residual astigmatism when compared to a calculator that does not consider PCA, with an expected reduction in mean residual astigmatism of 0.16 DC.14 Abulafia et al15 compared the older Alcon toric calculator (without considering PCA) and the Barrett toric calculator and found a mean centroid error difference of about 0.50 D in predicted residual astigmatism. Results were better with the Barrett calculator. The percentage of cases with absolute errors in predicted residual astigmatism ≤0.50 D and ≤1.00 D was higher for Barrett versus the older Alcon toric calculator (45% more and 10% more, respectively).15
The purpose of the current study was to determine the distribution of the residual refraction (sphere and cylinder) in the early postoperative period (40–70 days) for eyes undergoing cataract surgery with implantation of an AcrySof® toric IOL (Alcon, Fort Worth, TX) using the online Alcon–Barrett toric IOL calculator and to compare the residual refractive cylinder achieved to back-calculated theoretical results using the older online AcrySof Toric IOL calculator that does not consider ELP or PCA.
Patients and Methods
This was a prospective non-interventional single-site, single-arm clinical trial designed to evaluate the refractive and visual outcomes associated with using a toric intraocular lens based on the Alcon–Barrett toric calculator. Results were compared to those expected if the legacy Alcon toric calculator had been used for toric IOL planning using the same preoperative information. The study was approved by an institutional review board (Sterling IRB, Atlanta, GA, USA). This study was conducted in compliance with Good Clinical Practices (GCPs) and was consistent with the 1996 version of the Declaration of Helsinki. In addition, this study adhered to all applicable local, state, and federal requirements relevant to the use of study devices and materials. As a single-arm study involving patients being treated with the site’s usual standard of care, registration with clinicaltrials.gov was not required.
Subjects had to be at least 30 years of age, have cataracts, but have otherwise good ocular health with a potential acuity of 20/25 (0.1 logMAR) or better. They also had to have regular corneal astigmatism of 0.75D to 5.00D. To limit confounding variables, patients were excluded if they had any previous corneal refractive surgery or any anterior or posterior chamber surgery; corneal pathology, macular pathology and pupil abnormalities were also exclusion criteria, as was any condition that the surgeon deemed was likely to limit postoperative visual acuity. Any subject who experienced complications during surgery would remain in the study, but their results would be removed from the outcome analysis. No vulnerable subject populations were enrolled. All eligible subjects reviewed and signed an approved informed consent.
Eligible subjects were enrolled and assessed preoperatively, at 1-day and 40–70 days postoperatively. At the preoperative visit, clinical evaluations included measurement of visual acuity, manifest refraction, and biometry with the LENSTAR LS 900® biometer (Haag-Streit, Koeniz, Switzerland). The online Alcon–Barrett Toric Calculator was used to plan the axis of placement and cylinder power for the toric IOL with a printout of the toric calculator results made available. The surgically induced astigmatism value was 0.1D with the incision placement 15 degrees counter-clockwise from temporal. Any modification of the plan provided was documented on that printout (e.g. a surgeon may have elected a slightly higher or lower cylinder correction based on the expected residual refractive astigmatism predicted). Every IOL was selected based on achieving the least amount of residual “with the rule” astigmatism, even if the online Alcon–Barrett toric IOL calculator suggested a lens with residual “against the rule”. For oblique cases, the surgeon used the lens with the least amount of expected residual astigmatism, even if it flipped the axis. The Barret Universal II calculator was used to calculate the sphere power of the IOL.
The AcrySof® toric clear lens (SA6ATx, Alcon, Fort Worth, TX) was the only lens used, implanted by both surgeons (EY and AYC) in the same manner. All patients were marked at the slit lamp at 90 and 270 degrees before surgery. The surgeon used a 2.2 mm clear corneal incision made 15 degrees counter-clockwise from temporal orientation. A femtosecond laser system (LenSx®, Alcon) was used for the 5.1 mm anterior capsulotomy and for lens fragmentation on every eye. The steep meridian for toric IOL placement was identified using a Mendez marker intraoperatively. Any subjects experiencing any intraoperative adverse event were documented and discontinued from the study; they were followed with the clinic’s usual standard of care.
The postoperative regimen was the surgeon’s usual standard of care. During the postoperative visits, visual acuity was measured at 1-day and again at 40–70 days postop. At this final postoperative visit, the manifest refraction and keratometry readings using the same biometer were recorded, along with lens orientation as determined from the slit lamp.
Data were collected on appropriate case report forms and collated in Microsoft Excel, then imported into a Microsoft Access database for data checking and preliminary analysis (both Microsoft Corp., Redmond, WA, USA). Vector math was used to calculate differences between astigmatism values. Statistical analyses were performed using the Statistica data analysis software system, version 12 (TIBCO Software Inc., Palo Alto, CA, USA). Parametric comparisons of data were made using analysis of variance (ANOVA) while non-parametric analyses were based on the Chi-squared test, with a significance level of alpha = 0.05. Sample size calculations were based on the ability to detect a 0.25D difference in refractive astigmatism between the actual results from the Barrett calculator and theoretical results back-calculated. Using an alpha of 0.05 and a power of 0.80, and presuming a standard deviation of 0.4D for the postoperative refractive astigmatism (from previous research results), a minimum of 39 eyes were required.
The primary outcome measure was mean residual refractive cylinder (RRC) achieved with the online Alcon–Barrett calculator at 40–70 days postoperative. Secondary measures included the distribution of RRC, distance visual acuity (best-corrected and uncorrected) and the manifest refraction. Finally, comparisons of actual results and those suggested by the legacy toric IOL calculator were made. For this purpose, the vector differences between the actual refractive cylinder and the expected residual cylinder from the Alcon–Barrett toric IOL calculator were calculated. This was compared to the theoretical difference between the calculated refractive cylinder based on using the toric IOL suggested by the legacy toric IOL calculator and the expected residual cylinder from that calculator (theoretical back-calculations). The “remove and replace” algorithm described by Hill et al was used for this purpose, where theoretical astigmatism is based on the actual refractive astigmatism and the vector difference between the actual and legacy IOL powers and orientations.16
Results
There were 52 eyes of 38 subjects successfully enrolled in the study; 6 eyes of 4 subjects were lost to follow-up, leaving 46 eyes of 34 subjects for analysis. There were no adverse events and no safety concerns recorded. Postoperative keratometry data were not available for 4 eyes. Preoperative demographics and biometry for the eyes analyzed are shown in Table 1. Table 2 shows the distribution of IOL cylinder power and the average sphere power for each cylinder power. There was no statistically significant difference in the sphere power by IOL cylinder power (p = 0.69).
Table 1.
Subject Demographics (n = 46 Eyes of 34 Subjects, 22 Female)
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Age (years) | 68.7 | 9.2 | 46 | 88 |
| Average keratometry (D) | 44.4 | 1.38 | 42.02 | 46.52 |
| Corneal cylinder (D) | 1.53 | 0.64 | 0.75 | 3.59 |
| Axial length (mm) | 23.7 | 1.38 | 21.5 | 28.95 |
| Lens sphere power (D) | 20.18 | 4.1 | 6 | 28.5 |
Abbreviations: D, diopter; mm, millimeter; SD, standard deviation.
Table 2.
Toric Cylinder Power
| IOL | n | Average Sphere Power (D) | Nominal Cylinder Power at Corneal Plane (D) |
|---|---|---|---|
| T3 | 13 | 19 | 1.03 |
| T4 | 14 | 20.8 | 1.55 |
| T5 | 13 | 20.6 | 2.06 |
| T6 | 6 | 20.4 | 2.57 |
Abbreviation: D, diopter.
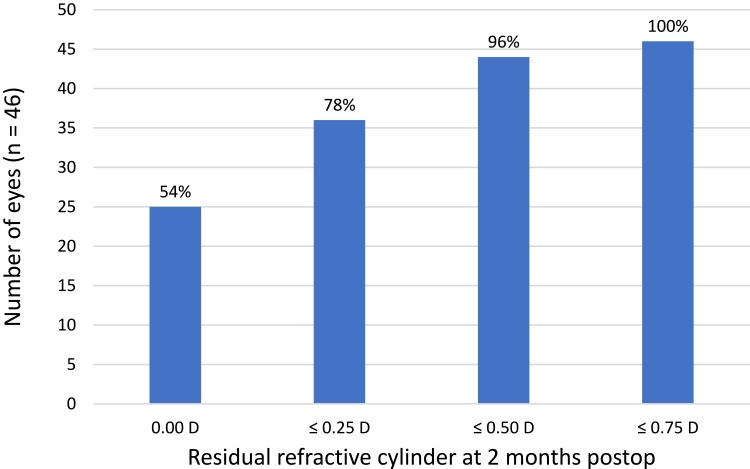
Two eyes of two subjects were targeted for monovision postoperatively, so their refraction was adjusted appropriately for analysis. All eyes (46/46) were within 0.50D of their intended spherical equivalent correction, with 85% of eyes (39/46) within 0.25D of intended. Of the 7 eyes not within 0.25D of the intended correction only two were under-corrected (manifest refraction spherical equivalent, MRSE 0.375D and 0.50D, respectively); the other 5 eyes were slightly over-corrected (MRSE −0.375D in 3 eyes and −0.50D in 2). Figure 1 shows the distribution of the actual residual refractive astigmatism achieved in this group of eyes. More than half of eyes in the study had no residual refractive cylinder, while 96% had 0.50D or less. No eye had more than 0.75D of residual refractive astigmatism.
Figure 1.
Actual residual refractive cylinder 40–70 days postoperative.
Abbreviation: D, diopter.
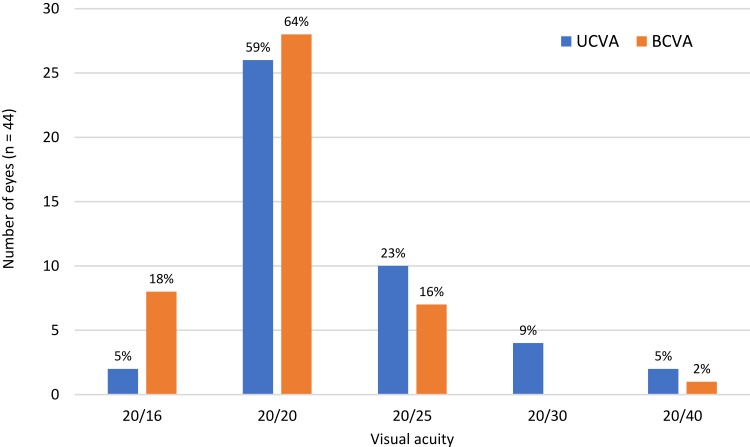
Figure 2 shows the resulting uncorrected and best-corrected distance visual acuity achieved in all eyes corrected for emmetropia (n = 44). As can be seen, 86% of eyes (38/44) had UCVA of 20/25 or better. While not shown, all but 3 eyes had a UCVA within 1 line of their BCVA (93%, 41/44). Only one eye could not be corrected to 20/25; in this eye the UCVA and BCVA were both 20/40.
Figure 2.
Postoperative visual acuity (n = 44, two monovision eyes not included).
Abbreviations: UCVA, uncorrected visual acuity; BCVA, best-corrected visual acuity.
Postoperative keratometry data were available for 42 eyes. The vector difference between the preoperative and postoperative anterior keratometry was considered a measure of the surgically induced corneal astigmatism; the mean vector magnitude was 0.43 ± 0.35D. The median value was 0.32D. Three eyes had a calculated difference of 1.0D or greater, with the highest being 1.50D. Despite those large anterior keratometry differences, two eyes had no residual refractive cylinder and one had a residual refractive cylinder of 0.50D. The magnitude of the vector centroid for all eyes was 0.03D.
Lens orientation data were available for 41 of 46 eyes at the postoperative visit; 93% of eyes (38/41) were within 5 degrees of the intended orientation, with only 3 eyes showing an orientation difference between 5 and 10 degrees. At 2 months postoperative, no eye had an orientation that was more than 10 degrees from intended.
As noted, the legacy toric IOL calculator was used to calculate the IOL cylinder power and orientation using the same biometry. Table 3 summarizes the differences in IOL cylinder power by orientation of the preoperative anterior keratometry. A lens was “lower” if the cylinder power designation of the implanted lens (based on the Alcon–Barrett calculator) was lower than for the lens suggested by the legacy calculator. For example, if the actual lens implanted was a T5 and the legacy calculator suggested a T3, the designation would be “2 higher”. In almost a third of cases, the difference between the legacy calculator and the Alcon–Barrett calculator was 2 lens steps (~1.50 D at the IOL plane, or ~1.00 D at the corneal plane).
Table 3.
Differences by Toric IOL Cylinder Power
| Power of Lens Implanted vs Legacy Calculation | n | Percent | ATR | OBL | WTR |
|---|---|---|---|---|---|
| 2 lower | 2 | 4% | 2 | ||
| 1 lower | 9 | 20% | 9 | ||
| Same | 4 | 9% | 2 | 2 | |
| 1 higher | 17 | 37% | 14 | 3 | |
| 2 higher | 14 | 30% | 13 | 1 |
Abbreviations: ATR, against the rule; OBL, oblique; WTR, with the rule.
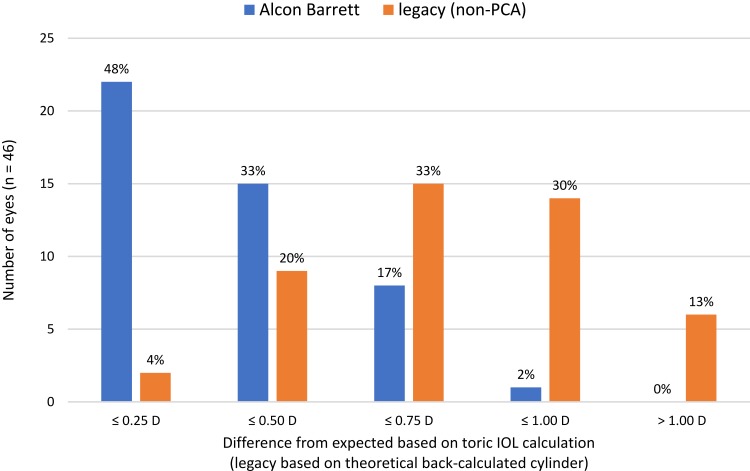
Figure 3 shows a histogram of the magnitude of the vector difference between the actual residual refractive astigmatism and the expected astigmatism from the Alcon–Barrett calculator. Also shown is the theoretical vector difference magnitude based on the IOL suggested by the legacy calculator (the theoretical back-calculation). The vector difference magnitude was significantly lower with the Alcon–Barrett (actual) suggested IOL relative to the theoretical result with the IOL suggested by the legacy (non-PCA) calculator (p < 0.001).
Figure 3.
Difference from expected for actual and theoretical (legacy) toric IOL calculations.
Abbreviation: D, diopter.
Discussion
The differences we found between the legacy calculator and the Alcon–Barrett calculator were larger than expected, with 34% of eyes having a calculated difference of 2 lens powers; this is without consideration of additional orientation effects. The differences are likely a function of PCA, ELP and the conservative nature of the legacy calculator, where the suggested lens never “flipped” the axis, even if the expected residual astigmatism would have been lower. However, the results in Figure 3 include consideration of the expected residual astigmatism from the two calculators, which compensates for that last factor. Differences seen in Figure 3 are therefore likely a function only of PCA and ELP.
Residual refractive cylinder results in the current study appear consistent with or slightly better than those achieved using toric IOL calculators that consider PCA.12,17,18 They are significantly better than those reported for earlier legacy calculators.3 The differences between the expected errors from the legacy calculator and the Alcon–Barrett calculator also appear consistent with findings from previous studies.6,12,15,19
The differences in the IOL cylinder powers calculated with the two different calculators are also consistent with reported findings in the past. It is expected that, because of the nature of PCA, with-the-rule astigmatism would be over-corrected and against-the-rule astigmatism would be under-corrected if it was not taken into account.7 Table 3 shows that considering PCA did lower the suggested cylinder power of the toric IOL in eyes with with-the-rule astigmatism and raised it in eyes with against-the-rule astigmatism. The authors were surprised to find that in more than a third of cases the difference in IOL power was two “step” increments, or about 1.00 D at the corneal plane.
As an alternative to using calculators that consider PCA in some fashion (through a proprietary algorithm such as the Alcon–Barrett calculator, or with a regression formula such as the Abulafia–Koch formula20), direct measurement of the total corneal astigmatism might be considered. Reported results have been good,21 though they have been reported to be variable, suggesting that the use of direct measurement may depend on improving the consistency of such measurements.22
There are several things of note related to SIA in the results. First is that the mean vector magnitude is significantly higher than the magnitude of the vector centroid. This has been noted in the past and it is important when surgeons are calculating their own SIA values; the magnitude of the vector centroid is the measure of interest. The vector centroid here was near-zero, and not much different than the 0.10D used for toric IOL planning purposes. It is also interesting to note that the only 3 eyes with a calculated SIA greater than 1.0D all had final lens orientations within 5 degrees of intended and a refractive cylinder of 0.50D or lower. This would seem to indicate that the SIA used (0.1D) in the calculations was appropriate, even in these eyes. The large SIA calculated in these eyes may have been due to measurement error (e.g. keratometric variability from dry eye),23 or it may be that anterior corneal keratometry does not adequately reflect the “true” SIA of the eye. Recent efforts to refine SIA calculation based on preoperative corneal astigmatism and postoperative refractive astigmatism have shown promise in this regard.24
There are limitations to the current study. While the sample size was calculated to be sufficient to demonstrate the differences between toric IOL calculations, larger sample sizes are always helpful. Since the Alcon–Barrett toric calculator was used for all eyes, there was no control eye or randomization present. However, with the evidence largely in favor of newer toric calculators, there was no ethical way to create a case–control study. One advantage of the study was its prospective nature; a high percentage of articles addressing this issue involve retrospective chart reviews.
Conclusion
In summary, the use of the Alcon–Barrett toric calculator, which includes consideration of PCA and ELP, produced a very high percentage of eyes with 0.5D or less of residual refractive astigmatism after surgery. Use of calculators that consider PCA is highly recommended.
Acknowledgments
Sarah Y. Makari, OD is a consultant to Science in Vision who received compensation for writing assistance in preparation of the manuscript. This study was supported by an investigator-initiated study grant from Alcon, Fort Worth, TX (IIT #33192747).
Disclosure
Dr. Elizabeth Yeu is a consultant to Alcon, Bausch + Lomb, iOptics, Johnson & Johnson Vision, LensAR, Mynosys, TopCon and Carl Zeiss Meditec. Dr. Albert Cheung is a consultant to Alcon. Dr. Richard Potvin is a consultant to Alcon. All authors report grants from Alcon, outside of the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Hoffmann PC, Hütz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg. 2010;36(9):1479–1485. doi: 10.1016/j.jcrs.2010.02.025 [DOI] [PubMed] [Google Scholar]
- 2.Kessel L, Andresen J, Tendal B, Erngaard D, Flesner P, Hjortdal J. Toric intraocular lenses in the correction of astigmatism during cataract surgery: a systematic review and meta-analysis. Ophthalmology. 2016;123(2):275–286. doi: 10.1016/j.ophtha.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 3.Visser N, Bauer NJ, Nuijts RM. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39(4):624–637. doi: 10.1016/j.jcrs.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 4.Savini G, Næser K. An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest Ophthalmol Vis Sci. 2015;56(2):827–835. doi: 10.1167/iovs.14-15903 [DOI] [PubMed] [Google Scholar]
- 5.Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: comparison of methodologies. J Cataract Refract Surg. 2016;42(2):217–225. doi: 10.1016/j.jcrs.2015.11.036 [DOI] [PubMed] [Google Scholar]
- 6.Koch DD, Jenkins RB, Weikert MP, Yeu E, Wang L. Correcting astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J Cataract Refract Surg. 2013;39(12):1803–1809. doi: 10.1016/j.jcrs.2013.06.027 [DOI] [PubMed] [Google Scholar]
- 7.Goggin M, Zamora-alejo K, Esterman A, van Zyl L. Adjustment of anterior corneal astigmatism values to incorporate the likely effect of posterior corneal curvature for toric intraocular lens calculation. J Refract Surg. 2015;31(2):98–102. doi: 10.3928/1081597X-20150122-04 [DOI] [PubMed] [Google Scholar]
- 8.Goggin M, van Zyl L, Caputo S, Esterman A. Outcome of adjustment for posterior corneal curvature in toric intraocular lens calculation and selection. J Cataract Refract Surg. 2016;42(10):1441–1448. doi: 10.1016/j.jcrs.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 9.Eom Y, Rhim JW, Kang SY, Kim SW, Song JS, Kim HM. Toric intraocular lens calculations using ratio of anterior to posterior corneal cylinder power. Am J Ophthalmol. 2015;160(4):717–724. doi: 10.1016/j.ajo.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 10.Davison JA, Potvin R. Refractive cylinder outcomes after calculating toric intraocular lens cylinder power using total corneal refractive power. Clin Ophthalmol. 2015;9:1511–1517. doi: 10.2147/OPTH.S88693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savini G, Hoffer KJ, Ducoli P. A new slant on toric intraocular lens power calculation. J Refract Surg. 2013;29(5):348–354. doi: 10.3928/1081597X-20130415-06 [DOI] [PubMed] [Google Scholar]
- 12.Canovas C, Alarcon A, Rosén R, et al. New algorithm for toric intraocular lens power calculation considering the posterior corneal astigmatism. J Cataract Refract Surg. 2018;44(2):168–174. doi: 10.1016/j.jcrs.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 13.Goggin M, Moore S, Esterman A. Outcome of toric intraocular lens implantation after adjusting for anterior chamber depth and intraocular lens sphere equivalent power effects. Arch Ophthalmol. 2011;129(8):998–1003. doi: 10.1001/archophthalmol.2011.188 [DOI] [PubMed] [Google Scholar]
- 14.Kern C, Kortüm K, Müller M, Kampik A, Priglinger S, Mayer WJ. Comparison of two toric IOL calculation methods. J Ophthalmol. 2018;2018:2840246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abulafia A, Hill WE, Franchina M, Barrett GD. Comparison of methods to predict residual astigmatism after intraocular lens implantation. J Refract Surg. 2015;31(10):699–707. doi: 10.3928/1081597X-20150928-03 [DOI] [PubMed] [Google Scholar]
- 16.Hill W, Osher R, Cooke D, et al. Simulation of toric intraocular lens results: manual keratometry versus dual-zone automated keratometry from an integrated biometer. J Cataract Refract Surg. 2011;37(12):2181–2187. doi: 10.1016/j.jcrs.2011.06.028 [DOI] [PubMed] [Google Scholar]
- 17.Solomon KD, Sandoval HP, Potvin R. Correcting astigmatism at the time of cataract surgery: toric IOLs and corneal relaxing incisions planned with an image-guidance system and intraoperative aberrometer versus manual planning and surgery. J Cataract Refract Surg. 2019;45(5):569–575. doi: 10.1016/j.jcrs.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 18.Gundersen KG, Potvin R. Clinical outcomes with toric intraocular lenses planned using an optical low coherence reflectometry ocular biometer with a new toric calculator. Clin Ophthalmol. 2016;10:2141–2147. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanavaty MA, Teeluck K, Bardan AS, Bedi KK, Ali S. Residual refractive astigmatism following toric intraocular lens implantation without consideration of posterior corneal astigmatism during cataract surgery with low anterior keratometric astigmatism up to 2.5 dioptres. Curr Eye Res. 2019;44(12):1399–1406. doi: 10.1080/02713683.2019.1638418 [DOI] [PubMed] [Google Scholar]
- 20.Abulafia A, Koch DD, Wang L, et al. New regression formula for toric intraocular lens calculations. J Cataract Refract Surg. 2016;42(5):663–671. doi: 10.1016/j.jcrs.2016.02.038 [DOI] [PubMed] [Google Scholar]
- 21.Park DY, Lim DH, Hwang S, Hyun J, Chung TY. Comparison of astigmatism prediction error taken with the Pentacam measurements, Baylor nomogram, and Barrett formula for toric intraocular lens implantation. BMC Ophthalmol. 2017;17(1):156. doi: 10.1186/s12886-017-0550-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira TB, Ribeiro P, Ribeiro FJ, O’neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017;33(12):794–800. doi: 10.3928/1081597X-20171004-03 [DOI] [PubMed] [Google Scholar]
- 23.Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672–1677. doi: 10.1016/j.jcrs.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 24.Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272–283. doi: 10.1016/j.jcrs.2018.09.028 [DOI] [PubMed] [Google Scholar]