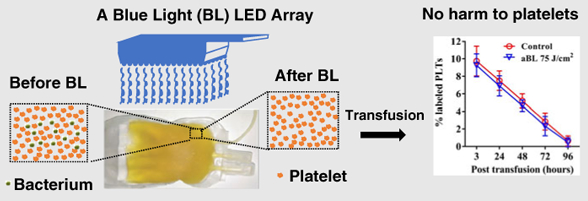
Abstract
Platelet (PLT) storage is currently limited to 5 days in clinics in the United States, in part, due to an increasing risk for microbial contamination over time. In light of well-documented antimicrobial activity of blue light (405–470 nm), we investigated potentials to decontaminate microbes during PLT storage by antimicrobial blue light (aBL). We found that PLTs produced no detectable levels of porphyrins or their derivatives, the chromophores that specifically absorb blue light, in marked contrast to microbes that generated porphyrins abundantly. The difference formed a basis with which aBL selectively inactivated contaminated microbes prior to and during the storage, without incurring any harm to PLTs. In accordance with this, when contamination with representative microbes was simulated in PLT concentrates supplemented with 65% of PLT additive solution in a standard storage bag, all “contaminated” microbes tested were completely inactivated after exposure of the bag to 405 nm aBL at 75 J/cm2 only once. While killing microbes efficiently, this dose of aBL irradiation exerted no adverse effects on the viability, activation or aggregation of PLTs ex vivo and could be used repeatedly during PLT storage. PLT survival in vivo was also unaltered by aBL irradiation after infusion of aBL-irradiated mouse PLTs into mice. The study provides proof-of-concept evidence for a potential of aBL to decontaminate PLTs during storage.
Keywords: activation, antimicrobial blue light, decontamination, function and survival, platelet storage
Graphical Abstract
1 |. INTRODUCTION
Platelet (PLT) transfusion is a critical first-line therapy for severe hemorrhages and the mainstay management of inherited and acquired thrombocytopenia of a variety of etiology [1]. Bacterial contamination of PLT concentrates during storage poses the greatest risk of bacterial sepsis and fatality in modern transfusion medicine due to unique storage conditions required for PLTs [2, 3]. While most of human blood products can be stored in refrigerators or freezers where microbial contamination can be well under control [4, 5], PLTs are uniquely stored at room temperature (20°C-24°C) with continuous agitation around 60 rpm/minute so as to sustain their survival and functionality [6–9]. However, room temperature favors the growth of various microorganisms, which is one of the main reasons to limit PLT storage for 5 days only in the United States, although PLTs are permitted to store for up to 7 days in the United Kingdom and Canada with pathogen inactivation technologies or in the United States in a 7-day container with bacterial detection devices approved by FDA [10].
Currently, light-based antimicrobial approaches to prevent from transfusion-associated bacterial sepsis include UVC irradiation and antimicrobial photodynamic inactivation. UVC irradiation can protect PLT concentrates from microbial contamination [2, 11, 12], which however adversely affects the function and viability of PLTs, as suggested by a decrease of ATP production, an increased expression of PLT surface marker P-selectin and disruption in S–S bonds of the integrin αIIbβ3 (GPIIb/IIIa) presumably because PLT proteins and mitochondrial nucleic acids can absorb UVC [11, 13–15]. Unlike UVC irradiation that does not alter the constituent of PLT concentrates directly, photodynamic inactivation of microorganisms requires exogenous photosensitizers, such as amotosalen, methylene blue and riboflavin. These photosensitizers can be excited by either UV light or visible light and lead to damages of the nucleic acids, thus preventing bacteria from growth [13, 16, 17]. Among these pathogen reduction technologies, only INTERCEPT Blood System (Cerus Corporation, Concord) was approved by FDA in the United States in 2014 and in Europe in 2002. The Mirasol Pathogen Reduction Technology system (Terumo BCT, Lakewood, Colorado) is now in phase III clinical trials in the United States, although it is approved in some European countries [16, 18, 19].
aBL represents a novel light-based approach and has attracted considerable attention lately due to its intrinsic antimicrobial effects without need of any exogenous compounds or photosensitizers [20–22]. The mechanism underlying the antimicrobial activity lies upon the ability of aBL to excite endogenous porphyrins that are abundantly generated by bacteria and fungi over mammalian cells [22]. aBL-mediated excitation of endogenous porphyrins resulted in the production of cytotoxic reactive oxygen species (ROS) that could be confined within the microbes following generation owing to their short-life and cell membrane impermeability [20,21]. aBL has been used clinically to treat jaundice in new-born infants for more than three decades with a long safety record [23] and recently for managing inflammatory acne owing its superior safety relative to UV irradiation [24–26]. However, a potential for aBL for inactivation of contaminated bacteria and fungi in PLT concentrates has not been explored so far to the best of our knowledge. Here, we report that aBL sufficiently inactivates five representative bacteria and one fungus frequently in association with PLT concentrate contamination, while not adversely affecting the viability, activation and aggregation of PLTs ex vivo and their in vivo survival after transfusion into mice.
2 |. RESULTS
2.1 |. No detectable porphyrins in human PLTs
Endogenous porphyrins were investigated in human PLTs, MRSA USA300, Pseudomonas aeruginosa ATCC 19660 and Candida albicans CEC 749 by fluorescence spectrometer and high-performance liquid chromatography (HPLC) (Figure 1). The characteristic fluorescence emission spectra of PLT and microbe lysates were obtained, along with reference hematoporphyrin in Figure 1A. The absorbance peaks of USA 300, P aeruginosa ATCC 19660 and C albicans CEC 749 samples appeared at 622, 628 and 624 nm, respectively, characteristic for the typical fluorescence emission porphyrins at an excitation of 405 nm [20,21]. In parallel, hematoporphyrin absorption peaked similarly at 624 nm. To our surprise, there was no absorption peak of porphyrins in PLT lysates. Apart from absorbance, principal emissions peaked at 7.0, 11.1, 18.5, 20.2 and 21.0 minutes (red arrows) for USA 300, 15.8 and 22.5 minutes (green arrows) for P aeruginosa ATCC 19660 and 12.9, 18.5, 20.2 and 21.0 minutes (orange arrows) for albicans CEC 749, respectively, as revealed by HPLC (Figure 1B). Once again, we did not observe any emission peak of porphyrins when PLT lysates were analyzed by HPLC in the parallel study (Figure 1B). The results suggest that multiple chromophores that can absorb 405 nm light are presented in these microbes, but no such chromophores are presented in human PLTs, making it possible to selectively inactivate contaminated bacteria and fungus by aBL without any harm to PLTs.
FIGURE 1.
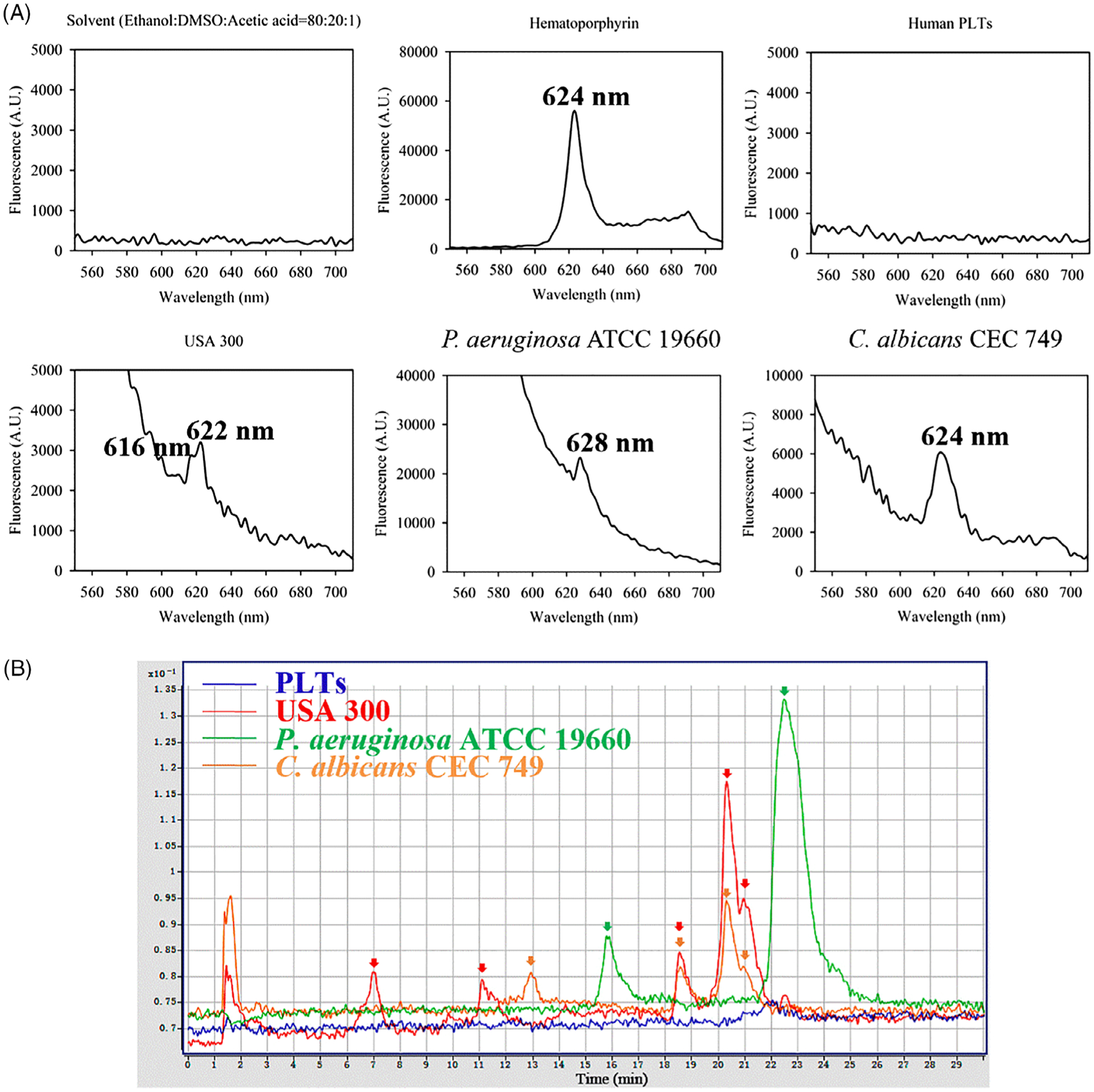
Determination of intracellular porphyrins in platelet (PLT) cells and representative microbes by fluorescence spectrometer (A) and high-performance liquid chromatography (HPLC) (B). A, Fluorescence emission spectra of solvent, positive control (hematoporphyrin), human PLT lysate and lysates of USA 300, Pseudomonas aeruginosa ATCC 19660 and Candida albicans CEC 749 at an excitation wavelength 405 nm and an emission width ranging from 560 to 700 nm. B, HPLC chromatograms of human PLT lysate (blue line) and lysates of USA 300 (red line), P aeruginosa ATCC 19660 (green line) and C albicans CEC 749 (orange line). Excitation wavelength was 405 nm, and emission wavelengths were 590, 610 and 630 nm
2.2 |. Microbes are more susceptible to 405 nm aBL than 470 nm
In an attempt to define a more sensitive aBL, we compared antimicrobial activities of 405 and 470 nm aBL irradiation against Bacillus cereus ATCC 14579, Staphylococcus epidermidis ATCC 35985, Streptococcus pyogenes, USA 300, P aeruginosa ATCC 19660 and C albicans CEC 749 in PLT additive solution (PAS), which represent frequently contaminated Gram-positive bacteria, Gram-negative bacteria and fungus [27–29]. As shown in Figure 2, all six microbes were more susceptible to 405 nm aBL irradiation than 470 nm and more than 2-log10 reductions of CFU/mL (99% inactivation) were achieved for all the microbial strains by 405 nm aBL irradiation at a fluence of 50 J/cm2. Among them, S pyogenes and P aeruginosa ATCC 19660 were most susceptible to 405 nm aBL and as high as 5-log10 CFU/mL bacteria could be completely eliminated by 405 nm aBL irradiation at 50 J/cm2. Although 470 nm aBL also killed the bacteria sufficiently, it was significantly inferior to 405 nm aBL.
FIGURE 2.
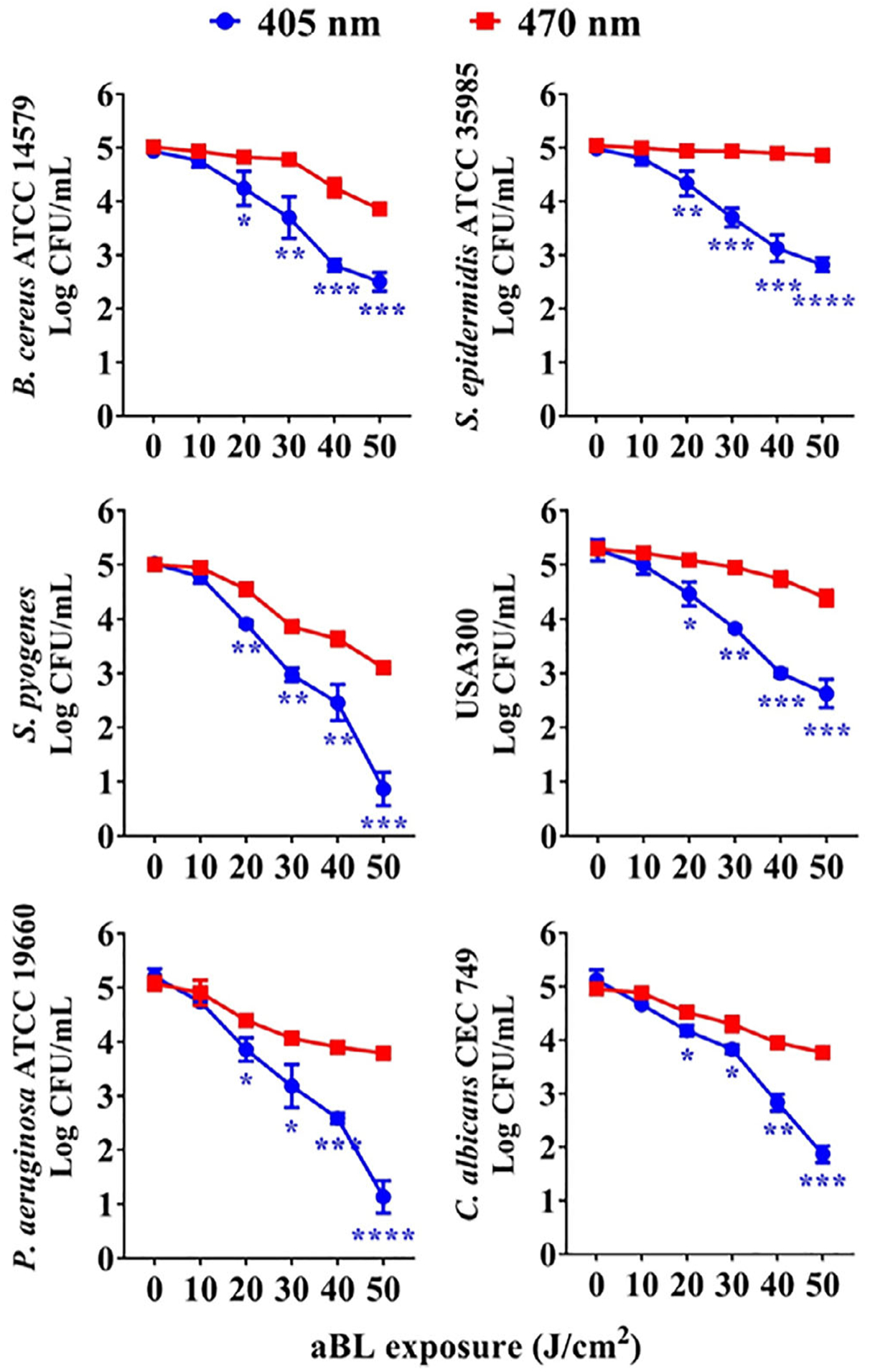
Comparison of antimicrobial activity of antimicrobial blue light (aBL) irradiation at 405 () and 470 nm (). Bacillus cereus ATCC 14579, Staphylococcus epidermidis ATCC 35985, Streptococcus pyogenes, USA 300, Pseudomonas aeruginosa ATCC 19660 and Candida albicans CEC 749 suspended in PLT additive solution (PAS) were illuminated with aBL at indicated fluences, after which colony-forming unit (CFU) was determined and expressed as mean ± SEM log CFU/mL, n = 5. *P < .05, **P < .01, ***P < .001 and ****P < .0001 between 405 and 470 nm aBL
2.3 |. aBL effectively inactivates bacteria and fungus in human PLT concentrates
Antimicrobial efficacies of 405 nm aBL irradiation against B cereus ATCC 14579, S epidermidis ATCC 35985, S pyogenes, USA 300, P aeruginosa ATCC 19660 and C albicans CEC 749 were next validated in human PLT concentrates supplemented with 65% PAS and stored in a standard PLT storage condition. All six microorganisms were inactivated in stored PLT concentrates after exposure of the bags to aBL irradiation at 75 J/cm2, and no microbial growth was seen after storage of the irradiated and “contaminated” PLT concentrates for 5 days. The bactericidal effects of aBL irradiation against all six microorganisms in PLT concentrates were closely correlated with those attained in PAS alone, with S pyogenes and P aeruginosa ATCC 19660 most susceptible to 405 nm aBL irradiation (Figure 3B). These two bacterial strains were eradicated completely by aBL irradiation at 50 J/cm2 as evidenced with no any bacterial growth in 5 days of storage after aBL exposure.
FIGURE 3.
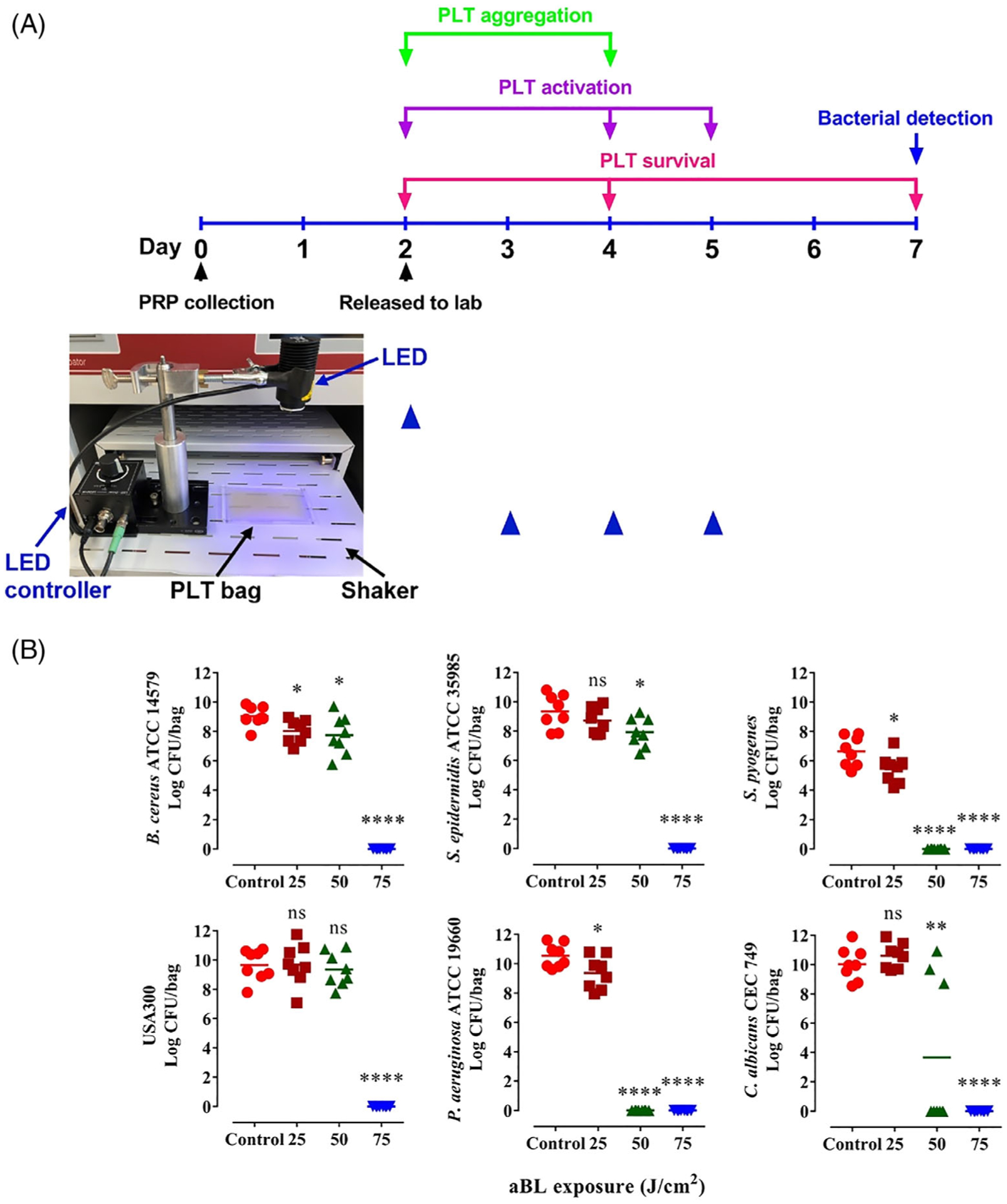
Depict of time lines of antimicrobial blue light (aBL) irradiation (A) and evaluation of its effects on platelets (PLTs) (B). A, The 2-day-old human PLT concentrates were irradiated with sham or 405 nm aBL once during storage or once a day for three consecutive days (from day 3 to day 5 after PLT collection) (blue triangle). The light-emitting diode (LED) was held firmly by a clamp on a support stand, and the distance between the LED and the PLT storage bag was fixed to obtain 55 mW/cm2 for the experiments (inset photo). The PLTs were irradiated at indicated times controlled by a LED controller while gently shaking on the shaker at 60 to 70 rpm. Residual bacteria in the PLT concentrates were determined on day 5 after aBL irradiation (day 7 after PLT collection). PLTs were counted to determine viability in 2 hours (day 2 after PLT collection), 2 days (day 4 after PLT collection) and 5 days (day 7 after PLT collection) after sham or aBL irradiation (red). PLT activations were analyzed in 2 hours (day 2 after PLT collection), 2 days (day 4 after PLT collection) and 3 days (day 5 after PLT collection) after sham or aBL irradiation (purple). PLT aggregations were assayed in 2 hours (day 2 after PLT collection) and 2 days (day 4 after PLT collection) (green). B, Bacillus cereus ATCC 14579, Staphylococcus epidermidis ATCC 35985, Streptococcus pyogenes, USA 300, Pseudomonas aeruginosa ATCC 19660 or Candida albicans CEC 749 was cultured overnight and added to PLT storage bags at 100 CFU/bag containing 3 mL PLT concentrates supplemented with 65% PLT additive solution (PAS). The bags each were exposed to sham light (●) or 405 nm aBL at 25 (■), 50 (▲) or 75 (▼) J/cm2 and then stored in a standard condition for 5 days. The number of residual bacteria in each bag was determined on day 5 after irradiation as Figure 2, n = 8. *P < .05, **P < .01 and ****P < .0001 in the presence or absence of aBL. ns, no significance
2.4 |. aBL does not affect PLT survival, activation or aggregation
The 2-day-old human PLT concentrates supplemented with 65% PAS at a ratio 35%:65% (vol/vol) of PLT concentrate to PAS in a storage bag at 3 mL/bag were irradiated by 405 nm aBL at a fluence of 25, 50 or 75 J/cm2, and then stored for 2 hours, 2 days or 5 days. There were no significant differences on PLT counts up to 5 days of storage, as measured by an ABX Hematology Analyzer, between sham and aBL irradiated-PLTs irrespective of aBL fluences employed (Figure 4). The results indicate that 405 nm aBL irradiation at 75 J/cm2 has no adverse effect on PLT survival, in good agreement with no porphyrins in PLTs.
FIGURE 4.
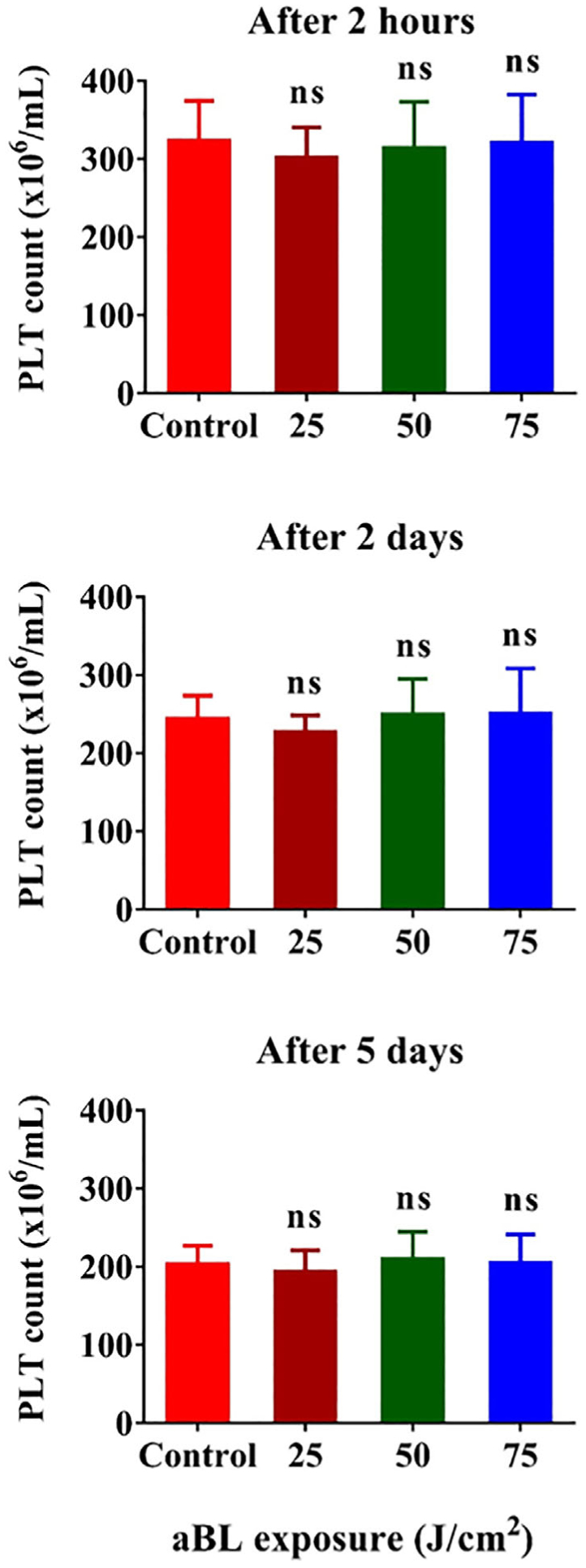
Effects of 405 nm antimicrobial blue light (aBL) irradiation on platelet (PLT) survival in vitro. The PLT concentrate was irradiated with aBL at 25, 50 or 75 J/cm2, respectively. PLT counts were determined in 2 hours or 2 or 5 days after aBL irradiation (red) as depicted in Figure 3A. Data represent mean ± SEM, n = 6. ns, no significance between sham- and aBL-treated samples
We went on to evaluate PLT activations with surface marker CD62P expression in the presence or absence of agonists thrombin receptor activator-6 (TRAP-6) or thrombin and expression of activated GPIIb/IIIa with or without agonist ADP. All agonists activated PLTs and vigorously increased the expression of CD62P or activated GPIIb/IIIa on the surface of PLTs whether the PLTs were examined in 2 hours, 2 days or 3 days after aBL irradiation (Figure 5). However, there were no differences in the expression of CD62P or activated GPIIb/IIIa in the presence or absence of 405 nm aBL irradiation at 75 J/cm2 (Figure 5A–C), even after three aBL treatments (Figure 5D). It was clear that aBL irradiation did not activate PLTs or interfere with agonist-mediated activation of PLTs. Likewise, when PLTs were stored for 2 hours (Figure 6A) or 2 days (Figure 6B) after 405 nm aBL irradiation at 75 J/cm2, their aggregation function remained intact when stimulated with phorbol myristate acetate (PMA), as suggested by overlapping aggregation curves of PLTs in the presence or absence of aBL irradiation. Flow cytometric analysis of increasing double-colored PLT aggregates over time is commonly used to determine clotting functions of PLT ex vivo [30]. The overlapping aggregation curves of PLTs in the presence or absence of aBL irradiation prior storage are consistent with little effect of the irradiance on PLT activation shown above, corroborating that aBL exerts no any significant adverse effect on PLT functions.
FIGURE 5.
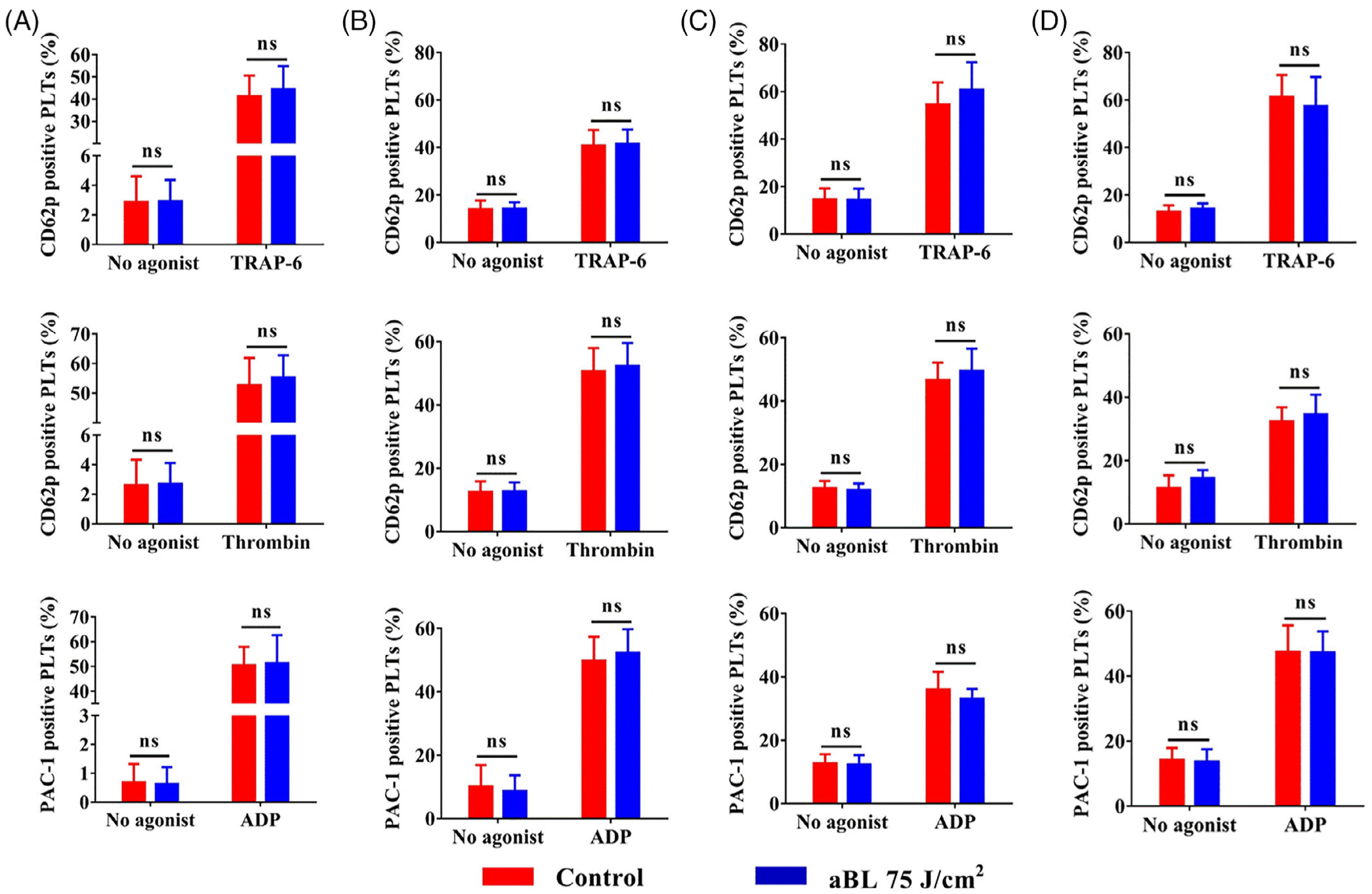
Effects of 405 nm antimicrobial blue light (aBL) on platelet (PLT) activation in the absence or presence of agonists. PLT concentrates were irradiated with sham or 405 nm aBL at 75 J/cm2 once and then evaluated for PLT activations in 2 hours (A), 2 days (B) or 3 days(C) after irradiation. Alternatively, the PLT concentrate was irradiated once a day for three consecutive days and determined for PLT activation in2 hours after the final irradiation (D). Percentages of PLTs positive for CD62P or GPIIb/IIIa expression in the absence or presence of an indicated agonist were evaluated by flow cytometry. The agonists tested were TRAP-6 at 10 μM, thrombin at 2 U/mL or ADP at 50 μM. Data represent mean ± SEM of six independent experiments with each assayed in triplicate. ns, no significance between sham- and aBL-treated samples
FIGURE 6.
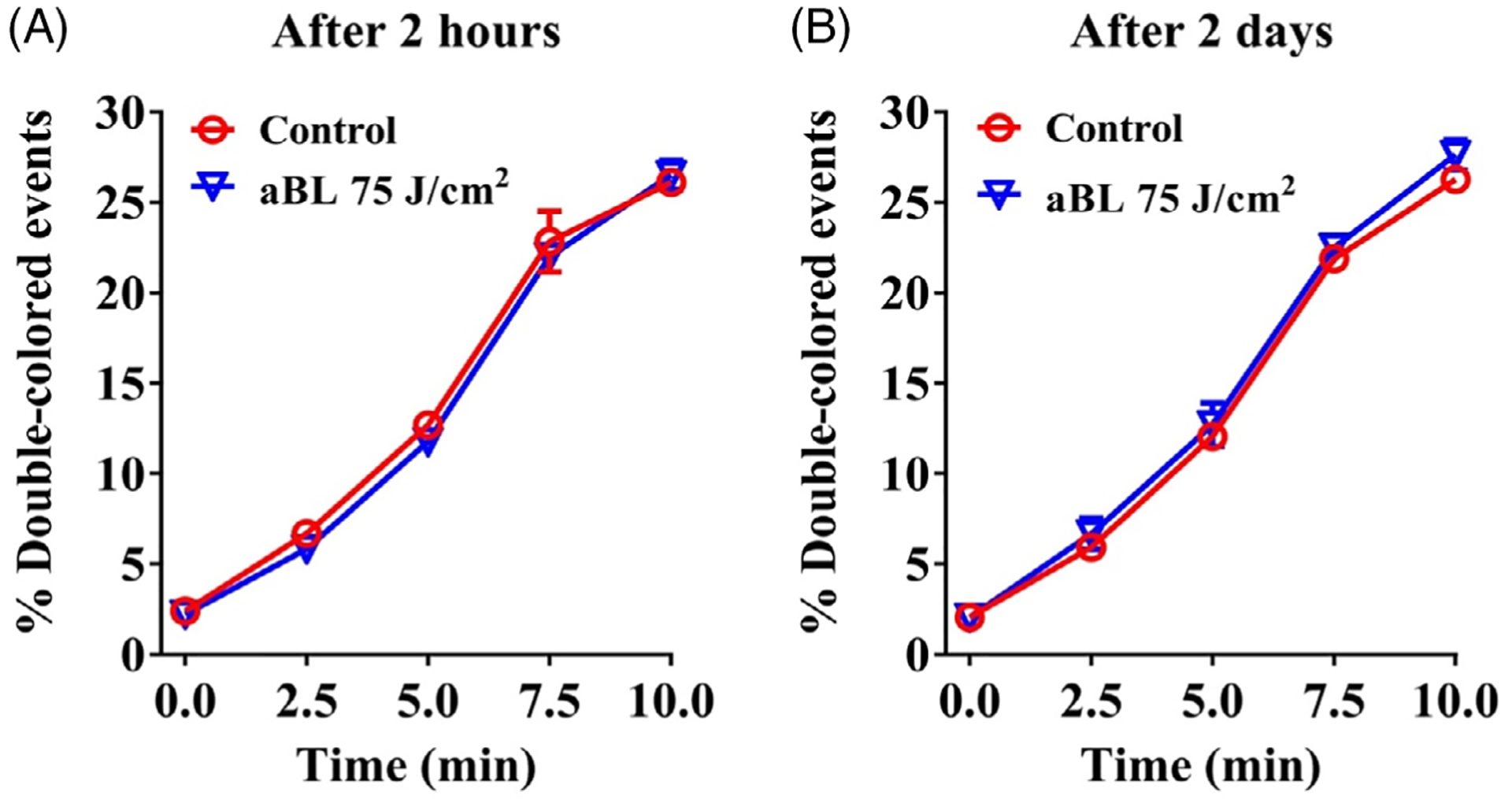
Effects of 405 nm antimicrobial blue light (aBL) irradiation on platelet (PLT) aggregation. PLTs were treated with sham or405 nm aBL at 75 J/cm2, and then stored for2 hours (A) or 2 days (B). PLTs were then divided into two groups, labeled with either PE-CD31 or APC-CD9, and mixed at a 1:1 ratio before stimulation. Aggregation was measured by flow cytometry over time after stimulation with PMA at 100 ng/mL. Double-colored events were quantified and presented as mean percentages ± SEM of six independent experiments with each assayed in triplicate
2.5 |. Similar recovery of mouse PLTs after infusion irrespective of 405 nm aBL irradiation
At last, freshly prepared mouse PLTs were treated with sham or 405 nm aBL at 75 J/cm2 and stored for 2 hours or 5 days in a standard storage condition. At indicated times of storage, the PLTs were fluorescently stained and infused into mice to determine their recovery in vivo because there are significant differences in the recovery of PLTs between in vivo and ex vivo, a lesson learnt from PLTs stored in 4°C [7, 10]. Infused PLTs declined in number over time and reached a lowest level in 96 hours for PLTs stored for 2 hours after aBL irradiation (Figure 7A,B) or 48 hours for 5-day-storaged PLTs (Figure 7C,D). The recovery of donor PLTs was however comparable in the presence or absence of aBL irradiation at 75 J/cm2, irrespective of whether the PLTs were assayed in 2 hours (Figure 7B) or 5 days of storage (Figure 7D) after aBL exposure. Representative flow cytometric profiles were presented in Figure 7A,C showing decreased donor PLTs that were labeled with calcein, a vital green fluorescent dye, relative to a total number of PLTs marked by anti-CD41 staining in the blood samples taken at indicated times after infusion.
FIGURE 7.
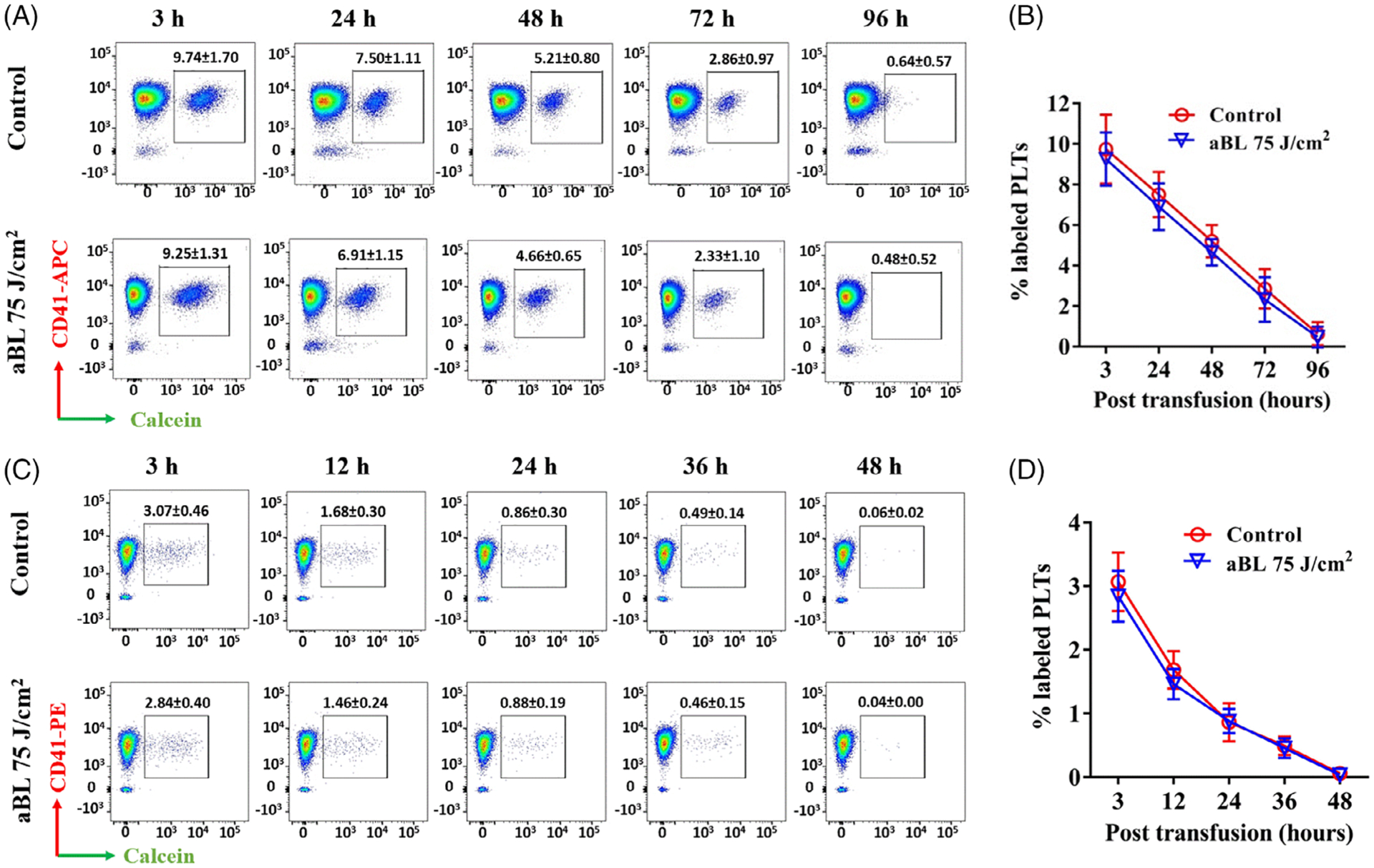
Antimicrobial blue light (aBL) did not affect recovery of mouse platelets (PLTs) following transfusion into mice. Concentrated PLTs were freshly prepared from pooled mouse blood samples, treated with sham or 405 nm aBL at 75 J/cm2 and stored for 2 hours (A and B) or5 days (C and D), after which the PLTs were labeled with calcein and intravenously infused into recipient mice. Blood was drawn at indicated times after infusion in alternative mice, labeled with anti-CD41 and analyzed by flow cytometry. A and C are representative dot plots of 2-hour- (A) and 5-day-storaged (C) PLTs from recipients labeled with calcein and CD41 at different time points after transfusion. B and D are changes of percentage double-labeled events in sham- and aBL irradiated-PLTs at indicated times after 2 hours (B) or 5 days (D) of storage. Results are shown as percent double-colored PLTs ± SEM with five mice in each time point
3 |. DISCUSSION
For the past few years, bacterial sepsis as a consequence of PLT concentrate contamination has been the greatest risk of transfusion-transmitted infections in the United States [31]. A great deal of effort has been made to decontaminate PLT concentrates during storage, including UV light-based INTERCEPT, MIRASOL and THERAFLEX. INTERCEPT and MIRASOL both require an exogenous photoactive sub-stance such as amotosalen or riboflavin in combination with UV light to inactivate pathogens, whereas UVC can directly kill contaminated microorganisms in THERAFLEX [32, 33]. While photosensitizers required for INTERCEPT and MIRASOL may complicate in PLT transfusion, UVC-based reduction of pathogens shows some adverse effects on PLT activation and in vivo recovery because UVC can be absorbed by proteins and mitochondrial DNA of PLTs [2, 11, 14]. We present here a potential of aBL-based approach for pathogen inactivation in PLT concentrates during storage, which does not require any additives and would not impose any adverse effects on the survival and functions of PLTs. aBL irradiation at 405 nm inactivates bacteria or fungi by exciting endogenous porphyrins within bacteria and generation of ROS resulting in the lethal oxidative damage to the microbes [22]. In contrast, PLTs do not produce porphyrins or their derivatives and are not sensitive to aBL, and thus, aBL can be potentially used repeatedly during PLT storage to minimize bacterial sepsis of PLT transfusion. In marked contrast, UVC can be applied to a PLT storage bag only once owing to its adverse effects on PLT activation and survival [2, 14]. However, whether aBL can be effective in viral decontamination of PLTs remains to be determined, although antiviral activity of 405 nm aBL has been shown [34–36]. aBL was also shown to be sufficient in bacterial decontamination of ex vivo stored plasma [37], but it may not be suitable for decontaminating full-size blood components or red blood cells because red blood cells contain hemoglobin that can absorb blue light and may generate significant heat upon aBL.
A number of studies have demonstrated the presence of endogenous porphyrins in clinical pathogens such as Gram-negative strains Acinetobacter baumannii [20, 21], Helicobacter pylori [38], P aeruginosa [21], Porphyromonas gingivalis [39] and Prevotella intermedia[39], Gram-positive strains MRSA [21, 40] and Propionibacterium acnes [41] and fungus C albicans [42]. In accordance with these investigations, we also found endogenous porphyrins in the strains of USA 300, P aeruginosa ATCC 19660 and C albicans CEC 749 by fluorescence spectrometer and HPLC. Porphyrins are believed necessary for the majority of pathogens to utilize heme as an iron source that is essential for survival and pathogenesis of these bacteria and fungi [43, 44], which explains the wide antimicrobial activities of blue laser spectrum (405–470 nm) [22, 45–47]. The abundance of porphyrins generated in microbial cells is the reason behind the more susceptibility of all six representative microbes to 405 nm aBL irradiation than 470 nm, in agreement with the previous study showing that foodborne pathogens Lactobacillus plantarum, S aureus and Vibrio parahaemolyticus were all more susceptible to 405 nm than 460 nm or 520 nm light irradiation [48]. Apart from the six microbes tested, Klebsiella pneumoniae and Escherichia coli were also commonly detected in contaminated PLT concentrates [49] and particularly sensitive to 405 nm aBL reported in the previous studies [45, 46, 50, 51].
PLT activation is one of the key adverse events associated with PLT storage, which can compromise their clotting and retraction function initiated by agonists in vivo. Our investigation demonstrates that aBL does not activate PLTs in the absence of an agonist or reduce their activation in the presence of a specific agonist. The expressions of two PLT activation biomarkers, CD62P and GPIIb/IIIa, were not affected by aBL irradiation at 75 J/cm2 [52, 53]. On the contrary, INTERCEPT or MIRASOL treatments have been shown to increase CD62P expression on PLTs [2, 16]. Likewise, UVC treatment elevated the expression of GPIIb/IIIa on PLTs owing to a reduction of the disulfide bonds of the receptors [14]. No aBL-mediated PLT activation was also corroborated by unaltered PLT aggregation that is directly associated with clotting function and retraction of stored PLTs [30]. These ex vivo observations were in good agreement with almost identical recovery between sham and aBL-treated PLTs in vivo after infusion of aBL-treated PLTs into mice, which is in sharp contrast to a reduced recovery of INTERCEPT-treated PLTs by 16% [54] or MIRASOL-treated PLTs by 25% [55].
Bacterial sepsis due to PLT concentrate contamination remains a public health concern despite current interventions, which is one of the major reasons to limit PLT storage for 5 days only. To minimize the risk, FDA permits extension of PLT storage to 7 days only if additional strategy is implemented to reduce a risk of bacterial sepsis. A simulation study finds that each additional day that PLT shelf life is extended may reduce PLT wastage by as much as 16% [56]. Recently we showed that near infrared low-level light could extend the shelf-life of stored PLTs beyond 7 days with improved quality and survial and the light also protected PLTs from apoptosis induced by anti-PLT antibody [57,58]. Thus, incorporation of both 405 nm and near infrared 810 nm light-emitting diode (LED) lights into the current PLT storage system may extend PLT shelf-life significantly. Our results indicate that the LED-based approach may offer an alternative to inactivate bacteria and fungi in PLT concentrates. However, further studies are needed to establish the clinical potential.
4 |. MATERIALS AND METHODS
4.1 |. Light source
An experimental device equipped with a LED with peak emission at 405 nm (Thorlabs, Newton, New Jersey) was used in this study (Figure 3A). The LED was held firmly by a clamp on a support stand, and the distance between the LED and the target surface was fixed to obtain 55 mW/cm2 as measured by a PM100D power/energy meter (Thorlabs, Newton, New Jersey) for all the experiments. The fluence was verified each time.
4.2 |. Microorganisms and cultures
Six representative microbial species associated with PLT concentrate contamination were selected in this study, among which four were Gram-positive bacteria, B cereus ATCC 14579, S epidermidis ATCC 35985, S pyogenes and methicillin-resistant S aureus USA 300 (MRSA USA300), one Gram-negative bacterium, P aeruginosa ATCC 19660 and one fungus, C albicans CEC 749 [16, 27–29]. These microbial strains were purchased from ATCC. The bacteria and fungus were grown in brain heart infusion (BHI) medium and yeast extract peptone dextrose (YPD) medium, respectively.
4.3 |. Fluorescence spectroscopy and high-performance liquid chromatography
Endogenous porphyrins in human PLTs, MRSA USA300,P. aeruginosa ATCC 19660 and C. albicans CEC 749 were determined by fluorescence spectroscopy and HPLC as previously described [20, 21, 42]. The PLT concentrates were obtained from Blood Donor Center at Massachusetts General Hospital with approval from Partners Human Research Ethics Committee as apheresis products of healthy de-identified volunteers and released to us after 2 days of sequestration to ensure no bacterial contamination as a standard clinical practice. The 2-day-old human PLT concentrates (1010 PLTs) or overnight bacterial and fungal cultures of 1010 colony-forming units (CFU) were centrifuged, washed twice with PBS and pelleted. The pellets were resuspended in extraction solvent composed of ethanol: dimethyl sulfoxide: acetic acid at a ratio of 80:20:1 (vol/vol/vol) and then stored at −80°C for 48 hours. The extracts were centrifuged, and the supernatants were collected and filtered through a Sep-Pak C18-Cartridge. Fluorescence of the PLT and microbe extracts was measured on a Fluoromax 3 (Horiba, Edison, New Jersey) with an excitation at 405 nm and an emission width ranging from 560 to 700 nm. The extracts were also analyzed for the presence of porphyrins by an Agilent’s 6430 Triple Quad LC/MS system (Agilent, Lexington, Massachusetts) in conjunction with a fluorescence detector at an excitation of 405 nm and the emission spectra of 590, 610 and 630 nm. All assays were performed in triplicate.
4.4 |. Comparison of antimicrobial activity of aBL at 405 nm vs 470 nm
To determine optimal laser parameters to kill the representative bacteria and fungus above, 3 mL of microbial suspensions at 105 CFU/mL in PAS (Fenwal, Lake Zurich, Illinois) was added to 35-mm Petri dishes and irradiated with aBL at 405 or 470 nm at an irradiance of 55 mW/cm2. Aliquots of 50 μL of the suspension were withdrawn at 0, 3, 6, 9, 12 and 15 minutes during aBL exposure, corresponding to 0, 10, 20, 30, 40 and 50 J/cm2, respectively. The resident microbes were quantified on BHI agar plates for bacteria or YPD agar plates for C. albicans CEC 749 as previously described [59]. All assays were performed in quintuplicate.
4.5 |. Microbial inactivation in PLT concentrates by aBL at 405 nm
The 2-day-old human PLT concentrates supplemented with PAS at a ratio 35%:65% (vol/vol) of PLT concentrate to PAS were split into PLT storage bags at 3 mL/bag using syringe with a 22 G needle, along with an estimate of 100 CFU/bag of indicated microbes to simulate PLT concentrate contamination. The storage bag was made in house with the polyvinylchloride (PVC)/tri-(2-ethylhexyl)- trimellitate (PVC-TEHTM) film that is currently used to fabricate standard PLT storage bags (a kind gift from American RENOLIT Corp. LA, California). The PVC-TEHTM film offers high oxygen and carbon dioxide permeability for PLT storage. The bags each were positioned under the LED and illuminated with 405 nm aBL for 0, 7.5, 15 or 22.5 minutes, corresponding to 0, 25, 50 and 75 J/cm2, respectively. A soft white light bulb irradiation was used as controls throughout the study. The irradiated PLT storage bags were stored for 5 days at 22°C with a gentle agitation of 60 to 70 rpm in a standard PLT storage incubator (Boekel Scientific, Pennsylvania). After storage, all contents in the PLT storage bag were removed, centrifuged and examined for resident microbes as above [59]. All assays were performed with eight independent experiments, each assayed in triplicate.
4.6 |. Effects of 405 nm aBL on human PLT survival, activation and aggregation
The 2-day-old human PLT concentrates supplemented with PAS were split into PLT storage bag as above and exposed to 0, 25, 50 or 75 J/cm2 405 nm aBL, respectively. PLTs were then counted to determine viability in 2 hours after aBL or 2 or 3 days after storage by a HemaTrue Veterinary Hematology Analyzer (Heska Corporation, Loveland, Colorado) as depicted in Figure 3A. All assays were performed with six independent experiments, each assayed in triplicate.
PLT activation was assessed by expression of activated integrin GPIIb/IIIa and CD62P, two common PLT activation biomarkers, at varying times after aBL irradiation [52, 60]. In some samples, the PLTs in a bag were exposed to 75 J/cm2 aBL or sham light on day 2. Around 200 μL of samples was taken in 2 hours after aBL or 2 or 3 days after storage to determine the PLT activation (Figure 3A). To verify whether repeated irradiations could be conducted safely during PLT storage to maximize the decontamination effect, the PLTs in a bag were also treated with aBL once a day for three consecutive days before PLT activation was evaluated on day 5 as depicted in Figure 3A. To evaluate PLT activation in the presence or absence of a specific stimulator, the PLTs were stained with FITC-PAC-1 antibody specific for activated GPIIb/IIIa (1:100; BD Bioscience, San Jose, California) and APC-antibody directed at CD62P (1:100; BD Bioscience, San Jose, California). FITC-mouse IgM and APC-IgG1 served as isotype controls. Alternatively, PLTs were activated with 100 μM TRAP-6, 2 U/mL thrombin, 50 μM ADP or vehicle control for 30 minutes at 25°C, after which the PLTs were fixed by 1 mL of 0.5% paraformaldehyde in PBS for 20 minutes and stained with specific anti-bodies against GPIIb/IIIa and CD62P as above, followed by flow cytometric analysis. The percentages of PLTs positive to either activated GPIIb/IIIa, CD62P or both were determined by FlowJo software. All assays were performed with six independent experiments, each assayed in triplicate.
To examine PLT aggregation, an important function for clotting, irradiant PLTs were collected at indicated times, diluted with HEPES/Tyrode’s solution to a final concentration of 50 × 106 cell/mL, and then divided into two parts equally: one was labeled with PE-anti-CD31 antibody and another with APC-anti-CD9 antibody as previously detailed [30, 61]. APC-mouse IgG1 and PE-mouse IgG1 served as isotype controls. After incubation for 30 minutes, the two populations of labeled PLTs were washed with HEP- ES/Tyrode’s solution and mixed at a 1:1 ratio, followed by activation with 100 ng/mL of PMA at 37°C while shaking at 500 rpm. At different times during the activation, samples were fixed by 0.5% paraformaldehyde in PBS for 20 minutes and analyzed by flow cytometry. The percentages of double-colored events were determined by FlowJo software as aggregated PLTs according to the reported method [30]. All assays were performed with six independent experiments, each assayed in triplicate.
4.7 |. Recovery of mouse PLTs in vivo after aBL irradiation
The animal protocol was approved by the subcommittee on Research Animal Care of the Massachusetts General Hospital in compliance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animal. C57BL/6 mice at 12 weeks of age were purchased from Charles River Laboratories. Blood samples were collected from mouse retro-orbital venous plexus into a tube containing 30 U/mL heparin in PBS and pooled. Concentrated PLTs were obtained by centrifugation of the blood samples at 200 g for 10 minutes to remove blood cells, followed by centrifugation at 1000 g to prepare 1 × 108 PLTs/mL. The concentrated PLTs were slowly injected into PLT storage bags using syringe with a 22 G needle. The bags containing concentrated PLTs were exposed to 75 J/cm2 aBL irradiation or sham light on day 0 and stored for 2 hours or 5 days under a standard storage condition. The PLTs were then stained with a vital fluorescent dye calcein at 5 μg/mL for 20 minutes, washed twice, resuspended in a modified Tyrode-HEPES buffer and intravenously infused into retro-orbital vein of mice at 5 × 106 PLTs per gram body weight. At indicated times after transfusion, blood samples were collected and labeled with APC-anti-CD41 or PE-anti-CD41 antibody. Percentages of calcein-positive PLTs relative to a total number of PLTs (CD41+) were quantified by flow cytometry to determine a recovery of infused PLTs.
4.8 |. Statistical analysis
Data are represented as mean ± SEM. Statistical analysis was performed with a paired Student t test between two groups or one-way ANOVA for multiple group comparisons. A P-value of < .05 is considered significant. All statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, California).
ACKNOWLEDGMENTS
We thank staff at the photopathology core of Wellman Center for Photomedicine for their assistant with flow cytometry and members in Dr. Wu’s group for stimulating discussion. We are very grateful for a kind gift of the PVC-TEHTM film from American RENOLIT Corp. LA, California. This work was supported by FA9550-17-1-0277, Department of Defense/Air Force Office of Scientific Research Military Photomedicine Program to MW and TD and Department funds to MW.
Funding information
U.S. Department of Defense, Grant/Award Number: FA9550-16-1-00173; Air Force Office of Scientific Research
REFERENCES
- [1].Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, Mumford AD, Stanworth SJ, Tinegate H, Br. J. Haematol 2017, 176, 365. [DOI] [PubMed] [Google Scholar]
- [2].Bashir S, Cookson P, Wiltshire M, Hawkins L, Sonoda L, Thomas S, Seltsam A, Tolksdorf F, Williamson LM, Cardigan R, Transfusion 2013, 53, 990. [DOI] [PubMed] [Google Scholar]
- [3].Ketter PM, Kamucheka R, Arulanandam B, Akers K, Cap AP, Transfusion 2019, 59, 1479. [DOI] [PubMed] [Google Scholar]
- [4].Loi MM, Kelher M, Dzieciatkowska M, Hansen KC, Banerjee A, West FB, Stanley C, Briel M, Silliman CC, Transfusion 2018, 58, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chaudhari CN, Med. J. Armed Forces India 2008, 65, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rumjantseva V, Grewal PK, Wandall HH, Josefsson EC, A. L, SÃ,rensen GL, Marth JD, Hartwig JH, Hoffmeister KM, Nat. Med 2009, 15, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hoffmeister KM, Felbinger TW, Falet H, Denis C,Bergmeier W, Mayadas TN, von Andrian UH, Wagner DD,Stossel TP, Hartwig JH, Cell 2003, 112, 87. [DOI] [PubMed] [Google Scholar]
- [8].Sarode R, Refaai MA, Matevosyan K, Burner JD, Hampton S, Rutherford C, Transfusion 2010, 50, 487. [DOI] [PubMed] [Google Scholar]
- [9].Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK, Cap AP, Shock 2014, 41, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wandall HH, Hoffmeister KM, Sørensen AL, Rumjantseva V, Clausen H, Hartwig JH, Slichter SJ, Blood 2008, 111, 3249. [DOI] [PubMed] [Google Scholar]
- [11].Sandgren P, Tolksdorf F, Struff WG, Gulliksson H, Vox Sang. 2011, 101, 35. [DOI] [PubMed] [Google Scholar]
- [12].Van Aelst B, Devloo R, Vandekerckhove P, Compernolle V, Feys HB, Transfusion 2015, 55, 2404. [DOI] [PubMed] [Google Scholar]
- [13].Prowse CV, Vox Sang. 2013, 104, 183. [DOI] [PubMed] [Google Scholar]
- [14].Verhaar R, Dekkers DWC, De Cuyper IM, Ginsberg MH, de Korte D, Verhoeven AJ, Blood 2008, 112, 4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Waters L, Padula MP, Marks DC, Johnson L, Transfusion 2019, 59, 2093. [DOI] [PubMed] [Google Scholar]
- [16].Marschner S, Goodrich R, Transfus. Med. Hemother 2011, 38, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Williamson LM, Cardigan R, Prowse CV, Transfusion 2003, 43, 1322. [DOI] [PubMed] [Google Scholar]
- [18].Levy JH, Neal MD, Herman JH, Crit. Care 2018, 22, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Irsch J, Lin L, Transfus. Med. Hemother 2011, 38, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR, Dai T, J Infect Dis 2014, 209, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Y, Wu X, Chen J, Amin R, Lu M, Bhayana B, Zhao J, Murray CK, Hamblin MR, Hooper DC, Dai T, J Infect Dis 2016, 213, 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang Y, Wang Y, Wang Y, Murray CK, Hamblin MR, Hooper DC, Dai T, Drug Resist. Updat 2017, 33–35, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stokowski LA, Adv. Neonatal Care 2006, 6, 303. [DOI] [PubMed] [Google Scholar]
- [24].Kleinpenning MM, Smits T, Frunt MHA, Van Erp PEJ, Kerkhof VD, Gerritsen RMJP, Photodermatol. Photo- immunol. Photomed 2010, 26, 16. [DOI] [PubMed] [Google Scholar]
- [25].Elman M, S. M, Harth Y, J. Cosmet. Laser Ther 2003, 5, 111. [PubMed] [Google Scholar]
- [26].Kawada A, Aragane Y, Kameyama H, Sangen Y, Tezuka T, J. Dermatol. Sci 2002, 30, 129. [DOI] [PubMed] [Google Scholar]
- [27].Jacobs MR, Good CE, Lazarus HM, Yomtovian RA, Clin. Infect. Dis 2008, 46, 1214. [DOI] [PubMed] [Google Scholar]
- [28].Sandgren P, Berlin G, Tynngard N, Transfusion 2016, 56, 1377. [DOI] [PubMed] [Google Scholar]
- [29].Yomtovian RA, Palavecino EL, Dysktra AH,Downes KA, Morrissey AM, Bajaksouzian S, Pokorny MA, Lazarus HM, Jacobs MR, Transfusion 2006, 46, 719. [DOI] [PubMed] [Google Scholar]
- [30].De Cuyper IM, Meinders M, van d. V., de Korte D, Porcelijn L, de Haas M, Eble JA, Seeger K, Rutella S, Pagliara D, Kuijpers TW, Verhoeven AJ, van d. B., Gutiérrez L, Blood 2013, 121, 70. [DOI] [PubMed] [Google Scholar]
- [31].Palavecino EL, Yomtovian RA, Jacobs MR, Transfus. Apher. Sci 2010, 42, 71. [DOI] [PubMed] [Google Scholar]
- [32].Tynngard N, Trinks M, Berlin G, Transfusion 2015, 55, 1169. [DOI] [PubMed] [Google Scholar]
- [33].Seghatchian J, de Sousa G, Transfus. Apher. Sci 2006, 35, 189. [DOI] [PubMed] [Google Scholar]
- [34].Tomb RM, Maclean M, Herron PR, Hoskisson PA,MacGregor SJ, Anderson JG, Bacteriophage 2014, 28, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tomb RM, Maclean M, Coia JE, Graham E, McDonald M, Atreya CD, MacGregor SJ, Anderson JG, Food Environ. Virol 2017, 9, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kingsley DH, Perez-Perez RE, Boyd G, Sites J, Niemira BA, J. Appl. Microbiol 2018, 124, 1017. [DOI] [PubMed] [Google Scholar]
- [37].Maclean M, Anderson JG, MacGregor SJ, White T, Atreya CD, J. Blood Transfus 2016, 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ, Antimicrob. Agents Chemother 2005, 49, 2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, Doukas AG, Goodson JM, Antimicrob. Agents Chemother 2005, 49, 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dai T, Gupta A, Huang Y-Y, Sherwood ME, Murray CK,Vrahas MS, Kielian T, Hamblin MR, Photomed. Laser Surg 2013, 31, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ashkenazi H, Malik Z, Harth Y, Nitzan Y, FEMS Immunol. Med. Microbiol 2003, 35, 17. [DOI] [PubMed] [Google Scholar]
- [42].Zhang Y, Zhu Y, Chen J, Wang Y, Sherwood ME, Murray CK, Vrahas MS, Hooper DC, Hamblin MR, Dai T, Virulence 2016, 7, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wilks A, Ikeda-Saito M, Acc. Chem. Res 2014, 47, 2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lewin A, Hederstedt L, Biochim. Biophys. Acta, Bioenerg 2016, 1857, 160. [DOI] [PubMed] [Google Scholar]
- [45].Gupta S, Maclean M, Anderson JG, MacGregor SJ, Meek RMD, Grant MH, Bone Joint J. 2015, 97, 283. [DOI] [PubMed] [Google Scholar]
- [46].Halstead FD, Thwaite JE, Burt R, Laws TR, Raguse M, Moeller R, Webber MA, Oppenheim BA, Appl. Environ. Microbiol 2016, 82, 4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Roh HJ, Kim A, Kang GS, Kim D-H, Fish. Aquat. Sci 2016, 19, 1. [Google Scholar]
- [48].Kumar A, Ghate V, Kim MJ, Zhou W, Khoo GH, Yuk HG, J. Appl. Microbiol 2016, 120, 49. [DOI] [PubMed] [Google Scholar]
- [49].Stormer M, Arroyo A, Brachert J, Carrero H, Devine D,Epstein JS, Gabriel C, Gelber C, Goodrich R,Hanschmann KM, Heath DG, Jacobs MR, Keil S, de Korte D, Lambrecht B, Lee CK, Marcelis J, Marschner S,McDonald C, McGuane S, McKee M, Muller T, Muthivhi H,T, Pettersson A, Radziwon P, Ramirez-Arcos S,Reesink H, Rojo W,J, Rood I, Schmidt M, Schneider CK,Seifried E, Sicker U, Wendel S, Wood EM, Yomtovian RA,Montag T Vox Sang. 2012, 102, 22–31. [DOI] [PubMed] [Google Scholar]
- [50].Barneck MD, Rhodes NLR, de la Presa M, Allen JP, Poursaid AE, Nourian MM, Firpo MA, Langell JT,J. Surg. Res 2016, 206, 316. [DOI] [PubMed] [Google Scholar]
- [51].Rhodes NL, de la Presa M, Barneck MD, Poursaid A, Firpo MA, Langell JT, Lasers Surg. Med 2016, 48, 311. [DOI] [PubMed] [Google Scholar]
- [52].Baker-Groberg S, Lattimore S, Recht M, McCarty OJT, Haley KM, J. Thromb. Haemost 2016, 14, 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lu Q, Malinauskas RA, Artif. Organs 2011, 35, 137. [DOI] [PubMed] [Google Scholar]
- [54].Snyder E, Raife T, Lin L, Cimino G, Metzel P,Rheinschmidt M, Baril L, Davis K, Buchholz DH, Corash L,Conlan MG, Transfusion 2004, 44, 1732. [DOI] [PubMed] [Google Scholar]
- [55].AuBuchon JP, Herschel L, Roger J, Taylor H, Whitley P,Li J, Edrich R, Goodrich RP, Transfusion 2005, 45, 1335. [DOI] [PubMed] [Google Scholar]
- [56].Blake JT, Transfusion 2017, 57, 3001. [DOI] [PubMed] [Google Scholar]
- [57].Zhang Q, Lu M, Wu MX, J. Biophotonics 2018, 90, 1. [Google Scholar]
- [58].Yang J, Zhang Q, Li P, Dong T, Wu MX, Sci. Rep 2016, 6, 38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jett BD, Hatter KL, Huycke MM, Gilmore MS, Bio- Techniques 1997, 23, 648. [DOI] [PubMed] [Google Scholar]
- [60].Manfredi AA, Baldini M, Camera M, Baldissera E,Brambilla M, Peretti G, Maseri A, Rovere-Querini P,Tremoli E, Sabbadini MG, Maugeri N, Ann. Rheum. Dis 2016, 75, 1511. [DOI] [PubMed] [Google Scholar]
- [61].Zhang Q, Dong T, Li P, Wu MX, Sci. Transl. Med 2016, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]


