Abstract
Objective.
American Indians experience substantial health disparities relative to the U.S. population, including vascular brain aging. Poorer cognitive test performance has been associated with cranial MRI findings in aging community populations, but no study has investigated these associations in elderly American Indians.
Methods.
We examined 786 American Indians aged 64 years and older from the Cerebrovascular Disease and its Consequences in American Indians study (2010-2013). Cranial magnetic resonance images were scored for cortical and subcortical infarcts, hemorrhages, severity of white matter disease, sulcal widening, ventricle enlargement, and volumetric estimates for white matter hyperintensities, hippocampus, and brain. Participants completed demographic, medical history, and neuropsychological assessments including testing for general cognitive functioning, verbal learning and memory, processing speed, phonemic fluency, and executive function.
Results.
Processing speed was independently associated with presence of any infarcts, white matter disease, and hippocampal and brain volumes, independent of socioeconomic, language, education, and clinical factors. Other significant associations included general cognitive functioning with hippocampal volume. Non-significant, marginal associations included general cognition with WMH and brain volume; verbal memory with hippocampal volume; verbal fluency and executive function with brain volume; and processing speed with ventricle enlargement.
Conclusions.
Brain-cognition associations found in this study of elderly American Indians are similar to those found in other racial/ethnic populations, with processing speed comprising an especially strong correlate of cerebrovascular disease. These findings may assist future efforts to define opportunities for disease prevention, to conduct research on diagnostic and normative standards, and to guide clinical evaluation of this underserved and overburdened population.
MESH TERMS: Neuropsychological Tests; Magnetic Resonance Imaging; Vascular brain injury; Cerebrovascular Disorders; Indians, North American; Health Status Disparities; Cognitive Aging; Cultural Diversity
INTRODUCTION
Vascular findings from cranial magnetic resonance imaging (MRI) are common in the elderly, with prevalence increasing with age, especially after 75 years (Morris, et al., 2009). Racial and ethnic minority populations experience disproportionate burden of diseases related to vascular aging (U.S. Department of Health and Human Services, 2014). American Indians, or individuals who have ancestry with any of the original peoples of North, Central, and South America or who maintain tribal affiliation or community attachment (Office of Management and Budget, 1997), are at especially high risk for stroke (Harris, Nelson, Muller, & Buchwald, 2015; Lee, et al., 1990; Y. Zhang, et al., 2008), and endure a greater burden of cerebral vascular injury risk, including from hypertension, diabetes mellitus, hypercholesterolemia, and obesity (Ayala, et al., 2001; Centers for Disease Control and Prevention, 2004; Gillum, 1995; Harwell, et al., 2005; Hutchinson & Shin, 2014; Lee, et al., 1990), compared with US general population (Ying Zhang, et al., 2008). However, American Indians are often not included in studies of vascular brain aging and dementia risk, even meta-analyses (Morris, et al., 2009).
Cerebral vascular injury or cerebral atrophy, detected by MRI, has been associated with increased risk of cognitive impairment, independent of age (Drag & Bieliauskas, 2010; Eyler, Sherzai, Kaup, & Jeste, 2011; Gorelick, et al., 2011). Specifically, associated deficits include frontal lobe-mediated functioning, especially executive function and processing speed (Erkinjuntti, et al., 2000; Jokinen, Kalska, Ylikoski, Madureira, Verdelho, Gouw, & al., 2009; Miralbell, et al., 2013). For example, in the Rotterdam Study of adults aged 60-90, brain infarcts, periventricular white matter hyperintensities (WMH), and overall brain atrophy were associated with poorer performance in both processing speed and executive function cognitive domains (Prins, et al., 2005). However, most previous research has focused on non-Hispanic whites, and some on African-Americans; the impact of cerebral vascular injury or cerebral atrophy on cognitive function in other ethnic populations is poorly understood, with the first estimates of population-based prevalence of cranial MRI findings in American Indians only very recently described (Suchy-Dicey, et al., 2016; Suchy-Dicey, et al., 2017). Whether American Indians differ in cognitive risk—and whether any such differences may be attributed to cerebral vascular injury or cerebral atrophy, excess burden of vascular comorbidities, predisposition to other adverse health disparities, variation in socio-demographics, or differences in functional testing performance itself—are yet unclear.
The prevailing theory has been that cognitive associations with cerebral vascular injury or atrophy in the corresponding brain regions are likely to be similar for non-Hispanic white populations as for other racial and ethnic populations, after accounting for differences in socio-demographics and clinical comorbidities and other confounders (DeCarli, et al., 2008). For example, both the Atherosclerosis Risk in Communities study and the Chicago Health and Aging project found no racial differences in cerebral vascular injury associations with cognition, when comparing African Americans with non-Hispanic whites (Aggarwal, et al., 2010; Mosley, et al., 2005). A more recent study comparing African-Americans, Hispanics, and non-Hispanic whites, the Washington Heights-Inwood Columbia Aging Project, reported a few differences; specifically, WMH were associated with processing speed and executive function among African-American participants and hippocampal volume was more strongly associated with memory among non-Hispanic white participants, compared with other participants (Zahodne, et al., 2015). The authors interpreted these findings as resulting from differences in environmental conditions, and not differences in underlying biology.
American Indians differ from the majority US population in many factors that can profoundly influence both cognitive function as well as cognitive testing performance, including educational attainment, socio-demographics, language, lifestyle, cultural traditions, and sociopolitical history (Suzuki, Naqvi, & Hill, 2013; Verney, Bennett, & Hamilton, 2015; Verney, et al., in press). Socioeconomic factors such as education and income are also associated with vascular risk and other chronic diseases (Gorelick, et al., 2011), although they may also constitute proxy variables for other exposure characteristics such as access to healthcare, nutrition, or social isolation. Use of Native languages, especially when reflective of bilingual status, and geographic region may represent proxy characteristics for environmental and cultural factors that affect cognitive function; although language does independently influence cognitive test performance. In addition to its influence on vascular risk, diabetes has been independently associated with cognitive impairment and dementia risk (Cholerton, Baker, & Craft, 2011; Luchsinger, 2010; Strachan, 2003), affecting processing speed, executive function, and memory domains (Sadanand, Balachandar, & Bharath, 2015; Tournoy, et al., 2010; van den Berg, et al., 2006; Zhao, et al., 2015). Understanding these biological, behavioral, sociocultural, and environmental differences that may explain cognitive and vascular health disparities in American Indians is critical to better serving this rapidly aging population, and an emerging public health priority (Hill, Perez-Stable, Anderson, & Bernard, 2015; Zahodne, et al., 2015).
This study aims to develop the first basis for better understanding of these relationships, with long-term potential to inform development of better prevention programs, risk quantification, and future mechanistic research. As the first report associating both vascular (infarcts; hemorrhages; WMH) and atrophy (sulcal and ventricle widening; hippocampal and brain volume) findings from MRI with cognitive testing performance across several cognitive domains including general cognitive functioning, processing speed, verbal learning and memory, verbal fluency, and executive functioning in elderly American Indians, this work primarily represents a descriptive, exploratory effort to examine whether and how basic cognition is related to a variety of cerebral changes using a cross-sectional analysis of data from a unique cohort of an understudied population. Although left-shifted neuropsychological or cognitive testing performance may be expected in elderly American Indians, compared with published normative data for the majority US population, the associations between cognitive performance and cerebral findings from MRI are expected to be similar as for other populations, once all relevant confounding factors are adequately considered.
METHODS
Setting and Participants
The Strong Heart Study is a population-based cohort of 4,549 adult American Indian members of 13 original tribes and communities from 3 geographic regions of the U.S., including the Northern Plains, Central Plains, and Southwest, which began recruitment for a baseline examination in 1989-1991 (Lee, et al., 1990). The parent cohort study defined American Indians as individuals who claim ancestry with any of the original peoples of North, Central, and South America and who maintain tribal affiliation or community attachment (Office of Management and Budget, 1997). A follow-up examination visit in 2010-2013, known as the Cerebrovascular Disease and its Consequences in American Indians (CDCAI) study (Suchy-Dicey, et al., 2016), recruited 1,033 surviving participants, all aged 64 and older, from the original baseline cohort for cranial MRI; neuropsychological, cognitive, and functional testing; and a clinic examination. CDCAI study procedures were designed to include protocols and instruments used by other large cohort studies of cerebral vascular injury; because participants were elderly and often frail, time constraints were also considered in selection of study components (Arnold, Psaty, Kuller, & al., 2005; Rosamond, Folsom, Chambless, & al., 1999). Tribal councils, the Indian Health Service, and Institutional Review Boards for participating communities and partner institutions approved all study procedures; all participants provided written informed consent. After data collections were complete, one community withdrew from ongoing research and analysis (n = 215). Some participants were additionally excluded from the current analyses based on incomplete or inadequate brain MRI scans (n = 29), incomplete cognitive testing (n = 3), or adjudicated prior stroke (n = 37), resulting in a final N=749 included in this cross-sectional analysis of data from the CDCAI study visit.
Cranial MRI Findings
Detailed MRI procedures have been described previously (Suchy-Dicey, et al., 2017). In brief, local Radiology centers at each field site used 1.5T MRI scanners to obtain six image sequences in contiguous slices, including sagittal T1-weighted localizer, co-registered 5 mm axial-T1, 5 mm axial-T2, and 5 mm axial-T2 * susceptibility-weighted in the anterior commissure/posterior commissure plane, 3 mm axial fluid-attenuated inversion recovery (FLAIR), and 1.5 mm sagittal T1-weighted volumetric gradient echo. Study neuroradiologists, blinded to participant characteristics, scored images for presence, number, and location of infarcts (>3mm) and hemorrhages as well as for graded severity (0-9) of WMH, sulcal widening, and ventricle enlargement. Infarct lesions were defined as lacunar if they were between 3 and 20 mm in maximum dimension and located within the caudate, lenticular nucleus, internal capsule, thalamus, brainstem, cerebellar white matter, centrum semiovale, or corona radiata. Neuroimaging technologists employed semi-automated software workflows (Suchy-Dicey, et al., 2017) to estimate volumetric measures, including WMH, hippocampus, brain, and intracranial volumes.
Cognitive Testing
Neuropsychological and cognitive examinations were administered at field sites by trained study staff, and scored and coded by study neuropsychologists. The test battery included the Modified Mini Mental State Exam (3MSE) (Teng & Chang Chui, 1987), California Verbal and Learning Test 2nd edition, Short Form (CVLT-II SF) (Delis, Kramer, Kaplan, & Ober, 2000), Wechsler Adult Intelligence Scale, 4th edition (WAIS-IV) Coding subtest (Wechsler, 2008), and the Controlled Oral Word Association (COWA) test (Benton & Hansher, 1976). The 3MSE is a global cognitive screening measure consisting of 40 questions, scored on a 100-point scale (Teng & Chui, 1987). The CVLT-II SF is a list learning test of nine words which provides indices of verbal learning and memory (Delis, et al., 2000) including short (30-second) delay free recall, and long delay (10-minute) free recall, with scoring based on the total number of words recalled (score 0-9). The WAIS-IV Coding test measures visuospatial processing speed and working memory, with scoring based on total number of symbols coded correctly over 120 seconds, ranging from 0 to 135 (Wechsler, 2008). The COWA is a measure of phonemic verbal fluency that also provides an index of executive functioning (Benton & Hansher, 1976), with scoring based on number of total correct words for the letters “F,” “A,” and “S” in each of three 60-second trials.
Other Measures
Trained study staff at each field site administered clinical examinations, including anthropometric measurements and blood and urine collections. Staff assessed height and weight, calculating body mass index (BMI, kg/m2); recorded blood pressure as the average of 3 seated electronic sphygmomanometer measures; and transcribed usual medications from bottle labels. Standard labs included fasting plasma glucose and blood lipids, including low density lipoprotein (LDL). Participants also completed questionnaires, self-reporting their year of birth, sex, years of formal education, annual household income, marital status, and ability to speak their traditional Native (“tribal”) language. Of note, language speaking capacity represents bilingual fluency because speaking English was a requirement of participation in the cohort.
Categorical variable definitions include sex (male, female), site (Northern Plains, Southern Plains, Southwest), education (up to or any high school, high school graduate, any college, college graduate), annual household income (<$10,000, $10,000-20,000, $20,000-35,000, >$35,000), marital status (single; married or partnered; divorced, separated or widowed), Native language speaking capacity (not at all, a little, moderately, very well), obese (BMI ≥30 kg/m2), diabetes (fasting plasma glucose ≥126 mg/dL or use of antihyperglycemic medications), hypertension (systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications), and hypercholesterolemia (LDL ≥130 mg/dL or use of statin medications).
Analytic Strategy
Descriptive summary of selected participant characteristics as of the time of the MRI examination included count and percent or mean and standard deviation. Histograms with gaussian overlay were used to graphically describe the distribution of neuropsychological test scores. Spearman rank correlation coefficients were estimated for each neuropsychological test with the others (3MSE, CVLT long delay free recall, WAIS coding, COWA) and for each MRI finding with the others (any infarct, hemorrhage, white matter disease grade, sulcal grade, ventricle grade, hippocampal volume, WMH volume, brain volume), to assess relationships among exposure and outcome variables.
Associations between MRI findings and neurocognitive performance test scores were examined using standardized linear regression models for each exposure and outcome combination. Independent (exposure) variables included presence of infarct, including lacunar or other type, or hemorrhage; ordinal grade for WMH, sulcal widening, or ventricle enlargement; or volumetric estimates for WMH, hippocampus, or brain (grey & white matter). Volumetric models were adjusted for intracranial space. Due to heavy skew in WMH volume, regression models used a log-transformed variable. In order to produce interpretable coefficients, brain volume was modeled in liter units. Dependent (outcome) variables included continuous raw scores for neurocognitive performance tests, including 3MSE, CVLT long delay free recall, WAIS-IV coding, and COWA. Regression models were adjusted using a nested, model-building approach, including unadjusted (Model 0), minimally-adjusted (Model 1), and fully-adjusted (Model 2), to assess the relative contribution of sets of confounders to the associations of primary interest. Model 1 adjusted for age, sex, site, education, income, marital status, and Native (“tribal”) language speaking capacity; Model 2 additionally adjusted for obesity, diabetes, hypertension, and high LDL. Adjustments were selected a priori based on having known association with both the vascular brain exposures and the cognitive function outcomes, including socioeconomic factors (education, income, and marital status), study field site (geographic region) and Native language use, and vascular risk factors (obesity, diabetes, hypertension, and high LDL), all evaluated as individual confounders and because of independent pathological or risk effect.
Primary inference was drawn from the fully-adjusted Model 2, as these confounders were stated a priori and the fully-adjusted model offers the strongest inference of independent effect between exposure and outcome. Robust standard errors were used to obtain unbiased errors from the ordinal least squares in the presence of heteroskedasticity. The Benjamini-Hochberg method for controlling the family-wise error rate, wherein the expected proportion of false discoveries is estimated amongst rejected null hypotheses, was used to account for the problem of multiple comparisons; false discovery rate (FDR)-corrected P-values, often termed Q-values, were presented (Benjamini, 1995). All analyses were done using Stata v.14 (StataCorp, 2014) or R version 3.3.1(Team, 2016).
RESULTS
The mean age of participants was approximately 73 years, and a majority were female (68%; Table 1). The majority of participants also tended to be divorced, separated, or widowed (56%); with at least some college education (55.8%, combined); and below $20,000 annual household income (58.7%, combined). Males and females were similar in most categories, with exception that more females were unmarried and low income; males had more findings of cerebral vascular injury or atrophy, which has been previously reported.(Suchy-Dicey, et al., 2017) Bilingual status was common, with many able to speak their Native language moderately or very well (38.6%, combined). Most were measured as or reported taking medications for high blood pressure (80%), diabetes (48.9%) and high LDL cholesterol (67.4%); and more than half (54.6%) were obese, with mean body mass index 31 kg/m2.
Table 1:
Selected participant characteristics among elderly American Indians from the Cerebrovascular Disease and its Consequences in American Indians study (2010-2013)
| Characteristic | Definition | All N=749 |
Female N=507 |
Male N=242 |
|---|---|---|---|---|
| Age | Years, mean (SD) | 72.8 (5.7) | 73.1 (6.0) | 72.2 (5.1) |
| Male sex | 242 (32.3 %) | - | - | |
| Study site (geographic region) | Northern Plains | 334 (44.6 %) | 228 (45.0%) | 106 (43.8%) |
| Southern Plains | 321 (42.9 %) | 208 (41.0%) | 113 (46.7%) | |
| Southwest | 94 (12.6 %) | 71 (14.0%) | 23 (9.5%) | |
| Marital status | Single | 40 (5.3 %) | 21 (4.1%) | 19 (7.9%) |
| Married/Partnered | 286 (38.2 %) | 148 (29.2%) | 138 (57.0%) | |
| Divorced / Widowed | 423 (56.5 %) | 338 (66.7%) | 85 (35.1%) | |
| Education | Up to / any high school | 141 (18.8 %) | 101 (19.9%) | 40 (16.5%) |
| Graduated high school | 190 (25.4 %) | 126 (24.9%) | 64 (26.4%) | |
| Attended some college | 304 (40.6 %) | 207 (40.8%) | 97 (40.1%) | |
| Graduated college | 114 (15.2 %) | 73 (14.4%) | 41 (16.9%) | |
| Annual household income | <$10,000 | 216 (28.8 %) | 154 (30.4%) | 62 (25.6%) |
| $10-20,000 | 224 (29.9 %) | 169 (33.3%) | 55 (22.7%) | |
| $20-35,000 | 167 (22.3 %) | 108 (21.3%) | 59 (24.4%) | |
| $35,000 | 142 (19.0 %) | 76 (15.0%) | 66 (27.3%) | |
| Ability to speak Native language | Not at all | 244 (32.6 %) | 163 (32.1%) | 81 (33.5%) |
| A little | 216 (28.8 %) | 138 (27.2%) | 78 (32.2%) | |
| Moderately | 96 (12.8 %) | 69 (13.6%) | 27 (11.2%) | |
| Very well | 193 (25.8 %) | 137 (27.0%) | 56 (23.1%) | |
| Obesity | BMI >30 kg/m2 | 406 (54.6 %) | 266 (53.0%) | 140 (57.9%) |
| Hypertension | SBP>140 mmHg, DBP>90 mmHg, medication | 599 (80.0 %) | 406 (80.1%) | 193 (79.8%) |
| Diabetes | FPG>126 mg/dL, medication | 366 (48.9 %) | 253 (49.9%) | 113 (46.7%) |
| High LDL cholesterol | LDL>100 mg/dL, medication | 519 (67.4 %) | 327 (66.2%) | 170 (71.1%) |
| Infarcts | ||||
| Lacunar infarcts * | Any | 164 (22.0 %) | 123 (24.4%) | 41 (16.9%) |
| Cortical infarcts * | Any | 141 (18.9 %) | 91 (18.1%) | 50 (20.7%) |
| Hemorrhage | Any | 40 (5.4 %) | 29 (5.8%) | 11 (4.5%) |
| WMH grade | Range 0-9, mean (SD) | 2.28 (1.16) | 2.36 (1.22) | 2.22 (1.08) |
| Sulcal grade | Range 0-9, mean (SD) | 3.06 (1.15) | 2.96 (1.13) | 3.31 (1.19) |
| Ventricle grade | Range 0-9, mean (SD) | 3.23 (1.41) | 3.06 (1.35) | 3.66 (1.42) |
| WMH volume | Unit: % IC volume†, mean (SD) | 0.57 (0.50) | 0.60 (0.55) | 0.60 (0.53) |
| Hippocampal volume | Unit: % IC volume†, mean (SD) | 0.55 (0.08) | 0.56 (0.08) | 0.52 (0.08) |
| Brain volume | Unit: % IC volume†, mean (SD) | 77.6 (4.6) | 78.3 (4.3) | 76.0 (4.7) |
| 3MSE | Score, mean (SD) | 88.6 (9.2) | 88.5 (9.4) | 88.8 (8.8) |
| CVLT long delay free recall | Score, mean (SD) | 5.5 (2.2) | 6.0 (4.0, 7.0) | 6.0 (4.0, 7.0) |
| WAIS-IV coding subtest | Score, mean (SD) | 44.5 (15.7) | 44.9 (16.2) | 43.6 (14.5) |
| COWA f,a,s test | Score, mean (SD) | 24.5 (11.5) | 24.9 (11.5) | 23.8 (11.5) |
Data given as n (%) unless otherwise noted; BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; FPG = fasting plasma glucose; LDL = low density lipoprotein; WMH: white matter hyperintensities; IC = intracranial; 3MSE: Modified Mini Mental State Examination; CVLT LF: California Verbal Learning Test 2nd Edition long delay free recall; WAIS-IV: Weschler Adult Intelligence Scale – 4th Edition (Coding sub-test); COWA: Controlled Oral Word Association test (F, A, S words)
Lacunar infarcts and cortical infarcts are not mutually exclusive categories; participants may have either or both.
Volumetric measures given as % of intracranial (IC) volume
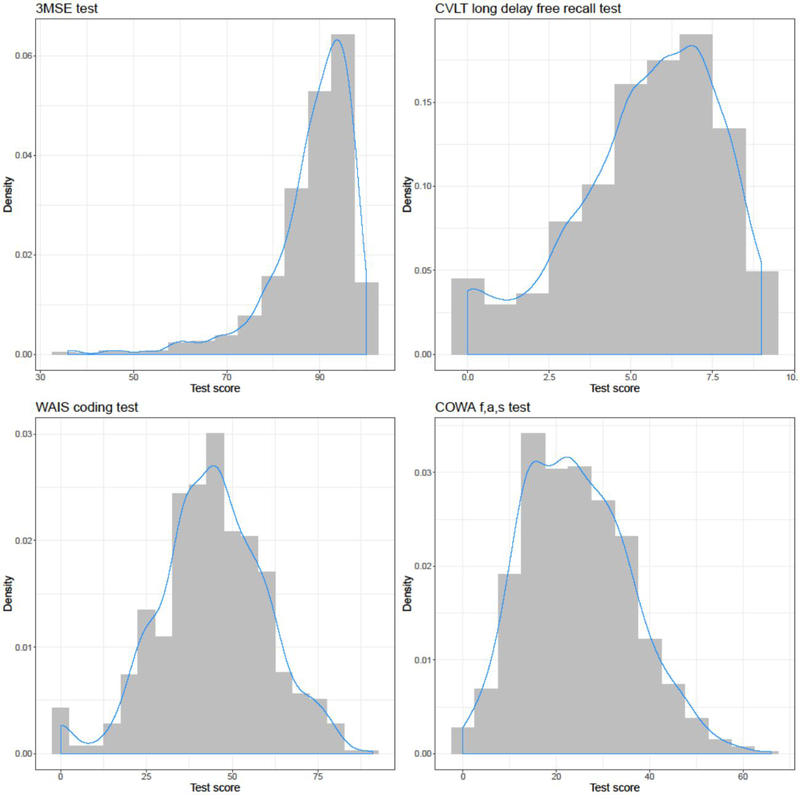
The distributions of scores for each cognitive test were visualized graphically using histograms and Gaussian curves (Figure). 3MSE was heavily right-skewed, with approximate mean score 88; CVLT long delay free recall test was slightly right-skewed, with mean score 5; WAIS coding subtest was somewhat normal, with mean score 44; COWA test was also somewhat normal, with mean score 24.
Figure:
Histograms of cognitive test scores including Modified Mini Mental Status Examination (3MSE), California Verbal Learning Test II (CVLT) short form long/free-recall test, Weschler Adult Intelligence Scale (WAIS) digit coding subtest, and Controlled Oral Word Association (COWA) f,a,s subtest among elderly American Indians, showing score density (Y-axis) with a Gaussian distribution overlay (blue curve). Mean scores overall were approximately: 3MSE (88), CVLT long free recall (5), WAIS coding (44), and COWA (24).
Spearman rank order correlation coefficients among cognitive test scores suggest that each test is significantly correlated with each other (Table 2-A). The strongest correlations were among 3MSE, WAIS, and COWA (ρ > 0.5); medium level correlations were detected for CVLT with 3MSE and WAIS (ρ ≈ 0.3); and smallest correlations were found for CVLT and COWA (ρ < 0.2). Similarly, Spearman rank order correlation coefficients among MRI findings indicated strong overlap among various cerebral vascular and atrophy type injuries (Table 2-B). Vascular lesions (infarct, hemorrhage) were significantly correlated with worse white matter disease grade and more white matter hyperintensities volume (ρ ≈ 0.1-0.3); as well as infarcts with worse sulcal grade, worse ventricle grade, and smaller brain volume (ρ ≈ 0.1). White matter disease grade and white matter hyperintensities volume were also significantly correlated with worse sulcal grade (ρ ≈ 0.2), worse ventricle grade (ρ ≈ 0.3-0.4), smaller hippocampal volume (ρ ≈ −0.2 to −0.3), smaller brain volume (ρ ≈ −0.2), and with each other (ρ >0.6). Graded and volumetric measures of atrophy were also intercorrelated—albeit not completely—notably, sulcal grade with ventricle grade (ρ ≈ 0.5); ventricle grade with smaller hippocampal volume (ρ ≈ −0.4); and both with brain volume (ρ ≈ −0.3 to −0.4).
Table 2-A:
Spearman’s rank order correlation coefficients (rho) and associated P-values for cognitive test scores in elderly American Indians from the Cerebrovascular Disease and its Consequences in American Indian study (2010-2013)
| CVLT LF Rho (p) P-value |
WAIS Rho (ρ) P-value |
COWA Rho (ρ) P-value |
|
|---|---|---|---|
| 3MSE | 0.312 P<0.001 |
0.546 P<0.001 |
0.516 P<0.001 |
| CVLT LF | - | 0.252 P<0.001 |
0.182 P<0.001 |
| WAIS coding | - | - | 0.548 P<0.001 |
3MSE = Mini Mental Status Examination; CVLT LF = California Verbal Learning Test II short form long delay free recall; WAIS = Weschler Adult Intelligence Scale coding subtest; COWA = Controlled Word Association f,a,s Test; N=737
Table 2-B:
Spearman’s rank order correlation coefficients (rho) and associated P-values for MRI findings in elderly American Indians from the Cerebrovascular Disease and its Consequences in American Indian study (2010-2013)
| Hemorrhage Rho (ρ) P-value |
WMG * Rho (ρ) P-value |
Sulci * Rho (ρ) P-value |
Ventricle * Rho (ρ) P-value |
HC volume † Rho (ρ) P-value |
WMH volume † Rho (ρ) P-value |
Brain volume † Rho (ρ) P-value |
|
|---|---|---|---|---|---|---|---|
| Infarcts | 0.072 P=0.067 |
0.310 P<0.001 |
0.084 P=0.032 |
0.120 P=0.002 |
−0.050 P=0.205 |
0.286 P<0.001 |
−0.118 P=0.002 |
| Hemorrhage | - | 0.144 P<0.001 |
0.099 P=0.011 |
0.078 P=0.046 |
0.016 P=0.685 |
0.105 P=0.008 |
−0.051 P=0.196 |
| WMG * | - | - | 0.200 P<0.001 |
0.330 P<0.001 |
−0.162 P<0.001 |
0.688 P<0.001 |
−0.201 P<0.001 |
| Sulci * | - | - | - | 0.461 P<0.001 |
−0.186 P<0.001 |
0.239 P<0.001 |
−0.344 P<0.001 |
| Ventricle * | - | - | - | - | −0.425 P<0.001 |
0.421 P<0.001 |
−0.413 P<0.001 |
| HC volume † | - | - | - | - | - | −0.268 P<0.001 |
0.437 P<0.001 |
| WMH volume † | - | - | - | - | - | - | −0.191 P<0.001 |
White matter grade (WMG), sulci, and ventricle grade coded as ordinal (grade 0-9)
Hippocampus (HC), white matter hyperintensities (WMH), and brain (grey & white matter) volumes coded as percent of intracranial volume N=654
Nested models evaluated influence of additional covariates (Supplemental Table). Some associations detected in Model 0 or Model 1 did not remain after adjustment for vascular comorbidities in Model 2. White matter grade was associated in Model 1 with lower 3MSE score (P=0.032) and ventricle enlargement grade was associated with lower COWA score (P=0.017). Individual adjustment for each clinical characteristic indicated that models for white matter grade and 3MSE may be limited in statistical power, as each individually adjusted model was still statistically significant (data not shown). Similar evaluation of associations between ventricle grade and COWA test were still significant after adjustment for each factor, except for diabetes. Graphical examination of residuals over fitted values, QQ plots, and residuals over leverage suggested that model assumptions were reasonably supported.
Standardized associations that did remain after full adjustment in Model 2 and accounting for multiple comparisons using false discovery rate (FDR; Table 3), were strongest for the WAIS-IV Coding subtest and the the 3MSE. Cerebral infarcts (β −0.10 95% CI −0.16, −0.05) and cortical infarcts (β −0.14 95% CI −0.19, −0.08), worse white matter grade (β −0.15 95% CI −0.21, −0.08), larger white matter hyperintensity volume (β −0.16 95% CI −0.24, −0.09), worse sulcal grade (β −0.10 95% CI −0.17, −0.04), worse ventricle grade (β −0.14 95% CI −0.20, −0.08), smaller hippocampus volume (β 0.13 95% CI 0.06, 0.21), and smaller brain volume (β 0.20 95% CI 0.03, 0.34) were all associated with lower scores on the WAIS-IV Coding subtest. Similarly, associations for lower 3MSE score included worse white matter grade (β −0.12, 95% CI 0.20, −0.04), white matter hyperintensity volume (β −0.14 95% CI −0.24, −0.05), ventricle grade (β −0.11 95% CI −0.18, −0.03), hippocampus volume (β 0.16 95% CI 0.07, 0.24), and brain volume (β 0.19 95% CI 0.03, 0.34). Negative associations for CVLT long delay free recall included findings for long-term recall with hippocampus volume (β 0.10 95% CI 0.02, 0.18) and for COWA with white matter hyperintensity volume (β −0.11 95% CI −0.19, 0.33), sulcal grade (β −0.08 95% CI −0.15, 0.02), and brain volume (β 0.19 95% CI 0.03, 0.34). Associations for other CVLT subtests, including short delay free recall, were similar to long delay free recall (data not shown). Direction and precision of these associations can be visually examined graphically in scatterplots with linear and loess fits of volumetric and graded MRI measures with cognitive test scores (Supplementary Figures).
Table 3:
Standardized beta coefficients with 95% confidence intervals (95%CI) and P-values from linear regressions of cognitive test scores with standardized MRI findings from elderly American Indians from the Cerebrovascular Disease and its Consequences in American Indian study (2010-2013)
| 3MSE | CVLT LF | WAIS-IV Coding | COWA | |
|---|---|---|---|---|
| β (95%CI) P-value FDR (Q-value) |
β (95%CI) P-value FDR (Q-value) |
β (95%CI) P-value FDR (Q-value) |
β (95%CI) P-value FDR (Q-value) |
|
| Any Infarct | −0.06 (−0.13, 0.01) P=0.079 Q=0.131 |
−0.03 (−0.10, 0.05) P=0.453 Q=0.503 |
−0.10 (−0.16, −0.05) P<0.001 Q=0.005 |
−0.03 (−0.10, 0.03) P=0.311 Q=0.377 |
| Lacunar infarct | −0.05 (−0.12, 0.02) P=0.160 Q=0.230 |
−0.02 (−0.09, 0.06) P=0.675 Q=0.730 |
−0.06 (−0.12, 0.001) P=0.054 Q=0.098 |
−0.003 (−0.07, 0.06) P=0.934 Q=0.951 |
| Cortical Infarct | −0.03 (−0.11, 0.04) P=0.410 Q=0.469 |
−0.05 (−0.12, 0.03) P=0.239 Q=0.299 |
−0.14 (−0.19, −0.08) P<0.001 Q=0.006 |
−0.04 (−0.11, 0.02) P=0.211 Q=0.272 |
| Hemorrhage | −0.03 (−0.10, 0.03) P=0.326 Q=0.384 |
−0.08 (−0.15, −0.003) P=0.040 Q=0.076 |
−0.01 (−0.06, 0.05) P=0.815 Q=0.858 |
0.002 (−0.06, 0.07) P=0.951 Q=0.951 |
| WMH Grade | −0.12 (−0.20, −0.04) P=0.002 Q=0.010 |
−0.09 (−0.17, −0.01) P=0.035 Q=0.070 |
−0.15 (−0.21, −0.08) P<0.001 Q=0.006 |
−0.07 (−0.14, 0.002) P=0.057 Q=0.099 |
| Sulcal Grade | −0.04 (−0.11, 0.02) P=0.195 Q=0.261 |
−0.05 (−0.13, 0.03) P=0.196 Q=0.261 |
−0.10 (−0.17, −0.04) P=0.003 Q=0.012 |
−0.08 (−0.15, −0.02) P=0.013 Q=0.035 |
| Ventricle Grade | −0.11 (−0.18, −0.03) P=0.006 Q=0.020 |
−0.07 (−0.15, 0.02) P=0.111 Q=0.178 |
−0.14 (−0.20, −0.08) P<0.001 Q=0.006 |
−0.06 (−0.13, 0.02) P=0.144 Q=0.222 |
| (log) WMH Volume (mL) * | −0.14 (−0.24, −0.05) P=0.003 Q=0.012 |
−0.11 (−0.21, −0.01) P=0.035 Q=0.070 |
−0.16 (−0.24, −0.09) P<0.001 Q=0.006 |
−0.11 (−0.19, −0.03) P=0.010 Q=0.031 |
| HC Volume (mL)* | 0.16 (0.07, 0.24) P<0.001 Q=0.006 |
0.10 (0.02, 0.18) P=0.012 Q=0.034 |
0.13 (0.06, 0.21) P<0.001 Q=0.006 |
0.05 (−0.02, 0.13) P=0.161 Q=0.230 |
| Brain Volume (L) * | 0.19 (0.03, 0.34) P=0.017 Q=0.043 |
0.16 (0.01, 0.31) P=0.035 Q=0.070 |
0.20 (0.06, 0.33) P=0.005 Q=0.018 |
0.19 (0.03, 0.34) P=0.019 Q=0.044 |
Comparison units for MRI findings (independent variable) include infarct, lacune, and hemorrhage present vs absent; WMH grade, sulcal grade, and ventricle grade per 1 point higher grade, range 0-9; log WMH volume, HC volume per 1 mL increase in volume; brain volume per 1 L increase in volume. Model adjusted for age, sex, site, marital status, income, education, Native language speaking ability (bilingual), obesity, diabetes, hypertension, hypercholesterolemia; 3MSE: Modified Mini Mental State Examination; CVLT LF: California Verbal Learning Test 2nd Edition Short Form long delay free recall; WAIS-IV: Weschler Adult Intelligence Scale 4th Edition (Coding sub-test); COWA: Controlled Oral Word Association test (F, A, S words); FDR (Q-value): false-discovery rate corrected P-value
Volumetric models adjusted for model 2, as well as for intracranial volume; volumetric beta coefficient & 95% CI estimates that are listed as 0 were calculated to 3 decimal places as <0.0001
DISCUSSION
Overview
Neuropsychological test performance was associated with findings from cranial MRI in this sample of American Indian elders, although some of these associations were diminished with the addition of sociodemographic, health, and behavioral characteristics in regression models. WAIS-IV Coding test, a measure of processing speed, was independently associated with several cranial MRI findings of both vascular injury and cortical and central atrophy. Processing speed has generally been the most consistent correlate of cerebral vascular injury in other large, community-based studies of elderly adults from other racial or ethnic populations, as well. Vascular injury has been generally associated with deficits in frontal lobe-mediated functioning— especially executive functioning and processing speed (Erkinjuntti, et al., 2000; Jokinen et al., 2009; Miralbell, et al., 2013), although processing speed has also been associated with white matter integrity (Aggarwal, et al., 2010; Longstreth, et al., 2005; Moseley & Linden, 2006; Prins, et al., 2005; Zahodne, et al., 2015), infarcts (Aggarwal, et al., 2010; Jokinen et al., 2009; Longstreth, et al., 2002; Mosley, et al., 2005; Prins, et al., 2005; Saczynski, et al., 2009), ventricle grade (Moseley & Linden, 2006), and brain atrophy (Aggarwal, et al., 2010; Prins, et al., 2005; Zahodne, et al., 2015).
Comparison with Other Populations: Infarct lesions
Other large studies of elderly adults of European or Asian descent, including middle-aged and elderly US non-Hispanic whites, have reported significant associations for neuropsychological tests of different cognitive domains with infarcts, WMH, cerebral atrophy, and cortical damage— often resulting from multiple, clinically-covert processes acting in concert (Dhamoon, et al., 2018; Knopman, et al., 2015). Cerebral infarcts, in particular, have been reported to increase risk of impairment and dementia across multiple cognitive domains including verbal fluency, memory, processing speed, executive function, motor function, and mood, independent of cardiovascular comorbidities, other lesions, and cerebral atrophy (Mosley, et al., 2005; Pantoni, Fierini, Poggesi, & Group, 2015; Saczynski, et al., 2009; Warren, et al., 2015), with more infarcts increasing the degree of risk (Saczynski, et al., 2009; Schnieder, et al., 2003; Vermeer, et al., 2003). Cerebral infarcts are often categorized by affected location, such as subcortical and cortical, with implications for different cognitive functions. Subcortical--thalamic, in particular--lesions have been associated with poor memory (Debette, et al., 2010; Saczynski, et al., 2009; Vermeer, et al., 2003), global cognitive functioning, motor speed, attention, executive functions, verbal fluency, and working memory (Jokinen et al., 2009); whereas a combination of both cortical and subcortical lesions has been associated with slower processing speed and poorer executive functioning.(Saczynski, et al., 2009)
In our study of American Indian elders, the presence of any infarcts, regardless of location, was associated with general cognitive function (3MSE) and processing speed (WAIS-IV Coding), but not with verbal memory or executive function (COWA, CVLT); after adjustment for multiple comparisons, significant associations included only any type of infarct, irrespective of location, with processing speed. Future research may be directed towards further elucidating these associations between location and number of lesions in association with various domains of cognitive dysfunction.
Comparison with Other Populations: Hemorrhages
Aneurysmal subarachnoid hemorrhage, the accumulation of blood in the subarachnoid space, impacts cognitive functioning depending on the location and extent of the hemorrhage, including memory, executive functioning, and language, or verbal memory.(Al-Khindi, Macdonald, & Schweizer, 2010) Both verbal and visual memory are predominantly mediated by lesions in the medial temporal lobes, whereas impairment in executive function is typically associated with lesions in the frontal lobes (Al-Khindi, et al., 2010). Even microbleeds—small hemorrhages characterized by homogeneous, round foci of low signal intensity on MRI gradient echo—can increase risk of larger hemorrhage, stroke, and dementia (Charidimou, et al., 2018), thus carrying possible therapeutic implications for prognosis in hypertension, diabetes, and stroke (Cordonnier, Al-Shahi Salman, & Wardlaw, 2007). Specific associations with microbleeds reported by large cohort studies include global neuropsychiatric burden, in particular symptoms of depression and disinhibition (Xu, et al., 2017), impairment in fluency, attention, and executive function domains (Valenti, et al., 2016), impairment in walking speed, independent of cognitive performance (Stijntjes, et al., 2016). Smaller clinical studies have identified possible mechanisms including reduction in resting state cerebral blood flow and chronic hypoperfusion (Gregg, et al., 2015) or hypertensive microangiopathy (Yamashiro, et al., 2014), with specific effects on cognition and function relating to specific regions affected.
Hemorrhage of any size was only associated with verbal memory (CVLT) in our study, and was not significant after adjustment for multiple comparisons; however, these limited findings may be due to the small number of elderly American Indian participants who had evidence of hemorrhage, and the broad definition in our measure of hemorrhage. Few studies have examined racial and ethnic differences in cerebral vascular injury, including both infarct and hemorrhage, or in the cognitive sequelae. One study has found no difference in microbleed presence and location, comparing African American and Caucasian ischemic stroke patients, but the presence of multiple microbleeds (≥5) was higher for those with African heritage (Shahjouei, et al., 2017).
Comparison with Other Populations: Small Vessel Disease
White matter lesions have been associated with poorer cognitive functioning in several cognitive domains in both middle-aged and elderly US non-Hispanic whites, including general cognitive functioning, processing speed, executive functioning, verbal fluency, and episodic memory (Longstreth, et al., 2005; Longstreth, et al., 2002; Mosley, et al., 2005). White matter lesion location may be critically important to identifying or evaluating risk of specific cognitive or functional consequences. Episodic memory was most associated with lesions in bilateral temporal-occipital lobes, right parietal periventricular space, and the left anterior limb of the internal capsule. Executive functioning was most associated with bilateral temporal-occipital and right parietal periventricular white matter lesions, and the anterior limb of the internal capsule bilaterally (Smith, et al., 2011). White matter disease burden was also associated with amnestic mild cognitive impairment in those aged 60 and older (Debette, et al., 2010). Previous reports have also found a greater burden of white matter lesions associated with hypertension, impaired glucose tolerance, and mood (Yamawaki, et al., 2015), and with verbal learning slope (Glazer, et al., 2015). The anterior thalamic radiation and forceps minor may also be strategic tracts in which white matter lesions are most strongly associated with cognitive impairment (Biesbroek, et al., 2016).
In our study, graded white matter disease was associated with processing speed (WAIS-IV Coding), but volumetric WMH measure was additionally associated with general cognition (3MSE), verbal memory (CVLT), verbal fluency and executive function (COWA), although these associations were not significant after adjustment for multiple comparisons. Future research should consider associations by brain structure and location, such as voxel-based volumetric analyses; as well as additional measures of episodic memory and verbal learning to more comprehensively examine the effect of small vessel disease in elderly American Indians.
Comparison with Other Populations: Cerebral Atrophy
Previous reports from the Strong Heart Study associated brain volume and hippocampus volume with processing speed (WAIS); brain volume and left hippocampus volume with verbal memory (CVLT); and left hippocampus volume with general cognition (3MSE), all independent of apolipoprotein E genotype (B. Cholerton, et al., 2017). Our study echoes these findings; hippocampus volume was associated with general cognition (3MSE), verbal memory (CVLT), and processing speed (WAIS-IC Coding); brain volume was associated with processing speed (WAIS-IV Coding) and verbal fluency (COWA)—although this last association was marginally significant after adjustment for multiple comparisons. Future research will benefit from longitudinal measures in order to directly examine atrophy in these and other brain regions.
In middle-aged US non-Hispanic whites, both sulcal and ventricular enlargement have been associated with poorer memory, and ventricle enlargement additionally with processing speed (Mosley, et al., 2005). In our study, both sulcal and ventricle grade were associated with processing speed (WAIS-IV Coding), and sulcal grade additionally with verbal fluency and executive function (COWA); however, these associations were not significant after adjustment for multiple comparisons. Some discrepancy between existing literature and our study may be due to differences in age of populations, as well as other socio-demographic and cultural disparities.
Effect Modification by Age and Sex
Age may present a particularly strong confounding or modifying influence on cognition. For example, WMH and hippocampal volume influenced cognitive decline in processing speed and executive functioning tasks, independent of age, in a sample of older participants aged 75-90 years (Papp, et al., 2014). Similarly, voxel-wise analyses revealed significant associations between WMH and both increasing age and processing speed and flexibility in regions throughout the brain (Birdsill, et al., 2014).
Sex may also modify the associations described. However, adjustment for characteristics that are in the causal pathway, that are caused by sex (e.g. smoking, alcohol use, heart conditions) would estimate only the partial effect of sex on cognition, i.e. the portion that is not mediated by the adjustment parameters. It is possible this estimate reflects some difference in biological sex, but it is also possible that it reflects some other effect for which sex is a proxy variable. Additionally, in previous work, our group found that only the CVLT and physical function tests were associated with participant sex in unadjusted analyses (Verney, et al., in press). Future research may examine these questions further through structured longitudinal models
Comparison with Other Populations: Cognitive Test Performance
Normative and diagnostic standards for cognitive tests are well-established for US non-Hispanic whites, including 3MSE (Jones & Gallo, 2002), WAIS-IV (Wisdom, Mignogna, & Collins, 2012), COWA (Tombaugh, Kozak, & Rees, 1999), and CVLT (Fine, Kramer, Lui, Yaffe, & Study of Osteoporotic Fractures Research, 2012). Compared with these normative standards, mean scores and overall distributions for our study were all left-shifted, suggesting need for formal neuropsychological test validation in this population. Education—both length and quality, language, culture, economic, social, and other factors can influence neuropsychological testing performance, and thus both normative and diagnostic scores, which limits interpretability of scores on an absolute scale in the absence of such validations. Future research to conduct domain and construct validation, with score calibration, will benefit clinical interpretability of these findings and support development of better understanding of cognitive impairment and dementia in elderly American Indians.
Limitations
This is a cross-sectional examination, so we were not able to directly evaluate temporal sequence in any associations. Also, although this examination includes the largest cohort study of American Indian elders from 3 major geographic regions, these findings may not be generalizable to other populations, including other American Indian or Alaska Native groups, or other indigenous populations. Additionally, because of the high degree of vascular risk in this population, participants may not represent the full range of exposures, including an “unexposed” group, which could limit the discoverability of associations. Further, inclusion of this study to survivors from study baseline could introduce bias in the form of differential selection if those who did not survive to participate had especially strong associations between cerebral vascular injury and cognitive status; such a limitation would tend to increase the possibility of Type II error, limiting the discoverability of true associations. However, previous analyses of differential survival from the baseline recruitment period (1989-1991) to the MRI examination (2010-2013) suggested that there were not major differences in vascular risk and comorbidities between those who did and did not participate in the follow-up visit (Suchy-Dicey, et al., 2018). It is possible that vascular comorbidities may be effect modifiers for the observed associations in this study. Future longitudinal research should examine these questions for diabetes, hypertension, and renal disease, all of which may have profound influence on cognitive function.
Summary
Several associations of MRI findings were detected among different cognitive domains, many similar to previous findings in non-Hispanic white and other populations, after adjustment for critically confounding characteristics. Some differences may represent effects from age, selection pressures, differences in neuropsychological test performance, or pathological discrepancies. Future research should examine these questions more fully. In summary, this is the first study of MRI-defined cerebral vascular injury and neuropsychological testing performance-defined cognitive function in a large cohort of American Indian elders, a population which experiences many chronic health and socio-demographic conditions that can lead to premature aging, increase risk of cerebral vascular injury and cerebral atrophy, and result in development of physical and cognitive impairment or dementia (Shiels, et al., 2017; Verney et al., 2015). Developing better understanding of the significant cultural, educational, economic, linguistic, and public health diversity among the 567 federally-recognized U.S. tribes, the many non-tribal indigenous communities, as well as other similarly-exposed minority populations, is critical to improving brain health and cognitive care in elderly peoples, in order to improve prevention efforts, inform risk and diagnostic assessments, and reduce health disparities overall.
Supplementary Material
Acknowledgements
None of the authors has any conflicts of interest to report. This work was supported by the National Heart Lung and Blood Institute (grants U01HL41642, U01HL41652, U01HL41654, U01HL65520, U01HL65521, R01HL109315, R01HL109301, R01HL109284, R01HL109282, R01HL109319, and R01HL093086); the National Institute on Aging (grants P50 AG005136 and R01AG049084); the National Institute on Minority Health and Health Disparities (2 U54 MD004811); and the Nancy and Buster Alvord Endowment (Thomas J. Montine). The opinions expressed in this manuscript are those of the authors, and do not necessarily reflect those of the Indian Health Service (IHS). The authors wish to thank all study communities, participants, and staff.
REFERENCES
- Aggarwal NT, Wilson RS, Bienias JL, De Jager PL, Bennett DA, Evans DA, & DeCarli C (2010). The association of magnetic response imaging measures with cognitive function in a biracial population sample. Arch Neurol, 67, 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khindi T, Macdonald RL, & Schweizer TA (2010). Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke, 41, e519–536. [DOI] [PubMed] [Google Scholar]
- Arnold AM, Psaty BM, Kuller LH, & al., e. (2005). Incidence of cardiovascular disease in older Americans: the cardiovascular health study. Journal of American Geriartrics Society, 53, 211–218. [DOI] [PubMed] [Google Scholar]
- Ayala C, Greenlund KJ, Croft JB, Keenan NL, Donehoo RS, Giles WH, … Marks JS (2001). Racial/ethnic disparities in mortality by stroke subtype in the United States, 1995-1998. Am J Epidemiol, 154, 1057–1063. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, H. Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B, 57, 289–300. [Google Scholar]
- Benton AL, & Hansher K (1976). Multilingual aphasia examination (2nd ed.). Iowa City, IO: AJA Associates. [Google Scholar]
- Biesbroek JM, Weaver NA, Hilal S, Kuijf HJ, Ikram MK, Xu X, … Chen CP (2016). Impact of Strategically Located White Matter Hyperintensities on Cognition in Memory Clinic Patients with Small Vessel Disease. PLoS One, 11, e0166261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsill AC, Koscik RL, Jonaitis EM, Johnson SC, Okonkwo OC, Herman BP, … Bendlin BB (2014). Regional white matter hyperintensities, aging, AD risk, and cognitive function. Neurobiol Aging, 35, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2004). Diabetes prevalence among American Indians and Alaska Natives and the overall population- United States, 1994-2002. Morb Mortal Wkly Rep, 52 (30), 702–704. [PubMed] [Google Scholar]
- Charidimou A, Shams S, Romero JR, Ding J, Veltkamp R, Horstmann S, … International, M.- M. I. (2018). Clinical significance of cerebral microbleeds on MRI: A comprehensive meta-analysis of risk of intracerebral hemorrhage, ischemic stroke, mortality, and dementia in cohort studies (v1). Int J Stroke, 13, 454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholerton B, Baker LD, & Craft S (2011). Insulin resistance and pathological brain ageing. Diabet Med, 28, 1463–1475. [DOI] [PubMed] [Google Scholar]
- Cholerton B, Omidpanah A, Madhyastha TM, Grabowski TJ, Suchy-Dicey AM, Shibata DK, Nelson LA, Verney SP, Howard BV, Longstreth WT Jr., Montine TJ, & Buchwald D (2017). Total Brain and Hippocampal Volumes and Cognition in Older American Indians: The Strong Heart Study. Alzheimer Dis Assoc Disord, 31, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C, Al-Shahi Salman R, & Wardlaw J (2007). Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain, 130, 1988–2003. [DOI] [PubMed] [Google Scholar]
- Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, … Seshadri S (2010). Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The Framingham Offspring Study. Stroke, 41, 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Reed BR, Jagust WJ, Martinez O, Ortega M, & Mungas D (2008). Brain behavior relationships amongst African Americans, Caucasians and Hispanics. Alzheimer Dis Assoc Disord, 22, 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). California Verbal Learning Test (CVLT-II) (2 ed.). US: The Psychological Corporation. [Google Scholar]
- Dhamoon MS, Cheung YK, Gutierrez J, Moon YP, Sacco RL, Elkind MSV, & Wright CB (2018). Functional Trajectories, Cognition, and Subclinical Cerebrovascular Disease. Stroke, 49, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drag LL, & Bieliauskas LA (2010). Contemporary Review 2009: Cognitive Again. J Geriatr Psychiatry Neurol, 23, 75–93. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, & al., e. (2000). Research criteria for subcortical vascular dementia in clinical trials. Journal of Neural Transmission Supplement, 59, 23–30. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Sherzai A, Kaup AR, & Jeste DV (2011). A review of functional brain imaging correlates of successful cognitive aging. Biol Psychiatry, 70, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine EM, Kramer JH, Lui LY, Yaffe K, & Study of Osteoporotic Fractures Research, G. (2012). Normative data in women aged 85 and older: verbal fluency, digit span, and the CVLT-II short form. Clin Neuropsychol, 26, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum RF (1995). The epidemiology of stroke in Native Americans. Stroke, 26, 514–521. [DOI] [PubMed] [Google Scholar]
- Glazer H, Dong C, Yoshita M, Rundek T, Elkind MS, Sacco RL, ., … Wright CB (2015). Subclinical cerebrovascular disease inversely associates with learning ability: The NOMAS. Neurology, 84, 2362–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, ., … Peterson RC (2011). Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 42, 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg NM, Kim AE, Gurol ME, Lopez OL, Aizenstein HJ, Price JC, ., … Klunk WE (2015). Incidental Cerebral Microbleeds and Cerebral Blood Flow in Elderly Individuals. JAMA Neurol, 72, 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R, Nelson LA, Muller C, & Buchwald D (2015). Stroke in American Indians and Alaska Natives: A Systematic Review. Am J Public Health, 105, e16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwell TS, Oser CS, Okon NJ, Fogle CC, Helgerson SD, & Gohdes D (2005). Defining disparities in cardiovascular disease for American Indians: trends in heart disease and stroke mortality among American Indians and whites in Montana, 1991 to 2000. Circulation, 112, 2263–2267. [DOI] [PubMed] [Google Scholar]
- Hill CV, Perez-Stable EJ, Anderson NA, & Bernard MA (2015). The National Institute on Aging Health Disparities Research Framework. Ethnicity & Disease, 25, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson RN, & Shin S (2014). Systematic review of health disparities for cardiovascular diseases and associated factors among American Indian and Alaska Native populations. PLoS One, 9, e80973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, Gouw A, & al., e. (2009). MRI-defined subcortical ischemic vascular disease: Baseline clinical and neuropsychological findings. The LADIS Study. Cerebrovascular Disease, 27, 336–344. [DOI] [PubMed] [Google Scholar]
- Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, Gouw A, ., … group, o. b. o. t. L. (2009). MRI-defined subcortical ischemic vascular disease: Baseline clinical and Neuropsychological findings. Cerebrovasc Dis, 27, 336–344. [DOI] [PubMed] [Google Scholar]
- Jones RN, & Gallo JJ (2002). Education and sex differences in the mini-mental state examination: effects of differential item functioning. J Gerontol B Psychol Sci Soc Sci, 57, P548–558. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, ., … & Investigators, A. N. (2015). Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke, 46, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, ., … Howard BV (1990). The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol, 132, 1141–1155. [DOI] [PubMed] [Google Scholar]
- Longstreth WT Jr., Arnold AM, Beauchamp NJ Jr., Manolio TA, Lefkowitz D, Jungreis C, ., … Furberg CD (2005). Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke, 36, 56–61. [DOI] [PubMed] [Google Scholar]
- Longstreth WT Jr., Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ Jr., O'Leary D, ., … Furberg CD (2002). Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke, 33, 2376–2382. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA (2010). Diabetes, related conditions, and dementia. J Neurol Sci, 299, 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralbell J, Lopez-Cancio E, Lopez-Oloriz J, Arenillas JF, Barrios M, Soriano-Raya JJ, ., … Mataro M (2013). Cognitive patterns in relation to biomarkers of cerebrovascular disease and vascular risk factors. Cerebrovascular Disease, 36, 98–105. [DOI] [PubMed] [Google Scholar]
- Morris Z, Whiteley WN, Longstreth WT Jr, Weber F, Lee Y-C, Tsushima Y, ., … Salman RA-S (2009). Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ, 339, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley JV, & Linden W (2006). Predicting blood pressure and heart rate change with cardiovascular reactivity and recovery: results from 3-year and 10-year follow up. Psychosom Med, 68, 833–843. [DOI] [PubMed] [Google Scholar]
- Mosley TH Jr., Knopman DS, Catellier DJ, Bryan N, Hutchinson RG, Grothues CA, ., … Szklo M (2005). Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology, 64, 2056–2062. [DOI] [PubMed] [Google Scholar]
- Office of Management and Budget. (1997). Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. In O. o. I. a. R. Affairs (Ed.). Washington DC: Federal Register Notice. [Google Scholar]
- Pantoni L, Fierini F, Poggesi A, & Group, L. S. (2015). Impact of cerebral white matter changes on functionality in older adults: An overview of the LADIS Study results and future directions. Geriatr Gerontol Int, 15 Suppl 1, 10–16. [DOI] [PubMed] [Google Scholar]
- Papp KV, Kaplan RF, Springate B, Moscufo N, Wakefield DB, Guttman CRG, & Wolfson L (2014). Processing speed in normal aging: Effects of white matter hyperintensities and hippocampal volume loss. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, 21, 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, ., … Breteler MMB (2005). Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain, 128, 2034–2041. [DOI] [PubMed] [Google Scholar]
- Rosamond WD, Folsom AR, Chambless LE, & al., e. (1999). Stroke incidence and survival among middle-aged adults: 9 year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke; a journal of cerebral circulation, 30, 736–743. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Jonsson PV, Garcia ME, ., … Launer LJ (2009). Cerebral infarcts and cognitive performance: Importance of location and number of infarcts. Stroke, 40, 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanand S, Balachandar R, & Bharath S (2015). Memory and executive functions in persons with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. [DOI] [PubMed] [Google Scholar]
- Schnieder JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, & Bennett DA (2003). Relation of cerebral infarctions to dementia and cognitive funciton in older persons. Neurology, 60, 1082–1088. [DOI] [PubMed] [Google Scholar]
- Shahjouei S, Tsivgoulis G, Singh M, McCormack M, Noorbakhsh-Sabet N, Goyal N, ., … Zand R (2017). Racial Difference in Cerebral Microbleed Burden among Ischemic Stroke Patients. J Stroke Cerebrovasc Dis, 26, 2680–2685. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Chernyavskiy P, Anderson WF, Best AF, Haozous EA, Hartge P, ., … Berrington de Gonzalez A (2017). Trends in premature mortality in the USA by sex, race, and ethnicity from 1999 to 2014: an analysis of death certificate data. Lancet, 389, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Salat DH, Jengs J, McCreary CR, Fischi B, Schmahmann JD, ., … Greenberg SM (2011). Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology, 76, 1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. (2014). Stata Statistical Software: Release 14. In. College Station, TX: StataCorp LP. [Google Scholar]
- Stijntjes M, de Craen AJ, van der Grond J, Meskers CG, Slagboom PE, & Maier AB (2016). Cerebral Microbleeds and Lacunar Infarcts Are Associated with Walking Speed Independent of Cognitive Performance in Middle-Aged to Older Adults. Gerontology, 62, 500–507. [DOI] [PubMed] [Google Scholar]
- Strachan MW (2003). Insulin and cognitive function. Lancet, 362, 1253. [DOI] [PubMed] [Google Scholar]
- Suchy-Dicey AM, Muller CJ, Madhyastha TM, Shibata D, Cole SA, Zhao J, ., … Buchwald D (2018). Telomere Length and Magnetic Resonance Imaging Findings of Vascular Brain Injury and Central Brain Atrophy: The Strong Heart Study. Am J Epidemiol, 187, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchy-Dicey AM, Shibata D, Best LG, Verney SP, Longstreth WT Jr., Lee ET, ., … Buchwald D (2016). Cranial Magnetic Resonance Imaging in Elderly American Indians: Design, Methods, and Implementation of the Cerebrovascular Disease and Its Consequences in American Indians Study. Neuroepidemiology, 47, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchy-Dicey AM, Shibata DK, Madhyastha TM, Grabowski TJ, Longstreth WT Jr., & Buchwald DS (2017). Findings of Vascular Brain Injury and Structural Loss from Cranial Magnetic Resonance Imaging in Elderly American Indians: The Strong Heart Study. Neuroepidemiology, 48, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki LA, Naqvi S, & Hill JS (2013). Assessing Intelligence in a Cultural Context In Leong FTL (Ed.), APA Handbook of Multicultural Psychology: Theory and Research (Vol. 1, pp. 247–266). Washington, DC: American Psychological Association. [Google Scholar]
- Team, R. C. (2016). R: A language and environment for statistical computing. In. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Teng EL, & Chang Chui H (1987). The Modified Mini-Mental (3MS) Examination. Journal Clinical Psychiatry, 48, 314–318. [PubMed] [Google Scholar]
- Teng EL, & Chui HC (1987). The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry, 48, 314–318. [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, & Rees L (1999). Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol, 14, 167–177. [PubMed] [Google Scholar]
- Tournoy J, Lee DM, Pendleton N, O'Neill TW, O'Connor DB, Bartfai G, ., … group, E. s. (2010). Association of cognitive performance with the metabolic syndrome and with glycaemia in middle-aged and older European men: the European Male Ageing Study. Diabetes Metab Res Rev, 26, 668–676. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2014). Healthy People 2020: Foundation Health Measures Archive on Disparities. In O. o. D. P. a. H. P. (ODPHP) (Ed.). Washington, DC. [Google Scholar]
- Valenti R, Del Bene A, Poggesi A, Ginestroni A, Salvadori E, Pracucci G, ., … Group, V. M.-T. S. (2016). Cerebral microbleeds in patients with mild cognitive impairment and small vessel disease: The Vascular Mild Cognitive Impairment (VMCI)-Tuscany study. J Neurol Sci, 368, 195–202. [DOI] [PubMed] [Google Scholar]
- van den Berg E, Kessels RP, Kappelle LJ, de Haan EH, Biessels GJ, & Utrecht Diabetic Encephalopathy Study, G. (2006). Type 2 diabetes, cognitive function and dementia: vascular and metabolic determinants. Drugs Today (Barc), 42, 741–754. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, & Breteler MMB (2003). Silent brain infarcts and the risk of dementia and cognitive decline. New England Journal of Medicine, 348. [DOI] [PubMed] [Google Scholar]
- Verney SP, Bennett J, & Hamilton JM (2015). Cultural Considerations in the Neuropsychologica Assessment of American Indians/Alaska Natives In Ferraro FR (Ed.), Minority and Cross-Cultural Aspects of Neuropsychological Assessment (2nd ed.). Lisse, Netherlands: Taylor & Francis. [Google Scholar]
- Verney SP, Suchy-Dicey A, Cholerton B, Calhoun D, Nelson L, Montine T, Ali T, Longstreth WJ, & Buchwald D (in press). The associations among sociocultural factors and neuropsychological functioning in older American Indians: the Strong Heart Study. Neuropsychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MW, Weiner MF, Rossetti HC, McColl R, Peshock R, & King KS (2015). Cognitive impact of lacunar infarcts and white matter hyperintensity volume. Dement Geriatr Cogn Dis Extra, 5, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2008). Wechsler Adult Intelligence Scale (4th ed.). In (4th ed.). San Antonio, TX: Pearson. [Google Scholar]
- Wisdom NM, Mignogna J, & Collins RL (2012). Variability in Wechsler Adult Intelligence Scale-IV subtest performance across age. Arch Clin Neuropsychol, 27, 389–397. [DOI] [PubMed] [Google Scholar]
- Xu X, Chan QL, Hilal S, Goh WK, Ikram MK, Wong TY, ., … Venketasubramanian N (2017). Cerebral microbleeds and neuropsychiatric symptoms in an elderly Asian cohort. J Neurol Neurosurg Psychiatry, 88, 7–11. [DOI] [PubMed] [Google Scholar]
- Yamashiro K, Tanaka R, Okuma Y, Shimura H, Ueno Y, Miyamoto N, ., … Hattori N (2014). Cerebral microbleeds are associated with worse cognitive function in the nondemented elderly with small vessel disease. Cerebrovasc Dis Extra, 4, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki M, Wada-Isoe K, Yamamoto M, Nakashita S, Uemura Y, Takahashi Y, ., … Nakashima K (2015). Association of cerebral white matter lesions with cognitive function and mood in Japanese elderly people: a population-based study. Brain Behav, 5, e00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne L, Manly JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, ., … Brickman AM (2015). Structural MRI predictors of late-life cogntiion differ across African Americans, Hispanics, and Whites. Current Alzheimer Research, 12, 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereaux RB, ., … Lee ET (2008). Incidence and Risk Factors for Stroke in American Indians: The Strong Heart Study. Circulation, 118, 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, ., … Lee ET (2008). Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation, 118, 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Roberts RO, Ding D, Cha R, Guo Q, Meng H, Luo J, ., … Petersen RC (2015). Diabetes is Associated with Worse Executive Function in Both Eastern and Western Populations: Shanghai Aging Study and Mayo Clinic Study of Aging. J Alzheimers Dis, 47, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.