Abstract
Natural killer (NK) cells are thought to play a key role in the successful establishment of a pregnancy by facilitating immunological adaptation of the semi-allogeneic developing embryo. The aim of this study was to explore the cell number, immunophenotypic characteristics, and activities of peripheral blood NK cells in women with repeated implantation failure (RIF). Peripheral blood was obtained from 27 women with RIF and 11 healthy, fertile controls during the middle luteal phase of the menstrual cycle. CD3- CD56+ NK cells were quantified and analyzed by flow cytometry for the expression of cytolytic molecules (granzyme B, granulysin, and perforin) as well as cell surface receptors responsible for NK cell activation or inhibition (NKG2D, NKp30, NKp46, CD158a, CD158b). NK cytotoxicity was measured at three effector-to-target cell ratios. Women with RIF and fertile controls did not differ significantly in the percentage of circulating CD3−CD56+ NK cells, or in the proportions of these cells that expressed granzyme B, granulysin, or perforin. The two groups also did not differ significantly in the proportions of NK cells expressing the receptors NKG2D, NKp30, NKp46, CD158a or CD158b. General linear model analysis showed that NK cytotoxicity increased with effector-to-target cell ratio. However, NK cytotoxicity did not differ significantly between patients with RIF and fertile controls. These results suggest that RIF is not associated with significant alterations in the number or function of peripheral blood NK cells.
Keywords: Activating receptor, Inhibitory receptor, NK cytotoxicity, Peripheral blood NK cells, Repeated implantation failure
Introduction
Infertility is an increasing social and medical problem that affects about 10% of child-bearing couples.1 Although assisted reproductive technology is effective for many infertile couples, there are still many patients who failed to conceive after several cycles of in vitro fertilization treatments (IVF). These patients are defined as having experienced repeated implantation failure (RIF).2
During implantation, recognition of the semi-allogeneic fetus by the maternal immune system is crucial for successful placentation and maintenance of normal pregnancy. NK cells constitute 5–10% of peripheral blood lymphocytes, and the main population of peripheral blood NK cells are CD56dimCD16+.3 One study suggested that the number and/or activity of peripheral blood NK cells may be elevated in women with RIF,4 suggesting that these parameters may be predictors of pregnancy loss. Similarly, another study showed increased NK cell cytotoxicity in women with a chromosomally normal pregnancy who miscarried.5 On the other hand, several studies have reported no significant difference in peripheral blood NK cell cytotoxicity between women with recurrent miscarriage or infertility and healthy controls,6, 7, 8 leading to the suggestion that elevated peripheral blood NK cell cytotoxicity may not be associated with pregnancy outcome.9 The role of peripheral blood NK cells during pregnancy remains controversial and further research is needed.
When an NK cell encounter with a target cell, they will release cytolytic granules containing granzymes, granulysin, and perforin to clear the target cell. In the first, positively charged granulysin can bind to a negatively charged cell membrane, leading to membrane damage.10 Granulysin exerts potent cytotoxic activity against a broad panel of microbial targets, tumor cells,11 and transplant cells.12 In the second pathway, perforin, a pore-forming protein, can bind to the target cell's membrane and contribute to the complement-mediated membrane attack complex. This allows granzymes to enter the cell and induce apoptosis by activating caspases.13 Slight elevation of perforin-positive uterine NK cells has been reported in human sporadic miscarriage.14 Whether levels of granulysin, perforin, or granzymes in peripheral blood NK cells are altered in women with RIF is unknown.
NK cytotoxicity against target cells is regulated by stimulatory and inhibitory receptors. Natural cytotoxicity receptors, which include NKp30, NKp44, and NKp46, activate and regulate NK cell cytotoxicity and cytokine production.15 The lectin-type NK cell receptor NKG2D also plays an important role in regulating NK cytotoxicity.16, 17 Inhibitory receptors such as CD158a and CD158b can inhibit activating signals through their immunoreceptor tyrosine-based inhibitory motifs.18 Little is known about the expression of stimulatory and inhibitory receptors on peripheral NK cells in women with RIF.
In this study, we aimed to analyze potential associations of RIF with the number and cytotoxicity of peripheral NK cells as well as the levels of cytotoxic molecules and NK receptors.
Materials and methods
Study population
The study was designed as a retrospective case-control study. Women who visited the Fertility Center of Shenzhen Zhongshan Urology Hospital from January 2016 to April 2017 were enrolled. The RIF group was composed of 27 women who experienced three or more IVF failures after transfer of at least one high-quality embryo per cycle.2, 19 A total of 11 healthy, fertile women who experienced at least one successful pregnancy were recruited to provide control samples.
A patient was excluded if she had (1) an abnormal chromosome karyotype; (2) a positive result on tests for human immunodeficiency virus, hepatitis B virus, hepatitis C virus, rapid plasma regain, Treponema pallidum particle assay, toxoplasma, rubella virus, cytomegalovirus, or herpes virus; (3) abnormal findings on ultrasonography and hysterosalpingogram/hysteroscopy; (4) abnormal basal levels of follicle-stimulating hormone, luteinizing hormone, estradiol, prolactin, or testosterone; or (5) male factor infertility. No patient was pregnant or on prescribed medication at the time of sample collection.
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Shenzhen Zhongshan Urology Hospital. All participants gave their informed consent for inclusion before they participated in the study.
Materials
K562 cells (China Center for Type Culture Collection, Wuhan, China) were used as target cells in the NK cytotoxicity assay. K562 cells were cultured in RPMI 1640 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA). 3,3′-Dioctadecyloxacarbocyanine perchlorate (DIO) (1911717; Invitrogen, San Diego, CA, USA) was dissolved in dimethyl sulfoxide (Sigma, St. Louis, MO, USA) to a concentration of 3 mM. Propidium iodide (PI; MKCB0899V; Sigma) was used to detect apoptosis.
Flow cytometry to identify NK cells and subpopulations expressing certain markers and effector molecules was performed using the following fluorophore-conjugated monoclonal mouse anti-human antibodies: PerCP-conjugated anti-CD3 (347344; BD Pharmingen, San Jose, CA, USA), PE-cy7-conjugated anti-CD56 (557747; BD Pharmingen), PE-conjugated anti-NKp30 (12–3379; eBioscience ThermoFisher, Waltham, MA, USA), APC-conjugated anti-NKp46 (17–3359; BD Pharmingen), FITC-conjugated anti-NKG2D (11–5878; eBioscience), FITC-conjugated anti-CD158a (556062; BD Pharmingen), PE-conjugated anti-CD158b (559785; BD Pharmingen), PE-conjugated anti-granzyme B (561142; BD Pharmingen), Alexa Fluor 647-conjugated anti-perforin (563576; BD Pharmingen), and Alexa Fluor 488-conjugated anti-granulysin (558254; BD Pharmingen).
Flow cytometric analysis of cytotoxic granules of NK cells
Heparinized whole blood was collected before 10 a.m. during the mid-luteal period of the menstrual cycle. Whole blood (100 μL) was incubated for 15 min with antibodies against the cell surface markers CD3 (5 μL) and CD56 (5 μL), then the blood was lysed using 1x FACS lysis solution (349202; BD Pharmingen). Cells were permeabilized with 1x Permeabilizing Solution 2 (340973; BD Pharmingen) for 10 min, then incubated for 30 min with antibodies against perforin, granzyme B and granulysin (10 μL each). Cells were analyzed on BD FACSCanto II flow cytometer using BD FACSDiva software.
Flow cytometric analysis of NK cell receptors
To assess surface expression of stimulatory NK cell receptors, an aliquot of whole blood (100 μL) collected as in section 2.3 was mixed with antibodies against CD3 (5 μL), CD56 (5 μL), NKp30 (10 μL), NKp46 (10 μL), and NKG2D (10 μL). To assess surface expression of inhibitory NK cell receptors, another blood aliquot was mixed with antibodies against CD3 (5 μL), CD56 (5 μL), CD158a (10 μL), and CD158b (10 μL). After 15-min incubation with the antibodies in the dark, the whole blood was lysed using 1×FACS lysis solution, then cells were analyzed on BD FACSCanto II flow cytometer using BD FACSDiva software.
NK cell cytotoxicity assay
NK cell cytotoxicity was measured as described.20 Briefly, peripheral blood mononuclear cells (PBMCs) as effector cells (E) were isolated by density gradient centrifugation using Ficoll-Paque Lymphoprep (Axis-Shield PoC As, Oslo, Norway). The target cells (T) were K562 leukemia cells, which were obtained from China Center for Type Culture Collection, Wuhan, China (GDC037), washed in phosphate-buffered saline (PBS) and stained with DIO. PBMCs and K562 cells were incubated at various E:T ratios in triplicate in 96-well Costar plates (Corning Incorporated, Corning, NY, USA) for 4 h at 37 °C and 5% CO2. After incubation, PI was added to each well to assess K562 cell viability. NK cytotoxicity was analyzed by flow cytometry and calculated as the percentage of K562 cells that were dead.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 (IBM, Chicago, IL, USA). A Kolmogorov-Smimov test was used to confirm the normal distribution of the data. Data were presented as mean ± SD or mean ± SE in the case of normally distributed data, or as median with quartiles in the case of skewed data. Inter-group differences in characteristics were examined using the independent t test in the case of normally distributed data, or the Mann-Whitney U test in the case of skewed data. Differences in NK cytotoxicity between the two groups at three E:T ratios were analyzed using a general linear model. Differences were analyzed using a Sphericity Assumed test when Mauchly's test of sphericity was associated with P > 0.05, or Pillai's Trace test when Mauchly's test was associated with P ≤ 0.05. A two-tailed P < 0.05 was considered statistically significant.
Results
Expression of cytotoxic granules in peripheral blood NK cells
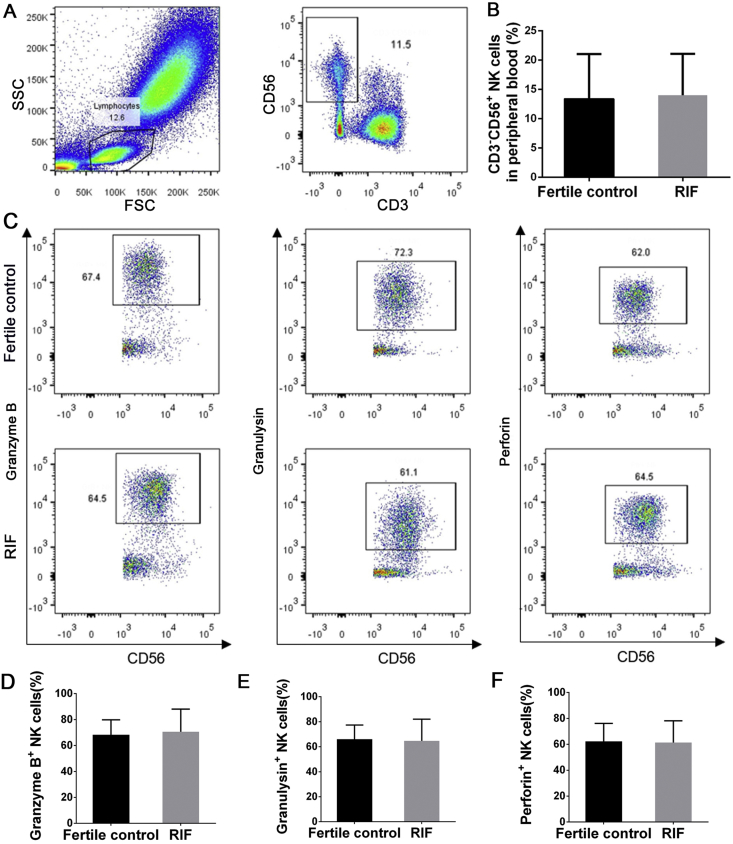
The total number of NK cells in peripheral blood and their expression of cytotoxic granules were investigated in the RIF and the fertile control groups. The two groups did not differ significantly in the percentage of circulating CD3−CD56+ NK cells (14.0% ± 7.1% vs 13.4% ± 7.6%, P = 0.82; Fig. 1A and B) or in the levels of cytotoxic granzyme B (70.6% ± 17.4% vs 68.3% ± 11.6%, P = 0.68), granulysin (64.7% ± 17.3% vs 66.1% ± 11.4%, P = 0.82), or perforin (61.4% ± 16.8% vs 62.3% ± 13.8%, P = 0.87) (Fig. 1C–F).
Figure 1.
Cytotoxic molecule expression by peripheral blood NK cells. (A) Representative flow cytometry plots of NK cells. (B) The percentage of peripheral NK cells in fertile controls and women with RIF. (C) Representative flow cytometry plots of granzyme B+, granulysin+, and perforin+ NK cells. Percentages of granzyme B+(D), granulysin+(E), and perforin+(F) NK cells are shown for fertile controls and women with RIF. Data are mean ± SD. Groups were compared using the independent t test.
Expression of stimulatory and inhibitory receptors on peripheral blood NK cells
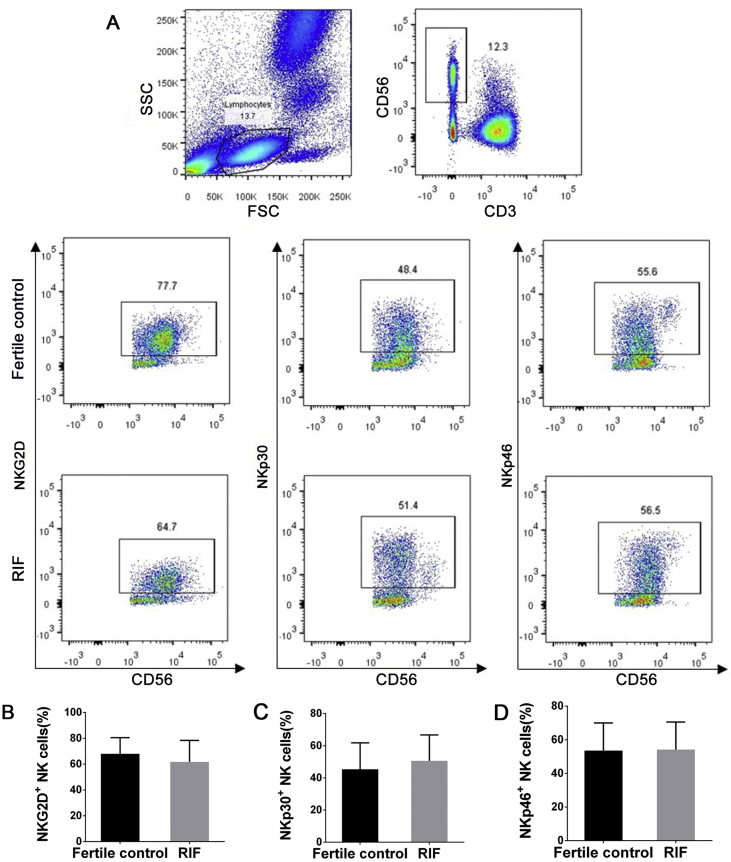
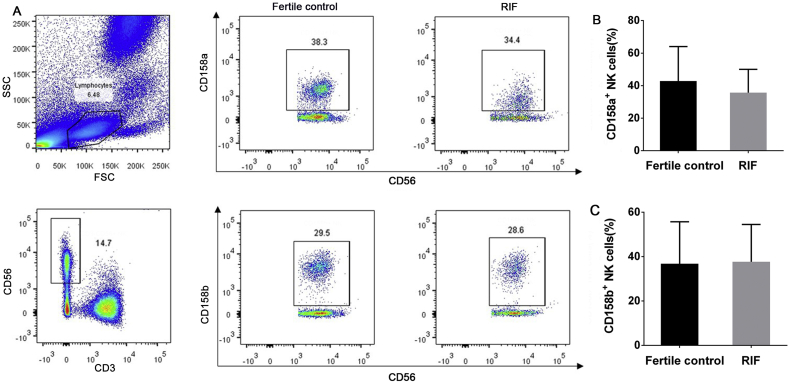
Women with RIF did not differ significantly from fertile controls in levels of NKG2D (61.7% ± 16.6% vs 67.9% ± 12.6%, P = 0.27), NKp30 (50.6% ± 16.1% vs 45.3% ± 16.5%, P = 0.37), or NKp46 (54.2% ± 16.4% vs 53.6% ± 16.4%, P = 0.92) (Fig. 2), or in the levels of CD158a (35.7% ± 14.3% vs 42.8% ± 21.2%, P = 0.23) or CD158b (37.7% ± 16.8% vs 36.8% ± 18.9%, P = 0.89) (Fig. 3).
Figure 2.
Analysis of stimulatory receptor expression on peripheral blood NK cells. (A) Flow dot plots represent the expression of stimulatory receptors NKG2D, NKp30, and NKp46. Numbers indicate the percentage of cells in the boxed area. Plots showing the difference in the frequency of NKG2D+(B), NKp30+(C), and NKp46+(D) NK cells between fertile controls (black) and women with RIF (gray). Small horizontal bars indicate mean ± SD. Groups were compared using the independent t test.
Figure 3.
Analysis of inhibitory receptor expression on peripheral blood NK cells. (A) Flow dot plots represent the expression of inhibitory receptors CD158a and CD158b. Numbers indicate the percentage of cells in the boxed area. Plots of the difference in the frequency of CD158a+(B) and CD158b+(C) NK cells between fertile controls (black) and women with RIF (gray). Small horizontal bars indicate mean ± SD. Groups were compared using the independent t test.
NK cytotoxicity assay
The general linear model showed that NK cytotoxicity increased with E:T ratio (P < 0.001) (Fig. 4). However, NK cytotoxicity did not differ significantly between patients with RIF and fertile controls at E:T ratios of 50:1 (40.9% ± 16.4% vs 45.1% ± 14.6%), 25:1 (31.8% ± 15.7% vs 35.7% ± 14.8%), or 12.5:1 (19.2% ± 12.1% vs 27.4% ± 10.2%) (P = 0.28).
Figure 4.
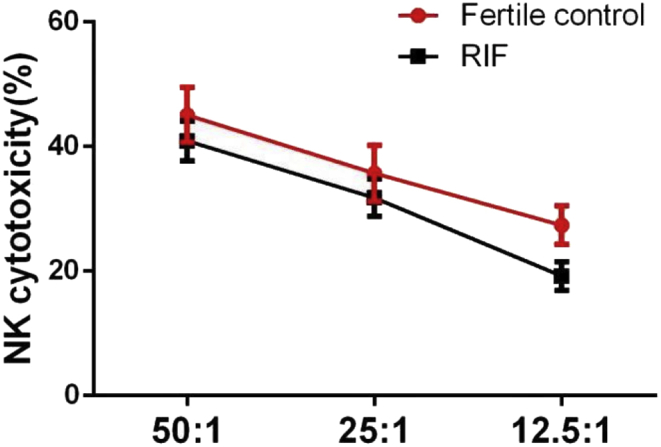
NK cytotoxicity assay. Cytotoxicity of peripheral blood NK cells from patients with RIF (red line) and from fertile controls (black line) at NK cell (E): K562 cell (T) ratios of 50:1, 25:1, and 12.5:1. Data are mean ± SE. Statistical analyses were performed using a general linear model.
Discussion
NK cells have been implicated in female reproductive performance. They have been associated with recurrent miscarriage or infertility due to either NK cell cytotoxicity or receptor expression.21 In the present study, we investigated the number, immunophenotypic characteristics, and cytotoxic capabilities of peripheral blood NK cells in women with RIF. Surprisingly, the data presented here suggest that RIF is not associated with significant alterations in the number of CD3−CD56+ NK cells; in the levels of granzyme B, granulysin, or perforin; in the expression of stimulatory or inhibitory receptors on NK cells; or NK cytotoxicity. These results provide evidence that peripheral blood NK cells may not be a clinically useful biomarker of immunological dysfunction in women with RIF.
During implantation, the embryo attaches to the luminal surface of the endometrium, then it migrates and invades in order to embed deeper in uterine tissue.22 The temporal and spatial distribution of uterine NK cells indicate that they play a key role in regulating trophoblast invasion in human pregnancy. In contrast, a potential contribution of peripheral blood NK cells to pathophysiology during pregnancy, such as in RIF, is still under debate. Several studies suggest that elevated peripheral blood NK cell number and/or their dysregulated function may contribute to RIF.23, 24 However, we failed to find any significant difference in the phenotypes or function of peripheral blood NK cells between women with RIF and fertile controls. Our findings are in agreement with previous work showing that peripheral blood NK cells are not related to recurrent failed IVF treatments, and may have no predictive value for pregnancy outcome.9
We did not observe differences in the expression of NK cytotoxic granules or NK surface receptors between women with RIF and fertile controls, but there are other pathways by which NK cells kill infected or transformed cells. For example, they can kill cells via pathways involving death receptors such as Fas and TRAIL.25, 26 Future work should examine the possibility that peripheral blood NK cells cause cytotoxicity by this or other pathways in women with RIF.
Against the tenets of transplantation immunology, the fetus, which is an allograft, survives in the maternal body during pregnancy. There is no direct contact between the mother and embryo; instead, the mother interacts only with the extraembryonic placenta after it implants in the uterus.27 During early pregnancy, including embryo implantation, immune tolerance is required, especially in the uterus to avoid rejection of the allogeneic embryo.28 As the most abundant immune cells, NK cells play an essential role in mediating local immunotolerance in uterine tissue.29 Uterine NK cells are believed to differentiate and proliferate from resident CD34+ hematopoietic stem cells,30 or to be recruited from peripheral blood and “re-programmed" to take on uterine phenotypes.31 Future work should pay more attention to the potential influence of uterine NK cells on embryo implantation and pregnancy outcomes, including RIF.
Our results should be interpreted with caution given several limitations. One is the retrospective nature of the study, and another is that we did not take into account several factors that can affect peripheral blood NK cell levels and their function, including stress,32 exercise,33, 34 menstrual period,35 and age.36 Although we excluded patients with conditions known to affect peripheral blood NK cells, it is possible that individuals may have had undetected autoimmune disease or infection that affected our results. Future investigations of peripheral blood NK cells and RIF should avoid these limitations.
In conclusion, the present study found that women with RIF and fertile controls did not differ significantly in the number of peripheral blood NK cells, their levels of cytotoxic granules or surface receptors, or their cytotoxicity. These findings suggest that RIF may not depend on the cytotoxic activity of peripheral blood NK cells. Larger prospective studies are needed to confirm and extend our results.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
This work was supported by the Shenzhen Healthcare Research Project, China (SZXJ2018004), Clinical Research Program of Chinese Medical Association, China (17020340703), and the Sanming Project of Medicine in Shenzhen, China (SZSM201502035).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Carp H.J., Selmi C., Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J Autoimmun. 2012;38(2-3):J266–J274. doi: 10.1016/j.jaut.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Margalioth E.J., Ben-Chetrit A., Gal M., Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21(12):3036–3043. doi: 10.1093/humrep/del305. [DOI] [PubMed] [Google Scholar]
- 3.Fu B., Tian Z., Wei H. Subsets of human natural killer cells and their regulatory effects. Immunology. 2014;141(4):483–489. doi: 10.1111/imm.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beer A.E., Kwak J.Y., Ruiz J.E. Immunophenotypic profiles of peripheral blood lymphocytes in women with recurrent pregnancy losses and in infertile women with multiple failed in vitro fertilization cycles. Am J Reprod Immunol. 1996;35(4):376–382. doi: 10.1111/j.1600-0897.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamada H., Kato E.H., Kobashi G. High NK cell activity in early pregnancy correlates with subsequent abortion with normal chromosomes in women with recurrent abortion. Am J Reprod Immunol. 2001;46(2):132–136. doi: 10.1111/j.8755-8920.2001.460203.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T., Takahashi Y., Kase N., Mori H. Proportion of CD56+3+ T cells in decidual and peripheral lymphocytes of normal pregnancy and spontaneous abortion with and without history of recurrent abortion. Am J Reprod Immunol. 1999;42(6):355–360. doi: 10.1111/j.1600-0897.1999.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 7.Katano K., Suzuki S., Ozaki Y., Suzumori N., Kitaori T., Sugiura-Ogasawara M. Peripheral natural killer cell activity as a predictor of recurrent pregnancy loss: a large cohort study. Fertil Steril. 2013;100(6):1629–1634. doi: 10.1016/j.fertnstert.2013.07.1996. [DOI] [PubMed] [Google Scholar]
- 8.Baczkowski T., Kurzawa R. Immunophenotypic profiles of peripheral blood lymphocytes on the day of embryo transfer in women undergoing in vitro fertilization. Folia histochemica et cytobiologica. 2007;45(suppl 1):S73–S77. [PubMed] [Google Scholar]
- 9.Thum M.Y., Bhaskaran S., Bansal A.S. Simple enumerations of peripheral blood natural killer (CD56+ NK) cells, B cells and T cells have no predictive value in IVF treatment outcome. Hum Reprod. 2005;20(5):1272–1276. doi: 10.1093/humrep/deh774. [DOI] [PubMed] [Google Scholar]
- 10.Kaspar A.A., Okada S., Kumar J. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol. 2001;167(1):350–356. doi: 10.4049/jimmunol.167.1.350. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z., Choice E., Kaspar A. Bactericidal and tumoricidal activities of synthetic peptides derived from granulysin. J Immunol. 2000;165(3):1486–1490. doi: 10.4049/jimmunol.165.3.1486. [DOI] [PubMed] [Google Scholar]
- 12.Sarwal M.M., Jani A., Chang S. Granulysin expression is a marker for acute rejection and steroid resistance in human renal transplantation. Hum Immunol. 2001;62(1):21–31. doi: 10.1016/s0198-8859(00)00228-7. [DOI] [PubMed] [Google Scholar]
- 13.Shresta S., Heusel J.W., Macivor D.M., Wesselschmidt R.L., Russell J.H., Ley T.J. Granzyme B plays a critical role in cytotoxic lymphocyte-induced apoptosis. Immunol Rev. 1995;146:211–221. doi: 10.1111/j.1600-065x.1995.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamada H., Shimada S., Morikawa M. Divergence of natural killer cell receptor and related molecule in the decidua from sporadic miscarriage with normal chromosome karyotype. Mol Hum Reprod. 2005;11(6):451–457. doi: 10.1093/molehr/gah181. [DOI] [PubMed] [Google Scholar]
- 15.Fukui A., Funamizu A., Yokota M. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol. 2011;90(1):105–110. doi: 10.1016/j.jri.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Wu J., Song Y., Bakker A.B. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285(5428):730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 17.Bauer S., Groh V., Wu J. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 18.Biassoni R., Cantoni C., Falco M. The human leukocyte antigen (HLA)-C-specific "activatory" or "inhibitory" natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med. 1996;183(2):645–650. doi: 10.1084/jem.183.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Liang P., Diao L. Thyroid autoimmunity is associated with decreased cytotoxicity T cells in women with repeated implantation failure. Int J Environ Res Publ Health. 2015;12(9):10352–10361. doi: 10.3390/ijerph120910352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Yin B., Lian R.C. Modulatory effects of vitamin D on peripheral cellular immunity in patients with recurrent miscarriage. Am J Reprod Immunol. 2016;76(6):432–438. doi: 10.1111/aji.12585. [DOI] [PubMed] [Google Scholar]
- 21.Kwak-Kim J., Gilman-Sachs A. Clinical implication of natural killer cells and reproduction. Am J Reprod Immunol. 2008;59(5):388–400. doi: 10.1111/j.1600-0897.2008.00596.x. [DOI] [PubMed] [Google Scholar]
- 22.Coughlan C., Ledger W., Wang Q. Recurrent implantation failure: definition and management. Reprod Biomed Online. 2014;28(1):14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Sacks G., Yang Y., Gowen E., Smith S., Fay L., Chapman M. Detailed analysis of peripheral blood natural killer cells in women with repeated IVF failure. Am J Reprod Immunol. 2012;67(5):434–442. doi: 10.1111/j.1600-0897.2012.01105.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang R., Yan G., Xing J. Abnormal ratio of CD57(+) cells to CD56(+) cells in women with recurrent implantation failure. Am J Reprod Immunol. 2017;78(5) doi: 10.1111/aji.12708. [DOI] [PubMed] [Google Scholar]
- 25.Orange J.S. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6(6):399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 26.Smyth M.J., Cretney E., Kelly J.M. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42(4):501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 27.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2(9):656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 28.Blois S.M., Ilarregui J.M., Tometten M. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 29.Fu B., Li X., Sun R. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci USA. 2013;110(3):E231–E240. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiossone L., Vacca P., Orecchia P. In vivo generation of decidual natural killer cells from resident hematopoietic progenitors. Haematologica. 2014;99(3):448–457. doi: 10.3324/haematol.2013.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mor G., Cardenas I., Abrahams V., Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salleh M.R. Life event, stress and illness. Malays J Med Sci. 2008;15(4):9–18. [PMC free article] [PubMed] [Google Scholar]
- 33.Fiuza-Luces C., Padilla J.R., Valentin J. Effects of exercise on the immune function of pediatric patients with solid tumors: insights from the PAPEC randomized trial. Am J Phys Med Rehabil. 2017;96(11):831–837. doi: 10.1097/PHM.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 34.Gustafson M.P., DiCostanzo A.C., Wheatley C.M. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J Immunother Cancer. 2017;5:30. doi: 10.1186/s40425-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berbic M., Fraser I.S. Immunology of normal and abnormal menstruation. Womens Health (Lond) 2013;9(4):387–395. doi: 10.2217/whe.13.32. [DOI] [PubMed] [Google Scholar]
- 36.Cooper M.A., Fehniger T.A., Caligiuri M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]