Abstract
Background
The direct anterior approach (DAA) for total hip arthroplasty (THA) has gained recent popularity, with 1 purported benefit being access to intraoperative fluoroscopy. However, there are limited data demonstrating improved component position with the use of intraoperative fluoroscopy. The aim of this study is to compare radiographic implant positioning on 2 consecutive cohorts of patients undergoing DAA THA performed by 1 surgeon either utilizing intraoperative fluoroscopy or not. We hypothesized that there would be no relevant radiographic differences between the cohorts.
Methods
Forty-two consecutive patients underwent DAA THA utilizing fluoroscopy (IFC), and 42 consecutive patients then underwent DAA THA without fluoroscopy (NFC). Using preoperative pelvis radiographs and 6-week postoperative pelvis radiographs, acetabular anteversion, inclination, femoral offset, and templated component sizes vs final intraoperatively selected sizes were recorded and compared between cohorts.
Results
Acetabular inclination was 45.0° for IFC and 45.6° for NFC (P = .629). Femoral offset difference preoperatively and postoperatively was 0.8 mm for IFC and 1.3 mm for NFC (P = .734). Number of hips within the so-called safe zone was 32 for IFC and 33 for NFC (P = .794). These all demonstrated no significant difference between the cohorts. However, acetabular anteversion was 13.7° for IFC and 11.2° for NFC (P = .02).
Conclusions
In this limited series, the routine use of intraoperative fluoroscopy did not improve implant positioning or sizing. This may be surgeon-specific or due to the result of the use of acetabular landmarks to guide placement of the components without fluoroscopy.
Keywords: Direct anterior approach, Total hip replacement, Fluoroscopy, Component positioning, Component safe zone
Introduction
The direct anterior approach (DAA) for total hip arthroplasty (THA) has enjoyed a recent increase in popularity [[1], [2], [3]]. Proponents note a number of purported benefits of the approach, including a modest early recovery advantage [4], low dislocation rate [5], and favorable radiographic component placement parameters [6]. One of the proposed technical advantages is that this approach is typically performed in the supine position, facilitating easy access to fluoroscopy, which theoretically can minimize errors in component placement and sizing to aid in re-establishing optimal hip biomechanics.
Fluoroscopy is routinely used during DAA THA to obtain the ideal anteversion and inclination of the acetabular component in particular [[7], [8], [9]]. However, fluoroscopy is not without some cost, including extraoperative time to obtain the images, as well as some concern that the sterile covering of the fluoroscopy arm may become contaminated during an operation [[10], [11], [12]]. It is also somewhat operator dependent, and if not employed correctly, can actually be misleading in case of some morphological variants or with differences in pelvic and/or fluoroscopy arm positioning, paradoxically leading to component malposition [13]. There are also conflicting data regarding exposure to radiation during the operation, though most investigations have not shown this to be of high concern [[14], [15], [16]].
Component placement is of paramount concern for successful THA, with much attention paid in particular to ideal acetabular component positioning. Optimal positioning of prosthetic components is necessary to successfully achieve the goals of THA, including re-establishment of femoral offset [2,17], restoration of hip biomechanics [18,19], improvement in postoperative stability [20,21], reduction in bearing wear [20,22], and reproduction of appropriate leg lengths [19,23]. In an attempt to optimize component positioning, a number of techniques have been developed, including the utilization of newer technology, such as robotics, computer navigation, and patient-specific positioning systems [[24], [25], [26], [27], [28]].
Controversy persists, however, over the ideal position to place components in THA. Historically, the so-called “safe-zone” has been promoted [29] as the ideal target for acetabular components so as to minimize postoperative instability. Although this has prevailed as a paradigm for many surgeons, recent work has brought this concept into question [30], with the realization that dislocation is more complex than only accounting for acetabular component angulation parameters [31]. Nonetheless, this so-called “safe-zone” remains the primary target for contemporary total hip replacement.
Advocates of the DAA THA tout the approach’s allowance of more anatomic placement of the components compared to alternative approaches [9,32]. In theory, this can allow the surgery to potentially be done more in reference to the patients’ native anatomic landmarks, creating the possibility to obviate the need for radiography during the operation. Although some investigations have been performed to investigate the utility of intraoperative fluoroscopy with DAA THA, no clear consensus has emerged. The purpose of the present study is to help evaluate whether there are differences in component position of THA performed via the DAA by 1 experienced surgeon (C.C.Y.) as determined by postoperative radiographs with or without the use of intraoperative fluoroscopy. The primary goal of this study is to compare the acetabular inclination and anteversion of components placed with and without the use of intraoperative fluoroscopic guidance. Secondary outcomes included femoral offset as well as differences between preoperatively planned templated sizes with intraoperatively selected component sizes. We hypothesized that there would be no significant difference with or without fluoroscopy use in any of these parameters.
Material and methods
Our institutional review board approved the study prior to its initiation. A power analysis was completed using acetabular inclination and anteversion as the primary endpoints in question. Using a power of 0.9 and published data [9] we concluded that to detect a difference in anteversion of 4° or inclination of 4°, we would need a sample size of at least 27 in each cohort. We therefore planned to include approximately 40 patients to allow for a generous dropout rate to ensure enough power to detect primary endpoint differences. Secondary endpoints included safe zone distribution, femoral offset differences, and differences in templated vs actual sizes for acetabular and femoral sizes.
All surgeries were performed by the senior author utilizing a DAA for THA on a regular operating room table utilizing identical components for all patients (Corail total hip stem, Pinnacle acetabular component; DePuy, J&J, Warsaw, IN). The initial cohort consisted of 42 consecutive patients whose surgeries were performed with the use of intraoperative fluoroscopy (IFC) to aid in component positioning. The experimental cohort consisted of the next 42 consecutive patients undergoing DAA THA, and who underwent surgery without the aid of intraoperative fluoroscopic guidance (NFC) of component positioning. No patients underwent formal radiographs taken while in the hospital. Patient demographic information was captured from the electronic medical record, noting patients’ age, sex, body mass index (BMI), and proximal femur morphology as described by Dorr et al. [33]. Radiographic parameters were calculated using Hip Analysis Software (Martell Hip Analysis Suite, Chicago, IL) utilizing their 6-week postoperative weight-bearing pelvis radiographs. Specific radiographic parameters that were considered included acetabular component inclination and anteversion, as well as femoral offset preoperatively and postoperatively, compared on pelvis radiographs. Femoral offset was defined as a horizontal distance measurement from the center of the femoral head to a proximal extension of the femoral shaft axis. Additionally, when available, we recorded preoperatively templated sizes of femoral and acetabular components as well as actual implanted sizes as recorded in the relevant operative notes. With regards to demographic data, student's t-test was employed to determine differences in continuous variables (eg, age, BMI), while the chi-squared test for association was used to test for differences in categorical variables (eg, gender, Dorr proximal femoral morphology). Student’s t-test was used to analyze differences between acetabular inclination, anteversion, as well as femoral offset preoperatively and postoperatively. Chi-squared test for association was used to determine if there were differences in the distribution of which acetabular components were present within the “safe zone,” as well as differences in templated sizes of acetabular and femoral components.
Results
Demographic data of the 2 cohorts are found in Table 1. No significant differences existed between the cohorts in terms of age, gender, BMI, or Dorr classification of proximal femoral morphology [33].
Table 1.
Descriptive analysis of patient demographics.
| Fluoroscopy group (N = 42) | No fluoroscopy group (N = 42) | P value | |
|---|---|---|---|
| Age | 65.2 ± 9.9 | 62.7 ± 8.2 | .223 |
| Gender (%) | Male: 21 (50) | Male: 17 (40.5) | .380 |
| Female: 21 (50) | Female: 25 (59.5) | ||
| BMI | 25.3 ± 3.7 | 26.0 ± 4.8 | .437 |
| Dorr type (%) | A: 30 (71.4) | A: 31 (73.8) | .807 |
| B: 12 (28.6) | B: 11 (26.2) |
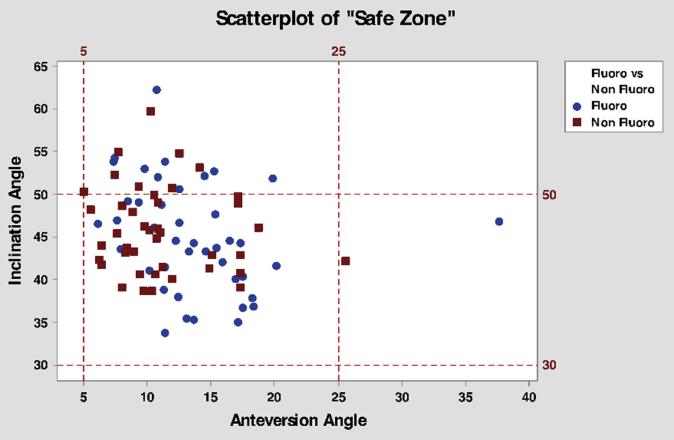
Radiographic parameters were also calculated, including acetabular component inclination angle and acetabular component anteversion angle, which, together, were used to calculate whether the components fell within the so-called “safe zone” [29]. The average inclination angle for the acetabular component in IFC was 45° ± 6.5° and in NFC was 46° ± 5.1° (P = .629). The anteversion of the acetabular components was found to be 13.6° ± 5.3° in IFC and 11.2° ± 4.2° in NFC (P = .02). The number of hips falling within the so-called “safe zone” was 32 (76.2%) in IFC, leaving 10 (23.8%) outside the safe zone. In NFC, there were 33 hips (78.6%) within the safe zone, and 9 (21.4%) outside the safe zone (P = .794). The table format for these data is found in Table 2, while the graphical depiction of the acetabular components is found in Figure 1.
Table 2.
Acetabular inclination and anteversion angles and inclusion within Lewinnek “safe zone.”
| Intraoperative fluoroscopy cohort (N = 42) | No fluoroscopy cohort (N = 42) | P value | |
|---|---|---|---|
| Inclination angle | 45.0 ± 6.5 | 45.6 ± 5.1 | .629 |
| Anteversion angle | 13.7 ± 5.3 | 11.2 ± 4.2 | .020 |
| Within “safe zone” | 32 (76.2%) | 33 (78.6%) | .794 |
| Outside “safe zone” | 10 (23.8%) | 9 (21.4%) |
Bolded value indicates the P value reached statistical significance (P < .05).
Figure 1.
Graphical depiction of acetabular inclination and anteversion, as well as grid demonstrating location of Lewinnek “safe zone.”
The 2 groups were also analyzed regarding differences in femoral offset, and ability to re-establish the preoperative measures. Comparing the 2 groups, the preoperative femoral offset measured in millimeters was 48.1 ± 5.6 in IFC and 47.4 ± 5.9 in NFC. Postoperatively, it was 48.8 ± 5.3 and 48.7 ± 6.7 mm, respectively. The difference in preoperative and postoperative femoral offset was 0.8 ± 5.3 mm in IFC and 1.3 ± 6.8 mm in NFC (P = .734), indicating no difference in change in offset between the 2 cohorts. These data are found in Table 3.
Table 3.
Comparison of cohort preoperative and postoperative femoral offset, measured in millimeters.
| Intraoperative fluoroscopy cohort (N = 41) | No fluoroscopy cohort (N = 42) | P value | |
|---|---|---|---|
| Preoperative femoral offset | 48.1 ± 5.6 | 47.4 ± 5.9 | .579 |
| Postoperative femoral offset | 48.8 ± 5.3 | 48.7 ± 6.7 | .912 |
| Difference between postoperative and preoperative femoral offset | 0.8 ± 5.3 | 1.3 ± 6.8 | .734 |
The senior author (C.C.Y.) routinely preoperatively templates total hips with the aid of digital templating software (OrthoView, Leuven, Belgium). All preoperative radiographs included a standardized marker ball for calibration. The preoperative templated sizes were reviewed and compared to the operative notes for all available patients in both cohorts. There were 4 patients in the NFC and 4 in the IFC for whom preoperative templated sizes were unavailable, leaving 38 remaining in each cohort available for review. In IFC, the templated femoral stem size was used in surgery 18 (47.4%) times, a size larger in 3 (7.9%) of cases, and a smaller size in 17 (44.7%) of cases. For NFC, the templated femoral stem size was used in 16 (42.1%) cases, a larger size in 1 (2.6%) case and a smaller size in 21 (55.3%) cases (P = .452), indicating no difference in distribution of femoral stem sizes from the preoperative template between cohorts. For acetabular cup sizes in IFC, the templated size was used in 12 (31.6%) cases, a larger size in 2 (5.3%) cases and a smaller size in 24 (63.1%) cases. For NFC, the templated size was used in 12 (31.6%) cases, a larger size in 5 (13.2%) cases and a smaller size in 21 (55.2%) cases (P = .466), indicating no difference in distribution of acetabular cup sizes from the preoperative template between the cohorts. These data regarding planned vs actual component sizes are found in Table 4.
Table 4.
Comparison of cohorts preoperatively and intraoperatively selected component sizes.
| Intraoperative fluoroscopy cohort (N = 38) | No fluoroscopy cohort (N = 38) | P value | |
|---|---|---|---|
| Same femoral size | 18 (47.4%) | 16 (42.1%) | .452 |
| Increase in femoral size | 3 (7.9) | 1 (2.6) | |
| Decrease in femoral size | 17 (44.7) | 21 (55.3) | |
| Same acetabular size | 12 (31.6) | 12 (31.6) | .466 |
| Increase in acetabular size | 2 (5.3) | 5 (13.2) | |
| Decrease in acetabular size | 24 (63.1) | 21 (55.2) |
Discussion
The question of whether to use fluoroscopy during THA has been broached in the literature previously. Beamer et al. [34] used a similar 2-consecutive cohort study design to look at the influence fluoroscopy had on radiographic parameters, with particular attention to the Lewinnek safe zone. In their series of similar number of patients, they found a nonstatistical increase in the percentage of patients within the safe zone parameters in the fluoroscopy cohort compared with the freehand technique. Their series included 2 surgeons, however, and this may have increased variability between their respective patients. Additionally, their series were done through a variety of surgical approaches (anterolateral, posterolateral, and extended iliofemoral), further diluting the uniformity of their cohort. Finally, their patients were placed in the lateral decubitus position, which, in our opinion, is less amenable to fluoroscopy use than placement in the supine position.
In another radiographic analysis, Lin et al. [6] reviewed consecutive cohorts of first posterior approach THAs followed by a series of a similar number of DAA THA patients. Postoperative radiographs were reviewed for acceptability according to established acetabular “safe zone” parameters as well as leg lengths and femoral offset. Their results indicated that compared to the posterior approach, the DAA THA had a higher rate of what was deemed radiographically acceptable acetabular inclination. However, there was no difference between anteversion, leg lengths, or offset parameters. The authors note that they employed fluoroscopy during all DAA THAs. The authors do make note that there may be different safe zones for different approaches, or that the “safe zone” may be “safer’ for certain approaches over others. Also of interest in their series, BMI was different between the differing approach cohorts.
Tischler et al. [35] reviewed 2 cohorts of patients undergoing primary THA with or without the use of fluoroscopy. They investigated the radiographic parameters of leg length discrepancy (LLD), femoral offset, and acetabular inclination angle. Secondarily, they reviewed the rate of complications following the surgery as well. The main difference they found was in terms of the operative time, with fluoroscopy adding additional time compared to without. No differences in the radiographic parameters were found. As distinguished from the current study, their operative approach was the modified Hardinge approach in the supine position. Additionally, their cohorts involved multiple surgeons, and did not include acetabular anteversion.
Rathod et al. [9] performed a radiographic investigation of fluoro vs no-fluoro THA groups. They examined 372 posterior THAs with 453 DAA THAs with fluoroscopy in terms of acetabular inclination and anteversion. They found that the DAA group with fluoroscopy had decreased variance in terms of acetabular inclination and anteversion. Additionally, they found that they were more likely to have the acetabular radiographic parameters fall into what they determined were desirable ranges with the DAA with fluoro cohort than in the posterior cohort. They were unable to determine if the improved radiographic parameters were owed to the approach or the use of fluoroscopy.
Bingham et al. [36] performed a recent investigation of 2 cohorts of patients undergoing THA via the DAA. They retrospectively reviewed radiographs for 3 primary parameters: LLD, acetabular inclination, and acetabular anteversion. There were approximately equal patients between the 2 cohorts, with 125 in the fluoro group and 140 in the no-fluoro group. No differences were detected in LLD or acetabular radiographic parameters. From a methodological standpoint, there were 2 key differences in this study’s cohorts: (1) the 2 cohorts were performed by 2 different surgeons and (2) the fluoro cohort was performed on a specialized fracture table and the no-fluoro cohort on a standard table. These methodological differences between cohorts may limit their equivalency. The anteversion measurements were made via a cross-through lateral radiograph, and they did not include femoral offset measurements, which distinguishes their study from the current investigation.
Finally, Jennings et al. [37] compared a series of 2 cohorts of DAA THA patients either using fluoroscopy (n = 98) or no fluoroscopy (n = 101). They reviewed the cohorts retrospectively for radiographic parameters looking at their 6-month postoperative films. Their primary radiographic endpoints were acetabular inclination and acetabular anteversion. They found that with the use of fluoroscopy, there was a difference in acetabular inclination, but none in the acetabular anteversion. Additionally, they found a higher percentage of patients within the “safe zone” with the use of fluoroscopy than those without (80% vs 63%). Their patients were historical, retrospective cohorts, but were performed by a single experienced surgeon.
Our study, like others of its kind in the literature, is not without limitations. First, and most apparently, is the relatively small numbers. Although similar in scope to some other studies, the number of patients in our investigation is rather modest. We selected our numbers based on a power calculation, but it is possible that given larger numbers, different conclusions might have been reached. Our cohorts were also taken sequentially, and not randomized, and therefore it is theoretically possible that relevant differences exist between them. Additionally, as a radiographic parameter investigation, our study takes no note of clinical outcomes. Anecdotally, neither cohort has had a dislocation nor other major complication in the short term, but the study is limited by its methodology in distinguishing longer term outcomes. Any differences or similarities in the cohorts remain in the realm of radiographic findings, as we do not have mid- or long-term follow-up for these patients yet, and as such, is a limitation to overall conclusions about the use of fluoroscopy in general. Additionally, there could be slight variations in our radiographs obtained. Obtaining pelvis radiographs is standardized at our institution to center the pubic symphysis over the coccyx and to obtain them standing with a marker ball. However, in spite of this, there could be slight differences in rotation between radiographs, giving some variability in our radiographic measurements. Finally, both cohorts demonstrated a similar distribution of change in size from templated to actual size used (Table 4), with an approximately equal number of patients in each cohort receiving similar vs decrease in templated size of femoral stem. There was a trend toward smaller sizes used compared to templated sizes. On radiographic follow-up there were no failures of the femoral components in the short term, and all components were thought to be of appropriate size during postoperative clinical follow-up. The particular trend toward using smaller than templated femoral sizes may be an idiosyncrasy unique to our institution’s method of placement of the calibration marker on preoperative radiographs. We hypothesize this due to the similar distribution of decrease in size of templated vs actual acetabular cup size, and the fact that the 2 cohorts were similar in this regard, indicating fluoroscopy was not causing a change in actual size of components used. Therefore, the particular trend of smaller than templated sizes was found in our study, but that exact finding might not be generalizable to other institutions.
We believe that the present study is of value to the existing literature. The DAA THA has gained significant footing in recent years, and it is currently experiencing refinement in terms of the best way to implement its use. Some have touted the ease of implementing the use of fluoroscopy as a potential benefit of the approach. Our results here indicate that there does seem to be a greater degree of anteversion with this approach with concomitant fluoroscopy. We postulate that the slightly higher degree of anteversion could be a result of the components’ fluoroscopic appearance, as conventional teaching advocated slightly more anteversion to protect against instability, and therefore the impression of the surgeon using fluoroscopic feedback may be to trend toward what he deems as acceptable radiographic anteversion. In the no fluoroscopy cohort, no such visual radiographic feedback was available, and placement was more gauged from anatomic landmarks, resulting in slightly less anteversion. We postulate that the modest degree of difference of acetabular anteversion is likely statistically, but not clinically significant. There is the potential that the difference is nonexistent when factoring in the degree of error in measuring anteversion. Even in spite of the decreased amount of anteversion in the no-fluoro cohort, there was no difference in terms of the numbers within the Lewinnek safe zone. We also use the added parameter of templated vs actual femoral stem and acetabular component size, which expands the scope of the current study compared to studies of similar investigations.
As previously noted [25,38], there is a documented learning curve associated with adopting the DAA for THA. Although not the focus of the present study, we hypothesize that the elimination of fluoroscopy during the operation may have more of an effect for less experienced surgeons undertaking these operations, and our results may not apply as directly to that clinical situation. However, there are potential advantages to the elimination of fluoroscopy during an operative procedure. In principle, it extends the length of an operation with multiple events of movement into and out of the field of the amplifier and receiver arm. Additionally, there is a real concern of contamination events during both the draping of the fluoroscope, as well as during the course of the procedure. If a breech occurs in the barrier, direct wound contamination could result, as the receiver typically is located directly over or in very close proximity to the wound, the surgeon’s hands, and retractors. Additionally, in some circumstances, it may be the case that fluoroscopy machines may not be readily available, whether by machine failure, or lack of universal availability. Additionally, since cementless components are made of materials with varying degrees of radio-opacity, assessment of anteversion using intraoperative fluoroscopy can be challenging and subject to imprecision. In these cases in particular, judgment of appropriate position may be especially suited to using native anatomy as guiding landmarks.
Finally, at many hospitals the use of fluoroscopy is not without direct costs associated with its use. Although this varies by location, the cost at the author’s institution exceeds several hundred dollars, and anecdotally, may amount to $1000 or more at some institutions, which is a significant increase in the cost of a procedure. The direct costs do not account for the possibility of additional costs in the form of increased operative times, which may also be substantial. In an increasingly cost-conscious healthcare landscape, the ability to eliminate unnecessary costs is becoming progressively more important.
Conclusions
In this series we compare two consecutive cohorts of patients undergoing DAA THA done by the same surgeon with or without the use of intraoperative fluoroscopy. Our results indicate there was no difference of component position within or outside of the so-called "safe zone," and that there was no difference in templated component sizes versus real implanted component size. There was a modest difference in anteversion between the cohorts that is likely clinically insignificant. The results here indicate that the use of intraoperative fluoroscopy during DAA THA may not be necessary to for appropriate component position or sizing.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2019.11.006.
Appendix A. Supplementary data
References
- 1.Kennon R.E., Keggi J.M., Wetmore R.S., Zatorski L.E., Huo M.H., Keggi K.J. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A(Suppl 4):39. doi: 10.2106/00004623-200300004-00005. [DOI] [PubMed] [Google Scholar]
- 2.Matta J.M., Shahrdar C., Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;441:115. doi: 10.1097/01.blo.0000194309.70518.cb. [DOI] [PubMed] [Google Scholar]
- 3.Kennon R., Keggi J., Zatorski L.E., Keggi K.J. Anterior approach for total hip arthroplasty: beyond the minimally invasive technique. J Bone Joint Surg Am. 2004;86-A(Suppl 2):91. doi: 10.2106/00004623-200412002-00013. [DOI] [PubMed] [Google Scholar]
- 4.Berend K.R., Lombardi A.V., Seng B.E., Adams J.B. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(Suppl 6):107. doi: 10.2106/JBJS.I.00525. [DOI] [PubMed] [Google Scholar]
- 5.Siguier T., Siguier M., Brumpt B. Mini-incision anterior approach does not increase dislocation rate: a study of 1037 total hip replacements. Clin Orthop Relat Res. 2004;(426):164. doi: 10.1097/01.blo.0000136651.21191.9f. [DOI] [PubMed] [Google Scholar]
- 6.Lin T.J., Bendich I., Ha A.S., Keeney B.J., Moschetti W.E., Tomek I.M. A comparison of radiographic outcomes after total hip arthroplasty between the posterior approach and direct anterior approach with intraoperative fluoroscopy. J Arthroplasty. 2017;32(2):616. doi: 10.1016/j.arth.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez A.M., Suarez J.C., Patel P., Benton E.G. Fluoroscopic imaging of acetabular cup position during THA through a direct anterior approach. Orthopedics. 2013;36(10):776. doi: 10.3928/01477447-20130920-06. [DOI] [PubMed] [Google Scholar]
- 8.Mirza A.J., Lombardi A.V., Morris M.J., Berend K.R. A mini-anterior approach to the hip for total joint replacement: optimising results: improving hip joint replacement outcomes. Bone Joint J. 2014;96-B(11 Supple A):32. doi: 10.1302/0301-620X.96B11.34348. [DOI] [PubMed] [Google Scholar]
- 9.Rathod P.A., Bhalla S., Deshmukh A.J., Rodriguez J.A. Does fluoroscopy with anterior hip arthroplasty decrease acetabular cup variability compared with a nonguided posterior approach? Clin Orthop Relat Res. 2014;472(6):1877. doi: 10.1007/s11999-014-3512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gershkovich G.E., Tiedeken N.C., Hampton D., Budacki R., Samuel S.P., Saing M. A comparison of three C-arm draping techniques to minimize contamination of the surgical field. J Orthop Trauma. 2016;30(10):e351. doi: 10.1097/BOT.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 11.Peters P.G., Laughlin R.T., Markert R.J., Nelles D.B., Randall K.L., Prayson M.J. Timing of C-arm drape contamination. Surg Infect (Larchmt) 2012;13(2):110. doi: 10.1089/sur.2011.054. [DOI] [PubMed] [Google Scholar]
- 12.Biswas D., Bible J.E., Whang P.G., Simpson A.K., Grauer J.N. Sterility of C-arm fluoroscopy during spinal surgery. Spine. 2008;33(17):1913. doi: 10.1097/BRS.0b013e31817bb130. [DOI] [PubMed] [Google Scholar]
- 13.James C.R., Peterson B.E., Crim J.R., Cook J.L., Crist B.D. The use of fluoroscopy during direct anterior hip arthroplasty: powerful or misleading? J Arthroplasty. 2018;33(6):1775. doi: 10.1016/j.arth.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Pomeroy C.L., Mason J.B., Fehring T.K., Masonis J.L., Curtin B.M. Radiation exposure during fluoro-assisted direct anterior total hip arthroplasty. J Arthroplasty. 2016;31(8):1742. doi: 10.1016/j.arth.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 15.McArthur B.A., Schueler B.A., Howe B.M., Trousdale R.T., Taunton M.J. Radiation exposure during fluoroscopic guided direct anterior approach for total hip arthroplasty. J Arthroplasty. 2015;30(9):1565. doi: 10.1016/j.arth.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 16.McNabb D.C., Jennings J.M., Levy D.L., Miner T.M., Yang C.C., Kim R.H. Direct anterior hip replacement does not pose undue radiation exposure risk to the patient or surgeon. J Bone Joint Surg Am. 2017;99(23):2020. doi: 10.2106/JBJS.17.00351. [DOI] [PubMed] [Google Scholar]
- 17.Boettner F., Zingg M., Emara A.K., Waldstein W., Faschingbauer M., Kasparek M.F. The accuracy of acetabular component position using a novel method to determine anteversion. J Arthroplasty. 2017;32(4):1180. doi: 10.1016/j.arth.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Ecker T.M., Tannast M., Murphy S.B. Computed tomography-based surgical navigation for hip arthroplasty. Clin Orthop Relat Res. 2007;465:100. doi: 10.1097/BLO.0b013e3181591c7d. [DOI] [PubMed] [Google Scholar]
- 19.Sculco P.K., Austin M.S., Lavernia C.J., Rosenberg A.G., Sierra R.J. Preventing leg length discrepancy and instability after total hip arthroplasty. Instr Course Lect. 2016;65:225. [PubMed] [Google Scholar]
- 20.Kennedy J.G., Rogers W.B., Soffe K.E., Sullivan R.J., Griffen D.G., Sheehan L.J. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13(5):530. doi: 10.1016/s0883-5403(98)90052-3. [DOI] [PubMed] [Google Scholar]
- 21.Komeno M., Hasegawa M., Sudo A., Uchida A. Computed tomographic evaluation of component position on dislocation after total hip arthroplasty. Orthopedics. 2006;29(12):1104. doi: 10.3928/01477447-20061201-05. [DOI] [PubMed] [Google Scholar]
- 22.Elkins J.M., Callaghan J.J., Brown T.D. The 2014 Frank Stinchfield Award: the “landing zone” for wear and stability in total hip arthroplasty is smaller than we thought: a computational analysis. Clin Orthop Relat Res. 2015;473(2):441. doi: 10.1007/s11999-014-3818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark C.R., Huddleston H.D., Schoch E.P., Thomas B.J. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38. doi: 10.5435/00124635-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Domb B.G., Bitar El Y.F., Sadik A.Y., Stake C.E., Botser I.B. Comparison of robotic-assisted and conventional acetabular cup placement in THA: a matched-pair controlled study. Clin Orthop Relat Res. 2014;472(1):329. doi: 10.1007/s11999-013-3253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamara E., Robinson J., Bas M.A., Rodriguez J.A., Hepinstall M.S. Adoption of robotic vs fluoroscopic guidance in total hip arthroplasty: is acetabular positioning improved in the learning curve? J Arthroplasty. 2017;32(1):125. doi: 10.1016/j.arth.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 26.Nodzo S.R., Chang C.-C., Carroll K.M. Intraoperative placement of total hip arthroplasty components with robotic-arm assisted technology correlates with postoperative implant position. Bone Joint J. 2018;100-B(10):1303. doi: 10.1302/0301-620X.100B10-BJJ-2018-0201.R1. [DOI] [PubMed] [Google Scholar]
- 27.Lee S., Kim J.-Y., Hong J., Baek S.-H., Kim S.-Y. CT-based navigation system using a patient-specific instrument for femoral component positioning: an experimental in vitro study with a sawbone model. Yonsei Med J. 2018;59(6):769. doi: 10.3349/ymj.2018.59.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1. doi: 10.4055/cios.2013.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewinnek G.E., Lewis J.L., Tarr R., Compere C.L., Zimmerman J.R. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg. 1978;60(2):217. [PubMed] [Google Scholar]
- 30.Abdel M.P., Roth P., Jennings M.T., Hanssen A.D., Pagnano M.W. What safe zone? The vast majority of dislocated THAs are within the Lewinnek safe zone for acetabular component position. Clin Orthop Relat Res. 2015;474(2):386. doi: 10.1007/s11999-015-4432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seagrave K.G., Troelsen A., Malchau H., Husted H., Gromov K. Acetabular cup position and risk of dislocation in primary total hip arthroplasty. Acta Orthop. 2017;88(1):10. doi: 10.1080/17453674.2016.1251255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakata K., Nishikawa M., Yamamoto K., Hirota S., Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698. doi: 10.1016/j.arth.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Dorr L.D., Faugere M.C., Mackel A.M., Gruen T.A., Bognar B., Malluche H.H. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993;14(3):231. doi: 10.1016/8756-3282(93)90146-2. [DOI] [PubMed] [Google Scholar]
- 34.Beamer B.S., Morgan J.H., Barr C., Weaver M.J., Vrahas M.S. Does fluoroscopy improve acetabular component placement in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3953. doi: 10.1007/s11999-014-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tischler E.H., Orozco F., Aggarwal V.K., Pacheco H., Post Z., Ong A. Does intraoperative fluoroscopy improve component positioning in total hip arthroplasty? Orthopedics. 2015;38(1):e1. doi: 10.3928/01477447-20150105-52. [DOI] [PubMed] [Google Scholar]
- 36.Bingham J.S., Spangehl M.J., Hines J.T., Taunton M.J., Schwartz A.J. Does intraoperative fluoroscopy improve limb-length discrepancy and acetabular component positioning during direct anterior total hip arthroplasty? J Arthroplasty. 2018;33(9):2927. doi: 10.1016/j.arth.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Jennings J.D., Iorio J., Kleiner M.T., Gaughan J.P., Star A.M. Intraoperative fluoroscopy improves component position during anterior hip arthroplasty. Orthopedics. 2015;38(11):e970. doi: 10.3928/01477447-20151020-04. [DOI] [PubMed] [Google Scholar]
- 38.Masonis J., Thompson C., Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(12 Suppl 2) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.