Abstract
Fructose, an essential biomolecule and it is a major ingredient of the modern diet across the globe. Excess consumption of fructose may be a key driver of many serious diseases such as obesity, heart diseases, type 2 diabetes and cancer. Understanding the metabolism of fructose, molecular mechanisms of its toxic nature will aid in the treatment of various diseases including cancer.
The diet plays a pivotal role in overall health and harmonious growth of every living organism. As the famous quote of Hippocrates goes “Let food be thy medicine, and let medicine be thy food” who was referred to as “Father of Medicine”.1 Though the quote is from ancient Greek and more than 2300-years-old, it is still relevant to the present generation of 21st century. Diet or food is a substance consumed to gain the essential nutritional support by an organism (organism may vary from unicellular bacteria to complex multicellular human beings).2 Dietary constituents of all the organisms are usually of plant (Vegan) or animal origin (Non vegan), and contains all the essential biomolecules and nutrients, such as carbohydrates, fats, proteins, nucleic acid, vitamins, or minerals. The dietary substance is ingested by an organism and absorbed by the organism's intestine or other organs or cells to provide energy in the form of ATPs, supports and maintains life, or stimulate growth and development of the organism. Carbohydrates are very important class of biomolecules which contain carbon, hydrogen and oxygen atoms and are present in almost all the human diet. The carbohydrates provide a lot of calories and act as main source of energy for metabolic activities. Fructose, a monosaccharide is one such carbohydrate present in many diets. Fructose is present in all the naturally occurring fruits we consume and is the molecule that is present in routinely used dietary table sugar making it sweet in taste.3 It is the compound present in high fructose corn syrup, maple syrup, agave nectar and virtually in almost all the high caloric sweetener and this makes us crave to consume different beverages or sweet dishes in everyday life.4 The consumption of fructose has dramatically increased globally in the last three to four decades. Until recently this compound is thought to be very good for overall health and a balanced diet is also likely to contain appreciable amount of fructose, even if it is confined to that found in only fruits and vegetables. Very sadly, it's the fructose, which is the major culprit behind obesity and the cause for various metabolic diseases and cancer.5 There are many elegant studies showing that excessive consumption of fructose can lead to increase in de novo lipogenesis, insulin resistance, type 2 diabetes, inflammation, and reactive oxygen species production.6 Glycolaldehyde, one of the products of fructose metabolism is a highly reactive molecule and it induces oxidative stress associated cellular damages.7 Another end product of fructose metabolism and food processing contaminant glyoxal promotes intestinal tumor growth in mouse model.8 This sweet sugar is also known to induce a change in the gut permeability and activate various signaling pathways and promote the release of harmful inflammatory factors to the various organs including liver, which has potential implications in increasing hepatic inflammation and hepatocellular carcinoma.5 All these studies show tumor promoting property of fructose molecules. This carcinogenic property of fructose has made this molecule as one of the connecting link or missing links between obesity, metabolism and cancer.
A recent and elegant study by Marcus D. Goncalves and collaborators revealed with strong evidence indicating that high-fructose corn syrup enhances intestinal tumor growth in mice.9 It is very clear that from last three to four decades increased consumption of high calorie western diet is associated with obesity, type 2 diabetes, fatty liver, cardiovascular disease throughout the globe.10,11 Fructose is a major component of high calorie western diet. It is also known that excessive consumption of soft beverages sweetened with high glucose and high-fructose corn syrup (HFCS) is associated with diet-induced obesity and various types of cancer including colorectal cancer.12,9 Along with previously known evidences this study clearly proves the oncogenic potential of fructose.
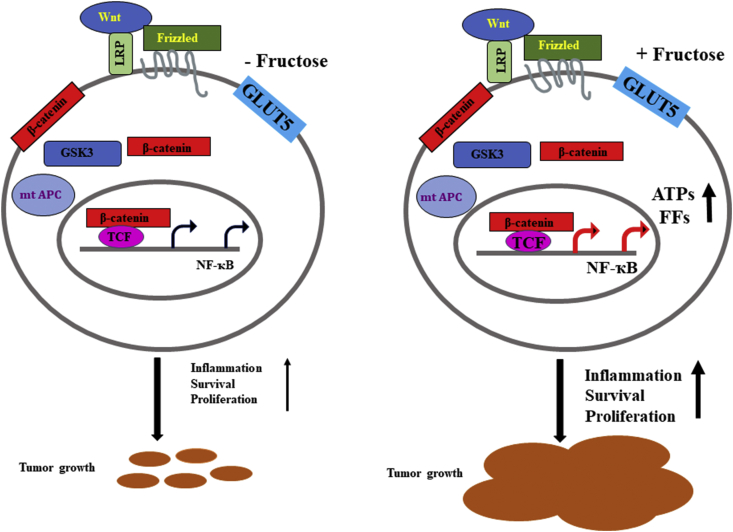
In this study authors have investigated and elucidated the effects of daily oral administration of HFCS in adenomatous polyposis coli (APC) mutant mice.9 The APC gene is a very well-studied tumor suppressor gene and a negative regulator of Wnt signaling and is frequently mutated (75–80%) in the early stages of colorectal cancer development.13 The product from this gene is known to suppress the NF-ҡB pathways and finally suppress the cell survival and induce apoptosis. The mutation in this gene would lead to the NF-ҡB activation and tumor growth.14 Therefore, these APC mutated mice are already genetically predisposed to develop intestinal tumors.9 The HFCS-consumed mice with initial small adenomatous polyps or small tumors get the sudden supply of hyper nutrition and this resulted in a rapid growth of the tumors and substantial increase in tumor size, tumor volume and tumor grade even in the absence of predisposed obesity and metabolic syndrome. Also, the consumption of HFCS increased the concentrations of fructose and glucose in the intestinal lumen and serum of these mice. The presence of high amount of glucose and fructose near the tumor microenvironment will help tumors to get more nutrition (Fig. 1). These tumors have showed the clear evidence of transported sugars inside their cytoplasm, which included both glucose and fructose. According to the author's experimental data, the treated fructose was converted to fructose-1-phosphate, leading to the activation of routine cellular housekeeping glycolytic pathways and also increased synthesis of free fatty acids (FFs) and ATPs that support tumor growth by providing raw materials for cell and nuclear membranes and other organelles. These clear and appropriate mouse data supported their proposed hypothesis that the combination of dietary glucose and fructose, even at a very low or moderate dose, can enhance and promote the development and progression of colon carcinogenesis or tumorigenesis.
Figure 1.
The possible molecular mechanism of high-fructose corn syrup enhanced intestinal tumor growth in mice.
The toxic effect of fructose is mainly due to the lack of cellular fructose homeostasis machinery. Fructose, unlike glucose is not regulated by either insulin or glucagon. It is very well-known fact that glucose is directly regulated by insulin.15 It is also reported that fructose-induces hepatocellular carcinoma (HCC) in mice lacking apoptosis inhibitor of macrophage (AIM).16 Furthermore, dietary fructose is also known to be involved in pathogenesis of nonalcoholic fatty liver disease and has association with development of triple-negative breast cancer incidence.17 Thus, more and more evidences provide experimental evidences that fructose consumption and oncogenesis has a direct connection.18 Here, this study proposes an additional hypothesis about oncogenesis.
Oncogenesis or tumorigenesis is a series of cellular biochemical events. There is a well-established hypothesis about oncogenesis according to which like all genes, tumor suppressor genes including APC may undergo a variety of mutations. These loss-of-function mutations that occur in only one allele of tumor suppressor gene or genes in a cell are recessive in nature. This recessive mutation in tumor suppressor gene or genes will fail to show any cancer phenotype or hallmarks of cancer. Thus, for a cell to become transformed cell or cancerous cell, both copies of cell's tumor suppressor genes must undergo functional mutation. The well-established “two-hit” hypothesis was proposed by American physician and geneticist Alfred Knudson in 1971.19 Until today, this hypothesis holds good and serves as the fundamental basis for researchers to understand the tumor suppressor genes and their oncogenic nature in driving cancer. Now, it is time to redefine this hypothesis and along with two hit there may be a third hit which is a possible triggering or promoting factor (Fructose) may play a major role in oncogenesis.
Ironically, fructose is a major constituent of our westernized modern diet throughout the globe and is evidently implicated as an underlying cause in the development of metabolic syndrome and various cancers.5 The GLUT5 (SLC2A5), a fructose transporter is required for intestinal fructose absorption, metabolism and is the target gene of Liver X receptor α (LXR- α).20 It is very well known that LXR-α is a ligand-activated transcription factor and therefore, it is tempting to speculate that LXRα might serve as a novel pharmacological target for selectively reprogramming fructose absorption via GLUT5 activity using its specific agonist (withaferin A) or antagonist (meso-dihydroguaiaretic acid) in the treatment of metabolic disease as well as cancers.
Therefore, this high impact study by Marcus D. Goncalves and his colleagues have shed the new torch light on the direct role of fructose in the form of high fructose corn syrup on the intestinal tumor growth in mice.9 This study can be extrapolated to get an idea on the toxic role of fructose in metabolic syndrome and cancers.
Acknowledgements
This work was supported by Ramalingaswami Re-entry fellowship, Department of Biotechnology, Govt. of India to the author.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Witkamp R.F., van Norren Let thy food be thy medicine… when possible. Eur J Pharmacol. 2018;836:102–114. doi: 10.1016/j.ejphar.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Dussutour A., Latty T., Beekman M., Simpson S.J. Amoeboid organism solves complex nutritional challenges. Proc Natl Acad Sci USA. 2010;107(10):4607–4611. doi: 10.1073/pnas.0912198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik V.S., Hu F.B. Fructose and cardiometabolic health: what the evidence from sugar-sweetened beverages tells us. J Am Coll Cardiol. 2015;66(14):1615–1624. doi: 10.1016/j.jacc.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lustig R.H. Fructose: it's “alcohol without the buzz”. Adv Nutr. 2013;4(2):226–235. doi: 10.3945/an.112.002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charrez B., Qiao L., Hebbard L. The role of fructose in metabolism and cancer. Horm Mol Biol Clin Investig. 2015;22(2):79–89. doi: 10.1515/hmbci-2015-0009. [DOI] [PubMed] [Google Scholar]
- 6.Samuel V.T. Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab. 2011;22(2):60–65. doi: 10.1016/j.tem.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Feng C.Y., Wong S., Dong Q. Hepatocyte inflammation model for cytotoxicity research: fructose or glycolaldehyde as a source of endogenous toxins. Arch Physiol Biochem. 2009;115(2):105–111. doi: 10.1080/13813450902887055. [DOI] [PubMed] [Google Scholar]
- 8.Svendsen C., Høie A.H., Alexander J., Murkovic M., Husøy T. The food processing contaminant glyoxal promotes tumour growth in the multiple intestinal neoplasia (Min) mouse model. Food Chem Toxicol. 2016;94:197–202. doi: 10.1016/j.fct.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves M.D., Lu C., Tutnauer J. High-fructose corn syrup enhances intestinal tumor growth in mice. Science. 2019;363(6433):1345–1349. doi: 10.1126/science.aat8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kearney J. Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci. 2010;365(1554):2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popkin B.M., Adair L.S., Ng S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schernhammer E.S., Hu F.B., Giovannucci E. Sugar-sweetened soft drink consumption and risk of pancreatic cancer in two prospective cohorts. Cancer Epidemiol Biomark Prev. 2005;14(9):2098–2105. doi: 10.1158/1055-9965.EPI-05-0059. [DOI] [PubMed] [Google Scholar]
- 13.Lamlum H., Ilyas M., Rowan A. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson's 'two-hit' hypothesis. Nat Med. 1999;5(9):1071–1075. doi: 10.1038/12511. [DOI] [PubMed] [Google Scholar]
- 14.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 15.Röder P.V., Wu B., Liu Y., Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48:e219. doi: 10.1038/emm.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozawa T., Maehara N., Kai T., Arai S., Miyazaki T. Dietary fructose-induced hepatocellular carcinoma development manifested in mice lacking apoptosis inhibitor of macrophage (AIM) Genes Cells. 2016;21(12):1320–1332. doi: 10.1111/gtc.12446. [DOI] [PubMed] [Google Scholar]
- 17.Strober J.W., Brady M.J. Dietary fructose consumption and triple-negative breast cancer incidence. Front Endocrinol. 2019;10:367. doi: 10.3389/fendo.2019.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng Y., Zhu J., Chen Z., Fu J., Zhang F. Fructose fuels lung adenocarcinoma through GLUT5. Cell Death Dis. 2018;9(5):557. doi: 10.1038/s41419-018-0630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segditsas S., Rowan A.J., Howarth K. APC and the three-hit hypothesis. Oncogene. 2009;28(1):146–155. doi: 10.1038/onc.2008.361. [DOI] [PubMed] [Google Scholar]
- 20.Zwarts I., van Zutphen T., Kruit J.K. Identification of the fructose transporter GLUT5 (SLC2A5) as a novel target of nuclear receptor LXR. Sci Rep. 2019;9(1):9299. doi: 10.1038/s41598-019-45803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]