Abstract
Transcervical resection of adhesion (TCRA) is the standard treatment for the intrauterine adhesions, but the recurrence of adhesions is a tough problem for the gynecologist. In addition, the therapeutic strategy after TCRA about prevention of recurrence remains controversial especially for the patients with moderate to severe intrauterine adhesions (IUAs). Hence, we designed this study to explore the safety and efficacy of fresh amnion grafts for preventing the recurrence after TCRA for patients with moderate to severe IUAs. One hundred patients with moderate to severe IUAs who presented with a history of hypomenorrhea, amenorrhea and infertility were included in the study from January 2015 to December 2017. Patients were divided into amnion group (52 patients) and chitosan group (48 patients). Fresh amnion grafts or intrauterine injections of chitosan were administered after TCRA. Transvaginal ultrasonography (TVUS) and hysteroscopy were performed at the first and third month after the operation. The surgical procedures for all patients were completed successfully without relevant complications. In amnion group, 8 patients exhibited relapse in the first month and 2 patients in three months after surgery; in chitosan group, 23 women exhibited relapse in the first month and 18 patients in three months after surgery. Statistical analysis revealed that the recurrence rate of adhesion in amnion group was significantly lower than those of chitosan group in the first and three months after surgery (P1 = 0.000, P2 = 0.000). After TCRA, fresh amnion graft plays a significant role in preventing further adhesions than injections of chitosan.
Keywords: Amnion graft, Asherman's syndrome, Intrauterine adhesions, Recurrence of adhesion, Transcervical resection of adhesion
Introduction
IUAs, which also known as Asherman's syndrome, have increased with expanded clinical awareness and availability of diagnostic testing.1 Hysteroscopy has been the criterion standard for diagnosis of IUAs,2 because it has the advantages of direct visualization of the location, extent, and morphological characteristics of adhesions as well as the quality of the endometrium with magnification accurately, hence, hysteroscopy has been established as the certain standard for the diagnosis of IUA at present which has higher sensitivity and specificity than hysterosalpingography (HSG) and TVUS.3, 4, 5 TCRA has become the conventional surgery for the treatment of IUA with the development of gynecological minimally invasive technology.6, 7 However, the affected endometrium can be further damaged by lesion excision and adhesion separation, and it takes some time for the endometrium to recover after TCRA. During this recovery period, it is still possible for IUA to develop. Current conventional methods for reducing the recurrence of IUA include the insertion of an intrauterine device (IUD) or Foley catheter or the use of estrogen therapy or adhesion barriers.8, 9 While these methods provide some protection against the postoperative regeneration of IUAs, they are not very effective for women with severe IUAs who need ancillary treatments to avoid the recurrence of IUAs.10 Hence, we need to find a new way to prevent the recurrence of IUAs for the women with moderate to severe IUAs after TCRA.
Amnion has obvious advantages compared with conventional methods: (1) as a biological barrier, amnion can inhibit inflammatory reaction by attaching to the surface of the wound;11 (2) it has multiple metabolic functions and contains kinds of growth factors such as epidermal growth factor, fibroblast growth factor, transforming growth factor-beta and platelet-derived growth factor12 which may be helpful to recovery of the injured endometrium; (3)it facilitates epithelial cell migration, and provides a scaffold that greatly helps the migration and growth of the endometrium;13 (4) immunosuppressive therapy is not necessary because amnion has low immunogenicity;14, 15 (5) amnion is rejected material after delivery, no ethical issues are involved in its use.16 We grafted amnion or injected chitosan into the uterine cavities of 100 women with moderate to severe IUA who underwent TCRA in our hospital from January 2015 to December 2017. Below, we report the results of this study.
Materials and methods
The study was conducted at the Department of Obstetrics and Gynecology, University-Town Hospital, Chongqing Medical University, in Chongqing, China. The study followed the principles set forth in the Helsinki Declaration of 1975, as revised in 2013. Approval of the Ethics Committee of ChongQing Medical University was obtained before the study, and all the participants gave informed consent. We reviewed the files of 100 women who were diagnosed with moderate to severe adhesions from January 2015 to December 2017 via hysteroscopy. The exclusion criteria were as follows: age >40 years, other medical conditions related to the uterus (for example, hysteromyoma, adenomyosis, and uterine malformation), other factors that affect pregnancy (for example, polycystic ovary syndrome), history of hormone therapy, personal or family history of thromboembolism disease, the finding of abnormal lumps on ultrasound, male infertility factors, and serious internal medicine diseases (for example, hyperthyroidism). This clinical trial has already registered (Chinese Clinical Trial Registry, www.chictr.org.cn, ChiCTR-ONC-16008210).
The participants were assigned to groups according to the level of adhesion based on the European Society of Gastrointestinal Endoscopy classification guidelines. One hundred patients were divided into amnion group (52 patients) and chitosan group (48 patients). The women in amnion group received fresh amnion grafts after TCRA, while the women in chitosan group received intrauterine injections of chitosan.
Prior to the surgery, informed consent forms should be collected from women before surgery. Performing complete check-ups and examinations for surgical contraindications. Inserting a disposable cervical dilator to soften the cervix at the day before surgery.
Collecting signed informed consent forms from women without communicable diseases (i.e., negative for hepatitis B virus, hepatitis C virus, HIV and syphilis) who are undergoing cesarean section and the amniotic fluid is not contaminated. These women should agree to donate their placentas voluntarily and provide their consent to the medical institution to use the amnion for medical science research. Removing the amnion out of the placenta via blunt separation under aseptic conditions, washing it repeatedly with normal saline (NS) to clean blood, and then soaking it with the cefuroxime. Then, using the amnion within 2–4 h.
Placing the patient in the lithotomy position, disinfecting routinely, administrating intravenous anesthesia, and dilating the cervix with Hegar cervical dilator (size 4.5 to size 10, increasing gradually) under the guidance of transabdominal ultrasonography to check the depth, size and direction of the uterine cavity. Closely observe the location, extent, and morphological characteristics of adhesions and the endometrial cavity under direct vision via hysteroscopy. Separating the adhesions with a needle electrode and fix the endometrium with a loop electrode to restore the normal shape and size of the uterine cavity, besides, the fallopian tubes are visible after TCRA.
In the study, a 16-Foley double balloon catheter covered with fresh amnion was transplanted into the uterine cavity of each woman after TCRA in amnion group, and five to ten milliliters of water were injected into the catheter and connected with a bag for continuous open drainage. The women in chitosan group received intrauterine injections of chitosan after TCRA.
All patients received continuous estrogen treatment with estradiol valerate (E2V) 3 mg per time thrice daily for 28 days after surgery, and cefoxitin were administered via intravenous infusion for 3 days to prevent infection. The balloon catheter was removed on the fifth day after surgery.
The women underwent examinations that included TVUS, hysteroscopy. If the hysteroscopy suggested that the uterus was a normal shape and size, no adhesions were present, and the fallopian tubes were visible, then dydrogesterone was administered at the dose of 10 mg per time twice a day for 7 days. If the hysteroscopy suggested the recurrence of adhesions, we performed another TCRA and fresh amnion graft or chitosan injection with the same follow-up procedures.
We used the statistical software SPSS version 20.0 to analyze the data. The measurement data are expressed as the mean and standard deviation, pairs of groups were compared using independent sample t test. Enumerative data are expressed as ratios, pairs of groups were compared using the chi-square test. A value of P < 0.05 was considered statistically significant.
Results
No complications occurred in all of the surgeries, such as uterine perforation, dilution hyponatremia, substantial hemorrhage, air embolism, and infection. The women's vital signs and chief complaints were monitored during the surgery, and no problems were found. All of the women had minor vaginal bleeding, which was the normal phenomenon after the surgery. The drainage bags contained no or minimal clear fluid, and the vaginal secretions had no peculiar smell. The white blood cell count and neutrophil percentages measured 3 days after the surgery revealed no significantly abnormities. None of the amnion and balloon catheter fell from the intrauterine cavity before the balloon catheter was taken out.
All of the women received the follow-up examinations for 3 months, including TVUS and hysteroscopy at the first and third month after surgery.
The age of the women and number of pregnancies were shown in Table 1, and no significant differences were observed among the amnion group and chitosan group (P > 0.05). There was no significant difference in possible etiological distribution between the two groups (P > 0.05) either, as shown in Table 2.
Table 1.
The general situation of the patients in the two groups ( ± s).
| Index | Amnion group | Chitosan group | The value of t | The value of P |
|---|---|---|---|---|
| Number of patients | 52 | 48 | ||
| Age | 29.04 ± 2.76 | 29.02 ± 3.10 | 0.030 | 0.976 |
| Number of pregnancies | 3.33 ± 0.47 | 3.35 ± 0.48 | −0.285 | 0.777 |
Table 2.
The comparison of the possible etiological distribution of patients with intrauterine adhesions between the two groups (%).
| Grouping | Artificial abortion (%) | Residue placenta (%) | Cesarean section (%) | Inflammation (%) | Total |
|---|---|---|---|---|---|
| Amnion group | 35 (67.3%) | 2 (3.8%) | 6 (11.5%) | 9 (17.3%) | 52 |
| Chitosan group | 34 (70.8%) | 2 (4.2%) | 5 (10.2%) | 7 (14.6%) | 48 |
| Total | 59 | 4 | 11 | 26 | 100 |
| The value of χ2 | 0.382 | ||||
| The value of P | 0.980 | ||||
In this study, we evaluated the preoperative and postoperative thickness of the endometrium comprehensively. The evaluation included quantitative detection with TVUS and qualitative detection under direct visualization of the endometrium via hysteroscopy. As Table 3 showed that there were no significant differences in the preoperative endometrial thicknesses between amnion group and chitosan group (P = 0.442). In contrast, the postoperative endometrial thickness in amnion group was significantly better than this of chitosan group (P = 0.000).
Table 3.
The average of the endometrial thickness before and after surgery in the two groups ( ± s).
| Index | Amnion group |
Chitosan group |
The value of t | The value of P |
|---|---|---|---|---|
| (n = 52) | (n = 48) | |||
| Endometrial thickness (mm) | ||||
| Preoperative | 3.12 ± 0.78 | 3.25 ± 0.96 | −0.772 | 0.442 |
| First month after surgery | 7.08 ± 0.76 | 6.50 ± 0.71 | 3.894 | 0.000 |
As shown in Table 4, Table 5, in amnion group, 8 patients exhibited relapse in the first month after surgery, 7 of these patients underwent a second operation, and just two of the fifty-two patients exhibited relapse in three months after surgery; in chitosan group, 23 women exhibited relapse in the first month after surgery, 21 of these patients underwent a second operation, and eighteen of the forty-eight patients exhibited relapse in three months after surgery. Statistical analysis revealed that the recurrence rate of adhesion in amnion group was significantly lower than those of chitosan group in the first and three months after surgery (P1 = 0.000, P2 = 0.000).
Table 4.
The comparison of the recurrence rate of adhesion in the first month after surgery in the two groups (%).
| Grouping | No adhesion (%) | Adhesion recurrence (%) | Total |
|---|---|---|---|
| Amnion group | 44 (84.6%) | 8 (15.4%) | 52 |
| Chitosan group | 25 (52.1%) | 23 (47.9%) | 48 |
| Total | 79 | 31 | 100 |
| The value of χ2 | 12.350 | ||
| The value of P1 | 0.000 | ||
Table 5.
The comparison of the recurrence rate of adhesion in three months after surgery in the two groups (%).
| Grouping | No adhesion (%) | Adhesion recurrence (%) | Total |
|---|---|---|---|
| Amnion group | 50 (96.2%) | 2 (3.8%) | 52 |
| Chitosan group | 30 (62.5%) | 18 (37.5%) | 48 |
| Total | 80 | 20 | 100 |
| The value of χ2 | 17.88 | ||
| The value of P2 | 0.000 | ||
Discussion
IUA is a condition caused by various factors, such as trauma and infection which make a great contribution to the fibrosis and formation of scar tissue in the endometrium because of the destruction of endometrial basal layer.3 The clinical symptoms of IUA are hypomenorrhea, amenorrhea, periodic hypogastralgia, infertility as well as obstetric complications which do great harm to women's reproductive health.17, 18 TCRA is the first choice for those patients who are diagnosed with moderate to severe intrauterine adhesions. However, how to reduce the recurrence of IUAs remains a tough problem for the gynecologists. A study19 had showed that the incidence of recurrence of IUAs after TCRA is 3.1%–23.5%, moreover, the rate of regeneration of severe IUAs have been reported as high as 62.5% of these cases. Another study revealed that recurrence of IUAs after surgery do great harm to the women's reproductive outcomes.20
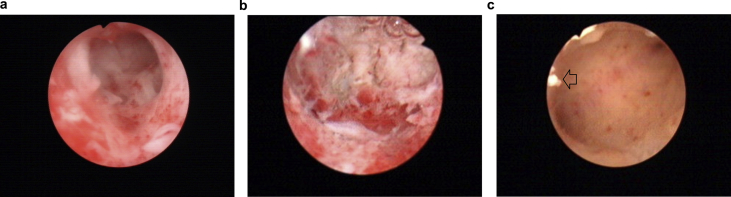
With the improvement of comprehensive management measures including the IUD, Foley catheter, estrogen therapy or adhesion barriers after TCRA, the recurrence rate of IUA had decreased to some degree;21, 22, 23 however, the results remain less than satisfactory, especially for the moderate to severe IUAs. In the present study, our results show a remarkable postoperative effect for woman with the severe IUA to prevent the recurrence of IUA after TCRA(see Fig. 1 for details).
Figure 1.
One Woman with Severe Intrauterine Adhesions. (a) Preoperative: adhesions and scarring had obliterated the uterine cavity. (b) Postoperative: restoration of the uterine cavity, with bilateral fallopian tubes visible. (c) One month after surgery: the uterine cavity had a normal shape and size, with no adhesions, and bilateral fallopian tubes were visible; residual amnion can be seen in the cavity (indicated by the arrow in the picture).
At present, in the field of Gynecology, few studies showed that amnion graft may be helpful for cervical reconstruction and vaginal reconstruction.24, 25 In recent years, few medical institution reported using amnion grafts to treat IUA. In 2006, Amer26 used amnion grafts to prevent the recurrence of IUA after surgery in 25 women with moderate to severe IUA. These women were examined via hysteroscopy during the fourth month after the operation. The findings indicated that no adhesions occurred in 12 women with moderate IUA and 1 woman with severe IUA. The remaining 12 women with severe IUA exhibited recurrence, although recurrence was mild in 10 of these 12 women (83.33%) and moderate in only 2 of these 12 women (16.67%). These results showed that more clinical trials are required to confirm the effectiveness of amnion. In 2010, the team of Amer27 performed homologous clinical research to determine the efficacy of fresh and dried amnion grafts after the hysteroscopic lysis of severe IUA for decreasing IUA recurrence and encouraging endometrial regeneration. The results showed that both methods were effective, but the fresh amnion graft performed better than the dried amnion graft.
In our study, we used fresh amnion grafts after TCRA combined with a balloon catheter to prevent the formation of new adhesions and estrogen treatment to stimulate the growth of the endometrium to treat and prevent IUA. Only 8 of 52 (15.4%) showed recurrence 1 month postsurgery. Among the recurrent women, there was one case of mild adhesion that was treated with blunt separation under hysteroscopy and followed for 3 months after surgery; 7 women had moderate adhesions who were treated with a second TCRA and fresh amnion graft. Just 2 of these 8 women showed relapse under hysteroscopy in the third month after the operation.
Our results show that the recurrence rate among women treated for moderate to severe IUA using fresh amnion grafts is far lower than that reported by the literature. The reasons may be as follows: (1) The amniotic epithelial layer outside covered the balloon catheter when we performed the fresh amnion graft, while the literature reported that the amniotic epithelial layer inside covered the balloon catheter, and this is probably the most important aspect. (2) The present study used continuous estrogen treatment in the first month after surgery, while the literature reported the use of artificial cycle therapy. Existing research has reported that the first month after surgery was the peak time of adhesion recurrence; therefore, continuous estrogen treatment can improve the cure rate of IUA more effectively than artificial cycle therapy. (3) The formation of IUA begins with a very slight adhesion, however, it looks similar with the scar's healing progresses, the adhesion becomes fibrotic and irreversible.28
The conceivable mechanisms of amnion's effectiveness in preventing IUA are as follows: (1) It provides a biological barrier and promotes epithelial hyperplasia: the amnion clinging to the uterine wall can provide an ideal basement membrane for the proliferation, differentiation and regeneration of new epithelium. Simultaneously, the basement membrane of the amnion can secrete many cytokines, such as epidermal growth factor, fibroblast growth factor and transforming growth factor-beta, which rapidly makes the bare area epithelial by stimulating the formation of epithelial cells, thus preventing the recurrence of adhesions and promoting the recovery of the endometrial wounds. (2) Amnion inhibits the inflammatory response. The amnion clinging to the surface of the wound can reduce the dead space between the wound and the amnion, thus inhibiting the proliferation and spread of bacteria. Amnion contains a variety of protease inhibitors, and the stroma of the amnion can eliminate inflammation by promoting the rapid apoptosis of inflammatory cells. (3) Amnion has anti-fibrosis effects. Research indicates women with IUAs whose stroma are largely replaced with fibrous tissue without vascular and the glands are always replaced by inactive cubocolumnar endometrial epithelium unresponsive to hormonal stimulation;29 moreover, transforming growth factor-β1 (TGF-β1) is highly expressed in the endometrium of the adhesion surface and is positively correlated with the degree of adhesion. The stroma of the amnion has a regulatory effect on the level of cytokines expressed by desmocytes; it can inhibit the expression of TGF-β1 mRNA and reduce the differentiation and proliferation of desmocytes, thus inhibiting the formation of fibrous scar tissue.30 (4) Containing stem cell like cells: Numerous studies show that endometrial stem cells, which are present in the basal layer of the endometrium, play a key role in the regeneration of functional layers in each menstrual cycle, and the basal layer damage is the main reason for the formation of intrauterine adhesions, therefore, a significant reduction in the number of endometrial stem cells may explain the lack of endometrial tissue in intrauterine adhesions.31, 32 Human amnion stem cells have the potential of multi-directional differentiation of stem cells, amnion mesenchymal stem cells produced by a whole amnion can reach 4 × 108,33 after TCRA, fresh amnion which is rich in human amnion stem cells were transplanted into the uterine cavity, these cells may pave the way for novel approaches in the development of tissue-engineered vascular grafts through the damaged endometrial surface, at the same time, under the stimulation of sex hormone, it was clonal expansion and differentiation into endometrial tissue, so as to promoting regeneration of the thin endometrial.
Studies indicate that the morbidity of IUA among infertile women is as high as 13%,34 which continues to increase annually due to greater awareness of IUA among clinicians and the increasing rate of mechanical suction dilation and curettage (D&C).35, 36 Morbidity due to IUA is mainly related to intrauterine surgeries for termination of pregnancy, such as D&C, caesarean section, which lead to most of IUA.36, 37, 38 Therefore, to prevent the recurrence of IUA and improve the effectiveness of IUA treatment, repeated artificial abortions should be avoided as much as possible.39, 40 Strengthening the sex education and popularizing their awareness of contraception for the women who are lack of sex education and contraception knowledge are the masterly ways to reduce the rates of unintended pregnancy and artificial abortion, and further decrease the incidence of IUAs in some ways.
The main strengths of this study are that it summarizes and improves the previous tests to further confirm and improve the effectiveness of fresh amnion grafts in preventing the postoperative recurrence of IUA.
We acknowledge that there are several limitations in the present study. The sample size is small, and the key about the treatment of IUA is the promotion of endometrial regeneration and the restoration of normal reproductive function. The pregnancy outcome is the most important evaluation index; therefore, a deficiency of this study is that the pregnancy outcomes have not been. Furthermore, the effect of optimal pregnancy time and pregnancy outcomes after surgery require long-term follow-up.
Conclusion
After TCRA, the use of amnion grafts is safe and effective than intrauterine injections of chitosan for the recurrence of IUAs, and it may make a great contribution to preventing further regeneration of adhesions.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgments
We thank all of the patients and the donators of placentas for their contribution to the study. Besides, we appreciate Shanbi Zhou for providing the way about how to prepare the fresh amnion. And the study was funded by the Medical Research Project of Chongqing (NO. 20141014) and the Medical Characteristic Professional Subjects Construction Project of Chongqing (NO. 2013-46).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2019.03.003.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.March C.M. Management of Asherman's syndrome. Reprod Biomed Online. 2011;23:63–76. doi: 10.1016/j.rbmo.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 2.March C., Israel R., March A. Hysteroscopic managment of intrauterine adhesions. Am J Obstet Gynecol. 1978;130:653–657. doi: 10.1016/0002-9378(78)90322-8. [DOI] [PubMed] [Google Scholar]
- 3.Deans R., Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;17:555–569. doi: 10.1016/j.jmig.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Robinson J.K., Colimon L.M., Isaacson K.B. Postoperative adhesiolysis therapy for intrauterine adhesions (Asherman's syndrome) Fertil Steril. 2008;90:409–414. doi: 10.1016/j.fertnstert.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 5.AAGL Advancing Minimally Invasive Gynecology Worldwide AAGL practice report: practice guidelines for management of intrauterine synechiae. J Minim Invasive Gynecol. 2010;17:1–7. doi: 10.1016/j.jmig.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Thomson A.J., Abbott J.A., Deans R., Kingston A., Vancaillie T.G. The management of intrauterine synechiae. Curr Opin Obstet Gynecol. 2009;21:335–341. doi: 10.1097/GCO.0b013e32832e07fc. [DOI] [PubMed] [Google Scholar]
- 7.Khan Z., Goldberg J.M. Hysteroscopic management of Asherman's syndrome. J Minim Invasive Gynecol. 2018;25:218–228. doi: 10.1016/j.jmig.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Healy M.W., Schexnayder B., Connell M.T. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:267–275. doi: 10.1016/j.ajog.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Salazar C.A., Isaacson K., Morris S. A comprehensive review of Asherman's syndrome: causes, symptoms and treatment options. Curr Opin Obstet Gynecol. 2017;29:249–256. doi: 10.1097/GCO.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 10.Salma U., Xue M., Md Sayed A.S., Xu D. Efficacy of intrauterine device in the treatment of intrauterine adhesions. Biomed Res Int. 2014;2014:589296. doi: 10.1155/2014/589296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King A.E., Paltoo A., Kelly R.W., Sallenave J.M., Bocking A.D., Challis J.R. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta. 2007;28:161–169. doi: 10.1016/j.placenta.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Rennie K., Gruslin A., Hengstschla ger M. Applications of amniotic membrane and fluid in stem cell biology and regenerative medicine. Stem Cell Int. 2012;2012:721538. doi: 10.1155/2012/721538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng S.C. Amniotic membrane transplantation for ocular surface reconstruction. Biosci Rep. 2001;21:481–489. doi: 10.1023/a:1017995810755. [DOI] [PubMed] [Google Scholar]
- 14.Li H., Niederkorn J.Y., Neelam S. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:900–907. doi: 10.1167/iovs.04-0495. [DOI] [PubMed] [Google Scholar]
- 15.Kubo M., Sonoda Y., Muramatsu R., Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539–1546. [PubMed] [Google Scholar]
- 16.Mamede A.C., Carvalho M.J., Abrantes A.M., Laranjo M., Maia C.J., Botelho M.F. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447–458. doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 17.March C. Asherman's syndrome. Semin Reprod Med. 2011;29:83–94. doi: 10.1055/s-0031-1272470. [DOI] [PubMed] [Google Scholar]
- 18.Song D., Liu Y., Xiao Y., Li T.C., Zhou F., Xia E. A matched cohort study comparing the outcome of intrauterine adhesiolysis for Asherman's syndrome after uterine artery embolization or surgical trauma. J Minim Invasive Gynecol. 2014;21:1022–1028. doi: 10.1016/j.jmig.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Yu D., Wong Y.M., Cheong Y., Xia E., Li T.C. Asherman syndrome-one century later. Fertil Steril. 2008;89:759–779. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 20.Yu D., Li T.C., Xia E., Huang X., Liu Y., Peng X. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman's syndrome. Fertil Steril. 2008;89:715–722. doi: 10.1016/j.fertnstert.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 21.Revaux A., Ducarme G., Luton D. Prévention des synéchies après hystéroscopie opératoire. Gynecol Obstet Fertil. 2008;36:311–317. doi: 10.1016/j.gyobfe.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Myers E.M., Hurst B.S. Comprehensive management of severe Asherman syndrome and amenorrhea. Fertil Steril. 2012;97:160–164. doi: 10.1016/j.fertnstert.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Johary J., Xue M., Zhu X., Xu D., Velu P.P. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J Minim Invasive Gynecol. 2014;21:44–54. doi: 10.1016/j.jmig.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Mhaskar R. Amniotic membrane for cervical reconstruction. Int J Gynaecol Obstet. 2005;90:123–127. doi: 10.1016/j.ijgo.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Seyed-Forootan K., Karimi H., Seyed-Forootan N.S. Autologous fibroblast-seeded amnion for reconstruction of neo-vagina in male-to-female reassignment surgery. Aesthet Plast Surg. 2018;42:491–497. doi: 10.1007/s00266-018-1088-z. [DOI] [PubMed] [Google Scholar]
- 26.Amer M.I., Abd-El-Maeboud K.H. Amnion graft following hysteroscopic lysis of intrauterine adhesions. J Obstet Gynaecol Res. 2006;32:559–566. doi: 10.1111/j.1447-0756.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 27.Amer M.I., Abd-El-Maeboud K.H., Abdelfatah I., Salama F.A., Abdallah A.S. Human amnion as a temporary biologic barrier after hysteroscopic lysis of severe intrauterine adhesions: pilot study. J Minim Invasive Gynecol. 2010;17:605–611. doi: 10.1016/j.jmig.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Shokeir T.A., Fawzy M., Tatongy M. The nature of intrauterine adhesions following reproductive hysteroscopic surgery as determined by early and late follow-up hysteroscopy: clinical implications. Arch Gynecol Obstet. 2008;277:423–427. doi: 10.1007/s00404-007-0475-5. [DOI] [PubMed] [Google Scholar]
- 29.Foix A., Bruno R., Davison T., Baltasar L. The pathology of postcurettage adhesions. Am J Obstet Gynecol. 1966;96:1027–1033. doi: 10.1016/0002-9378(66)90452-2. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.B., Li D.Q., Tan D.T., Meller D.C., Tseng S.C. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–334. [PubMed] [Google Scholar]
- 31.Gargett C.E., Nguyen H.P., Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13:235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]
- 32.Gargett C.E., Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98:11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Alviano F., Fossati V., Marchionni C. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in virto. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panayotidis C., Weyers S., Bosteels J., van Herendael B. Intrauterine adhesions (IUA): has there been progress in understanding and treatment over the last 20 years? Gynecol Surg. 2009;6:197–211. [Google Scholar]
- 35.Dalton V.K., Saunders N.A., Harris L.H., Williams J.A., Lebovic D.I. Intrauterine adhesions after manual vacuum aspiration for early pregnancy failure. Fertil Steril. 2006;85 doi: 10.1016/j.fertnstert.2005.11.065. 1823.e1-1283.e3. [DOI] [PubMed] [Google Scholar]
- 36.Gilman A.R., Dewar K.M., Rhone S.A., Fluker M.R. Intrauterine adhesions following miscarriage: look and learn. J Obstet Gynaecol Can. 2016;38:453–457. doi: 10.1016/j.jogc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Salzani A., Yela D.A., Gabiatti J.R., Bedone A.J., Monteiro I.M. Prevalence of uterine synechia after abortion evacuation curettage. Sao Paulo Med J. 2007;125:261–264. doi: 10.1590/S1516-31802007000500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedler S., Margalioth E.J., Kafka I., Yaffe H. Incidence of post-abortion intra-uterine adhesions evaluated by hysteroscopy—a prospective study. Hum Reprod. 1993;8:442–444. doi: 10.1093/oxfordjournals.humrep.a138068. [DOI] [PubMed] [Google Scholar]
- 39.Sedgh G., Henshaw S.K., Singh S., Bankole A., Drescher J. Legal abortion worldwide: incidence and recent trends. Perspect Sex Reprod Health. 2007;39:216–225. doi: 10.1363/3921607. [DOI] [PubMed] [Google Scholar]
- 40.Casey P.R. Abortion among young women and subsequent life outcomes. Best Pract Res Clin Obstet Gynaecol. 2010;24:491–502. doi: 10.1016/j.bpobgyn.2010.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.