Abstract
Bone morphogenetic protein 9 (BMP9) (or GDF2) was originally identified from fetal mouse liver cDNA libraries. Emerging evidence indicates BMP9 exerts diverse and pleiotropic functions during postnatal development and in maintaining tissue homeostasis. However, the expression landscape of BMP9 signaling during development and/or in adult tissues remains to be analyzed. Here, we conducted a comprehensive analysis of the expression landscape of BMP9 and its signaling mediators in postnatal mice. By analyzing mouse ENCODE transcriptome datasets we found Bmp9 was highly expressed in the liver and detectable in embryonic brain, adult lungs and adult placenta. We next conducted a comprehensive qPCR analysis of RNAs isolated from major mouse tissues/organs at various ages. We found that Bmp9 was highly expressed in the liver and lung tissues of young adult mice, but decreased in older mice. Interestingly, Bmp9 was only expressed at low to modest levels in developing bones. BMP9-associated TGFβ/BMPR type I receptor Alk1 was highly expressed in the adult lungs. Furthermore, the feedback inhibitor Smads Smad6 and Smad7 were widely expressed in mouse postnatal tissues. However, the BMP signaling antagonist noggin was highly expressed in fat and heart in the older age groups, as well as in kidney, liver and lungs in a biphasic fashion. Thus, our findings indicate that the circulating BMP9 produced in liver and lungs may account for its pleiotropic effects on postnatal tissues/organs although possible roles of BMP9 signaling in liver and lungs remain to be fully understood.
Keywords: BMP9/GDF2, Bone morphogenetic proteins (BMPs), Hepatic metabolism, Mesenchymal stem cells, Neurogenesis, Osteogenic differentiation, Pulmonary arterial hypertension, Tumorigenesis
Introduction
Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily and play important roles in embryogenesis, organogenesis, cell proliferation, stem cell differentiation, and adult tissue homeostasis.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Disruptions in BMP signaling cause skeletal and extraskeletal anomalies.1, 4, 11 At least 14 types of BMPs in humans and rodents have been identified.1, 5, 9, 12, 13 BMPs were initially discovered when demineralized bone was found to induce de novo bone formation.14, 15
Different BMPs exert distinct but overlapping biological functions. BMP9 (also known as growth differentiation factor 2 or GDF-2) represents a less characterized member of the BMP family.4, 9, 13 Through a comprehensive analysis of human BMPs in mesenchymal stem cells (MSCs), we demonstrated that BMP9 is one of the most potent BMPs among the 14 types of BMPs in inducing osteogenic/odontogenic differentiation of MSCs.9, 10, 16, 17, 18, 19 We also found that BMP9 is resistant to the naturally occurring antagonist noggin,20 and demonstrated that TGFβ/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in MSCs.21 As BMP9 is one of the least studied BMP, we performed mechanism-based studies and identified several early downstream targets,9, 10, 22, 23, 24, 25, 26, 27, 28 and demonstrated that BMP9 signaling has extensive cross-talks with other signaling pathways, especially Wnt and Notch signaling.9, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37
It has also been reported that BMP9 plays important roles in inducing and maintaining basal forebrain cholinergic neurons, inhibiting hepatic glucose production, inducing the expression of key enzymes of lipid metabolism, stimulating hepcidin 1 expression, regulating angiogenesis, and modulating tumorigenesis.10, 38 BMP9 has further been implicated in the pathogenesis of pulmonary arterial hypertension.38, 39
Originally identified from fetal mouse liver cDNA libraries, BMP9 is highly expressed in the developing mouse liver.4, 9, 40 Increasing evidence indicates that BMP9 exerts diverse and pleiotropic biological functions during postnatal development and in maintaining adult tissue homeostasis. Thus, it is conceivable that a postnatal expression landscape of BMP9 and its important signaling mediators would provide important insights into the potential functions of BMP9 signaling in major organs and/or tissues. However, the expression landscape of BMP9 and its signaling mediators during development and/or in adult tissues has yet to be fully investigated.
In this study, we sought to conduct a comprehensive analysis of the expression landscape of BMP9 and its signaling mediators in postnatal mice. We first analyzed the mouse ENCODE transcriptome data sets and found that Bmp9 was highly expressed in liver although Bmp9 expression was detectable in embryonic brain, adult lungs and adult placenta. However, the ENCODE datasets are limited in scope and depth. Thus, we conducted a comprehensive qPCR analysis of the RNAs isolated from major mouse tissues/organs at various ages. Our results demonstrated that, although at relatively lower levels at birth, Bmp9 was highly expressed in the liver and lung tissues of young adult mice, but decreased in older mice. Bmp9 expression was also detectable in 2-week spleen, brain and fat tissues and muscle samples. Interestingly, Bmp9 was only expressed at modest to low levels in developing bones. Alk1 was highly expressed in the adult lungs, while Alk2 was expressed at relatively higher levels in fat and kidney tissues. Furthermore, the feedback inhibitors Smad6 and Smad7 of BMP signaling were widely expressed in mouse postnatal tissues, including developing bones. However, the BMP signaling antagonist noggin was expressed highly in fat and heart tissues in the older age groups, as well as in kidney, liver and lung tissues in a biphasic fashion. Collectively, our findings indicate that the circulating BMP9 produced in liver and lungs may account for its pleiotropic effects on multiple postnatal tissues/organs although the functional roles of BMP9 signaling in liver and lungs remain to be fully understood.
Materials and methods
Mouse ENCODE transcriptome data analysis
The RNA profiling data sets were generated by the Mouse ENCODE project PRJNA66167 as described.41 The data sets included 30 mouse samples derived from embryonic and adult tissues, and are publically available through NCBI website (https://www.ncbi.nlm.nih.gov/gene/). The acquired reads per kilobase per million reads (RPMKs) for Bmp9, Alk1, Alk2, Smad6 and Smad7 in the 30 mouse samples were graphed by using the Microsoft Excel software.
Animal tissue samples
The use of mouse tissues was approved by the Institutional Animal Care and Use Committee and all experimental procedures involved in harvesting mouse tissues were performed according to the approved guidelines. Briefly, C57BL/6J mice at newborn (NB), 2-week, 4-week, 3-month, 8-month and 18-month old (male, n = 5) were obtained from The University of Chicago Transgenic Core Facility.
Total RNA isolation from mouse tissues and reverse transcription
For total RNA isolation, mouse brain, fat (inguinal region), heart, kidney, liver, lung, muscle, spleen, femur and parietal bone (PB) at various ages were harvested immediately after sacrificing the animals, placed in RNase-free mortars containing NucleoZOL Reagent (Takara Bio USA, Mountain View, CA) and liquid nitrogen, and crashed with RNase-free pestles in a RNase-free biosafety cabinet. Total RNA was subsequently isolated according to the manufacturer's introduction, and subjected to reverse transcription with hexamer and M-MuLV (New England Biolabs, Ipswich, MA). The cDNA products were diluted as templates for qPCR.
Touchdown quantitative real-time PCR (TqPCR)
The qPCR primers were designed by using Primer3 Plus program.42 SYBR Green-based quantitative real-time PCR analysis was performed by following our previously optimized TqPCR protocol.43 The qPCR reactions were done in triplicate. All expression values were normalized to the reference gene Gapdh expression by using the 2–ΔΔCt method.44, 45, 46, 47 The qPCR primer sequences are listed in Table 1.
Table 1.
List of qPCR Primers.
| Gene | Accession No. | Forward | Reverse |
|---|---|---|---|
| Bmp9/Gdf2 | NM_019506.4 | TGAGTCCCATCTCCATCCTC | ACCCACCAGACACAAGAAGG |
| Alk1 | NM_001277255.1 | ACCTGGGACTGGCTGTGA | GCAGTCTGTGCGGATGTG |
| Alk2 | NM_001110204.1 | GTGGCTCCGGTCTTCCTT | AGCGACATTTTCGCCTTG |
| Smad6 | NM_008542.3 | ATCACCTCCTGCCCCTGT | CTGGGGTGGTGTCTCTGG |
| Smad7 | NM_001042660.1 | AAGATCGGCTGTGGCATC | CCAACAGCGTCCTGGAGT |
| Nogging | NM_008711.2 | GCGGCCAGCACTATCTACA | GGGGCGAAGTAGCCATAAA |
| Gapdh | NM_008084 | GAAGGTCGGTGTGAACGGAT | ACTGTGCCGTTGAATTTGCC |
Results and discussion
Mouse ENCODE transcriptome data indicate Bmp9 is highly expressed in liver while Bmp9-associated BMP type I receptor Alk1 is highly expressed in lungs
As one of the least studied BMPs, BMP9 was originally identified from mouse embryonic liver tissue.40 However, the expression patterns of BMP9 and its signaling mediators during development and/or in adult tissues have not been investigated. The recently completed ENCODE transcriptome projects have offered a glimpse of the expression patterns for a given gene.
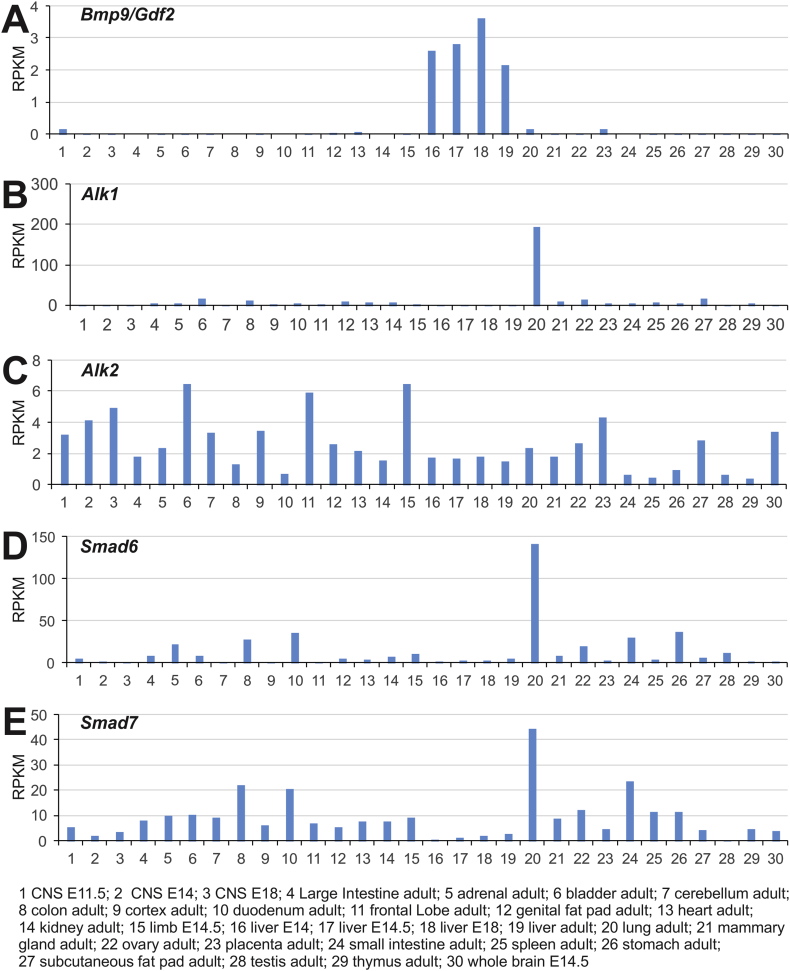
Here, we took advantage of the mouse ENCODE database and examined the expression patterns of Bmp9 and its signaling mediators in 30 mouse samples. Consistent with earlier reports,40 Bmp9 expression level was apparently high in liver E14, E14.5, E18 and adult liver samples, while Bmp9 expression was also detected in CNS E11.5, adult lung, and adult placenta (Fig. 1A). Surprisingly, Bmp9 associated BMP type I receptor Alk1 was highly expressed in adult lung tissue although several tissues (such as adult bladder, colon, genital fat pad, heart, kidney, mammary gland, ovary, and subcutaneous fat pad) expressed detectable levels of Bmp9 (Fig. 1B). Conversely, another Bmp9-associated type I receptor Alk2 exhibited a broad range, even though many of those tissues exhibited medium to lower levels of Bmp9 expression (Fig. 1C).
Figure 1.
Across-tissue expression of Bmp9 and its signaling mediators revealed by mouse ENCODE transcriptome analysis. The RNA profiling data sets (n = 30 samples) were generated by the Mouse ENCODE project, PRJNA66167, as described in.41 The tabulated reads per kilobase per million reads (RPMKs) for Bmp9 (A), Alk1 (B), Alk2 (C), Smad6 (D) and Smad7 (E) in the 30 mouse samples were graphed. The individual samples were delineated at the bottom of the graphs.
We also analyzed the ENCODE data for BMP signaling feedback inhibitor Smads (or I-Smads), Smad6 and Smad7. Surprisingly, adult lung tissue has the highest expression for both I-Smads, followed by various GI tissues, although Smad7 is more widely expressed than Smad6 (Fig. 1D & E). Taken together, the mouse ENCODE transcriptome data indicate that Bmp9 expression is relatively restricted to liver, and to a lesser extent, lungs, while the Bmp9 signaling mediators, especially Alk1, Smad6 and Smad7, exhibit high expression levels in adult lungs, suggesting that BMP9 may play an important role in regulating biological functions and tissue homeostasis of the lungs.
Bmp9 is highly expressed in postnatal liver and lung tissues
Even though the ENCODE database has offered a quick overview of the expression of Bmp9 and its signaling mediators, those data are limited in scope and depth. On the other hand, the currently available antibodies against BMP9 lack either specificity and/or reactivity. Furthermore, it is relatively difficult to quantify immunostaining results. Thus, we decided to carry out qPCR analysis of the postnatal expression of Bmp9 and related signaling mediators. We collected total RNA from the major mouse tissues/organs, such as whole brain, fat (inguinal region), heart, kidney, liver, lungs, muscle, spleen, femur, and parietal bone (PB) at various ages, including newborn (NB), 2-week, 4-week, 3-month, 8-month and 18-month old mice.
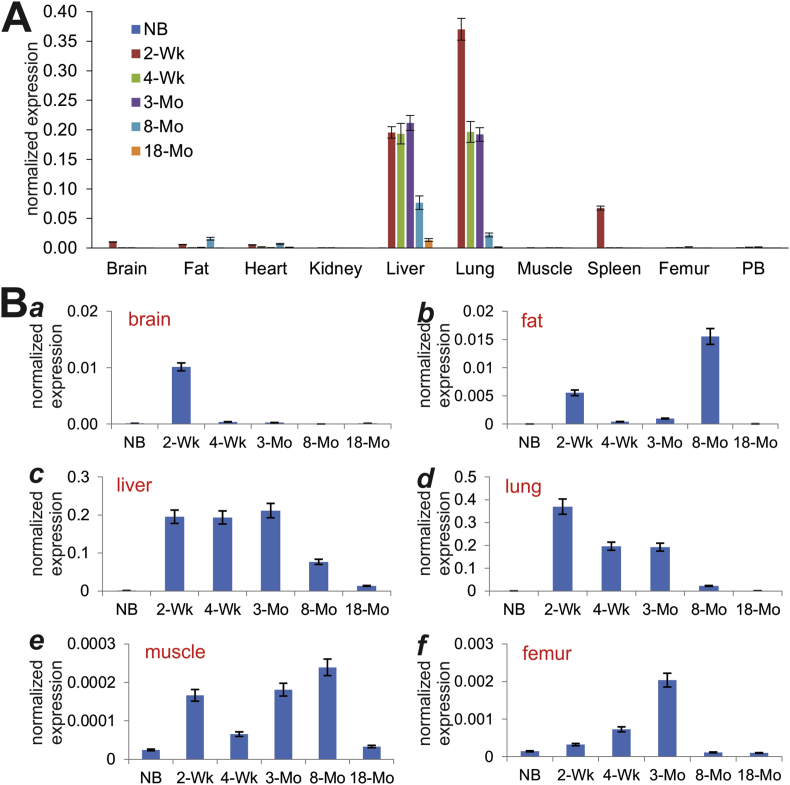
Using our previously optimized touchdown qPCR (or TqPCR) technique,43 we analyzed the expression of Bmp9 in these tissues. At age groups, Bmp9 was shown to express at much higher levels in liver and lung tissues, although its expression showed an age-dependent trend in decrease (Fig. 2A). Interestingly, a significant expression peak of Bmp9 was found in 2-week spleen tissue (Fig. 2A). A detailed analysis indicates that Bmp9 expression was readily detectable in 2-week brain, 2-week and 8-month fat tissue, and in muscle at most of the analyzed time points (Fig. 2B, panels a, b, and e). Interestingly, even though highly expressed at later time points (e.g., 2-week, 4-week and 3-month), Bmp9 was expressed at relatively low levels in both liver and lung tissues at birth (Fig. 2B, panels c & d). In bone tissue such as femur, Bmp9 expression increased over the time from newborn to 3-month (Fig. 2B, panel f), indicating BMP9 may play an important role in bone and skeletal development.
Figure 2.
Expression of Bmp9 in postnatal mouse tissues determined by TqPCR analysis. Total RNA was isolated from mouse brain, fat (inguinal region), heart, kidney, liver, lung, muscle, spleen, femur, and parietal bone (PB) at various ages: newborn (NB), 2-week, 4-week, 3-month, 8-month and 18-month old. The RT cDNA products were subjected to TqPCR analysis of Bmp9 expression in these tissues.
Bmp9-associated TGFβ/BMPR type I receptor Alk1 is highly expressed in the lungs, while Alk2 expressed relatively higher in fat and kidney tissues
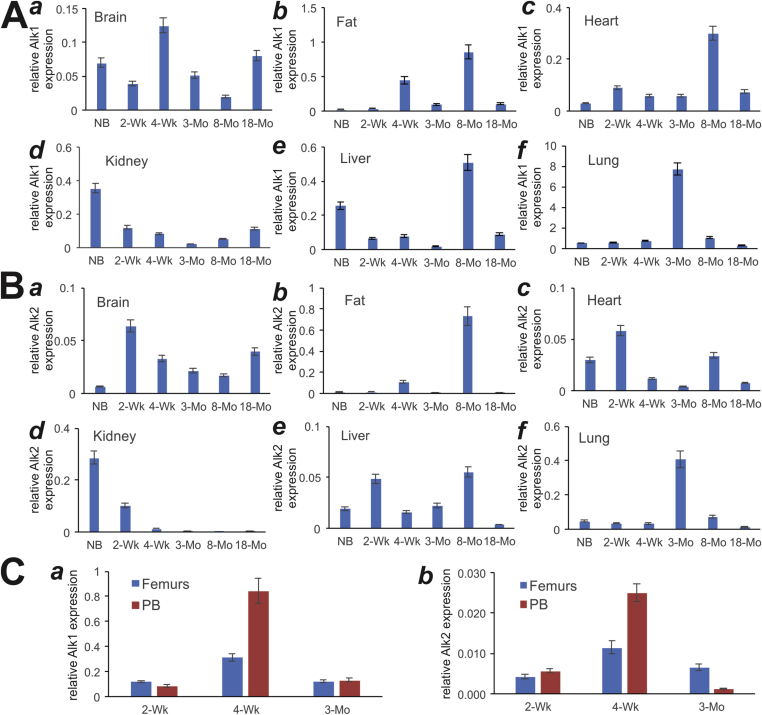
We previously demonstrated that BMP9 initiates its signaling through interactions with TGFβ/BMPR type I receptor Alk1 and Alk221. Our results indicated that Alk1 was modestly expressed in various age groups of the brain, heart, kidney and liver tissues (Fig. 3A, panels a, c, d, and e). However, the highest expression levels of Alk1 were found in the lungs, especially in 3-month lungs, although Bmp9 was also highly expressed in 8-month fat tissue (Fig. 3A, panels c & f). On the other hand, Alk2 was expressed modestly, but more broadly in the analyzed tissues, including brain, fat, heart, kidney, liver and lungs, although the highest level of Alk2 expression was found in 8-week fat tissue sample (Fig. 3B, panels a to f).
Figure 3.
Expression of BMP9-associated receptors Alk1 and Alk2 in postnatal mouse tissues determined by TqPCR analysis. Total RNA was isolated from mouse brain, fat (inguinal region), heart, kidney, liver, lung, muscle, spleen, femur, and parietal bone (PB) at various ages: newborn (NB), 2-week, 4-week, 3-month, 8-month and 18-month old. The RT cDNA products were subjected to TqPCR analysis of Alk1 and Alk2 expression in these tissues.
Lastly, we examined the expression of Alk1 and Alk2 in the developing bone tissues, femurs and parietal bone (PB). We found that the expression of Alk1 and Alk2 peaked at the 4-week time point in both bone samples (Fig. 3C, panels a & b). However, Alk1 was expressed at much higher levels than Alk2 at the three age groups, suggesting Alk1 may play a more important role in developing bones.
The negative regulators of BMP9 signaling, Smad6, Smad7 and noggin, are widely expressed in mouse postnatal tissues
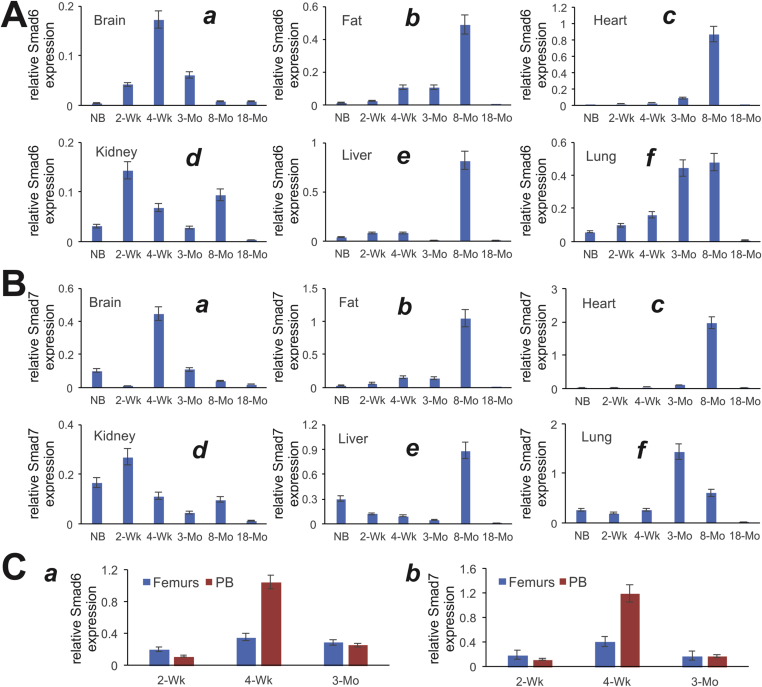
As the feedback inhibitors Smad6 and Smad7 (or so-called I-Smads) can serve as indirect indicators of the status of BMP signaling activities including BMP9, we further analyzed the expression patterns of Smad6 and Smad7 in the mouse tissues. In brain tissue, the expression of both Smad6 and Smad7 was shown highest in the 4-week group (Fig. 4A panel a and Fig. 4B panel a). On the other hand, both Smad6 and Smad7 peaked their expression in the 8-month age group in fat, heart, and liver tissues (Fig. 4A panels b, c, & e, and Fig. 4B panels b, c, & e). Smad6 and Smad7 also exhibited similar expression patterns in kidney samples with the highest expression at the 2-week age group (Fig. 4A panel d, and Fig. 4B panel d). However, the expression of Smad6 and Smad7 in the lungs seemingly increased with ages within the first 8 and 3 months after birth, respectively (Fig. 4A, panel f; and Fig. 4B panel f).
Figure 4.
Expression of BMP9 signaling feedback inhibitors, Smad6 and Smad7, in postnatal mouse tissues determined by TqPCR analysis. Total RNA was isolated from mouse brain, fat (inguinal region), heart, kidney, liver, lung, muscle, spleen, femur, and parietal bone (PB) at various ages: newborn (NB), 2-week, 4-week, 3-month, 8-month and 18-month old. The RT cDNA products were subjected to TqPCR analysis of Smad6 and Smad7 expression in these tissues.
We further analyzed the expression of I-Smads in developing bone tissues and found that both Smad6 and Smad7 peaked their expression in femurs and parietal bone at the 4-week age group (Fig. 4C panels a & b), suggesting that BMP signaling, which is negatively regulated by Smad6 and Smad7, may play an important role in developing bones.
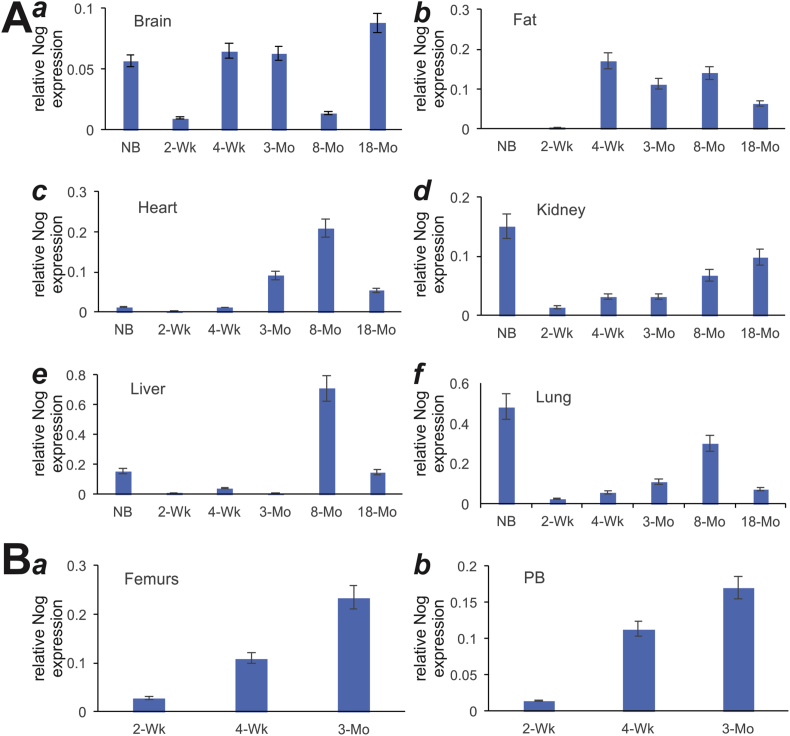
Lastly, we analyzed the expression of the BMP signaling antagonist noggin. We found that noggin was expressed at low levels in the brain tissues (Fig. 5A panel a). In fat and heart tissues, noggin was shown to express at older age groups (e.g., 4-week to 8-month) (Fig. 5A, panels b & c). On the other hand, in kidney, liver and lung tissues noggin showed biphasic expression, expressed at high levels at birth and then dropped sharply with gradual increases with age (Fig. 5A, panels d, e, & f). However, in the developing bone tissues noggin expression increased with ages, from 2-week, 4-week to 3-month in femur and parietal bone tissues (Fig. 5B, panel a & b). Taken together, these results indicate that negative regulators of BMP9 signaling, Smad6, Smad7 and noggin, are widely expressed in mouse postnatal tissues.
Figure 5.
Expression of BMP9 signaling inhibitor, Noggin, in postnatal mouse tissues determined by TqPCR analysis. Total RNA was isolated from mouse brain, fat (inguinal region), heart, kidney, liver, lung, muscle, spleen, femur, and parietal bone (PB) at various ages: newborn (NB), 2-week, 4-week, 3-month, 8-month and 18-month old. The RT cDNA products were subjected to TqPCR analysis of Noggin expression in these tissues.
BMP9 exhibits pleiotropic biological functions and yet is primarily expressed in postnatal liver and lungs
Increasing evidence indicates that BMP9 plays diverse arrays of biological functions in development and adult tissue homeostasis. However, little is known about the in vivo expression landscape of BMP9 and its signaling mediators. We analyzed the mouse ENCODE transcriptome dataset, which contains 30 mouse samples of embryonic and adult tissues, and found that Bmp9 was highly expressed in E14, E14.5, E18 liver and adult liver, although Bmp9 expression was detectable in E11.5 brain, adult lungs and adult placenta. The ENCODE dataset further revealed that BMP9 receptor Alk1 was highly expressed in adult lung tissue while Alk2 was more broadly expressed, and that adult lung tissue had the highest expression for both I-Smads, followed by various GI tissues, although Smad7 is more widely expressed than Smad6. Thus, the mouse ENCODE transcriptome data suggest that Bmp9 expression may be relatively restricted to liver, and to a lesser extent, lungs, while the Bmp9 signaling mediators, especially Alk1, Smad6 and Smad7, exhibit high expression levels in adult lungs.
To overcome the ENCODE's limitations in scope and depth, we conducted a transcriptomic analysis of BMP9 signaling in major mouse organs including brain, fat, heart, kidney, liver, lungs, muscle, spleen, femur, and parietal bone at different ages ranging from newborn, 2-week, 4-week, 3-month, 8-month to 18-month old mice. Our results demonstrated that, although at relatively low levels at birth, Bmp9 was highly expressed in the liver and lung tissues of young adult mice, but decreased in older mice. Bmp9 expression was also detectable in 2-week spleen, brain and fat tissues and muscle samples. Interestingly, Bmp9 was only expressed at low to modest levels in developing bones.
Furthermore, BMP9-specific BMP-R type I receptor Alk1 was highly expressed in the adult lungs, while Alk2 was expressed at relatively higher levels in fat and kidney tissues. The I-Smads Smad6 and Smad7 were widely expressed in mouse postnatal tissues, including developing bones. The naturally occurring antagonist of BMP signaling, noggin, was expressed highly in fat and heart tissues at older age groups, as well as in kidney, liver and lung tissues in a biphasic fashion. It seems that the expression patterns of noggin were independent of that of BMP9, Alk1 and/or I-Smads in adult mice, which is consistent with our earlier findings about the refractory nature of BMP9 against noggin.20
Emerging evidence indicates that BMP9 exerts diverse and pleiotropic biological functions during postnatal development and in maintaining adult tissue homeostasis. The postnatal expression landscape of BMP9 and its important signaling mediators should provide important insights into the potential functions of BMP9 signaling in major organs and/or tissues. We have recently conducted a comprehensive transcriptomic profiling of the MSCs stimulated by the 14 types of BMPs at the early stage, which allows us to identify the immediate early transcriptomic changes in MSCs.48 The present study indicates that the circulating BMP9 produced in liver and lungs may account for its pleiotropic effects on multiple postnatal tissues/organs. Furthermore, the expression profiling analysis strongly suggests that BMP9 may play important roles in regulating hepatic metabolism and tissue homeostasis and pulmonary endothelial cells of the lungs,10, 38, 39 which warrants thorough future investigations.
Conflict of interest
The authors declare no competing interest.
Acknowledgments
The reported work was supported in part by research grants from the National Institutes of Health (CA226303 to TCH), the U.S. Department of Defense (OR130096 to JMW), the Scoliosis Research Society (TCH and MJL). This project was also supported in part by The University of Chicago Cancer Center Support Grant (P30CA014599) and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000430. TCH was also supported by the Mabel Green Myers Research Endowment Fund and The University of Chicago Orthopaedics Alumni Fund. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Hogan B.L. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10(13):1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 2.Reddi A.H. Bone morphogenetic proteins: an unconventional approach to isolation of first mammalian morphogens. Cytokine Growth Factor Rev. 1997;8(1):11–20. doi: 10.1016/s1359-6101(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 3.Attisano L., Wrana J.L. Signal transduction by the TGF-beta superfamily. Science. 2002;296(5573):1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 4.Wang R.N., Green J., Wang Z. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Gene Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Z.L., Sharff K.A., Tang N. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.H., Liu X., Wang J. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis. 2013;5(1):13–31. doi: 10.1177/1759720X12466608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denduluri S.K., Idowu O., Wang Z. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Gene Dis. 2015;2(1):13–25. doi: 10.1016/j.gendis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teven C.M., Farina E.M., Rivas J., Reid R.R. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Gene Dis. 2014;1(2):199–213. doi: 10.1016/j.gendis.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamplot J.D., Qin J., Nan G. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells. 2013;2(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Luther G., Wagner E.R., Zhu G. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther. 2011;11(3):229–240. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- 11.Zhao G.Q. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35(1):43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 13.Luu H.H., Song W.X., Luo X. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 14.Urist M.R. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 15.Wozney J.M., Rosen V., Celeste A.J. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 16.Cheng H., Jiang W., Phillips F.M. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Kang Q., Sun M.H., Cheng H. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11(17):1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 18.Huang X., Wang F., Zhao C. Dentinogenesis and tooth-alveolar bone complex defects in BMP9/GDF2 knockout mice. Stem Cells Dev. 2019;28(10):683–694. doi: 10.1089/scd.2018.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F., Song J., Zhang H. Wnt and BMP signaling crosstalk in regulating dental stem cells: implications in dental tissue engineering. Gene Dis. 2016;3(4):263–276. doi: 10.1016/j.gendis.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Hong S., Li M. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res. 2013;31(11):1796–1803. doi: 10.1002/jor.22427. [DOI] [PubMed] [Google Scholar]
- 21.Luo J., Tang M., Huang J. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 2010;285(38):29588–29598. doi: 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Y., Kang Q., Cheng H. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem. 2003;90(6):1149–1165. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- 23.Peng Y., Kang Q., Luo Q. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(31):32941–32949. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 24.Luo Q., Kang Q., Si W. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(53):55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 25.Sharff K.A., Song W.X., Luo X. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J Biol Chem. 2009;284(1):649–659. doi: 10.1074/jbc.M806389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang E., Zhu G., Jiang W. Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J Bone Miner Res. 2012;27(7):1566–1575. doi: 10.1002/jbmr.1622. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J., Weng Y., Liu X. Endoplasmic reticulum (ER) stress inducible factor cysteine-rich with EGF-like domains 2 (Creld2) is an important mediator of BMP9-regulated osteogenic differentiation of mesenchymal stem cells. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0073086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu N., Jiang D., Huang E. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci. 2013;126(Pt 2):532–541. doi: 10.1242/jcs.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang N., Song W.X., Luo J. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med. 2009;13(8B):2448–2464. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W., Deng Z.L., Chen L. Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L., Jiang W., Huang J. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25(11):2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X., Qin J., Luo Q. Cross-talk between EGF and BMP9 signalling pathways regulates the osteogenic differentiation of mesenchymal stem cells. J Cell Mol Med. 2013;17(9):1160–1172. doi: 10.1111/jcmm.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H., Wang J., Deng F. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs) Biomaterials. 2015;39:145–154. doi: 10.1016/j.biomaterials.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R., Zhang W., Cui J. Targeting BMP9-promoted human osteosarcoma growth by inactivation of notch signaling. Curr Cancer Drug Targets. 2014;14(3):274–285. doi: 10.2174/1568009614666140305105805. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H., Li L., Dong Q. Activation of PKA/CREB signaling is involved in BMP9-induced osteogenic differentiation of mesenchymal stem cells. Cell Physiol Biochem. 2015;37(2):548–562. doi: 10.1159/000430376. [DOI] [PubMed] [Google Scholar]
- 36.Liao J., Wei Q., Zou Y. Notch signaling augments BMP9-induced bone formation by promoting the osteogenesis-angiogenesis coupling process in mesenchymal stem cells (MSCs) Cell Physiol Biochem. 2017;41(5):1905–1923. doi: 10.1159/000471945. [DOI] [PubMed] [Google Scholar]
- 37.Liao J., Yu X., Hu X. lncRNA H19 mediates BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) through Notch signaling. Oncotarget. 2017;8(32):53581–53601. doi: 10.18632/oncotarget.18655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mostafa S., Pakvasa M., Coalson E. The Wonders of BMP9: from mesenchymal stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism to regenerative medicine. Gene Dis. 2019;6(3):201–223. doi: 10.1016/j.gendis.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toshner M. BMP9 morphs into a potential player in portopulmonary hypertension. Am J Respir Crit Care Med. 2019;199(7):819–821. doi: 10.1164/rccm.201810-1886ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song J.J., Celeste A.J., Kong F.M., Jirtle R.L., Rosen V., Thies R.S. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136(10):4293–4297. doi: 10.1210/endo.136.10.7664647. [DOI] [PubMed] [Google Scholar]
- 41.Yue F., Cheng Y., Breschi A. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515(7527):355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Untergasser A., Cutcutache I., Koressaar T. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Q., Wang J., Deng F. TqPCR: a touchdown qPCR assay with significantly improved detection sensitivity and amplification efficiency of SYBR green qPCR. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao C., Zeng Z., Qazvini N.T. Thermoresponsive citrate-based graphene oxide scaffold enhances bone regeneration from BMP9-stimulated adipose-derived mesenchymal stem cells. ACS Biomater Sci Eng. 2018;4(8):2943–2955. doi: 10.1021/acsbiomaterials.8b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan J., Wei Q., Liao J. Noncanonical Wnt signaling plays an important role in modulating canonical Wnt-regulated stemness, proliferation and terminal differentiation of hepatic progenitors. Oncotarget. 2017;8(16):27105–27119. doi: 10.18632/oncotarget.15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu X., Li L., Yu X. CRISPR/Cas9-mediated reversibly immortalized mouse bone marrow stromal stem cells (BMSCs) retain multipotent features of mesenchymal stem cells (MSCs) Oncotarget. 2017;8(67):111847–111865. doi: 10.18632/oncotarget.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao C., Jiang W., Zhou N. Sox9 augments BMP2-induced chondrogenic differentiation by downregulating Smad7 in mesenchymal stem cells (MSCs) Gene Dis. 2017;4(4):229–239. doi: 10.1016/j.gendis.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Luo Q., Shu Y. Transcriptomic landscape regulated by the 14 types of bone morphogenetic proteins (BMPs) in lineage commitment and differentiation of mesenchymal stem cells (MSCs) Gene Dis. 2019 doi: 10.1016/j.gendis.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]