Abstract
Background
Recent evidence describes which interventions are driving insurance payments in the management of osteoarthritis (OA) before total knee arthroplasty (TKA); however, relatively little is known about how these costs are distributed among patients.
Methods
We reviewed the Humana claims database for patients who underwent primary TKA from 2009 to 2016. Insurance payments for treatment, imaging, and evaluation and management were calculated from OA diagnosis to TKA, the distribution of payments was determined, and a high-payment group was identified by determining the point at which patients began to account for a disproportionate percentage of payments. This group of high-payment patients was compared with remaining patients (low-payment patients) based on demographic factors and nonarthroplasty payments and utilization.
Results
The top 30% of patients accounted for more than 70% of nonarthroplasty payments. High-payment patients were more likely to be younger, female, and more comorbid. Median time from diagnosis to TKA for high-payment patients was 3 times longer than that for low-payment patients (654 days [320-1191] vs 204 days [68-582], P < .001), and median payment per patient was more than 5 times higher ($1891 [1405-2782] vs $362 [198-613], P < .001).
Conclusions
Identification of high-payment patients in the management of knee OA may allow for targeted care pathways and cost-reduction strategies in the nonarthroplasty period, although additional studies are necessary to further characterize this population and efficiently recognize appropriate TKA candidates and timing.
Keywords: Osteoarthritis, Total knee arthroplasty, Health insurance, Reimbursement payments, Cost distribution
Introduction
Osteoarthritis (OA) is a highly prevalent disease and a leading cause of disability in the United States [1,2]. In addition to its effects on individuals through pain and disability, OA also accounts for a significant economic burden [3]. Annual direct and indirect OA costs from 2008 to 2011 averaged $62 billion and $80 billion, respectively [4], and the economic impact of OA is projected to grow as the population ages and obesity becomes increasingly prevalent [5]. Knee OA in particular accounts for the majority of symptomatic OA and 83% of the global OA disease burden [6,7].
Current management of knee OA involves a number of interventions directed at reducing pain and improving function. Numerous clinical practice guidelines have been developed to standardize management of knee OA [[8], [9], [10]]. However, despite a general consensus on major management strategies across guidelines [11], there remains wide variation in practice patterns with low adherence to recommendations [12,13]. Furthermore, most knowledge on cost and care delivery patterns in knee OA focuses on the end-stage treatment of joint arthroplasty [12,14,15]. However, OA is a chronic condition, and optimal disease-based care will require a deeper understanding of the entire nonarthroplasty treatment episode, especially as novel care and payment models for OA continue to emerge [16,17].
Several recent studies describe which interventions are driving insurance payments in the management of knee OA before total knee arthroplasty (TKA) [12,14]. Still, relatively little is known about how these payments are distributed among patients. General health care expenditures in the United States are concentrated in a relatively small percentage of the population, with 5% of patients accounting for 50% of costs [18]. Nonarthroplasty payments for knee OA may be expected to mirror this marked variation in patient utilization, which could present opportunities for cost reduction and improved care delivery among distinct patient populations.
In this study, we seek to determine which diagnostic and treatment modalities make up the majority of insurance payments from OA diagnosis to TKA and characterize the distribution of nonarthroplasty knee OA payments among patients. We believe the majority of payments will be concentrated among a small proportion of patients and that these patients will have distinct demographic characteristics and utilization patterns compared with other patients.
Material and methods
This review was conducted using the Humana subset of the PearlDiver Patient Record Database (www.pearldiverinc.com; PearlDiver, Inc., West Conshohocken, PA). The PearlDiver database is commercially available and contains deidentified and Health Insurance Portability and Accountability Act–compliant patient records and billing codes. The Humana division of the database contains 22 million patients with claims from 2007 through 2016 and undergoes regular internal and external review processes to ensure data validity.
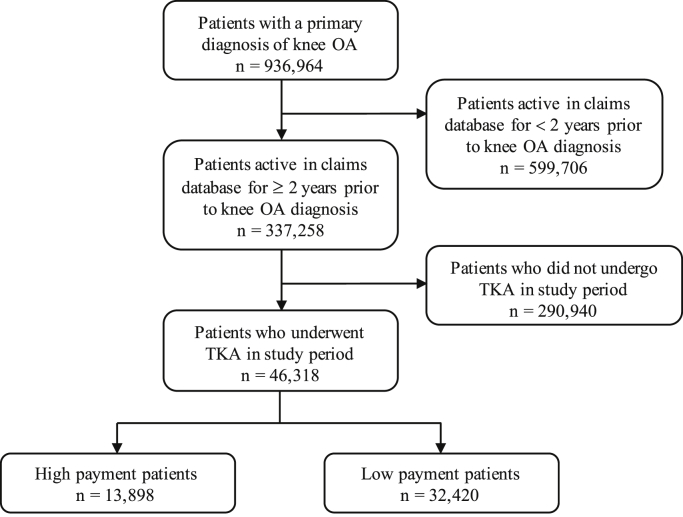
Patients with a diagnosis of knee OA were identified using International Classification of Diseases, 9th Revision (ICD-9) and 10th Revision (ICD-10) codes (Appendix A). Only patients active in the Humana database for 2 years before their initial documented knee OA diagnosis were included. Patients who underwent primary TKA were identified using Current Procedural Terminology (CPT), ICD-9, and ICD-10 codes (Appendix B). A total of 46,318 patients who underwent primary TKA met inclusion criteria. Figure 1 details the process of patient selection for the study. Patient billing records were tracked from initial knee OA diagnosis to the day before TKA and filtered for reimbursement payments associated with a primary ICD-9 or ICD-10 diagnosis of knee OA or knee pain (Appendix A).
Figure 1.
Flowchart outlining selection of patients from database.
Based on treatments addressed in the American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines [9], the following treatment modalities were analyzed: physical therapy (PT), knee braces, prescription nonsteroidal antiinflammatory drugs (NSAIDs), tramadol, opioids, corticosteroid (CS) injections, hyaluronic acid (HA) injections, and arthroscopic debridement. All interventions were covered by Humana over the course of the study period. Imaging modalities analyzed included radiography, computed tomography (CT), and magnetic resonance (MR) imaging. Records associated with evaluation and management (E/M) were also evaluated. All treatment, imaging, and E/M records were identified using CPT codes (Appendix B), and only those specifically associated with a primary diagnosis of knee pain or OA were included in the analysis (Appendix A). For injections, the payment for the procedure and the injected medication was analyzed. For medications, prescriptions filled on the same day or the day after an encounter for knee pain or OA were included. For overall nonarthroplasty knee OA payments, we calculated the number of patients, total payment, payment per event, payment per patient, and percentage of overall nonarthroplasty payment for each service. For treatments, we also report the AAOS recommendation status of each modality.
To determine the distribution of payments, patients were ranked in descending order based on total payments incurred for knee pain or OA from diagnosis to TKA. Aggregate spending was calculated incrementally for each 5th percentile of the population, and a group of high-payment patients was identified by determining the point at which patients began to account for a disproportionately high percentage of payments (ie, when 5% of the cohort began accounting for more than 5% of total payments). This group of high-payment patients was compared with remaining patients based on demographic factors, comorbidities, and nonarthroplasty payments. The Elixhauser Comorbidity Index was calculated for each group, as well as specific comorbidities, including hypertension, diabetes, peripheral vascular disease (PVD), depression, and obesity. Validated Elixhauser ICD-9 and ICD-10 comorbidity codes were used to identify patients with documentation of these comorbidities during the examined time period [19]. For each group, we also calculated the number of patients receiving each service, per-patient payment for each patient receiving the service, and the median and average number of events per patient receiving the service.
Normality of data distributions was assessed using the Shapiro-Wilk test. Parametric data were analyzed using the t-test and expressed as averages ± standard deviation. Nonparametric data were analyzed using the Mann-Whitney U-test and expressed as medians [interquartile range]. Nominal data were analyzed using the Chi-squared test and expressed as percentages. All tests were two-sided and considered significant at a P value < .05.
Results
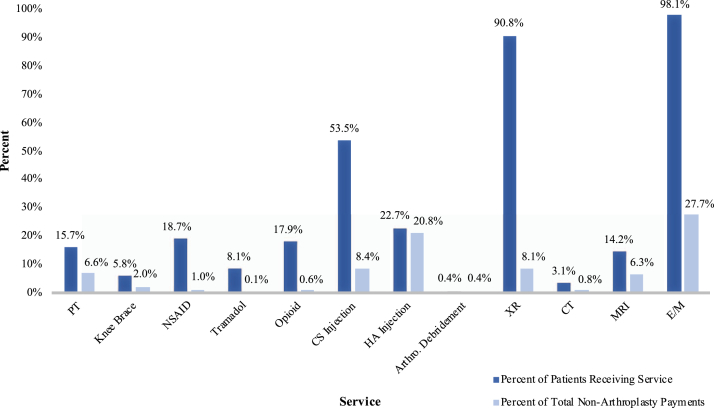
The overall drivers of payment in the nonarthroplasty period were E/M, HA injections, CS injections, radiography, PT, and MR imaging (Table 1, Fig. 2). Total nonarthroplasty payments from diagnosis to TKA were $49,845,016, and the total payments for all services analyzed accounted for 83% ($41,186,234) of these payments. Other payments were related to inpatient claims where OA was listed as a diagnosis but was not the primary reason for admission.
Table 1.
Nonarthroplasty payment breakdown for all patients.
| Service | Number of patients (%) | Total payment for service | Average payment per event | Average payment per patient | Percentage of total nonarthroplasty knee OA payments | AAOS knee OA clinical practice guidelines | Strength of recommendation |
|---|---|---|---|---|---|---|---|
| Treatment | |||||||
| PT | 7282 (15.7) | $3,279,921 | $85 | $450 | 6.6% | Recommend | Strong |
| Knee Brace | 2706 (5.8) | $986,324 | $341 | $365 | 2.0% | Unable to recommend | Inconclusive |
| NSAID | 8670 (18.7) | $495,872 | $42 | $57 | 1.0% | Recommend | Strong |
| Tramadol | 3754 (8.1) | $36,902 | $8 | $10 | 0.1% | Recommend | Strong |
| Opioid | 8275 (17.9) | $307,122 | $24 | $37 | 0.6% | Unable to recommend | Inconclusive |
| CS Injection | 24,782 (53.5) | $4,176,209 | $85 | $169 | 8.4% | Unable to recommend | Inconclusive |
| HA Injection | 10,536 (22.7) | $10,387,890 | $308 | $986 | 20.8% | Cannot recommend | Strong |
| Arthroscopic debridement | 163 (0.4) | $216,587 | $1388 | $1329 | 0.4% | Cannot recommend | Strong |
| Imaging | |||||||
| Radiography | 42,036 (90.8) | $4,016,167 | $53 | $96 | 8.1% | ||
| CT | 1419 (3.1) | $376,388 | $259 | $265 | 0.8% | ||
| MRI | 6587 (14.2) | $3,117,206 | $434 | $473 | 6.3% | ||
| Evaluation and management | |||||||
| E/M | 45,417 (98.1) | $13,789,646 | $84 | $304 | 27.7% | ||
| Total | $41,186,234 | 82.6% | |||||
Figure 2.
Percent of all patients receiving each service and percent of total nonarthroplasty payments for each service.
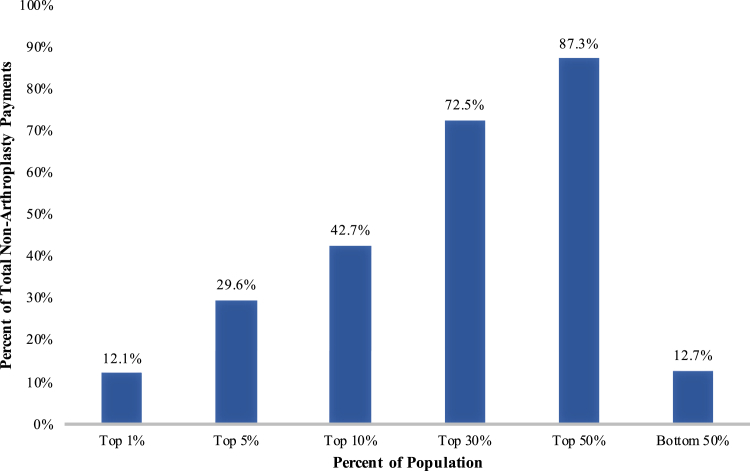
Nonarthroplasty knee OA payments were not equally distributed among patients. The top 5% of patients by payment accounted for 30% of total payments, and the bottom 50% of patients by payment accounted for only 13% of total payments (Fig. 3). We identified the 30th percentile as the point at which patients began to account for a disproportionately high percentage of payments. These high-payment patients accounted for more than 70% of nonarthroplasty payments, while patients in the bottom 70th percentile (low-payment patients) accounted for less than 30% of nonarthroplasty payments.
Figure 3.
Distribution of nonarthroplasty knee OA payments by payment percentile.
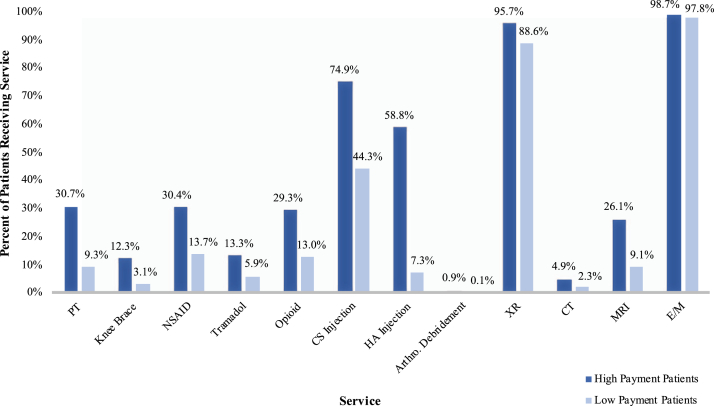
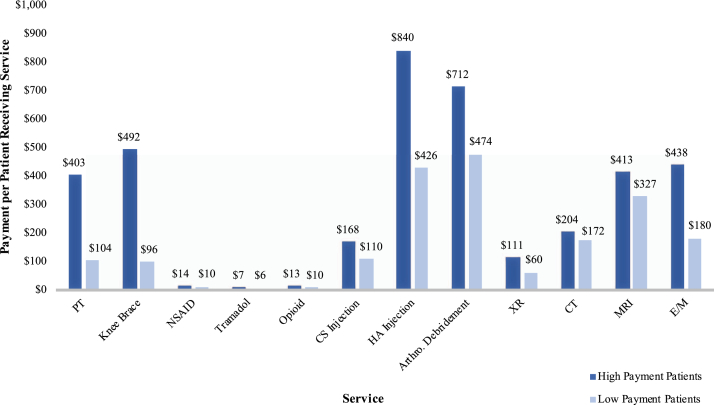
High-payment patients were more likely to be younger, female, from the southern United States, more comorbid, and treated for longer before TKA. They also received more of almost every service analyzed than did low-payment patients. Hypertension (81.4% vs 71.8%, P < .001), diabetes (32.6% vs 29.4% P < .001), PVD (20.5% vs 14.7%, P < .001), depression (21.8% vs 11.9%, P < .001), and obesity (32.6% vs 21.8%, P < .001) were all more common among high-payment patients (Table 2). The Elixhauser comorbidity score was slightly higher for high-payment patients (8.6 ± 4.4 vs 8.3 ± 4.4, P < .001). Median time from diagnosis to TKA for high-payment patients was 3 times longer than for low-payment patients (654 days [320-1191] vs 204 days [68-582], P < .001), and median per-patient payment was more than 5 times higher ($1891 [1405-2782] vs $362 [198-613], P < .001). For every service analyzed, there was a higher proportion of patients in the high-payment group who received it (Fig. 4). The difference was especially pronounced in the case of HA injections, in which 60% of high-payment patients and only 7% of low-payment patients received the service (P < .001). Furthermore, median per-patient payment for patients who received a given service was greater in the high-payment group for all observed services (Fig. 5), and the high-payment group received a greater number of per-patient service events for almost every service, with the exceptions of arthroscopic debridement and CT imaging (Table 3). The increased number of per-patient service events in the high-payment group was greatest for PT (5 [1-10] vs 1 [1-4] events, P < .001) and E/M (5 [4-7] vs 2 [2-4] events, P < .001) visits.
Table 2.
Patient characteristic and payment comparison for high- and low-payment patients.
| High-payment patients (n = 13,898) | Low-payment patients (n = 32,420) | P value | |
|---|---|---|---|
| Age at diagnosis, years | |||
| <50 | 2.6% | 1.1% | P < .001 |
| 50-59 | 11.7% | 7.0% | |
| 60-69 | 30.0% | 28.7% | |
| 70-79 | 44.8% | 50.5% | |
| 80-89 | 9.9% | 11.6% | |
| ≥90 | 1.0% | 1.2% | |
| Sex | |||
| Female | 64.4% | 60.1% | P < .001 |
| Male | 35.6% | 39.9% | |
| Region | |||
| NE | 2.1% | 2.5% | P < .001 |
| MW | 29.7% | 29.4% | |
| WE | 8.7% | 10.4% | |
| SO | 59.5% | 57.7% | |
| Elixhauser Comorbidity Index | 8.6 ± 4.4 | 8.3 ± 4.4 | P < .001 |
| Comorbidities | |||
| Hypertension | 81.4% | 71.8% | P < .001 |
| Diabetes | 32.6% | 29.4% | P < .001 |
| PVD | 20.5% | 14.7% | P < .001 |
| Depression | 21.8% | 11.9% | P < .001 |
| Obesity | 32.6% | 21.8% | P < .001 |
| Nonarthroplasty payments | $36,142,052 | $13,702,964 | |
| Percentage of total nonarthroplasty payments | 72.5% | 27.5% | |
| Nonarthroplasty payment per patient (median [IQR]) | $1891 [1405-2782] | $362 [198-613] | P < .001 |
| Days from OA diagnosis to TKA (median [IQR]) | 654 [320-1191] | 204 [68-582] | P < .001 |
Figure 4.
Percent of patients receiving each service in high- and low-payment groups. There was a significant difference (P < .001) between high- and low-payment patients for each service.
Figure 5.
Median payment per-patient receiving each service in high- and low-payment patients. There was a significant difference (P ≤ .001) between high- and low-payment patients for each service.
Table 3.
Service events per patient for high- and low-payment patients.
| Service events | High-payment patients (n = 13,898) | Low-payment patients (n = 32,420) | P value |
|---|---|---|---|
| Physical therapy | |||
| Events per patient (median [IQR]) | 5 [1-10] | 1 [1-4] | P < .001 |
| Events per patient (average ± SD) | 7.3 ± 8.7 | 2.9 ± 3.7 | |
| Knee brace | |||
| Events per patient (median [IQR]) | 1 [1-1] | 1 [1-1] | P < .001 |
| Events per patient (average ± SD) | 1.1 ± 0.3 | 1.0 ± 0.2 | |
| NSAID | |||
| Events per patient (median [IQR]) | 1 [1-2] | 1 [1-1] | P < .001 |
| Events per patient (average ± SD) | 1.5 ± 0.9 | 1.2 ± 0.5 | |
| Tramadol | |||
| Events per patient (median [IQR]) | 1 [1-1] | 1 [1-1] | P < .001 |
| Events per patient (average ± SD) | 1.3 ± 0.7 | 1.1 ± 0.5 | |
| Opioid | |||
| Events per patient (median [IQR]) | 1 [1-2] | 1 [1-1] | P < .001 |
| Events per patient (average ± SD) | 1.8 ± 1.6 | 1.3 ± 0.7 | |
| CS injection | |||
| Events per patient (median [IQR]) | 2 [1-3] | 1 [1-2] | P < .001 |
| Events per patient (average ± SD) | 2.7 ± 2.2 | 1.6 ± 1.1 | |
| HA injection | |||
| Events per patient (median [IQR]) | 3 [2-5] | 2 [1-3] | P < .001 |
| Events per patient (average ± SD) | 3.5 ± 2.8 | 2.4 ± 1.6 | |
| Arthroscopic debridement | |||
| Events per patient (median [IQR]) | 1 [1-1] | 1 [1-1] | P = .320 |
| Events per patient (average ± SD) | 1.0 ± 0.2 | 1.0 ± 0.0 | |
| Radiography | |||
| Events per patient (median [IQR]) | 2 [2-3] | 1 [1-2] | P < .001 |
| Events per patient (average ± SD) | 2.4 ± 1.3 | 1.6 ± 0.8 | |
| CT imaging | |||
| Events per patient (median [IQR]) | 1 [1-1] | 1 [1-1] | P = .981 |
| Events per patient (average ± SD) | 1.0 ± 0.2 | 1.0 ± 0.2 | |
| MR imaging | |||
| Events per patient (median [IQR]) | 1 [1-1] | 1 [1-1] | P < .001 |
| Events per patient (average ± SD) | 1.2 ± 0.4 | 1.0 ± 0.2 | |
| Evaluation and management | |||
| Events per patient (median [IQR]) | 5 [4-7] | 2 [2-4] | P < .001 |
| Events per patient (average ± SD) | 5.9 ± 3.6 | 2.8 ± 2.0 | |
Discussion
An understanding of payment and utilization patterns in nonarthroplasty OA management is important to inform the development of health policy and alternative care models; however, an emphasis on averages across the OA population overlooks the high degree of variation present in patient utilization. Our study demonstrates that the majority of nonarthroplasty OA payments before TKA are concentrated among a relatively small proportion of patients.
The main drivers of insurance payments in the nonarthroplasty period included E/M, HA injections, CS injections, and radiography. Recent evidence demonstrates significant gaps between clinical practice guidelines and practice patterns in the management of knee OA [12,13]. Similarly, we found that treatments such as HA injections, CS injections, and opioids were highly utilized, especially among the high-payment group. HA injections are not recommended by the AAOS, while recommendations for CS injections and opioids are inconclusive. Almost 60% of high-payment patients received at least one HA injection, while less than 8% of low-payment patients did. Furthermore, HA injections were a significant driver of payment, accounting for more than 20% of total nonarthroplasty payments. Recommended treatments such as PT, NSAIDs, and tramadol each had utilization under 20% in the overall cohort, and together, they accounted for less than 10% of nonarthroplasty payments. Our results are consistent with studies detailing utilization patterns 1 to 2 years before TKA [12,14]. One study [14] assessed interventions in the 2-year period before TKA, which found a high percentage of nonarthroplasty payments, including injections and arthroscopy, occur in close proximity to TKA when they may not be clinically or cost-effective. A similar study [12] examined outpatient OA interventions in the year preceding TKA and found high use of HA injections, CS injections, and opioids. Our results support these findings and extend them to the entire nonarthroplasty care episode from diagnosis to TKA, further underscoring the economic implications of care that is either not recommended or inconclusive in accordance with current AAOS clinical practice guidelines for knee OA.
We identified a group of high-payment patients, which comprised 30% of patients and more than 70% of nonarthroplasty payments. This group had substantially higher per-patient payments and utilized significantly more of almost every service analyzed before TKA. Such variation in utilization is likely the result of multiple factors, including patient need for and access to health care, appropriateness for and willingness to undergo TKA [20,21], and inconsistent practice patterns among providers. This disparity in service provision presents opportunity for targeted efforts to reduce or redistribute payments associated with inappropriate or excessive treatment and increase the equity, appropriateness, and value of services delivered to the population seeking care for knee OA. This information can also be used to counsel patients on the cost of nonarthroplasty knee OA management, especially with rising enrollment in high-deductible health plans and an increasingly cost-minded patient population [22].
Patients in the high-payment group were more likely to be younger, female, and located in the southern United States. Our results are consistent with those of other reports that females incur higher costs for OA care than males, which may be due to increased disease severity observed in female patients [23,24]. Younger patients likely receive a higher level of nonarthroplasty management in an attempt to avoid or delay the need for TKA due to concerns about lower implant survival in younger patients [25]. This point is further reflected in the longer time period from diagnosis to TKA for high-payment patients. There was also a higher prevalence of comorbidities in the high-payment group. Increased comorbidity has been shown to increase individual healthcare costs for a number of conditions [26]. In addition, certain comorbidities such as depression are associated with increased pain in patients with OA [27], which may result in higher resource utilization. Clinicians may also be more likely to utilize nonarthroplasty interventions for comorbid patients in an attempt to avoid surgery or optimize preoperative variables such as body mass index or HbA1c [28,29]. In our study, the increased time of nonarthroplasty management for younger and more comorbid patients suggests the interventions analyzed may be at least partially effective in delaying the need for TKA, although we are unable to comment on the efficacy of these interventions in symptom relief or their healthcare value.
Nonetheless, our study provides a characterization of different patient populations in the management of knee OA who may be most effectively served by distinct care pathways. The majority of patients in the low-payment group underwent TKA within a year of OA diagnosis. These patients were generally older and had fewer comorbidities than those in the high-payment group, likely making them better candidates for early surgical intervention. High-payment patients, on the other hand, were treated for longer before TKA and had a higher prevalence of comorbidities. Patients who are anticipated to incur high payments for nonarthroplasty OA management, for example, younger patients with diabetes or depression, could be directed toward separate pathways that emphasize appropriate and targeted longitudinal care. Important aspects of these models may include an emphasis on recommended clinical practice guidelines, exercise and behavior change aimed at managing comorbid obesity and diabetes, and psychological support such as pain coping or cognitive behavioral therapy. These services have been shown to improve outcomes in patients with OA and hold the potential to reduce resource utilization elsewhere in the system [30,31].
Moreover, proceeding with arthroplasty earlier may help decrease payments associated with nonarthroplasty management, particularly in high-payment patients. Several studies have demonstrated the potential for payment reduction with earlier TKA [32,33]. One study [32] showed decreased direct and indirect costs and increased quality-adjusted life years gained for patients undergoing early TKA compared with those receiving nonarthroplasty treatment for 2 years before TKA. Still, decisions regarding arthroplasty are complex and must balance potential economic benefits with various clinical and patient factors. One report [34] estimates a third of TKA procedures in the United States may be performed for reasons that do not meet published appropriate use criteria. Future efforts to aid in timely recognition of appropriate TKA candidates will be critical moving forward.
Our study is subject to the limitations of retrospective analysis. Results are dependent on accurate documentation and coding, and as a result, we likely underrepresent service delivery and the payments thereof for knee OA. We also restricted our analysis to records associated specifically with a primary diagnosis of knee OA or knee pain, but patients with knee OA may have received services billed under ICD-9 or ICD-10 codes for other knee pathologies. Specifically, arthroscopic debridement before TKA has been reported at much higher rates than we observed when other indications are included in analysis [13,14]. Moreover, several treatments such as NSAIDs and knee braces are available over the counter and likely not fully represented in administrative claims databases. In addition, we were unable to provide information on OA severity, an important factor in the approach to management. We attempted to control for differences in disease severity at the time of diagnosis by limiting our analysis to patients who had been active with the insurance provider for 2 years before an initial diagnosis of knee OA. Still, we are unable to comment on the role of disease severity in our analysis. Another factor that can contribute to costs in the management of knee OA is bilaterality of symptoms, which we lacked the ability to determine in this analysis.
Conclusions
OA payments before TKA were unequally distributed among patients, with 30% of patients responsible for more than 70% of payments. These high-payment patients were more likely to be younger, female, more comorbid, and treated for longer before TKA. This group also received high levels of nonrecommended care. Early identification of high-payment patients may allow for targeted care pathways and cost-reduction strategies in the nonarthroplasty period, although additional studies are necessary to further characterize this population and efficiently recognize appropriate TKA candidates and timing.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field whichmay be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2019.11.008.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.artd.2019.11.008.
Supplementary data
References
- 1.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence R.C., Felson D.T., Helmick C.G. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 4.Hootman J.M., Helmick C.G., Barbour K.E., Theis K.A., Boring M.A. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015-2040. Arthritis Rheum. 2016;68(7):1582. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Bone and Joint Initiative . 3rd ed. U.S. Bone and Joint Initiative; 2014. The burden of musculoskeletal diseases in the United States (BMUS)https://www.boneandjointburden.org/docs/The%20Burden%20of%20Musculoskeletal%20Diseases%20in%20the%20United%20States%20(BMUS)%203rd%20Edition%20(Dated%2012.31.16).pdf [Google Scholar]
- 6.Neogi T., Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39(1):1. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vos T., Flaxman A.D., Naghavi M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAlindon T.E., Bannuru R.R., Sullivan M.C. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22(3):363. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Jevsevar D.S. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg M.C., Altman R.D., April K.T. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 11.Nelson A.E., Allen K.D., Golightly Y.M., Goode A.P., Jordan J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014;43(6):701. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Bedard N.A., Dowdle S.B., Anthony C.A. The AAHKS clinical research award: what are the costs of knee osteoarthritis in the year prior to total knee arthroplasty? J Arthroplasty. 2017;32(9s):S8. doi: 10.1016/j.arth.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Dhawan A., Mather R.C., 3rd, Karas V. An epidemiologic analysis of clinical practice guidelines for non-arthroplasty treatment of osteoarthritis of the knee. Arthroscopy. 2014;30(1):65. doi: 10.1016/j.arthro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J.R., Bradley A.T., Lieberman J.R. Preoperative interventions and charges before total knee arthroplasty. J Arthroplasty. 2016;31(12):2730. doi: 10.1016/j.arth.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 15.Bozic K.J., Stacey B., Berger A., Sadosky A., Oster G. Resource utilization and costs before and after total joint arthroplasty. BMC Health Serv Res. 2012;12:73. doi: 10.1186/1472-6963-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen K.D., Choong P.F., Davis A.M. Osteoarthritis: models for appropriate care across the disease continuum. Best Pract Res Clin Rheumatol. 2016;30(3):503. doi: 10.1016/j.berh.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Hunter D.J., Neogi T., Hochberg M.C. Quality of osteoarthritis management and the need for reform in the US. Arthritis Care Res. 2011;63(1):31. doi: 10.1002/acr.20278. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell E.M., Machlin S.R. Statistical brief (medical expenditure panel survey (US)) Agency for Healthcare Research and Quality (US); Rockville, MD: 2017. Concentration of health expenditures and selected characteristics of high spenders, U.S. Civilian noninstitutionalized population, 2015. [PubMed] [Google Scholar]
- 19.Healthcare Cost and Utilization Project . Agency for Healthcare Research and Quality; 2017. HCUP elixhauser comorbidity software.https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#diagnoses [PubMed] [Google Scholar]
- 20.Fraenkel L., Benjamin Nowell W., Stake C.E. Impact of information presentation format on preference for total knee replacement surgery. Arthritis Care Res. 2019;71(3):379. doi: 10.1002/acr.23605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riddle D.L., Golladay G.J., Hayes A., Ghomrawi H.M. Poor expectations of knee replacement benefit are associated with modifiable psychological factors and influence the decision to have surgery: a cross-sectional and longitudinal study of a community-based sample. Knee. 2017;24(2):354. doi: 10.1016/j.knee.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen R.A., Zammitti E.P. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief. 2018;(317):1. [PubMed] [Google Scholar]
- 23.Kotlarz H., Gunnarsson C.L., Fang H., Rizzo J.A. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60(12):3546. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 24.Srikanth V.K., Fryer J.L., Zhai G., Winzenberg T.M., Hosmer D., Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis and cartilage. 2005;13(9):769. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Rand J.A., Trousdale R.T., Ilstrup D.M., Harmsen W.S. Factors affecting the durability of primary total knee prostheses. J Bone Joint Surg Am. 2003;85(2):259. doi: 10.2106/00004623-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Cortaredona S., Ventelou B. The extra cost of comorbidity: multiple illnesses and the economic burden of non-communicable diseases. BMC Med. 2017;15(1):216. doi: 10.1186/s12916-017-0978-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma A., Kudesia P., Shi Q., Gandhi R. Anxiety and depression in patients with osteoarthritis: impact and management challenges. Open Access Rheumatol. 2016;8:103. doi: 10.2147/OARRR.S93516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fournier M.N., Hallock J., Mihalko W.M. Preoperative optimization of total joint arthroplasty surgical risk: obesity. J Arthroplasty. 2016;31(8):1620. doi: 10.1016/j.arth.2016.02.085. [DOI] [PubMed] [Google Scholar]
- 29.Harris A.H., Bowe T.R., Gupta S., Ellerbe L.S., Giori N.J. Hemoglobin A1C as a marker for surgical risk in diabetic patients undergoing total joint arthroplasty. J Arthroplasty. 2013;28(8 Suppl):25. doi: 10.1016/j.arth.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Dixon K.E., Keefe F.J., Scipio C.D., Perri L.M., Abernethy A.P. Psychological interventions for arthritis pain management in adults: a meta-analysis. Health Psychol. 2007;26(3):241. doi: 10.1037/0278-6133.26.3.241. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy C.J., Mills P.M., Pullen R. Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis. Health Technol Assess. 2004;8(46):iii. doi: 10.3310/hta8460. [DOI] [PubMed] [Google Scholar]
- 32.Mather R.C., 3rd, Hug K.T., Orlando L.A. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi: 10.1186/1471-2474-15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bedair H., Cha T.D., Hansen V.J. Economic benefit to society at large of total knee arthroplasty in younger patients: a Markov analysis. J Bone Joint Surg Am. 2014;96(2):119. doi: 10.2106/JBJS.L.01736. [DOI] [PubMed] [Google Scholar]
- 34.Riddle D.L., Jiranek W.A., Hayes C.W. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheum. 2014;66(8):2134. doi: 10.1002/art.38685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.