Graphical abstract
Keywords: Wheat bran, Box-Behnken design, Waste newspaper hydrolysate, Starch hydrolysates, Lignocellulose
Highlights
-
•
Box-Behnken Design is used for optimization of process parameters.
-
•
Physical and chemical factors are used as influential parameters.
-
•
Valorization of liquid/solid waste is the sustainable and cost effective approach.
-
•
Bioconversion of waste biomass into energy is the part of waste management.
Abstract
Lignocellulosic biomass is a promising feedstock for future renewable fuels. It constitutes a substantial renewable substrate for the production of value-added chemicals. Lignocellulosic materials mostly contain a mixture of polymers such as cellulose, hemicelluloses, and lignin. Utilizing waste lignocellulosic materials such as agricultural residues, grasses, forestry wastes can significantly reduce the cost of raw materials. Optimization of significant process parameters is also a very important stage to develop an efficient and cost-effective bioprocess. The present paper describes the Box-Behnken design based optimization to evaluate the effects of various process parameters viz.temperature, pH, inoculum dosages, particle size, moisture percentage and incubation period on the production of cellulases by T. reesei NCIM 1186 and N. crassa NCIM 1021 under wheat bran based solid-state fermentation. It also portrays the utility of various plant polysaccharide hydrolysates such as boiled bagasse, bagasse, wheat straw, waste newspaper as well as starch hydrolysates in cellulase production.
1. Introduction
Lignocellulosic biomass is the most abundant renewable resource produced by photosynthesis on earth and has been considered as a sustainable feedstock [1]. Biomass-derived products can reduce the current dependency on fossil-based products, however, there are still many technical and economical tasks facing the utilization of this material [2]. Among these lignocellulosic biomasses, agricultural wastes are more important as this can be converted into products that are of commercial importance such as fuels, chemicals, enzymes, and high-value products and single cell protein [3,4]. The biodegradation of this lignocellulosic biomass is limited by several factors like the crystallinity of cellulose, available surface area and lignin content [1,5]. Major portions of lignocellulosic biomass are cellulose and hemicellulose. Cellulose is a homopolymer of glucose, while hemicellulose is a heteropolymer composed of mainly mannose, galactose, xylose and arabinose [6].
Trichoderma ressei is one of the most extensively studied and best-known cellulase producing organisms having a complete set of cellulases [7]. Neurospora crassa was recently reported to produced high yields of CMCase and β-glucosidase when growing in the solid substrate [8].
Wheat (Triticum aestivum) is a top cereal crop which is mainly utilized for human consumption and livestock feed. A wheat kernel comprises three principal fractions–bran, germ, and endosperm. The outer layers are all parts of the bran. The bran fraction is a by‐product of milling and has food and nonfood applications [9]. To protect the grain and endosperm material, more than half the bran comprises water-insoluble fiber (53 %). It contains mostly cellulose, xylose, and arabinose, which are tightly bound to proteins [10]. Bran is particularly rich in dietary fibers, minerals, bioactive compounds and contains significant quantities of starch, proteins, and vitamin [9,11]. A high amount of protein present in the wheat bran may also reduce the cellulase biosynthesis but the soluble oligosaccharides, starch, and cellulose present in the wheat bran significantly impact on the cellulase production [12]. Wheat bran due to its nutritional content and large surface area serves to be an excellent carbon source without any supplementary carbon source for the production of lignocellulolytic enzymes. Additionally, no prior pretreatment is necessary for the utilization of wheat bran in enzyme production [13].
The solid substrate act as a source of carbons, nitrogens, minerals as well as growth factors. The high product concentration, higher productivity, lower downstream processing, direct use of the fermented product as crude enzymes and utilization of byproduct make solid-state fermentation, a promising technology [14,15]. Literature reported that A.fumigatus UAM2 produces cellulase (0.24FPU/mL) under oil palm decanter cake based solid-state fermentation [16]. Verma et al. (2011) utilized pea peel waste as a solid substrate for cellulase (2.86 FPU/mL) production by T. reesei NCIM 1186 [3].
The statistical technique was an important tool to solve and to optimize numerous parameters, to find relativeness among the factors, to find the best combination of parameters and prediction of responses [17]. Designed experiments are less time consuming and less expensive than haphazard ones. This method is more satisfactory and effective than other methods, such as classical, one at a time or mathematical methods. In most bioprocesses such as fermentation, there are no true theoretical or mathematical models that can describe the whole process with 100 % certainty. The optimization of fermentation conditions, particularly physical and chemical parameters are of primary importance in the development of any fermentation process [18]. Box-Behnken designs are response surface designs specially made to require three levels, coded as -1, 0, +1. A Box-Behnken design was employed to analyze the interactive effects of these parameters and to arrive at an optimum. The base points for the design were selected from a single parameter study [[18], [19], [20]]. In Box-Behnken design the treatment combinations are at the midpoints of edges of the process space and the center [21]. Pasha et al. (2013) [22] investigated the Box-Behnken design based optimization for glucose production from oil palm empty fruit bunch.
Literature has been reported on the utilization of lignocellulosic hydrolysate for the growth and production system of microbes. Depending on the operational conditions acidic hydrolysates contained sugars such as xylose, glucose, and arabinose as well as decomposition products of hemicellulose (such as acetic acid, furfural, 5-hydroxymethylfurfural (HMF) and dehydration product of pentoses and hexoses) [[23], [24], [25], [26]].
The use of the hydrolysates as a soluble inducing substance for cellulase production deserves more study. Acid hydrolysis of raw materials to obtain xylose solutions has a double consequence, the elimination of waste and the generation of a value-added product such as xylitol [27,28]. The nonglucose carbohydrate portion of the lignocellulosic biomass is much more sensitive to acid hydrolysis than the glucose portion. Dilute acid pretreatment targets the hemicellulose fraction liberating pentose sugars while redistributing lignin and disrupting the crystalline structure of cellulose fibrils [29].
During pretreatment of lignocellulosic, in addition to the sugars, aliphatic acids (acetic, formic and levulinic acid) furan derivatives furfural and HMF (2-furaldehyde and 5-hydroxymethyl-2-furaldehyde) and phenolic compounds are formed. These compounds are known to affect microbial growth and product fermentation performance. Furfural could be generated as a degradation product from pentoses [24,25,30,31]. Another inhibitory substance founded in lignocellulolsic is acetic acid. Acetic acid can be generated when the hydrolysis reaction takes place at the acetyl group of hemicelluloses. Several detoxification methods like neutralization, overliming with calcium hydroxide, activated charcoal, ion exchange resins [[32], [33], [34]] and enzymatic detoxification using laccase [35] are known for removing various inhibitory compounds from lignocellulosic hydrolysates. Overliming the hydrolysate has been effective as a detoxification process due to partial removal of toxic inhibitors, such as furfural and 5-hydroxymethylfurfural. Another potential drawback of overliming is sugar loss due to hydroxide-catalyzed degradation reactions and the conversion of sugars into unfermentable compounds [36]. Chandel et al. [35] have also demonstrated that acetic acid concentration is not altered using overliming but this method led to the removal of furans (45.8 %) and phenolics (35.87 %). After detoxification, the concentrations of all those toxic compounds were lower than the reported levels causing, lesser inhibition of microorganism metabolism [36]. Biglow and Wyman (2002) [37] stated that few microbial strains were also able to consume or convert essentially all furans, aldehydes and acetic acid which released in the hydrolysates after hydrolysis process.
Starch is a polymeric carbohydrate comprising of a large number of glucose units joined by glycosidic bonds and contains two major macromolecular components amylose and amylopectin. Amylose is the most linear (1→4)-linked β-glucan and can have a degree of polymerization (DP) as high as 600. Amylopectin, β(1→4)-linked β-glucan with β-(1→6) branch points [38,39].Potatoes are the world's fourth-largest food crop followed by rice, wheat and maize [40]. Starch molecules arrange themselves in the plant in semi-crystalline granules. Each plant species has a unique starch granular size. Rice starch is relatively small (about 2 μm) while potato starch has larger granules (up to 100 μm). Wheat starch grains are bimodal in size with smaller B-starch and larger A-starch granules. B-starch is always highly tainted with pentosans, fine fibers, lipids, and protein [[41], [42], [43]]. Wheat starch hydrolysate contained nutrients along with glucose-rich solution serves as a prospective supplement for lignocellulosic hydrolysates [44]. As the literature suggested that the water- soluble acid hydrolyzed starch is an excellent inducer for growth and cellulase production, which induces the enzymes to the same extent as pure cellulose [45,46].
Paper, is one of the largest constituents of solid waste, has become a severe problem for disposal. It is a very important and challenging task of managing solid waste. Newspaper based cellulosic feedstock has emerged as an attractive option for the production of value-added chemicals [47]. Cellulose of waste paper is somehow difficult to hydrolyze enzymatically because it is associated with hemicelluloses and lignin. Dilute-acid hydrolysis is a fast and easy way to pretreat these lignocellulosic materials [48]. The wastepaper hydrolysate was found to have cellulase inducing capability and to induce a complete set of cellulase components. The hydrolysate was also concluded to be a better soluble inducer than sophorose [49].
The present paper describes the utility of wheat bran waste biomass as raw material; Illustrates the Box- Behnken design based optimization of different process parameters such as temperature, pH, moisture percentage, particle size, inoculum dosages and incubation period for cellulase production by Trichoderma and Neurospora. It also portrays the efficacy of various plant polysaccharide hydrolysates such as bagasse, wheat straw, waste newspaper as well as starchy hydrolysates.
2. Materials and methods
2.1. Materials
All the chemicals and reagents used to perform the experimental work were of Himedia, Sigma Aldrich, and Merck make. T. reesei NCIM 1186 and N. crassa NCIM 1021 were procured from National Chemical Laboratory, Pune. Raw materials were collected from a local market. Separate sets of batch experiments were carried out in 250 mL Erlenmeyer flasks containing sieved wheat bran biomass as the raw material for the growth and production of organisms impregnated with the following production media in (g/L) Urea, 0.3; (NH4)2SO4, 1.4; KH2PO4, 2.0; MgSO4.7H2O, 0.3; Peptone, 1.0; Tween 80, 0.2; FeSO4.7H2O, 0.005; MnSO4.7H2O, 0.0016; ZnSO4.7H2O; 0.0014; CaCl2.2H2O; CoCl2.6H2O, 0.02. Wheat bran biomass-based bed soaked with basal salt media were autoclaved and then inoculated with a specific volume of cell dry weight (g/L) T. reesei and N. crassa respectively. The production flasks were placed in an incubator at a different set of process parameters. A separate set of batch experiments was performed to investigate the effect of baggase, wheat straw, waste newspaper and starch hydrolysate. All the hydrolysates based experimental flasks inoculated with culture solution were placed in an incubator at optimized conditions, to study the effect of hydrolysates.
2.2. Experimental setup
2.2.1. Estimation of holocellulose content in wheat bran biomass
This method determines the total carbohydrate contents of non-wood materials. Holocellulose in non-wood raw materials was estimated by the TM1-A-9 test method mentioned in the laboratory manual of Central Pulp and Paper Research Institute (CPPRI) Saharanpur, U.P [50].
2.2.2. Estimation of lignin content in wheat bran biomass
Acid-insoluble lignin was estimated as per the TM1-A-7 test method mentioned in the laboratory manual of CPPRI [50].
2.2.3. Determination of ash content
5g of ground OD sample (passed through mesh size 40) were taken in a sintered crucible and then placed inside a muffle furnace at 600 °C for two hours, followed by cooling in a desiccator. Ash content was calculated by the following expression [50].
Ash % = W2 (weight of ash with crucible) – W1 (weight of the only crucible) x 100/OD weight of the sample
2.2.4. Measurement of moisture content
The moisture content of the medium was estimated by drying 5 g of the wet sample to a constant weight at 105 °C, and the dry weight was recorded.
2.2.5. Experimental design
To optimize different process parameters for cellulase production, Box-Behnken design (BBD) was used for optimization study. This design was the most suitable for the quadratic response surface and generated the second-order polynomial regression model. Three different levels were studied for each independent variable. A total of 13 experiments (sets of combination) were conducted for three independent variables and FPA (IU/mL) obtained was taken as a dependent variable or response (Y1). All the experiments were performed in a 250 ml Erlenmeyer flask containing wheat bran biomass as a solid bed. The independent variables used were process temperature (°C) (X1) process pH (X2) and inoculum dosages (X3) and their levels were mentioned in Table 3. After achieving the optimized process conditions, another trail was run of 13 experiments with another three independent variables. The independent variables used in another trail were particle size of wheat bran (μm) (X1), Moisture percentage(X2) and incubation period (X3) and their levels were mentioned in Table 5. The quadratic response function for n variables with interaction terms was considered for the mathematical relationship between independent and dependent variables. The relation between independent and dependent variables actual was described by the following equation.
| Y1=β0+β1 × 1+β2 × 2+β3 × 3+β11 × 1+β22 × 2+β33 × 3+ β12 × 1X2+β13 × 1X3+β23 × 2X3 |
Table 3.
Comparative experimental and predicted values of cellulase activity (IU/mL) achieved by Trichoderma under different set of combinations using particle size, moisture percentage and incubation period as parameters for the response of cellulase activity.
| Medium code | Particle size | MP | IP | FPA (IU/mL) E | Particle size | MP | IP | FPA (IU/mL)P |
|---|---|---|---|---|---|---|---|---|
| A | 450 | 61 | 3 | 2.69 | −1 | 0 | −1 | 3.618463 |
| B | 850 | 55 | 3 | 2.17 | 0 | −1 | −1 | 2.881086 |
| C | 850 | 66 | 3 | 2.05 | 0 | 1 | −1 | 3.088698 |
| D | 1250 | 61 | 3 | 3.19 | 1 | 0 | −1 | 4.013295 |
| E | 450 | 55 | 6 | 2.34 | −1 | −1 | 0 | 3.006647 |
| F | 450 | 66 | 6 | 2.18 | −1 | 1 | 0 | 3.232605 |
| G | 850 | 61 | 6 | 4.72 | 0 | 0 | 0 | 5.516143 |
| H | 1250 | 55 | 6 | 2.85 | 1 | −1 | 0 | 3.371527 |
| I | 1250 | 66 | 6 | 2.65 | 1 | 1 | 0 | 3.561757 |
| J | 450 | 61 | 9 | 2.96 | −1 | 0 | 1 | 3.81397 |
| K | 850 | 55 | 9 | 2.49 | 0 | −1 | 1 | 3.026628 |
| L | 850 | 66 | 9 | 2.37 | 0 | 1 | 1 | 3.235203 |
| M | 1250 | 61 | 9 | 3.53 | 1 | 0 | 1 | 4.109922 |
E: Experimental value (exp); P: Predicted value (pre), MP: Moisture percentage, IP: Incubation period (days).
Table 5.
Comparative experimental and predicted values of cellulase activity FPA (IU/mL) achieved by Neurospora under different set of combinations using particle size, moisture percentage and incubation period as parameters for the response of cellulase activity.
| Medium code | Particle size | MP | IP | FPA(IU/mL)E | Particle size | MP | IP | FPA(IU/mL)P |
|---|---|---|---|---|---|---|---|---|
| A | 450 | 66 | 5 | 1.65 | −1 | 0 | −1 | 1.784503 |
| B | 850 | 61 | 5 | 1.85 | 0 | −1 | −1 | 2.018746 |
| C | 850 | 71 | 5 | 1.58 | 0 | 1 | −1 | 1.696059 |
| D | 1250 | 66 | 5 | 1.72 | 1 | 0 | −1 | 2.316303 |
| E | 450 | 61 | 10 | 1.81 | −1 | −1 | 0 | 1.632143 |
| F | 450 | 71 | 10 | 1.14 | −1 | 1 | 0 | 1.069268 |
| G | 850 | 66 | 10 | 2.36 | 0 | 0 | 0 | 1.753605 |
| H | 1250 | 61 | 10 | 1.51 | 1 | −1 | 0 | 0.768943 |
| I | 1250 | 71 | 10 | 1.42 | 1 | 1 | 0 | 0.686068 |
| J | 450 | 66 | 15 | 1.05 | −1 | 0 | 1 | 1.006908 |
| K | 850 | 61 | 15 | 0.98 | 0 | −1 | 1 | 0.086339 |
| L | 850 | 71 | 15 | 0.87 | 0 | 1 | 1 | −0.23672 |
| M | 1250 | 66 | 15 | 1.07 | 1 | 0 | 1 | −0.81629 |
E: Experimental value (exp); P: Predicted value (pre), MP: Moisture percentage, IP: Incubation period (days).
Y1 was the response; X1, X2 and X3 were the independent variables; β0 was the intercept; β1, β2 and β3 were linear coefficients; β11, β22 and β33 were square coefficients; and β12, β13 and β23 were interaction coefficients.
2.2.6. Preparation of bagasse & wheat straw hydrolysates
Raw materials were collected from a local market. Chopped and grounded bagasse and wheat straw of definite particle size were used for the acid pretreatment. Acid treatment of raw materials has been performed with 5, 10 and 20 % H2SO4 (v/v) solution with maintaining solid-liquid ratio of about 1:20. Further, these solutions were kept at room temperature for 1 h and then subjected to steam treatment under pressure of 15 lb at 121 °C for 1 h duration. After steam treatment solutions, were cooled and filtered with a muslin cloth. Due to acid treatment at a higher temperature several toxic components were released in the hydrolysates. To minimize the effect of toxic substances, overliming process has been conducted. Calcium salt solution was used to perform overliming process. The hydrolysates were added into calcium salt solution and boiled for 1 h and kept at room temperature for the next 1 h. The resulting detoxified hydrolysates suspension was diluted to 100 mL with distilled water and make up the resulting solution at pH 7.0 with diluted NaOH solution. The resulting solution which has been obtained under these treatments was used in the production medium.
2.2.7. Preparation of waste news paper hydrolysates
The hydrolysates of waste newspaper were prepared by acid digestion. Old newspaper (Times of India) were collected form the local market. Before acid pretreatment, the deinking process of the chopped and grounded waste newspaper has been performed by using effective chemicals and surfactants (Tween-80). Deinked waste newspapers were further used for the acid pretreatment. Acid treatment of WNP (Waste newspaper) has been performed with a 20–70 % H2SO4 (v/v) solution with maintaining the definite solid-liquid ratio. Waste newspaper was soaked in acid, at room temperature for one hour. These solutions were cooled out, filtered and made up the resulting solution pH 7.0 by dilute NaOH solution. The resulting hydrolyzates were used in the production medium.
2.2.8. Preparation of starch hydrolysates
Acid pretreatment of starch was carried out by using 2 %, 5 %, and 10 % HCl (v/v) solution. 10 g of wheat, potato and rice starch powdered biomass were taken separately, further 40 mL of diluted HCl solution with specific strength was added to maintain the slurry of about 25 %. Afterward, starch slurries were subjected to steam treatment under a pressure of 15 psi at 121 °C for 1 and 3 h time duration. The treated starch slurries were used in the production medium as pure hydrolyzates.
2.2.9. Inoculum development
T. reesei NCIM 1186 and N. crassa NCIM 1021 were procured from National Chemical Laboratory (NCL), Pune, India. For inoculum development separate experiments were performed in 250 mL Erlenmeyer flasks containing 100 mL of potato dextrose broth (PDB) medium (In g/L peeled potato, 200; dextrose, 20; and yeast extract, 0.1) and M2 broth medium (In g/L Glucose, 10; Glycerine, 10; Yeast extract, 5; KH2PO4, 0.3; MgSO4.7H2O, 0.1) each, in which 5 loopfull cultures of mycelial conidia were added and shaken at 180 rpm at 30 °C in an incubator shaker for 3–4 days [51]. A definite volume of prepared cultures in broth suspension was used as inoculum for further production studies.
2.2.10. Dry weight determination
5.0 mL of culture solution was taken from potato dextrose and M2 broth medium. It was then filtered on a dried and preweighed Whatman filter paper No 1. Further, the biomass and other solids collected were washed thoroughly with distilled water and further with 5.0 mL of 0.9 % sterile saline solution. Then filtered mycelium was dried for 24 h at 105 °C until attainment of constant weight and weighed. The determination of fungal growth by cell dry weight was expressed as the mean of three independent readings.
2.2.11. Preparation of production media
Three types of production medium were used for production studies. (I) Normal basal salt media was used for production studies having the following constituents (g/L): Urea, 0.3; (NH4)2SO4, 1.4; KH2PO4, 2.0; MgSO4.7H2O, 0.3; Peptone, 1.0; Tween 80, 0.2; FeSO4.7H2O, 0.005; MnSO4.7H2O, 0.0016; ZnSO4.7H2O; 0.0014; CaCl2.2H2O; CoCl2.6H2O, 0.02 (II) Modified basal salt media, In which 10 %(v/v) dosages of boiled bagasse, bagasse hydrolysate, wheat straw hydrolysate and waste newspaper hydrolysate solution were incorporated in the earlier described production media separately. (III) Modified basal salt media, In which 2 and 5 % (v/v) dosages of potato, wheat and rice starch hydrolysate solution were incorporated in the earlier described production media separately.
2.2.12. Preparation of raw material
Wheat bran biomass was dried then ground and sieved with a mesh screen. The ground raw material was used as a solid substrate for cellulase production studies.
2.2.13. Solid state fermentation process
A separate set of fermentation experiments were carried out in 250 mL Erlenmeyer flasks containing sieved wheat bran as a carbon source, which was impregnated with the earlier discussed production mediums. Raw material soaked with normal as well as modified basal salt medium was autoclaved, cooled, and then inoculated with a specific volume of culture broth solution of T.reesei and N.crassa respectively. The autoclaved and inoculated flasks were placed in an incubator.
2.3. Product analysis
2.3.1. Extraction and assay of enzyme
Distilled water was added to the fermented samples (in a 1:5 proportion) in Erlenmeyer flasks, and the extraction was done after shaking in a shaker at 150 rpm for 1 h. The sample was then filtered and the extract obtained was centrifuged at 6000 rpm. The resulting supernatant was stored and used as an enzyme source. All extractions were conducted in duplicate.
2.3.2. Total cellulase activity (filter paper activity)
Filter paper activity (FPA) was determined by the method recommended by Ghose (1987) [52].
3. Results and discussions
3.1. Proximate analysis of wheat bran biomass
To determine the suitability and effectiveness of wheat bran for cellulase production, proximate analysis has been executed.
Table 1, portrays the major constituents in wheat bran as holocellulose whereas lignin was present in a lesser amount. The rest of the valuable constituents present maybe starch, protein and antioxidants.
Table 1.
Content of Major Constituents of Ground, Sieved and Oven-dried wheat bran.
| Constituents | % composition |
|---|---|
| Holocellulose | 57.35 ± 2.96 |
| Lignin | 7.12 ± 1.18 |
| Ash | 1.20 ± 0.56 |
| Moisture | 2.13 ± 0.67 |
Data are reported as mean ± standard deviation based on the repeated trails.
Based on earlier studies of XRD and FTIR analysis of wheat bran, it has been observed that XRD pattern of wheat bran showed reduced peak height with flat and larger area indicates least crystalline nature of cellulose. The FTIR spectra of wheat bran also showed the presence of a lesser amount of lignin and phenolic components which provides a condition for effortless uptake of cellulose by the fungal system. Therefore it can be suggested that cellulose present in wheat bran is easily available for the microbial attack [53].
3.2. Optimization of process parameters used in cellulase production by Trichoderma and Neurospora under wheat bran based solid bed
To determine the much effective and optimum conditions as well as to detect the frequent interactions between two or more factors statistical optimization methodology has been used. Separate sets of the batch experiments have been performed using design-based optimization with each microbial source. Design based optimization for the fermentation process could overcome the limitations of classical empirical methods and has been proved to be a powerful tool for the optimization of cellulase production as well as to determine the optimum operating conditions necessary for the scale-up of the process by reducing the number and cost of experiments. Single variable optimization methods are not only tedious but also can lead to misinterpretation of results, especially because the interaction between different factors is overlooked. Designed based experiments use a small set or combination of carefully planned experiments. The present section describes the Box-Behnken design of experiments for cellulase production by Trichoderma and Neurospora fungal strains to get their effective and valuable conditions.
3.2.1. Optimization of physical and chemical parameters for cellulase production by T. reesei
Temperature, pH, initial moisture content of the substrate, inoculums size of fungal strains, particle sizes of raw material, incubation period were identified as the most influential among physical and chemical parameters. A Box Behnken design was used to analyze the interactive effect of these parameters and to arrive at an optimum. The base points for the design were selected from a single parameter study (data not shown). Different sets of combinations have been used for cellulase production by T. reesei.
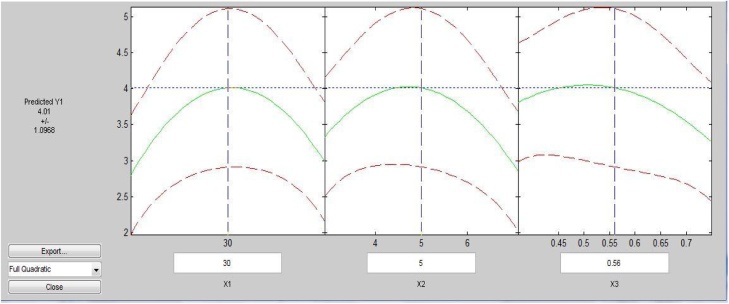
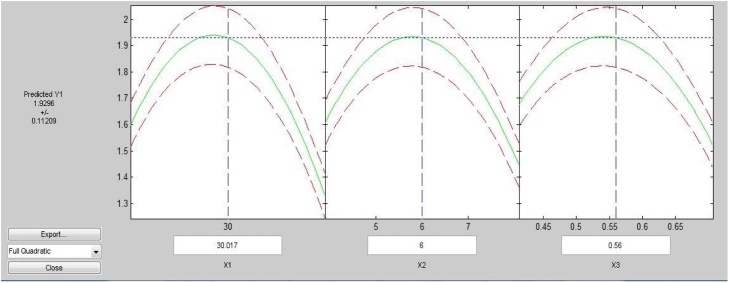
It was observed from Fig. 1, Fig. 2 that temperature 30 °C, pH 5 and inoculums dosages of 0.56 g/L was found a quite effective set of combination for cellulase activity (4.01 IU/mL) as compared to other.
Fig. 1.
Cellulase activity achieved by Trichoderma as a response (Y1) of three parameters temperature(X1), pH(X2) and Inoculum dosage(X3).
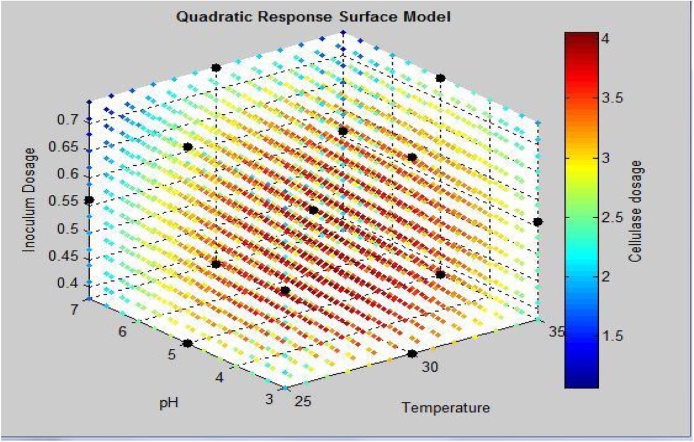
Fig. 2.
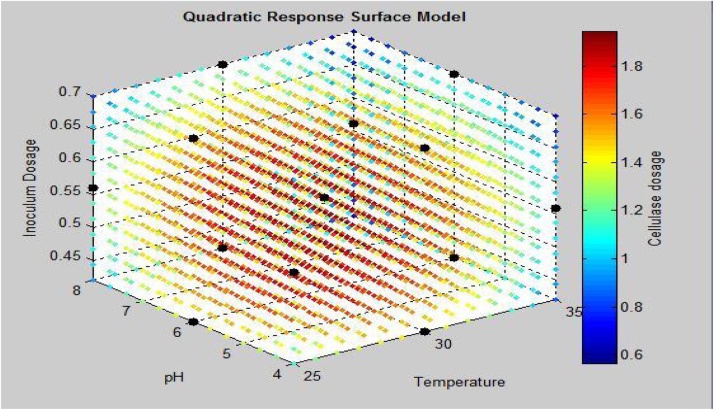
Quadratic response surface model with cellulase activity attained by Trichoderma as a response of parameters temperature, pH and Inoculum dosage. Cellulase dosage represents the cellulase activity: FPA(IU/mL).
It was also observed from Fig. 1 that the rate of decrement in enzyme activity was somewhat lower towards process temperature more than 30 °C, pH lesser than 5 and lower dosages of inoculum than 0.56 g/L.
At higher temperature enzyme biosynthesis decreases due to thermal deactivation enzymes and microbes [54]while at lower temperature (25 °C) the affinity of substrates for microbial system in the cells is lowered, because of the stiffening of lipids of the membrane and due to this the microbial enzyme production capability is decreased [55]. Operational pH induces stress response and triggering a pH signal pathway to regulate the expression [56].
It has also been observed from Fig. 2 that the higher cellulase activity region lies in the center of the graph which proves the significantly better optimization of process parameters.
The observed and predicted values are quite close to each other which represents the good correlation as shown in Table 2.
Table 2.
Comparative experimental and predicted values of cellulase activity (IU/mL) achieved by Trichoderma under different set of combinations using temperature, pH and inoculum dosage as parameters for the response of cellulase activity.
| Medium code | T(0C) | pH | Inoculum dosages(g/l) | FPA (IU/mL)E | T(0C) | pH | Inoculum dosages (g/l) | FPA (IU/mL)P |
|---|---|---|---|---|---|---|---|---|
| A | 25 | 3 | 0.56 | 2.36 | −1 | −1 | 0 | 1.258606 |
| B | 25 | 5 | 0.38 | 3.10 | −1 | 0 | −1 | 4.023431 |
| C | 25 | 5 | 0.74 | 1.69 | −1 | 0 | 1 | 1.477622 |
| D | 25 | 7 | 0.56 | 2.32 | −1 | 1 | 0 | 2.437406 |
| E | 30 | 3 | 0.38 | 3.24 | 0 | −1 | −1 | 4.367511 |
| F | 30 | 3 | 0.74 | 1.58 | 0 | −1 | 1 | 1.277202 |
| G | 30 | 5 | 0.56 | 4.01 | 0 | 0 | 0 | 4.174276 |
| H | 30 | 7 | 0.38 | 3.48 | 0 | 1 | −1 | 4.564311 |
| I | 30 | 7 | 0.74 | 1.63 | 0 | 1 | 1 | 1.438002 |
| J | 35 | 3 | 0.56 | 2.51 | 1 | −1 | 0 | 2.221106 |
| K | 35 | 5 | 0.38 | 3.35 | 1 | 0 | −1 | 4.548431 |
| L | 35 | 5 | 0.74 | 1.14 | 1 | 0 | 1 | 0.877622 |
| M | 35 | 7 | 0.56 | 1.97 | 1 | 1 | 0 | 2.399906 |
E: Experimental value (exp), P: Predicted value (pre).
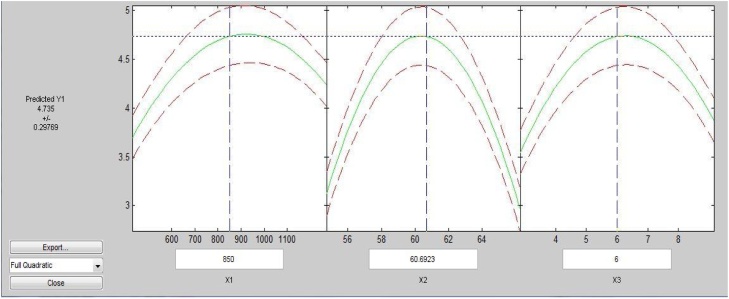
Different sets of combinations based on process parameters such as particle size, moisture percentage and incubation period, have been used for cellulase production by T.reesei. It was observed in Table 2 as well as Fig. 3, Fig. 4 that particle size of 850 μm, moisture percentage 61 and incubation period of 6 days was found a quite effective set of combinations for cellulase activity (4.72 IU/mL) as compared to other.
Fig. 3.
Cellulase activity achieved by Trichoderma as a response (Y1) of three parameters particle size(X1), moisture percentage (X2) and incubation period (X3).
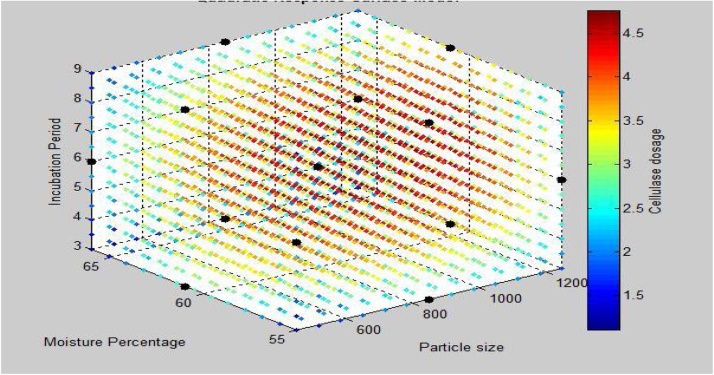
Fig. 4.
Quadratic response surface model with cellulase activity attained by Trichoderma as a response of three parameters particle size, moisture percentage and incubation period. Cellulase dosage represents the cellulase activity: FPA(IU/mL).
It was also observed from Fig. 3 that the rate of decrement in enzyme activity was somewhat lower towards particle size bigger than 850 μm, higher as well as lower moisture percentage than 61, more than 6 days of the incubation period. Moisture content is a critical factor in SSF processes because this variable has an influence on the microbial growth, biosynthesis as well as secretion of enzymes. According to researchers higher moisture levels can cause a reduction in the enzyme yields due to the steric hindrance of the growth of producer strain by the reduction in the porosity (interparticle space of the matrix), thus interfering oxygen transfer [57]. Alam et al. [58] reported the optimal moisture content in the solid substrate appears to be at 50 %, under this condition a cellulase activity of 0.0433 units was obtained.
It has also been observed from Fig. 4 that higher cellulase activity region lies in the nearby center of the graph which proves significantly good optimization of process parameters.
The observed and predicted values of cellulase activity are very close to each other which represents a better correlation as observed from Table 3.
3.2.2. Optimization of physical and chemical parameters for cellulase production by Neurospora crassa
A separate set of design experiments has been performed to study the optimization of process parameters.
Different sets of combinations based on process parameters such as temperature, pH and inoculum dosages, have been used for cellulase production by N. Crassa. It was observed in Table 4 as well as Fig. 5, Fig. 6 that temperature at 30 °C, pH at 6 and inoculums dosages of 0.56 g/L was found a quite effective set of combinations for cellulase activity (1.93 IU/mL) as compared to other.
Table 4.
Comparative experimental and predicted values of cellulase activity (IU/mL) achieved by Neurospora under different set of combinations using temperature, pH and inoculum dosage as parameters for the response of cellulase activity.
| Medium code | T(0C) | pH | Inoculum dosages(g/L) | FPA (IU/mL) E | T(0C) | pH | Inoculum dosages(g/L) | FPA (IU/mL) P |
|---|---|---|---|---|---|---|---|---|
| A | 25 | 4 | 0.56 | 1.35 | −1 | −1 | 0 | 1.691181 |
| B | 25 | 6 | 0.42 | 1.40 | −1 | 0 | −1 | 1.630877 |
| C | 25 | 6 | 0.70 | 1.28 | −1 | 0 | 1 | 1.650396 |
| D | 25 | 8 | 0.56 | 1.18 | −1 | 1 | 0 | 1.45307 |
| E | 30 | 4 | 0.42 | 1.43 | 0 | −1 | −1 | 1.733673 |
| F | 30 | 4 | 0.70 | 1.26 | 0 | −1 | 1 | 1.784189 |
| G | 30 | 6 | 0.56 | 1.93 | 0 | 0 | 0 | 2.313885 |
| H | 30 | 8 | 0.42 | 1.26 | 0 | 1 | −1 | 1.51956 |
| I | 30 | 8 | 0.70 | 1.11 | 0 | 1 | 1 | 1.558081 |
| J | 35 | 4 | 0.56 | 1.08 | 1 | −1 | 0 | 1.57468 |
| K | 35 | 6 | 0.42 | 1.15 | 1 | 0 | −1 | 1.507376 |
| L | 35 | 6 | 0.70 | 1.00 | 1 | 0 | 1 | 1.576895 |
| M | 35 | 8 | 0.56 | 0.946 | 1 | 1 | 0 | 1.37257 |
E = Experimental values, P = Predicted values.
Fig. 5.
Cellulase activity as a response (Y1) of three parameters temperature (X1), pH (X2) and Inoculum dosage (X3).
Fig. 6.
Quadratic response surface model with cellulase activity attained by Neurospora as a response of parameters temperature, pH and Inoculum dosage. Cellulase dosage represents the cellulase activity: FPA(IU/mL).
It was also observed from Fig. 4 that rate of decrement in enzyme activity was somewhat lower towards temperature lower than 30 °C, pH lower than 6 and inoculum dosages lower than 0.56 g/L.
The size of inoculum seems to have a profound effect on microbial growth and enzyme production. The effect of inoculum size on the enzyme activity was also studied by Dhillon et al. [59] they stated that maximum enzyme activity was observed using 5 % inoculum. An increase in inoculum size from 5 % showed a progressive decrease in enzyme activity reaching the lowest at 20 % inoculums. A higher inoculum concentration becomes favorable, probably because of the reduction in the lag phase caused by highly concentrated inocula. A decrease in its production on increasing the inoculum size could be due to competition between microorganism colonies for nutrients and probably the non-availability of nutrients for the large population limits the fungal growth [60]. Therefore a suitable and appropriate inoculums size or dosages required for healthier fungal propagation and their enzyme production.
It has also been observed from Fig. 6 that higher cellulase activity region lies in the center of the graph which proves the significantly better optimization of process parameters.
The observed and predicted values of cellulase activity are very close to each other which represents the good correlation as observed from Table 4.
Different sets of combinations based on process parameters such as particle size, moisture percentage and incubation period, have been used for cellulase production by N. crassa.
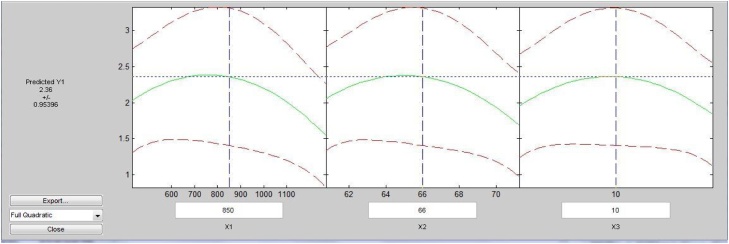
It was observed from Table 5 as well as Fig. 7, Fig. 8 that particle size of 850 μm, moisture percentage 66, and incubation period of 10 days was found a quite effective set of combinations for cellulase activity (2.36 IU/mL) produced by N. crassa as compared to other. It was also observed from Fig. 7 that the rate of decrement in enzyme activity was somewhat lower towards smaller particle size than 850 μm, lower moisture percentage than 66 and smaller incubation period than 10 days.
Fig. 7.
Cellulase activity achieved by Neurospora as a response (Y1) of three parameters particle size(X1), moisture percentage (X2) and incubation period (X3).
Fig. 8.
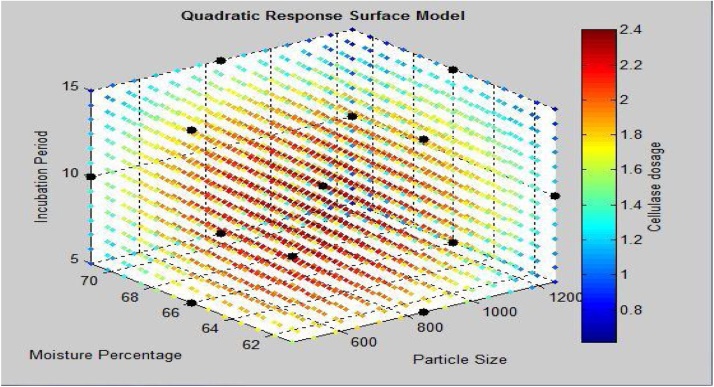
Quadratic response surface model with cellulase activity attained by Neurospora as a response of parameters particle size, moisture percentage and incubation period. Cellulase dosage represents the cellulase activity: FPA(IU/mL).
As literature suggested that in medium particle-sized bed, both inter-particle porosity and surface area is high, which provides an encouraging situation for growth and enzyme production by microbes due to better mass and heat transfer. The too smaller substrate particle sizes may result in substrate accumulation, which may interfere with microbial respiration/aeration and therefore result in poor growth [61]. On the other hand at larger particle sizes, interparticle porosity is high, but the surface area is low [62].
It has also been observed from Fig. 8 that higher cellulase activity region lies in the nearly center of the graph which proves the significantly good optimization of process parameters.
The observed and predicted values of cellulase activity are nearly close to each other which represents the good correlation as observed from Table 5.
Various coefficients of experimental set up 1 and 2 as well as both trails in each set up are shown in Table 6. It can be concluded that in each sets particular group (a combination of parameters) based optimization was found quite effective, as well as in most of the cases experimental and predicted values were very close or nearby close to each other, representing the better correlation between them. When compared to the cellulases produced by Trichoderma and Neurospora, higher FPA (4.72IU/mL) achieved by Trichoderma as compared to Neurospora (2.36IU/mL) strain under optimized condition. Optimized sets of the condition have been achieved and which were used in further production studies.
Table 6.
Coefficient values under different set of conditions.
|
T. reesei(Set 1) |
N. crassa (Set 2) |
||
|---|---|---|---|
| Trail 1 | Trail 2 | Trail 1 | Trail 2 |
| Temp, pH, inoculums dosages | Particle size, moisture percentage, incubation period | Temp, pH, inoculums dosages | Particle size, moisture percentage, incubation period |
| Coefficients (β0,β1,β2,β3,β11,β22,β33,β12,β13,β23) | |||
| −42.2368 | −188.437 | −20.0693 | −72.7166 |
| 2.46125 | 0.008198 | 0.99295 | 0.001051 |
| 2.141875 | 6.143066 | 1.02425 | 2.25315 |
| 15.73225 | 1.282604 | 16.73036 | 0.347988 |
| −6.59E-16 | −4.06E-06 | 0.00090 | 6.00E-05 |
| −0.0250 | 1.46E-05 | −0.01071 | −3.75E-06 |
| −0.3125 | −2.06E-05 | 0.017857 | −0.00030 |
| −0.0405 | −4.35E-06 | −0.01697 | −3.27E-06 |
| −0.20813 | −0.05085 | −0.09169 | −0.0177 |
| −13.2716 | −0.10347 | −15.2168 | −0.0168 |
Wheat bran was found as a suitable raw material for cellulase production under solid-state fermentation which might be due to the presence of soluble oligosaccharides, starches as well as easily available celluloses which significantly induces the cellulase production. Oligosaccharides present in wheat bran may also be converted into strong inducer such as sophrose and gentiobiose by transglucosylation for cellulase production. As the literature reported that wheat bran is a good source of nitrogen (due to high protein content) and hemicellulose, Altogether it is a good source of inducer for cellulolytic enzyme system [63]. Although the cellulose percentage in wheat bran is low [12] but it is easily utilizable by microbes, this was proved by XRD pattern of wheat bran. Lesser number of peaks with smaller peak height in the XRD pattern of wheat bran [53] which suggests that cellulose present are easily available for microbial hydrolysis.
3.3. Effect of boiled bagasse syrup, bagasse hydrolysate and wheat straw hydrolysate on cellulase production under wheat bran based solid-state fermentation
To explore the effect of boiled bagasse syrup, bagasse hydrolysate and wheat straw hydrolysate on cellulase production under wheat bran based solid-state fermentation separate set of the experiment have been performed. Maximum cellulase activities (IU/mL) in terms of FPA attained by T.reesei and N.crassa were 5.07 ± 0.03,5.03 ± 0.03, 5.00 ± 0.06; 2.41 ± 0.02, 2.43 ± 0.04, 2.38 ± 0.07 under boiled bagasse syrup, 10 % acid treated bagasse hydrolysate and 5 % acid treated wheat straw hydrolysate containing wheat bran based solid-state fermentation respectively. It was observed from Table 7, that fungal strain produces significantly better cellulase activities under boiled bagasse syrup, 10 % acid treated bagasse hydrolysate and 5 % acid treated wheat straw hydrolysate as compared to others. The higher cellulase activity produced by fungal strains under wheat bran bed incorporating with boiled bagasse syrup and 10 % acid treated bagasse hydrolysate and 5 % acid treated wheat straw hydrolysate as compared to wheat bran bed solely, which may be due to the inclusion of some others inductive nature of sugars as well as lesser inhibitory environment of wheat bran, all these situations generate favorable condition for growth and enzyme production.
Table 7.
Comparative enzyme activity achieved by various fungal strains under boiled bagasse syrup(BBS), bagasse hydrolysates(BH) and wheat straw hydrolysates(WSH) containing wheat bran based SSF at 30 °C and pH 5.
| Lignocellulosic Hydrolysates | T. reesei | N. crassa | |
|---|---|---|---|
| FPA(IU/mL) | FPA(IU/mL) | ||
| BBS | 5.07 ± 0.03 | 2.41 ± 0.02 | |
| BH | 10 % H2SO4 treated | 5.03 ± 0.03 | 2.43 ± 0.04 |
| 20 % H2SO4treated | 4.78 ± 0.09 | 2.32 ± 0.06 | |
| WSH | 5 % H2SO4treated | 5.00 ± 0.06 | 2.38 ± 0.07 |
| 10 % H2SO4 treated | 4.86 ± 0.11 | 2.35 ± 0.03 | |
3.4. Effect of waste news paper hydrolysates on cellulase production under wheat bran based solid state fermentation
To explore the effect of acid treated waste newspaper hydrolysate on cellulase production under wheat bran based solid state fermentation separate set of the experiment has been performed using 20, 30, 40, 50 and 70 % H2SO4 treated waste newspaper hydrolysate. It has been observed from Table 8, that fungal strain produces better cellulase activity under wheat bran solid bed incorporated with 40 % acid treated waste newspaper hydrolysate. Maximum cellulase activities (IU/mL) in terms of FPA attained by T. reesei, and N.crassa were 5.15 ± 0.09 and 2.47 ± 0.03 under 40 % acid treated waste newspaper hydrolysate containing wheat bran bed. Improvement in the cellulase activities may be due to the release of sophrose, cellobiose, xylose and glucose sugars, which induces the cellulase production by fungal strains. On the other hand, lesser inhibitory condition provided by wheat bran bed generates favorable condition for fungal growth and activity enhancement under such condition.
Table 8.
Comparative enzyme activity achieved by various fungal strains under waste newspaper hydrolysates containing wheat bran based SSF at 30 °C and pH 5.
| Waste News Paper Hydrolysates | T. reesei | N. crassa |
|---|---|---|
| FPA(IU/mL) | FPA(IU/mL) | |
| WNP(20 % acid treated) | 4.87 ± 0.06 | 2.38 ± 0.02 |
| WNP(30 % acid treated) | 4.96 ± 0.03 | 2.41 ± 0.04 |
| WNP(40 % acid treated) | 5.15 ± 0.09 | 2.47 ± 0.03 |
| WNP(50 % acid treated) | 4.82 ± 0.17 | 2.38 ± 0.06 |
| WNP(70 % acid treated) | 4.69 ± 0.03 | 2.33 ± 0.03 |
3.5. Effect of Starch hydrolysates on cellulase production under wheat bran based solid state fermentation
To investigate the role of starch hydrolysates in cellulase production under wheat bran based solid state fermentation, a separate set of experiments were performed. Pretreatment of starches was performed by using 2 and 5 % HCl with 1 h of pretreatment time. Higher cellulase activities (IU/mL) in terms of FPA attained by T.reesei and N.crassa were 5.74 ± 0.07 and 2.61 ± 0.05 respectively under 5 %(v/v) dosages of 2 % acid hydrolyzed wheat starch hydrolysate based solid state fermentation. This might be due to the release of some dimeric sugars (sophorose) in the acid hydrolysate starches. It was also observed from Table 9, that fungal strain produces significantly higher cellulase activities under 2 % HCl treated wheat starch hydrolysate based fermentation medium as compared to 5 % HCl treated one, which may be due to the fact that upon increasing the acid strength overhydrolysis of starch is taken place thereby other byproducts may be generated, which might have served as inhibitors for cellulase production [47]. When compared the effectiveness of wheat starch hydrolysates for various used fungal strains than it has been observed that all the strains performed much better in terms of cellulase activity under wheat starch hydrolysate containing wheat bran based solid state fermentation. N. crassa (2.63 ± 0.05) performed well also under rice starch hydrolysate media base solid state fermentation. Potato starch hydrolysate was found less effective for cellulase activity enhancement by both fungal strains under wheat bran based solid state fermentation.
Table 9.
Comparative enzyme activities achieved by different fungal strains under various starch hydrolysates incorporated wheat bran based SSF at 30 °C and pH 5.
| Starch hydrolysates (SH) | T. reesei | N. crassa | |
|---|---|---|---|
| FPA(IU/mL) | FPA(IU/mL) | ||
| 2 %HCl WSH | 2 %(v/v) | 5.10 ± 0.03 | 2.54 ± 0.03 |
| 5 %(v/v) | 5.74 ± 0.07 | 2.61 ± 0.05 | |
| 5 %HCl WSH | 2 %(v/v) | 4.31 ± 0.09 | 2.20 ± 0.10 |
| 5 %(v/v) | 4.27 ± 0.04 | 2.16 ± 0.03 | |
| 2 %HCl PSH | 2 %(v/v) | 4.86 ± 0.07 | 2.44 ± 0.15 |
| 5 %(v/v) | 5.09 ± 0.05 | 2.55 ± 0.03 | |
| 5 %HCl PSH | 2 %(v/v) | 4.28 ± 0.15 | 2.25 ± 0.11 |
| 5 %(v/v) | 4.20 ± 0.03 | 2.18 ± 0.09 | |
| 2 %HCl RSH | 2 %(v/v) | 5.02 ± 0.07 | 2.59 ± 0.12 |
| 5 %(v/v) | 5.25 ± 0.11 | 2.63 ± 0.05 | |
| 5 %HCl RSH | 2 %(v/v) | 4.34 ± 0.03 | 2.28 ± 0.02 |
| 5 %(v/v) | 4.37 ± 0.02 | 2.31 ± 0.04 | |
It can be concluded that wheat starch hydrolysate was found quite effective for cellulase induction capability. We can also suggest that raw material composition also affects the performance of starch hydrolysates or in other words they utilized better under less inhibitory environment.
4. Conclusions
The bioconversion of waste biomass into valuable energy is the part of waste management and sustainable approach. The utilization of lignocellulosic solid, as well as liquid wastes under optimized conditions, has proven as the main contender to overcome the economical and waste management problem to a great extent. To optimize the process parameters of cellulase production from Trichoderma and Neurospora were investigated by Box–Behnken design (BBD), an effective and reliable tool for finding the optimal conditions of process parameters used in cellulase production. Various lignocellulosic, as well as starchy hydrolysates, were used in cellulase production under wheat bran based solid-state fermentation. Fungal strains performed well under bagasse as well as wheat starch hydrolysates based fermentation.
CRediT authorship contribution statement
Nitin Verma: Conceptualization, Data curation, Investigation, Resources, Writing - original draft, Writing - review & editing. Vivek Kumar: Supervision, Software, Formal analysis, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
Authors gratefully acknowledged the ministry of human resource and development, India for providing fellowship to carry out present research work.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00416.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Farinas C.S. Current developments in biotechnology and bioengineering. Curr. Adv. Solid-State Ferment. 2017:169–183. Chapter 9. [Google Scholar]
- 2.Liu Y., Yi N., Lu X., Zhang X., He H., Pan F., Zhou L., Liu X., Ji X., Zhang S. Cascade utilization of lignocellulosic biomass to high-value products. Green Chem. 2019;(Issue):13. [Google Scholar]
- 3.Verma N., Bansal M.C., Kumar V. Pea peel waste: a lignocellulosic waste and its utility in cellulase production by Trichoderma reesei under solid state cultivation. Bioresources. 2011;6(2):1505–1519. [Google Scholar]
- 4.Verma N., Kumar V., Bansal M.C. Utility of Luffa cylindrica and Litchi chinensis peel, an agricultural waste biomass in cellulase production by Trichoderma reesei under solid state cultivation. Biocatal. Agric. Biotechnol. 2018;16:483–492. [Google Scholar]
- 5.Maeda R.N., Barcelos C.A., Anna L.M.M.S., Jr, Pereira N. Cellulase production by Penicillium funiculosum and its application in the hydrolysis of sugar cane bagasse for second generation ethanol production by fed batch operation. J. Biotechnol. 2013;163:38–44. doi: 10.1016/j.jbiotec.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Singh L.K., Chaudhary G., Majumder C.B., Ghosh S. Utilization of hemicellulosic fraction of lignocellulosic biomaterial for bioethanol production. Adv. Appl. Sci. Res. 2011;5:508–521. [Google Scholar]
- 7.Reczey K., Szengyel Z., Eklund R., Zacchi G. Cellulase production by T. reesei. Bioresour. Technol. Rep. 1996;57:25–30. [Google Scholar]
- 8.Macris B.J., Kekos D., Evangelidou X. Simple and inexpensive method for cellulase and β-glucosidase production by Neurospora crassa. Appl. Microbiol. Biotechnol. 1989;31:150–155. [Google Scholar]
- 9.Onipe O.O., Jideani A.I.O., Beswa D.C. Composition and functionality of wheat bran and its application in some cereal food. Int. J. Food Sci. Technol. 2015;50:2509–2518. [Google Scholar]
- 10.Šramková Z., Gregová E., Šturdík E. Chemical composition and nutritional quality of wheat grain. Acta Chim. Slovaca. 2009;2(1):115–138. [Google Scholar]
- 11.http://en.wikipedia.org/wiki/Bra.
- 12.Sun X., Liu Z., Qu Y., Li X. The effects of wheat bran composition on the production of biomass hydrolyzing enzymes by Penicilium decumbens. Appl. Biochem. Biotechnol. 2008;146:119–128. doi: 10.1007/s12010-007-8049-3. [DOI] [PubMed] [Google Scholar]
- 13.Sharma D., Garlapati V.K., Goel G. Bioprocessing of wheat bran for the production of lignocellulolytic enzyme cocktail by Cotylidia pannosa under submerged conditions. Bioengineered. 2016;7(2):88–97. doi: 10.1080/21655979.2016.1160190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey A., Soccol C.R., Nigam P., Soccol V.T. Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresour. Technol. Rep. 2000;74:69–80. [Google Scholar]
- 15.Nigam P. Processing of agriculture wastes in solid state fermentation for cellulolytic enzyme production. JSIR. 1996;55:457–463. [Google Scholar]
- 16.Razak M.N.A., Ibrahim M.F., Yee P.L., Hassan M.A., Abd-Aziz S. Utilization of oil palm decanter cake for cellulase and polyoses production. Biotechnol. Bioprocess Eng. 2012;17(Issue-3):547–555. [Google Scholar]
- 17.Ahsan T., Chen J., Wu Y., Irfan M. Application of response surface methodology for optimization of medium components for the production of secondary metabolites by Streptomyces diastatochromogenes KX852460. AMB Express. 2017;7:96. doi: 10.1186/s13568-017-0388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis F., Sabu A., Nampoothiri K.M., Ramachandran S., Ghosh S., Szakacs G., Pandey A. Use of response surface methodology for optimizing process parameters for the production of α- amylase by Aspergillus oryzae. Biochem. Eng. J. 2003;15:107–115. [Google Scholar]
- 19.Aktas N., Boyaci I.H., Mutlu M., Tanyolac A. Optimization of lactose utilization in deproteinated whey by Kluveromyces marxianus using response surface methodology (RSM) Bioresour. Technol. Rep. 2006;97:2252–2259. doi: 10.1016/j.biortech.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Zhi W., Song J., Ouyang F., Bi J. Application of response surface methodology to the modeling of α- amylase purification by aqueous two phase systems. J. Biotechnol. 2005;118:157–165. doi: 10.1016/j.jbiotec.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 21.2019. Engineering Statstic Hand Book, Response Surface Design. http//www.itl.nist.gov/div898/handbook/pri/section 3/pri335.htm. [Google Scholar]
- 22.Pasma S.A., Daik R., Maskat M.Y., Hassan O. Application of box-behnken design in optimization of glucose production from oil palm empty fruit bunch cellulose. Int. J. Polym. Sci. 2013;2013 [Google Scholar]
- 23.Gonzalez G., Lopez-Santin J., Caminal G., Sola C. Dilute acid hydrolysis of wheat straw hemicellulose at moderate temperature. Biotechnol. Bioeng. 2004;28:288–293. doi: 10.1002/bit.260280219. [DOI] [PubMed] [Google Scholar]
- 24.Cardona C.A., Quintero J.A., Paz I.C. Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour. Technol. Rep. 2010;101:4754–4766. doi: 10.1016/j.biortech.2009.10.097. [DOI] [PubMed] [Google Scholar]
- 25.Talebnia F., Karakashev D., Angelidaki I. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. Rep. 2010;101:4744–4753. doi: 10.1016/j.biortech.2009.11.080. [DOI] [PubMed] [Google Scholar]
- 26.Pessoa Jr A., Mancilha I.M., Sato S. Acid hydrolysis of hemicellulose from sugarcane bagasse. Braz. J. Chem. Eng. 1997;14(3) [Google Scholar]
- 27.Guerra-Rodríguez E., Portilla-Rivera O.M., Jarquín-Enríquez L., Ramírez J.A., Vázquez M. Acid hydrolysis of wheat straw: a kinetic study. Biomass Bioenergy. 2012;36:346–355. [Google Scholar]
- 28.Zhuang J., Liu Y., Wu Z., Sun Y., Lin L. Hydrolysis of wheat straw hemicellulose and detoxification of the hydrolysate for xylitol production. Bioresources. 2009;4(2) [Google Scholar]
- 29.Paniagua A.I., Diez-Antolinez R., Hijosa-Valsero M., Sanchez M.E., Coca M. Response surface optimization of dilute sulfuric acid pretreatment of switchgrass (Panicum virgatum l.) for fermentable sugars production. Chem. Eng. Trans. 2016;49:223–228. [Google Scholar]
- 30.Sun J., Shi J., Zhao M., Xue S., Ren J.J., Jiang Y. A comparative analysis of property of lychee polyphenoloxidase using endogenous and exogenous substrates. Food Chem. 2008;108:818–823. doi: 10.1016/j.foodchem.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 31.Sun R., Lawther J.M., Banks W.B. A tentative chemical structure of wheat straw lignin. Ind. Crops Prod. 1997;6:1–8. [Google Scholar]
- 32.Chowdhary N.A., Monruzzaman M., Nahar N., Chowdhary N. Production of celllulase and saccharification of lignocellulosic by A. Micromonospora sp. World J. Microbiol. Biotechnol. 1991;7:603–606. doi: 10.1007/BF00452840. [DOI] [PubMed] [Google Scholar]
- 33.Pattra S., Sangyoka S., Boonmee M., Reungsang A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int. J. Hydrogen Energy. 2008;33:5256–5265. [Google Scholar]
- 34.Canilha L., Rodrigues Rde C.L.B., Antunes F.A.F., Chandel A.K., Milessi T.S.D.S., Felipe M.D.G.A., Silvio Silvério da Silva S.S.D. 2013. Bioconversion of Hemicellulose from Sugarcane Biomass Into Sustainable Products., Sustainable Degradation of Lignocellulosic Biomass - Techniques, Applications and Commercialization. [Google Scholar]
- 35.Chandel A.K., Kapoor R.K., Singh A., Kuhad R.C. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM3501. Bioresour. Technol. Rep. 2007;98:1947–1950. doi: 10.1016/j.biortech.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 36.Roberto I.C., Lacis L.S., Barbosa M.F.S., Demancilha I.M. Utilization of sugarcane bagasse hemicellulose hydrolysate byPichia stipitis for the production of ethanol. Process Biochem. 1991;26:15–21. [Google Scholar]
- 37.Biglow M., Wyman C.E. Cellulase production on bagasse pretreated with hot water. Appl. Biochem. Biotechnol. 2002:98–100. doi: 10.1385/abab:98-100:1-9:921. [DOI] [PubMed] [Google Scholar]
- 38.Serge Pérez S., Baldwin P.M., Daniel J., Gallant D.J. third edition. Elsevier Inc.; 2009. Structural Features of Starch Granules I., Starch: Chemistry and Technology. [Google Scholar]
- 39.Razdan N., Kocher G.S. Utilization of damaged and spoiled wheat grains for bioethanol production Agricultural Communication. Biosci. Biotech. Res. Comm. 2018;11(4):658–673. [Google Scholar]
- 40.http://en.wikipedia.org/wiki/Wheat.
- 41.http://en.wikipedia.org/wiki/Potato.
- 42.2019. International Starch Institute, Denmark, Technical Memorandum on Wheat Starch.http://www.starch.dk/isi/starch/tm33wheat.asp [Google Scholar]
- 43.http://en.wikipedia.org/wiki/Rice.
- 44.Cornejo-Ramírez Y.I., Martínez-Cruz O., Toro-Sánchez C.L.D., Wong-Corral F.J., Borboa-Flores J., Cinco-Moroyoqui F.J. The structural characteristics of starches and their functional properties. CyTA – J. Food. 2018;16(1):1003–1017. [Google Scholar]
- 45.Wayman M., Chen S. Cellulase production by Trichodermma reesei using whole wheat flour as a carbon source. Enzyme Microbial Technol. 1992;14:825–831. [Google Scholar]
- 46.Wang C.H., Hseu T.H., Huang C.M. Induction of cellulases by cellooligosaccharides in Trichoderma koninghii G-39. J. Biotechnol. 1988;9:47–60. [Google Scholar]
- 47.Byadgi S.A., Kalburgi P.B. Production of bioethanol from waste newspaper. Procedia Environ. Sci. 2016;35:555–562. [Google Scholar]
- 48.Htway W.M., Thein T.Z., Myat Y. Bioethanol production from office waste paper. Int. J. Sci. Eng. Appl. 2018;7(08):166–168. [Google Scholar]
- 49.Ju L.K., Afolabi O.A. Waste paper hydrolysate as soluble inducing substrate for cellulase production in continous culture of Trichoderma reesei. Biotechnol. Prog. 1999;5:91–97. doi: 10.1021/bp980116n. [DOI] [PubMed] [Google Scholar]
- 50.Laboratory Manual . Estimation of Acid Insoluble Lignin in wood/nonwood,” TM1A-7; “Estimation of Acid Soluble Lignin in wood/nonwood,” TM1A-8; “Estimation of Holocellulose in wood/nonwood”; TM1-A9. 2001. Laboratory manual of Central pulp and paper research institute, analysis of fibrous raw materials, proximate chemical analysis, Saharanpur, 247001(U.P.), India. [Google Scholar]
- 51.Domingues F.C., Queinoz J.A., Cabral J.M.S., Fonseca L.P. The influence of culture conditions on mycelial structure and cellulase production by Trichoderma reesei RUT C 30. Enzyme Microbial Technol. 2004;26 doi: 10.1016/s0141-0229(99)00166-0. [DOI] [PubMed] [Google Scholar]
- 52.Ghose T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987;59(2):257–268. [Google Scholar]
- 53.Verma N., Kumar V., Bansal M.C. Utilisation of egg shell waste in cellulase production by Neurospora crassa under wheat bran based solid state cultivation. Polish J. Environ. Stud. 2012;21(2):491–497. [Google Scholar]
- 54.Widowati E., Utami R., Mahadjoeno E., Saputro G.P. Effect of temperature and pH onpolygalacturonase production by pectinolyticbacteria Bacillus licheniformis strain GD2a insubmerged medium from Raja Nangka (Musaparadisiaca var. Formatypica) banana peel waste. IOP Conf. Ser.: Mater. Sci. Eng. 2017 [Google Scholar]
- 55.Nedwell D.B. Effect of low temperature on microbial growth: lowered affinity for substrates limits growth at low temperature. FEMS Microbiol. Ecol. 1999;30(2):101–111. doi: 10.1111/j.1574-6941.1999.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 56.Virgilio S., Cupertino F.B., Bernardes N.E., Freitas F.Z., Takeda A.A.S., Rd M., Fontes M. Molecular components of the neurospora crassa pH signaling pathway and their regulation by pH and the PAC-3 transcription factor. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pasanen A.L., Kalliokoski P., Pasanen P., Jantunen M.J., Nevalainen A. Laboratry studies on the relationship between fungal growth and atmospheric temperature and humidity. Environ. Int. 1991;17(4):225–228. [Google Scholar]
- 58.Alam Md Z., Muhammad N., Mahmat M.E. Production of cellulose from oil palm biomass as substrate by solid state bioconversion. Am. J. Appl. Sci. Res. 2005;2(2):569–572. [Google Scholar]
- 59.Dhillon S.S., Gill R.K., Gill S.S., Singh M. Studies on the utilization of citrus peel for pectinase production using fungus Aspergillus niger. Int. J. Environ. Stud. 2004;61(2):199–210. [Google Scholar]
- 60.Mandhania S., Jain V., Malhotra S.P. Culture optimization for enhanced production of microbial pectin methylesterase under submerged conditions. Asian J. Biochem. Pharm. Res. 2010;5:12–22. [Google Scholar]
- 61.Vanajakshi J., Subhaker C., Jetty A. Media engineering for the production of cellulases by a novel strain Aspergillus sp IICT-F 141 using wheat bran as raw materials. J. Pure Appl. Microbiol. 2009;3(2):477–483. [Google Scholar]
- 62.Pandey A., Selvakumar P., Soccol C.R., Nigam P. Solid state fermentation for the production of industrial enzymes. Curr. Sci. 1999;77:149–162. [Google Scholar]
- 63.Brijwani K., Oberoi H.S., Vadlani P.V. Production of cellulolytic enzyme system in mixed culture solid state fermentation of soyabean hulls supplemented with wheat bran. Process Biochem. 2010;45(1):120–128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.