Abstract
Primary health care (PHC) providers are at the front line of asthma management. To evaluate how planned asthma follow-up occurred in PHC and whether lung function tests were used, 203 patients were followed for 12 years as part of a real-life asthma cohort Seinäjoki Adult Asthma Study (SAAS). A total of 152 patients had visits in PHC attending on average to four planned contacts during 12-year follow-up corresponding to one visit every third year. National guideline recommends annual visits. Patients with ≥4 contacts seemed to have more difficult asthma and better adherence to inhaled corticosteroid medication. Lung function tests were performed on average in 87.5% of annual planned follow-up contacts. Spirometry was performed in 70%, 71% and 97% of all contacts depending on whether it was a contact to GP, nurse or both. Overall, the frequency of follow-up contacts was insufficient but PHC adherence to lung function testing was excellent.
Subject terms: Asthma, Health policy, Clinical trial design, Rehabilitation, Population screening
Introduction
Asthma is a common, heterogeneous disease, causing considerable morbidity affecting all age groups1. Adherence to international and national guidelines in asthma seems to be highly variable2–5. It is logical to assume that if clinical guidelines were better adopted it would also lead to better patient outcomes.
Asthma prevalence is still increasing also in Finland1,6, and the most of asthma cases are diagnosed at adult age7,8. Remission of adult-onset asthma is rare9,10. There are many possible reasons for poor asthma control and high symptom burden such as allergic or chronic rhinitis, smoking, comorbidities, obesity and low initial lung function as well as problems in inhalation techniques and adherence to asthma medication1,11,12. Patients with both systemic inflammation and comorbidity have been shown to have the poorest outcome in asthma13. To improve asthma control and outcomes, it is crucial that the routine follow-up contacts in primary health care (PHC) are performed according to a high standard, and there is a need to pay attention to the quality of these contacts2,14.
Finland was one of the first countries to implement a national asthma programme15. The main goals of the Finnish Asthma Programme (1994–2004) were to improve national asthma management, prevent an increase in costs and decrease the burden of asthma to individuals and society16,17. One of the main objectives of the programme was to strengthen the role of PHC in the prevention, diagnosis and long-term therapy of asthma15–19. The Finnish Asthma Programme emphasized measures to confirm asthma diagnosis by lung function tests, to follow patients regularly and to monitor asthma control also by lung function tests intermittently15,16. To achieve these objectives, nurses in the PHC were trained to perform spirometry and general practitioners (GP) to interpret the result. In 2001, spirometry was available in 95% of Finnish health care centres20.
To our knowledge, no previous long-term follow-up studies exist on the occurrence of planned asthma follow-up contacts in PHC and use of lung function tests during the long-term follow-up of asthma. Thus the main aim of this study was to describe how planned asthma follow-up contacts occurred in PHC and to evaluate the use of objective lung function tests (spirometry and peak flow monitoring) in the long-term follow-up of asthma patients. The second aim was to evaluate the use of lung function tests depending on who encounters the patient: GP, nurse, or both.
Results
Characteristics of the study population
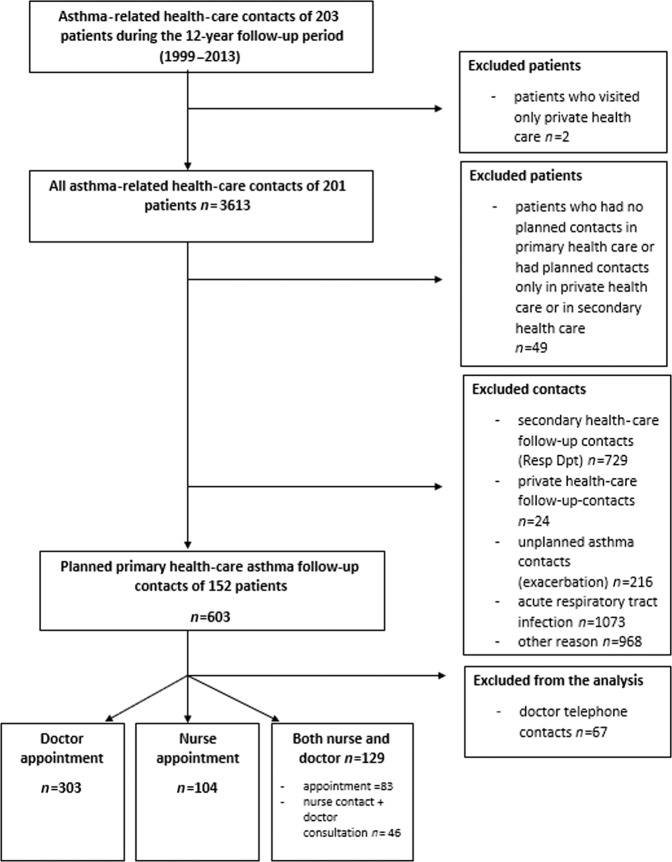
The current study is a part of the real-life adult asthma cohort, Seinäjoki Adult Asthma Study (SAAS), in which 203 patients were followed for 12 years (1999–2013) after diagnosis of new-onset adult asthma21. The exclusion and inclusion criteria of the SAAS study are shown in eTable 1. Out of the total of 203 patients, 152 participated in planned PHC asthma follow-up contacts. Forty-nine patients were excluded because of not having planned follow-ups in PHC or having them only in private health care or in respiratory department (Fig. 1). Most of the patients with planned PHC asthma follow-up contacts were females (Table 1). At follow-up visit, mean age was 59 years and every second patient had smoking history. Approximately one third of the patients had uncontrolled asthma according to Global Initiative for Asthma (GINA) 201022. The main characteristics of the study population at follow-up visit are shown in Table 1.
Fig. 1. Study profile.
The flowchart of the study.
Table 1.
Basic characteristics of patients having planned asthma follow-up contacts in primary care at 12-year follow-up visit.
| Patients having asthma follow-up contacts in primary care | |
|---|---|
| Number of patients | 152 |
| Female, n (%) | 96 (63.2) |
| Age | 59 (13) |
| BMI | 28.5 (5.9) |
| Smokers (ex or current), n (%) | 76 (50.0) |
| Atopic, n (%)a | 51 (37.2) |
| Rhinitis, n (%) | 107 (71.8) |
| Uncontrolled asthma, n (%)b | 46 (30.3) |
| Daily LABA in use, n (%) | 78 (51.3) |
| Daily add-on drug in use, n (%) | 85 (83.3) |
| Daily ICS in use, n (%) | 125 (81.2) |
| Daily SABA in use, n (%) | 21 (13.8) |
| ≥1 oral corticosteroid course during 12-year follow-up, n (%) | 50 (33.6) |
| Pre-BD FEV1 (%) | 87 (17) |
| Post-BD FEV1 (%) | 91 (17) |
| Pre-BD FEV1/FVC | 0.74 (0.67–0.79) |
| Post-BD FEV1/FVC | 0.76 (0.70–0.80) |
| FeNO (ppb) | 11 (5–19) |
| Blood neutrophils (×109/l) | 3.7 (2.8–4.7) |
| Blood eosinophils (×109/l) | 0.15 (0.10–0.27) |
| Total IgE (kU/l) | 61 (23–154) |
| ACO (post-FEV1/FVC < 0.7 and pack-years ≥10), n (%) | 19 (12.6) |
| ACT score | 21 (19–24) |
If not otherwise mentioned, data shown are mean (SD) or median (25th–75th percentiles).
BMI Body Mass Index, LABA long-acting β2-agonist, Add-on drug long-acting β2-agonist, leukotriene receptor antagonist, theophylline and/or tiotropium in daily use, ICS inhaled corticosteroid, SABA short-acting β2-agonist, BD bronchodilator, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, FeNO fraction of NO in exhaled air, ACO asthma–COPD overlap, ACT asthma control test.
aAt least one positive skin prick test of common allergens.
bAssessment of asthma control was performed according to the Global Initiative for Asthma (GINA) 2010 report.
The distribution of the planned follow-up contacts in primary care
The number of all planned asthma follow-up contacts in PHC was 603. Thus, on average, each patient (n = 152) had approximately four planned contacts during the 12-year follow-up period. During the years 1–12 after diagnosis, annual number of planned contacts varied from 21 to 67 (Fig. 2). The annual average of planned contacts was 50, i.e. every third patient attended a planned visit each year.
Fig. 2. The distribution of planned contacts in primary care during 12-year follow-up.
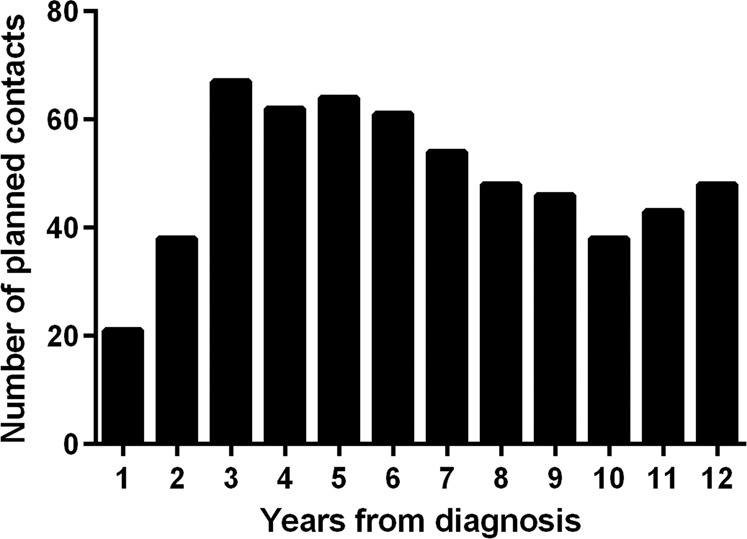
Total number of planned contacts was 603.
Differences between patients having <4 or ≥4 planned contacts
The patients participating in planned follow-ups (n = 152) were divided into two groups according to the number of planned asthma follow-up contacts in PHC (<4 vs. ≥4 follow-up contacts): 84 patients had <4 [median 1 (interquartile range (IQR) 1–2)] and 68 patients had at least 4 [median 6 (IQR 4–8)] planned follow-up contacts during the 12-year follow-up period. The groups with <4 vs. ≥4 follow-up visits showed no difference regarding gender, age, smoking, lung function, markers of inflammation [blood eosinophils, neutrophils, immunoglobulin E (IgE) or fraction of NO in exhaled air (FeNO)] or proportion of severe asthma according to ERS/ATS 201423 (Table 2). Approximately one third of the patients in both groups had uncontrolled asthma according to GINA 2010 (Table 2)22. Patients with higher number of planned follow-up visits (≥4) had more often inhaled corticosteroid (ICS) medication in daily use and their adherence to ICS medication over 12 years was higher. This group had also higher number of all asthma-related health care visits and were more often in working life (Table 2). No significant differences were found in lung function or other parameters at the baseline (eTable 2).
Table 2.
Characteristics of the study groups at 12-year follow-up visit.
| Planned PHC follow-up contacts ≥4 | Planned PHC follow-up contacts <4 | P value | |
|---|---|---|---|
| Number of patients | 68 | 84 | |
| Female, n (%) | 43 (63.2) | 53 (63.1) | 0.986 |
| Age | 59 (12.8) | 60 (13.4) | 0.641 |
| BMI | 27.3 (23.6–30.9) | 28.1 (25.1–31.7) | 0.152 |
| Smokers (ex/current), n (%) | 30 (44.1) | 46 (54.8) | 0.192 |
| Pack-years | 19 (9–32) | 15 (4–28) | 0.233 |
| Rhinitis, n (%) | 48 (72.7) | 59 (71.1) | 0.825 |
| Uncontrolled asthma, n (%)a | 21 (30.9) | 25 (29.8) | 0.510 |
| Severe asthma, n (%)b | 5 (7.4) | 4 (4.8) | 0.501 |
| Daily ICS in use, n (%) | 63 (92.6) | 62 (73.8) | 0.003 |
| ICS dose of daily users (budesonide eq. µg) | 800 (400–1000) | 800 (400–1000) | 1.000 |
| ICS, n (%) | |||
| At high dose | 23 (39.7) | 18 (25.0) | 0.074 |
| At medium dose | 16 (27.6) | 13 (18.1) | 0.194 |
| Total adherence in ICS medication during 12 years | 82.1 (34.7) | 68.1 (37.3) | 0.025 |
| Daily LABA in use, n (%) | 40 (58.8) | 38 (45.2) | 0.096 |
| Daily SABA in use, n (%) | 9 (13.2) | 12 (14.3) | 0.852 |
| Daily add-on drug in use, n (%) | 43 (63.2) | 42 (50.0) | 0.102 |
| ≥1 oral corticosteroid course for asthma during 12-year follow-up, n (%) | 24 (35.8) | 26 (31.7) | 0.597 |
| Hospitalizations ≥1, n (%) | 17 (25.0) | 22 (26.2) | 0.867 |
| ACO (post-FEV1/FVC < 0.7 and pack-years ≥10), n (%) | 7 (10.4) | 12 (14.3) | 0.480 |
| ACT score | 21 (19–24) | 22 (20–24) | 0.726 |
| Blood eosinophils (×109/l) | 0.15 (0.09–0.27) | 0.16 (0.10–0.29) | 0.429 |
| Blood neutrophils (×109/l) | 3.9 (2.7–4.7) | 3.6 (2.8–4.7) | 0.564 |
| Total IgE (kU/l) | 71 (26–161) | 52 (22–150) | 0.485 |
| FeNO (ppb) | 11 (5–19) | 12 (5–19) | 0.467 |
| Pre-BD FVC (%) | 97.5 (14.7) | 99.6 (14.3) | 0.388 |
| Pre-BD FEV1 (%) | 85.5 (18.0) | 88.8 (16.3) | 0.240 |
| Post-BD FVC (%) | 98.4 (15.0) | 101.2 (14.6) | 0.243 |
| Post-BD FEV1 (%) | 88.5 (17.9) | 92.5 (15.8) | 0.149 |
| Post-BD FEV1/FVC | 0.74 (0.69–0.80) | 0.77 (0.71–0.81) | 0.197 |
| Annual change in lung function from Max0–2.5 to follow-up | |||
| FEV1 (ml/year) | −45.6 (37.2) | −46.0 (29.1) | 0.939 |
| FEV1 %/year | −0.53 (1.09) | −0.44 (0.89) | 0.565 |
| Comorbidities | 1.0 (0–2.0) | 1.0 (0–3.0) | 0.103 |
| In working life, n (%) | 36 (52.9) | 30 (35.7) | 0.033 |
| Time of education ≥12 years, n (%) | 23 (33.8) | 17 (20.2) | 0.059 |
| All asthma-related health care visits during 12-year follow-up | 19 (13–26) | 14 (9–20) | 0.001 |
| Unplanned visits | 3.5 (1–11) | 4.0 (1–10) | 0.945 |
If not otherwise mentioned, data shown are mean (SD) or median (25th–75th) percentiles. Statistically significant P values are presented in bold. Annual change in FEV1 or FVC from point of maximal lung function within 2.5 years after start of therapy to the 12-year follow-up visit.
PHC primary health care, BMI Body Mass Index, ICS inhaled corticosteroid, LABA long-acting β2-agonist, SABA short-acting β2-agonist, Add-on drug long-acting β2-agonist, leukotriene receptor antagonist, theophylline and/or tiotropium in daily use, ACO asthma–COPD overlap, ACT asthma control test, FeNO fraction of NO in exhaled air, BD bronchodilator, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s.
aAssessment of asthma control was performed according to the Global Initiative for Asthma (GINA) 2010 report.
bAssessment of asthma severity was performed according to the ERS/ATS severe asthma guideline 2014.
Lung function tests in planned follow-up contacts
To evaluate whether spirometry or peak flow monitoring were used in the follow-up of asthma as suggested by the guidelines, we collected information from the planned follow-up visits (n = 603). We excluded 67 follow-up contacts related to planned GP telephone contacts only. Thus, out of the total 603 contacts, we included 536 planned PHC follow-up contacts where patient encountered GP, nurse or both. Spirometry, peak flow monitoring or both were performed in 87.5% of these contacts. During the 12-year follow-up, peak flow monitoring was carried out in 51.7% of the contacts and spirometry in 76.1% of the contacts. Incomplete peak flow monitoring was excluded. The annual percentages of performed lung function tests in planned follow-up contacts (n = 536) are shown in Fig. 3. There was no sign of a decrease in performance of lung function testing during the 12-year follow-up.
Fig. 3. Percentage of lung function tests performed in planned follow-up contacts in primary health care.
The data are presented as percentage of all annual planned contacts. Total amount of planned contacts during 12-year follow-up was 536.
Lung function tests in planned follow-up contacts according to the health care professional
To evaluate whether differences exist in the use of lung function tests according to who encounters the patient in the follow-up contact, we divided the total amount of the follow-up contacts (n = 536) into three groups (Fig. 1). Out of all the planned follow-up contacts, 303 were GP contacts, 104 were asthma-nurse contacts and in 83 contacts patient met first nurse and GP thereafter. In 46 contacts, nurse met patient and then consulted GP, and these contacts were included to the last group (total number of combined GP and nurse contacts n = 129).
We found that peak flow monitoring, spirometry or both were done in 98.4% of all planned asthma contacts if patient encountered both nurse and GP. Spirometry was done more often than peak flow monitoring through the whole follow-up period irrespective of who encountered the patient in the planned follow-up contact. Lung function tests were performed more often if patient met both doctor and nurse when compared to encountering either alone (Fig. 4).
Fig. 4. Percentage of lung function tests performed according to the health care professional encountering the patient in primary health care.
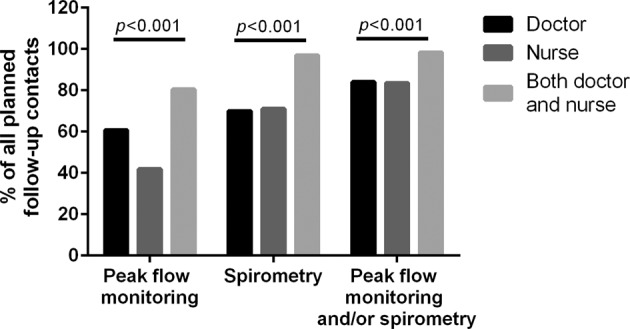
Percentage of performed lung function tests in planned contacts according to professionals during the 12-year follow-up period. Number of contacts with GP was 303, 104 with nurse and 129 with both doctor and nurse.
Discussion
To the best of our knowledge, no previous long-term follow-up studies exist on the occurrence of planned asthma follow-up contacts in PHC and use of lung function tests during the long-term follow-up of asthma. In this 12-year real-life follow-up study, we found that each patient had on average 4 planned asthma contacts in PHC during the follow-up period corresponding to a frequency of 1 visit every third year while the national guideline recommended annual contacts with nurse or GP. Adherence to lung function tests, especially to spirometry, as a part of assessing asthma control was excellent. Spirometry, peak flow monitoring or both were performed in 87.5% of all planned contacts, spirometry in 76.1% and peak flow monitoring in 51.7% of contacts. If both professionals were involved in follow-up visit, lung function tests were done in almost every planned asthma contact. These results suggest that in Finland the frequency of asthma follow-up contacts is insufficient but the PHC adherence to lung function test performance is at high level.
According to Finnish guidelines16,24 and current GINA report, asthma patient should have regular review by health care provider1. In many studies, it has been suggested that adherence to recommended regular follow-up is insufficient5,17,25, and many patients are lost to follow-up26. In these studies, conclusions have mostly been made based on relatively short follow-up or based on asthma-related visits or planned contacts in PHC during the previous year. The recommendation of the Finnish Asthma Programme15,16 was that patients should continue visits with health care professionals yearly even if asthma is controlled. We found that the given recommendation on asthma follow-up contact frequency was not followed even if patients were informed about the importance to continue long-term visits in PHC. In our study, 49 out of 203 patients were excluded because of not having planned follow-ups in PHC and 29 of these patients did not have any follow-up contacts during the 12-year follow-up period. Total of 152 patients participated in planned asthma contacts mostly in PHC but it is possible that some of the patients had also additional asthma contacts in respiratory department or in private health care. The first two follow-up contacts after asthma diagnosis were mainly done in the respiratory department explaining why there were fewer planned contacts in PHC during the first 2 years of the follow-up period. After the first 2 years, the number of planned asthma contacts increased and slightly decreased in the middle of the follow-up period until new increase towards the end of the 12-year follow-up. The patients who had planned follow-up contacts in PHC had on average four contacts, but when using this amount as a threshold value we found that most patients (n = 84) had less than four planned contacts during the 12-year follow-up period showing that most of the asthma patients are not regularly visiting a doctor or nurse. Our finding is supported by the previous Swedish observational cohort study25 where on average every third patient visited primary care doctor because of asthma irrespective of disease severity. Previous Finnish cross-sectional study showed that in 2010 69% of asthma patients reported a scheduled visit to a physician compared to 73% in 200117. Scheduled appointments to nurse reduced similarly from 28% in 2001 to 23% in 2010 while health care services had essentially remained the same17. However, study consisted of patients visiting pharmacies17, indicating that more therapy and planned follow-up adherent patients might have been selected. Recent American study showed that across all age groups 22.2% of the patients had no asthma-related visits to the primary care in the previous year. The visits that were actualized due to asthma were made for evaluation of acute symptoms, but planned asthma care visits were not found5. The previous and our results suggest that nonadherence to follow-up is a worldwide phenomenon. Because remission of adult-onset asthma is rare unlike in childhood asthma9,10,27,28, missing regular follow-up cannot be assumed to be harmless. It can be claimed that even four planned contacts during a 12-year follow-up period is too few.
Planned asthma management with systematic approach in general practice has been shown to improve asthma control29. We were not able to find studies considering occurrence of long-term planned follow-up in PHC and how planned contacts affect asthma control in long-term period. It is logical to assume that patients with more planned asthma contacts are having better asthma control and that regular long-term follow-up improves outcome. In our study, one third of the patients in both groups (<4 or ≥4 planned contacts) had uncontrolled asthma according to GINA 201022, and there was no difference in the proportion of severe asthma according to ERS/ATS 201423 and no differences in asthma control according to asthma control test (ACT) scores or lung function. These findings suggest that the frequency of asthma contacts had no effect on the level of asthma control. The group with four or more planned contacts had also more other asthma-related health care contacts. This result combined with tendency to increased use of high ICS dose and add-on drugs suggests that these patients had more persistent and difficult disease. They also had better adherence to ICS medication. Thus our results suggest that patients with more difficult asthma are more likely to participate regularly in planned follow-up contacts and they also have better adherence to medication. One could also speculate that with more regular follow-up it is possible to treat more persistent asthma to the same level with milder ones because the two contact groups did not have any significant differences in lung function, markers of inflammation or asthma control at the end of the follow-up. To support this, a Danish study showed that systematic approach in planned follow-up contacts increased the level of well-controlled asthma by 20% and reduced uncontrolled asthma by 14%29. Advantages of more frequent contacts were also reported recently with type 2 diabetes patients who had stopped to attend to follow-up in diabetes clinics as prescribed: with more frequent contacts, they succeeded to improve their glycaemic control in primary diabetes health care30. Previous results support the assumption that with regular follow-up it is possible to improve control of a persistent disease. However, we were not able to assess whether medically correct actions were taken in planned asthma follow-up contacts or whether the good adherence to ICS medication was due to more regular follow-up or more difficult asthma or both of them. Previous study of the SAAS cohort showed that cumulative dose of ICS increased during the 12-year follow-up period and prescription discontinuation was rare31. Good adherence to asthma therapy has been suggested to improve the clinical outcomes and to lessen health care costs32.
Access to asthma follow-up visits has shown significant regional variation in Finland depending for example on the municipal service system and resources33. In our study, patients with four or more planned contacts were more often in working life even though the mean age of the two groups was similar. One explanation may be that employees may have had better access to PHC services in Finland, as previously suggested34,35, because of the ability to use both occupational and PHC services. There are probably many patient-related issues affecting adherence to asthma follow-up including attitudes, personal resources, ability and asthma symptoms. Many patients with asthma do not regard themselves as sick and are not concerned about their condition36, and it could also be one reason to miss follow-up in our study. In previous studies, patients lost to follow-up have been younger and have had clinical features of less severe asthma at the time of diagnosis, with similar findings also in studies concerning adherence to asthma medications26,32,37. In our study, age, sex or lung function at baseline was not associated with less frequent follow-up. In the group of less than four planned contacts, almost 74% of the patients reported daily ICS use but the median daily ICS dose was 800 µg indicating that most of the patients using ICS were treated with moderate-to-high doses.
Guidelines recommend that assessment of asthma should include evaluation of symptom control, future risk of adverse outcomes, treatment issues such as inhaler technique and adherence, side effects, smoking and comorbidities1,24. There is no universal common consensus about all aspects and contents of asthma control visits for example for lung function testing. Current GINA report recommends objective lung function measurements as necessary for initial diagnosis of asthma as well as long-term monitoring of asthma1. Previous studies have shown that reliance on patient-reported clinical symptoms38–41 or ACT score can lead to overestimation of asthma control41,42. Inclusion of spirometry in the assessment guarantees more accurate monitoring of asthma control38–41 without input from secondary care43. Objective lung function measurements are not comprehensively used in asthma diagnostics44 and monitoring2,3,45, despite several studies38–41 and guidelines1 supporting their use. A Swedish study showed that one third of the patients with asthma visiting PHC during initial visits and approximately half of the patients during follow-up visits had a clinical evaluation, including spirometry or peak flow monitoring, in agreement with recommendations2. In Germany, 57% of physicians used spirometry as a part of assessing asthma control when proportion was 46% in France, 47% in Australia, 28% in Canada, 54% in China and 24% in Japan3. In contrast, our results show that in Finland spirometry was performed in >76% of all scheduled contacts.
To the best of our knowledge, there are no previous studies investigating the longitudinal lung function follow-up of adult asthma patients in PHC. We found that spirometry, peak flow monitoring or both were performed in almost 88% of all planned follow-up contacts. When both professionals took part in the visit, lung function tests were carried out in almost every planned contact. Utilization of spirometry was higher compared with peak flow monitoring during the entire follow-up. In Finland due to the law of special reimbursement for chronic asthma medication, it has been crucial for decades to confirm asthma diagnosis by objective lung function tests, but continuous follow-up of lung function tests has not been required for the reimbursement. In the PHC, the use of spirometry increased significantly after introduction of both the national programmes of asthma (1994–2004) and chronic obstructive pulmonary disease (COPD; 1997–2007) and the current asthma care guideline (2000–)46. To enhance the implementation of the asthma programme, regional guidance was also available in 79% of the Finnish health care centres in 200120,47. The quality of the Finnish PHC spirometry curves has been found good in 78–80% of cases48. As shown also in the previous study of pre-diagnostic lung function tests in the same area49, the current study of the post-diagnostic use of lung function tests support adherence to the national and regional asthma guidelines.
The Current Finnish Guideline recommends that asthma patient should have an annual planned contact with nurse or GP if asthma control is otherwise good and that the appointment with GP should be at least every third or fifth year24. Based on the evaluation of the results of the Finnish Asthma Programme, it was recommended that the role of asthma nurses should be further strengthened so that educated nurses could perform most of the annual asthma follow-up contacts16. Our study showed that this was not reached while only approximately 17% (n = 104) of all planned contacts were nurses’ and most of the patients had overall less than four planned contacts during the follow-up period. Similarly, in previous studies most of the planned visits of asthma patients were doctor appointments17,25. According to a previous Finnish study, respiratory nurses in PHC tend to lack appropriate time in relation to number of respiratory patients when they also take care of other patients and tasks33. In our study, spirometry, peak flow monitoring or both was performed in almost every planned contact if patient encountered both nurse and GP. This suggests that planned asthma follow-up contact may benefit from the involvement of both professionals50. In a Danish study29, planned asthma management by both nurse and doctor participating with systematic approach improved asthma control. In a previous review, nurse-led care did not have any differences when compared to physician-led management of asthma51, but because the review included only one study with uncontrolled patients and was based on relatively small number of studies that the results cannot be directly applied to primary care practice where patients are often multimorbid and have often uncontrolled disease.
Our study has several strengths. The diagnosis of asthma was made by a respiratory physician and the diagnosis was based on typical symptoms and objective lung function measurements showing reversibility of airway obstruction. Smokers and patients with comorbidities were not excluded. Therefore, this study population well represents a typical PHC population with asthma21. Possible weakness of our study is that our results may not represent entire Finland. There may be regional imbalance for example in the frequency of spirometry or planned follow-up contacts. We were not able to assess what kind of conclusions were made based on the lung function tests and how these conclusions affected on therapy and asthma control. Also, skills of GPs to interpret spirometry were not estimated. We were not able to assess how often spirometry revealed a clinical issue that was not emerged by measuring asthma control with ACT because in Finland ACT was gradually introduced around 2010.
Evidence-based medicine and guidelines have improved the quality of health care, but still suboptimal adherence to care guidelines is a common worldwide problem seen not only with asthma2–5 and chronic obstructive pulmonary disease52–54 but also with other common chronic conditions, such as cardiovascular diseases and diabetes55–59. GPs generally deal with multimorbid patients. It could be argued that asthma may lack appropriate attention and follow-up with patients with multimorbidity, as recently found with COPD54. Based on our results, it is essential to pay more attention to asthma follow-up not only when the frequency of planned contacts is insufficient but also when many patients choose not to participate in follow-up. In the Finnish health care system, arranging the follow-up contact is primarily the patients’ responsibility as most often no recall systems are used in PHC. It is essential to pay more attention to occurrence of planned follow-up contacts during the routine prescribing or dispensing. Adequate resources, including respiratory nurses, in PHC should be guaranteed because it has influence both on management of regular follow-up of asthma and other chronic conditions and on availability of health care services. The role of respiratory nurses should be strengthened so that they could focus more on respiratory patients and their follow-up. It can be argued whether every patient needs an annual asthma follow-up contact if asthma is mild and otherwise in control. In the future, identification of asthma phenotype may enable to determine the optimal follow-up frequency for different patients12. More research is needed to evaluate how other essential factors such as smoking and comorbidities associated with asthma control are managed in follow-up contacts in long-term period.
In conclusion, we showed that PHC adherence to lung function measurements, especially to spirometry, as a part of assessing asthma control is good in Finland. The frequency of asthma follow-up contacts in PHC is insufficient when only every third patient was attending a planned visit each year. We showed that adherence to therapy may be better if patients have more planned contacts. In the future, it is necessary to pay more attention to asthma follow-up and characterize the population who is at a risk to drop out of asthma follow-up.
Methods
Study design, inclusion and exclusion criteria
The present study was a part of SAAS, which is a single-centre (Department of Respiratory Medicine, Seinäjoki Central Hospital, Seinäjoki, Finland) 12-year real-life follow-up study of patients with new-onset asthma diagnosed at adult age (≥15 years). The details of the SAAS study protocol with specific diagnostic criteria has been published separately previously21. This study is registered at www.ClinicalTrials.gov with identifier number NCT02733016.
In the original study, cohort patients (n = 257) were recruited between October 1999 and April 2002 from the diagnostic visit in Seinäjoki Central Hospital respiratory department. Diagnosis of new-onset asthma was made by a respiratory physician based on typical symptoms and was confirmed by objective lung function measurements9,12,21. Smokers and patients with concomitant COPD or other comorbidities were not excluded (Supplementary Table 1). After the diagnosis was confirmed and the medication started, the patients were treated and monitored by their personal physicians mostly in PHC according to the Finnish National Asthma Programme15,16.
After 12 years (mean 12.2, range 10.8–13.9), a total of 203 patients completed a follow-up visit in respiratory department. Asthma status, disease control, comorbidities and medication were evaluated using structured questionnaires (Airways Questionnaire 20 (AQ20) and ATC), and lung function was measured. The participants of the follow-up visit gave written informed consent to the study protocol approved by the Ethics committee of Tampere University Hospital, Tampere, Finland (R12122). In addition to the data gathered at these visits, all data of asthma-related health care contacts during 12-year period was collected from PHC, occupational health care, private clinics and hospitals as previously prescribed9,12,21. The flowchart of the study is shown in Supplementary Fig. 1.
In the present study, all asthma-related health care contacts of the 203 patients during the 12-year follow-up period were explored. Two of the patients were excluded in the beginning because they visited only in private health care. The rest 201 patients had 3616 asthma-related health care contacts. Of those, we included planned PHC (public health care centres and occupational health care) asthma follow-up contacts of 152 patients, the total number of contacts being 603 (Fig. 1). Out of the rest 49 patients, 20 arranged their follow-up in private health care and 29 patients did not have any planned follow-up between the diagnostic visit and the year 2013 follow-up visit in the respiratory department. The data of 152 patients and the data gathered from their planned asthma contacts in PHC were evaluated. During the SAAS study period, all health care centres in our region had respiratory nurses and coordinator–GP responsible for the asthma management in the health care centre, yet every GP managed their own asthma patients.
Lung function, computation of adherence, inflammatory parameters and other clinical measurements
Lung function measurements were performed with a spirometer according to international recommendations60. Only complete 2-week peak flow monitoring was included when evaluating the use of lung function tests. Prescribed medications and dose calculations were carried out based on the data obtained from planned asthma contacts and the dispensed ICS doses were obtained from the Finnish Social Insurance Institution that records all purchased medication from any Finnish pharmacy. Adherence to ICS medication was evaluated by comparing the patient’s dispensed doses to the prescribed doses for the whole 12-year period. Shortly, we converted all prescribed and dispensed ICS doses to budesonide equivalents and based on that information calculated annual and total 12-year adherence for each patient61. FeNO was measured with a portable rapid-response chemiluminescent analyser according to American Thoracic Society standards62 (flow rate 50 mL/s; NIOX System, Aerocrine, Solna, Sweden). Venous blood was collected, and white blood cell differential counts were determined. Total IgE levels were measured by using ImmunoCAP (Thermo Scientific, Uppsala, Sweden). Laboratory assays were performed in an accredited laboratory (SFS-EN ISO/IEC 17025:2005 and ISO 15189:2007) of Seinäjoki Central Hospital. Patients completed AQ2063 and ACT. Assessment of asthma control was performed according to the GINA 2010 report22.
Definition of PHC
In Finland health care services are divided into PHC and specialized medical care. The country is divided into 21 hospital districts, which provide specialist medical care for the population in their area. Finland has approximately 160 health care centres and many of these consist of several branches, especially in cities. In addition, employers have an obligation to provide occupational health care for their employees. The primary aim of occupational health care is to maintain and improve work ability64. For example, an adult working person who has a new-onset asthma diagnosed at specialized medical care may have the ability to use either PHC services or occupational health care. In this study, we considered both planned follow-up contacts in health care centres and in occupational health care as the PHC follow-up contacts.
Statistical analysis
Continuous data are expressed as mean (SD) for variables with normal distribution and, if skewed distribution, shown as median and 25th–75th percentiles. Shapiro–Wilk test was used to assess normality. Two-group comparisons were performed by using Student’s t test for continuous variables with normal distribution, Mann–Whitney test for continuous variables with skewed distribution or Pearson Chi-square test for categorized variables. Statistical analyses were performed using the SPSS software, version 25 (IBM SPSS, Armonk, NY). A P value < 0.05 was regarded as statistically significant. Two-sided P values were used.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Mrs. Aino Sepponen, RN, is gratefully acknowledged for her help through all the stages of this work. This study was supported by The Research Foundation of the Pulmonary Diseases (Helsinki, Finland), Tampere Tuberculosis Foundation (Tampere, Finland), the Finnish Anti-Tuberculosis Association Foundation (Helsinki, Finland), the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (VTR, Tampere, Finland) and the Medical Research Fund of Seinäjoki Central Hospital (Seinäjoki, Finland). None of the sponsors had any involvement in the planning, execution, drafting or write-up of this study.
Author contributions
This study is a part of the Seinäjoki Adult Asthma Study. J.T. analysed and interpreted the data with help of P.I., wrote the manuscript and contributed to the study design. L.E.T. and H.K. designed the study and guided the work. P.I. contributed to the study design, interpretation of the data and provided statistical advice. P.I. also helped with drawing pictures to this article. I.V. contributed to the computation of adherence. O.N. contributed to the laboratory analyses. All authors accept full conduct of the study and critically revised the manuscript and accepted the final version.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information File). According to ethical permission and patient data protection laws of Finland, single patient data cannot be made available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41533-020-0166-2.
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2019 GINA report. https://ginasthma.org/gina-reports/ (2019).
- 2.Weidinger P, Nilsson JL, Lindblad U. Adherence to diagnostic guidelines and quality indicators in asthma and COPD in Swedish primary care. Pharmacoepidemiol. Drug Saf. 2009;18:393–400. doi: 10.1002/pds.1734. [DOI] [PubMed] [Google Scholar]
- 3.Chapman KR, et al. Physician perspectives on the burden and management of asthma in six countries: the Global Asthma Physician Survey (GAPS) BMC Pulm. Med. 2017;17:153. doi: 10.1186/s12890-017-0492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cloutier MM, et al. Clinician agreement, self-efficacy, and adherence with the Guidelines for the Diagnosis and Management of Asthma. J. Allergy Clin. Immunol. Pract. 2018;6:886–894. doi: 10.1016/j.jaip.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yawn BP, Rank MA, Cabana MD, Wollan PC, Juhn YJ. Adherence to asthma guidelines in children, tweens, and adults in primary care settings: a practice-based network assessment. Mayo Clin. Proc. 2016;91:411–421. doi: 10.1016/j.mayocp.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jousilahti P, Haahtela T, Laatikainen T, Mäkelä M, Vartiainen E. Asthma and respiratory allergy prevalence is still increasing among Finnish young adults. Eur. Respir. J. 2016;47:985–987. doi: 10.1183/13993003.01702-2015. [DOI] [PubMed] [Google Scholar]
- 7.Kankaanranta H, Tuomisto LE, Ilmarinen P. Age-specific incidence of new asthma diagnoses in Finland. J. Allergy Clin. Immunol. Pract. 2017;5:189–191. doi: 10.1016/j.jaip.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Sood A, et al. Adult-onset asthma becomes the dominant phenotype among women by age 40 years. The longitudinal CARDIA study. Ann. Am. Thorac. Soc. 2013;10:188–197. doi: 10.1513/AnnalsATS.201212-115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuomisto LE, et al. A 12-year prognosis of adult-onset asthma: Seinäjoki Adult Asthma Study. Respir. Med. 2016;117:223–229. doi: 10.1016/j.rmed.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Rönmark E, Lindberg A, Watson L, Lundbäck B. Outcome and severity of adult onset asthma – report from the obstructive lung disease in northern Sweden studies (OLIN) Respir. Med. 2007;101:2370–2377. doi: 10.1016/j.rmed.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Clatworthy J, Price D, Ryan D, Haughney J, Horne R. The value of self-report assessment of adherence, rhinitis and smoking in relation to asthma control. Prim. Care Respir. J. 2009;18:300–305. doi: 10.4104/pcrj.2009.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilmarinen P, et al. Cluster analysis on longitudinal data of patients with adult-onset asthma. J. Allergy Clin. Immunol. Pract. 2017;5:967–978. doi: 10.1016/j.jaip.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Ilmarinen P, et al. Comorbidities and elevated IL-6 associate with negative outcome in adult onset asthma. Eur. Respir. J. 2016;48:1052–1062. doi: 10.1183/13993003.02198-2015. [DOI] [PubMed] [Google Scholar]
- 14.Pinnock H, et al. Setting the standard for routine asthma consultations: a discussion of the aims, process and outcomes of reviewing people with asthma in primary care. Prim. Care Respir. J. 2010;19:75–83. doi: 10.4104/pcrj.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haahtela T, Laitinen LA. Asthma Programme in Finland 1994-2004. Report of a Working Group. Clin. Exp. Allergy. 1996;26:1–24. doi: 10.1111/j.1365-2222.1996.tb02572.x. [DOI] [PubMed] [Google Scholar]
- 16.Haahtela T, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61:663–670. doi: 10.1136/thx.2005.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauppi P, et al. Reduced severity and improved control of self-reported asthma in Finland during 2001-2010. Asia Pac. Allergy. 2015;5:32–39. doi: 10.5415/apallergy.2015.5.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haahtela T, et al. The Finnish experience to save asthma costs by improving care in 1987-2013. J. Allergy Clin. Immunol. 2017;139:408–414. doi: 10.1016/j.jaci.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Burki TK. Asthma control: learning from Finland’s success. Lancet Respir. Med. 2019;7:207–208. doi: 10.1016/S2213-2600(19)30030-X. [DOI] [PubMed] [Google Scholar]
- 20.Erhola M, et al. The Asthma Programme of Finland: an evaluation survey in primary health care. Int. J. Tuberc. Lung Dis. 2003;7:592–598. [PubMed] [Google Scholar]
- 21.Kankaanranta H, Ilmarinen P, Kankaanranta T, Tuomisto LE. Seinajoki Adult Asthma Study (SAAS): a protocol for a 12-year real-life follow-up study of new-onset asthma diagnosed at adult age and treated in primary and specialised care. NPJ Prim. Care Respir. Med. 2015;25:15042. doi: 10.1038/npjpcrm.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Heart, Lung and Blood Institutes of Health. Global Initiative for Asthma, Global Strategy for Asthma Management and Prevention, 1995 updated 2010. https://ginasthma.org/wp-content/uploads/2019/01/2010-GINA.pdf (2010).
- 23.Chung KF, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 24.Haahtela, T. et al. Current care guidelines. Asthma. https://www.kaypahoito.fi/en/ccs00016 (2013). [PubMed]
- 25.Larsson K, et al. Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR) Respir. Res. 2018;19:12. doi: 10.1186/s12931-018-0719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang M, et al. Lost to follow-up in asthmatics does not mean treatment failure: causes and clinical outcomes of non-adherence to outpatient treatment in adult asthma. Allergy Asthma Immunol. Res. 2013;5:357–364. doi: 10.4168/aair.2013.5.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? Eur. Respir. Rev. 2013;22:44–52. doi: 10.1183/09059180.00007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess JA, et al. Factors influencing asthma remission: a longitudinal study from childhood to middle age. Thorax. 2011;66:508–513. doi: 10.1136/thx.2010.146845. [DOI] [PubMed] [Google Scholar]
- 29.Backer V, Bornemann M, Knudsen D, Ommen H. Scheduled asthma management in general practice generally improve asthma control in those who attend. Respir. Med. 2012;106:635–641. doi: 10.1016/j.rmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Kauppila T, Erikson JG, Honkasalo M, Raina M, Laine MK. Relationship between number of contacts between previous dropouts with type 2 diabetes and healthcare professionals on glycaemic control: a cohort study in public primary health care. Prim. Care Diabetes. 2019;13:468–473. doi: 10.1016/j.pcd.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Vähätalo I, Ilmarinen P, Tuomisto LE, Niemelä O, Kankaanranta H. Inhaled corticosteroids and asthma control in adult-onset asthma: 12-year follow-up study. Respir. Med. 2018;137:70–76. doi: 10.1016/j.rmed.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir. Med. 2013;107:1481–1490. doi: 10.1016/j.rmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Aine T, Puolanne M, Vasankari T. The status of asthma and COPD care in Finland today as perceived by professionals. Finnish Med. J. 2017;15-16:1015–1020. [Google Scholar]
- 34.Tolvanen E, Koskela TH, Mattila KJ, Kosunen E. Analysis of factors associated with waiting times for GP appointments in Finnish health centres: a QUALICOPC study. BMC Res. Notes. 2018;11:220. doi: 10.1186/s13104-018-3316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virtanen P, Kivimäki M, Vahtera J, Koskenvuo M. Employment status and differences in the one-year coverage of physician visits: different needs or unequal access to services? BMC Health Serv. Res. 2006;6:123. doi: 10.1186/1472-6963-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price D, Flecher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim. Care Respir. Med. 2014;24:14009. doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams LK, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J. Allergy Clin. Immunol. 2007;120:1153–1159. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Cowie RL, Underwood MF, Field SK. Asthma symptoms do not predict spirometry. Can. Resp. J. 2007;14:339–342. doi: 10.1155/2007/816132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan A, Stanbrook M. Must family physicians use spirometry in managing asthma patients? Can. Fam. Physician. 2010;56:126–128. [PMC free article] [PubMed] [Google Scholar]
- 40.Jain VV, et al. Impact of an integrated disease management program in reducing exacerbations in patients with severe asthma and COPD. Respir. Med. 2014;108:1794–1800. doi: 10.1016/j.rmed.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Park SY, et al. Factors affecting discrepant correlation between asthma control test score and pulmonary function. Allergy Asthma Immunol. Res. 2015;7:83–87. doi: 10.4168/aair.2015.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munoz-Cano R, et al. Follow-up of patients with uncontrolled asthma: clinical features of asthma patients according to the level of control achieved (the COAS study) Eur. Respir. J. 2017;49:1501885. doi: 10.1183/13993003.01885-2015. [DOI] [PubMed] [Google Scholar]
- 43.Walker PP, Mitchell P, Diamantea F, Warburton CJ, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur. Respir. J. 2006;28:945–952. doi: 10.1183/09031936.06.00019306. [DOI] [PubMed] [Google Scholar]
- 44.Gershon AS, Victor JC, Guan J, Aaron SD, To T. Pulmonary function testing in the diagnosis of asthma. Chest. 2012;141:1190–1196. doi: 10.1378/chest.11-0831. [DOI] [PubMed] [Google Scholar]
- 45.To T, et al. Quality of asthma care under different primary care models in Canada: a population-based study. BMC Fam. Pract. 2015;16:19. doi: 10.1186/s12875-015-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasankari T, Pietinaho A, Lertola K, Junnila SY, Liippo K. Use of spirometry and recording of smoking habits of COPD patients increased in primary health care during national COPD programme. BMC Fam. Pract. 2011;12:97. doi: 10.1186/1471-2296-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somppi, M.-L., Talvikunnas, S.-L., Tuomisto, L., & Leisti, S. Etelä-Pohjanmaan malli, julkaisu 21. Etelä-Pohjanmaan sairaanhoitopiiri (in Finnish) [Regional Asthma Programme of adult asthma patient.] (1997).
- 48.Tuomisto LE, et al. Asthma Programme in Finland: the quality of primary care spirometry is good. Prim. Care Respir. J. 2008;17:226–231. doi: 10.3132/pcrj.2008.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuomisto LE, Kaila M, Erhola M. Asthma programme in Finland: comparison of adult asthma referral letters in 1994 and 2001. Respir. Med. 2007;101:595–600. doi: 10.1016/j.rmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Lisspers K, Ställberg B, Hasselgren M, Johansson G, Svärdsudd K. Primary health care centres with asthma clinics: effects on patients’ knowledge and asthma control. Prim. Care Respir. J. 2010;19:37–44. doi: 10.4104/pcrj.2009.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuethe MC, Vaessen-Verberne AAPH, Elbers RG, Van Aalderen WMC. Nurse versus physician-led care for the management of asthma (Review) Cochrane Database Syst. Rev. 2013;28:CD009296. doi: 10.1002/14651858.CD009296.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Overington JD, et al. Implementing clinical guidelines for chronic obstructive pulmonary disease: barriers and solutions. J. Thorac. Dis. 2014;6:1586–1596. doi: 10.3978/j.issn.2072-1439.2014.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrara R, et al. Improvement in the management of chronic obstructive pulmonary disease following a clinical educational program: results from a prospective cohort study in the Sicilian general practice setting. NPJ Prim. Care Respir. Med. 2018;23:10. doi: 10.1038/s41533-018-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandelowsky H, et al. COPD management by Swedish general practitioners – baseline results of the PRIMAIR study. Scand. J. Prim. Health Care. 2018;1:5–13. doi: 10.1080/02813432.2018.1426148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill MN, Miller NH, DeGeest S. AHS Posion paper: Adherence and persistence with taking medication to control high blood pressure. J. Clin. Hypertens. 2010;12:757–764. doi: 10.1111/j.1751-7176.2010.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gyberg V, et al. Patients with coronary artery disease and diabetes need improved management: a report from the EUROASPIRE IV survey: a registry from the EuroObservational Research Programme of the European Society of Cardiology. Cardiovasc. Diabetol. 2015;14:133. doi: 10.1186/s12933-015-0296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eder S, et al. Guidelines and clinical practice at the primary level of healthcare in patients with type 2 diabetes mellitus with and without kidney disease in five European countries. Diabetes Vasc. Dis. Res. 2019;16:47–56. doi: 10.1177/1479164118795559. [DOI] [PubMed] [Google Scholar]
- 58.Giezeman M, Arne M, Theander K. Adherence to guidelines in patients with chronic heart failure in primary health care. Scand. J. Prim. Health Care. 2017;35:336–343. doi: 10.1080/02813432.2017.1397253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geary L, Hasselström J, Carlsson AC, Eriksson I, von Euler M. Secondary prevention after stroke/transient ischemic attack: a randomized audit and feedback trial. Acta Neurol. Scand. 2019;140:107–115. doi: 10.1111/ane.13109. [DOI] [PubMed] [Google Scholar]
- 60.Miller MR, et al. Standardisation of spirometry. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 61.Vähätalo I, et al. 12-year adherence to inhaled corticosteroids in adult-onset asthma. Eur. Respir. J. 2019;54:PA4186. doi: 10.1183/23120541.00324-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.American Thoracic SocietyEuropean Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 63.Barley EA, Quirk FH, Jones PW. Asthma health status measurement in clinical practice: validity of a new short and simple instrument. Respir. Med. 1998;92:1207–1214. doi: 10.1016/S0954-6111(98)90423-1. [DOI] [PubMed] [Google Scholar]
- 64.Ministry of Social Affairs and Health. Health care in Finland. http://urn.fi/URN:ISBN:978-952-00-3395-8 (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information File). According to ethical permission and patient data protection laws of Finland, single patient data cannot be made available.