Abstract
Background
A Mediterranean-style eating pattern is consistently associated with a decreased diabetes risk in Mediterranean and European populations. However, results in U.S. populations are inconsistent. The objective of this study was to assess whether a Mediterranean-style eating pattern would be associated with diabetes risk in a large, nationally representative U.S. cohort of black and white men and women.
Methods
Participants from the Atherosclerosis Risk in Communities study prospective cohort without diabetes, cardiovascular disease, or cancer at baseline (visit 1, 1987–1989; n = 11,991) were included (mean age 54 years, 56% female, 75% white). Alternate Mediterranean Diet scores (aMed) were calculated using the mean dietary intake self-reported at visit 1 and visit 3 (1993–1995) or visit 1 only for participants censored before visit 3. Participants were followed from visit 1 through 31 December 2016 for incident diabetes. We used Cox regression models to characterize associations of aMed (quintiles as well as per 1-point higher) with incident diabetes adjusted for energy intake, age, sex, race and study center, and education (Model 1) for all participants then stratified by race and body mass index (BMI). Model 2 included potential mediating behavioral and clinical measures associated with diabetes. Results are presented as hazard ratios and 95% confidence intervals.
Results
Over a median follow-up of 22 years, there were 4024 incident cases of diabetes. Higher aMed scores were associated with lower diabetes risk [Model 1: 0.83 (0.73–0.94) for Q5 vs Q1 (p-trend < 0.001) and 0.96 (0.95–0.98) for 1-point higher]. Associations were stronger for black vs white participants (interaction p < 0.001) and weaker for obese vs normal BMI (interaction p < 0.01). Associations were attenuated but statistically significant in Model 2.
Conclusions
An eating pattern high in fruits, vegetables, whole grains, legumes, nuts, and fish, and moderate in alcohol was associated with a lower risk of diabetes in a community-based U.S. population.
Subject terms: Epidemiology, Risk factors
Introduction
A Mediterranean-style eating pattern (Mediterranean pattern) is one of the eating patterns recommended by the American Heart Association, American College of Cardiology, and the Dietary Guidelines for Americans to reduce chronic disease risk1,2. While there are cultural variations in the foods and beverages included in a Mediterranean pattern, overall it is largely plant-based, relatively high in olive oil and seafood, but low in dairy, red meat, and refined grains1,3,4. Observational and experimental studies suggest that higher adherence to a Mediterranean pattern is associated with improved cardiovascular disease risk factors5–7, reduced risk of cardiovascular events8,9, and reduced cardiovascular-related mortality9,10. The American Diabetes Association recommends adherence to a Mediterranean pattern, as one of several potential strategies, to prevent cardiovascular complications in individuals with diabetes. However, evidence regarding how Mediterranean pattern adherence can improve diabetes risk for U.S. populations is inconsistent11.
It is unclear if a Mediterranean pattern, independent of a Mediterranean lifestyle, can reduce diabetes risk in US adult populations11. Mediterranean and European populations tend to be less racially and ethnically heterogeneous, have lower body mass indexes (BMI), be less sedentary, have larger social networks, and place a greater emphasis on rest and sleep compared to the general U.S. population12,13. In Mediterranean and other European settings, adherence to a Mediterranean pattern is consistently associated with a reduced risk of diabetes14–16. Yet, research about Mediterranean pattern adherence and diabetes risk in U.S. populations is limited and inconsistent11. Research is needed to assess whether potential benefits of a Mediterranean pattern are translatable to heterogeneous populations which place less emphasis on ideologies and behaviors of Mediterranean-like cultures. Further, benefits may vary by race and BMI. Being of a minority race and having a BMI > 25 kg/m2 are among the top recognized risk factors for diabetes in the U.S. and eating habits differ within these groups17.
The purpose of this study was to assess associations between Mediterranean pattern scores and incident diabetes in a U.S. community-based population of adults. We also explored consistency of associations within racial and BMI subgroups17.
Methods
Study population
We used data collected from participants in the Atherosclerosis Risk in Communities (ARIC) study. The primary aim of the ARIC study was to investigate atherosclerosis etiology and clinical sequelae as well as to assess how cardiovascular disease risk factors differ by race, sex, place, and time18. Investigators originally recruited middle-aged adults (45–65 years, n = 15,792) from four U.S. communities (Washington County, Maryland; suburban Minneapolis, Minnesota; Jackson, Mississippi; and Forsyth County, North Carolina) and performed baseline assessments in 1987–89 (visit 1). Participants returned for follow-up visits in 1990–92 (visit 2), 1993–95 (visit 3), 1996–98 (visit 4), 2011–13 (visit 5), 2016–17 (visit 6), and 2018–18 (visit 7). Each participating institution received ethical approval from an institutional review board and all participants provided informed consent at each visit. This study has been registered with clinicaltrials.gov (NCT00005131).
The total sample size for this analysis was 11,991 participants (Supplementary Fig. S1) at visit 1. Participants were excluded for the following reasons: (1) if they had prevalent or history of cardiovascular disease, diabetes, or cancer (n = 3318); (2) implausible energy intake (<600 or >4200, and <500 or >3600 kcal for males and females, respectively) derived from food frequency questionnaire (FFQ) responses or ≥10 missing FFQ items (n = 270); (3) participants identified as Asian (n = 28) or Indian (n = 14) due to small sample size; (4) participants identified as black from Maryland (n = 25) and Minnesota (n = 19) due to inability to decipher the influence of geographic region versus race with small samples19; (5) missing baseline covariates (n = 20); (6) missing follow-up time; and (7) missing food items needed to calculate the Mediterranean pattern score (n = 63).
Dietary intake assessment
A trained interviewer administered a 66-item semi-quantitative FFQ and alcohol consumption-related questions to participants at visits 1 and 3. The ARIC FFQ was adapted from a previously validated FFQ20 to ascertain more details pertaining to fish intake, cooking fats, and baked goods19. Participants reported average food intake frequency of a pre-specified portion size of various food items during the previous year. Nutrient estimates of assessed food and beverage items were obtained from linking to the United States Department of Agriculture’s Food Composition Databases21 at Harvard Medical School’s Channing Laboratory20. Repeated measurement of dietary and alcohol intakes from visit 1 and visit 3 were averaged for all analyses to increase precision. Visit 1 intake data were used if participants developed diabetes or were censored before visit 3 or if they did not attend visit 3.
Mediterranean-style eating pattern scoring system
Alternate Mediterranean Diet (aMed) scores, previously developed to assess Mediterranean pattern adherence in U.S. populations22, were calculated using the FFQ data. The aMed scoring system assigns 1 point for self-reported consumption above the cohort’s sex-specific median for intakes of total vegetables, total fruit, whole grains, nuts, legumes, fish, and ratio of monounsaturated fatty acids to saturated fatty acids (MUFA:SFA), 1 point for self-reported consumption below the cohort’s sex-specific median intake for red and processed meat, and 1 point for self-reported alcohol intake between 5–15 g/day for females and 10–25 g/day for males. Scores range from 0 to 9 points; higher scores presume higher adherence to a Mediterranean pattern. Designation of ARIC FFQ items for the aMed scoring system is provided in Supplementary Table S1.
Incident diabetes ascertainment
Incident diabetes cases were identified according to the following criteria: (1) self-reported physician diagnosis, (2) self-reported use of diabetes-related medication during previous 2 weeks, (3) measured fasting blood glucose concentration ≥126 mg/dL, or (4) measured non-fasting blood glucose concentration ≥200 mg/dL. Self-reported physician diagnosis or self-reported diabetes medication usage was ascertained at study visits and via annual follow-up phone calls between study visits and after visit 4. This definition of incident diabetes has high specificity23. Participants who did not develop diabetes were censored for death, loss to follow-up, or administratively censored on 31 December 2016.
Covariates
Age, race, education level (less than high school, high school or GED/vocational equivalent, and more than high school), smoking (current, former, never), and family history of diabetes (including both maternal and paternal) were ascertained via questionnaires at visit 1. A race-center variable was derived to represent whites and blacks at each study center. Physical activity was measured via the Baecke questionnaire, which converts self-reported physical activity from sports, leisure, and work into a 1–5 point score24,25. Height and weight were measured via standard protocols and BMI was calculated as body weight in kilograms divided by height in meters squared. Seated blood pressure was measured in triplicates separated by resting for 5 minutes with a random-zero sphygmomanometer. The mean of the second and third measurements was used in the analysis. Hypertension status was determined using the 2003 National Heart, Lung, and Blood Institute guidelines26, defined as systolic blood pressure ≥140, diastolic blood pressure ≥90 mm Hg, or current use of anti-hypertensive medication. All biomarkers measurement methods have been previously described and all ARIC protocols are available at https://sites.cscc.unc.edu/aric/cohort-manuals.
Statistical methods
We compared visit 1 participant characteristics and eating patterns according to aMed quintiles. We used Cox proportional hazards regression models to estimate hazard ratios (HR) and corresponding 95% confidence intervals (CI) for associations between aMed scores (according to quintiles and per 1-point higher) and incident diabetes. We tested for linear trends across quintiles by modeling quintiles as an ordinal variable. We tested for linear splines (a knot at 2 points was significant) to visually depict the shape of the relationship between aMed scores and risk of incident diabetes across the full range of aMed scores. In addition to analyzing overall aMed scores, associations between individual aMed components and incident diabetes were assessed. Follow-up time in days from study visit 1 was used as the time metric. The proportional hazard assumption was assessed via log-log plots and Schoenfeld’s residual tests. Stata version 15 statistical software was used for all analyses (StataCorp, College Station, Texas, USA).
Three covariate structures were used in the Cox regression analyses. Model 1 was adjusted for energy intake and demographic variables of age, sex, race-center, and education level. Model 2 included all variables in Model 1 plus behavioral variables associated with risk of developing diabetes, including smoking status and physical activity. Model 3 included all variables in Model 2 plus potential clinical mediators of diabetes including fasting glucose (mg/dL), hypertension status (yes/no), low-density lipoprotein cholesterol (mg/dL), BMI category (normal, overweight, or obese), and family history of diabetes (yes/no) to test for a potential direct association between aMed scores and incident diabetes.
Race27 and baseline BMI28 were chosen a priori as potential effect modifiers. Sex and education level effect modifiers were assessed post hoc. Likelihood ratio tests were used to assess interaction by categorical BMI (excluding underweight participants, n = 117), race (with adjustment for center rather than race-center), educational level, and sex on associations between continuous aMed scores and incident diabetes as well as quintiles of aMed and incident diabetes. If the p-value for interactions were significant (p < 0.05) using Model 1, we then conducted stratified analyses with Models 1, 2, and 3.
Results
Participants in higher aMed score quintiles were qualitatively more likely to have a higher education, have a higher physical activity level, were less likely to be current smokers and less likely to be obese compared to participants in lower aMed score quintiles (Table 1). Age, sex, race, fasting glucose, hypertension status, and LDL cholesterol were similar across quintiles.
Table 1.
Baseline characteristics according to Alternate Mediterranean Diet (aMed) score quintiles for participants in the Atherosclerosis Risk in Communities study.
| Quintiles of aMed score: aMed score range (n participants) | |||||
|---|---|---|---|---|---|
| Baseline characteristic | Quintile 1: 0–2 (n = 2,340) | Quintile 2: 3–4 (n = 4,573) | Quintile 3: 5 (n = 2,152) | Quintile 4: 6 (n = 1,589) | Quintile 5: 7–9 (n = 1,247) |
| Age (years) | 53 ± 5.6 | 54 ± 5.7 | 54 ± 5.6 | 54 ± 5.8 | 54 ± 5.6 |
| Female | 1,365 (56%) | 2,558 (56%) | 1,226 (57%) | 928 (58%) | 678 (54%) |
| White | 1,872 (77%) | 3,407 (75%) | 1,566 (73%) | 1,206 (76%) | 955 (76%) |
| Education | |||||
| Less than high school | 637 (26%) | 1,094 (24%) | 417 (19%) | 236 (15%) | 163 (13%) |
| High school or equivalent | 1,079 (43%) | 1,888 (41%) | 896 (42%) | 619 (39%) | 468 (38%) |
| More than high school | 714 (29%) | 1,591 (35%) | 839 (39%) | 734 (46%) | 616 (49%) |
| Smoking status | |||||
| Current | 774 (32%) | 1,294 (28%) | 495 (23%) | 299 (19%) | 237 (19%) |
| Former | 700 (29%) | 1,372 (30%) | 700 (32%) | 562 (36%) | 446 (36%) |
| Never | 955 (39%) | 1,906 (42%) | 957 (45%) | 724 (46%) | 564 (45%) |
| Body mass index (kg/m2) | 27.5 ± 5.4 | 27.3 ± 5.1 | 27.6 ± 5.2 | 27.2 ± 5.0 | 26.7 ± 4.7 |
| BMI categories | |||||
| Normal (18.5 to <25) | 839 (35%) | 1,563 (34%) | 703 (33%) | 560 (35%) | 482 (39%) |
| Overweight (25 to <30) | 940 (37%) | 1,829 (40%) | 875 (41%) | 655 (41%) | 491 (39%) |
| Obese (≥30) | 632 (26%) | 1,126 (25%) | 558 (26%) | 359 (23%) | 262 (21%) |
| Fasting glucose (mmol/L) | 5.5 ± 0.55 | 5.5 ± 0.52 | 5.5 ± 0.53 | 5.4 ± 0.52 | 5.4 ± 0.50 |
| Hypertensivea | 690 (29%) | 1,413 (31%) | 706 (33%) | 479 (30%) | 349 (28%) |
| LDL-cholesterol (mmol/L) | 3.5 ± 0.99 | 3.5 ± 1.01 | 3.6 ± 1.04 | 3.5 ± 1.02 | 3.5 ± 0.99 |
| Physical activity scoreb | 2.3 ± 0.74 | 2.4 ± 0.77 | 2.5 ± 0.79 | 2.6 ± 0.81 | 2.7 ± 0.85 |
Results are presented as mean ± standard deviation for continuous variables and n (%) for categorical variables. Column totals may not add up to 100% due to rounding.
aHypertension status determined if systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-reported anti-hypertension medication usage26.
bPhysical activity score (1-lowest to 5-highest) calculated based on intensity and time of leisure sport and exercise24,25.
Participants in higher quintiles of aMed scores had qualitatively higher intakes of total energy, percent of energy from carbohydrates, and percent of energy from protein compared to participants in lower aMed score quintiles (Table 2). Percent of energy from total and saturated fat was lower for higher quintiles of aMed scores, while percent of energy from monounsaturated and polyunsaturated fats were similar across quintiles. Fiber, sodium, potassium, and magnesium were higher for higher quintiles and cholesterol was lower at higher quintiles. The aMed components of fruits, vegetables, nuts, whole grains, legumes, and fish were higher at higher quintiles of aMed scores but MUFA:SFA and red and processed meats were similar. The number of alcoholic drinks consumed per week was higher at higher quantiles.
Table 2.
Dietary intake according to Alternate Mediterranean Diet (aMed) score quintiles in the Atherosclerosis Risk in Communities study.
| Quintile of aMed Score: aMed score range (n participants) | |||||
|---|---|---|---|---|---|
| Nutrient | Quintile 1: 0–2 (n = 2,430) | Quintile 2: 3–4 (n = 4,573) | Quintile 3: 5 (n = 2,152) | Quintile 4: 6 (n = 1,589) | Quintile 5: 7–9 (n = 1,247) |
| Energy intake (kcal/day) | 1,432 ± 520.0 | 1,566 ± 537.5 | 1,709 ± 553.0 | 1,803 ± 564.7 | 1,877 ± 521.8 |
| Macronutrients | |||||
| %Ea carbohydrate | 47 ± 9.0 | 49 ± 8.7 | 50 ± 7.9 | 51 ± 7.8 | 52 ± 7.1 |
| %E protein | 17 ± 3.8 | 18 ± 3.7 | 18 ± 3.7 | 19 ± 3.5 | 19 ± 3.3 |
| %E fat | 35 ± 6.3 | 33 ± 6.0 | 32 ± 5.8 | 31 ± 5.7 | 30 ± 5.4 |
| %E saturated fat | 13 ± 2.7 | 12 ± 2.5 | 11 ± 2.3 | 11 ± 2.3 | 10 ± 2.1 |
| %E monounsaturated fat | 13 ± 2.7 | 13 ± 2.7 | 12 ± 2.6 | 12 ± 2.6 | 12 ± 2.5 |
| %E polyunsaturated fat | 5 ± 1.2 | 5 ± 1.2 | 5 ± 1.2 | 5 ± 1.1 | 5 ± 1.1 |
| Fiber (g/1000 kcal) | 8 ± 2.7 | 10 ± 3.2 | 12 ± 3.4 | 13 ± 3.4 | 14 ± 3.7 |
| Cholesterol (mg/1000 kcal) | 163 ± 55.8 | 154 ± 52.4 | 151 ± 49.3 | 146 ± 44.0 | 138 ± 42.78 |
| Micronutrients | |||||
| Sodium (mg/1000 kcal) | 877 ± 185.2 | 905 ± 182.7 | 929 ± 171.5 | 951 ± 166.5 | 963 ± 162.4 |
| Potassium (mg/1000 kcal) | 1,510 ± 378.8 | 1,630 ± 378.4 | 1,724 ± 364.1 | 1,774 ± 336.0 | 1,815 ± 317.3 |
| Magnesium (mg/1000 kcal) | 142 ± 35.1 | 155 ± 34.6 | 166 ± 34.3 | 172 ± 32.6 | 180 ± 33.1 |
| Calcium (mg/1000 kcal) | 398 ± 177.1 | 401 ± 167.7 | 411 ± 151.1 | 418 ± 145.3 | 410 ± 135.4 |
| aMed score components | |||||
| Vegetables (cups/day) | 1.3 ± 0.81 | 2.1 ± 1.34 | 3.0 ± 1.76 | 3.6 ± 2.04 | 4.0 ± 2.49 |
| Fruits (servings/day) | 1.2 ± 0.88 | 1.8 ± 1.26 | 2.4 ± 1.45 | 2.8 ± 1.65 | 3.1 ± 1.38 |
| Nuts (ounces/week) | 1.1 ± 1.80 | 2.1 ± 2.89 | 2.9 ± 3.36 | 3.4 ± 3.40 | 4.4 ± 4.10 |
| Whole grains (servings/day) | 0.5 ± 0.55 | 0.8 ± 0.82 | 1.1 ± 0.92 | 1.3 ± 0.92 | 1.6 ± 0.94 |
| Legumes (cups/day) | 0.7 ± 0.50 | 1.0 ± 0.75 | 1.3 ± 0.88 | 1.5 ± 1.07 | 1.8 ± 1.12 |
| Fish (servings/week) | 1.0 ± 0.92 | 1.7 ± 1.48 | 2.5 ± 2.21 | 3.1 ± 2.92 | 3.7 ± 2.44 |
| MUFA:SFAb | 1.0 ± 0.31 | 1.1 ± 0.15 | 1.1 ± 0.15 | 1.1 ± 0.15 | 1.2 ± 0.15 |
| Red and processed meat (servings/day) | 1.1 ± 0.70 | 1.1 ± 0.69 | 1.1 ± 0.78 | 1.0 ± 0.85 | 0.9 ± 0.72 |
| Alcohol (g/day)c | 0 (0–4) | 0 (0–6) | 0 (0–6) | 0 (0–7) | 0 (0–10) |
| Drinks per weekd | 2.5 (0–7.5) | 2.5 (0–7.0) | 2.5 (0–7.0) | 3.0 (0.5–7.0) | 4.0 (1.0–8.0) |
Results are presented as mean ± standard deviation, unless noted otherwise. Dietary intakes are self-reported means of visit 1 and visit 3 via self-reported via a food frequency questionnaire. Visit 1 dietary intake was used if incident diabetes or censoring occurred before visit 3. A detailed description of portion sizes is available in Supplementary Table S1.
a%E; percent of total energy.
bMonounsaturated to saturated fat ratio.
cMedian (25th percentile–75th percentile) reported for alcohol in units of g/day. A standard drink contains about 14 g of pure alcohol.
dMedian and (25th percentile–75th percentile) reported for servings (drinks of alcohol) per week.
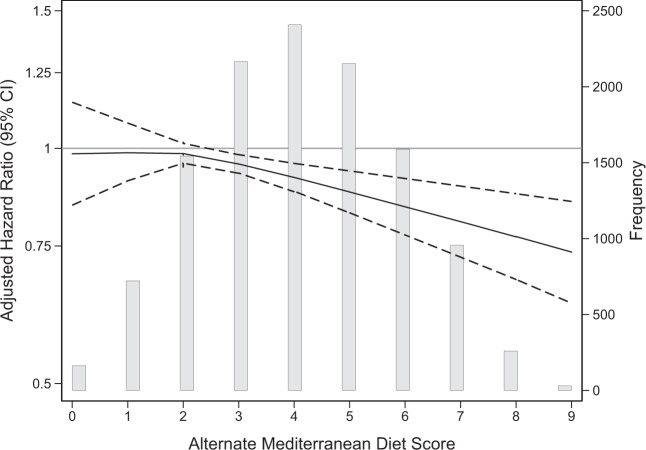
During a median of follow-up of 22 years, there were 4,024 incident cases of diabetes among the 11,991 participants. The overall incidence rate during follow-up was 1.7 diabetes cases per 100 person-years. Higher quintiles of aMed scores were associated with lower incident diabetes risk (trend p < 0.001) after controlling for energy intake and socio-demographic factors [Model 1 HR (95% CI) for quintile 5 vs 1: 0.83 (0.73–0.94); Fig. 1; Supplementary Table S2). The HR for quintile 5 vs. 1 was attenuated after adjusting for physical activity and smoking [Model 2 HR (95% CI): 0.88 (0.77–0.99)]. After further adjusting for clinical measures, there was no direct association between aMed score quintiles and diabetes [Model 3 HR (95% CI): 0.94 (0.82–1.07)]. Trends across quintiles remained statistically significant for Models 2 and 3 (p = 0.005 and p = 0.03, respectively; Supplementary Table S2). A 1-point higher aMed score was associated with a lower risk of incident diabetes after adjusting for energy intake and socio-demographic factors [Model 1 HR (95% CI): 0.96 (0.95–0.98); Fig. 1], behavioral risk factors [Model 2 HR (95% CI): 0.97 (0.96–0.99], and clinical measures [Model 3 HR (95% CI): 0.98 (0.96–0.99); Supplementary Table S2]. There was a linear inverse relationship between aMed scores and incident diabetes risk for aMed scores at and above a score of 2-points (p < 0.001; Fig. 2).
Fig. 1. Adjusted hazard ratios for Alternate Mediterranean Diet (aMed) scores and incident diabetes for the overall population and according to race and BMI categories in the Atherosclerosis Risk in Communities study.
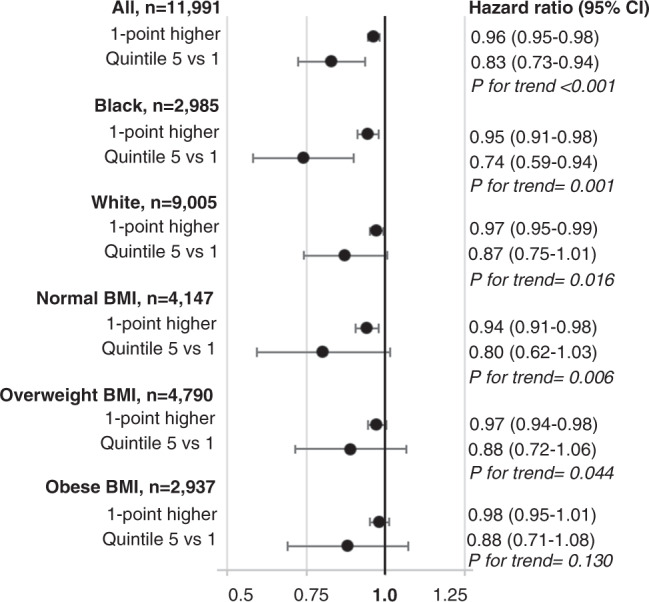
Results are represented as hazard ratios and 95% confidence intervals from Cox regression models adjusted for total energy intake, age, sex, race and study center (center only for the race-specific analyses), and education level. The first point estimate within each subgroup represents the risk of incident diabetes per 1-point higher in aMed scores. The second point estimate within each subgroup represents the risk of incident diabetes for those in the fifth quintile vs the first quintile (reference group). P-values for trend were calculated from Wald tests modeling aMed quintiles as an ordinal variable.
Fig. 2. Alternate Mediterranean Diet (aMed) score distribution and adjusted hazard ratios for incident diabetes in the Atherosclerosis Risk in Communities study.
The solid line indicates the hazard ratio for incident diabetes estimated via a Cox regression model with Alternate Mediterranean Diet scores modeled continuously as a linear spline with a knot at a score of 2 points, adjusted for total energy intake, age, sex, race and study center, and education level. The dashed lines indicate the 95% confidence interval. The grey bars represent the number of participants with each alternate Mediterranean diet score.
There were stronger inverse associations between aMed scores and diabetes risk for black compared to white participants (Fig. 2; Supplementary Table S3; p value for interaction <0.001). Associations were significant for participants who had a normal BMI, attenuated for participants who were overweight, and not significant for participants who were obese (Fig. 1; Supplementary Table S4; interaction p value < 0.01). The relationship between aMed scores and incident diabetes did not differ by sex or education level (interaction p values > 0.05).
Higher component scores for nuts [Model 1 HR (95% CI): 0.92 (0.86–0.98] and legumes [Model 1 HR (95% CI): 0.92 (0.86–0.98)], representing intake above sex-specific medians, and higher component scores for alcohol [Model 1 HR (95% CI): 0.83 (0.75–0.91)], representing moderate intake, were associated with a lower risk of incident diabetes after adjusting for total energy intake and socio-demographic factors. Higher component scores for red and processed meat (Model 1 HR (95% CI): 0.91 (0.84–0.97)], indicating intake below the sex-specific median, was also associated with lower risk of incident diabetes. Component scores for whole grains, vegetables, fruits, MUFA:SFA, and fish were not independently associated with diabetes risk. Results were similar for Model 2 but only alcohol remained significant in Model 3 [HR (95% CI): 0.81 (0.74–0.90)]. Results were similar when all aMed components were included in the models.
Discussion
Higher Mediterranean-style eating pattern scores were inversely associated with risk of incident diabetes in this prospective cohort study of 11,991 middle-aged U.S. adults followed for a median of 22 years. The results were attenuated but persisted after additional adjustment for health behaviors and clinical factors related to diabetes risk. Associations between aMed scores and incident diabetes were stronger among black compared to white participants, were stronger for normal weight compared to overweight or obese participants, and were not different for males and females. Higher nut and legume intake, moderate alcohol intake, and lower red and processed meat intake were the main drivers of the noted associations.
Associations between Mediterranean pattern scores and diabetes risk in U.S. cohorts are inconsistent. We found that Mediterranean pattern scores are associated with a lower risk of diabetes in a U.S. population of blacks and whites by up to 17%. Diabetes risk reductions (up to 25%) associated with Mediterranean pattern scores were previously noted in a cohort of mostly white high socioeconomic U.S. men (Health Professionals Follow-up Study; n = 41,615)29. In a smaller but more ethnically diverse U.S. cohort, the Multi-Ethnic Study of Atherosclerosis (n = 5,290), there was no association between Mediterranean pattern scores and diabetes risk in the overall population or within racial subgroups30. The larger sample size of the ARIC study allowed for more precise white vs black comparisons than these prior studies. We found stronger associations between Mediterranean pattern adherence and incident diabetes for black compared to white participants. This is contrary to previous research which showed inverse associations between a Dietary Approaches to Stop Hypertension-style eating pattern and diabetes risk only for white individuals in a cohort that was comprised largely of black and Hispanic individuals31. There is a need for follow-up research regarding the finding that black individuals may particularly benefit from adherence to a Mediterranean pattern because of the high risk of diabetes in this racial subgroup27.
Unlike data from U.S. cohorts, associations between higher Mediterranean pattern scores and reduced diabetes risk are consistent from Mediterranean/European cohorts14–16. One hypothesis is that this may be due to cultural practices of a Mediterranean lifestyle. Lifestyle and food choices have changed in the Mediterranean region since Ancel Key’s discovery of the cardioprotective Mediterranean pattern12. Modernized farming practices and industrialization of the food supply have led to increased BMI and higher cardiovascular disease risk in the same geographic regions that Keys first mapped out32. However, daily physical activity, close social networks, shared family meals, adequate rest, and abundant social exchanges of a Mediterranean lifestyle still persist as protective disease factors into the twenty-first century13. Aside from physical activity, these factors were not measured in ARIC so it is difficult to determine whether these behaviors would modify associations between Mediterranean pattern adherence and chronic disease risk in our population.
Obtaining and maintaining a healthy body weight is recognized as the most influential modifiable risk factor for type 2 diabetes prevention17. We found that associations between aMed score and incident diabetes was stronger among participants with a normal BMI at baseline. The results of this analysis suggest that the detrimental health implications of being overweight or obese override potential health benefits of a Mediterranean pattern. This theme is previously noted in the ARIC study33,34, as well as other U.S. populations27, for associations between healthy eating patterns and various chronic disease outcomes. Additionally, previous controlled feeding trials in individuals without diabetes showed little improvement in fasting markers of glycemic control when adopting a weight-maintenance Mediterranean pattern7 or other heart healthy eating pattern33–37 in the absence of body weight reductions >2 kilograms. However, markers of glycemic control can improve regardless of eating pattern composition in trials that prescribe intentional weight loss for participants38,39. Therefore, our results align with those of randomized controlled trials which suggest that adopting a healthy eating pattern in the absence of weight loss may not meaningfully reduce diabetes risk or associated risk factors in overweight or obese populations. While adherence to a healthy eating pattern high in fruits, vegetables, and whole grains could potentially result in lower diabetes risk among those with normal BMI, discussion of calorie restriction to achieve and maintain a healthy body weight should remain at the forefront of diabetes prevention.
The ARIC FFQ was not a priori designed to assess adherence to a Mediterranean pattern. The FFQ contains no questions about olive oil intake, which is the main staple of a Mediterranean pattern. Further, limited variation in fruit, vegetable, and whole-grain eating habits of U.S. populations, as well as the lack of adequate whole-grain related FFQ questions, may not be sufficient to correctly identify independent associations with diabetes. Due to the aMed rank-based scoring system used to assess Mediterranean pattern adherence, no quintile met commonly recommended food group intake thresholds of a more traditional Mediterranean patterns for nuts, fats, vegetables, or whole grains1,3. Further, a traditional Mediterranean pattern is commonly recognized to be high in total and/or monounsaturated fat (up to 40% and 20% of total energy, respectively)3,4. The higher quintiles in this study reported lower total fat and saturated fat intakes than the lower quintiles with limited variability in mono- and polyunsaturated fat across quintiles. To note, about half of monounsaturated fatty acids consumed by U.S. populations come from red meat, not olive oil. The associations noted between aMed scores and diabetes risk in this study are not applicable to a traditional high total and monounsaturated fat (from olive oil) Mediterranean pattern. Switching to a high-fat eating pattern of any kind could potentially cause weight-gain and increase diabetes risk because all fat types are energy dense40.
Our study can address previously noted gaps in the literature about eating patterns and chronic disease risk. The observed inverse associations between Mediterranean pattern adherence and incident diabetes may be more precise and generalizable to the U.S. population than previous studies because the ARIC study is a large cohort of both men and women, and blacks and whites, representing four different U.S. regions. We also used a highly sensitive measure of incident diabetes (doctor diagnosis, medication usage, and blood glucose concentrations), although largely self-reported23. Although type 1 vs type 2 diabetes cases were indistinguishable via these ascertainment methods, 90–95% of diabetes cases in the U.S. are type 2 diabetes41. Further, type 2 diabetes most often presents in individuals over the age of 4541. We excluded diabetes cases at baseline and participant age at enrollment was >45 years. Therefore, it is assumed that incident diabetes cases in this sample are largely type 2 diabetes. Further, while dietary intake assessments were self-reported, using the average of two measurements (visit 1 and visit 3) is expected to reduce measurement error and increase precision of exposure-outcome associations42. The temporal nature of assessing Mediterranean pattern adherence at baseline with a median of 22 follow-up years is another strength of this study.
Unmeasured and residual confounding in observational studies can remain after multivariable adjustments. Specifically, associations between Mediterranean pattern adherence and diabetes were negated in participants who were overweight or obese. However, energy intake was highest in the fifth quintile with limited variation in BMI and physical activity across quintiles. Energy calculations from food frequency questionnaires tend to be unreliable and there was no objective measure of energy intake (such as doubly labeled water) used in the ARIC study to validate the energy intake reported in Table 2. To address this source of potential confounding, the multivariable regression models were adjusted for total energy intake. However, more generally, individuals in the U.S. who adhere to a healthy eating pattern often have other healthy lifestyle behaviors such as being physically active, refraining from smoking, and have financial and educational means to make healthier lifestyle choices. This extent of residual confounding after adjusting for these factors is unknown.
An eating pattern high in fruits, vegetables, whole grains, legumes, nuts, and fish, and moderate in alcohol, similar to the Mediterranean-style eating pattern assessed in this analysis and recommended by the 2015–2020 Dietary Guidelines for Americans, was associated with an overall lower risk of diabetes in a community-based U.S. population. This association was particularly strong for black and normal weight individuals but was absent for individuals who were overweight or obese. Increased awareness and promotion of healthy eating patterns for diabetes prevention in predominantly black communities may reduce disease burden. Future research is needed to assess if a calorically restrictive Mediterranean-style eating pattern, resulting in clinically meaningful weight loss, can reduce future diabetes risk in individuals who are overweight or obese. Adhering to a healthy eating pattern is an important component of an overall healthy and active lifestyle to obtain and maintain a healthy body weight and to reduce risk of adult-onset diabetes for the U.S. population.
Supplementary information
Supplemental Table S1-S4, Supplemental Figure S1
Acknowledgements
We thank the staff and participants of the ARIC study for their important contributions. The ARIC study has been funded in part by contracts from the National Heart, Lung, and Blood Institute, National Institutes of Health, and Department of Health and Human Services (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). C.M.R. is supported by a mentored research scientist development award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782) and a grant from the National Heart, Lung, and Blood Institute (R21 HL143089). E.S. was supported by NIH/NIDDK grant K24DK106414. E.A.H. is supported by a grant from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (training grant T32 HL007024).
Author contributions
L.E.O. and C.M.R. designed the research; L.E.O. analyzed the data with assistance from E.A.H.; L.E.O. wrote the paper with editorial assistance from all coauthors; and C.M.R. is the guarantor and had primary responsibility for final content. All authors read and approved the final paper.
Data availability
Data are available upon request from the National Heart, Lung, and Blood Institute Biologic Specimens and Data Repository Information Coordinating Center (BioLINCC) (accession number: HLB00020019b).
Code availability
Statistical code is available upon request from the corresponding author.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41387-020-0113-x).
References
- 1.United States Department of Health and Human Services and Department of Agriculture. Dietary Guidelines for Americans. (US Government Printing Office, Washington, DC, 2015).
- 2.Van Horn L, et al. Recommended Dietary Pattern to Achieve Adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: a Scientific Statement From the American Heart Association. Circulation. 2016;134:e505–e529. doi: 10.1161/CIR.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 3.PREDIMED Study Investigators. PREDIMED Study Mediteranean diet in the primary prevention of cardiovascular disease: Research Protocol. Version 1. http://www.predimed.es/uploads/8/0/5/1/8051451/_1estr_protocol_olf.pdf (2003). Accessed 18 May 2018.
- 4.Willett WC, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am. J. Clin. Nutr. 1995;61(6 Suppl):1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 5.Estruch R, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann. Intern. Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 6.de Lorgeril M, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.CIR.99.6.779. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor LE, Paddon-Jones D, Wright AJ, Campbell WW. A Mediterranean-style eating pattern with lean, unprocessed red meat has cardiometabolic benefits for adults who are overweight or obese in a randomized, crossover, controlled feeding trial. Am. J. Clin. Nutr. 2018;108:33–40. doi: 10.1093/ajcn/nqy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liyanage T, et al. Effects of the Mediterranean diet on cardiovascular outcomes-a systematic review and meta-analysis. PLoS ONE. 2016;11:e0159252. doi: 10.1371/journal.pone.0159252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estruch R, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 10.Buckland G, et al. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Br. J. Nutr. 2011;106:1581–1591. doi: 10.1017/S0007114511002078. [DOI] [PubMed] [Google Scholar]
- 11.United States Department of Health and Human Services and Department of Agriculture. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. (US Government Printing Office, Washington, DC, 2015).
- 12.Bach-Faig, A. et al. Mediterranean Diet Foundation Expert Group. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 14(12A), 2274–2284 (2011). [DOI] [PubMed]
- 13.Georgousopoulou EN, et al. Mediterranean lifestyle and cardiovascular disease prevention. Cardiovasc Diagn. Ther. 2017;7(Suppl 1):S39–S47. doi: 10.21037/cdt.2017.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Gonzalez MA, et al. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336:1348–1351. doi: 10.1136/bmj.39561.501007.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi M, et al. Mediterranean diet and glycaemic load in relation to incidence of type 2 diabetes: results from the Greek cohort of the population-based European Prospective Investigation into Cancer and Nutrition (EPIC) Diabetologia. 2013;6:2405–2413. doi: 10.1007/s00125-013-3013-y. [DOI] [PubMed] [Google Scholar]
- 16.InterAct Consortium, et al. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: the InterAct project. Diabetes Care. 2011;34:1913–1918. doi: 10.2337/dc11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Insitute of Diabetes and Digestive and Kidney Diseases. Risk factors for type 2 diabetes and preventing type 2 diabetes. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-type-2-diabetes (2016).
- 18.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am. J. Epidemiol. 1989;129:687–702. doi: 10.1093/oxfordjournals.aje.a115184. [DOI] [PubMed] [Google Scholar]
- 19.Stevens J, et al. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutr. Res. 1996;16:735–745. doi: 10.1016/0271-5317(96)00064-4. [DOI] [Google Scholar]
- 20.Willett WC, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Shimakawa T, et al. Dietary intake patterns and sociodemographic factors in the atherosclerosis risk in communities study. ARIC Study Investigators. Prev. Med. 1994;23:769–780. doi: 10.1006/pmed.1994.1133. [DOI] [PubMed] [Google Scholar]
- 22.Fung TT, et al. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvin E, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR, et al. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci. Sports Exerc. 1997;29:901–909. doi: 10.1097/00005768-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Chobanian AV, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 27.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann. Intern. Med. 2014;106:517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes. Rev. 2014;15:504–515. doi: 10.1111/obr.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Koning L, et al. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34:1150–1156. doi: 10.2337/dc10-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abiemo EE, et al. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA) Br. J. Nutr. 2013;109:1490–1497. doi: 10.1017/S0007114512003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liese AD, Nichols M, Sun X, D’Agostino RB, Jr., Haffner SM. Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care. 2009;32:1434–1436. doi: 10.2337/dc09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vardavas CI, Linardakis KM, Hatzis CM, Saris WH, Kafatos AG. Cardiovascular disease risk factors and dietary habits of farmers from Crete 45 years after the first description of the Mediterranean diet. Eur. J. Cardiovasc Prev. Rehabil. 2010;17:440–446. doi: 10.1097/HJR.0b013e32833692ea. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, et al. Plant-based diets and incident CKD and kidney function. Clin. J. Am. Soc. Nephrol. 2019;14:682–691. doi: 10.2215/CJN.12391018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebholz CM, et al. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am. J. Kidney Dis. 2016;68:853–861. doi: 10.1053/j.ajkd.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor L. E., Li J., Sayer R. D., Hennessy J. E., Campbell W. W. Short-term effects of healthy eating pattern cycling on cardiovascular disease risk factors: pooled results from two randomized controlled trials. Nutrients. 10.3390/nu10111725 (2018). [DOI] [PMC free article] [PubMed]
- 36.Roussell MA, et al. Beef in an Optimal Lean Diet study. Effects on lipids, lipoproteins, and apolipoproteins. Am. J. Clin. Nutr. 2012;95:9–16. doi: 10.3945/ajcn.111.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnan S, et al. A randomized controlled-feeding trial based on the Dietary Guidelines for Americans on cardiometabolic health indexes. Am. J. Clin. Nutr. 2018;108:266–278. doi: 10.1093/ajcn/nqy113. [DOI] [PubMed] [Google Scholar]
- 38.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, ornish, weight watchers, and zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 39.Hill AM, Harris Jackson KA, Roussell MA, West SG, Kris-Etherton PM. Type and amount of dietary protein in the treatment of metabolic syndrome: a randomized controlled trial. Am. J. Clin. Nutr. 2015;102:757–770. doi: 10.3945/ajcn.114.104026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rice Bradley BH. Dietary fat and risk for Type 2 diabetes: a review of recent research. Curr. Nutr. Rep. 2018;7:214–226. doi: 10.1007/s13668-018-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Type 2 Diabetes. https://www.cdc.gov/diabetes/basics/type2.html (2015).
- 42.Hu FB, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am. J. Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1-S4, Supplemental Figure S1
Data Availability Statement
Data are available upon request from the National Heart, Lung, and Blood Institute Biologic Specimens and Data Repository Information Coordinating Center (BioLINCC) (accession number: HLB00020019b).
Statistical code is available upon request from the corresponding author.