Abstract
Purpose of Review
The importance of the posterolateral corner (PLC) with respect to knee stability, particularly in the setting of anterior cruciate ligament (ACL) deficiency, has become more apparent in recent years. The purposes of this article are to review the current concepts of PLC injuries and to address their role in the ACL-deficient and ACL-reconstructed knee.
Recent Findings
Recent literature demonstrates that a single staged, combined reconstruction is optimal. Studies further provide more thorough insight into avoidance of tunnel collision during the multiligament reconstruction. In total, reconstruction procedures have demonstrated successful outcomes in over 90% of patients.
Summary
In summary, we report that in the setting of suspected concomitant PLC and ACL injury, it is essential to address both injuries; appreciating the local anatomy, diagnostic modalities, and surgical techniques are each crucial to achieving desirable clinical outcomes.
Keywords: Posterolateral corner, Lateral (fibular) collateral ligament, Anterior cruciate ligament, Multiligament injury, Ligament reconstruction
Introduction
With an estimated 175,000 anterior cruciate ligament (ACL) reconstructions performed annually, the ACL is the most frequently reconstructed ligament in the human knee [1]. Long-term outcome analysis of ACL reconstructions has demonstrated at best an 80–90% return to preinjury function, and there is a reported 15% risk of reinjury to the reconstructed graft [2, 3]. Concomitant ligament damage in acute knee injuries can be difficult to assess secondary to patient guarding due to swelling and poor visualization on routine magnetic resonance imaging (MRI) scans, and, as such, surgical challenges increase significantly with multiple ligament involvement. Further improvements in ACL reconstruction outcomes may be dependent on proper diagnosis of concomitant ligament damage at initial presentation and an improved understanding and implementation of current surgical techniques.
The posterolateral corner (PLC) was once considered the “dark side” of the knee due to the relatively poor understanding of its local anatomy, subjective clinical exam findings, unvalidated diagnostic imaging findings, and a lack of evidence-based approaches for reconstruction. Partly because of an evolving appreciation for its relationship with the ACL, recent literature on the topic has improved our understanding of the PLC and has paved the way for biomechanically validated surgical reconstruction techniques that are supported by very successful clinical outcomes. PLC injuries have been reported to account for up to 16% of all knee ligament injures and are commonly associated with cruciate ligament injuries, with only 28% of all PLC injuries occurring in isolation [4–6]. The purposes of this article were to review the current concepts of PLC injuries and to address their role in the ACL-deficient and ACL-reconstructed knee.
Anatomy and Function
The PLC consists of three major static stabilizing structures and several secondary dynamic and static stabilizers which collectively provide both the primary restraint against varus translation and also resist posterolateral rotation of the tibia relative to the femur. The three major static stabilizers are the fibular collateral ligament (FCL), the popliteus tendon (PLT), and the popliteofibular ligament (PFL) [7].
Fibular (Lateral) Collateral Ligament (FCL)
The FCL is the primary varus stabilizer of the knee. The femoral attachment is located slightly proximal (1.4 mm) and posterior (3.1 mm) to the lateral epicondyle in a small bony depression (Figs. 1 and 2). This attachment site is approximately 18.5 mm proximal and posterior to the PLT attachment site when the knee is at 70°; this relationship is important to appreciate in anatomic reconstruction techniques. The primary distal attachment site is located in a bony depression on the fibular head: 8.2 mm posterior to the anterior margin of the fibular head and 28.4 mm distal to the tip of the fibular styloid process. The remaining portion of the distal insertion blends with the peroneus longus fascia. On average, the FCL is 69.6 mm in length [7].
Fig. 1.
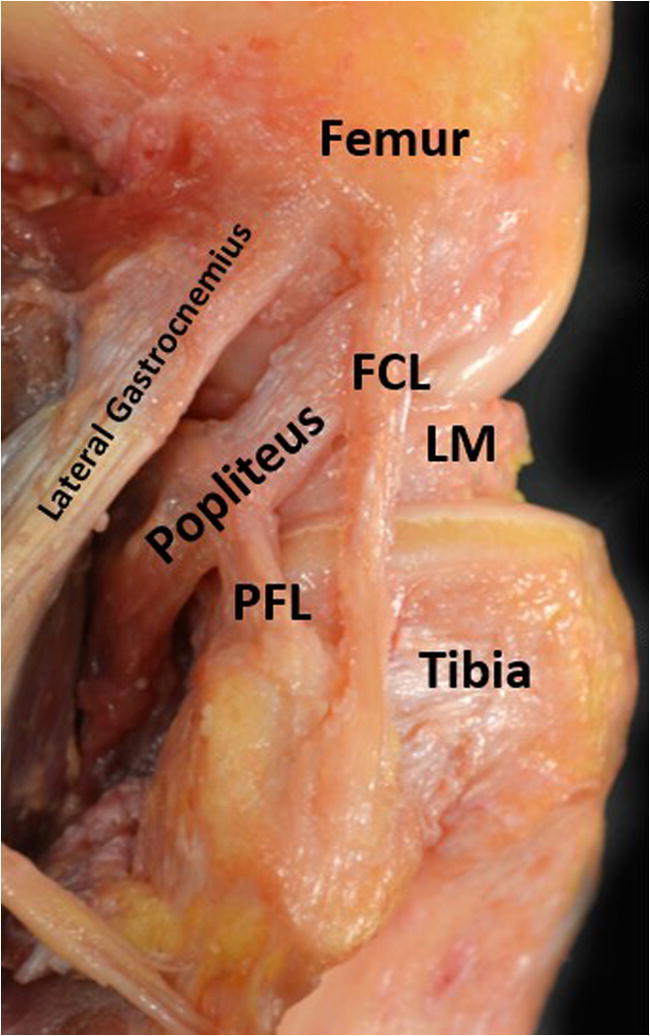
Dissection of the major structures of the PLC from a lateral perspective with the long head of the biceps femoris resected. PFL, patellofemoral ligament; FCL, fibular collateral ligament; LM, lateral meniscus
Fig. 2.
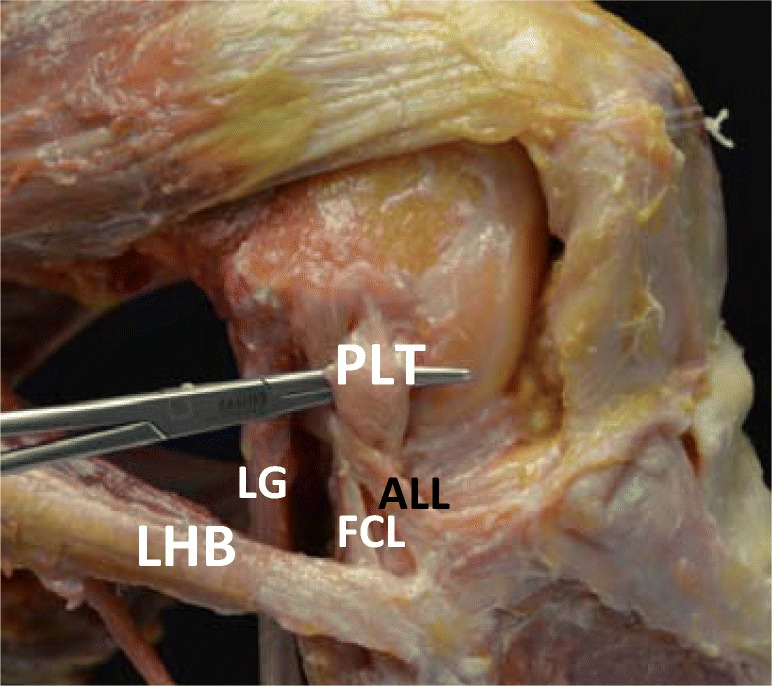
Dissection of the major structures of the PLC with the knee at 90° flexion. PLT, popliteus tendon; LG, lateral gastrocnemius; FCL, fibular collateral ligament; ALL, anterolateral ligament; LHB, long head of biceps
Popliteus Tendon (PLT)
The femoral insertion of the PLT constitutes the most anterior femoral insertion of the PLC (Fig. 2). The popliteus muscle originates on the lateral aspect of the femur and extends posterior and distally in an oblique fashion to insert at a broad attachment site on the posteromedial aspect of the tibia. This femoral attachment footprint is located posterior to the margin of the lateral femoral condyle articular cartilage and at the anterior fifth of the popliteal sulcus. It becomes tendinous in the lateral third of the popliteal fossa and intra-articular as it courses deep to the FCL. The average total length of the PLT is 54.5 mm [7].
Popliteofibular Ligament (PFL)
The PFL, formerly called the arcuate ligament, has distinct anterior and posterior divisions and anchors the musculotendinous junction of the popliteus muscle to the fibular head (Figs. 1 and 2) [8]. The distolateral attachment of the anterior division is located on the anterior downslope of the medial aspect of the fibular styloid process. Similarly, the posterior division attaches at the apex and posteromedial aspect of the fibular styloid process. The posterior division (5.8 mm) has a larger width than the anterior division (2.8 mm). Significant for anatomic reconstruction, the PFL and PLT form an 83° angle, on average, at their junction [7].
Secondary Structures—Dynamic and Static Stabilizers
Secondary structures help stabilize the knee in a static and dynamic manner. From deep to superficial, these structures include the mid-third lateral capsular and anterolateral ligament, coronary ligament, lateral gastrocnemius tendon, fabellofibular ligament, long head of the biceps femoris, iliotibial band, and the anterolateral ligament.
The midthird lateral capsular ligament is a thickening of the lateral capsule. It attaches to the femur near the lateral epicondyle, has a capsular attachment to the lateral meniscus, and attaches to the tibia just distal to the lateral articular cartilage between the posterior border of Gerdy’s tubercle and the anterior edge of the popliteal hiatus. It is composed of two subcomponents: meniscofemoral and meniscotibial ligaments [9, 10].
The coronary ligament of the lateral meniscus is defined as the meniscotibial portion of the posterolateral joint capsule. It begins laterally at the tibial attachment of the posterior cruciate ligament and forms the medial border of the popliteal hiatus [11, 12].
The lateral gastrocnemius tendon arises from the most lateral portion of the gastrocnemius muscle belly at or near the posterior aspect of the supracondylar process of the distal femur. The femoral attachment site is an average of 13.8 mm posterior to the FCL attachment site. The tendon courses distally to fuse with the medial gastrocnemius and the solus muscles to form the sural triceps (Figs. 1 and 2) [7].
The fabellofibular ligament is the distal thickening of the capsular arm of the short head of the biceps femoris. In the majority of patients, it extends vertically from the fabella at the lateral head of the gastrocnemius to the lateral aspect of the fibular styloid process. Of note, the fabella is a sesamoid bone in the minority of cases, and more often a cartilaginous analog, that is found within the proximal lateral gastrocnemius tendon [7, 13, 14].
The long head of the biceps femoris originates at the ischial tuberosity of the pelvis and extends distally through the posterior and lateral aspects of the thigh until it attaches using both a direct and anterior arm. The direct arm attaches laterally to the fibular styloid on the lateral aspect of the fibular head. The anterior arm attaches laterally to the FCL fibular attachment on the fibular head. Between the two arms’ attachment sites lies the biceps bursa, or FCL-biceps bursa, which must be accessed in order to assess the distal FCL attachment [7].
The iliotibial band (ITB) is the most superficial layer of the lateral aspect of the knee. It originates at the anterolateral external lip of the iliac crest and extends distally to the anterolateral aspect of the tibia at Gerdy’s tubercle. Throughout its course, there are numerous peripheral attachments. Significantly, during an open PLC procedure, the ITB must be incised longitudinally to properly assess the FCL and PLT attachment sites [7].
The anterolateral ligament (ALL) comes under tension during internal rotation of the tibia when the knee is at 30° of flexion. The femoral attachment is located posterior and proximal to the lateral femoral epicondyle and the FCL; the anterolateral tibia attachment is approximately midway between the center of Gerdy’s tubercle and the anterior margin of the fibular head [15]. Studies have shown that Segond fractures can occur from the tibial attachment site of the ALL [16].
Other Important Components
The common peroneal nerve innervates the anterior and lateral compartments of the lower extremity and is supplied by spinal nerve roots L4-S2. It emerges from a bifurcation of the sciatic nerve in the posterior thigh and courses along the biceps femoris and around the neck of the fibula until it splits into the superficial and deep peroneal nerve. The peroneal nerve is injured in 13–16.7% of PLC injures; the injury mechanism is most likely secondary to the initial traction injury on the nerve with a hyperextension or varus force and also due to hematoma formation and subsequent nerve compression (Fig. 3) [6, 17, 18].
Fig. 3.
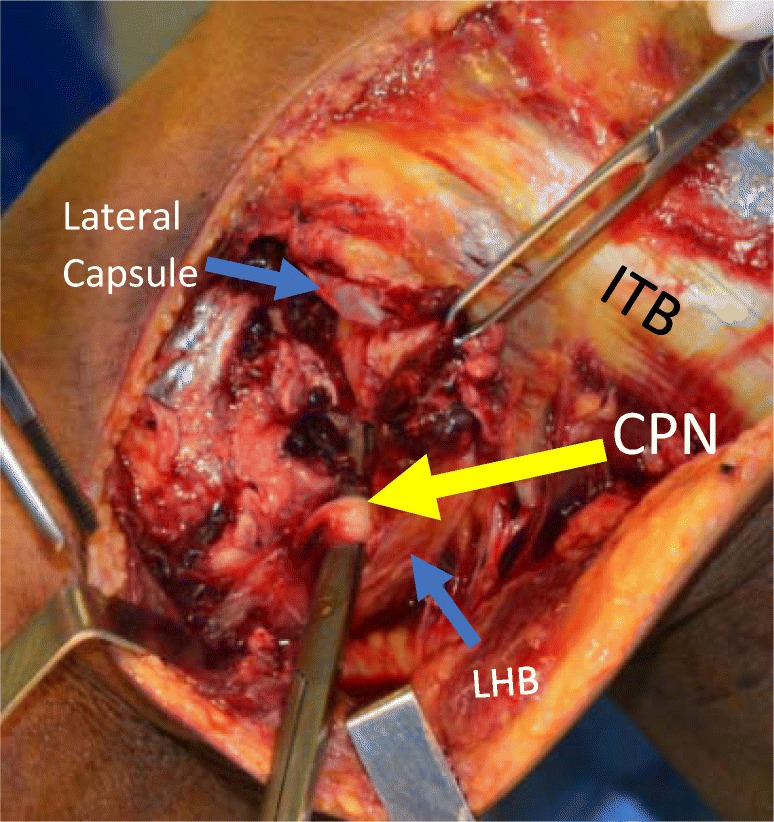
Dissection of a left lateral knee at 90° of flexion, identifying the common peroneal nerve (CPN). Important to identify and protect intraoperatively. ITB: Iliotibial band; LHB: long head of the biceps femoris
The lateral inferior genicular artery emerges from the popliteal artery and courses extra-articularly along the lateral joint capsule. Along the lateral aspect of the knee, the artery winds anteriorly, coursing anterior to the fabellofibular ligament and posterior to the PFL. It is important to identify this artery during PLC procedures because it can serve as both an aid in anatomical identification of important structures and because bleeding from this artery can cause hematoma formation and transient peroneal neuropraxia [19].
Evaluation
Mechanism
The most common mechanisms of injury to the PLC involve a posterolateral-directed force to the anteromedial tibia, knee hyperextension, and/or severe external rotation of the tibia while the knee is partially flexed. This most commonly occurs in the setting of athletic trauma, motor vehicle accident, and falls [20]. Only 28% of PLC injuries occur in isolation and are typically associated with ACL or posterior cruciate ligament (PCL) tears [5, 6].
Presentation
Patients with acute injuries of the PLC most frequently present with pain over the posterolateral aspect of the knee, perceived side-to-side instability near extension, posterolateral rotary instability, difficulty walking on uneven ground, ecchymosis and swelling, and/or foot drop. Chronic injures present with instability with side-to-side activities, an inadvertent hyperextension or a varus thrust gait, difficulty maintaining full extension, and a limited ability to resume sports [21]. Current literature reports injury to the common peroneal nerve in 16.7% of patients with isolated PLC injuries and 13–16% in combined PLC-cruciate ligament injuries [17, 22, 23]. Localized swelling and pain can limit the diagnostic capabilities of the clinical exam.
Physical Exam
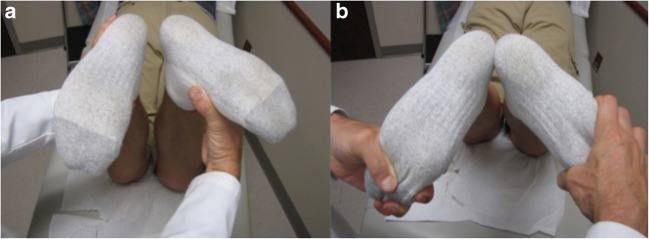
Upon presentation of a possible PLC injury, a thorough physical examination should include the Lachman test, pivot shift, dial test at 30° and 90° (Fig. 4), posterolateral drawer test with the knee at 90° of flexion and 30° of external rotation, heel height differences compared to the contralateral knee and varus stress examination at 0° and 30°. When the presentation is suspicious for a multi-ligament knee injury, it is essential to also perform the external recurvatum test (measured via heel height differences in cm) and the reverse pivot shift test [6, 23, 24]. The literature demonstrates that increased recurvatum suggests a combined PLC or FCL and ACL injury [19]. The reverse pivot shift test is performed with the knee flexed to approximately 80–90°, with a valgus and external rotational force applied. In this position, a positive test would be identified if the tibia became subluxed posterolaterally. The knee is then extended; if the tibia is posterolaterally subluxed, the ITB will reduce it as it goes from functioning as a flexor to an extender of the knee and a visible reduction of the tibia on the femur can occur. However, a positive reverse pivot shift test can be found in 35% of normal physiologically lax knees, highlighting the importance of performing the clinical knee exam on the contralateral knee for comparison and also considering the physical exam finding in the setting of the injury as a whole [25].
Fig. 4.
Demonstration of the dial test with the knee at 30°. a The patient at neutral tibial rotation on left and increased tibial external rotation on the right. b > 10° difference on side-to-side comparison, significant for a positive dial test and suggestive of PLC injury
Imaging
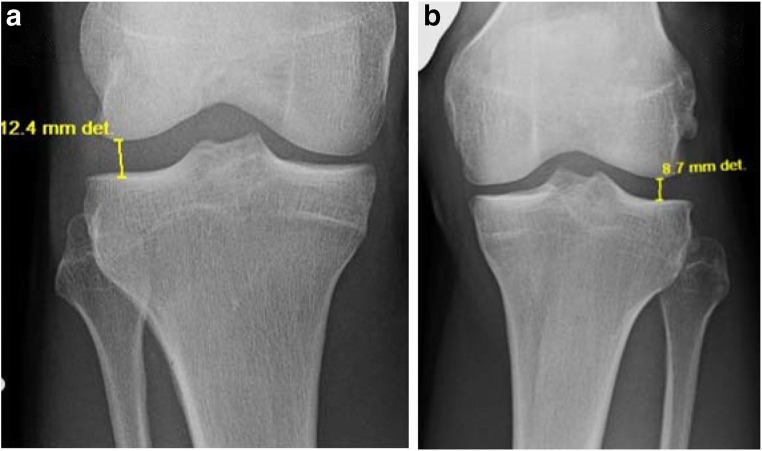
Acute PLC injuries are difficult to identify on standard anteroposterior and bent knee patellofemoral x-ray radiographs. Thus, it is essential to obtain bilateral varus stress radiographs for a reliable diagnosis of the objective amount of side-to-side differences in lateral compartment gapping. The literature demonstrates that varus stress radiographs are both a reliable and reproducible method to evaluate the severity of PLC lesions [26]. Varus stress radiographs should be performed with the knee at 20° flexion. Lateral compartment gapping is determined by measuring the shortest distance between the subchondral bone surface of the most distal aspect of the lateral femoral condyle and the corresponding lateral tibial plateau. LaPrade et al. have reported that an isolated, complete FCL tear can be identified by a spatial difference of 2.7–4.0 mm (Fig. 5), while a side-to-side difference of greater than 4 mm corresponds to an associated grade III PLC injury [26]. It should be noted that a 2016 biomechanical study suggested that an isolated FCL tear could be identified by a side-to-side increase of 1.99 mm on varus stress radiographs and a 2.71 mm increase in an ACL-deficient knee [27].
Fig. 5.
Varus stress radiographs showing a likely FCL tear according to the accepted values by LaPrade et al. [26]. a The affected knee, while b the healthy knee. There is a side-to-side difference of 3.7 mm
Additionally, Chahla et al. conducted an expert consensus study on injuries to the PLC and concluded that experts believe that MRI should always be performed in the assessment of suspected acute posterolateral corner injuries [28]. The MRI should be a minimum 1.5 T in magnetic power. MRI has been reported to have 90% sensitivity and specificity for IT band, biceps tendon, FCL, and popliteus tendon injury (Fig. 6). The only PLC structure with poor reported diagnostic accuracy on MRI was the PFL, with 68.8% sensitivity and 66.7% specificity [4, 29, 10]. Among patients with an ACL injury diagnosed on MRI, 19.7% were found to have a concomitant PLC injury [30].
Fig. 6.
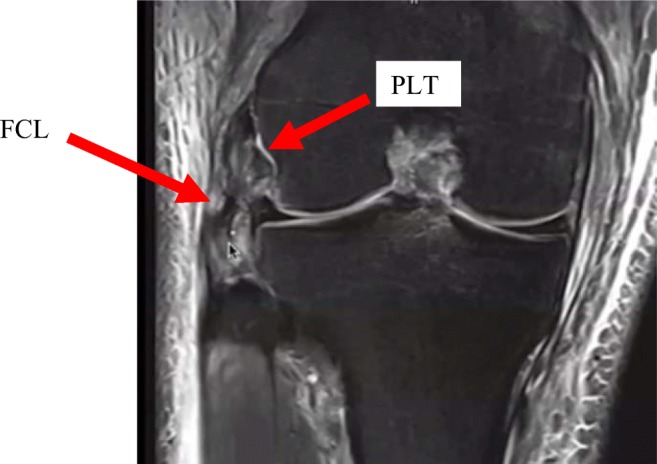
This magnetic resonance image of a right knee shows a complete tear of the fibular collateral ligament (FCL). It further shows increased signal intensity at the femoral attachment site of the popliteus tendon (PLT) suggesting PLT injury as well
As previously discussed, PLC injuries in the setting of an acute ACL tear are often misdiagnosed or missed completely. As such, the conclusion reached by Geeslin et al. in 2010 about the implication of MRI bone bruises is significant. They showed that bone bruises were frequently found in patients with both acute isolated and combined PLC injuries. Among patients with combined ACL and PLC injuries, 50% had anteromedial femoral condyle bone bruises, 39.5% had posterolateral tibial plateau bone bruises, and 28.9% had posteromedial tibial plateau bone bruises identified on MRI. Thus, in the setting of an ACL tear, when the presence of an anteromedial femoral condyle bone bruise is identified on MRI, there should be an increased level of suspicion for a concurrent PLC knee injury (Fig. 7) [31•].
Fig. 7.
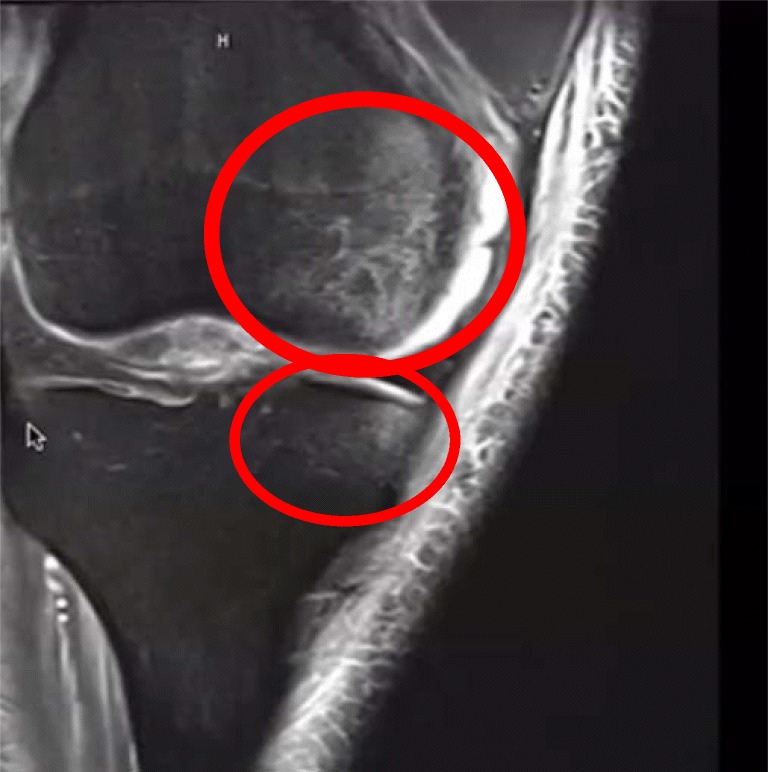
This magnetic resonance image of a right knee demonstrates the classical bone bruise pattern associated with PLC injuries. This image shows an anteromedial femoral condyle and tibial plateau bone bruising pattern
Classification
Posterolateral corner injuries are most commonly classified by either the Hughston scale, which is a subjective classification system that considers the amount of perceived varus stress opening when compared to the contralateral limb, or the Fanelli classification system, which stratifies by the degree of combined posterolateral rotational instability noted [23] (Table 1).
Table 1.
PLC classification systems
| Classification | Rating scale | Finding suggestion |
|---|---|---|
| Fanelli Classification | Type A: 10° increase in external rotation of the tibia. | PFL, PLT |
| Type B: 10° increase in external rotation of the tibia. Slight varus relaxation (5–10 mm in varus load test) | PFL, PLT, FCL | |
| Type C: 10° increase in external rotation of the tibia. Severe varus relaxation (> 10 mm in varus load test) | PFL, PLT, FCL, capsular avulsion, cruciate ligament | |
| Hughston scale | Grade I: 0–5 mm or 0–5° | -Minimal ligament tearing with no abnormal motion |
| Grade II: 6–10 mm; 6–10° | -Partial tearing with slight/moderate abnormal motion | |
| Grade III: > 10 mm or > 10° | -Complete tearing with marked abnormal movements |
Treatment
When the workup indicates a combined acute PLC and ACL injury, reconstruction or a combined hybrid repair should ideally be performed within 3 weeks of injury [32]. There are two primary reasons to ensure that the surgery is performed within this time frame: (1) failure to address the PLC immediately leaves the ACL graft under increased tension and (2) acute reconstruction/repair allows for the native anatomic landmarks to be properly identified for most anatomic reconstructions.
Non-surgical management of PLC injuries is not robustly described in the literature, but positive results with conservative management and early-mobilization of acute grade I or II isolated PLC injuries have been reported [33, 34].
Surgical options to address a combined PLC and ACL injury include either repair or reconstruction of the PLC. Westermann et al. concluded that similar outcomes can be achieved with either repair or reconstruction of PLC injuries treated concurrently with ACL reconstruction at 6-year follow-up [35]. However, the majority of current literature suggests that reconstruction techniques are associated with significantly superior patient outcomes. Historically, end-to-end isolated midsubstance repair of the injured PLC structures was only applied to acute cases; however, due to the higher failure rates in comparison to reconstruction (40% versus 6% in one cohort and 37% versus 9% in another cohort, respectively), PLC repair is not recommended [32, 36, 37]. A systematic review by Moulton et al. that included 456 knees described a 10% total failure rate using reconstruction techniques in chronic injuries [38••]. Currently, repairs are reserved for [1] injuries that involve avulsions, especially of structures avulsed off the fibular head (i.e., FCL, the PFL, and the biceps tendon) or PLT, which need to be reattached to the bone, and (2) injuries involving the capsule and the lateral meniscocapsular ligaments that are anchored and sutured to the underlying bone [5].
Some authors are proponents of the multistage ligament reconstruction approach, suggesting that multiligamentous injuries to the PCL and/or posterolateral corner should be repaired 6 weeks prior to the ACL reconstruction due to the reported length of the surgery itself [37], while other studies provide evidence to support a single staged approach. A systematic review reported that the repair of acute grade III PLC injuries with multi-staged reconstruction was associated with a 38% failure rate, whereas a more robust, single-stage reconstruction technique for PLC injuries with concurrent reconstruction of cruciate injuries resulted in an overall 9% failure rate [38••].
The authors of the current review are proponents of a single stage surgery for all acute knee injuries which involve PLC injuries. In addition to the aforementioned reasons, a single staged procedure allows for all torn structures to be addressed in one operation, leading to a decreased risk of attenuation of surgically reconstructed structures due to untreated or unrecognized structures which are codependent on each other. Further, a single stage surgery allows the patient to begin rehabilitation and have the opportunity to resume activities sooner. A prospective study described equivalent post-operative clinical outcomes using an early partial weight-bearing protocol when compared to a non-weight-bearing protocol following either an isolated FCL reconstruction or a combined FCL and ACL reconstruction [39]. Further, a rehabilitation program focused on early knee motion can help to prevent the arthrofibrosis that has been associated with single-stage multiple ligament reconstruction procedures [40••].
The current authors do support a multistaged reconstructive technique for chronic ACL/PLC injuries in the setting of concurrent genu varus malalignment. Genu varus in the setting of a chronic PLC injury leads to increased tension on the PLC and is associated with a significant risk of PLC graft failure if not properly addressed. The first stage should be a proximal tibial osteotomy, which in isolation has been shown to address the PLC laxity in 38% of cases- particularly those cases with low velocity knee injuries and isolated chronic PLC injuries [41]. When the proximal tibial osteotomy does not adequately address the laxity, a combined PLC/ACL reconstruction should be performed at least 6 months after the first procedure and after the osteotomy has healed [40••].
Surgical Technique
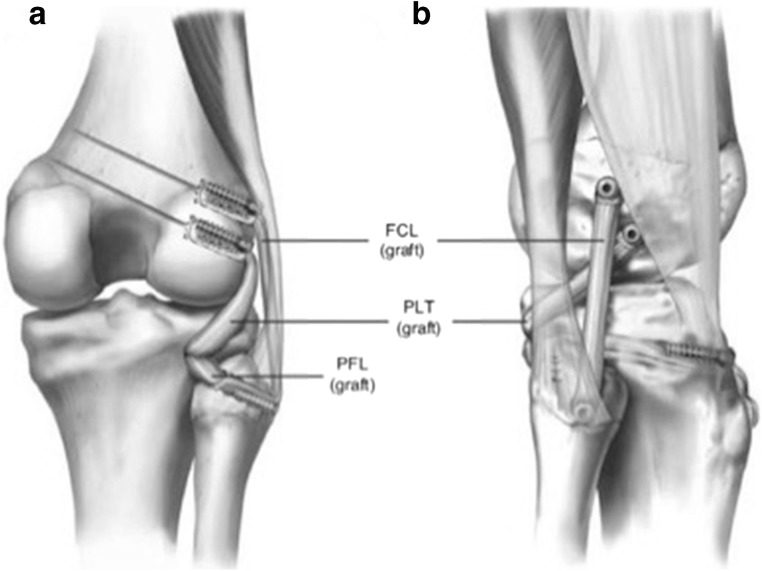
The current authors recommend the use of an anatomic PLC reconstruction for grade III tears that was derived from a combination of quantitative analysis and biomechanical studies. Ideally, the reconstruction is performed with an Achilles tendon allograft or semitendinosus autografts, because these grafts have demonstrated favorable outcomes in clinical studies [41]. The preferred technique begins with a hockey stick incision along the lateral aspect of the knee followed by a neurolysis of the common peroneal nerve, and subsequently a dissection of the posterolateral knee (Fig. 8). From there, the remnant of the FCL and its attachment site on the lateral aspect of the fibular head are identified. The fibular head tunnel is reamed first, followed by the tibial tunnel and passing sutures are placed. The femoral insertion of the FCL and PLT are then identified and eyelet pins are drilled through the center of their attachment sites. After confirmation of the 18.5 mm distance between these two femoral attachments, the FCL and PLT tunnels are drilled, passing sutures are placed, and the grafts are fixed proximally before being passed distally. Finally, the distal ends of the grafts are fixed (fibular head first followed by the tibia) (Fig. 9) [42].
Fig. 8.
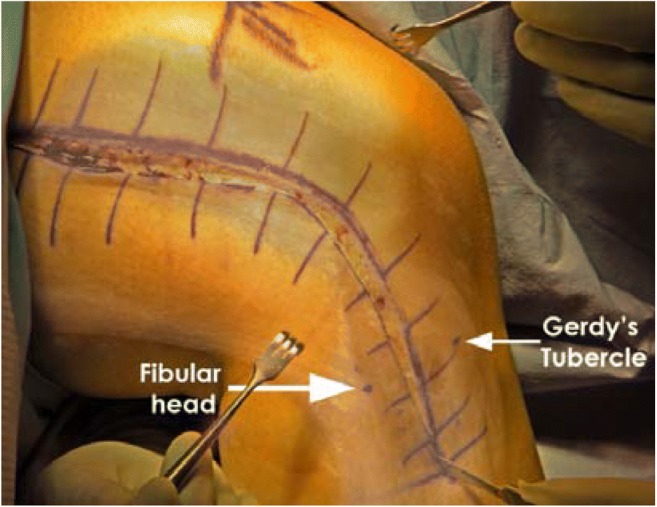
A right knee lateral view showing a hockey stick incision extending from the femoral shaft and lateral femoral condyle to the area between Gerdy’s tubercle and fibula head is performed to develop a posterior-based skin flap
Fig. 9.
Right knee posterior (a) and lateral (b) view demonstrating the anatomic reconstruction. One can see the restoration of the native anatomy in this reconstruction technique
An isolated PLC reconstruction has been reported to produce similar outcomes when compared to combined single stage PLC and ACL reconstructions [43••]. Some studies suggest that the PLC structures should be reconstructed and tensioned prior to the ACL reconstruction to prevent a surgically induced external tibia rotation deformity [44, 45•]. Conversely, a biomechanical study by Moatshe et al. concluded that the PLC should be tensioned last in a multiligament reconstruction in order to avoid tibial internal rotation at lower flexion angles [46••].
A potential complication for single staged anatomical reconstruction techniques is the risk of collision between the FCL and PLB-ACL tunnels [47, 48]. Camarda et al. reported that the risk of tunnel collision can best be avoided by limiting the proximal angulation of the FCL tunnel and directing the tunnel anteriorly with an axial angulation between 20 and 40°. Further, the study demonstrated that tunnel collision can be avoided by reaming parallel to the tangent line to the distal ends of the medial and lateral femoral condyle [49•]. Similarly, Moatshe et al. concluded that to avoid convergence with the ACL tunnel, the FCL and PLT tunnels should be aimed 35° anteriorly and 0° proximally on the lateral aspect of the knee [50]. This study also showed a 100% tunnel collision rate when the FCL tunnel was aimed 0° in the axial plane and 0° in the coronal plane.
PLC in ACL-Deficient Knees
The functional diversity of the PLC structures becomes apparent in the ACL-deficient knee. In a healthy knee, the PLC has a minimal role in the prevention of anterior tibial translation [51]. However, in an ACL-deficient knee, the medial meniscus and the PLC function as secondary stabilizers, with the PLC acting to prevent anterior translation mostly in the early degrees of flexion.
In 2000, Kanamori and colleagues conducted a biomechanical study that utilized a robotic/universal force-moment sensor testing system to analyze the forces on the PLC structures after transection of the ACL [52]. They showed that in the ACL-deficient knee, the in situ forces on the PLC structures increased by 123% at full extension and 413% at 15° of flexion. They also concluded that the PLC has a minor role in resisting anterior tibial loads. This biomechanical analysis was supported by the clinical study from Noyes et al. who described patients with ACL deficiency and varus malalignment. They reported that these patients have increased tension of the PLC, which was noted by observing an increased laxity of the structures during manual testing [53]. They also suggested that in the setting of a triple-varus knee (deformity of the joint/bone plus lateral laxity and hyperextension/external rotation), chronic ACL deficiency can theoretically cause secondary PLC injury. Further biomechanical studies have shown minimal difference in external rotation after the transection of the ACL, supporting the assertion that the PLC structures are the primary stabilizers for external rotation [54].
PLC in ACL-Reconstructed Knees
Biomechanical data about the role of the PLC in a native knee has demonstrated the intimate relationship between the PLC and ACL [44]. When the static structures of the PLC were sectioned in cadaveric knees, a significant increase in force on the ACL was found when varus moments and a coupled varus-internal rotation moments were applied to the joint; specifically, these forces were greatest when the knee was at 30° of flexion. Thus, when the static structures of the PLC remain deficient after ACL reconstruction, one can expect increased tension on the ACL reconstruction graft and thus an increased risk for both acute and chronic graft failure. Additionally, a study by Plaweski et al. demonstrated similar results; their study initially analyzed the native knee biomechanics, then sectioned both the static PLC structures and the ACL, before ultimately reconstructing the ACL [55]. Once the ACL reconstruction was complete, the authors showed increased varus and external rotation displacement. From there, the study demonstrated a return to native kinematics after reconstruction of the PLC static structures.
This conclusion about the role of the PLC in ACL reconstructions has also been supported in clinical practice. A study examined ACL reconstructions with concomitant PLC injuries treated conservatively. In this study, both PLC injuries with ≥ 10° of increased tibial external rotation compared to the normal knee at 30° of flexion and those injuries with ≥ 10° of increased tibial external rotation with varus opening of 5–10 mm and a firm endpoint at 30° of knee flexion were managed conservatively. They showed that those knees with additional varus opening did far worse, suggesting that conservative management of more severe PLC injuries significantly impacts the reconstructed ACL graft [56]. Meanwhile, Fanelli et al. demonstrated successful outcomes in 97.1% of patients and a mean Lysholm score of 91.8 in patients who underwent concomitant ACL and PLC reconstructions [57].
Conclusions
The posterolateral corner of the knee has garnered an increase in academic interest in recent years because of its relationship to the ACL and its role in providing stability to the knee. Biomechanical data has demonstrated the intimate relationship that these structures share and the reliance that ACL grafts have on PLC stability. Further, surgical reconstruction techniques to restore the native anatomy have been both validated biomechanically, and also supported with strong clinical outcome studies. With these results, recent literature is encouraging for the up to 15% of ACL injury patients with concomitant PLC injury who are indicated for concurrent ACL and PLC reconstruction.
Compliance with Ethical Standards
Conflict of Interest
Robert Dean declares that he has no conflict of interest.
Robert LaPrade reports grants and personal fees from Arthrex, Inc., grants from Linvatec, grants and personal fees from Ossur, grants and personal fees from Smith & Nephew, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on ACL: Risk Factors, Outcomes, Preventions
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Robert S. Dean, Email: robertdean@TCOmn.com
Robert F. LaPrade, Email: laprademdphd@gmail.com
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Frank RM, Verma NN. Graft selection in revision ACL reconstruction. In: Bach BR Jr, Provencher MT, editors. ACL surgery: How to get it right the first time and what to do if it fails. SLACK. NJ: Thorofare; 2010. pp. 217–229. [Google Scholar]
- 2.Chen T, Zhang P, Chen J, Hua Y, Chen S. Long-Term Outcomes of Anterior Cruciate Ligament Reconstruction Using Either Synthetics With Remnant Preservation or Hamstring Autografts: A 10-Year Longitudinal Study. Am J Sports Med. 2017;45(12):2739–2750. doi: 10.1177/0363546517721692. [DOI] [PubMed] [Google Scholar]
- 3.Kay J, Memon M, Marx RG, Peterson D, Simunovic N, Ayeni OR. Over 90% of children and adolescents return to sport after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1019–1036. doi: 10.1007/s00167-018-4830-9. [DOI] [PubMed] [Google Scholar]
- 4.Laprade RF, Wentorf FA, Fritts H, Gundry C, David Hightower C. A Prospective Magnetic Resonance Imaging Study of the Incidence of Posterolateral and Multiple Ligament Injuries in Acute Knee Injuries Presenting With a Hemarthrosis. Arthroscopy. 2007;23(12):1341–1347. doi: 10.1016/j.arthro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Geeslin AG, LaPrade RF. Outcomes of Treatment of Acute Grade-III Isolated and Combined Posterolateral Knee Injuries. J Bone Joint Surg Am. 2011;93(18):1672–1683. doi: 10.2106/JBJS.J.01639. [DOI] [PubMed] [Google Scholar]
- 6.Laprade RF, Terry GC. Injuries to the Posterolateral Aspect of the Knee Association of Anatomic Injury Patterns with Clinical Instability. Am J Sports Med. 1997;25(4):433–438. doi: 10.1177/036354659702500403. [DOI] [PubMed] [Google Scholar]
- 7.LaPrade Robert F., Ly Thuan V., Wentorf Fred A., Engebretsen Lars. The Posterolateral Attachments of the Knee. The American Journal of Sports Medicine. 2003;31(6):854–860. doi: 10.1177/03635465030310062101. [DOI] [PubMed] [Google Scholar]
- 8.LaPrade RF, Griffith CJ, Coobs BR, Geeslin AG, Johansen S, Engebretsen L. Improving outcomes for posterolateral knee injuries. J Orthop Res. 2014;32(4):485–491. doi: 10.1002/jor.22572. [DOI] [PubMed] [Google Scholar]
- 9.Chahla J, Moatshe G, Dean CS, LaPrade RF. Posterolateral Corner of the Knee: Current Concepts. Arch Bone Joint Surg. 2016;4(2):97–103. [PMC free article] [PubMed] [Google Scholar]
- 10.LaPrade Robert F., Gilbert Thomas J., Bollom Timothy S., Wentorf Fred, Chaljub Gregory. The Magnetic Resonance Imaging Appearance of Individual Structures of the Posterolateral Knee. The American Journal of Sports Medicine. 2000;28(2):191–199. doi: 10.1177/03635465000280020901. [DOI] [PubMed] [Google Scholar]
- 11.James EW, LaPrade CM, LaPrade RF. Anatomy and Biomechanics of the Lateral Side of the Knee and Surgical Implications. Sports Med Arthrosc Rev. 2015;23(1):2–9. doi: 10.1097/JSA.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 12.Stannard JP, Brown SL, Robinson JT, McGwin G, Volgas DA. Reconstruction of the Posterolateral Corner of the Knee. Arthroscopy. 2005;21(9):1051–1059. doi: 10.1016/j.arthro.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Terry GC, LaPrade RF. The Biceps Femoris Muscle Complex at the Knee. Am J Sports Med. 1996;24(1):2–8. doi: 10.1177/036354659602400102. [DOI] [PubMed] [Google Scholar]
- 14.Kawashima T, Takeishi H, Yoshitomi S, Ito M, Sasaki H. Anatomical study of the fabella, fabellar complex and its clinical implications. Surg Radiol Anat. 2007;29(8):611–616. doi: 10.1007/s00276-007-0259-4. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy MI, Claes S, Fuso FAF, Williams BT, Goldsmith MT, Turnbull TL, et al. The Anterolateral Ligament: An Anatomic, Radiographic, and Biomechanical Analysis. Am J Sports Med. 2015;43(7):1606–1615. doi: 10.1177/0363546515578253. [DOI] [PubMed] [Google Scholar]
- 16.Segond, P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progres Med. 1879;7:297–299, 319–321, 340–341.
- 17.Delee JC, Riley MB, Rockwood CA. Acute posterolateral rotatory instability of the knee. Am J Sports Med. 1983;11(4):199–207. doi: 10.1177/036354658301100403. [DOI] [PubMed] [Google Scholar]
- 18.Girolami M, Galletti S, Montanari G, Mignani G, Schuh R, Ellis S, et al. Common Peroneal Nerve Palsy due to Hematoma at the Fibular Neck. J Knee Surg. 2013;26(S 01):S132–S135. doi: 10.1055/s-0032-1330055. [DOI] [PubMed] [Google Scholar]
- 19.LaPrade RF, Ly TV, Griffith C. The External Rotation Recurvatum Test Revisited: Reevaluation of the Sagital Plane Tibiofemoral Relationship. Am J Sports Med. 2008;36(4):709–712. doi: 10.1177/0363546507311096. [DOI] [PubMed] [Google Scholar]
- 20.Lunden JB, Bzdusek PJ, Monson JK, Malcomson KW, Laprade RF. Current Concepts in the Recognition and Treatment of Posterolateral Corner Injuries of the Knee. J Orthop Sport Phys Ther. 2010;40(8):502–516. doi: 10.2519/jospt.2010.3269. [DOI] [PubMed] [Google Scholar]
- 21.Porrino J, Sharp JW, Ashimolowo T, Dunham G. An Update and Comprehensive Review of the Posterolateral Corner of the Knee. Rad Clin North Am. 2018;56(6):935–961. doi: 10.1016/j.rcl.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Krukhaug Y, Mølster A, Rodt A, Strand T. Lateral ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 1998;6(1):21–25. doi: 10.1007/s001670050067. [DOI] [PubMed] [Google Scholar]
- 23.Hughston JL, Norwood LA. The posterolateral drawer test and external rotation recurvatum test for posterolateral rotatory instability of the knee. Clin Orthop. 1980;147:82–87. [PubMed] [Google Scholar]
- 24.Qureshi MZ, Gorczyca JT, Doyle AJ, Gestring ML. Posterior sternoclavicular joint dislocation: A rare manifestation of seatbelt injury. Surgery. 2016;162(4):958–960. doi: 10.1016/j.surg.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 25.Cooper D E. Tests for posterolateral instability of the knee in normal subjects. Results of examination under anesthesia. The Journal of Bone & Joint Surgery. 1991;73(1):30–36. doi: 10.2106/00004623-199173010-00005. [DOI] [PubMed] [Google Scholar]
- 26.LaPrade RF, Heikes C, Bakker AJ, Jakobsen RB. The reproducibility and repeatability of varus stress radiographs in the assessment of isolated fibular collateral ligament and grade-III posterolateral knee injuries. An in vitro biomechanical study. J Bone Joint Surg Am. 2008;90(10):2069–2076. doi: 10.2106/JBJS.G.00979. [DOI] [PubMed] [Google Scholar]
- 27.McDonald LS, Waltz RA, Carney JR, Dewing CB, Lynch JR, Asher DB, et al. Validation of varus stress radiographs for anterior cruciate ligament and posterolateral corner knee injuries: A biomechanical study. Knee. 2016;23(6):1064–1068. doi: 10.1016/J.KNEE.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Chahla J, Murray IR, Robinson J, Lagae K, Margheritini F, Fritsch B, et al. Posterolateral corner of the knee: an expert consensus statement on diagnosis, classification, treatment, and rehabilitation. Sports Traumatology, Arthroscopy: Knee Surgery; 2018. pp. 1–10. [DOI] [PubMed] [Google Scholar]
- 29.LaPrade RF, Bollom TS, Wentorf FA, Wills NJ, Meister K. Mechanical Properties of the Posterolateral Structures of the Knee. Am J Sports Med. 2005;33(9):1386–1391. doi: 10.1177/0363546504274143. [DOI] [PubMed] [Google Scholar]
- 30.Temponi EF, de Carvalho Júnior LH, Saithna A, Thaunat M, Sonnery-Cottet B. Incidence and MRI characterization of the spectrum of posterolateral corner injuries occurring in association with ACL rupture. Skelet Radiol. 2017;46(8):1063–1070. doi: 10.1007/s00256-017-2649-y. [DOI] [PubMed] [Google Scholar]
- 31.Geeslin Andrew G., LaPrade Robert F. Location of Bone Bruises and Other Osseous Injuries Associated with Acute Grade III Isolated and Combined Posterolateral Knee Injuries. The American Journal of Sports Medicine. 2010;38(12):2502–2508. doi: 10.1177/0363546510376232. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy M, Ridley TJ, Bollier M, Cook S, Wolf B, Amendola A. Posterolateral Knee Reconstruction Versus Repair. Iowa Orthopedic J. 2015;35:20–25. [PMC free article] [PubMed] [Google Scholar]
- 33.Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med. 1989;17(1):83–88. doi: 10.1177/036354658901700114. [DOI] [PubMed] [Google Scholar]
- 34.Krukhaug Y, Mølster A, Rodt A, Strand T. Lateral ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 1998;6(1):21–25. doi: 10.1007/s001670050067. [DOI] [PubMed] [Google Scholar]
- 35.Westermann RW, Spindler KP, Huston LJ, Wolf BR. Posterolateral Corner Repair versus Reconstruction: 6-year Outcomes from a Prospective Multicenter Cohort. Orthop J Sports Med. 2017;5(7_suppl6):2325967117S0026. doi: 10.1177/2325967117S00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy BA, Dajani KA, Morgan JA, Shah JP, Dahm DL, Stuart MJ. Repair versus Reconstruction of the Fibular Collateral Ligament and Posterolateral Corner in the Multiligament-Injured Knee. Am J Sports Med. 2010;38(4):804–809. doi: 10.1177/0363546509352459. [DOI] [PubMed] [Google Scholar]
- 37.Stannard JP, Brown SL, Farris RC, McGwin G, Volgas DA. The posterolateral corner of the knee: Repair versus reconstruction. Am J Sports Med. 2005;33(6):881–888. doi: 10.1177/0363546504271208. [DOI] [PubMed] [Google Scholar]
- 38.Moulton Samuel G., Geeslin Andrew G., LaPrade Robert F. A Systematic Review of the Outcomes of Posterolateral Corner Knee Injuries, Part 2. The American Journal of Sports Medicine. 2015;44(6):1616–1623. doi: 10.1177/0363546515593950. [DOI] [PubMed] [Google Scholar]
- 39.LaPrade Robert F., DePhillipo Nicholas N., Cram Tyler R., Cinque Mark E., Kennedy Mitchell I., Dornan Grant J., O’Brien Luke T. Partial Controlled Early Postoperative Weightbearing Versus Nonweightbearing After Reconstruction of the Fibular (Lateral) Collateral Ligament: A Randomized Controlled Trial and Equivalence Analysis. The American Journal of Sports Medicine. 2018;46(10):2355–2365. doi: 10.1177/0363546518784301. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy Nicholas I., LaPrade Christopher M., LaPrade Robert F. Surgical Management and Treatment of the Anterior Cruciate Ligament/Posterolateral Corner Injured Knee. Clinics in Sports Medicine. 2017;36(1):105–117. doi: 10.1016/j.csm.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 41.LaPrade RF, Spiridonov SI, Coobs BR, Ruckert PR, Griffith CJ. Fibular Collateral Ligament Anatomical Reconstructions. Am J Sports Med. 2010;38(10):2005–2011. doi: 10.1177/0363546510370200. [DOI] [PubMed] [Google Scholar]
- 42.Serra Cruz Raphael, Mitchell Justin J., Dean Chase S., Chahla Jorge, Moatshe Gilbert, LaPrade Robert F. Anatomic Posterolateral Corner Reconstruction. Arthroscopy Techniques. 2016;5(3):e563–e572. doi: 10.1016/j.eats.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moulton Samuel G., Matheny Lauren M., James Evan W., LaPrade Robert F. Outcomes following anatomic fibular (lateral) collateral ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;23(10):2960–2966. doi: 10.1007/s00167-015-3634-4. [DOI] [PubMed] [Google Scholar]
- 44.LaPrade Robert F., Resig Scott, Wentorf Fred, Lewis Jack L. The Effects of Grade III Posterolateral Knee Complex Injuries on Anterior Cruciate Ligament Graft Force. The American Journal of Sports Medicine. 1999;27(4):469–475. doi: 10.1177/03635465990270041101. [DOI] [PubMed] [Google Scholar]
- 45.Wentorf Fred A., LaPrade Robert F., Lewis Jack L., Resig Scott. The Influence of the Integrity of Posterolateral Structures on Tibiofemoral Orientation When an Anterior Cruciate Ligament Graft is Tensioned. The American Journal of Sports Medicine. 2002;30(6):796–799. doi: 10.1177/03635465020300060701. [DOI] [PubMed] [Google Scholar]
- 46.Moatshe Gilbert, Chahla Jorge, Brady Alex W., Dornan Grant J., Muckenhirn Kyle J., Kruckeberg Bradley M., Cinque Mark E., Turnbull Travis Lee, Engebretsen Lars, LaPrade Robert F. The Influence of Graft Tensioning Sequence on Tibiofemoral Orientation During Bicruciate and Posterolateral Corner Knee Ligament Reconstruction: A Biomechanical Study. The American Journal of Sports Medicine. 2018;46(8):1863–1869. doi: 10.1177/0363546517751917. [DOI] [PubMed] [Google Scholar]
- 47.Gali Julio Cesar, Bernardes Adilio de Paula, dos Santos Leonardo Cantarelli, Ferreira Thiago Carrazone, Almagro Marco Antonio Pires, da Silva Phelipe Augusto Cintra. Tunnel collision during simultaneous anterior cruciate ligament and posterolateral corner reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy. 2014;24(1):195–200. doi: 10.1007/s00167-014-3363-0. [DOI] [PubMed] [Google Scholar]
- 48.Neven E, D’Hooghe P, Bellemans J. Double-Bundle Anterior Cruciate Ligament Reconstruction: A Cadaveric Study on the Posterolateral Tunnel Position and Safety of the Lateral Structures. Arthroscopy. 2008;24(4):436–440. doi: 10.1016/J.ARTHRO.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Camarda Lawrence, D′Arienzo Michele, Patera Giovanni Palermo, Filosto Leone, LaPrade Robert F. Avoiding tunnel collisions between fibular collateral ligament and ACL posterolateral bundle reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy. 2010;19(4):598–603. doi: 10.1007/s00167-010-1299-6. [DOI] [PubMed] [Google Scholar]
- 50.Moatshe Gilbert, Brady Alex W., Slette Erik L., Chahla Jorge, Turnbull Travis Lee, Engebretsen Lars, LaPrade Robert F. Multiple Ligament Reconstruction Femoral Tunnels: Intertunnel Relationships and Guidelines to Avoid Convergence. The American Journal of Sports Medicine. 2016;45(3):563–569. doi: 10.1177/0363546516673616. [DOI] [PubMed] [Google Scholar]
- 51.Crespo B, James EW, Metsavaht L, Laprade RF. Injuries to posterolateral corner of the knee: a comprehensive review from anatomy to surgical treatment. Rev Bras Ortop. 2015;50:363–370. doi: 10.1016/j.rboe.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanamori, A., Sakane, M., Zeminski, J., Rudy, T. W., & Woo, S. L.-Y. (2000). In-situ force in the medial and lateral structures of intact and ACL-deficient knees. J Orthop Sci. 5(6), 567–571.Retrieved from 10.1007/s007760070007.pdf [DOI] [PubMed]
- 53.Noyes FR, Barber-Westin SD, Hewett TE. High Tibial Osteotomy and Ligament Reconstruction for Varus Angulated Anterior Cruciate Ligament-Deficient Knees. Am J Sports Med. 2000;28(3):282–296. doi: 10.1177/03635465000280030201. [DOI] [PubMed] [Google Scholar]
- 54.Wroble Randall R., Grood Edward S., Cummings John S., Henderson Joel M., Noyes Frank R. The role of the lateral extraarticular restraints in the anterior cruciate ligament-deficient knee. The American Journal of Sports Medicine. 1993;21(2):257–263. doi: 10.1177/036354659302100216. [DOI] [PubMed] [Google Scholar]
- 55.Plaweski Stephane, Belvisi Baptiste, Moreau-Gaudry Alexandre. Reconstruction of the Posterolateral Corner After Sequential Sectioning Restores Knee Kinematics. Orthopaedic Journal of Sports Medicine. 2015;3(2):232596711557056. doi: 10.1177/2325967115570560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhillon Mandeep, Akkina Narendranadh, Prabhakar Sharad, Bali Kamal. Evaluation of outcomes in conservatively managed concomitant Type A and B posterolateral corner injuries in ACL deficient patients undergoing ACL reconstruction. The Knee. 2012;19(6):769–772. doi: 10.1016/j.knee.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Fanelli G, Fanelli D, Edson C, Fanelli M. Combined Anterior Cruciate Ligament and Posterolateral Reconstruction of the Knee Using Allograft Tissue in Chronic Knee Injuries. J Knee Surg. 2014;27(05):353–358. doi: 10.1055/s-0034-1382786. [DOI] [PubMed] [Google Scholar]