Abstract
BACKGROUND:
Some studies have shown that there is a certain rotation of the eye in the sitting and lying position of the patient. The Visumax system used for the Refractive Lenticule Extraction-Small Incision Lenticule Extraction (ReLEx SMILE) surgery lacks the rotation of eye control function. So, is the ReLEx SMILE surgery for patients with astigmatism safe and effective?
AIM:
To evaluate the outcomes of the ReLEx SMILE surgery in cases with myopic astigmatism.
METHODS:
The case series included 120 eyes with myopic astigmatism undergoing ReLEx SMILE surgery from January 2018 to November 2018. The distribution of patients for two subgroups based on the power of astigmatism, low astigmatic group (≤ 1.50D) and high astigmatic group (> 1.50D). All patients were measured UDVA, CDVA, refractive sphere, astigmatism and sphere equivalent before and after surgery one week, one month and three months carefully. The astigmatic correction was evaluated by the vectorial analysis Alpins.
RESULTS:
The mean efficacy index of the low and high astigmatic group was 1.035 and 1.082 (respectively); the mean safety index was 1.113 and 1.215 (respectively). 93% of eyes in the low astigmatic group had an angle of error (AE) within ± 15 degrees and 100% in high astigmatic group. There was an undercorrection in astigmatic treatment. No complications during and after surgery were recorded.
CONCLUSION:
ReLEx SMILE surgery for the myopic astigmatic treatment was safe and effective.
Keywords: ReLEx SMILE, Astigmatism, Myopia
Introduction
The development history of refractive surgery has gone through three generations. The first generation is photorefractive keratectomy (PRK) surgery. The second generation is LaserAssisted In Situ Keratomileusis (LASIK) surgery that creates a corneal flap. And now with the introduction of the femtosecond laser, in 2011, the third generation of refractive surgery occurred and have been called Refractive Lenticule Extraction-Small Incision Lenticule Extraction (ReLEx SMILE) with a no-flap technique [1]. Many researches have been indicated that the ReLEx SMILE had excellent results. This technique was extremely safe and effect because of avoiding the complications concerning to corneal flap. Moreover, the studies indicated that it had excellent predictability and stability for the correction of myopia. Together, the rate of dry eye syndrome and the amount of corneal aberration were also decreased [2], [3], [4], [5]. However, the Visumax system (Carl Zeiss Meditec, Germany) that has been used for this surgery lacks the rotation of eye control function. That has made the surgeons had to control the rotational eyes completely by hand and personal experience. Some surgeons have worried about the capability of this technique for the treatment of moderate or high astigmatism compared to LASIK surgery while at present, many excimer laser modern machines have cyclotorsion control function. Anyway, few papers report the results of the astigmatic correction by vector analysis, and very little researches have been still concerned with those patient’s relative high astigmatism (especially > 3.0D). On the other hand, we have not found any reports about this issue in Vietnam. This study would like to assess the results of the ReLEx SMILE surgery in cases with myopic astigmatism.
Materials and Methods
The design of the study
The study method is case series. Selection criteria were patients with myopic astigmatism undergoing the ReLEx SMILE surgery from January 2018 to November 2018 at Vietnam National Institute of Ophthalmology (VNIO), Vietnam. Exclusion criteria were patients with combined systemic diseases or other problems of the eye. Distribution of patients for two subgroups was based on the power of astigmatism: low astigmatic group (astigmatism preoperative ≤ 1.50D) and high astigmatic group (astigmatism preoperative > 1.50D). The study was accepted by the institutional ethics committee of VNIO. All patients understood and voluntarily participated in this study.
Preoperative and postoperative examinations
If the patients wear contact lens, they need to discontinue at least two weeks with the soft contact lens or at least four weeks with the rigid contact lens before the initial examination.
All patients were examined carefully before surgery, including slit-lamp biomicroscopy, tonometry, uncorrected and corrected distance visual acuity (UDVA and CDVA), fundus evaluation, keratometry, non-contact specular microscopy, and corneal topography. Automated refraction was implemented before and after instilling cycloplegic solution (Cyclogyl 1%). Refractive indices were carefully measured several separated times before surgery to indicate the final refractive result for the treatment program.
After surgery one week, one month and three months, the UDVA and CDVA, tonometry, slit-lamp biomicroscopy and measurement of corneal topography were repeated. The refractive sphere, refractive astigmatism, the axis of astigmatism, manifest refraction spherical equivalent (MRSE) were also measured. The complications were recorded.
Astigmatic correction
The astigmatic correction was based on the method of Alpins, which allowed to analyze astigmatic vectors. Through the amount of astigmatic treatment, they could evaluate the efficacy of astigmatic correction. The first vector had been determined was the target induced astigmatism vector (TIA), which was manifest initial astigmatism, and needed to treat. The second vector had been known was the surgically induced astigmatism vector (SIA), which was the amount of astigmatic correction caused by surgery. The third vector was the difference between the TIA and the SIA, called the difference vector (DV) [6], [7]. Based on these above three vectors, many other indices have been identified to evaluate the characteristics of astigmatic correction further. The index of success (IOS) [8] would like to indicate the proportion of residual astigmatism, and was the value obtained by the DV division for the TIA; the ideal value was zero. The correction index (CI) was the ratio achieved by the SIA division for the TIA. If the CI was higher than 1.0, the treatment had shown overcorrection, while CI was lower than 1.0, the procedure had been under correction. The magnitude of error (ME) was the arithmetic difference between the magnitude of the SIA and the TIA. If the ME was positive the treatment had been an overcorrection, and if the ME was negative, the treatment had occurred under correction. The angle of error (AE) was the arithmetic difference between the angle of the SIA and the TIA. If the TIA was positive, the correction had shown counter clockwise with the initial astigmatic axis, and if the TIA was negative, the treatment had defined clockwise with the initial astigmatic axis.
Surgical technique
The procedure was performed by using the VisuMax system (Carl Zeiss Meditec, Germany) which emits femtosecond laser beam with near-infrared wavelengths in an extremely short time (10-15 seconds). The diameter of lenticule was set between 6.3 and 7.0 mm; the thickness of cap that was established 110 µm or 120 µm depends on the power of spherical refraction equivalent. The length of the incision was set 2 mm. In all cases, the patient was lying on the operation bed and align the head and the body. It was necessary to avoid head tilt. Topical anesthesia was achieved with one drop proparacaine hydrochloride (Alcaine, Alcon) every three minutes for three times before surgery. The time of treatment laser was 23 seconds. Then, a hook was used to separate two planes of the lenticule. The first, the surgeon delineated the upper interface and then was the lower interface. When both interfaces had been completely separated, the lenticule was extracted through the small incision. Finally, one drop antibiotic solution (Ofloxacin 0.3%, Santen) was instilled.
Statistical analysis
Data were analyzed using SPSS 16.0. The number of data was presented as X ± SD, and the percentage of data were shown in %. A p-value less 0.05 was considered statistically significant. Student’s t-test was used to compare refractive indices, UDVA, CDVA, MRSE, corneal thickness, average keratometry, TIA, SIA, DV, ME, AE, CI, IOS…between two groups (low and high astigmatism). To compare proportions, using Fisher’s exact test if the number of data was too small to do the Chi-square test.
Results
The Table 1 showed the characteristics of patients were presented, and no significant differences about the patient’s age as well as the MRSE, refractive sphere, corneal thickness and average keratometry were found in both groups (low and high astigmatism) with p > 0.05.
Table 1.
Preoperative patient characteristics
| Characteristics | Total (74 patients) | Low Astigmatism (≤ -1.50D) | High Astigmatism (> -1.50D) | P |
|---|---|---|---|---|
| Female/male sex (n) | 50/24 | 40/20 | 10 /4 | |
| Patients (n) | 74 | 60 | 14 | |
| Eyes (n) | 120 | 97 | 23 | |
| Age (X ± SD) | 21.21 ± 3.84 | 21.30 ± 3.66 | 20.83 ± 4.56 | 0.648 |
| CDVA (X ± SD) | 0.02 ± 0.06 | 0.004 ± 0.019 | 0.08 ± 0.12 | < 0.001 |
| MRSE (D) | -4.31 ± 1.74 | -4.20 ± 1.59 | -4.77 ± 2.25 | 0.262 |
| Refractive Sphere (D) [9] | -3.75 ± 1.68 (-0.50 to -8.00) | -3.77 ± 1.59 (-0.50 to -6.50) | -3.66 ± 2.07 (-0.50 to -8.00) | 0.826 |
| Refractive Astigmatism (D) [9] | -1.14 ± 0.67 (-0.50 to -4.50) | -0.88 ± 0.33 (-0.50 to -1.50) | -2.22 ± 0.64 (-1.75 to -4.50) | < 0.001 |
| Corneal Thickness (μm) | 544.10 ± 24.47 | 544.45 ± 23.73 | 542.61 ± 27.89 | 0.771 |
| Average Keratometry (D) | 43.74 ± 1.15 | 43.73 ± 1.15 | 43.75 ± 1.18 | 0.957 |
Note: CDVA = corrected distance visual acuity; MRSE = manifest refraction spherical equivalent.
The corrected distance visual acuity (CDVA) in the group of patients with low astigmatic myopia was worse than that in the group of patients with high astigmatic myopia (p < 0.001). The average refractive astigmatism preoperative was -0.88 ± 0.33D in the low astigmatic group and -2.22 ± 0.64D in the high astigmatic group. The significant difference was less than 0.001. The minimum astigmatic value was -0.05D and maximum was -4.50D.
Some technical parameters during surgery were showed in table 2. The popular cap thickness was 120 µm in 97% of cases. The mean of the lenticule central thickness was 70 ± 36 µm. The average of the optical zone was 6.4 ± 0.3 mm with ranged from 6.3 mm to 7.0 mm.
Table 2.
Parameters during surgery
| Parameter | Value |
|---|---|
| Thickness of cap (%) | |
| 120 μm | 97 |
| 110 μm | 3 |
| Central thickness of lenticule (μm) | 70 ± 36 |
| Minimum lenticule thickness (μm) | 13 ± 3 |
| Optical zone (mm) [9] | 6.4 ± 0.3 (6.3 to 7.0) |
The refractive outcomes of both groups were presented in Table 3. A significant difference was found in the UDVA logMar postoperative between the low astigmatic group and high astigmatic group (0.004 ± 0.105; 0.057 ± 0.099, respectively, p = 0.031). However, the CDVA logMar postoperative in both groups had no significant difference with p = 0.136. Most of the refractive outcomes had not statistic significant between 2 groups (p > 0.05), such as the sphere, cylinder, MRSE, efficacy index, safety index, DV [10], MA, and AE. The TIA and the SIA in the low astigmatic group were less than those in the high astigmatic group (p < 0.001). The CI and IOS [9] were also significantly lower in the low astigmatic group than in the high astigmatic group with p = 0.026.
Table 3.
Comparison of refractive outcomes between low and high astigmatic groups
| Characteristics | Low Astigmatism (≤ -1.50D) | High Astigmatism (> -1.50D) | P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| UDVA LogMar | 0.004 | 0.105 | 0.057 | 0.099 | 0.031 |
| CDVA LogMar | -0.03 | 0.093 | 0.004 | 0.098 | 0.136 |
| Sphere (D) | 0.214 | 0.317 | 0.087 | 0.298 | 0.078 |
| Cylinder (D) | -0.271 | 0.548 | -0.207 | 0.209 | 0.366 |
| MRSE (D) | 0.093 | 0.313 | -0.015 | 0.312 | 0.145 |
| Efficacy index | 1.035 | 0.264 | 1.082 | 0.219 | 0.384 |
| Safety index | 1.113 | 0.235 | 1.215 | 0.221 | 0.057 |
| TIA | 0.879 | 0.335 | 2.217 | 0.641 | < 0.001 |
| SIA | 0.608 | 0.615 | 2.011 | 0.725 | < 0.001 |
| DV | 0.271 | 0.548 | 0.207 | 0.209 | 0.366 |
| CI | 0.656 | 0.504 | 0.896 | 0.116 | 0.026 |
| ME | -0.271 | 0.548 | -0.207 | 0.209 | 0.366 |
| AE | -2.99 | 7.265 | -1.087 | 6.735 | 0.238 |
| IOS | 0.344 | 0.504 | 0.104 | 0.116 | 0.026 |
Note: UDVA = uncorrected distance visual acuity; CDVA = corrected distance visual acuity; MRSE = manifest refractive spherical equivalent; TIA = target induced astigmatism; SIA = surgically induced astigmatism; DV = difference vector; CI = correction index; ME = magnitude of error; AE = angle of error; IOS = index of success
Figure 1 illustrated the UDVA postoperative in both groups. The UDVA postoperative was better than or equal to 20/20 Snellen in 75.3% (with the low astigmatic group) and in 69.6% (with the high astigmatic group). The UDVA of 20/30 or less in the low astigmatic group was approximately half of that in the high astigmatic group (8.3% and 17.4%, respectively).
Figure 1.
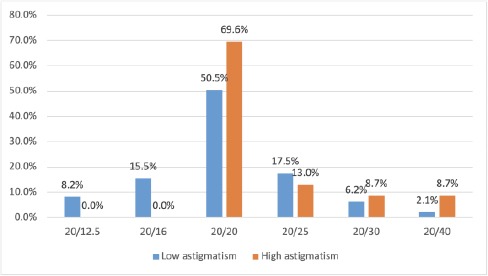
Uncorrected distance visual acuity postoperative
Result of comparison of UDVA between the low astigmatic group and the high astigmatic group was indicated in Table 4. Value of Pearson Chi-Square with 0.575 which meaned that the percentages of UDVA (≥ 20/20 and < 20/20) between two groups were similar.
Table 4.
Comparison of Uncorrected distance visual acuity between two groups
| UDVA | Low astigmatism (≤ 1.50D) | High astigmatism (> 1.50D) | Pearson Chi-Square |
|---|---|---|---|
| ≥ 20/20 | 73 (75.3%) | 16 (69.6%) | 0.575 |
| < 20/20 | 24 (24.7%) | 7 (30.4%) |
The correlation between the TIA and the SIA were indicated in Figure 2. This was a high positive correlation. We could see that most of the values were in undercorrection area that suggested the SIA was less than TIA, and there was an undercorrection in astigmatic treatment.
Figure 2.
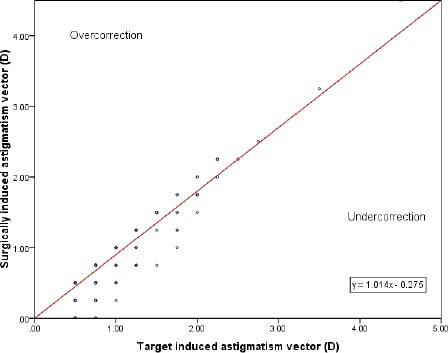
Target induced astigmatism and surgically induced astigmatism
Angle of Error (AE) was showed in Figure 3 and Table 5. The average absolute AE was 6.227 ± 4.758 and 5.348 ± 4.086 degrees (in the low astigmatic and the high astigmatic group). The result was not statistically different with p = 0.375.
Figure 3.
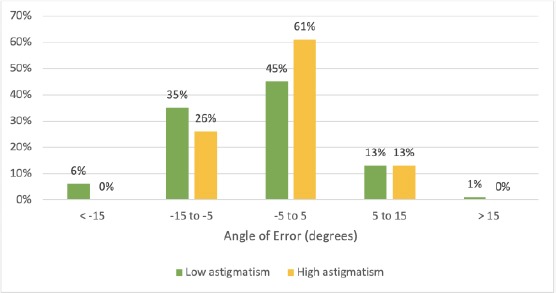
Refractive Astigmatism Angle of Error
Table 5.
Average of Angle of Error
| Angle of Error (degrees) | Low astigmatism (≤ 1.50D) | High astigmatism (> 1.50D) | p |
|---|---|---|---|
| Arithmetic | -2.99 ± 7.265 | -1.08 ± 6.73 | 0.238 |
| Absolute | 6.227 ± 4.758 | 5.348 ± 4.086 | 0.375 |
In the low astigmatic group, 93% of eyes had the AE within ± 15 degrees, and 100% was the rate of the AE within ± 15 degrees in the high astigmatic group. The difference in arithmetic and the absolute mean of the AE were not significantly (p = 0.238 and 0.375, respectively) (Figure 3).
There were not any cases which occurred a suction loss or other complications (such as an incomplete lenticule extraction) during the procedure. No eyes of corneal ectasia postoperative were recorded. This study did not also find keratitis after surgery.
Discussion
Some studies indicated that there was a certain rotation of the eye in the sitting and lying position. With the patients undergoing refractive surgery, the preoperative measurement refraction in the sitting position and the surgical intervention in the lying position may exist the rotational eye motions which could lead to reducing the efficacy of laser procedure, especially the eyes with high astigmatism [6]. Nowadays, many modern excimer laser systems have cyclotorsional control function that helps to increase the quality of treatment significantly by LASIK surgery [7]. Through the comparison between the iris images in the sitting and lying position, the laser system automatically analyzes to define the rotational eye. Then, the machine calculates and compensates for cyclotorsional eye motions. In the ReLEx SMILE surgery, Visumax machine has not the cyclotorsional control function that is considered the main limitation of this method. As a result, we would like to evaluate the outcomes of the ReLEx SMILE surgery for the eyes with myopic astigmatism (power of astigmatism ≥ 0.50D). In our study, we distributed the patients to two groups based on the power of astigmatism, which were the low astigmatic group (≤ 1.50D) and the high astigmatic group (> 1.50D). To minimizing the cyclotorsional eyes motions, we attempted to put the patients straight on the bed so that the patient’s head aligns with his body, avoiding head tilt.
The outcomes of the ReLEx SMILE in this study was generally very good. In particular, the UDVA postoperative was better than or equal to 20/20 Snellen in 75.3% (with the low astigmatic group) and in 69.6% (with the high astigmatic group). The UDVA of 20/30 or less in the low astigmatic group was approximately half of that in the high astigmatic group (8.3% and 17.4%, respectively). However, when we compared the percentages of UDVA (≥ 20/20 and < 20/20) between the low astigmatic group and the high astigmatic group, we recognized that there was no difference between two groups (p = 0.575). It that means ReLEx SMILE surgery provided good outcomes of UDVA in both two groups which did not depend on the power of astigmatism preoperative. This result was consistent with the study of Zhang et al., who presented the UDVA postoperative of 20/20 Snellen or more was in 79.6% [8]. One another research also showed the UDVA after the ReLEx SMILE surgery two years was better than or equal to 20/20 in 90% [9]. The authors all confirmed that ReLEx SMILE surgery gave good result of visual acuity in patient with astigmatic myopia.
To assess whether this surgery was effective and safe, we based on the efficacy index and the safety index. The efficacy index was the proportion between the UDVA decimal postoperative and the CDVA decimal preoperative, and the safety index was the proportion between the CDVA decimal postoperative and CDVA decimal preoperative. In our study, the efficacy index was basically 1.035 ± 0.264 with the low astigmatic group and 1.082 ± 0.219 with the high astigmatic group. There was no significant difference in the efficacy index between the two groups (p = 0.384). This index was similar with the result of Lin et al. (1.04 ± 0.20) [5]. Similarly, the safety index of this study was 1.113 ± 0.235 and 1.215 ± 0.221 in the low astigmatic group and the high astigmatic group, respectively, with no significant difference (0.057). This index was also approximately to result of Lin et al., (1.01 ± 0.05) [5]. Another study of Chan et al., showed the efficacy of ReLEx SMILE surgery with 79.6% of eyes had a UDVA of 20/20 or better, and the safety with 57.1% of eyes gained one line of visual acuity, only one eye lost one line, no case lost two or more lines [10]. The outcomes provided evidence to the consideration that ReLEx SMILE surgery was extremely effective and safe in correcting low to moderate astigmatism [10].
The MRSE postoperative was generally good and no statistic difference between two groups (Table 3). Residual astigmatism postoperative (equal to the difference of vector) in the low astigmatic group and the high astigmatic group was 0.271 ± 0.548 and 0.207 ± 0.209D, respectively, with no significant difference (0.366). However, the correction index (CI) and index of success (IOS) [11] were 0.896 and 0.104 (respectively) in the high astigmatic group and significantly higher than those in the low astigmatic group (p = 0.026). This difference may be correlated with the results of the angle of error (AE). In the low astigmatic group, the AE within ± 5 degrees was only accounted in 45%, and 61% in the high astigmatic group. Moreover, 93% of eyes had an AE within ± 15 degrees in the low astigmatism group, and 100% was the rate of the AE within ± 15 degrees in the high astigmatic group (Figure 3). In our results (Table 5), although the difference of the AE was not statistically significant, the lower value of high astigmatic group may occur due to pay special attention to aligning that was occupied less than in the low astigmatic group.
We observed the undercorrection in this study (Figure 2). Several previous researches also showed a trend toward undercorrection [8], [11]. There was a study that compared the astigmatic treatment between two methods (the LASIK and the ReLEx SMILE) indicated excellent results with both techniques. However, an undercorrection postoperative was found in both surgeries in the eyes with high astigmatism (> 2.25 D) that agreed with our results. The high AE values might be the reason for those undercorrections [12].
To compensate for the rotational eye movements, Ganesh introduced a manual technique [13]. They used a pen to mark the horizontal axis of the limbal cornea when the patient sat up straight. After the patient lay down on the operation bed, the docking and suction progress were implemented through the contact glass. The surgeon looked in the microscope eyepiece and corrected the horizontal axis of the centering grid (inside of the microscope eyepiece) to coincide with the marked axis on the patient’s eye by manual rotation contact glass. The authors of this study indicated the average cyclotorsion was 5.64 ± 2.55 degrees in 82% of eyes and the rotational contact glass technique compensated well for it. However, this method still existed some drawbacks. The first, the initial marking might be not incorrect. The second, the marking might lead the damage of the cornea, which could make the patients uncomfortable during and after surgery. The third, loss of suction might occur because of the above discomfort or the manual rotation contact glass. The final, the ink marks or the lesions could appear on the cornea might result due to the black spots after laser performance.
However, we did not implement corneal marking before surgery because we had only four eyes with real high astigmatism (≥ 2.50D) in this study. Therefore, we have just aligned exactly the patient’s body and head, central fixation of eyes, we have achieved good astigmatic correction.
In conclusion, although the Visumax machine has not any modern eye-tracking systems as well as the cyclotortional control function, the ReLEx SMILE surgery still has had high safety and efficacy index. The most important in treatment for myopic astigmatism undergoing SMILE surgery is to carefully align the patient’s body and head, central fixation of eyes during the procedure.
Results of the ReLEx SMILE surgery might be improved with cyclotorsional control systems in the newer generations in the future.
Ethical approval
Our study was accepted by the institutional ethics committee of the Vietnam National Institute of Ophthalmology. All patients understood and voluntarily participated in this study.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Sekundo W, Kunert KS, Blum M. Blum, Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism:results of a 6 month prospective study. Br J Ophthalmol. 2011;95(3):335–9. doi: 10.1136/bjo.2009.174284. https://doi.org/10.1136/bjo.2009.174284 PMid:20601657. [DOI] [PubMed] [Google Scholar]
- 2.Ivarsen A, Asp S, Hjortdal J. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmology. 2014;121(4):822–8. doi: 10.1016/j.ophtha.2013.11.006. https://doi.org/10.1016/j.ophtha.2013.11.006 PMid:24365175. [DOI] [PubMed] [Google Scholar]
- 3.Kim JR, et al. Efficacy, predictability, and safety of small incision lenticule extraction:6-months prospective cohort study. BMC Ophthalmol. 2014;14:117. doi: 10.1186/1471-2415-14-117. https://doi.org/10.1186/1471-2415-14-117 PMid:25280533 PMCid:PMC4192335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denoyer A, et al. Dry eye disease after refractive surgery:comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology. 2015;122(4):669–76. doi: 10.1016/j.ophtha.2014.10.004. https://doi.org/10.1016/j.ophtha.2014.10.004 PMid:2545∃. [DOI] [PubMed] [Google Scholar]
- 5.Lin F, Xu Y, Yang Y. Comparison of the visual results after SMILE and femtosecond laser-assisted LASIK for myopia. J Refract Surg. 2014;30(4):248–54. doi: 10.3928/1081597X-20140320-03. https://doi.org/10.3928/1081597X-20140320-03 PMid:24702576. [DOI] [PubMed] [Google Scholar]
- 6.Chernyak DA. Cyclotorsional eye motion occurring between wavefront measurement and refractive surgery. J Cataract Refract Surg. 2004;30(3):633–8. doi: 10.1016/j.jcrs.2003.08.022. https://doi.org/10.1016/j.jcrs.2003.08.022 PMid:15050260. [DOI] [PubMed] [Google Scholar]
- 7.Prakash G, et al. Comparison of laser in situ keratomileusis for myopic astigmatism without iris registration, with iris registration, and with iris registration-assisted dynamic rotational eye tracking. J Cataract Refract Surg. 2011;37(3):574–81. doi: 10.1016/j.jcrs.2010.11.025. https://doi.org/10.1016/j.jcrs.2010.11.025 PMid:21333879. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, et al. Vector analysis of low to moderate astigmatism with small incision lenticule extraction (SMILE):results of a 1-year follow-up. BMC Ophthalmol. 2015;15(1):8. doi: 10.1186/1471-2415-15-8. https://doi.org/10.1186/1471-2415-15-8 PMid:25618419 PMCid:PMC4328987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobashi H, et al. Two-years results of small-incision lenticule extraction and wavefront-guided laser in situ keratomileusis for Myopia. Acta Ophthalmol. 2018;96(2):e119–e126. doi: 10.1111/aos.13470. https://doi.org/10.1111/aos.13470 PMid:2↷305. [DOI] [PubMed] [Google Scholar]
- 10.Chan TC, et al. Vector analysis of astigmatic correction after small-incision lenticule extraction and femtosecond-assisted LASIK for low to moderate myopic astigmatism. Br J Ophthalmol. 2016;100(4):553–9. doi: 10.1136/bjophthalmol-2015-307238. https://doi.org/10.1136/bjophthalmol-2015-307238 PMid:26206791. [DOI] [PubMed] [Google Scholar]
- 11.Khalifa MA, et al. Vector analysis of astigmatic changes after small-incision lenticule extraction and wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg. 2017;43(6):819–824. doi: 10.1016/j.jcrs.2017.03.033. https://doi.org/10.1016/j.jcrs.2017.03.033 PMid:28732617. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Wang Y, Chen X. Comparison of moderate-to high-astigmatism corrections using WaveFront-guided laser in situ Keratomileusis and small-incision Lenticule extraction. Cornea. 2016;35(4):523–30. doi: 10.1097/ICO.0000000000000782. https://doi.org/10.1097/ICO.0000000000000782 PMid:26890662. [DOI] [PubMed] [Google Scholar]
- 13.Ganesh S, Brar S, Pawar A. Results of Intraoperative Manual Cyclotorsion Compensation for Myopic Astigmatism in Patients Undergoing Small Incision Lenticule Extraction (SMILE) J Refract Surg. 2017;33(8):506–512. doi: 10.3928/1081597X-20170328-01. https://doi.org/10.3928/1081597X-20170328-01 PMid:287∯4. [DOI] [PubMed] [Google Scholar]


