Abstract
Background
Cervical spondylosis causes pain and disability by compressing the spinal cord or roots. Surgery to relieve the compression may reduce the pain and disability, but is associated with a small but definite risk. .
Objectives
To determine whether: 1) surgical treatment of cervical radiculopathy or myelopathy is associated with improved outcome, compared with conservative management and 2) timing of surgery (immediate or delayed pending persistence/progression of relevant symptoms and signs) has an impact on outcome.
Search methods
We searched CENTRAL, MEDLINE, and EMBASE to 1998 for the original review. A revised search was run in CENTRAL (The Cochrane Library 2008, Issue 2), MEDLINE, EMBASE, and CINAHL (January 1998 to June 2008) to update the review.
Authors of the identified randomised controlled trials were contacted for additional published or unpublished data.
Selection criteria
All randomised or quasi‐randomised controlled trials allocating patients with cervical radiculopathy or myelopathy to 1) "medical management" or "decompressive surgery (with or without fusion) plus medical management" 2) "early decompressive surgery" or "delayed decompressive surgery".
Data collection and analysis
Two authors independently selected trials, assessed risk of bias and extracted data.
Main results
Two trials (N = 149) were included. In both trials, allocation concealment was inadequate and arrangements for blinding of outcome assessment were unclear.
One trial (81 patients with cervical radiculopathy) found that surgical decompression was superior to physiotherapy or cervical collar immobilization in the short‐term for pain, weakness or sensory loss; at one year, there were no significant differences between groups.
One trial (68 patients with mild functional deficit associated with cervical myelopathy) found no significant differences between surgery and conservative treatment in three years following treatment. A substantial proportion of cases were lost to follow‐up.
Authors' conclusions
Both small trials had significant risks of bias and do not provide reliable evidence on the effects of surgery for cervical spondylotic radiculopathy or myelopathy. It is unclear whether the short‐term risks of surgery are offset by long‐term benefits. Further research is very likely to have an impact on the estimate of effect and our confidence in it.
There is low quality evidence that surgery may provide pain relief faster than physiotherapy or hard collar immobilization in patients with cervical radiculopathy; but there is little or no difference in the long‐term.
There is very low quality evidence that patients with mild myelopathy feel subjectively better shortly after surgery, but there is little or no difference in the long‐term.
Plain language summary
Surgery for cervical radiculomyelopathy
Cervical spondylosis, or degeneration (wear and tear of the bones and discs in the neck), is a very common condition affecting most of us at some point in our lives. It is frequently related to strain of the supporting muscles or wear and tear of the discs that connect the individual bones (vertebrae) that form the spine, resulting in neck pain. Radiculopathy is pain, weakness or reduced reflexes that follow the path of nerves that come from the neck region. Myelopathy is spasticity and weakness in the lower limbs with or without "numb and clumsy" hands.
Most people with degeneration in the neck area may have no symptoms. In 10% to 15% of cases, the condition worsens to the extent that surgery is recommended. Surgery is aimed at improving these problems, but it is unclear which type of surgical procedure is best and how effective it is.
This review of two trials with 149 patients found no conclusive evidence to support surgical treatment for people with degeneration, radiculopathy or myelopathy. Possible limitations of this review include the the lack of large trials and the risk of bias associated with these studies. Further research is very likely to change the estimate of effects and our confidence in the results.
Future large‐scale randomised trials with better methods are needed to provide clear evidence on the balance between risk and benefit from surgery for individuals with cervical degeneration with radiculopathy or myelopathy.
Background
Size of the problem: Cervical degenerative disease is an almost universal concomitant of human aging. Over half of the middle‐aged population has radiological or pathological evidence of cervical spondylosis (Hughes 1965; Irvine 1965; Pallis 1954). This condition is often asymptomatic, but in 10% to 15% of the cases, it is associated with, or progresses to, root or cord compression (Bednarik 2004; Teresi 1987)
Prognosis of conservatively treated cases: The natural history of symptomatic cervical degenerative disease is uncertain because traditionally, some patients have been treated surgically. However, the idea that the conservatively treated individuals will necessarily develop progressive disability is not supported by reliable evidence (Bednarik 2004; Bradshaw 1957; Campbell 1960; Clarke 1956; King 2005; Lees 1963). The disease may remain static for lengthy periods; sometimes patients with severe disability can also improve without surgery (Lees 1963; Nurick 1972). The widespread belief that patients with radicular symptoms will eventually develop overt myelopathy is also not based on good evidence.
Surgical morbidity rates: Surgical procedures for cervical radiculo‐myelopathy can be associated with complications, including death (Apfelbaum 2000; Berge Henegouwen 1991; Bertalanffy 1989; Burke 2005; Ebershold 1995; Herkowitz 1989; Polkey 1984; Rowland 1992; Saunders 1991). Reported death rates varied from zero to 1.8%. Non‐fatal complications, including oesophageal perforation, carotid or vertebral artery injuries, or injury to the neural structures occurred in 1% to 8% of patients. The therapeutic effects of surgery are not always satisfactory (King 2005; Lunsford 1980) and the overall outcome may be similar to conservative management (Persson 1997).
Choice of surgical procedure: The best type of surgical procedure for this condition is not known. Decompression of the cord or nerve root is the principal aim, which can be achieved with a variety of anterior cervical approaches or posterior cervical laminectomies. Anterior cervical decompression is traditionally combined with fusion of the decompressed segment (Chagas 2005), although evidence exists that this may not be necessary (Martins 1976). Artificial materials have also been used to avoid postoperative pain in the graft donor site. They were met with initial enthusiasm and subsequent disappointment due to failure of fusion (Hafez 1997), post‐operative pseudarthrosis, or disease progression adjacent to the level of the arthrodesis (Hilibrand 1999). However, promising results have been reported lately with the use of hydroxyapatite, ceramic, carbon, and polyetheretherketone (PEEK) cages, and more recently with cervical artificial disks (Cho D‐Y 2002; Coric 2006; Frederic 2006).
The need for a systematic review: In view of these uncertainties, it is not surprising that there are substantial variations in the proportion of patients with cervical spondylotic myelopathy or radiculopathy who are referred for surgery (Harland 1998). Furthermore, among patients selected for surgery, there are substantial variations between centres in the choice of operative procedure. There is clearly a need for a systematic review of the randomised trials comparing "surgery" with "no surgery". A systematic review of the trials comparing one type of surgical approach with another will be the subject of a separate review (Jacobs 2004). This update was necessary, since no concrete evidence on the role of surgery in the treatment of cervical radiculo‐myelopathy was identified in the original review.
Objectives
To determine whether: 1) surgical treatment of cervical radiculopathy or myelopathy is associated with improved outcome, compared to conservative management and 2) timing of surgery (immediate or delayed upon persistence/progression of relevant symptoms and signs) has an impact on outcome.
Methods
Criteria for considering studies for this review
Types of studies
All randomized or quasi‐randomised controlled trials allocating patients with cervical radiculopathy or myelopathy to: 1) "Medical management" or "Decompressive surgery (with or without some form of fusion) plus medical management" or 2) "Early decompressive surgery" or "Delayed decompressive surgery".
No language restrictions were implemented.
Types of participants
Patients with a clinical diagnosis of cervical radiculopathy (pain along the cutaneous distribution of one or more cervical roots, sometimes associated with weakness and hyporeflexia), or myelopathy (spasticity and weakness in the lower limbs with or without "numb and clumsy" hands), and supported by appropriate radiological findings.
Patient characteristics such as age, disability at presentation, duration of symptoms, cord diameter, cord area and altered cord signal on MRI (Mehalic 1990; Singh 2001; Suri 2003), increased cervical spinal mobility (Barnes 1984; Sampath 2000) and the presence of a congenitally narrow spinal canal (Clifton 1990; McCormack 1996) which may influence outcome were also considered where available.
Types of interventions
Any form of surgical decompression in the cervical spine, with or without fusion, designed to alleviate the symptomatic cord or root compression.
Types of outcome measures
Information on the following outcome measures was independently obtained by two review authors for each trial where available: i) Surgical morbidity (neurological deficit, oesophageal or recurrent laryngeal nerve injury, deep seated infection or repeat surgery) and mortality (within four weeks of surgery) ii) Pain intensity (in the neck, head or limbs) measured by: visual analogue scale, or other measure of pain severity. iii) Functional performance of the arms or legs measured by: nine‐hole peg task, 10‐metre walk, NCSS, Sickness Impact Profile, Odom's and Ranawat's criteria, Nurick's scale (Nurick 1972). iv) Mood measured by: Mood Adjective Check List and Hospital Anxiety and Depression (HAD) scale, or other validated questionnaire. v) Quality of life measured by: SF‐36, Health Assessment Questionnaire (HAQ) or EuroQol.
Physical examination or other symptoms were not included as outcomes, since these are highly subjective and are not validated measures of outcome.
Search methods for identification of studies
The Trials Search Co‐ordinator of the Cochrane Back Review Group oversaw the search for the review update. This was run three times, in October 2006 (from January 1998, with the original search strategy), August 2007 (from January 1998 with a revised search strategy) and June 2008, in CENTRAL (The Cochrane Library 2008, Issue 2), MEDLINE, EMBASE and CINAHL. See Appendix 1. The search strategy was revised for the update so that is was more in line with the search strategy recommended by the Cochrane Back Review Group (Furlan 2009)
For the original review, an electronic search was performed in the Cochrane Central Register of Controlled Trials, MEDLINE (between 1966 and 1998) and EMBASE (between 1980 and 1998). The search strategy was developed with the aid of the Co‐ordinator of the Cochrane Stroke Trial Search Group. See Appendix 2;
Finally, all references in the identified randomised controlled trials were checked and authors contacted to detect any additional published or unpublished data.
Data collection and analysis
Titles and abstracts identified from the database were checked by two authors (IPF and PFXS for the original review and IPF and IN for the update). The assessment was not blinded for authors, institutions or journal since there is little evidence from the Cochrane Methods Group that this makes a measurable improvement in the quality of reviews. The full texts of all studies of possible relevance were obtained for independent assessment by both authors. The authors decided which trials fit the inclusion criteria, and graded their methodological quality. Any disagreements were resolved by discussion between the authors. Authors were contacted for clarification where necessary.
Two review authors independently re‐assessed the risk of bias in the updated review using the 12 criteria recommended in the updated method guidelines for systematic reviews in the Cochrane Back Review Group (Furlan 2009; Appendix 3), and met to reach consensus. We decided not to blind studies for authors, institution or journal because the review authors who assessed the risk of bias were familiar with the literature. Criteria were marked as 'yes' if they were met, 'no' if not, and 'unsure' if there was insufficient information provided to make a judgment. The results of our assessment are in the Risk of Bias Tables for each included study.
Two authors performed data extraction independently and the authors of trials were contacted to provide missing data where possible. This was necessary in one report originally published in Czech, the author of which provided a translation into English upon our request. Data were checked and entered onto the computer by one author.
A weighted treatment effect (using random effects) was calculated across trials using the Cochrane statistical package, RevMan version 5.1. The results were expressed as odds ratio (OR and 95% confidence intervals (CI)) and risk difference (RD with 95% CI) for dichotomous outcomes and weighted mean difference (WMD and 95% CI) for continuous outcomes. When data were inadequate, no quantitative comparisons were reported.
Sensitivity analyses were to be performed on the basis of methodological quality and to test for heterogeneity in the results. In addition, sub‐group analyses were to be used to investigate possible differences between patients with different characteristics, as they may influence the response to treatment, as explained earlier. If data were available, sub‐group analyses were to be performed to investigate the effect of age, disability at presentation, cord diameter, cord area and altered cord signal on MRI, increased cervical spinal mobility and presence of congenitally narrow spinal canal on outcome measures. However, given the paucity of data, it became impossible to perform detailed sensitivity analyses.
Quality of Evidence
The quality of evidence for each outcome was assessed using the GRADE approach (Furlan 2009). Each outcome was assessed on five domains: limitation of study design, inconsistency, indirectness (inability to generalize), imprecision (insufficient or imprecise data) of results, and publication bias (Appendix 4). The overall quality of evidence for each outcome is the result of the combination of the assessments in all domains. High quality evidence was provided by RCTs with low risk of bias that provided consistent, direct and precise results with no other considerations for potential bias for the outcome. The quality was reduced by a level for each of the domains not met. The following lists the five levels of evidence for the GRADE approach:
High quality of evidence: Further research is very unlikely to change our confidence in the estimate of effect. There are consistent findings among 75% of RCTs with low risk of bias that are generalizable to the population in question. There are sufficient data, with narrow confidence intervals. There are no known or suspected reporting biases. (All of the domains are met.)
Moderate quality of evidence: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. (One of the domains is not met.)
Low quality of evidence: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. (Two of the domains are not met.)
Very low quality of evidence: We are very uncertain about the estimate. (Three of the domains are not met.)
No evidence: there were no trials that measured this outcome
Results
Description of studies
We found four trials after screening over 13,000 citations for the original review published in 1998. Only two were included in the review. One trial recruited 81 patients with cervical radiculopathy (Persson 1997). One trial recruited 68 patients with cervical myelopathy Bednarik 1999.
The results for the January 1998 to August 2007 search were screened and 43 RCTs were selected. The results for the August 2007 to June 2008 search were screened and 19 RCTs were selected. The sets were combined, and two duplicates were removed, leaving a total of 60 papers. Of these, most reported trials comparing the efficacy of different surgical techniques (e.g. discectomy versus fusion) or devices (e.g. implant versus grafting) and all of the recruited patients were treated surgically. A few studies compared surgical treatment to non‐surgical treatment. Two of these involved prospective clinical trials. A number of other papers reported updates from the two studies included in the initial review (Persson 1997; Bednarik 1999). Summary details of the trials are given in the Characteristics of included studies section; note we updated Bednarik 1999 to Kadanka 2002, to reflect the most recent report on follow‐up.
Patients with clinical suspicion of cervical radiculopathy, in whom no radiological confirmation (with CT‐myelography or MRI) was evident (N = 19) were excluded in the study by Persson 1997. Similarly, patients with clinical and radiological evidence of cervical cord compression (N = 10) were excluded from the same study. Kadanka 2002 excluded patients with severe cervical myelopathy (modified Japanese Orthopaedic Association score less than 12, N = 12).
Surgically treated patients were operated on through a variety of anterior or posterior cervical approaches.
Functional status was assessed in Kadanka's study with a modified Japanese Orthopaedic Association scale (Benzel 1991), and a gait analysis employing the authors' own scale (Kadanka 1997). Briefly, the gait scale scores were as follows: patients able to walk for 5 km scored 10 points, those who were able to walk for 1 km scored 9 points, those who could walk for 500 m scored 8 points, those who walked for 100 m scored 7 points, those who walked for 25 m scored 6 points, those who could walk for 100 m with the aid of a walking stick scored 5 points, those who could walk 25 m with the help of a walking stick scored 4 points, those who could walk 10 m with a walking stick scored 3 points, those who could only mobilize around their bed scored 2 points, whereas bedridden patients scored 1 point. In addition, evaluation of daily activities by video recording (the recordings showed how the patients buttoned their shirts, brushed their hair and teeth, performed diadochokinesis, put on their shoes, walked and ran, and went up and down stairs), performed by two physicians blinded to the type of treatment was undertaken. Finally, patient self‐evaluation using the same scale as that used in video recording was reported.
Kadanka's study from 2000 used the same cohort reported by the same group in Kadanka 2002, with a limited (two‐year) follow‐up.Two studies by Kadanka in 2005 provided no new information. The aim of those reports was to identify subgroups of patients that may benefit from surgery without presenting any reliable data in favour of surgery in the first place. Finally, a paper by Persson in 2001 reported the same information already included in the original review from Persson 1997.
In relation to the other randomized control trials, Nardi 2005 did not provide data to support the conclusion that percutaneous cervical nucleoplasty is better than conservative treatment for radiculopathy (attempts to contact the authors through e‐mail, fax, in writing and telephone, for further clarification, were unsuccessful).The same applied to Li 2004, where no data to support the statement that micro‐endoscopic discectomy is superior to conservative treatment were provided. Therefore, these two trials were excluded.
Risk of bias in included studies
Both studies were small and therefore prone to small study bias. Issues related to selection bias (Kadanka 2002, performance bias (Persson 1997), attrition bias (Kadanka 2002) and detection bias (in both trials, inherent to studies including surgically‐treated patients) were identified (refer to Risk of bias tables and Figure 1).
1.
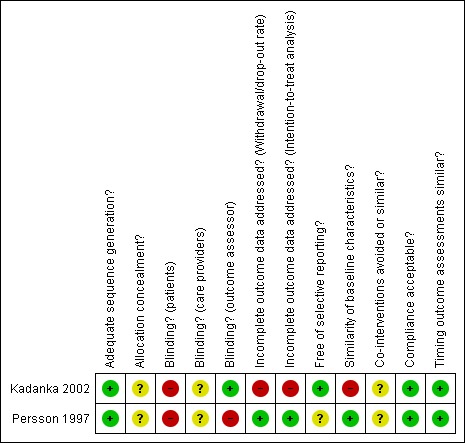
Risk of bias assessment: review authors' judgements about each risk of bias item for each included study.
Allocation
A coin toss was used for randomisation in Kadanka 2002, and sealed opaque envelopes were used in Persson 1997.
Blinding
In one trial, clinical evaluation was provided by a physiotherapist not taking part in the physiotherapy treatment (Persson 1997). The assessor was not blinded to the treatment modality (the patients were wearing a collar during the assessment). In Kadanka 2002, all patients were wearing a collar, therefore blinding the assessment.
Incomplete outcome data
In Persson 1997, 16 months after the onset of treatment, one patient in the surgery group had moved and was lost at follow‐up. In addition, one patient in the collar group did not keep her appointment because, according to the authors, she had completely recovered. In Kadanka 2002, 19 patients were lost to follow‐up between 24 and 36 months. Five surgically‐treated patients died during this follow‐up period, but the authors state that their deaths were physically unrelated to the surgery.
Effects of interventions
A) Surgical morbidity or mortality‐number of procedures per patient
No major adverse events or deaths related to surgery were reported in the trials. In Persson 1997, one surgically‐treated patient had a graft infection and was operated on again (between three months and one year from the time of the original enrolment). Another surgically‐treated patient had an exploration of the brachial plexus and a further six patients had surgery in adjacent levels during the same period. In Kadanka 2002, five patients died, at least two years from the time of the initial enrolment.
B) RADICULAR PAIN in cervical radiculopathy
One trial included randomised data from 81 patients and evaluated radicular pain in cervical radiculopathy (Persson 1997). The pain intensity was assessed by means of a visual analogue scale (VAS). Current pain and worst pain during the preceding week was reported. This was repeated eight to12 days later and the mean value was used for statistical analysis.
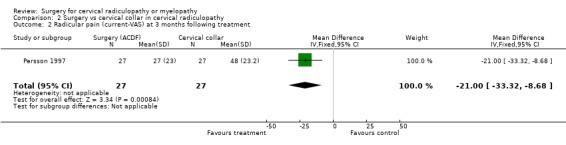
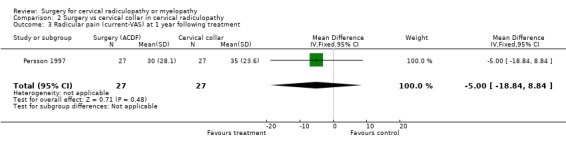
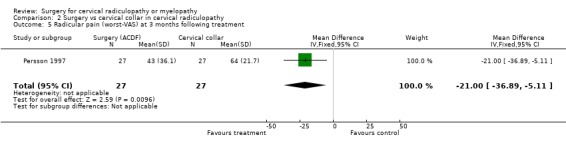
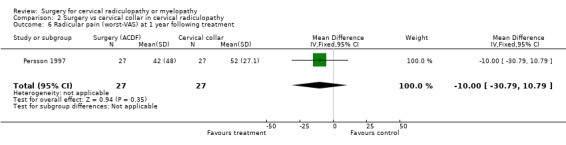
There is low quality evidence (unable to generalize, sparse data) from one trial (N = 81), that at three months, surgically treated patients had significantly less pain than those treated with physiotherapy (MD ‐14, 95% CI ‐27.84 to ‐0.16) and a cervical collar (MD ‐21, 95% CI ‐33.32 to ‐8.68), but at one year, there was no statistical difference between the groups (Physio: MD ‐9, 95% CI ‐23.39 to 5.39; Collar:(MD ‐5, 95% CI ‐18.84 to 8.84)). There was also low quality evidence (unable to generalize, sparse data) from one trial (N = 81), that at three months, the 'worst pain in the preceding week' reported by surgically‐treated patients was significantly less than those treated with a collar at three months ((MD ‐21, 95% CI ‐36.89 to ‐5.11), but there were no significant differences between the groups at one year (MD ‐10, 95% CI ‐30.79 to 10.79).
C) Functional performance in cervical myelopathy
Surgery was compared with conservative treatment (intermittent cervical immobilization (soft collar), NSAIDs, intermittent bed rest) in one study with a high risk of bias (Kadanka 2002; N = 68). Patients with mild deficits were randomised.
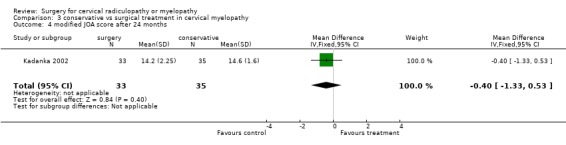
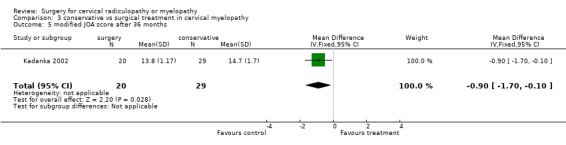
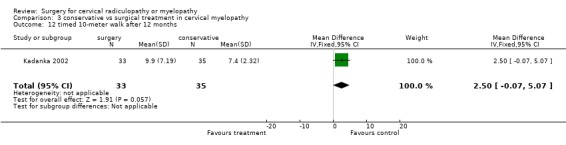
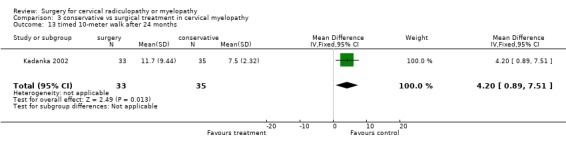
There is low quality evidence from one trial (N = 68 patients) that at six and thirty‐six months, there was no significant difference in function (assessed with a modified JOA scale) between patients treated conservatively and those who underwent surgery (MD ‐1.2, 95% CI ‐2.18 to ‐0.22; MD ‐0.9, 95% CI ‐1.70 to ‐0.10, respectively). At 12 and twenty‐four months, those who were treated conservatively were significantly better (MD ‐0.7, 95% CI ‐1.73 to 0.33; MD ‐0.9, 95% CI ‐1.70 to ‐0.10, respectively) than those who had undergone surgery.
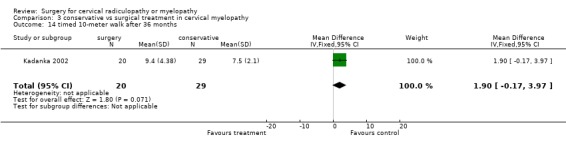
At thirty‐six months, those who were treated conservatively scored better on the 10‐meter walk (MD 1.90, 95% CI ‐0.17, 3.97) than those who had undergone surgery
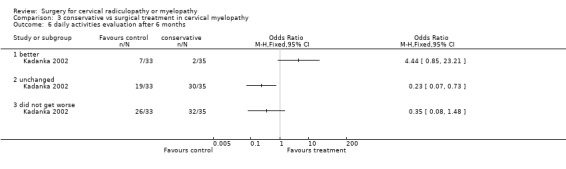
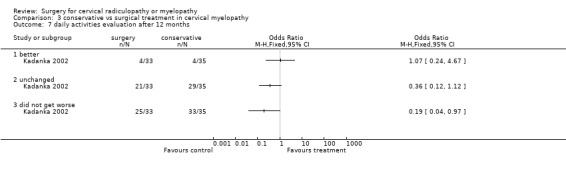
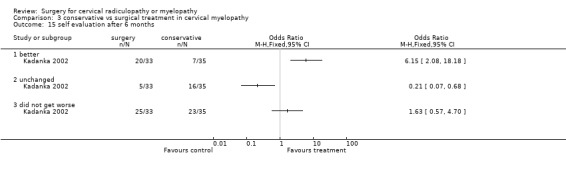
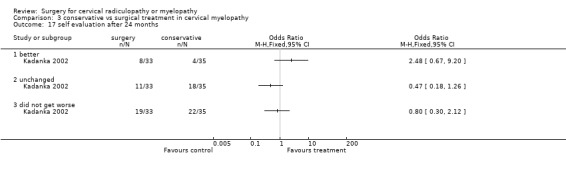
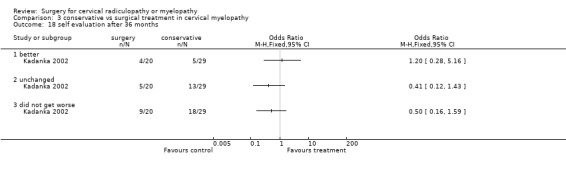
At six months, significantly more surgically treated patients (20 out of 33) felt better, compared to the conservatively treated cohort (7/35). No differences were found in those who were unchanged (5/33 in the surgical and 16/35 in the conservative group) or did not get worse (25/33 in the surgical and 23/35 in the conservative group). However, by thirty‐six months, there were no significant differences between the surgically treated patients (4 out of 20) who felt better, compared to the conservatively treated cohort (5/29). No differences were found in those who were unchanged (5/20 in the surgical and 13/29 in the conservative group) or did not get worse (9/20 in the surgical and 18/29 in the conservative group).
In summary, there is very low quality evidence (high risk of bias, inability to generalize, sparse data) from one trial (N = 68) that there is little or no difference in function and quality of life in the long‐term (thirty‐six months) between those with mild myelopathy who had received surgery and those who had received conservative treatment.
D) Other outcomes (limb paraesthesia and sensory loss) in cervical radiculopathy
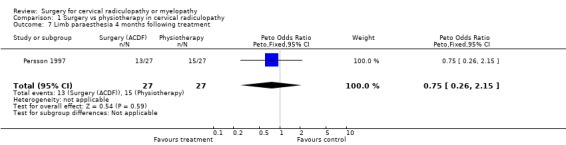
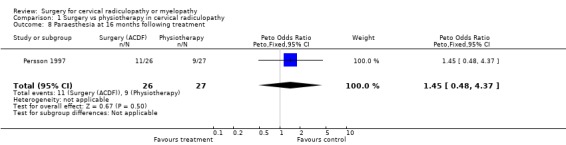
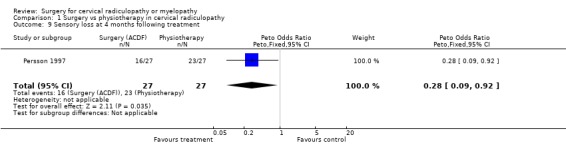
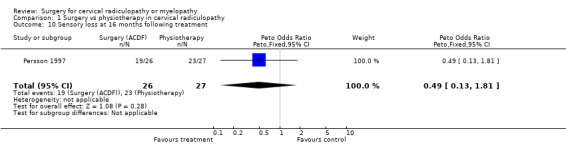
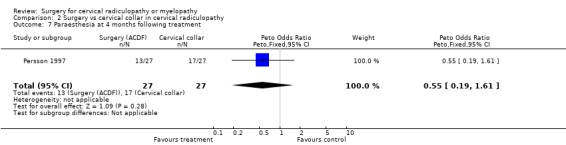
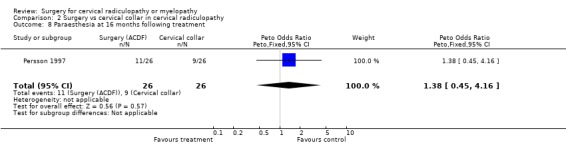
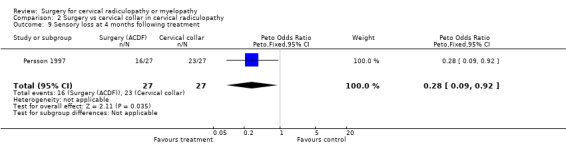
Almost all of the patients (98%) had paraesthesia at presentation (Persson 1997). At four months post‐treatment, no differences were noted between surgically‐treated patients and those treated with physiotherapy, in relation to those patients who improved and those in whom the paraesthesia remained unchanged or became worse (Odds Ratio (OR) 0.75, 95% CI 0.26 to 2.15). In addition, no differences between groups were noted at 16 months post‐treatment (OR 1.45, 95% CI 0.48 to 4.37). When comparing surgery with cervical collar, no differences were noted at four months post‐treatment, in relation to those patients who improved and those in whom the paraesthesia remained unchanged or became worse (OR 0.55, 95% CI 0.19 to 1.61). Finally, no differences between groups were noted at 16 months post‐treatment (OR 1.38, 95% CI 0.45 to 4.16). Sensory loss was noted in 54% of patients at presentation (Persson 1997). It is not clear whether it was equally distributed between the different groups of patients. At four months post‐treatment, the surgically treated patients fared better (had more patients who improved than those who remained unchanged or became worse) than those treated with physiotherapy, (OR 0.28, 95% CI 0.09 to 0.92). However, no differences between groups were noted at 16 months post‐treatment (OR 0.49, 95% CI 0.13 to 1.81). Similarly, when comparing surgery with cervical collar, differences were noted at four months post‐treatment, with the surgically treated patients demonstrating more favourable results (OR 0.28, 95% CI 0.09 to 0.92). However, no differences between groups were noted at 16 months post‐treatment (OR 0.51, 95% CI 0.14 to 1.90).
In summary, there is low quality evidence (unable to generalize, sparse data) from one trial (N = 81), that there is little or no difference in limb paraesthesia or sensory loss in the long‐term (16 months) between patients with cervical radiculopathy who were treated surgically and those who were treated with either a cervical collar or physiotherapy.
Discussion
A) ROLE OF SURGERY IN CERVICAL SPONDYLOTIC RADICULOPATHY
We found a single prospective randomised controlled trial, comparing surgical and conservative treatment for cervical radiculopathy (Persson 1997). At three months, surgery resulted in superior results in terms of pain (reduction of 29% in VAS), compared to physiotherapy (19% reduction in VAS) or hard collar immobilization (4% reduction in VAS). However, at one year, there were no significant differences in measurements among groups, with a 30% reduction in pain in the surgically‐treated patients, 17% in the patients treated with physiotherapy and 16% in those treated with a hard collar. Similar results were obtained in relation to sensory loss, where at four months post‐treatment, surgically‐treated patients had better outcomes, but at 16 months there were no significant differences between groups. These results confirm a short‐term benefit from surgery, compared with physiotherapy or immobilization in terms of pain relief, which was not present at one year after the onset of treatment. There were a considerable number of cross‐overs in the patients and the total number of participants was very small. The apparent lack of treatment benefit at one year in this study may represent a Type II error, so a larger number of patients may be needed to provide more reliable evidence on long‐term effects of surgery. However, the results of this study are in line with a community‐based epidemiological survey of 561patients from Rochester, where a spontaneous symptomatic improvement within five years from the onset of symptoms was reported in 75% of patients with cervical radiculopathy (Radhakrishnan 1994). The long‐term benefit from surgery has been questioned, and the early improvement may sometimes be followed by accelerated clinical deterioration due to progression of the pathological process in the treated or adjacent levels of the cervical spine. The lack of influence upon the natural history of the condition has also been speculated in large retrospective reviews, where late functional deterioration manifested in patients who exhibited early improvement following surgical intervention (Ebershold 1995).
Surgically treated patients had better results in relation to muscle strength compared to the two conservatively treated groups at four months according to the authors (data inadequate for comparisons). At one year, there were no differences between groups. These findings may well represent the improvement in pain control, which is closely related to muscle strength.
Finally, an important consideration in association with the findings of this study underlies the importance of recruiting an independent assessor of outcome. The functional improvement following surgery was much more modest (40% reduction in pain in the visual analogue scale) compared to the majority of reports (in which the surgeon's bias is not eliminated) which estimate an improvement in 80% to 90% of patients (Ebershold 1995; Henderson 1983; Martins 1976). Of equal interest, a prospective, non‐randomized, multi‐centre investigation of 246 patients with cervical radiculopathy and blinded assessment reported significant improvement in pain in the medically‐treated patients, and persistent excruciating pain in 26% of the surgically treated patients (Sampath 1999).
B) ROLE OF SURGERY IN CERVICAL SPONDYLOTIC MYELOPATHY
A small study of 68 patients with mild or moderate myelopathy were allocated by coin toss to surgery or conservative treatment (Kadanka 2002). Age and sex ratio were similar between the two groups. The slight imbalance of mJOA scores and the somewhat greater imbalance of gait scores, both favouring the groups allocated to conservative treatment, suggest that either the treatment allocation may have been biased or that the imbalance arose by chance, because the method of randomisation was not stratified by baseline mJOA score. The mJOA and gait scores were better amongst conservatively managed patients at six months, but by two years no differences were noted between the two groups in terms of functional disability.
Despite the lack of current epidemiological data about the natural history of cervical myelopathy, earlier studies suggest that untreated individuals do not necessarily develop progressive disability (Bradshaw 1957; Campbell 1960; Clarke 1956; Lees 1963). In addition, the disease may remain static for lengthy periods and sometimes patients with severe disability can also improve without treatment (Lees 1963; Nurick 1972). Even recent reports of prospectively studied patients fail to show surgery‐related benefit (King 2005).
An additional problem related to patients with myelopathy is that functional disability and quality of life may be more important than pain. Unfortunately, no outcome measures have been validated for this pathological entity, although efforts to achieve this are currently being exercised (Casey 1996; Singh 1999). Certainly, the poor specificity of the mJOA scoring scale together with the small number of randomised patients may account for the lack of any lasting beneficial effect of surgery upon the natural history of cervical myelopathy.
A non‐validated video recording measure (assessing hand dexterity and gait) at six months appeared to favour surgery, in contrast to the mJOA and timed 10‐meter walk scores which favoured conservative management (Kadanka 2002).
A large number of patients were lost to follow‐up between 24 and 36 months. Patients who crossed over were excluded from the analyses and the baseline functional status was almost statistically worse in patients undergoing surgery in Kadanka's study. All these issues may have masked a potential benefit from surgery, but this is certainly speculative.
C) GENERAL COMMENTS
Despite the fact that more than 4,000 operations are performed annually in the United Kingdom for conditions related to cervical spondylosis, we found no conclusive evidence to support surgical treatment for cervical spondylotic radiculo‐myelopathy (CSRM). It is possible that CSRM is a heterogeneous condition in which there may be subgroups of patients who will benefit from surgery. Many factors have been implicated in the propensity to develop CSRM, including advanced age, disability at presentation, cord diameter, cord area, altered cord signal on MRI (T2 and T1 weighted images) (Singh 2001; Suri 2003), increased cervical spinal mobility (Barnes 1984; Sampath 2000) and the presence of a congenitally narrow spinal canal. The same factors may determine the response to surgery either positively (increased cervical mobility) or negatively (advanced age, congenitally narrow spinal canal).
Potential complications of surgical procedures are not negligible (Apfelbaum 2000; Berge Henegouwen 1991; Bertalanffy 1989; Burke 2005; Ebershold 1995; Polkey 1984; Saunders 1991). Although incapacitating adverse effects are not very frequent, their occurrence in a disease process with a potentially benign course is of undoubted gravity. Reassuringly, no major adverse events were reported in the included trials. It is also of interest that over 50% of the patients treated surgically who did not get better had MRI evidence of an inadequate decompression (Clifton 1990). However, as stated earlier, the radiological criteria of cervical root or cord compression on MR imaging are subjective (Mehalic 1990; Singh 2001) and it is not certain that they correlate with the clinical symptomatology. The presence of altered intramedullary signal on MRI associated with cord compression and its association with the presence and significance of clinical symptomatology and semiology as well as its resolution following decompression (in T2+/‐ T1 images) was scrutinized by several investigators (Suri 2003). However, no correlation between the presence and its regression following surgery and clinical outcome has been established (Singh 2001).
The randomized studies analysed in this review were probably too small to identify any serious complications associated with the surgical treatment of cervical radiculopathy or myelopathy. It is also of importance that the excluded "randomized" studies did not provide any meaningful data to support the authors' conclusions
Authors' conclusions
Implications for practice.
The available small randomised trials do not provide reliable evidence on the effects of surgery for cervical spondylotic radiculopathy or myelopathy.
There is low quality evidence from one trial (81 patients) that surgery appears to provide pain relief faster in patients who suffer with cervical radiculopathy, compared to physiotherapy or hard collar immobilization. There is low quality evidence from the same trial that the long term effectiveness of physiotherapy or hard collar immobilization is possibly equally effective. There is very low quality evidence from one trial (68 patients) that patients with mild myelopathy feel subjectively better following surgery, but there was no evidence from objective testing of any benefit for up to three years. It is not clear whether the short‐term risks of surgery are offset by any long‐term benefits.
Implications for research.
Large‐scale randomised trials, preferably employing blinded assessment of outcome with reliable and validated assessment tools, are needed to provide clear evidence on the potential benefit from surgery for cervical spondylosis. The focus in radiculopathy should include the long‐term effectiveness of artificial cervical discs, compared to non‐surgical modalities. In relation to the myelopathic patients, separating the relevant subgroups, radiological (single/multilevel disease, congenitally narrow canal, hypermobility) as well as functional, may help us rethink our future surgical strategies.
What's new
| Date | Event | Description |
|---|---|---|
| 27 October 2009 | New citation required but conclusions have not changed | Substantive methodological amendment following updated literature search between 1998 and 2008. Conclusions not changed. |
| 25 June 2008 | New search has been performed | Searches updated to June 2008 and additional publications reporting longer follow‐up to original trials were identified and included. No new trials identified. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 16 June 2008 | Amended | Converted to new review format. |
| 3 February 2006 | Amended | Feb 3/06 ‐ contact author infomed that review will be tagged as 'withdrawn' in this month's submission. It will be re‐instated once the update has been submitted and approved for publication. Literature search last done in 1998. VEP |
| 30 April 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to express our gratitude to Brenda Thomas and Hazel Frazer for their guidance during the preparation of the original review.
In addition, we are equally indebted to Victoria Pennick, Managing Editor, and Rachel Couban, Trials Search Co‐ordinator of the Cochrane Back Review Group for their guidance during the preparation of the update.
Appendices
Appendix 1. Search Strategies for Review Update, 2008
MEDLINE 1 Clinical Trial.pt. 2 randomized.ab,ti. 3 placebo.ab,ti. 4 dt.fs. 5 randomly.ab,ti. 6 trial.ab,ti. 7 groups.ab,ti. 8 or/1‐7 9 Animals/ 10 Humans/ 11 9 not (9 and 10) 12 8 not 11 13 neck muscles.sh. 14 exp Neck/ 15 whiplash injuries.sh. 16 neck.ti,ab. 17 exp Cervical Vertebrae/ 18 or/13‐17 19 exp Spinal Cord Compression/ 20 exp Spinal Osteophytosis/ 21 exp Spinal Nerve Roots/ 22 exp Radiculopathy/ 23 radiculopathy.mp. 24 myelopathy.mp. 25 radiculomyelopathy.mp. 26 myeloradiculopathy.mp. 27 or/19‐26 28 exp Surgery/ 29 exp Surgical Procedures, Operative/ 30 surgery.mp. 31 surgical.mp. 32 or/28‐31 33 12 and 18 and 27 and 32
EMBASE 1 Clinical Article/ 2 exp Clinical Study/ 3 Clinical Trial/ 4 Controlled Study/ 5 Randomized Controlled Trial/ 6 Major Clinical Study/ 7 Double Blind Procedure/ 8 Multicenter Study/ 9 Single Blind Procedure/ 10 Phase 3 Clinical Trial/ 11 Phase 4 Clinical Trial/ 12 crossover procedure/ 13 placebo/ 14 or/1‐13 15 allocat$.mp. 16 assign$.mp. 17 blind$.mp. 18 (clinic$ adj25 (study or trial)).mp. 19 compar$.mp. 20 control$.mp. 21 cross?over.mp. 22 factorial$.mp. 23 follow?up.mp. 24 placebo$.mp. 25 prospectiv$.mp. 26 random$.mp. 27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp. 28 trial.mp. 29 (versus or vs).mp. 30 or/15‐29 31 14 and 30 32 human/ 33 Nonhuman/ 34 exp ANIMAL/ 35 Animal Experiment/ 36 33 or 34 or 35 37 32 not 36 38 31 not 36 39 37 and 38 40 38 or 39 41 neck muscles.mp. 42 exp NECK/ 43 whiplash injuries.mp. 44 neck.mp. 45 exp Neck Muscle/ 46 exp Cervical Spine/ 47 exp Cervical Spondylosis/ 48 or/41‐47 49 Spinal Cord Compression/ 50 exp Cervicobrachial Neuralgia/ 51 exp "spinal root"/ 52 exp Radiculopathy/ 53 radiculopathy.mp. 54 myelopathy.mp. 55 exp Myeloradiculopathy/ 56 radiculomyelopathy.mp. 57 exp "nerve root"/ 58 or/49‐57 59 exp surgery/ 60 surgery.mp. 61 surgical.mp. 62 or/59‐61 63 40 and 48 and 58 and 62
CINAHL 1 Randomized Controlled Trials.mp. 2 clinical trial.pt. 3 exp Clinical Trials/ 4 (clin$ adj25 trial$).tw. 5 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 6 exp PLACEBOS/ 7 placebo$.tw. 8 random$.tw. 9 exp Study Design/ 10 (latin adj square).tw. 11 exp Comparative Studies/ 12 exp Evaluation Research/ 13 Follow‐Up Studies.mp. 14 exp Prospective Studies/ 15 (control$ or prospective$ or volunteer$).tw. 16 Animals/ 17 or/1‐15 18 17 not 16 19 neck muscles.mp. or exp Neck Muscles/ 20 exp NECK/ 21 exp Neck Pain/ 22 exp Cervical Vertebrae/ 23 exp Whiplash Injuries/ 24 or/19‐23 25 exp Nerve Compression Syndromes/ 26 exp Spinal Cord Compression/ 27 exp Spinal Osteophytosis/ 28 exp RADICULOPATHY/ 29 radiculopathy.mp. 30 radiculomyelopathy.mp. 31 myelopathy.mp. 32 myeloradiculopathy.mp. 33 or/25‐32 34 exp Surgery, Operative/ 35 surgery.mp. 36 surgical.mp. 37 exp DECOMPRESSION, SURGICAL/ 38 or/34‐37 39 18 and 24 and 33 and 38
CENTRAL #1 MeSH descriptor Neck, this term only #2 MeSH descriptor Neck Injuries explode all trees #3 MeSH descriptor Whiplash Injuries, this term only #4 MeSH descriptor Neck Muscles, this term only #5 MeSH descriptor Neck Pain, this term only #6 (neck) #7 (whiplash) #8 MeSH descriptor Cervical Vertebrae explode all trees #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Spinal Cord Compression explode all trees #11 MeSH descriptor Spinal Osteophytosis explode all trees #12 MeSH descriptor Spinal Nerve Roots explode all trees #13 MeSH descriptor Radiculopathy explode all trees #14 Radiculopathy #15 myelopathy #16 radiculomyelopathy #17 myeloradiculopathy #18 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17) #19 MeSH descriptor Surgery explode all trees #20 MeSH descriptor Surgical Procedures, Operative explode all trees #21 surgery #22 surgical #23 (#19 OR #20 OR #21 OR #22) #24 (#9 AND #18 AND #23)
Appendix 2. Search strategies for the Original Review (1998)
CENTRAL 1. cervical vertebrae/ 2. intervertebral disk/ 3. neck/ 4. neck pain/ 5. nerve compression syndromes/ 6. spinal cord compression/ 7. spinal cord diseases/ 8. exp spinal diseases/ 9. cervical$. tw. 10. neck$. tw. 11 spinal$. tw. 12. spine. tw. 13. spondyl$. tw. 14. (nerve adj compression adj syndrome$). tw. 15. or/1‐14 16. 33. surg$. tw. 17. 15 and 16 18. cervical vertebrae/ su 19. neck/ su 20. neck pain/ su 21. nerve compression syndromes/ su 22. spinal cord compression/ su 23. spinal cord diseases/ su 24. exp spinal diseases/ su 25. intervertebral disk/ su 26. or/17‐25
MEDLINE 1. randomized controlled trial. pt. 2. randomized controlled trials/ 3. controlled clinical trial. pt. 4. controlled clinical trials/ 5. random allocation/ 6. double‐blind method/ 7. single‐blind method/ 8. clinical trial. pt. 9. exp clinical trials/ 10. (clin$ adj25 trial$). tw. 11. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)). tw. 12. placebos/ 13. placebo$. tw. 14. random$. tw. 15. research design/ 16. volunteer$. tw. 17. or/ 10‐16 18. cervical vertebrae/ 19. intervertebral disk/ 20. neck/ 21. neck pain/ 22. nerve compression syndromes/ 23. spinal cord compression/ 24. spinal cord diseases/ 25. exp spinal diseases/ 26. cervical$. tw. 27. neck$. tw. 28. spinal$. tw. 29. spine. tw. 30. spondyl$. tw. 31. (nerve adj compression adj syndrome$). tw. 32. or/18‐31 33. surg$. tw. 34. 32 and 33 35. cervical vertebrae/ su 36. neck/ su 37. neck pain/ su 38. nerve compression syndromes/ su 39. spinal cord compression/ su 40. spinal cord diseases/ su 41. exp spinal diseases/ su 42. intervertebral disk/ su 43. or/34‐42 44. 17 and 43 45. animal/ not (human/ and animal/) 46. 44 not 45
EMBASE 1. clinical trial/ 2. multicenter study/ 3. phase 2 clinical trial/ 4. phase 3 clinical trial/ 5. phase 4 clinical trial/ 6. randomized controlled trial/ 7. controlled study/ 8. meta analysis/ 9. crossover procedure/ 10. double blind procedure/ 11. single blind procedure/ 12. randomization/ 13. major clinical study/ 14. placebo/ 15. drug comparison/ 16. clinical study/ 17. "0197".tg. (Controlled Study) 18. "0150". tg. (Major Clinical Study) 19. "03738".dc. (Placebo) 20. (clin$ adj25 trial$).tw. 21. ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw. 22. placebo$.tw. 23. random$.tw. 24. control$.tw. 25. or/ 1‐24 26. cervical spinal cord/ 27. cervical spine/ 28. intervertebral disk/ 29. exp neck/ 30. spinal cord/ 31. spinal nerve/ 32. spine/ 33. cervicobrachial neuralgia/ 34. ligament lesion/ 35. neck injury/ 36. neck pain/ 37. shoulder pain/ 38. spinal cord disease/ 39. exp spinal cord injury/ 40. exp spine disease/ 41. nerve decompression/ 42. exp spinal cord surgery/ 43. cervical$. tw. 44. neck$. tw. 45. spinal$. tw. 46. spine$. tw. 47. spondyl$. tw. 48. (nerve adj compression adj syndrome$). tw. 49. or/26‐48 50. surg$. tw. 51. 49 and 50 52. cervicobrachial neuralgia/ su 53. neck injury/ su 54. neck pain/ su 55. shoulder pain/ su 56. spinal cord disease/ su 57. exp spinal cord injury/ su 58. exp spine disease/ su 59. nerve decompression/ su 60. or/51‐59 61. 60 and 25 62. human/ 63. "888".tg. 64. 62 or 63 65. nonhuman/ 66. "777".tg. 67. 65 or 66 68. 64 and 67 69. 67not 68 70. 61 not 69
Appendix 3. Criteria for a judgment of yes for the sources of risk of bias
1. Was the method of randomization adequate?
A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for studies with two groups), rolling a dice (for studies with two or more groups), drawing of balls of different colours, drawing of ballots with the study group labels from a dark bag, computer‐generated random sequence, pre‐ordered sealed envelops, sequentially‐ordered vials, telephone call to a central office, and pre‐ordered list of treatment assignments
Examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study, and hospital registration number
2. Was the treatment allocation concealed?
Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient.
Was knowledge of the allocated interventions adequately prevented during the study?
3. Was the patient blinded to the intervention?
This item should be scored “yes” if the index and control groups are indistinguishable for the patients or if the success of blinding was tested among the patients and it was successful.
4. Was the care provider blinded to the intervention?
This item should be scored “yes” if the index and control groups are indistinguishable for the care providers or if the success of blinding was tested among the care providers and it was successful.
5. Was the outcome assessor blinded to the intervention?
Adequacy of blinding should be assessed for the primary outcomes. This item should be scored “yes” if the success of blinding was tested among the outcome assessors and it was successful or:
for patient‐reported outcomes in which the patient is the outcome assessor (e.g., pain, disability): the blinding procedure is adequate for outcome assessors if participant blinding is scored “yes
for outcome criteria assessed during scheduled visit and that supposes a contact between participants and outcome assessors (e.g., clinical examination): the blinding procedure is adequate if patients are blinded, and the treatment or adverse effects of the treatment cannot be noticed during clinical examination
for outcome criteria that do not suppose a contact with participants (e.g., radiography, magnetic resonance imaging): the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed when assessing the main outcome
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g., co‐interventions, hospitalization length, treatment failure), in which the care provider is the outcome assessor: the blinding procedure is adequate for outcome assessors if item “4” (care provider) is scored “yes”
for outcome criteria that are assessed from data of the medical forms: the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed on the extracted data
Were incomplete outcome data adequately addressed?
6. Was the drop‐out rate described and acceptable?
The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and drop‐outs does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and does not lead to substantial bias a 'yes' is scored. (N.B. these percentages are arbitrary, not supported by literature).
7. Were all randomized participants analysed in the group to which they were allocated?
All randomized patients are reported/analyzed in the group they were allocated to by randomization for the most important moments of effect measurement (minus missing values) irrespective of non‐compliance and co‐interventions.
8. Are reports of the study free of suggestion of selective outcome reporting?
In order to receive a ‘yes’, the review author determines if all the results from all pre‐specified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the report, or in the absence of the protocol, assessing that the published report includes enough information to make this judgment.
Other sources of potential bias:
9. Were the groups similar at baseline regarding the most important prognostic indicators?
In order to receive a “yes”, groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of patients with neurological symptoms, and value of main outcome measure(s).
10. Were co‐interventions avoided or similar?
This item should be scored “yes” if there were no co‐interventions or they were similar between the index and control groups.
11. Was the compliance acceptable in all groups?
The reviewer determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s). For example, physiotherapy treatment is usually administered over several sessions; therefore it is necessary to assess how many sessions each patient attended. For single‐session interventions (for ex: surgery), this item is irrelevant.
12. Was the timing of the outcome assessment similar in all groups?
Timing of outcome assessment should be identical for all intervention groups and for all important outcome assessments.
Appendix 4. Grading the quality of evidence ‐ definition of domains
Limitations within Study Design (Quality) refers to the 12 risk of bias criteria noted in Appendix 3.
Consistency (heterogeneity) refers to the similarity of results across studies. When all studies are included in the meta‐analysis, ‘consistency’ is defined as absence of statistical heterogeneity. In the case that not all studies are combined in a meta‐analysis, ‘consistency’ is defined when all studies for the specific outcome lead to the same decision or recommendation, and ‘inconsistency’ is present if the results of two or more studies lead to clinically different decisions or recommendations. Authors use their judgment to decide if there is inconsistency when only one study leads to clinically different decision or recommendation.
Directness (generalizability) refers to the extent to which the people, interventions and outcome measures are similar to those of interest.
Precision of the evidence relates to the number of studies, patients and events for each outcome. Imprecise data is defined as:
Only one study for an outcome, regardless of the sample size or the confidence interval
Multiple studies combined in a meta‐analysis: the confidence interval is sufficiently wide that the estimate is consistent with conflicting recommendations. For rare events one should consider the confidence interval around the risk difference rather than the confidence interval around the relative risk
Multiple studies not combined in a meta‐anlaysis: the total sample size is underpowered to detect a clinically significant difference between those who received the index intervention compared to those who received the control intervention. In this case, a post‐hoc sample size calculation should be performed to determine the adequate sample size for each outcome
Reporting (Publication) bias should only be considered present if there is actual evidence of reporting bias rather than only speculation about reporting bias. The Cochrane Reporting Bias Methods Group describes the following types of Reporting Bias and Definitions:
Publication Bias: the publication or non publication of research findings, depending on the nature and direction of the results.
Time Lag Bias: the rapid or delayed publication of research findings, depending on the nature and direction of the results.
Language Bias: the publication of research findings in a particular language, depending on the nature and direction of the results.
Funding Bias: the reporting of research findings, depending on how the results accord with the aspirations of the funding body.
Outcome Variable Selection Bias: the selective reporting of some outcomes but not others, depending on the nature and direction of the research findings.
Developed Country Biases: the non publication or non indication of findings, depending on whether the authors were based in developed or in developing countries.
Data and analyses
Comparison 1. Surgery vs physiotherapy in cervical radiculopathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Radicular pain (current‐VAS) at presentation | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐15.39, 9.39] |
| 2 Radicular pain (current‐VAS) at 3 months following treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐12.00 [‐27.84, ‐0.16] |
| 3 Radicular pain (current‐VAS) at 1 year following treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐23.39, 5.39] |
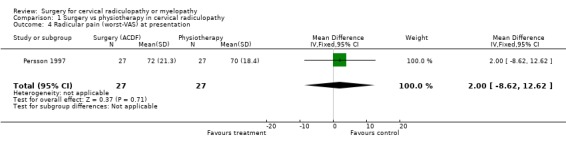
| 4 Radicular pain (worst‐VAS) at presentation | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐8.62, 12.62] |
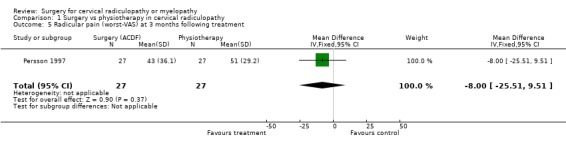
| 5 Radicular pain (worst‐VAS) at 3 months following treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐25.51, 9.51] |
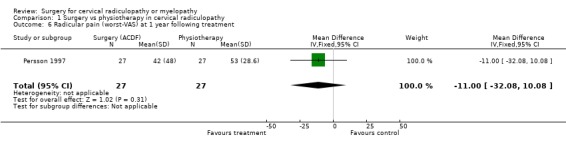
| 6 Radicular pain (worst‐VAS) at 1 year following treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐32.08, 10.08] |
| 7 Limb paraesthesia 4 months following treatment | 1 | 54 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.26, 2.15] |
| 8 Paraesthesia at 16 months following treatment | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [0.48, 4.37] |
| 9 Sensory loss at 4 months following treatment | 1 | 54 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.09, 0.92] |
| 10 Sensory loss at 16 months following treatment | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.13, 1.81] |
1.1. Analysis.
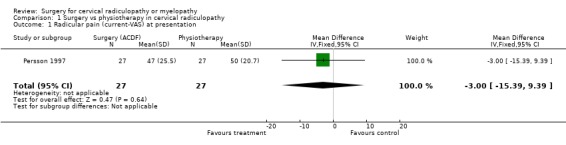
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 1 Radicular pain (current‐VAS) at presentation.
1.2. Analysis.
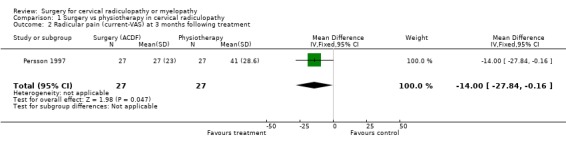
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 2 Radicular pain (current‐VAS) at 3 months following treatment.
1.3. Analysis.
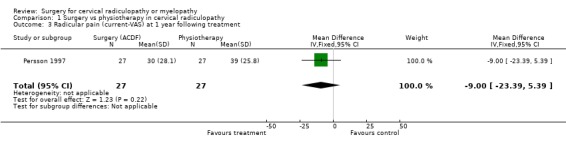
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 3 Radicular pain (current‐VAS) at 1 year following treatment.
1.4. Analysis.
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 4 Radicular pain (worst‐VAS) at presentation.
1.5. Analysis.
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 5 Radicular pain (worst‐VAS) at 3 months following treatment.
1.6. Analysis.
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 6 Radicular pain (worst‐VAS) at 1 year following treatment.
1.7. Analysis.
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 7 Limb paraesthesia 4 months following treatment.
1.8. Analysis.
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 8 Paraesthesia at 16 months following treatment.
1.9. Analysis.
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 9 Sensory loss at 4 months following treatment.
1.10. Analysis.
Comparison 1 Surgery vs physiotherapy in cervical radiculopathy, Outcome 10 Sensory loss at 16 months following treatment.
Comparison 2. Surgery vs cervical collar in cervical radiculopathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
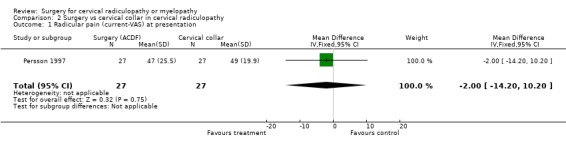
| 1 Radicular pain (current‐VAS) at presentation | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.20, 10.20] |
| 2 Radicular pain (current‐VAS) at 3 months following treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐21.0 [‐33.32, ‐8.68] |
| 3 Radicular pain (current‐VAS) at 1 year following treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐18.84, 8.84] |
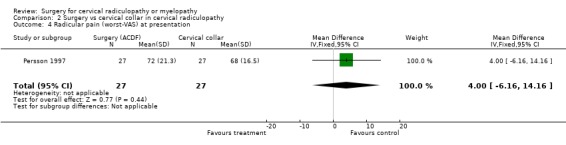
| 4 Radicular pain (worst‐VAS) at presentation | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐6.16, 14.16] |
| 5 Radicular pain (worst‐VAS) at 3 months following treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐21.0 [‐36.89, ‐5.11] |
| 6 Radicular pain (worst‐VAS) at 1 year following treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐30.79, 10.79] |
| 7 Paraesthesia at 4 months following treatment | 1 | 54 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.19, 1.61] |
| 8 Paraesthesia at 16 months following treatment | 1 | 52 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.45, 4.16] |
| 9 Sensory loss at 4 months following treatment | 1 | 54 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.09, 0.92] |
| 10 Sensory loss at 16 months following treatment | 1 | 52 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.14, 1.90] |
2.1. Analysis.
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 1 Radicular pain (current‐VAS) at presentation.
2.2. Analysis.
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 2 Radicular pain (current‐VAS) at 3 months following treatment.
2.3. Analysis.
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 3 Radicular pain (current‐VAS) at 1 year following treatment.
2.4. Analysis.
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 4 Radicular pain (worst‐VAS) at presentation.
2.5. Analysis.
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 5 Radicular pain (worst‐VAS) at 3 months following treatment.
2.6. Analysis.
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 6 Radicular pain (worst‐VAS) at 1 year following treatment.
2.7. Analysis.
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 7 Paraesthesia at 4 months following treatment.
2.8. Analysis.
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 8 Paraesthesia at 16 months following treatment.
2.9. Analysis.
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 9 Sensory loss at 4 months following treatment.
2.10. Analysis.
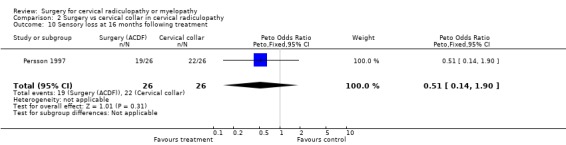
Comparison 2 Surgery vs cervical collar in cervical radiculopathy, Outcome 10 Sensory loss at 16 months following treatment.
Comparison 3. conservative vs surgical treatment in cervical myelopathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 modified JOA score at presentation | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.28, 0.28] |
| 2 modified JOA score after 6 months | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.62, 0.22] |
| 3 modified JOA score after 12 months | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.92, ‐0.08] |
| 4 modified JOA score after 24 months | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.33, 0.53] |
| 5 modified JOA score after 36 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.70, ‐0.10] |
| 6 daily activities evaluation after 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 better | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 unchanged | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 did not get worse | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 daily activities evaluation after 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 better | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 unchanged | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 did not get worse | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 daily activities evaluation after 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 better | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 unchanged | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 did not get worse | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 daily activities evaluation after 36 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 better | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 unchanged | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 did not get worse | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 timed 10‐meter walk at presentation | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.77, 1.77] |
| 11 timed 10‐meter walk after 6 months | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [0.04, 2.96] |
| 12 timed 10‐meter walk after 12 months | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐0.07, 5.07] |
| 13 timed 10‐meter walk after 24 months | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [0.89, 7.51] |
| 14 timed 10‐meter walk after 36 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐0.17, 3.97] |
| 15 self evaluation after 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1 better | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 unchanged | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 did not get worse | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 self evaluation after 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1 better | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 unchanged | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 didi not get worse | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 self evaluation after 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1 better | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 unchanged | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 did not get worse | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 self evaluation after 36 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1 better | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 unchanged | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 did not get worse | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
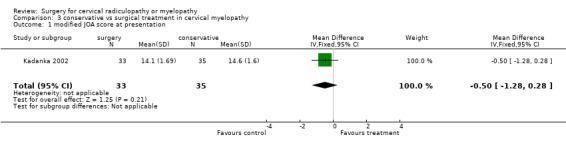
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 1 modified JOA score at presentation.
3.2. Analysis.
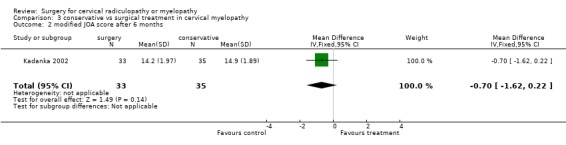
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 2 modified JOA score after 6 months.
3.3. Analysis.
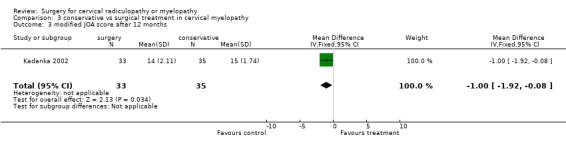
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 3 modified JOA score after 12 months.
3.4. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 4 modified JOA score after 24 months.
3.5. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 5 modified JOA score after 36 months.
3.6. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 6 daily activities evaluation after 6 months.
3.7. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 7 daily activities evaluation after 12 months.
3.8. Analysis.
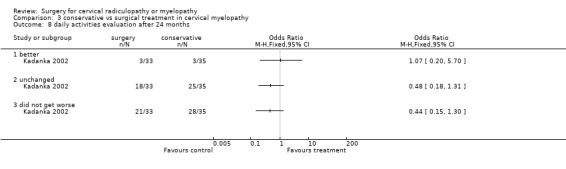
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 8 daily activities evaluation after 24 months.
3.9. Analysis.
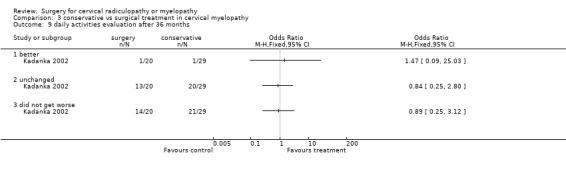
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 9 daily activities evaluation after 36 months.
3.10. Analysis.
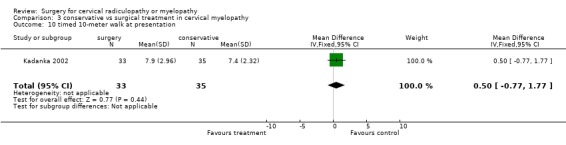
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 10 timed 10‐meter walk at presentation.
3.11. Analysis.
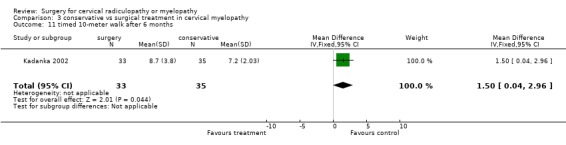
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 11 timed 10‐meter walk after 6 months.
3.12. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 12 timed 10‐meter walk after 12 months.
3.13. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 13 timed 10‐meter walk after 24 months.
3.14. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 14 timed 10‐meter walk after 36 months.
3.15. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 15 self evaluation after 6 months.
3.16. Analysis.
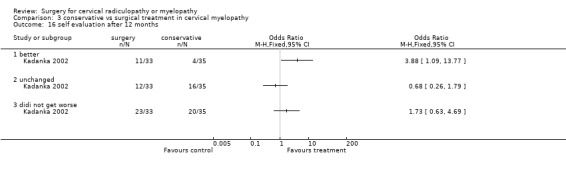
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 16 self evaluation after 12 months.
3.17. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 17 self evaluation after 24 months.
3.18. Analysis.
Comparison 3 conservative vs surgical treatment in cervical myelopathy, Outcome 18 self evaluation after 36 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kadanka 2002.
| Methods | C: Coin toss
Outcome assessor blind
Exclusions during trial: 2 (patients initially allocated to the conservative group, but underwent surgery) Losses to FU: 19 between 24 and 36 months. In addition 5 patients who underwent surgery died during the same period |
|
| Participants | Czech Republic 68 patients (48 men and 20 women) age<75 Mild to moderate myelopathy (mJOA score>12) |
|
| Interventions | Rx :22 anterior decompression (with autograft, 15 had additional plating), 6 corpectomy and 5 laminoplasty Conservative treatment: intermittent cervical immobilization (soft collar), NSAIDs, intermittent bed rest |
|
| Outcomes | modified JOA score at onset, 6,12, 24 and 36 months after treatment evaluation of daily activities by video recording at onset, 6,12 ,24 and 36 months after treatment timed 10‐meter walk at onset, 6,12, 24 and 36 months after treatment self‐evaluation at onset, 6,12, 24 and 36 months after treatment |
|
| Notes | Ex: severe myelopathy (mJOA <12) FU: 3 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | coin toss |
| Allocation concealment? | Unclear risk | not stated |
| Blinding? patients | High risk | |
| Blinding? care providers | Unclear risk | not stated, but unlikely |
| Blinding? outcome assessor | Low risk | "They were evaluated by two physicians blinded to the type of treatment" |
| Incomplete outcome data addressed? Withdrawal/drop‐out rate | High risk | Five surgically treated patients died during the follow‐up period, but their deaths were physically unrelated to the surgery 19 patients were lost to follow‐up between 24 and 36 months |
| Incomplete outcome data addressed? Intention‐to‐treat analysis | High risk | Two patients allocated to the conservative group underwent surgery and they were excluded from the analysis |
| Free of selective reporting? | Low risk | |
| Similarity of baseline characteristics? | High risk | mJOA score substantially‐almost significantly‐ different between surgically and conservatively treated groups |
| Co‐interventions avoided or similar? | Unclear risk | A variety of non surgical interventions were administered to the conservatively treated patients |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Low risk | |
Persson 1997.
| Methods | C: sealed envelopes outcome assessor not blind Exclusions during trial: None Losses to FU: 2 | |
| Participants | Sweden 81 patients (46% women) Mean age 47 years Clinical and radiological (MRI or CT‐myelography) evidence of cervical radiculopathy | |
| Interventions | Rx: ACDF (with allograft, n=26) or cervical laminectomy Control: physiotherapy or cervical collar | |
| Outcomes | Pain (VAS) paraesthesia and sensory loss at onset, 3, 12 and 16 months after treatment | |
| Notes | Ex: spinal cord compression, absence of radiological evidence of root compression
FU: 16 months
Cross‐overs (patients allocated in conservative groups undergoing surgery): 6 (between 3 and 12 months); 1 allocated to physiotherapy and 5 allocated to collar group 3 patients allocated to the surgical group refused surgery because of spontaneous improvement."Intention to treat principle" was applied throughout. 8 surgically‐treated patients underwent a second operation between 3 and 12 months (6 in adjacent levels, 1 because of graft infection, 1 had exploration of the brachial plexus) 11 patients in the surgery group and 12 in the collar group received physiotherapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'randomized by the use of sealed envelopes' |
| Allocation concealment? | Unclear risk | not stated |
| Blinding? patients | High risk | patients aware of intervention group |
| Blinding? care providers | Unclear risk | not stated, but unlikely |
| Blinding? outcome assessor | High risk | 'the same physiotherapist [who completed the baseline assessment], who did not take part in the treatment, also administered the post‐treatment measurements'; but patients in the collar group were wearing their collars. |
| Incomplete outcome data addressed? Withdrawal/drop‐out rate | Low risk | |
| Incomplete outcome data addressed? Intention‐to‐treat analysis | Low risk | 'allocation to the surgical group was retained in accordance with the 'intention to treat' principle. In the physiotherapy and cervical collar groups, all patients carried out the allocated treatment'. |
| Free of selective reporting? | Unclear risk | muscle strength, function (assessed by Sickness Impact Profile) and mood (assessed by Mood Adjective Check List) were investigated but data inadequate for quantitative comparisons |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | Unclear risk | 11 patients in the surgery group and 12 in the collar group received physiotherapy; '1 patient in the physiotherapy group and 5 patients in the collar group underwent surgery because the result of the conservative therapies was unsatisfactory' |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Low risk | |
C‐Concealment of allocation; Rx‐Treatment; JOA‐Japanese Orthopaedic Association; ACDF‐Anterior Cervical Decompression and Fusion; VAS‐Visual Analogue Scale; Ex‐Exclusion Criteria; C‐inadequate; B‐unclear
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Li 2004 | Incomplete data |
| Nardi 2005 | Incomplete data. Authors were approached through e‐mail, telephone messaging and in writing, but no response was obtained |
Contributions of authors
Ioannis Fouyas wrote the protocol with help from Peter Sandercock and Patrick Statham. Ioannis Fouyas and Patrick Statham did the searches and extracted data. Patrick Statham and Chris Lynch (Neurologist, Dunedin, New Zealand) commented on the protocol, the analysis and helped to edit the original review.
Ioannis Nikolaidis wrote the update with the assistance of Ioannis Fouyas. Patrick Statham and Peter Sandercock helped with editing the final manuscript.
Declarations of interest
None Known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Kadanka 2002 {published data only}
- Bednařík J, Kadaňka Z, Vohâňka S, Stejskal L, Vlach O, Schroder R. The value of somatosensory‐and motor evoked potentials in predicting and monitoring the effect of therapy in spondylotic cervical myelopathy. Prospective randomized study. Spine 1999;24(15):1593‐8. [DOI] [PubMed] [Google Scholar]
- Kadanka Z, Bednarik J, Vohanka S, Vlach O. Spondylotic cervical myelopathy: conservative vs surgical treatment. Scripta Medica (Brno) 1997;70(7):317‐327. [Google Scholar]
- Kadaňka Z, Bednařik J, Vohâňka S, Vlach O, Stejskal L, Chaloupka R, et al. Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. European Spine Journal 2000;9:538‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaňka Z, Mareš M, Bednařík J, Smrčka V, Krbec M, Stejskal L, et al. Approaches to spondylotic cervical myelopathy. Conservative versus Surgical Results in a 3‐year Follow up Study. Spine 2002;27(20):2205‐11. [DOI] [PubMed] [Google Scholar]
- Kadaňka Z, Mareš M, Bednařík J, Smrčka V, Krbec M, Stejskal L, et al. Predictive factors for spondylotic cervical myelopathy treated conservatively or surgically. European Journal of Neurology 2005;12(1):16‐24. [DOI] [PubMed] [Google Scholar]
- Kadaňka Z, Mareš M, Bednařík J, Smrčka V, Krbec M, Stejskal L, et al. Predictive factors for spondylotic cervical myelopathy treated conservatively or surgically. European Journal of Neurology 2005;12(1):55‐63. [DOI] [PubMed] [Google Scholar]
Persson 1997 {published data only}
- Persson L, Karlberg M, Magnusson M. Effects of different treatments on postural performance in patients with cervical root compression. A randomized prospective study assessing the importance of the neck in postural control. Journal of Vestibular Research 1996;6(6):439‐53. [PubMed] [Google Scholar]
- Persson LCG, Carlsson C‐A, Carlsson JY. Long‐lasting cervical radicular pain managed with surgery, physiotherapy, or a cervical collar. A prospective, randomized study. Spine 1997;22(7):751‐8. [DOI] [PubMed] [Google Scholar]
- Persson LCG, Lilja A. Pain, coping, emotional state and physical function in patients with chronic radicular neck pain. A comparison between patients treated with surgery, physiotherapy or neck collar: A blinded, prospective randomized study. Disability and Rehabilitation 2001;23(8):325‐35. [DOI] [PubMed] [Google Scholar]
- Persson LCG, Moritz U, Brandt L, Carlsson C‐A. Cervical radiculopathy: pain, muscle weakness and sensory loss in patients with cervical radiculopathy treated with surgery, physiotherapy or cervical collar. A prospective, controlled study. European Spine Journal 1997;6:256‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Li 2004 {published data only}
- Li R, Zhang X‐M, Xu Y‐G, Ding P‐M, Dong W‐Y, Shen L, et al. Neural functional restoration and stability of cervical vertebra in cervical syndrome patients after micro‐endoscopic discectomy. Zhongguo Linchuang Kangfu 2004;8(20):3938‐9. [Google Scholar]
Nardi 2005 {published data only}
- Nardi PV, Cabezas D, Cesaroni A. Percutaneous cervical nucleoplasty using coblation technology. Clinical results in fifty consecutive cases. Acta Neurochirurgica ‐ Supplement 2005;92:73‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Apfelbaum 2000
- Apfelbaum RI, Kriskovich MD, Haller JR. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine 2000;25(22):2906‐12. [DOI] [PubMed] [Google Scholar]
Barnes 1984
- Barnes MP, Saunders M. The effect of cervical mobility on the natural history of cervical spondylotic myelopathy. Journal of Neurology Neurosurgery and Psychiatry 1984;47:17‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bednarik 1999
- Bednařík J, Kadaňka Z, Vohâňka S, Stejskal L, Vlach O, Schroder R. The value of somatosensory‐and motor evoked potentials in predicting and monitoring the effect of therapy in spondylotic cervical myelopathy. Prospective randomized study. Spine 1999;24(15):1593‐8. [DOI] [PubMed] [Google Scholar]
Bednarik 2004
- Bednařík J, Kadaňka Z, Dusek L, Novothy O, Surelova D, Urbanek I, et al. Presymptomatic Spondylotic Cervical Cord Compression. Spine 2004;29(20):2260‐9. [DOI] [PubMed] [Google Scholar]
Benzel 1991
- Benzel EC, Lancon J, Kesterson L, Haden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. Journal of Spinal Disorders 1991;4:286‐95. [DOI] [PubMed] [Google Scholar]
Berge Henegouwen 1991
- Berge Henegouwen DP, Roukema JA, Nie JC, vd Werken C. Esophageal perforation during surgery on the cervical spine. Neurosurgery 1991;29:766‐8. [DOI] [PubMed] [Google Scholar]
Bertalanffy 1989
- Bertalanffy H, Eggert H. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wein) 1989;99:41‐50. [DOI] [PubMed] [Google Scholar]
Bradshaw 1957
- Bradshaw P. Some aspects of cervical spondylosis. Q J Med 1957;26:177‐208. [PubMed] [Google Scholar]
Burke 2005
- Burke JP, Gerszten PC, Welch WC. Iatrogenic vertebral artery injury during anterior cervical spine surgery. Spine J 2005;5(5):508‐14. [DOI] [PubMed] [Google Scholar]
Campbell 1960
- Campbell AMG, Phillips DG. Cervical disc lesions with neurological disorder. Differential diagnosis, treatment, and prognosis. BMJ 1960;2:481‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casey 1996
- Casey ATH, Bland JM, Crockard HA. Development of a functional scoring system for rheumatoid arthritis patients with cervical myelopathy. Annals of Rheumatic Diseases 1996;55:901‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chagas 2005
- Chagas H, Domingues F, Aversa A, Fonseca ALV, Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surgical Neurology 2005 July;64(Supplement 1):30‐5. [DOI] [PubMed] [Google Scholar]
Cho D‐Y 2002
- Cho D‐Y, Liau W‐R, Liu J‐T, Sheu P‐C, et al. Prelimanary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery 2002;51(6):1343‐50. [PubMed] [Google Scholar]
Clarke 1956
- Clarke E, Robinson PK. Cervical myelopathy: a complication of cervical spondylosis; a complication of cervical spondylosis. Brain 1956;79:483‐510. [DOI] [PubMed] [Google Scholar]
Clifton 1990
- Clifton AG, Stevens JM, Whitear P, Kendall BE. Identifiable causes for poor outcome in surgery for cervical spondylosis. Post‐operative computed myelography and MR imaging. Neuroradiology 1990;32:450‐5. [DOI] [PubMed] [Google Scholar]
Coric 2006
- Coric D, Finger F, Boltes P. Prospective randomized controlled study of the Bryan Cervical Disc:early clinical results from a single investigational site. Journal of Neurosurgery Spine 2006;4(1):31‐5. [DOI] [PubMed] [Google Scholar]
Ebershold 1995
- Ebershold MJ, Pare MC, Quast LM. Surgical treatment for cervical spondylitic myelopathy. Journal of Neurosurgery 1995;82:745‐51. [DOI] [PubMed] [Google Scholar]
Frederic 2006
- Frederic S, Benedict R, Payer M. Implantation of an empty carbon fiber cage or a tricortical iliac crest autograft after cervical discectomy for a single‐level disc herniation:a prospective comparative study. Journal of Neurosurgery Spine 2006;4(4):292‐9. [DOI] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M, Editorial Board, Cochrane Back Review Group. 2009 Updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Hafez 1997
- Hafez R, Crockard H. Failure of osseous conduction with cervical interbody BOP graft. Br J Neurosurg 1997;11:57‐9. [DOI] [PubMed] [Google Scholar]
Harland 1998
- Harland SP, Laing RJ. A survey of the peri‐operative management of patients undergoing anterior cervical discectomy in the U.K. and Eire. Br J Neurosurg 1998;12:113‐7. [DOI] [PubMed] [Google Scholar]
Henderson 1983
- Henderson CM, Hennessy RG, Shuey Jr HM, Shackelford EG. Posterior‐lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: A review of 846 consecutively operated cases. Neurosurgery 1983;5:504‐12. [DOI] [PubMed] [Google Scholar]
Herkowitz 1989
- Herkowitz HN. Surgical management of cervical spondylotic radiculopathy and myelopathy. Clin Orthop 1989;239:94‐108. [PubMed] [Google Scholar]
Hilibrand 1999
- Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. Journal of Bone and Joint Surgery 1999 April;81(4):519‐28. [DOI] [PubMed] [Google Scholar]
Hughes 1965
- Hughes JT, Brownell B. Necropsy observations on the spinal cord in cervical spondylosis. Riv Patol Nerv Ment 1965;86:196‐204. [PubMed] [Google Scholar]
Irvine 1965
- Irvine DH, Foster JB, Newell DJ, Klukvin RN. Prevalence of cervical spondylosis in a general practice. Lancet 1965;2:1089‐92. [DOI] [PubMed] [Google Scholar]
Jacobs 2004
- Jacobs W, Anderson PG, Limbeek J, Willems P, Pavlov P. Single or double‐level anterior interbody fusion techniques for cervical degenerative disc disease. Cochrane Database of Systematic Reviews 2004, Issue 4. [CD004958. DOI: 10.1002/14651858.CD004958] [DOI] [PubMed] [Google Scholar]
Kadanka 1997
- Kadaňka Z, Bednařík J, Vohanka S, Vlach O. Spondylotic cervical myelopathy: conservative vs surgical treatment. Scripta Medica (Brno) 1997;70(7):317‐27. [Google Scholar]
King 2005
- King Jr JT, Moossy JJ, Tsevat J, Robert MS. Multimodal assessment after surgery for cervical spondylotic myelopathy. Journal of Neurosurgery Spine 2005;2(5):526‐34. [DOI] [PubMed] [Google Scholar]
Lees 1963
- Lees F, Turner JW. Natural history and prognosis of cervical spondylosis. Br Med J 1963;2(5373):1607‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lunsford 1980
- Lunsford LD, Bissonette DJ, Zorub DS. Anterior surgery for cervical disc disease. Part 2. Treatment of cervical spondylotic myelopathy in 32 cases. J Neurosurg 1980;53:12‐9. [DOI] [PubMed] [Google Scholar]
Martins 1976
- Martins AN. Anterior cervical discectomy with and without interbody bone graft. J Neurosurg 1976;44:290‐5. [DOI] [PubMed] [Google Scholar]
McCormack 1996
- McCormack BM, Weinstein PR. Cervical spondylosis ‐ An update. West J Med 1996;165:43‐51. [PMC free article] [PubMed] [Google Scholar]
Mehalic 1990
- Mehalic TF, Pezzuti RT, Applebaum BI. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery 1990;26:217‐27. [DOI] [PubMed] [Google Scholar]
Nurick 1972
- Nurick S. Natural history and results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain 1972;95:101‐8. [DOI] [PubMed] [Google Scholar]
Pallis 1954
- Pallis C, Jones AM, Spillane JD. Cervical spondylosis. Incidence and implications. Brain 1954;77:274‐89. [DOI] [PubMed] [Google Scholar]
Polkey 1984
- Polkey CE. Immediate effects of cervical and upper dorsal laminectomy. Proc Soc Brit Neurosurg 1984;47:106. [Google Scholar]
Radhakrishnan 1994
- Radhakrishnan K, Litchy WJ, O' Fallon M, Kurland LT. Epidemiology of cervical radiculopathy. A population‐based study from Rochester, Minnesota, 1976 through 1990. Brain 1994;117:325‐35. [DOI] [PubMed] [Google Scholar]
Rowland 1992
- Rowland LP. Surgical treatment of cervical spondylotic myelopathy: time for a controlled trial. Neurology 1992;42:5‐13. [DOI] [PubMed] [Google Scholar]
Sampath 1999
- Sampath P, Bendebba M, Davis JD, Ducker T. Outcome in patients with cervical radiculopathy. Prospective, multi‐center study with independent clinical review. Spine 1999;24(6):591‐7. [DOI] [PubMed] [Google Scholar]
Sampath 2000
- Sampath P, Bendebba M, Davis J, Ducker T. Outcome of patients treated for cervical myelopathy: a prospective, multicenter study with independent clinical review. Spine 2000 March 15;25(6):670‐676. [DOI] [PubMed] [Google Scholar]
Saunders 1991
- Saunders RL, Bernini PM, Shiffeffs TG, Reeves AG. Central corpectomy for cervical spondylotic myelopathy; a consecutive series with long‐term follow‐up evaluation. J Neurosurg 1991;74:163‐70. [DOI] [PubMed] [Google Scholar]
Singh 1999
- Singh A, Crockard HA. Quantitative assessment of cervical spondylotic myelopathy by a simple walking test. Lancet 1999;354:370‐3. [DOI] [PubMed] [Google Scholar]
Singh 2001
- Singh A, Crockard HA, Platts A, Stevens J. Clinical and radiological correlates of severity and surgery‐related outcome in cervical spondylosis. Journal Neurosurgery 2001 April;94(2):189‐198. [DOI] [PubMed] [Google Scholar]
Suri 2003
- Suri A, Chabbra RPS, Mehta VS, Gaikwad S, Pandey RM. Effects of intamedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine Journal 2003;3(1):33‐45. [DOI] [PubMed] [Google Scholar]
Teresi 1987
- Teresi LM, Lufkin RB, Reicher MA, Moffit BJ, Vinuela FV, Wilson GM, et al. Asymptomatic degenerative disc disease and spondylosis of the cervical spine: MR imaging. Radiology 1987;164:83‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fouyas 2001
- Fouyas IP, Statham PF, Sandercock PA, Lynch C. Surgery for cervical radiculomyelopathy. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001466.pub2] [DOI] [PubMed] [Google Scholar]
Fouyas 2002
- Fouyas IP, Statham PF, Sandercock PA. Cochrane review on the role of surgery in cervical spondylotic radiculomyelopathy. Spine 2002;27(7):736‐47. [DOI] [PubMed] [Google Scholar]


