Abstract
Background
Benign prostatic hyperplasia (BPH) is a nonmalignant enlargement of the prostate, which can lead to obstructive and irritative lower urinary tract symptoms (LUTS). The pharmacologic use of plants and herbs (phytotherapy) for the treatment of LUTS associated with BPH is common. The extract of the berry of the American saw palmetto, or dwarf palm plant, Serenoa repens (SR), which is also known by its botanical name of Sabal serrulatum, is one of several phytotherapeutic agents available for the treatment of BPH.
Objectives
This systematic review aimed to assess the effects and harms of Serenoa repens in the treatment of men with LUTS consistent with BPH.
Search methods
We searched for trials in general and in specialized databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE®, EMBASE, CINAHL®, Web of Science, SCOPUS, BIOSIS Previews®, LILACS, ClinicalTrials.gov, Controlled‐Trials.com, World Health Organization (WHO), and Google Scholar. We also handsearched systematic reviews, references, and clinical practice guidelines. There were no language restrictions.
Selection criteria
Trials were eligible if they randomized men with symptomatic BPH to receive preparations of SR (alone or in combination) for at least four weeks in comparison with placebo or other interventions, and included clinical outcomes, such as urologic symptom scales, symptoms, and urodynamic measurements. Eligibility was assessed by at least two independent observers (JT, RM).
Data collection and analysis
One review author (JT) extracted Information on patients, interventions, and outcomes which was then checked by another review author (RM). The main outcome measure for comparing the effectiveness of SR with active or inert controls was change in urologic symptom‐scale scores, with validated scores taking precedence over non validated ones. Secondary outcomes included changes in nocturia and urodynamic measures. The main outcome measure for harms was the number of men reporting side effects.
Main results
In a meta‐analysis of two high quality long‐term trials (n = 582), Serenoa repens therapy was not superior to placebo in reducing LUTS based on the AUA (mean difference (MD) 0.25 points, 95% confidence interval (CI) ‐0.58 to 1.07). A 72 week trial with high quality evidence, using the American Urological Association Symptom Score Index, reported that SR was not superior to placebo at double and triple doses. In the same trial the proportions of clinical responders (≥ three‐point improvement) were nearly identical (42.6% and 44.2% for SR and placebo, respectively), and not significant (RR 0.96, 95% CI 0.76 to 1.22).
This update, which did not change our previous conclusions, included two new trials with 444 additional men, an 8.5% (5666/5222) increase from our 2009 updated review, and a 28.8% (1988/1544) increase for our main comparison, SR monotherapy versus placebo control (17 trials). Overall, 5666 men were assessed from 32 randomized, controlled trials, with trial lengths from four to 72 weeks. Twenty‐seven trials were double blinded and treatment allocation concealment was adequate in 14.
In a trial of high quality evidence (N = 369), versus placebo, SR did not significantly decrease nightly urination on the AUA Nocturia scale (range zero to five) at 72 weeks follow‐up (one‐sided P = 0.19).
The three high quality, moderate‐to‐long term trials found peak urine flow was not improved with Serenoa repens compared with placebo (MD 0.40 mL/s, 95% CI ‐0.30 to 1.09).
Comparing prostate size (mean change from baseline), one high quality 12‐month trial (N = 225) reported no significant difference between SR and placebo (MD ‐1.22 cc, 95% CI ‐3.91 to 1.47).
Authors' conclusions
Serenoa repens, at double and triple doses, did not improve urinary flow measures or prostate size in men with lower urinary tract symptoms consistent with BPH.
Plain language summary
Serenoa repens for benign prostatic hyperplasia
Benign prostatic hyperplasia (BPH) is the nonmalignant enlargement of the prostate gland that is caused by an increase in volume of epithelial (top layer of tissue that line cavities and surfaces of the body) and stromal (connective tissue) cells. This increase in cells can, over time, create fairly large, discrete nodules in the periurethral region of the prostate, and in turn can restrict the urethral canal causing partial or complete blockage.
The use of plants and herbs (phytotherapy) for the treatment of lower urinary tract symptoms associated with BPH is common and has been growing steadily in most Western countries. The extract of the berry of the American saw palmetto, or dwarf palm plant, Serenoa repens (SR), which is also known by its botanical name of Sabal serrulatum, is one of several phytotherapeutic agents available for the treatment of BPH.
The update of this review included 32 randomized controlled trials involving 5666 men.
Compared with placebo, Serenoa repens, at double and triple the usual dose, provides no improvement for nocturia, peak urine flow, and symptom scores for men with benign prostatic hyperplasia.
Summary of findings
Summary of findings for the main comparison. SR compared to placebo for benign prostatic hyperplasia.
| Serenoa repens compared to placebo for benign prostatic hyperplasia | ||||||
| Patient or population: men with benign prostatic hyperplasia Settings: clinic Intervention: SR Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | SR | |||||
| AUA total score, mean change from baseline American Urological Association Symptom Score. Scale from: 0 to 35. Follow‐up: 52 to 72 weeks | The mean AUA total score, mean change from baseline ranged across control groups from ‐0.72 to ‐2.99 points | The mean AUA total score, mean change from baseline in the intervention groups was 0.25 higher (0.58 lower to 1.07 higher) | 582 (2 studies) | ⊕⊕⊕⊕ high | ||
| Peak urine flow, mean change from baseline millilitres/second Follow‐up: 26 to 72 weeks | The mean peak urine flow, mean change from baseline in the control groups was millilitres/second | The mean peak urine flow, mean change from baseline in the intervention groups was 0.40 higher (0.30 lower to 1.09 higher) | 667 (3 studies) | ⊕⊕⊕⊕ high | ||
| Clinical responders ≥ 3 point improvement in AUA Follow‐up: mean 72 weeks | 442 per 1000 | 424 per 1000 (336 to 539) | RR 0.96 (0.76 to 1.22) | 357 (1 study) | ⊕⊕⊕⊕ high | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
In the hyperplastic prostate, as compared to a healthy one, cell proliferation (epithelial and stromal cells) and cell death have achieved disequilibrium, causing a net increase of cells in the organ. This process is not well understood, but the evidence suggests an interplay among "[a]ndrogens, estrogens, stromal‐epithelial interactions, growth factors, and neurotransmitters[,] … either singly or in combination, in the etiology of the hyperplastic process" (see Table of key terms (Table 6)) (Campbell‐Walsh Urology 2010). This increase in cells can, over time, create fairly large, discrete nodules in the periurethral region of the prostate, and in turn, can restrict the urethral canal causing partial or complete blockage.
1. Table of key terms.
| AUA | The American Urological Association Symptom Score Index, and the same score as the IPSS. These are self‐rated, validated (i.e., symptoms that are confirmed clinically) questionnaires that measure the severity of irritative and obstructive urination symptoms. There are seven questions with each question scaled from 0 to 5. A higher score indicates worse symptoms. There are seven questions with each question scaled from 0 to 5. A higher score indicates worse symptoms. |
| BPH | Benign prostatic hyperplasia (BPH) is the nonmalignant enlargement of the prostate gland that is caused by an increase in volume of epithelial (top layer of tissue that line cavities and surfaces of the body) and stromal (connective tissue) cells into discrete, fairly large nodules in the periurethral (surrounding the urethra) region. These nodules in turn can restrict the urethral canal causing partial or complete blockage. |
| Hyperplasia | The proliferation of cells (for BPH, the epithelial and stromal cells) within an organ beyond the ordinary. |
| IPSS | International Prostate Symptom Score. IPSS is scored precisely like the AUA. See above. |
| Peak urine flow | The maximum rate of urine as measured by a uroflowmeter. Also known as Qmax. |
| Phytosterols | Steroidal alcohols that occur naturally in plants. |
| Phytotherapy | The use of plants, or plant extracts for medicinal purposes. |
| Serenoa repens | A small palm native to the American Southeast, SR is popularly known as Saw palmetto. When used as a phytotherapy, it is often called Sabal serrulatum. It is the extract of its berries, the fatty acids and phytosterols, that is used in the treatment of BPH. |
| TURP | Transurethral resection of the prostate. A catheter is inserted into the urethra up to the prostate to remove tissue by electrocautery or sharp dissection. |
Histological evidence of the prevalence of BPH is found in more than 40% of men in their fifties and nearly 90% of men in their eighties (Berry 1984). Absolute prevalence rates of BPH differ widely in a number of multinational, longitudinal, population‐based studies (Meigs 2001; Platz 2002), although they are strikingly consistent in age‐related increases that parallel Berry's reporting in his biopsy and cadaver study (Berry 1984). In 2000 in the US there were approximately 4.5 million visits to physicians that resulted in a primary diagnosis of BPH; in the same year there were nearly 8 million visits that resulted in a primary or secondary diagnosis (Urologic Diseases in American 2007). In our 2002 update (Wilt 2002), we reported 300,000 prostatectomies for BPH annually (McConnell 1994), and in 2009 (Tacklind 2009), we reported slightly more than 87,000 prostatectomies for BPH (Urologic Diseases in American 2007). This more than three‐fold decrease in transurethral resections of the prostate (TURPs) ‐ formerly the gold standard of practice for severe symptomatic BPH ‐ is negatively correlated to the medical management of BPH (Lepor 1996; McConnell 2003). Complementing this trend, phytotherapy has been growing steadily in most Western countries. Phytotherapeutic agents represent nearly half of the medications dispensed for BPH in Italy, compared with 5% for α‐blockers and 5% for 5‐ARIs (5α‐reductase inhibitors) (Di Silverio 1993). In Germany and Austria, phytotherapy is the first‐line treatment for mild to moderate urinary obstructive symptoms and represents more than 90% of all drugs prescribed for the treatment of BPH (Buck 1996). In the United States its use has also markedly increased. In a 2002 survey, SR was used by 2.5 million adults, "often for BPH" (Barnes 2002). A fairly recent survey demonstrated that one third of men choosing non surgical therapy for BPH were using herbal preparations alone or in combination with prescription medications (Bales 1999).
For the clinical indication of BPH, i.e., BPH + symptoms (retention, inability to empty bladder, overactive bladder), the link between them is less secure now than it was, and it is now thought that "a significant portion of LUTS is due to age‐related detrusor dysfunction" (Campbell‐Walsh Urology 2010). LUTS is also seen in men with sleep disorders and polyuria. Nevertheless, there is significant epidemiologic evidence that progressive BPH is associated with LUTS (Roehrborn 2001).
Description of the intervention
There are about 30 phytotherapeutic compounds available for the treatment of BPH, and one of the most widely used is an extract from the berry of the American saw palmetto or dwarf palm plant, Serenoa repens, which is also known by its botanical name of Sabal serrulatum. While the purported mechanism of its relief of LUTS secondary to BPH is unknown, some of those proposed are:
alteration in cholesterol metabolism (Christensen 1990);
antiestrogenic and antiandrogenic effects (Dreikorn 1990; Marwick 1995), with SR (Permixon®) acting as a weak surrogate 5‐ARI inhibiting the conversion of testosterone to dihydrotestosterone (DHT) (Dedhia 2008);
anti‐inflammatory effects (Buck 2004; McGuire 1987) by a decrease in available sex hormone‐binding globulin (Di Silverio 1993);
pro‐apoptotic properties and inhibition of cellular proliferation (Buck 2004; Vacherot 2000; Vela‐Navarrete 2005);
the dependent inhibition of 5‐ARI in the stroma and epithelium of the prostate (Weisser 1996);
the relaxation of smooth muscles of the detrusor and the prostate via α1‐adrenergic receptors (Campbell‐Walsh Urology 2010);
placebo effect (Campbell‐Walsh Urology 2010).
How the intervention might work
The causes of LUTS related to BPH are not entirely known, although it is theorized that a combination of prostatic cellular proliferation (BPH) and smooth muscle dysfunction are likely reasons (Campbell‐Walsh Urology 2010). The efficacy of SR, if it exists, is dependent on one, both, or an unknown pathway.
Why it is important to do this review
In the West, phytotherapies, and in particular SR, are widely used for the relief of urinary symptoms attributed to BPH. It is therefore useful to patients, clinicians, and health policy makers, to determine the comparative effectiveness and harms of SR.
Objectives
The main outcomes were the efficacy of SR versus placebo or control in improving urologic symptom‐scale scores or global report of urinary symptoms (improved versus stable or worsened), and side effects. Secondary outcomes included changes in nocturia, prostate size, and peak urine flow.
Methods
Criteria for considering studies for this review
Types of studies
Randomized, controlled clinical trials.
Types of participants
Men with lower urinary tract symptoms (LUTS) consistent with benign prostatic hyperplasia (BPH).
Types of interventions
Comparison of preparations of SR with placebo or medical therapies for BPH with a treatment duration of at least 30 days.
Types of outcome measures
Primary outcomes
Urologic symptom scores (Boyarsky, American Urologic Association Symptom Index (AUA), and the International Prostate Symptom Score (IPSS)), with validated scores taking precedence over non validated ones. Both the AUA and IPSS use an identical scale of zero to 35, with mild symptoms scored 1 to 8, medium 9 to 18, and severe ≥ 19.
Secondary outcomes
Change in peak urine flow (mL/s).
Change in prostate size (measured in cubic centimeters (cc)).
Nocturia (times/evening).
Overall physician or patient assessment of urinary symptoms.
Adverse events (harms).
Search methods for identification of studies
See Appendices.
Electronic searches
We electronically searched the Cochrane Central Register of Controlled trials (CENTRAL), (including the database of the Cochrane Prostatic Diseases and Urologic Cancers Group) MEDLINE® (from 2008 to 2011), EMBASE (from 2001 to 1 January 2012), Web of Science®, Scopus, BIOSIS Previews®, LILACS, http://clinicaltrial.gov/, http://www.controlled‐trials.com/, and http://www.who.int/ictrp/en/.
Searching other resources
We handsearched systematic reviews, references, clinical‐practice guidelines, and conference abstracts.
Data collection and analysis
We assessed mean urologic symptom scores (IPSS, AUA), nocturia (# times), peak urine flow (mL/s), and prostate size (cc). The number and per cent of men reporting specific harms were also evaluated.
For the primary analysis (of the stated primary and secondary outcomes), all trials including SR in mono preparations and in combination were analyzed separately (e.g., SR versus placebo or active controls, SR + Urtica dioica versus placebo or controls). We pooled studies that were deemed clinically similar and provided sufficient information.
Selection of studies
In this update, two review authors (JT, RM) decided on eligibility.
Data extraction and management
Two review authors (JT, RM) independently assessed study characteristics and extracted data. Missing or additional information was sought from authors/sponsors. Extracted data were reviewed by the principal review author and discrepancies were resolved by discussion.
Assessment of risk of bias in included studies
As a measure of overall methodologic study quality – and bias – we assessed scales and criteria developed by Schulz and The Cochrane Collaboration (Cochrane Handbook 2011; Schulz 1995). The following seven criteria were addressed.
Selection bias I (Was there an articulated rule for allocating interventions based on chance?).
Selection bias II (Was there any foreknowledge of the allocation of interventions by anyone?).
Blinding bias I (During the course of the trial were study participants and personnel blinded to the knowledge of who received which intervention?).
Blinding bias II (Were the outcome assessors blinded to who received the intervention and who did not?).
Attrition bias (Did the trial assess all patients, or account for those not assessed?).
Reporting bias (Were outcomes selectively reported?).
Other bias (Were arms assessed differently?).
Each criterion was answered by 'low risk', 'unclear risk', and 'high risk', and summarized here (Figure 1; Figure 2). For the main outcome, we also assessed the quality of evidence in the Table 1 using GRADEPro (GRADEPro 2008).
1.
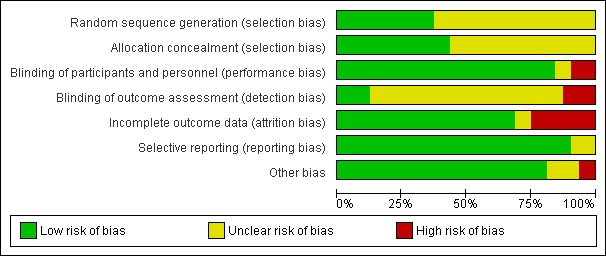
Risk of bias graph: review authors' judgements about each risk of bias item presented as per cents across all included studies.
2.
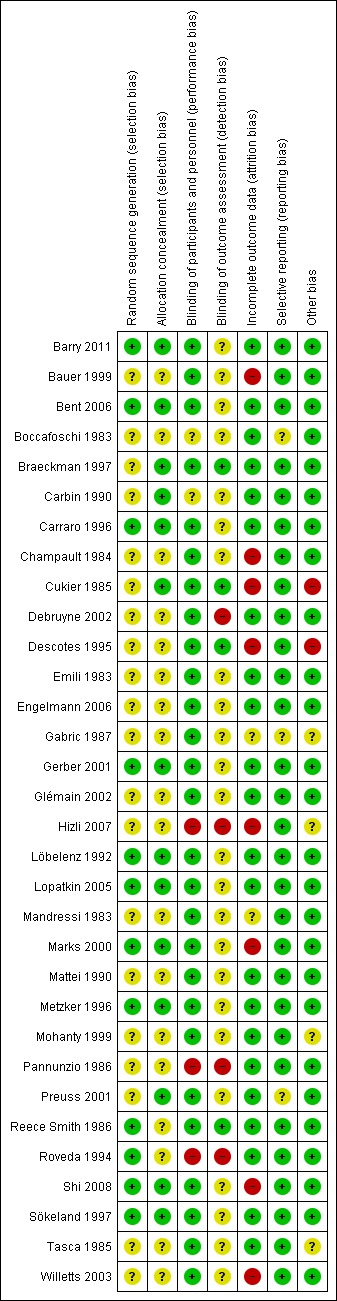
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We performed our statistical analysis according to the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook 2011). Dichotomous outcomes were expressed using risk ratios (RR) or absolute risk reductions (RD), using the Mantel‐Haenszel method. Continuous outcomes were expressed as mean differences (MD), or, for unequal scales, standardized mean differences (SMD). To minimize the uncertainty of the pooled‐effect estimate, we used an inverse variance method, which allowed larger trials with smaller SEM (standard error of the mean) more weight over smaller trials with larger SEM.
All outcome measures utilized 95% confidence intervals (CI), with a P value of ≤ 0.05 considered to be statistically significant.
Unit of analysis issues
Quasi‐randomized trials were not included.
Dealing with missing data
To assess the per cent change of patients' urologic symptoms, a modified intention‐to‐treat (ITT) was performed (i.e., men who dropped out or were lost to follow‐up were considered to have had worsening symptoms) (Lavori 1992). The denominator for the modified ITT analysis included the number randomized to treatment at baseline and the numerator included the number completing the trial and showing improvement.
Assessment of heterogeneity
We assessed for heterogeneity by using the I₂ statistic. If a meta‐analysis had a an I₂ of > 50%, we conducted a descriptive sensitivity analysis.
Assessment of reporting biases
To minimize reporting bias, we cross‐referenced trials and their protocols, compared systematic reviews to their included studies, and contacted authors.
Data synthesis
Assuming some level of unexplained heterogeneity among trials, we used a random‐effects model to adjust for effect size inconsistency.
Subgroup analysis and investigation of heterogeneity
We conducted no a priori analyses by subgroup. For investigation of heterogeneity, see Assessment of heterogeneity.
Sensitivity analysis
Results
Description of studies
This updated review (2012) found two new trials (n = 444), an 8.5% increase from our last update in 2009. Overall, 5666 men were assessed,
Overall (32 studies), weighted mean follow‐up was 29.2 weeks, and ranged from 4 to 72 weeks. The weighted mean age of all enrollees was 64.6 years (25 studies). The age of participants from reporting studies ranged from 40 to 90 years (21 studies). The percentage of men who dropped out or were lost to follow‐up was 10.4% (590/5666) and ranged from 0% to 21.4% (32 studies). Only one trial assessed compliance (Shi 2008), which was, after 12 weeks, 98% for the verum arm, and 100% for the placebo arm.
Fifteen trials (Barry 2011; Bauer 1999; Bent 2006; Carraro 1996; Debruyne 2002; Engelmann 2006; Gerber 2001; Glémain 2002; Hizli 2007; Lopatkin 2005; Marks 2000; Metzker 1996; Preuss 2001; Shi 2008; Sökeland 1997) provided efficacy outcomes using validated, identical, self‐reporting questionnaires (AUA, IPSS), and which were graded on a zero ("Never") to five ("Almost always") scale, for a total score of 35 points.
Of the included trials, 12 used Permixon®, a commercialized extract of the fruit of SR. Of these 12, seven compared Permixon® with placebo; the remaining trials compared Permixon®, either alone or in combination (e.g., Permixon® + tamsulosin), to finasteride, tamsulosin, and Depostat. Five studies compared another standardized combination of SR and (160 mg) and Urtica dioica extracts (120 mg), and which is known by the commercial name Prostagutt® forte, or PRO 160/120. Of these, three compared PRO 160/120 with placebo, one with finasteride, and one with tamsulosin. Fourteen trials used generic SR alone or in combination with other phytotherapies (pumpkin seeds, vitamins A and E, nettle root, Pygeum africanum).
Twenty‐two of the 32 included trials reported racial data. Ninety‐five per cent (3511/3678) of participants were White, 1.5% (55/3678) were African American, 1.2% (43/3678) were Hispanic, and 3.1% (115/3678) were Asian/Pacific Islanders. All studies reported regional affiliations; accumulatively, all save four could be dichotomized between Europe and the United States. Nearly 84% (4400/5267) of study participants were European, and 16.5% (867/5267) American.
The weighted mean baseline PSA (nine trials) was 2.8 ng/mL, and ranged from 1.7 ng/mL to 3.4 ng/mL. The weighted mean baseline prostate volume (12 trials) was 43.6 cc, and ranged from 33 cc to 57 cc.
Overall, symptom‐scale score results were reported in 23 studies (Barry 2011; Bauer 1999; Bent 2006; Braeckman 1997; Carbin 1990; Carraro 1996; Champault 1984; Debruyne 2002; Descotes 1995; Engelmann 2006; Gabric 1987; Gerber 2001; Glémain 2002; Hizli 2007; Lopatkin 2005; Mandressi 1983; Marks 2000; Metzker 1996; Mohanty 1999; Preuss 2001; Reece Smith 1986; Sökeland 1997; Willetts 2003), but only 14 reported the IPSS/AUA validated scores. Results for nocturia were reported in 15 studies (Barry 2011; Boccafoschi 1983; Carbin 1990; Carraro 1996; Champault 1984; Cukier 1985; Debruyne 2002; Descotes 1995; Emili 1983; Mandressi 1983; Mattei 1990; Mohanty 1999; Pannunzio 1986; Preuss 2001; Reece Smith 1986), but only 12 reported data that permitted pooling. Pannunzio presented per cent with nocturia at baseline and endpoint, but without defining nocturia. Debruyne reported per cent improvement, but did not provide baseline values.
Peak urine flow was reported in 27 studies (Barry 2011; Bauer 1999; Bent 2006; Boccafoschi 1983; Braeckman 1997; Carraro 1996; Champault 1984; Debruyne 2002; Descotes 1995; Emili 1983; Engelmann 2006; Gabric 1987; Gerber 2001; Glémain 2002; Hizli 2007; Löbelenz 1992; Lopatkin 2005; Marks 2000; Metzker 1996; Mohanty 1999; Pannunzio 1986; Preuss 2001; Reece Smith 1986; Shi 2008; Sökeland 1997; Tasca 1985; Willetts 2003).
Data on prostate size were reported in 13 trials (Bauer 1999; Bent 2006; Braeckman 1997; Carraro 1996; Debruyne 2002; Emili 1983; Hizli 2007; Marks 2000; Mattei 1990; Pannunzio 1986; Roveda 1994; Shi 2008; Sökeland 1997). Eight trials reported endpoints (Braeckman 1997; Carraro 1996; Debruyne 2002; Hizli 2007; Mattei 1990; Roveda 1994; Shi 2008; Sökeland 1997), one reported mean change (Bent 2006), and two reported per cent change from baseline (Emili 1983; Pannunzio 1986).
(See Characteristics of included studies and Description of studies).
The comparisons were as follows.
SR versus placebo (Barry 2011; Bauer 1999; Bent 2006; Boccafoschi 1983; Braeckman 1997; Champault 1984; Cukier 1985; Descotes 1995; Emili 1983; Gerber 2001; Löbelenz 1992; Mandressi 1983; Mattei 1990; Mohanty 1999; Reece Smith 1986; Shi 2008; Tasca 1985; Willetts 2003)
-
SR versus other phytotherapeutic control
Permixon® versus Pygeum africanum (Mandressi 1983)
SR orally versus SR rectally (Roveda 1994)
-
SR versus active control
SR versus gestonorone caproate (Pannunzio 1986)
Permixon® versus tamsulosin (Debruyne 2002; Glémain 2002; Hizli 2007)
SR versus finasteride (Carraro 1996)
-
SR + phytotherapeutic agent versus placebo
Curbicin (Sabal serrulata 80 mg and Cucurbita pepo L. (pumpkin seeds) 80 mg) versus placebo (Carbin 1990)
Prostagutt® forte versus placebo (Gabric 1987; Lopatkin 2005; Metzker 1996)
Permixon® + Pygeum africanum versus placebo (Mandressi 1983)
SR + nettle root extract + pumpkin seed oil extract + vitamin A versus placebo Marks 2000)
Cerniton AF™ (SR + phytosterol + ß‐sitosterol + vitamin E) (Preuss 2001)
-
SR + phytotherapeutic agent versus active control
SR + Urtica dioica versus finasteride (Sökeland 1997)
SR + Urtica dioica versus tamsulosin (Engelmann 2006)
-
SR + active agent versus active control
Permixon® + tamsulosin versus tamsulosin (Glémain 2002; Hizli 2007)
Serenoa repens alone or in combination versus placebo
There were 25 trials comparing SR, alone or in combination, with placebo (Barry 2011; Bauer 1999; Bent 2006; Boccafoschi 1983; Braeckman 1997; Champault 1984; Cukier 1985; Descotes 1995; Emili 1983; Gabric 1987; Gerber 2001; Löbelenz 1992; Lopatkin 2005; Mandressi 1983 (Mandressi was a three‐arm trial with placebo and active controls); Marks 2000; Mattei 1990; Metzker 1996; Mohanty 1999; Preuss 2001; Reece Smith 1986; Shi 2008; Tasca 1985; Willetts 2003). Nine trials (Barry 2011; Bauer 1999; Bent 2006; Gerber 2001; Lopatkin 2005; Marks 2000; Metzker 1996; Preuss 2001; Shi 2008) reported baseline values for IPSS/AUA total score for a weighted mean of 15.7 points, indicating moderately severe symptoms. Twelve trials reported baseline nocturia in some form (Barry 2011; Boccafoschi 1983; Champault 1984; Cukier 1985; Descotes 1995; Emili 1983; Mandressi 1983; Mattei 1990; Mohanty 1999; Preuss 2001; Reece Smith 1986; Tasca 1985), with 11 trials poolable (Barry 2011; Boccafoschi 1983; Champault 1984; Cukier 1985; Descotes 1995; Emili 1983; Mandressi 1983; Mattei 1990; Mohanty 1999; Preuss 2001; Reece Smith 1986) for a weighted mean of 2.1 incidents per night. Barry and Preuss reported AUA Nocturia; the weighted mean was 2.3, which correlates well with the other nine trials. Sixteen trials reported baseline peak urine flow (Barry 2011; Bauer 1999; Bent 2006; Boccafoschi 1983; Braeckman 1997; Descotes 1995; Emili 1983; Gerber 2001; Löbelenz 1992; Lopatkin 2005; Marks 2000; Metzker 1996; Mohanty 1999; Shi 2008; Tasca 1985; Willetts 2003) for a weighted mean of 12.4 mL/s. This compares favorably to Abrams' and Griffiths' definition of intravesical obstruction as a peak urine flow of ≤ 10 mL/s (Abrams 1979). The most commonly used dose of SR was 160 mg twice daily. The anomalies were Champault, who reported 80 mg twice daily, Gabric "20 drops" thrice daily, Löbelenz 100 mg once daily, Marks 106 mg twice daily, Pruess (SR + ß‐sitosterol) 286 mg twice daily, and Barry, whose dose‐response trial titrated 320 mg to 960 mg of SR.
Serenoa repens alone or in combination versus active control
Of 10 trials comparing SR, alone or in combination, with a control (Carbin 1990; Carraro 1996; Debruyne 2002; Engelmann 2006; Glémain 2002; Hizli 2007; Mandressi 1983; Pannunzio 1986; Roveda 1994; Sökeland 1997), five reported a weighted mean, baseline IPSS total score (Carraro 1996; Debruyne 2002;Engelmann 2006; Hizli 2007; Sökeland 1997) of 15.3 points. Three trials reported nocturia at baseline (Carbin 1990; Mandressi 1983; Pannunzio 1986), but only two trials had poolable data (Carbin 1990; Mandressi 1983) for a weighted mean of 1.93 nocturnal visits. Reported in seven trials (Carraro 1996; Debruyne 2002; Engelmann 2006; Glémain 2002; Hizli 2007; Pannunzio 1986; Sökeland 1997), the weighted mean, baseline peak urine flow was 11.0 mL/s. These results indicate that on average men had urinary symptoms consistent with moderate BPH, with moderate defined as IPSS/AUA total score eight to 19.
Most trials reported doses of SR equal to 160 mg twice daily, with the exceptions of Carbin (160 mg thrice daily), Roveda (160 mg 4 times daily), and Engelmann (160 mg daily). Barry 2011 was a dose‐finding trial of 320 mg, 640 mg, and 960 mg.
Five studies reported baseline prostate volumes (Carraro 1996; Debruyne 2002; Hizli 2007; Roveda 1994; Sökeland 1997), but only four were able to be pooled. The weighted mean baseline prostate size for the four studies (Carraro 1996; Debruyne 2002; Hizli 2007; Sökeland 1997) was 44.5 cc.
Results of the search
This search (January 2012) discovered two new trials that met inclusion criteria.
Included studies
Excluded studies
Risk of bias in included studies
Allocation
In 43.8% of trials (14/32) treatment allocation concealment was considered adequate (see Figure 1 and Figure 2).
Blinding
Eighty‐four per cent (27/32) of studies were double blinded. In four trials (12.5%) outcome assessors were blinded (Figure 1; Figure 2).
Incomplete outcome data
Nearly sixty‐nine per cent (22/32) of trials reported adequate attrition bias (Figure 1; Figure 2).
Selective reporting
Ninety per cent (29/32) of trials described adequate reporting bias (Figure 1; Figure 2).
Other potential sources of bias
Eighty‐one per cent (26/32) of trials were judged adequate for other sources of bias (Figure 1; Figure 2).
Effects of interventions
See: Table 1
Serenoa repens versus placebo
(17 trials)
Urinary symptom scores
Eleven trials (two long‐term (> one year), two moderate‐term (six to 12 months), 13 short‐term (< six months) reported outcomes for urinary symptom‐scale scores comparing Serenoa repens monotherapy with placebo, but only five studies utilized the validated AUASI/IPSS indices (Barry 2011; Bauer 1999; Bent 2006; Gerber 2001; Willetts 2003). Long‐ or moderate‐term treatment with Serenoa repens did not improve LUTS compared with placebo based on mean changes from baseline in the AUASI/IPSS (MD ‐0.16 points. 95% CI ‐1.45 to 1.14) (Barry 2011; Bent 2006; Gerber 2001). There was substantial heterogeneity between studies (I₂ = 52%), but this was changed (I₂ = 0%) when the moderate‐termed (six months) trial by Gerber 2001 was removed from the analysis. One trial reported clinically noticeable relief (≥ 3‐point improvement) for the SR arm (‐4.4 points) but not the placebo arm (‐2.2 points) (Lepor 1996). Bent 2006 found no noticeable relief for either arm (‐0.68 points versus 0.72 points, respectively). Barry 2011, which reported clinically meaningful improvements (≥ 3 points) for SR and placebo of 42.6% and 44.2%, respectively, found a RR of 0.96 (95% CI 0.76 to 1.22; P = 0.76). Willetts 2003 compared IPSS total scores from an unequal baseline (t‐test, P = 0.028), and reported improved symptoms for both arms, (treatment effect 1.74, 95% CI ‐0.54 to 4.03).
Braeckman 1997 (N = 238), who compared an unidentified, non validated urinary symptom score (scale 0 to 19) at endpoint favoring SR (MD ‐1.41, 95% CI ‐2.22 to ‐0.60; P = 0.0002). Mohanty 1999 (N = 75) reported symptom improvements for SR and placebo (81.4% and 64.3%, respectively) using a non validated, global, modified Boyarsky scale (range zero to 27, with a higher score indicating worse symptoms). The absolute risk reduction was 17% favoring SR. Two trials used a physician‐assessed, symptom‐improvement score, and found no difference between SR and placebo (Analysis 1.6), although with high heterogeneity (I₂ = 91%). In a patient‐rated survey, a meta‐analysis of four trials favored SR (Analysis 1.5), but also with substantial heterogeneity (I₂ = 86%). Reece Smith 1986, in a 12‐week trial, compared 11 symptom assessments from both physicians and patients; there were no significant inter group differences for any symptom in either physician or patient assessments.
1.6. Analysis.
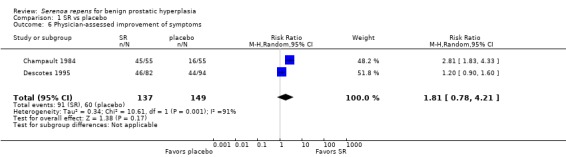
Comparison 1 SR vs placebo, Outcome 6 Physician‐assessed improvement of symptoms.
1.5. Analysis.
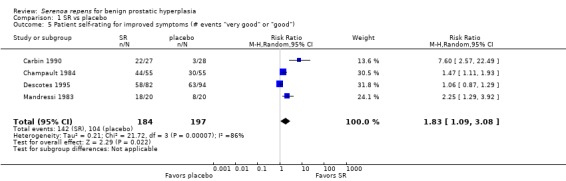
Comparison 1 SR vs placebo, Outcome 5 Patient self‐rating for improved symptoms (# events "very good" or "good").
Nocturia
On the validated AUA score, nocturia was not significantly improved versus placebo.
Ten trials (Barry 2011; Boccafoschi 1983; Champault 1984; Cukier 1985; Descotes 1995; Emili 1983; Mandressi 1983; Mattei 1990; Mohanty 1999; Reece Smith 1986) compared data for nocturia. Mohanty's short‐term trial (two months) reported an absolute risk reduction of 11.24% favoring SR. An initial meta‐analysis of nine trials (Boccafoschi 1983; Champault 1984; Cukier 1985; Descotes 1995; Emili 1983; Mandressi 1983; Mattei 1990; Reece Smith 1986; Tasca 1985) significantly favored SR (Analysis 1.2), but with substantial heterogeneity (I₂ = 76%). (We were unable to conduct a plausible sensitivity analysis.) Barry 2011, at 72 weeks follow‐up, found SR not superior to placebo in the AUA Nocturia score (range zero to 5, with a higher score indicating worse nocturia) (1‐sided P value = 0.19).
1.2. Analysis.
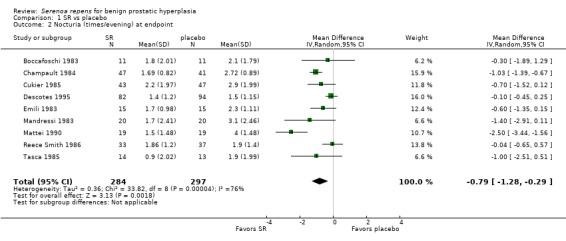
Comparison 1 SR vs placebo, Outcome 2 Nocturia (times/evening) at endpoint.
Peak urine flow
Moderate or long‐term Serenoa repens therapy did not improve peak urine flow rates compared with placebo based on mean changes from baseline (MD 0.40 mL/s, 95% CI ‐0.30 to 1.09; I₂ = 0%) (Analysis 1.3).
1.3. Analysis.
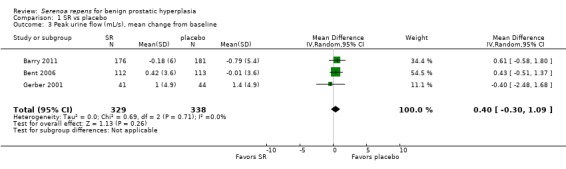
Comparison 1 SR vs placebo, Outcome 3 Peak urine flow (mL/s), mean change from baseline.
Fourteen trials (Barry 2011; Bauer 1999; Bent 2006; Boccafoschi 1983; Braeckman 1997; Champault 1984; Descotes 1995; Emili 1983; Gerber 2001; Löbelenz 1992; Mohanty 1999; Reece Smith 1986; Tasca 1985; Willetts 2003) presented data for peak urine flow. Three trials reported data that were not poolable (Bauer 1999; Löbelenz 1992; Willetts 2003). Bauer 1999 and Löbelenz 1992 found a 12% and 5.2% absolute improvement favoring SR, respectively. Willetts 2003 reported placebo improved flows significantly better than SR (t‐test, P < 0.001). Our meta‐analysis of six trials comparing endpoints found no difference (MD 0.35, 95% CI ‐1.05 to 1.76), and with little heterogeneity (I₂ = 21%) (Analysis 1.4). A sensitivity analysis (the low quality short term Mohanty 1999 trial was eliminated) of mean change (Analysis 1.3) would seem to confirm the same.
1.4. Analysis.
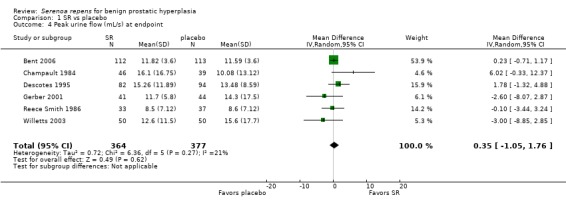
Comparison 1 SR vs placebo, Outcome 4 Peak urine flow (mL/s) at endpoint.
Prostate size
One long‐term trial reported no significant reduction in prostate volume following treatment with Serenoa repens versus placebo (‐1.22 mL, 95% CI ‐3.90 to 1.47) (Bent 2006).
Six trials (Bauer 1999; Bent 2006; Braeckman 1997; Emili 1983; Mattei 1990; Mohanty 1999) reported data for prostate size; four were poolable (Bent 2006; Braeckman 1997; Mohanty 1999; Mattei 1990). Bauer 1999 (N = 101), with a follow‐up of six months, reported slight increases for SR and placebo (1.4% (34.5 cc to 35 cc) and 1.5% (31.7 cc to 32.2 cc), respectively). Emili 1983 (N = 30), with a four‐week follow‐up, reported (in a qualitative scale) 26.6% reduction for the SR arm, and no change for the placebo arm. Two meta‐analyses, one comparing endpoints and the other mean changes, found no significant differences between arms (Analysis 1.7 and Analysis 1.8, respectively).
1.7. Analysis.
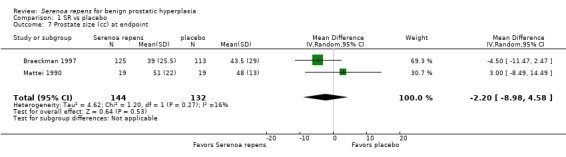
Comparison 1 SR vs placebo, Outcome 7 Prostate size (cc) at endpoint.
1.8. Analysis.
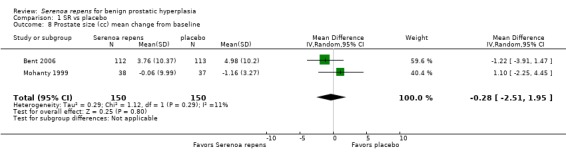
Comparison 1 SR vs placebo, Outcome 8 Prostate size (cc) mean change from baseline.
Adverse events/Adverse effects
Adverse events, or harms, of SR, were few and mild, and compared with placebo, not statistically significant.
A meta‐analysis of four trials and "any" adverse event found no difference between arms (Analysis 1.10). Barry 2011 reported 530 ("any") adverse events in 136 men who received SR, and 476 events in 137 men who received placebo. The comparison was not significant (Fisher exact test, P = 0.80). For common, possibly drug‐related effects (asthenia, decrease in libido, diarrhea, dizziness, ejaculation disorders, GI (gastrointestinal) distress, headache, postural hypotension), Barry reported only GI distress, with 52 events in 38 men who received SR and 58 events in men who received placebo. This comparison was not significant as well (Fisher exact test, P > 0.99).
1.10. Analysis.
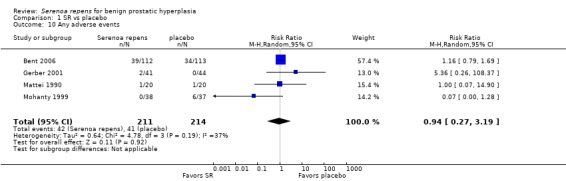
Comparison 1 SR vs placebo, Outcome 10 Any adverse events.
SR (Permixon®) versus finasteride
(One trial)
Urinary symptom scores
Carraro 1996 (N = 1098) found no difference between SR and finasteride in the IPSS total score at endpoint (MD 0.40 points, 95% CI ‐0.57 to 1.37).
Nocturia
At endpoint, SR was not superior to finasteride (MD ‐0.05 nocturnal visits, 95% CI ‐0.49 to 0.39).
Peak urine flow
Peak urine flow improved for both arms but was not significantly different (MD ‐0.50 mL/s, 95% CI ‐1.91 to 0.91).
Prostate size
For Permixon® versus finasteride, prostate size decreased ‐6% (43.0 cc to 41.5 cc) and ‐18% (44.0 cc to 36.7 cc), respectively, and favored finasteride (MD 4.80 cc, 95% CI 1.42 to 8.18).
SR (Permixon®) versus tamsulosin
(Two trials)
Urinary symptom scores
Comparing mean change of the IPSS total score (tamsulosin dose for both trials was 0.4 mg daily), these trials showed comparable efficacy, but one was not superior to the other (Analysis 3.1). A caveat: I₂ was 50%.
3.1. Analysis.
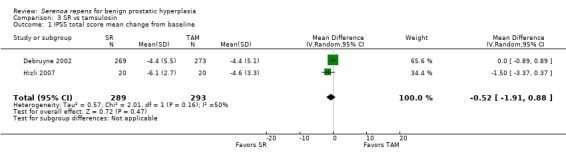
Comparison 3 SR vs tamsulosin, Outcome 1 IPSS total score mean change from baseline.
Nocturia
The risk ratio favored Permixon® but was not significant (RR 0.91%, 95% CI 0.66 to 1.27) (Debruyne 2002).
Peak urine flow
Debruyne 2002 found slight increases in peak urine flow and Hizli 2007 slight decreases, but in the meta‐analysis there was no significant difference (Analysis 3.2).
3.2. Analysis.
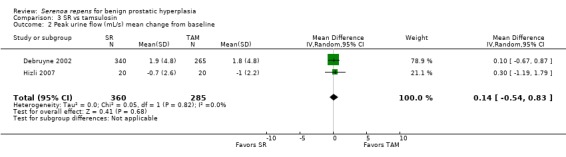
Comparison 3 SR vs tamsulosin, Outcome 2 Peak urine flow (mL/s) mean change from baseline.
Prostate size
Both trials reported shrinking prostates for all arms save a single tamsulosin arm. No difference was found in the meta‐analysis (Analysis 3.3).
3.3. Analysis.

Comparison 3 SR vs tamsulosin, Outcome 3 Prostate size (cc) mean change from baseline.
SR (Permixon®) versus gestonorone caproate
(One trial)
Peak urine flow
Pannunzio 1986 (N = 60) reported a significant difference in mean change, favoring SR (MD 2.00 mL/s, 95% CI 1.49 to 2.51).
Prostataplex™ versus placebo
(One trial)
(Prostataplex™ is SR, soybean oil, beeswax, soy lecithin, gelatin, glycerin, de‐ionized water, titanium dioxide, carmine red, natural vanilla flavor.)
Urinary symptom score
Shi 2008 (N = 94) considered, a priori, an intra group decrease of three points of the IPSS total score to be clinically significant. After a three‐month follow‐up, the Prostataplex™ arm decreased a mean of 2.02 points, and the placebo arm decreased a mean of 0.33 (Student's t‐test, P < 0.001). The comparison of endpoints (14.83 versus 14.13, respectively) was not significant (Student's t‐test, P = 0.545).
Peak urine flow
Shi 2008 reported a significant difference at endpoint favoring Prostataplex™ (MD 2.33 mL/s, (95% CI 1.51 to 3.15).
Prostate size
Shi 2008 reported slight decreases at endpoint (Prostataplex™ = 2.1 cc; placebo = 2.48 cc) but no significant difference between them (MD ‐0.28 cc, 95% CI ‐10.38 to 9.82).
Cernitin™ + SR + ß‐sitosterol + vitamin E versus placebo
(One trial)
Urinary symptom scores
Preuss 2001 (N = 144) reported a significant difference in the AUA total score (MD ‐2.93 points, 95% CI ‐5.06 to ‐0.80) favoring combination therapy.
Nocturia
Preuss 2001 found a significant difference between the two arms in the AUA nocturia sub scale (0 to 5; '0' is no trips, '5' is 5 trips or more) and favoring Cernitin™ (MD ‐0.70, 95% CI ‐1.07 to ‐0.33).
Peak urine flow
Combination therapy was superior to placebo at endpoint (MD ‐1.30 mL/s, 95% CI ‐1.61 to ‐0.99).
SR + Urtica dioica versus placebo
(Three trials)
Urinary symptom scores
Metzker 1996 (N = 40) found a significant difference in IPSS endpoint (40‐week follow‐up) that favored combination therapy (MD ‐3.50 points, 95% CI ‐6.75 to ‐0.25). Lopatkin 2005 (N = 257), comparing mean change, did not (MD ‐1.00 points, 95% CI ‐2.13 to 0.13). Gabric 1987 (N = 30) compared the combination Prostagutt® forte with placebo, and included a physician evaluated global symptom score (scale one to three; one = no change, two = satisfactory change, three = excellent change) at six‐week endpoint. The median (extrapolated from graph) for the verum arm was 1.3 and for the placebo arm 2.2 (P < 0.05).
Peak urine flow
Lopatkin 2005, comparing mean change, reported positive mean changes of about 2 mL/s for both arms, but the comparison was not significant (MD ‐0.10 mL/s, 95% CI ‐1.22 to 1.02). Gabric 1987 (N = 30) and Metzker 1996 found a significant difference at endpoint (Analysis 2.1), but it was marginal (P = 0.05).
2.1. Analysis.
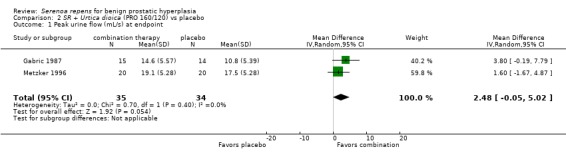
Comparison 2 SR + Urtica dioica (PRO 160/120) vs placebo, Outcome 1 Peak urine flow (mL/s) at endpoint.
SR + Urtica dioica versus finasteride
(One trial)
Urinary symptom scores
Sökeland 1997 (N = 543) found no significant difference in IPSS total score at 12‐week endpoint (MD 0.30 points, 95% CI ‐1.28 to 1.88).
Peak urine flow
Sökeland 1997 reported increases of 2.7 mL/s and 3.2 mL/s for SR + Urtica dioica and placebo, respectively, but the comparison was not significant (MD ‐0.80 mL/s, 95% CI ‐1.98 to 0.38, P > 0.05).
Prostate size
Sökeland 1997 (mean prostate size 43.3 cc) reported declines in prostate volume for both arms at the end of 12‐week follow‐up. The PRO 160/120 arm decreased 0.7% (42.7 cc to 42.4 cc), and the finasteride arm, 15.5% (44.0 cc to 37.2 cc), for an absolute improvement of 14.8% favoring finasteride.
SR + Urtica dioica versus tamsulosin
(One trial)
Urinary symptom scores
Engelmann 2006 (N = 140), which reported responders to treatment (defined as IPSS ≤ 7 at endpoint), reported a non significant risk ratio favoring tamsulosin (RR 1.16, 95% CI 0.69 to 1.94).
SR + tamsulosin versus tamsulosin
(Two trials)
Urinary symptom scores
Glémain 2002 and Hizli 2007, comparing IPSS total scores, found improvements in all arms, but the difference was not significant (Analysis 4.1).
4.1. Analysis.
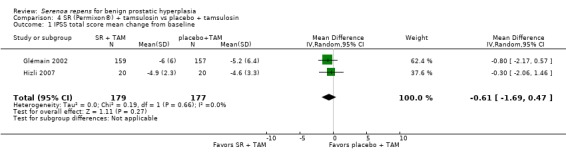
Comparison 4 SR (Permixon®) + tamsulosin vs placebo + tamsulosin, Outcome 1 IPSS total score mean change from baseline.
Peak urine flow
Glémain 2002 (N = 329) and Hizli 2007 (n = 40 in this comparison) reported positive changes in all arms, but no statistical difference (Analysis 4.2).
4.2. Analysis.
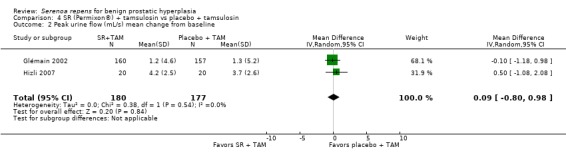
Comparison 4 SR (Permixon®) + tamsulosin vs placebo + tamsulosin, Outcome 2 Peak urine flow (mL/s) mean change from baseline.
Prostate size
Hizli 2007i (n = 40 for these comparisons), in a 24‐week study, also reported no significant difference in mean change (MD 0.20 cc, 95% CI ‐1.10 to 1.50).
SR versus SR + tamsulosin
(One trial)
Urinary symptom scores
Hizli 2007 reported no difference in IPSS total score mean change (MD ‐1.20 points, 95% CI ‐2.75 to 0.35).
Peak urine flow
There was no significant difference in mean change (MD ‐1.00 mL/s, 95% CI ‐2.46 to 0.46).
Prostate size
Although both treatments decreased prostate volume, SR monotherapy was not significantly better than combination therapy (MD 0.10 cc, 95% CI ‐1.34 to 1.54).
Adverse effects/adverse events
We assessed adverse effects associated with SR and active controls (α‐blockers and 5α‐reductase inhibitors). For the 19 trials reporting, adverse effects were generally mild. The most common adverse effects associated with SR, finasteride, and tamsulosin were asthenia (abnormal loss of strength), decreased libido, diarrhea, dizziness, ejaculation disorders, gastrointestinal distress, headaches, and postural hypotension. no arm reported an incidence increase of adverse effects greater than 5%. None of the comparisons was statistically significant (Table 7).
2. Summary table of adverse effects (SR versus placebo).
| Serenoa repens | Placebo | (Significance, P ≤ 0.05) | |
| 5 trials | n/N (%) | ||
| Diarrhea, n = 3 | 2/191 (1.0) | 3/194 (1.5) | P = 0.67 |
| Dizziness, n = 1 | 0/38 (0) | 1/37 (2.7) | P = 0.49 |
| GI distress, n = 5 | 9/315 (2.9) | 3/312 (1.0) | P = 0.10 |
| Headache, n = 2 | 0/115 (0) | 2/114 (1.8) | P = 0.29 |
Denominator is number in arm. Per cents are rounded to the nearest tenth.
Incidences of asthenia, decrease in libido, dizziness, ejaculation disorders, headache, postural hypotension were most commonly reported in the trials with tamsulosin. Ejaculation disorders were nearly statistically significantly greater (P = 0.06) in the tamsulosin arm compared to the SR arm (35% versus 0%) (Table 8). The risk ratio for any adverse event favored SR, but was not statistically significant (RR 0.98, 95% CI 0.89 to 1.09). Hizli 2007 reported no adverse events.
3. Summary table of adverse effects (SR versus tamsulosin).
| Permixon® | Tamsulosin | (Significance, P ≤ 0.05) | |
| 1 trial | n/N (%) | ||
| Asthenia | 0/20 (0) | 2/20 (10) | P = 0.29 |
| Decrease in libido | 0/20 (0) | 4/20 (20) | P = 0.13 |
| Dizziness | 0/20 (0) | 2/20 (10) | P = 0.29 |
| Ejaculation disorders | 0/20 (0) | 7/20 (35) | P = 0.06 |
| Postural hypotension | 0/20 (0) | 1/20 (0.5) | P = 0.49 |
Denominator is number in arm. Per cents are rounded to the nearest tenth.
Compared with finasteride, reported adverse effects included decrease in libido, diarrhea, gastrointestinal distress, and headache. The most common adverse effect reported for SR was decreased libido (2.2%). The most common adverse effect for finasteride was also decreased libido (3.0%). There were more headaches in the Permixon arm than the finasteride (1.3% versus 0.4%, respectively). No comparisons were significant (Table 9).
4. Summary table of adverse effects (SR versus finasteride).
| Permixon® | Finasteride | (Significance, P ≤ 0.05) | |
| 1 trial | n/N (%) | ||
| Decrease in libido | 12/551 (2.2) | 16/542 (3.0) | P = 0.42 |
| Diarrhea | 5/551 (1) | 6/542 (1) | P = 0.74 |
| Gastrointestinal distress | 10/551 (1.8) | 15/542 (2.8) | P = 0.30 |
| Headache | 7/551 (1.3) | 2/542 (0.4) | P = 0.12 |
Denominator is number in arm. Per cents are rounded to the nearest tenth.
Serious adverse events (i.e., events with no necessary causal association with the intervention) were reported in the Bent trial comparing SR with placebo (Bent 2006). These events included cardiovascular event, elective orthopedic surgery, gastrointestinal bleeding, bladder cancer, colon cancer, elective hernia repair, hematoma, melanoma, prostate cancer, shortness of breath, and rhabdomyolysis. The SR arm had eight (7%) serious adverse events, and 18 (16%) were reported for the placebo arm (P = 0.05). In a meta‐analysis (five studies) of "any" adverse event, and which included both causal and non‐causal harms, the risk ratio favored SR but not significantly (Analysis 1.10).
Study withdrawals
All 32 trials reported some data for losses to follow‐up. For the main comparison, SR monotherapy versus placebo, there were 17 trials reporting but only 14 were poolable (Analysis 1.9). The comparison was not significant. The three trials that were unable to be analyzed did not report sufficient data to make a determination (Cukier 1985; Descotes 1995; Mandressi 1983). Three other trials reported zero withdrawals and thus were not included (Boccafoschi 1983; Emili 1983; Löbelenz 1992). For SR monotherapy versus tamsulosin (two trials), the comparison was not statistically significant (Analysis 3.4; versus finasteride (one trial), the comparison was significant, and favored finasteride (RR 1.39, 95% CI 1.02 to 1.89).
1.9. Analysis.
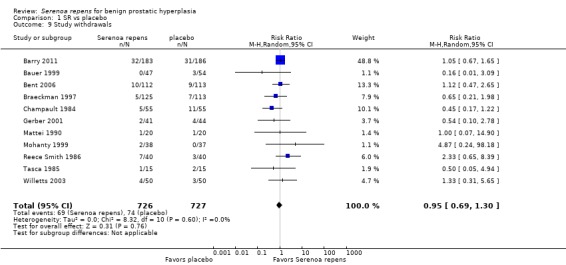
Comparison 1 SR vs placebo, Outcome 9 Study withdrawals.
3.4. Analysis.
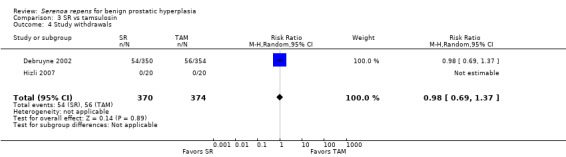
Comparison 3 SR vs tamsulosin, Outcome 4 Study withdrawals.
Discussion
Summary of main results
In 1998, 2000, and 2002, we reported, after evaluating mostly under‐powered, short‐term trials with variable study design, outcomes, and non validated symptom‐scale scores, that Serenoa repens (SR) provided mild improvement of urologic symptoms. In 2008 we reversed course and reported SR was not superior to placebo for symptom scores (MD ‐0.77 points, 95% CI ‐2.88 to 1.34). Heterogeneity was high (I₂ = 63%) but we thought the high quality Bent 2006 trial carried the day. In 2012, with Barry 2011, we now have another adequately powered, high quality, long‐term (follow‐up 72 weeks), dose‐finding trial (320 mg/d, 640 mg/d, 960 mg/d), which found SR not superior to placebo at 24, 48, and 72 weeks, respectively. In the meta‐analysis of this trial with the other high quality trial from the 2009 update, there was no significant difference between arms (MD ‐0.25 points, 95% CI ‐0.58 to 1.07) and heterogeneity was nonexistent (I₂ = 0%). Barry 2011 also reported responders (men with ≥ three‐point improvement) and found the risk ratio favored placebo, but not significantly (0.96 ,95% CI 0.76 to 1.22).
Overall completeness and applicability of evidence
The overall completeness of the evidence was good in 2012, and much better than it was at the inauguration of this review in 1998. For example, of the 14 trials that were placebo controlled and compared with SR monotherapy, seven utilized the commercialized Permixon®. Sixteen of the 32 trials used a validated score ‐ the AUA or IPSS ‐ to assess symptoms. Fifteen of 16 of those trials provided baseline or endpoint IPSS or AUA scores, although not all gave measures of variance (10 did). Of 15 trials with baseline or endpoint data for nocturia, 12 provided means, one provided "per cent with nocturia," and two the AUA nocturia score. Twenty‐three trials reported baseline and endpoint data for peak urine flow, and seven reported mean differences. Eleven of 32 trials provided data at baseline or endpoint for prostate size, and four provided mean change with measures of variance.
Quality of the evidence
Perhaps the outstanding problem in our first published review (1998) was underpowered trials. Of 18, only six randomized 100 men or more. In this updated review of 32 trials, 15 randomized 100 men or more (range 100 to 1098). This trend toward higher powered trials ‐ and with corresponding smaller CI ‐ yields better statistical evidence. Another significant problem was the use of non validated symptom scores. In 1998 17% (3/18) of trials used validated scores; in this update 50% (16/32) do. In this update, 14 trials reported nocturia data at baseline and endpoint, most with measures of variance, but only two reported mean changes and variances, a better statistical metric that yields smaller confidence intervals and standard errors and thus a truer estimate of effect. Data reporting for prostate size was slightly better; five trials reported baselines and endpoints for prostate size, and four reported mean changes with variances. Peak urine flow was reported best: 15 trials reported baselines and endpoints; seven reported mean changes with variances.
Potential biases in the review process
From our very first review in 1998 we were sensitive to biases among trials and decided a priori to report outcomes via a random‐effects model, which is a more conservative estimate of treatment effect. By the end of the 14 following years, we have seen a dramatic improvement in the methodological quality of reporting trials (Barry 2011; Bent 2006). Of the seven risk criteria (see Assessment of risk of bias in included studies), all were reported 'low risk of bias' save one ('Blinding of outcome assessment'), which was reported as 'unknown'.
Agreements and disagreements with other studies or reviews
Boyle 2004, a systematic review of proprietary Permixon®, reported improvements in the IPSS for SR and placebo (4.78 and 4.54 points, respectively), but the comparison was not significant (P > 0.05), as well as indirect. Boyle also reported both arms improved peak urine flow (Permixon® 1.02 mL/s versus placebo 1.20 mL/s) from baseline, but the comparison favored placebo (P = 0.04). In our meta‐analysis of four trials (none of which used Permixon®) and 736 men, the comparison favored placebo as well (MD 0.18 mL/s, 95% CI ‐0.54 to 0.89; I₂ = 0%) but was not significant. Both Boyle 2004 and Maccagnano 2006 claimed the efficacy of Permixon® by its comparative effectiveness to α‐blockers and 5α‐reductase inhibitors. We believe the Barry and Bent trials have shown Serenoa repens', if not necessarily Permixon's, non‐superiority to placebo.
Authors' conclusions
Implications for practice.
Serenoa repens is a widely used in Europe and the US to treat lower urinary tract symptoms associated with benign prostatic hyperplasia. Our conclusion that SR, even at escalating doses, is not superior to placebo, is based on two high quality, clinical trials, one with a follow‐up of six years.
Implications for research.
We do not know if our conclusions are generalizable to proprietary products of Serenoa repens, such as Permixon® or Prostagutt® forte. Nonstandarization is a long‐recognized problem of phytotherapeutic products, and that includes SR (Habib 2004; Lowe 1996). Future research needs are that RCTs using branded SR have a follow‐up of at least one year, are methodologically sound, well powered, use validated, symptom‐scale scores, and most importantly, have a placebo arm.
Feedback
Anna Rita Bilia, et al, 31 August 2009
Summary
Feedback: Quality of a herbal medicinal product is essential. Both the safety profile and the efficacy of a multi‐component herbal medicinal product are irrevocably linked to quality. Quality should be assessed according to the monographs reported in the European Pharmacopoeia or in other Pharmacopoeias or pharmaceutical reference books [1, 2]. These record the methods to define the quality of multi‐component herbal drugs and also of defined selected extracts, according to classification of active constituents, pharmacologically active markers and quality markers [3, 4]. Additionally, pharmacopeial methods are fully validated to perform correctly under the given analytical proceedings irrespectively of the environment where they are performed (ICH guideline Q2(R1); www.ich.org).
Quality of a defined multi‐component herbal extract is strictly related to the quality of the botanical source (herbal drug) defined by the botanical name of the plant according to the binomial system (genus, species, variety and author) and the part used (e.g. leaf, root or fruit). In addition other factors should be considered such as the method of preparation (extraction process, solvents used; solubility and stability of the plant constituents), the drug extract ratio (DER), time and temperature operations, which could be crucial not only for safety but also for the efficacy of the product [5‐8]. Ideally, in analogy with the analytical procedures for testing, also the production‐process should be fully validated, in order to guarantee consistency of the final product, as far as possible.
For these reasons the final mix of constituents in a multi‐component extract may exert different activities and in some circumstances, may even have a different safety profile from another type of extract, that is derived from the identical herb. These facts are taken into consideration and documented for well‐defined herbal extracts in a new series of published European Community Monographs, authorised by the EMEA [9]. It is noteworthy that, among the various types of plant products, e.g. food and botanical products, on the world market, only Herbal Medicinal Products are produced under rigid quality systems, such as Good Sourcing Practices (GSP), Good Agricultural Practices (GAP), Good Field Collection Practices (GFCP), Good Processing Practices (GPP), as well as Good Manufacturing Practices (GMP). As a consequence the quality can be assessed and the final product can be considered reproducible.
According to the above arguments, it is crucial to realise, that the identical botanical source cannot guarantee the bioequivalence of its various multi‐component extracts and of the resulting different Herbal Medicinal Products. The situation in the Cochrane review on Serenoa repens [10] leaves no doubt, that various different Serenoa extracts (not always defined) and their subsequently varying final medicinal products, have been summarised, then analysed, in order to obtain the final conclusions of the review. This left the reader to assume, that both comparable (bioequivalent) and non‐comparable products were included and compared in this study, in spite of the fact, that they might have exerted different, e.g. non‐comparable safety and/or efficacy profiles.
Considering statements and definitions mentioned above, the Cochrane Review on Serenoa repens [10] has been evaluated by the contributors to the present 'Letter to the editor.' Four in part‐related problems were encountered. In the following four comments, these problems have been addressed.
Comment 1. Problem, missing conclusion regarding studies with a positive control Serenoa alone, was compared in 4 of the 30 investigated clinical trials with known BPH drugs, such as Finasteride, Tamsulosin and Gestonorone caproate as positive controls. Reported in the review were a few minor differences and many comparable results for the various evaluated symptoms and no difference for the overall urinary symptom scores, between treatments with either Serenoa extract or these BPH drugs in different studies with up to 1098 patients. This apparently demonstrated, that the efficacy of these drugs was not different from that of the Serenoa products. Selected results are shown here, to exemplify the commentary: 1 study compared Serenoa to finasteride (MD, mean difference, ‐0,40 Points, 95% CI ‐0.57 to 1.37, P > 0.05); 2 studies compared Serenoa to tamsulosin (WMD, weighted mean difference, ‐0.52 points, 95% CI ‐1.91 to 0.88, P > 0.05).
The reader of the review, even without being in the position to repeat the full statistical analysis, could conclude, that efficacy of Serenoa should be similar or comparable to these BPH drugs. The final statement of the authors, that "Sereoa is not different from placebo", is in clear contradiction to these reports. This contradiction has not been addressed, nor discussed, by the authors of the review.
Conclusion to comment 1
Contradictions described here, unless resolved, prohibit a final conclusion about the efficacy of Serenoa repens products.
Comment 2. Problem, chemical complexity of a multi‐component plant‐extract; 'non‐equivalence' of analysed products The authors of the review appear to have treated the various Serenoa fruit preparations, derived from different extracts, used in the 30 clinical trials, which they analysed, as if these extracts were identical single chemical entities. i.e. the authors appear not to have considered in their analysis, that components of different multi‐component preparations vary, according to their extraction procedure, the solvent used, the drug‐extract‐ratio, the total constituents probably vary, the co‐active constituents probably vary and the standardization can vary. Thus the doses can vary.
The authors have stated in the review, that "of the 15 trials (in true only 14 appeared to have been actually analysed in the review), that were placebo‐controlled and compared to Serenoa repens monotherapy, 7 utilized the commercialized Permixon®, which assured that our comparators were equivalent" [page 13]. Thus the reader may conclude, that 7 out of 14 placebo‐controlled trials were included in this analysis, that were 'non‐equivalent' (i.e. 50% of the comparators). This causes concern about the validity of the authors' statement as well as the authors' conclusions.
Comment 3. Problem, variation of dose
The dosage relates directly to the composition of a multi‐component extract (see comment 2 as well). Thus, the dosage between studies can vary, even if identical amounts are given. Naturally, the dosage must also vary, if the administered amount differs. The 'daily dose' of an extract administered, varied from study to study, in the 30 studies analysed: from 20 drops, 100 mg, 160 mg, 212 mg, 286 mg, 320 mg (a number of studies), 480 mg up to 640 mg, mostly applied in two portions. There was no statistical evaluation in the review taking these different dosages that were used in the 30 clinical trials, into account.
The BPH treatments using non‐identical Serenoa preparations at strongly varying dosages, were summarized and investigated in the review, as if identical treatments with defined dosages, had been used. This is, as if one would assume, that apples, pears and even lemons will taste the same, merely because they are round.
Conclusion to comments 2 and 3
The statistical comparative analysis by the authors of the review, focuses on clinical symptom‐scores of BPH in 30 trials, but they have omitted to fully address the consequences of analysing heterogenous Serenoa preparations administered in heterogenous dosage schemes, in those trials. For example the 7 'non‐equivalent' placebo‐controlled trials should not have been considered as a valid part of a comparative clinical analysis of the placebo‐controlled studies.
Comment 4. Problem, studies conducted with Serenoa‐containing combination products.
Nine (9) of 30 analysed studies, were conducted with combination products containing Serenoa repens extracts, besides one or more other potentially active phytotherapeutic agent (there was no consideration of the dosage, the various extracts were not defined, in the Cochrane review).
Conclusion to comment 4
These studies do not give evidence concerning the efficacy of Serenoa. Any efficacy or lack of efficacy cannot be attributed to Serenoa, such as would be the case in mono‐therapy, but could be influenced by the other plant components in each product. The reader may conclude, that these studies do not qualify for a comparative analysis and cannot support a conclusive statement concerning the activity of Serenoa repens.
Summarising conclusions from comments 1‐4
‐Of 30 analysed studies, 7 placebo‐controlled studies with "non‐comparable" Serenoa products and 9 studies with combination products, could be deleted for good reasons, possibly leaving 14 studies for a revision of the comparative analysis.
‐The authors final conclusion in this review "Sereoa is not different from placebo," does not appear to have been corroborated by rigorous scientific reasoning. Even without repeating the full statistical evaluation (which appears to be necessary as well), the authors final conclusion regarding the efficacy of Serenoa repens, needs to be reconsidered.
References
Saw Palmetto Fruit. Sabalis serrulatae fructus. European Pharmacopoeia. Council of Europe.
Saw palmetto. USP, United States Pharmacopeia.
Guideline on declaration of herbal substances and herbal preparations in herbal medicinal products/traditional herbal medicinal products in the SPC (Summary of product characteristics). EMEA adopted guideline. London, 26 July 2007 Doc. Ref: EMEA/HMPC/CHMP/CVMP/287539/2005
Reflection papers on markers used for quantitative and qualitative analyses of herbal medicinal products and traditional herbal medicinal products. EMEA adopted guideline. London, 15 July 2008. Doc. Ref. EMEA/HMPC/253629/2007
Gaedcke F, Steinhoff B. In: Herbal medicinal products. Scientific and regulatory basis for development, quality assurance and marketing authorisation. CRC Press, Boca Raton, 2003.
Guideline on specifications: test procedures and acceptance criteria for herbal substances, herbal preparations and herbal medicinal products/traditional herbal medicinal products. EMEA adopted guideline. London 30 March 2006. CPMP/QWP/2819/00 Rev 1. EMEA/CVMP/814/00 Rev 1
Guideline on quality of herbal medicinal products/traditional herbal medicinal products. London, 30 March 2006. EMEA adopted guideline. CPMP/QWP/2819/00 Rev 1. EMEA/CVMP/814/00 Rev 1.
Vlietinck A, Pieters L, Apers S. Legal requirements for the quality of herbal substances and herbal preparations for the manufacturing of herbal medicinal products in the European Union. Planta Med 2009;75:683‐8.
a) about European Community Monographs http://www.emea.europa.eu/htms/human/hmpc/hmpcmonographs.htm ; b) homepage of HMPC (Herbal Medicinal Product Committee of EMEA) http://www.emea.europa.eu/htms/human/hmpc/index.htm; c) about HMPC documents (Herbal Medicinal Product Committee of EMEA) http://www.emea.europa.eu/htms/human/hmpwp/working.htm.
Tacklind J, MacDonald R, Rutks I, Wilts TJ. Serenoa repens for benign prostatic hyperplasia (Review). The Cochrane Collaboration, The Cochrane Library 2009, Issue 2, pp.1‐56. Publishers John Wiley & Sons, Ltd.
Submitter agrees with default conflict of interest statement:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Thank you for your comments.
The reviewer remarks that we are acting non‐scientifically by lumping, for example, Permixon® and generic Serenoa repens, is mistaken. Others have made claims that Serenoa repens (whether generic or as Permixon®) alleviates symptoms associated with BPH. We have merely tested their hypothesis.
The reviewer makes two excellent points on bio equivalency and dosages, and in a forthcoming update we will address both. However, we disagree with the reviewers' suggestion that we should have utilized only the Permixon® trials. We conducted a systematic review of the evidence related to all of these products.
The Permixon® trials, which the reviewer urges us to use exclusively, are of almost uniformly poor quality. For example, the 7 RCTs that compared Permixon® to placebo had study populations of 22, 30, 60, 80, 110, 168, and 215. These trials were conspicuously underpowered, with the possible exception of the last two. Follow‐up for the 7 Permixon®‐versus‐placebo trials, measured in weeks, was 4, 4, 4, 4, 8.5, 10 and 12. Only one of the six Permixon® trials that were compared to an active control (or combination therapy with either Permixon® or the active control) utilized a placebo arm.
The reviewer states: "The reader of the review, even without being in the position to repeat the full statistical analysis, could conclude that efficacy of Serenoa should be similar or comparable to these BPH drugs. The final statement of the authors that 'Serenoa is not different from placebo,' is in clear contradiction to these reports." We are not contradictory, but the evidence is ambiguous, as we put it in the review. For example, Carraro [1] (Permixon® versus finasteride) reported a decrease in IPSS symptom scores for both arms (‐37% versus ‐39%, respectively); unfortunately, he did not include a placebo arm. Carraro's trial was certainly well powered (N = 1098), but follow‐up was only 26 weeks.
The consequence of the reviewers' recommendation to use only the Permixon® trials would eliminate the highest quality trial of the thirty, and Bent’s NEJM trial [2] (Serenoa repens versus placebo) is methodologically superior to all of the other twenty‐nine. Bent writes "these studies [previous RCTs] are limited by the small numbers of subjects enrolled, their short duration, their failure to use standard outcome measures, and the lack of information from participants concerning how effectively the placebo was blinded."
After 12‐month follow‐up Bent reported "[b]oth groups also had a small decrease in the AUASI score . . . : the score decreased by 0.68 in the saw palmetto group (95 percent confidence interval, ‐1.37 to 0.01) and by 0.72 in the placebo group (95 percent confidence interval, ‐1.40 to ‐0.04) ('Table 2'). There was, however, no significant difference between groups in the mean change in AUASI scores over time (difference in mean change, 0.04 point; 95 percent confidence interval, ‐0.93 to 1.01)."
Can these results be extrapolated to European populations using Permixon®? We think so. Nevertheless, we welcome an equivalent European trial utilizing Permixon® when it becomes available.
References
Carraro J‐C, Raynaud J‐P, Koch G, Chisholm GD, Di Silverio F, Teillac P, Da Silva FC, Cauquil J, Chopin DK, Hamdy FC, et al. Comparison of phytotherapy (Permixon®) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. The Prostate 1996;29:231‐40.
Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, Avins AL. Saw palmetto for benign prostatic hyperplasia. The New England Journal of Medicine 2006;354(6):557‐66.
Contributors
The consumers
Corresponding author: Prof. Dr. Anna Rita Bilia, Phytolab, University of Florence, Department of Pharmaceutical Sciences, via Ugo Schiff 6, I‐50019 Sesto Fiorentino (Florence), Italy. E‐mail: ar.bilia@unifi.it.
Co‐contributors names and addresses:
S. F.A.J. Horsten, PhD., Steenmarteakker 1, NL‐3994 GE Houten;
S. Sturm, PhD., University of Innsbruck, Inst. of Pharmacy/Pharmacognosy, A‐6020 Innsbruck;
M. Frater‐Schröder, PhD., Schwand, CH‐9642 Ebnat‐Kappel.
The authors
James Tacklind
Rod MacDonald
Indy Rutks
Tim Wilt
(2009)
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2012 | New citation required but conclusions have not changed | Search updated and byline changed; conclusions not changed |
| 31 October 2012 | New search has been performed | Search updated 27 January 2012; two new studies included |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 6 May 2011 | Amended | For this update (2012) we added adverse events (harms) to Secondary outcomes. |
| 4 March 2010 | Amended | Under 'Feedback/1 Anna Rita Bilia, et al, 31 August 2009/Summary/Reply', a clause read: "and by 0.72 in the placebo group (95 percent confidence interval, ‐1.40 to ‐0.04) ('Table 2')." It has been changed to "and by 0.72 in the placebo group (95 percent confidence interval, ‐1.40 to ‐0.04) ('Table 2')." |
| 29 September 2008 | New citation required and conclusions have changed | We have modified our findings of the efficacy of Serenoa repens. |
| 10 July 2008 | New search has been performed | This is a substantial update with 9 new trials. |
| 25 March 2008 | Amended | Converted to new review format. |
| 21 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
This work was partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant Number 1R01 DK063300‐01A2. Authors are employees of the US Department of Veterans Affairs. The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, the National Institutes of Health, or the Department of Veterans Affairs. We also wish to thank Maurizio Tiso, Margaret Haugh, Rich Crawford, Tatyana Shamilyan, Philipp Dahm, Yelena Slinin, and Joan Barnes for their work in translating and abstracting data from the non‐English language studies.
Appendices
Appendix 1. Search strategies
A. We searched Google Scholar using all combinations for the following categories:
prostatic hyperplasia OR bph OR benign prostatic hyperplasia;
serenoa repens OR s. repens OR sabal serrulata OR saw palmetto;
rct OR randomized controlled trial OR randomised controlled trial.
Restrictions were by years (2008 to 2011) and category (Medicine, Pharmacology, and Veterinary Science).
B. We searched Ovid MEDLINE® from 2008 to 2011 by crossing an optimally sensitive search strategy for trials from The Cochrane Collaboration with the following MeSH search terms.
prostatic hyperplasia.mp.
phytosterols.mp.
plant extracts.mp.
sitosterols.mp.
serenoa repens.mp.
sabal serrulata.mp.
saw palmetto.mp.
or/2‐7
1 and 8
limit 9 to randomized controlled trial
limit 11 to yr="2008 ‐ 2011"
We included all subheadings (Dickersin 1994).
C. We also searched the following using the same key terms we used for the Ovid MEDLINE® search and limited by the dates 2008 to 2011:
The Cochrane Library, including the database of the Cochrane Prostatic Diseases and Urologic Cancers Group, the Cochrane Field for Complementary Medicine, and the Cochrane Central Register of Controlled Trials (CENTRAL);
Web of Science®;
CINAHL®;
BIOSIS Previews®;
LILACS;
D. EMBASE was searched from 2001 to 1 January 2012 using the following strategy.
| Set | Items | Description |
| S1 | 8684 | PROSTATIC (W) HYPERPLASIA |
| S2 | 7071 | BPH/TI,AB |
| S3 | 240 | SAW (W) PALMETTO |
| S4 | 179 | SERENOA (W) REPENS |
| S5 | 150 | PERMIXON |
| S6 | 31 | SABAL (W) SERRULATA |
| S7 | 252 | (S1 OR S2) AND (S3 OR S4 OR S5 OR S6) |
| S8 | 276370 | RANDOMIZED (W) CONTROLLED (W) TRIAL? OR RANDIMISED (W)CONTROLLE‐D (W) TRIAL? |
| S9 | 11351 | RCT? |
| S10 | 51 | S7 AND (S8 OR S9) |
| S11 | 37 | S10/2001:2011 |
There were no language restrictions.
Data and analyses
Comparison 1. SR vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 AUA total score, mean change from baseline (0 to 35 (35 most severe)) | 2 | 582 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.58, 1.07] |
| 2 Nocturia (times/evening) at endpoint | 9 | 581 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.28, ‐0.29] |
| 3 Peak urine flow (mL/s), mean change from baseline | 3 | 667 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.30, 1.09] |
| 4 Peak urine flow (mL/s) at endpoint | 6 | 741 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐1.05, 1.76] |
| 5 Patient self‐rating for improved symptoms (# events "very good" or "good") | 4 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.09, 3.08] |
| 6 Physician‐assessed improvement of symptoms | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.78, 4.21] |
| 7 Prostate size (cc) at endpoint | 2 | 276 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐8.98, 4.58] |
| 8 Prostate size (cc) mean change from baseline | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐2.51, 1.95] |
| 9 Study withdrawals | 11 | 1453 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.69, 1.30] |
| 10 Any adverse events | 4 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.27, 3.19] |
1.1. Analysis.
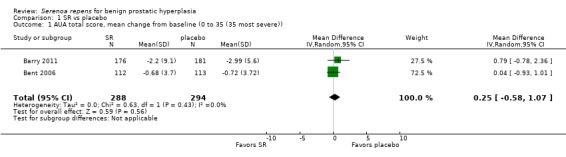
Comparison 1 SR vs placebo, Outcome 1 AUA total score, mean change from baseline (0 to 35 (35 most severe)).
Comparison 2. SR + Urtica dioica (PRO 160/120) vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peak urine flow (mL/s) at endpoint | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 2.48 [‐0.05, 5.02] |
Comparison 3. SR vs tamsulosin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IPSS total score mean change from baseline | 2 | 582 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.91, 0.88] |
| 2 Peak urine flow (mL/s) mean change from baseline | 2 | 645 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.54, 0.83] |
| 3 Prostate size (cc) mean change from baseline | 2 | 579 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐1.44, 1.13] |
| 4 Study withdrawals | 2 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.37] |
Comparison 4. SR (Permixon®) + tamsulosin vs placebo + tamsulosin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IPSS total score mean change from baseline | 2 | 356 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.69, 0.47] |
| 2 Peak urine flow (mL/s) mean change from baseline | 2 | 357 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.80, 0.98] |
| 3 Study withdrawals | 2 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.40] |
4.3. Analysis.
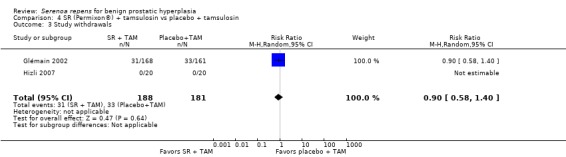
Comparison 4 SR (Permixon®) + tamsulosin vs placebo + tamsulosin, Outcome 3 Study withdrawals.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barry 2011.
| Methods | Multisite trial Randomization: 1:1 Participants, caregivers and investigators blinded |
|
| Participants | Geographic region: see 'Study setting' below Study setting: 11 N. American clinical sites N = 369 Baseline AUA: saw palmetto 14.4; placebo 14.7 Baseline prostate size: NR Mean age (range): 61.0 (NR) years Race: non‐Hispanic White 79.6%; Black 11.5%; Hispanic, Latino; other 9.0% Diagnostic criteria: peak urine flow of at least 4 mL/s; AUA 8 to 24 at 2 visits. | |
| Interventions | Control: matching placebo Treatment: SR 320 mg once daily for 24 weeks, followed by 640 mg once daily for 24 weeks, followed by 960 mg once daily for 24 weeks Follow‐up: 72 weeks Lost to follow‐up: n = 12 | |
| Outcomes | AUA NIH CPSI (National Institute of Health, Chronic Prostatitis Symptom Index) Urinary symptom scale BPH Inpact Index IPSS QoL NIH CPSI QoL scale AUA Nocturia Peak urine flow Erectile/ejaculatory function ICS male incontinence |
|
| Notes | Exclusions
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | attrition documented |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Bauer 1999.
| Methods | Number of sites unknown Randomization: unclear Blinding: patients, providers | |
| Participants | Geographic regions: Germany/Italy Study setting: community N = 101 Baseline IPSS: Sabal extract 9.6; placebo 8.9 Baseline prostate volume: Sabal extract 34.5 cc; placebo 31.7 cc Mean age (range): 66.1 (NR (no record)) years Race: White Diagnostic criteria: confirmed diagnosis of BPH with enlargement of the prostate, symptoms of obstruction and a maximum flow of < 15 mL/s | |
| Interventions | Control: matching placebo Treatment: Sabal extract (LG166/S) 160 mg twice daily Study duration: 6 months Lost to follow‐up: n = 3(?) | |
| Outcomes | IPSS symptom score Peak urine flow Prostate volume Sexual function Dropouts due to side effects: n = 0 | |
| Notes | Exclusions: patients treated for BPH within 1 month of the trial start; prostate cancer; acute urinary tract infection; chronic prostatitis; neurogenic bladder. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | per protocol outcomes |
| Selective reporting (reporting bias) | Low risk | outcomes not selectively reported |
| Other bias | Low risk | arms were assessed equally |
Bent 2006.
| Methods | Dual site and surrounding community Randomization: computer generated Blinding: patients, providers | |
| Participants | Geographic region: Northern California Study setting: VA Hospital/Kaiser Permanente and community N = 225 Baseline AUA: SR 15.7; placebo 15.0 Baseline prostate volume: SR 34.7 cc; placebo 33.9 cc Mean age (range): 63.0 (NR) years Race: White 82%; Black 5%; Asian/Pacific Islander 7%; Hispanic 5%; other 1% Diagnostic criteria: moderate‐to‐severe symptoms of BPH (AUA ≥ 8); Peak urine flow < 15 mL/s | |
| Interventions | Control: matching placebo Treatment: Sabal extract 160 mg twice daily Study duration: 12 months Lost to follow‐up: n = 9 | |
| Outcomes | AUA symptom score BPH Impact Index Peak urine flow | |
| Notes | Exclusions: < 49 years old; less than moderate symptoms of BPH (AUA < 8); peak urine flow < 4 mL/s or residual volume > 250 mL after voiding; history of prostate cancer; surgery for BPH; urethral stricture; neurogenic bladder; creatinine > 2 mg/dL; PSA > 4 ng/dL; medications known to affect urination; severe concomitant disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | stated |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | likely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Boccafoschi 1983.
| Methods | Single‐site study Randomization: sealed envelopes Blinding: patients, providers | |
| Participants | Geographic region: Italy Study setting: community N = 22 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): 68.0 (54 to 78) years Race: White Diagnostic criteria: Men with symptomatic BPH not in need of surgery | |
| Interventions | Control: matching placebo Treatment: Permixon® 160 mg twice daily Study duration: 8.5 weeks Lost to follow‐up: n = 0 | |
| Outcomes | Dysuria (4‐point scale) Peak urine flow Mean urine flow Voiding time Total voided volume Pollachiuria Dropouts due to side effects: not reported | |
| Notes | Exclusions: cancer; currently on other medication; urinary tract infection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Unclear risk | unclear |
| Other bias | Low risk | adequate |
Braeckman 1997.
| Methods | Number of sites unknown Randomization: sequentially numbered sealed opaque envelopes Blinding: patients, providers | |
| Participants | Geographic region: Belgium Study setting: community N = 238 Baseline symptom score: NR Baseline prostate volume: Prostaserene® 44 cc, placebo 45 cc Mean age (range): 65 (57 to 73) years Race: White Diagnostic criteria: peak urine flow 5 to 15 mL/s; residual urine volume ≤ 60 mL; personal score list 0 to 4; no global physician assessment. | |
| Interventions | Control: matching placebo Treatment: Prostaserene® 160 mg twice daily Study duration: 12 weeks Lost to follow‐up: 5% | |
| Outcomes | Symptom improvement Peak urine flow Mean urine flow Total voided volume Bladder residual volume Prostate size Dropouts due to side effects: < 1% | |
| Notes | Exclusions: Age > 80 years; prostate/other cancers ; urine flow < 5 mL/s or > 15 mL/s; residual volume > 60 mL; currently on medications; urinary tract infection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not mentioned |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Carbin 1990.
| Methods | Multisite study Randomization: random allocation according to a centrally controlled code list Blinding: patients, providers | |
| Participants | Geographic region: Sweden and Denmark Study setting: community N = 55 Baseline symptom score: NR Baseline prostate volume: NR Mean age (range): 61.6 (51.0 to 72.0) years Race: White Diagnostic criteria: The presence of BPH on the basis of history, clinical examination of the prostate and acid phosphatase determination | |
| Interventions | Control: matching placebo Treatment: Combination phytotherapy (Curbicin (Sabal serrulata 80 mg and Cucurbita pepo L. (pumpkin seeds) 80 mg) 2 tablets thrice daily) Study duration: 12 weeks Lost to follow‐up: 4% | |
| Outcomes | Dysuria Mean urine flow Voiding time Bladder residual volume Nocturia Patient self‐evaluation Dropouts due to side effects: n = 0 | |
| Notes | Exclusions: Need of imminent surgery due to symptom severity; bladder residual urine > 300 mL; previous treatment with Curbicin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Carraro 1996.
| Methods | Multisite study Randomization: computer‐generated randomization code Blinding: patients, providers | |
| Participants | Geographic region: Nine European countries Study setting: community N = 1098 Baseline IPSS: Permixon® 15.7; finasteride 15.7 Baseline prostate volume: Permixon® 43.0 cc; finasteride 44.0 cc Mean age (range): 64.5 (49 to 88) years Race: White Diagnostic criteria: BPH diagnosed by digital rectal exam (DRE); International Prostate Symptom Score (IPSS) > 6; maximum urinary flow between 4 to 15 mL/s (with a urine volume at least 150 mL, and a postvoid residue of < 200 mL); prostate size > 25 mL; serum prostate‐specific antigen (PSA) < 10 ng/mL (prostates less than or equal to 60 mL) or 15 ng/mL (prostates > 60 mL); good mental and physical condition. | |
| Interventions | Control: finasteride 5 mg (PROSCAR®) + placebo (morning) and two placebos (evening) Treatment: Permixon® 160 mg + placebo twice daily Study duration: 26 weeks Lost to follow‐up: 13.4% | |
| Outcomes | Symptom improvement ‐ IPSS symptom score (0 to 35 points) Quality of life score (0 to 6 points) Sexual function score (0 to 20 points) Peak urine flow Mean urine flow Total voided volume Bladder residual volume Prostate size (volume) Serum PSA Dropouts due to side effects: 4% (Permixon® n = 28, finasteride n = 14) | |
| Notes | Exclusions: Prostate cancer; bladder disease; abnormal liver function; diuretics or drugs with antiandrogenic or alpha‐receptor properties in the preceding 3 months; urogenital infections; disease potentially affecting micturition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | adequate |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Champault 1984.
| Methods | Number of sites unknown Randomization: unclear Blinding: patients, providers | |
| Participants | Geographic region: France Study setting: community N = 110 Baseline IPSS: NR Baseline prostate size: NR Mean age (range): NR (NR) years Race: White Diagnostic criteria: peak urine flow; mean urine flow; residual urine volume (no details given) | |
| Interventions | Control: matching placebo Treatment: Permixon® 80 mg twice daily Average follow‐up: 4 weeks Lost to follow‐up: 15% | |
| Outcomes | Dysuria Mean urine flow Bladder residual volume Nocturia Patient self‐rating Physician self‐rating Dropouts due to side effects: NR | |
| Notes | Exclusions: prostate cancer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not mentioned |
| Allocation concealment (selection bias) | Unclear risk | not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | per protocol analysis |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Cukier 1985.
| Methods | Multisite study Randomization: numbered or coded identical containers administered sequentially Blinding: patients, providers | |
| Participants | Geographic region: France Study setting: community N = 168 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): 69 (NR) years Race: White Diagnostic criteria: Patients with "prostatism" or for whom surgery was not indicated (no mechanical or infectious complications). | |
| Interventions | Control: matching placebo Treatment: Permixon® 160 mg twice daily Study duration: 10 weeks Lost to follow‐up: 13% | |
| Outcomes | Symptom score (# of daily mictions) Dysuria (4‐point scale) Bladder residual volume Nocturia Dropouts due to side effects: NR | |
| Notes | Exclusions: Symptoms for at least 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | per protocol analysis |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | High risk | number randomized to each arm was not described |
Debruyne 2002.
| Methods | Multisite study Randomization: not described Blinding: patients, providers | |
| Participants | Geographic region: 11 European countries Study setting: 98 community centers N = 704 Baseline IPSS: Permixon® 15.5; tamsulosin 15.2 Baseline prostate size: Permixon® 48.0 cc; tamsulosin 47.7 cc Mean age (range): 64.9 (50.0 to 85.0) years Race: NR Diagnostic criteria: IPSS ≥ 10; Peak urine flow 5 to 15 mL/s; voided volume at least 150 mL; post‐voiding volume < 150 mL; prostate volume ≥ 25 cc; serum PSA < 4 ng/mL (men with PSA 4 to 10 ng/mL required a free/total PSA ratio of at least 15%) | |
| Interventions | Control: tamsulosin 0.4 mg daily (capsules were matched in color, smell, size) Treatment: Permixon® 320 mg daily Follow‐up: 12 months Lost to follow‐up: n = 110 | |
| Outcomes | IPSS total score Nocturia Peak urine flow Dropouts due to side effects: tamsulosin n = 8; Permixon® n = 3 | |
| Notes | Exclusions: history of bladder disease likely to affect micturition; urethral stenosis; PC; pelvic radiotherapy; repeated infection of the urinary tract; chronic bacterial prostatitis; any disease likely to cause urinary problems; patients with clinically significant cardiovascular diagnosis; hematuria, insulin‐dependent diabetes mellitus; history severe hepatic failure; abnormal liver function tests; concomitant medication likely to interfere with study medication; known hypersensitivity to study drugs; participation in other clinical trial in previous 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | not adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Descotes 1995.
| Methods | Multisite study Randomization: noted but method not stated Blinding: patients, providers | |
| Participants | Geographic region: France Study setting: community N = 215 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): 66.3 (NR) years Race: White Diagnostic criteria: mild‐moderate (stages I or II) BPH; dysuria (daytime and nocturnal urinary frequency (> 2 nocturnal micturitions, excluding those at bedtime and on awakening) of at least 8 weeks); maximum urinary flow > or equal to 5 mL/s. | |
| Interventions | Control: matching placebo Treatment: Permixon® 160 mg twice daily Study duration: 4 weeks Lost to follow‐up: 18% | |
| Outcomes | Dysuria Peak urine flow Mean change in daytime urinary frequency Nocturia Patient‐based global efficacy Physician‐based global efficacy Dropouts due to side effects: 1 (complaints of fatigue, depression and stomach upset) | |
| Notes | Exclusions: Excessively mild or severe symptoms of BPH including incontinence, bladder distension, urine flow< 5 mL/s; cancer; prior treatment for BPH; urogenital infection; hematuria; diabetes; any prior surgery that could induce dysuria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | number randomized to arms not described nor losses to each |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | High risk | In first phase of trial placebo responders were eliminated. |
Emili 1983.
| Methods | Single‐site study Randomization: noted but method not stated Blinding: patients, providers | |
| Participants | Geographic region: Italy Study setting: community N = 30 Baseline symptom score: NR Baseline prostate volume: NR Mean age (range): NR (44 to 78) years Race: White Diagnostic criteria: Men with manageable BPH | |
| Interventions | Control: matching placebo Treatment: Permixon® 160 mg twice daily Study duration: 4 weeks Lost to follow‐up: n = 0 | |
| Outcomes | Peak urine flow Mean urine flow Bladder residual volume Prostate size (qualitative scale used) Nocturia Dropouts due to side effects: none | |
| Notes | Exclusions: Prior treatment for BPH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Engelmann 2006.
| Methods | Multisite study Randomization: noted but not described Blinding: patients, providers | |
| Participants | Geographic region: Germany Study setting: private out‐patient centers N = 140 Baseline IPSS: Prostagutt® forte 20.0; tamsulosin 21.0 Baseline prostate volume: NR Mean age (range): 65.0 (NR) years Race: NR Diagnostic criteria: maximum urinary flow rate ≤ 12 mL/s at a urinary volume ≥ 150 mL. | |
| Interventions | Control: tamsulosin 0.4 mg daily Treatment: Prostagutt® forte (sabal fruit extract+urtica root extract) twice daily Study duration: 60 weeks Lost to follow‐up: n = 3 (a total of 121 completed the trial at week 60) | |
| Outcomes | IPSS total score IPSS QoL CEDQ (Cologne Erectile Dysfunction Questionnaire) Peak urine flow Mean urine flow Mean urine volume Duration of flow increase Ultrasound residual volume | |
| Notes | Exclusions: Patients whose peak urinary volume changed by more than 3 mL/s during a 2‐week period; < 50 yrs old; IPSS < 13 and < 3 for the IPSS QoL; residual urinary volume < 150 mL; congested urinary tract passages; an indication of BPH surgery; urinary tract infection; prostate carcinoma; diabetes; neurogenic or bladder dysfunction; previous treatment with 5ARI; concomitant medication that could interfere with treatment efficacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Gabric 1987.
| Methods | Multisite study Randomization: unclear Blinding: patients, providers | |
| Participants | Geographic region: Croatia Study setting: community N = 30 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): 65 (40 to 82) years Race: White Diagnostic criteria: BPH, Stages I, II (Vahlensieck) | |
| Interventions | Control: placebo Treatment: Prostagutt® forte (SR + Urtica dioica) 20 drops thrice daily Study duration: 6 weeks Lost to follow‐up: none | |
| Outcomes | Physician rating of improvement Peak urine flow Bladder residual volume Dropouts due to side effects: none | |
| Notes | Exclusions: Stage IV prostate adenoma; bacterial prostatitis; cystitis; urethritis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unclear |
| Selective reporting (reporting bias) | Unclear risk | adequate |
| Other bias | Unclear risk | adequate |
Gerber 2001.
| Methods | Multisite or single‐site: NR Randomization: computer number table Blinding: patients, providers | |
| Participants | Geographic region: USA Study setting: community N = 85 Baseline IPSS: SR 16.7; placebo 15.8 Baseline prostate volume: NR Mean age (range): 65.0 (≥ 45) years Race: NR Diagnostic criteria: IPSS score ≥ 8 | |
| Interventions | Control: placebo Treatment: SR 160 mg twice daily Study duration: 6 months Lost to follow‐up: 7% (SR n = 2, placebo n = 4) | |
| Outcomes | Symptom improvement ‐ IPSS symptom score Quality of Life score Peak urine flow Dropouts due to side effects: 1% (SR n = 1) | |
| Notes | Exclusions: prostate surgery; history of prostate cancer or urethral stricture; treated with finasteride, saw palmetto or other alternative therapy (past 6 months); or treated with alpha‐blocker (within 1 month). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | adequate |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Glémain 2002.
| Methods | Multisite study Randomization noted but not described Blinding: patients, providers (unsure if assessors blinded) | |
| Participants | Geographic region: France Study setting: 47 regional settings N = 329 Baseline IPSS: tamsulosin + SR (Permixon®) 16.2; tamsulosin 16.3 Baseline prostate volume: NR Mean age (range): 65 (NR) years Race: NR Diagnostic criteria: IPSS ≥ 13, Peak urine flow 7 to 15 mL/s | |
| Interventions | Control: tamsulosin daily + placebo twice daily Treatment: tamsulosin daily + SR (Permixon®) twice daily Study duration: 52 weeks Lost to follow‐up: n = 64 | |
| Outcomes | Symptom improvement‐ IPSS total score IPSS QoL & UROLIFE© BPH QoL9 Peak urine flow Dropouts due to side effects: none | |
| Notes | Exclusions: previous surgery on the prostate, vesicle collar or pelvic area; residual post‐urine volume of >300 mL; prostate cancer; urine infection; α/ß‐blockers, α‐agonists, cholinergics or anticholinergics were prohibited; hepatic insufficiency; cardiovascular event or cerebrovascular event; allergy to intervention drugs Treatments for BPH (such as α‐blockers) stopped at least 15 days before randomization; other treatments, such as plant extracts and finasteride, were stopped 1 month before randomization. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not clear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Hizli 2007.
| Methods | Single or multisite: NR Randomization: NR Blinding: not described | |
| Participants | Geographic region: Turkey Study setting: unknown N = 60 Baseline IPSS: SR (Permixon®) 16.2; tamsulosin 18.0; SR (Permixon®) + tamsulosin 15.6 Baseline prostate volume: SR (Permixon®) 35.2 cc; tamsulosin 38.6 cc; SR (Permixon®) + tamsulosin 31.2 cc Mean age (range): 58.6 (43 to 73) years Race: NR Diagnostic criteria: IPSS ≥ 10; Peak urine flow 5 to 15 mL; prostate volume ≥ 25 cc; PSA ≤ 4 ng/mL | |
| Interventions | Control 1: tamsulosin 0.4 mg daily Control 2: SR (Permixon®) 320 mg daily + tamsulosin 0.4 mg daily Treatment: SR (Permixon®) 320 mg daily Study duration: 24 weeks Lost to follow‐up: n = 0 | |
| Outcomes | IPSS total score IPSS QoL Prostate volume PSA Post‐void residual volume | |
| Notes | Exclusions: cardiovascular disease; hematuria; insulin dependent diabetes; prostate cancer; concomitant medications likely to interfere with study medications; hypersensitivity to study drugs; concomitant medications likely to interfere with study medications; hypersensitivity to study drugs; pelvic radiotherapy; UT repeated infection; chronic bacterial prostatitis; any other disease that causes urinary problems; history of severe hepatic failure; abnormal liver function; history of bladder disease likely to affect micturition; urethral stenosis; and participating in clinical trial in last 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | high |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Unclear risk | unclear |
Lopatkin 2005.
| Methods | Multisite study Randomization: random number generator program Blinding: patients, providers | |
| Participants | Geographic region: Europe Study setting: NR N = 257 Baseline IPSS: PRO 160/120 (Prostagutt® forte) 18.0; placebo 18.0 Baseline prostate volume: PRO 160/120 44.9 cc; placebo 46.4 cc Mean age (range): PRO 160/120 68 (NR) (n = 127); placebo 67 (NR) (n = 126) Diagnostic criteria: Peak urine flow (voiding volume): <15 mL/s; change in max urinary flow between screening and of run‐in period ? 3 mL/s; urinary output at baseline: > 100 mL; IPSS total score ≥ 14; IPSS QoL ≥ 4. | |
| Interventions | Control: matching placebo Treatment: PRO 160/120 160 mg SR + 120 mg Urtica dioica twice daily Study duration: 24 weeks Lost to follow‐up: n = 7 | |
| Outcomes | IPSS total score IPSS QoL Peak urine flow | |
| Notes | Exclusions: age < 50; PSA >10 ng/mL; PC; large residual urine > 350 mL; concomitant medications affecting micturition (α‐blockers); previous surgery on pelvis, urinary tract, urethral stricture or pelvic radiation; symptomatic urinary tract infection; chronic bacterial prostatitis; serious health risks; diabetes; diabetic neuropathy; mental condition to restrict informed consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | adequate |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Löbelenz 1992.
| Methods | Multisite study Randomization: computer‐generated randomization code Blinding: patients, providers | |
| Participants | Geographic region: Germany Study setting: community N = 60 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): NR (48 to 82) years Race: White Diagnostic criteria: BPH, Stages I, II; peak urine flow < 20 mL/s. | |
| Interventions | Control: matching placebo Treatment: Sabal extract 100 mg daily Study duration: 6 weeks Lost to follow‐up: n = 0 | |
| Outcomes | Peak urine flow Mean urine flow Dropouts due to side effects: n = 0 | |
| Notes | Exclusions: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | adequate |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Mandressi 1983.
| Methods | Number of sites unknown Randomization: Identical packaging Blinding: patients, providers | |
| Participants | Geographic region: Italy Study setting: community N = 60 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): NR (50 to 80) years Race: White Diagnostic criteria: men with symptomatic BPH confirmed on rectal examination | |
| Interventions | Control 1: matching placebo Control 2: Pygeum africanum extract (dose not given) Treatment: Permixon® 320 mg daily Study duration: 4 weeks Lost to follow‐up: unclear | |
| Outcomes | Patient self‐rating Dysuria (pain on voiding) Urgency Tenesmus (straining) Difficult urination Post‐voiding residual Pollachiuria Nocturia Dropouts due to side effects: none |
|
| Notes | Exclusions: details not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not described |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Marks 2000.
| Methods | Single‐site study Randomization: table of random numbers Blinding: patients | |
| Participants | Geographic region: USA Study setting: community N = 44 Baseline IPSS: Saw palmetto herbal blend 18.4; placebo 16.4 Baseline prostate volume: Saw palmetto herbal blend 58.5 cc; placebo 55.6 cc Mean age (range): 64 (45 to 80) years Race: White 73%, Black 7%, Asian 11% Diagnostic criteria: moderate to severe BPH with enlarged prostate (DRE), IPSS score of 9 or greater, PSA < 15 ng/mL, prostate volume 30 cc or greater. | |
| Interventions | Control: placebo Treatment: Saw palmetto herbal blend (saw palmetto 106 mg, nettle root extract 80 mg, pumpkin seed oil extract 160 mg, vitamin A 190 mg) thrice daily Study duration: 6 months Lost to follow‐up: 7% | |
| Outcomes | Symptom improvement ‐ IPSS symptom score Peak urine flow Post‐void residual volume PSA Prostate volume Dropouts due to side effects: None | |
| Notes | Exclusions: concurrent use of α‐blockers; use of finasteride, phytotherapy within last 18 months or α‐blockers within last month; chronic prostatitis; previous bladder or prostate surgery; neurogenic bladder | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | adequate |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | per protocol analysis |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Mattei 1990.
| Methods | Single‐site study Randomization: noted but method not stated Blinding: patients, providers | |
| Participants | Geographic region: Italy Study setting: community N = 40 Baseline symptom score: NR Baseline prostate volume: Talso (SR extract) 36 mm (diameter); placebo 37 mm Mean age (range): NR (45 to 72) years Race: White Diagnostic criteria: Men with manageable BPH | |
| Interventions | Control: matching placebo Treatment: Talso (SR extract) 160 mg twice daily Study duration: 13 weeks Lost to follow‐up: 5% | |
| Outcomes | Dysuria (symptom score 0 to 4) Bladder residual volume (incomplete emptying ‐ symptom score 0 to 4) Discomfort (Pollachiuria ‐ symptom score 0 to 4) Daytime frequency Nocturia Prostate size Dropouts due to side effects: 1 patient from each group due to "stomach pains." Unclear relation to therapy. | |
| Notes | Exclusions: Urogenital disease, prostate cancer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Metzker 1996.
| Methods | Single‐site study Randomization: computer‐generated randomization code Blinding: patients, providers | |
| Participants | Geographic region: Germany Study setting: community N = 40 Baseline IPSS: Prostagutt® forte 18.6; placebo 19.0 Baseline prostate volume: NR Mean age (range): 65.5 (52 to 84) years Race: White Diagnostic criteria: BPH, Alken stages I, II | |
| Interventions | Control: matching placebo Treatment: Combination phytotherapy: Prostagutt® forte (SR 160 mg and Urtica dioica 120 mg) 1 capsule twice daily Study duration: 48 weeks Lost to follow‐up: 7.5% | |
| Outcomes | Symptom improvement ‐ IPSS symptom score Peak urine flow Bladder residual volume Patient self‐evaluation Dropouts due to side effects: none | |
| Notes | Exclusions: Age < 50 years; cancer; taking other prostate medications/contraindicated medications; infections; recent or current urinary tract operations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | adequate |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Mohanty 1999.
| Methods | Site not described Randomization not described Blinding: double blinded |
|
| Participants | Geographic region: NR Study setting: clinic N = 75 Baseline modified Boyarsky: NR Baseline prostate volume: SR 28.78 mL; placebo 29.89 mL Mean age (range): NR (40 to 90) years Race: NR Diagnostic criteria: BPH grades I or II; symptomatic; without surgical indication; took no BPH drug treatment for last 30 days. | |
| Interventions | Control: matching placebo, 1 capsule twice daily Treatment: SR, 1 capsule twice daily Study duration: 2 months Lost to follow‐up: SR n = 2; placebo n = 0 | |
| Outcomes | Modified Boyarsky (range 0 to 27, with a higher score indicating worse symptoms) Frequency Nocturia Peak urine flow Residual volume Prostate size (ultrasound) Adverse events |
|
| Notes | Exclusions: men with prostate conditions other than BPH (carcinoma of the prostate, infective prostatitis); serious renal, hepatic and cardiac conditions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | none |
| Other bias | Unclear risk | no description of baseline comparability of the two arms other than for age |
Pannunzio 1986.
| Methods | Single‐site study Randomization: noted but method not stated Blinding: none | |
| Participants | Geographic region: Italy Study setting: community N = 60 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): NR (44 to 78) years Race: White Diagnostic criteria: Men with BPH without prior treatment; bladder residual volume of < 150 mL | |
| Interventions | Control: Depostat (gestonorone caproato 200 mg) intramuscularly every week for 8 weeks Treatment: Permixon® 160 mg twice daily Study duration: 8 weeks Lost to follow‐up: none | |
| Outcomes | Dysuria (% of men with symptoms) Pollachiuria Nocturia Peak urine flow Voiding time Prostate size Dropouts due to side effects: none | |
| Notes | Exclusions: cancer; urogenital infections | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Preuss 2001.
| Methods | Multisite study Randomization: by cluster method Blinding: patients, providers | |
| Participants | Geographic region: Washington, DC, Florida, Idaho N = 144 Baseline AUA: Cerniton AF™ 18.9; placebo 17.7 Baseline prostate volume: NR Mean age: NR (NR) years Race: NR Diagnostic criteria: dx of BPH; maximal urinary flow rate of 5 to 15 mL/s for a voided volume in excess of 100 mL | |
| Interventions | Control: Placebo Treatment: Cerniton AF™ (378 mg), saw palmetto complex, phytosterol, ß‐sitosterol (286 mg), and vitamin E (100 IU (international units)) twice daily Study duration: 3 months Lost to follow‐up: 17 | |
| Outcomes | AUA Emptying Frequency Hesitancy Urgency Weak stream Straining Nocturia Adverse events Bladder volume Mean flow rate Maximal flow rate | |
| Notes | Exclusions: > 80 yrs old; presence of any tumor, malformation, or infection of the genitourinary tract; any severe or concomitant medical condition that would make it difficult to participate; severe lab abnormalities (WHO grades 2 to 4); medical treatment for BPH with finasteride within last 4 weeks; men treated with antibiotics for genitourinary tract infections. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | cluster randomization |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Unclear risk | adequate |
| Other bias | Low risk | adequate |
Reece Smith 1986.
| Methods | Single‐site trial Randomization: random allocation with numbered folders Blinding: patients, providers | |
| Participants | Geographic region: United Kingdom Study setting: community N = 80 Baseline symptom score: NR Baseline prostate volume: NR Mean age (range): 66.6 (55.0 to 80.0) years Race: White Diagnostic criteria: Men with symptomatic BPH with symptoms scored by an investigator and symptoms scored with a self‐assessment questionnaire | |
| Interventions | Control: matching placebo Treatment: Permixon® 160 mg twice daily Study duration: 12 weeks Lost to follow‐up: 12.5% | |
| Outcomes | Mean urine flow Bladder residual volume Investigator assessment (symptom score 0 to 2) Patient self‐assessment data Libido Dropouts due to side effects: 2 patients from the treatment group (nausea and vomiting) | |
| Notes | Exclusions: malignant disease or "whose symptoms not fulfilling entry criteria" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | adequate |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Roveda 1994.
| Methods | Single‐site study Randomization: random allocation using tables of random numbers Blinding: none | |
| Participants | Geographic region: Italy Study setting: community N = 30 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): 62.9 (55 to 76) years Race: White | |
| Interventions | Control: SR 640 mg rectal capsule once daily Treatment: SR 160 mg oral capsules 4 times daily Study duration: 4 weeks Lost to follow‐up: n = 0 | |
| Outcomes | Dysuria Bladder residual volume Prostate size Pollachiuria Overall effect of treatment summary Dropouts due to side effects: none | |
| Notes | Exclusions: age < 50 and > 80; on current medication; prior treatment for BPH. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | adequate |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Shi 2008.
| Methods | Multisite study Randomization: envelope selection Blinding: patients, providers | |
| Participants | Geographic region: Shangai, China Study setting: urology clinic and community hospital N = 94 Baseline IPSS: NR Baseline prostate volume: Prostataplex™ 47.7 cc; placebo 48.4 cc Mean age (range): 65 (62 to 68) years Race: Chinese Diagnostic criteria: newly diagnosed LUTS associated with BPH based on urological symptoms, including nocturia, incomplete emptying, urinary frequency, intermittence, weak stream, straining and urgency. | |
| Interventions | Control: matching placebo Treatment: Prostataplex™ 2 pills/daily Study duration: 3 months Lost to follow‐up: n = 2 | |
| Outcomes | Maximum urinary flow rate | |
| Notes | Exclusion: history of prostate cancer and the use of any drugs, herbs or other nonprescription preparations for LUTS associated with BPH within 4 weeks of screening, including finasteride, α or ß‐blockers, diuretics, calcium channel blockers and anticholinergic drugs. Abnormal laboratory parameters, including PSA > 4 ng/mL, serum creatinine more than 160 µmol/la urine bacterial count greater than 100,000/ml, BUN > 8 mg/dL, MFR > 15 mL/s and voiding volume < 150 mL, were also grounds for exclusion. Additional exclusion criteria were patient inability to understand or follow the study protocol, current or previous participation in another clinical trial, BPH judged by a urologist to require surgical treatment, previous bladder or prostate surgery, micturition problems associated with an identified bladder pathology (neurogenic bladder, bladder neck stenosis, lithiasis or bladder cancer), urethral stricture, recurrent urinary tract infections, known renal, hepatic or cardiac insufficiency, diabetes mellitus, recent MI, known alcohol abuse, known sensitivity to the ingredients in the product, significant depression or other psychiatric disease noted during the initial screening, any other cancer in the last 5 years except skin cancer and being on anticoagulation therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "blindly randomized" |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | per protocol analysis |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Sökeland 1997.
| Methods | Multisite study Randomization: computer‐generated randomization code Blinding: patients, providers | |
| Participants | Geographic region: Germany Study setting: community N = 543 (516 therapy trial) Baseline IPSS: PRO 160/120 11.3; finasteride 11.8 Baseline prostate volume: PRO 160 / 120 42.7 cc; finasteride 44.0 cc Mean age (range): NR (50 to 88) years Race: White Diagnostic criteria: BPH, Stages I, II (Alken) | |
| Interventions | Control: finasteride 5 mg plus placebo (2 capsules per day in a double dummy design) Treatment: Combination phytotherapy: PRO 160 / 120 (Sabal extract 160 mg and Urtica extract 120 mg) 2 capsules daily Study duration: 12 weeks Lost to follow‐up: 5% (Data from 489 participants were used in therapy effect analysis and data from 516 participants used for side effects analysis) | |
| Outcomes | Symptom improvement‐IPSS symptom score Quality of life ‐ American Urological Association Score Peak urine flow Bladder residual volume Prostate size (volume) Dropouts due to side effects: (no details given) | |
| Notes | Exclusions: < 50 years of age; BPH III or > (Alken); PSA > 10 ng/mL; cancer; taking other prostate medications; infections; severe concomitant disease that warrants therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | adequate |
| Allocation concealment (selection bias) | Low risk | adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not clear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Tasca 1985.
| Methods | Single‐site trial Randomization: noted but method not stated Blinding: patients, providers | |
| Participants | Geographic region: Italy Study setting: community N = 30 Baseline symptom score: NR Baseline prostate volume: NR Mean age (range): 61.5 (49 to 81) years Race: White Diagnostic criteria: Stage I and Stage II prostatic adenomas | |
| Interventions | Control: matching placebo Treatment: PA109 (Permixon®) 160 mg twice daily Study duration: 8 weeks Lost to follow‐up: 10% | |
| Outcomes | Dysuria (% reporting) Peak urine flow Mean urine flow Total voided volume Pollachiuria‐daytime (% reporting) Pollachiuria‐nocturnal (% reporting) Urgency (% reporting) Dropouts due to side effects: 1 patient from the treatment group | |
| Notes | Exclusions: Details not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | adequate |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Unclear risk | adequate |
Willetts 2003.
| Methods | Single‐site study Randomization: randomized using balanced‐blocks, where each block was for 6 men. Randomization codes were concealed in sealed envelopes and opened only after last man had completed treatment Blinding: patients, providers | |
| Participants | Geographic region: Sydney, Australia Study setting: community N = 100 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): 63.9 (NR) years Race: NR Diagnostic criteria: at least 3 symptoms of prostatism: 1) increased frequency; 2) hesitancy; 3) nocturia; 3) hesitancy; 4) dribbling and poor stream. | |
| Interventions | Control: matching placebo (paraffin oil in identical capsules, twice daily) Treatment: SR 160 mg of CO₂ extract, twice daily Study duration: 12 weeks Lost to follow‐up: n = 7 | |
| Outcomes | IPSS Peak urine flow IIEF Questionnaire | |
| Notes | Exclusions: ≥ 80; no significant medical condition: insulin‐dependent diabetes; severe cardiopulmonary disease; significant CNS disease; androgens in previous 4 weeks; 5ARI; α‐blockers; herbals for urinary problems; history of PC or adenomas; urethral, bladder, renal abnormalities; urogenital surgery; renal stones; strictures or scarring; acute urinary retention or allergy to study treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | yes but significant difference between arms in IPSS at baseline |
| Allocation concealment (selection bias) | Unclear risk | not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | per protocol analysis |
| Selective reporting (reporting bias) | Low risk | adequate |
| Other bias | Low risk | adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adriazola Semino 1992 | SR versus active control (Prazosin). No indication of randomization. |
| Al‐Shukri 2000 | Not randomized. |
| Aliaev 2009 | Men had chronic abacterial prostatitis. |
| Authié 1987 | Not an RCT. |
| Comar 1986 | Study duration unknown. |
| Di Silverio 1992 | Tissue study investigating the antiestrogenic effect of SR versus placebo. |
| Gerber 1998 | Open‐label study, no control group. |
| Giannakopoulos 2002 | No control. |
| Grasso 1995 | Average treatment duration < than 1 month. |
| Pavone 2010 | Not an RCT. |
| Pecoraro 2004 | No relevant outcomes. |
| Popa 2005 | Re‐analysis of the included study Metzker 1996. Wirksamkeit eines sabal‐urtica‐kombinationspraparates bei der behandlung der benignen prostatahyperplasie (BPH). Der Urologe B 1996;36(4):292‐300. |
| Sinescu 2011 | Not an RCT. |
| Sivkov 2001 | Same as Lopatkin 2005. |
| Stepanov 1999 | No control. |
| Strauch 1994 | Enzyme study (inhibition of 5 alpha‐reductase) comparing SR versus finasteride in a 1 week, open, randomized, active‐controlled study. |
| Vela‐Navarrete 2003 | Dual publication. |
| Vela‐Navarrete 2005 | No relevant outcomes comparing Permixon® to control. |
| Veltri 2002 | No clinical outcomes. |
| Vinarov 2010 | No active or placebo control. |
| Weisser 1997 | Enzyme study investigating the influence of Sabal serrulata (versus placebo) on epithelial and stromal enzyme activities of BPH tissue. |
Differences between protocol and review
For this update (2012) we added adverse events (harms) to Secondary outcomes.
Contributions of authors
JT searched for trials and wrote the analysis. RM and TW edited the manuscript. IR and JUS wrote the search strategy.
Sources of support
Internal sources
Management Decision and Research Center‐Department of Veterans Affairs HSRD, USA.
Minneapolis/VISN‐13 Center for Chronic Diseases Outcomes Research (CCDOR), USA.
External sources
-
The Cochrane Complementary Medicine Field bursary award, USA.
The Cochrane review group Prostatic Diseases and Urologic Cancers received a one‐time payment from the Cochrane Complementary Medicine Field to update this review.
-
Grant no. 5R01DK63300‐4, USA.
Editing support was in part provided by the National Institutes of Health (NIH), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Declarations of interest
None declared.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barry 2011 {published data only}
- Barry MJ, Meleth S, Lee JY, Kreder KJ, Avins AL, Nickel JC, et al. Effect of increasing doses of Saw palmetto extract on lower urinary tract symptoms. JAMA 2011;306(12):1344‐51. [clinicaltrials.gov ID: NCT00603304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauer 1999 {published data only}
- Bauer HW. Casarosa C. Cosci M. Fratta M. Bleβmann G. Saw palmetto fruit extract for treatment of benign prostatic hyperplasia. Results of a placebo‐controlled double‐blind study [Behandlung der benignen Prostatahyperplasie mit Sabalfrucht‐Extrakt: Wirksamkeit und Verträglichkeit ‐ Ergebnisse einer plazebokontrollierten Doppelblindstudie]. MMW Fortschritte der Medizin 1999;141(25):127‐32. [PubMed] [Google Scholar]
Bent 2006 {published data only}
- Bent S, Kane C, Shinohara K, Neuhaus J, Hudes E, Goldberg H, et al. Saw palmetto for benign prostatic hyperplasia. The New England Journal of Medicine 2006;354(6):557‐66. [DOI] [PubMed] [Google Scholar]
Boccafoschi 1983 {published data only}
- Boccafoschi C, Annoscia S. Serenoa repens extract and placebo in prostatic benign hyperplasia: clinical results [Confronto fra estratto di serenoa repens e placebo mediate prova clinica controllata in pazienti con adenomatosi prostatica]. Urologia 1983;50:1257‐68. [Google Scholar]
Braeckman 1997 {published data only}
- Braeckman J. The extract of Serenoa repens in the treatment of benign prostatic hyperplasia: a multicenter open study. Current Therapeutic Research 1994;55(7):776‐85. [Google Scholar]
- Braeckman J, Bruhwyler J, Vanderkerckhove K, Géczy J. Efficacy and safety of the extract of Serenoa repens in the treatment of benign prostatic hyperplasia: therapeutic equivalence between twice and once daily dosage forms. Phytotherapy Research 1997;1:558‐63. [Google Scholar]
- Braeckman J, Denis L, Leval J, Keuppens F, Cornet A, De Bruyne, et al. A double‐blind, placebo‐controlled study of the plant extract Serenoa repens in the treatment of benign hyperplasia of the prostate. European Journal of Clinical Research 1997;9:247‐59. [Google Scholar]
Carbin 1990 {published data only}
- Carbin BE, Larsson B, Lindahl O. Treatment of benign prostatic hyperplasia with phytosterols. British Journal of Urology 1990;66(6):639‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carraro 1996 {published data only}
- Carraro JC, Raynaud JP, Koch G, Chisholm GD, Silverio F, Teillac P, et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. The Prostate 1996;29(4):231‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Champault 1984 {published data only}
- Champault G, Patel JC, Bonnard AM. A double‐blind trial of an extract of the plant Serenoa repens in benign prostatic hyperplasia. British Journal of Clinical Pharmacology 1984;18:461‐2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cukier 1985 {published data only}
- Cukier J, Ducassou J, Guillou M, Leriche A, Lobel B, Toubol J. [Permixon versus placebo; resultats d'une étude multicentrique]. Comptes Rendus de Therapeutique et de Pharmacologie Clinique 1985;4:15‐21. [Google Scholar]
Debruyne 2002 {published data only}
- Debruyne F, Boyle P, Calais Da Silva F, Gillenwater JG, Hamdy FC, Perrin P, et al. Evaluation of the clinical benefit of Permixon and tamsulosin in severe BPH patients‐‐PERMAL study subset analysis [Evaluation du benefice clinique de Permixon® et de la tamsulosine dans le traitement de l'hypertrophie bénigne de la prostate (HBP) sévére: Analyse d'une sous‐groupe de l'étude Permal]. European Urology 2004;45:773‐80. [DOI] [PubMed] [Google Scholar]
- Debruyne F, Koch G, Silva FC, Gillenwater JG, Hamdy FC, Perrin P, et al. Comparison of a phytotherapeutic agent (Permixon®) with an a‐blocker (tamsulosin) in the treatment of benign prostatic hyperplasia: a 1‐year randomized international study [Comparaison d'un produit de phytothérapie (Permixon®) et d'un alpha‐bloquant (tamsulosine) dans le traitement de l'hypertrophie bénigne de la prostate: étude internationale randomisée d'une durée de 12 mois]. Progrès en Urologie 2002;41:497‐507. [PubMed] [Google Scholar]
Descotes 1995 {published data only}
- Descotes JL, Rambeaud JJ, Deschaseaux P, Faure G. Placebo‐controlled evaluation of the efficacy and tolerability of Permixon® in benign prostatic hyperplasia after the exclusion of placebo responders. Clinical Drug Investigation 1995;5:291‐7. [Google Scholar]
Emili 1983 {published data only}
- Emili E, Lo Cigno M, Petrone U. [Risultati clinici su un nuovo farmaco nella terapia dell'ipertofia della prostata (Permixon)]. Urologia 1983;50:1042‐8. [Google Scholar]
Engelmann 2006 {published data only}
- Engelmann U, Walther C, Bondarenko B, Funk P, Schläfke S. Efficacy and safety of a combination of sabal and urtica extract in lower urinary tract symptoms. A randomized, double‐blind study versus tamsulosin. Arzneimittel‐Forschung 2006;56(3):222‐9. [DOI] [PubMed] [Google Scholar]
Gabric 1987 {published data only}
- Gabric V, Miskic H. [Behandlung des benignen prostata‐adenoms und der chronischen prostatatitis]. Therapiewoche 1987;37:1775‐88. [Google Scholar]
Gerber 2001 {published data only}
- Gerber GS, Kuznetsov D, Johnson BC, Burstein JD. Randomized, double‐blind, placebo‐controlled trial of saw palmetto in men with lower urinary tract symptoms. Urology 2001;58(6):960‐4. [DOI] [PubMed] [Google Scholar]
Glémain 2002 {published data only}
- Glémain P, Coulange C, Billebaud T, Gattegno B, Muszynski R, Loeb G, et al. Tamsulosin with or without Serenoa repens in benign prostatic hyperplasia: the OCOS trial [Tamsulosine avec ou sans Serenoa repens dans l'hypertrophie bénigne de la prostate: l'essai OCOS]. Progrès en Urologie 2002;12:395‐403. [PubMed] [Google Scholar]
Hizli 2007 {published data only}
- Hizli F, Uygur MC. A prospective study of the efficacy of Serenoa repens, tamsulosin, and Serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. International Urology and Nephrology 2007;39:879‐86. [DOI] [PubMed] [Google Scholar]
Löbelenz 1992 {published data only}
- Löbelenz J. [Extractum Sabal fructus bei benigner prostatahyperplasie (BPH). klinische prufung im stadium I und II]. Therapeutikon 1992;6(1‐2):34‐7. [Google Scholar]
Lopatkin 2005 {published data only}
- Lopatkin N, Sivkov A, Walther C, Schläfke S, Medvedev A, Avdeichuk J, et al. Combined extract of sabal palm and nettle in the treatment of patients with lower urinary tract symptoms in the double blind, placebo‐controlled trial. World Journal of Urology 2005;23(2):139‐46. [DOI] [PubMed] [Google Scholar]
Mandressi 1983 {published data only}
- Mandressi S, Tarallo U, Maggioni A, Tombolini P, Rocco F, Quadraccia. Medical treatment of benign prostatic hyperplasia: efficacy of the extract of Serenoa repens (Permixon®) compared to that of the extract of Pygeum africanum and a placebo [Terapia medica dell'adenoma prostatico: confronto della efficacia dell'estratto di serenoa repens (Permixon®) versus l'estratto di pigeum africanum e placebo]. Urologia 1983;50(4):752‐8. [Google Scholar]
Marks 2000 {published data only}
- Marks LS, Partin AW, Epstein JI, Tyler VE, Simon I, Macairan ML, et al. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. The Journal of Urology 2000;163(5):1451‐6. [PubMed] [Google Scholar]
Mattei 1990 {published data only}
- Mattei FM, Capone M, Acconcia A. [Medikamentose therapie der benignen prostatahyperplasie mit einem extrakt der sagepalme]. TW Urologie Nephrologie 1990;2:346‐50. [Google Scholar]
Metzker 1996 {published data only}
- Metzker H, Kieser M, Holscher U. [Wirksamkeit eines Sabal‐Urtica‐kombinationspraparates bei der behandlung der benignen prostatahyperplasie (BPH)]. Der Urologe B 1996;36(4):292‐300. [Google Scholar]
Mohanty 1999 {published data only}
- Mohanty NK, Jha RJ, Dutt C. Randomized double‐blind controlled clinical trial of Serenoa repens versus placebo in the management of patients with symptomatic Grade I to Grade II benign prostatic hyperplasia (PBH). Indian Journal of Urology 1999;16(1):26‐31. [Google Scholar]
Pannunzio 1986 {published data only}
- Pannunzio E, D’Ascenzo R, Giardinetti F, Civili P, Persichelli E. [Serenoa repens vs. gestonorone caproato nel trattamento dell’ipertofia prostatica benigna: Studio randomizzato]. Urologia 1986;53(5):696‐705. [Google Scholar]
Preuss 2001 {published data only}
- Preuss HG, Marcusen C, Regan J, Klimberg IW, Welebir TA, Jones WA. Randomized trial of a combination of natural products (Cernitin, saw palmetto, ß‐sitosterol, vitamin E) on symptoms of benign prostatic hyperplasia (BPH). International Urology and Nephrology 2001;33:217‐25. [DOI] [PubMed] [Google Scholar]
Reece Smith 1986 {published data only}
- Reece Smith H, Memon A, Smart CJ, Dewbury K. The value of Permixon in benign prostatic hypertrophy. British Journal of Urology 1986;58:36‐40. [DOI] [PubMed] [Google Scholar]
Roveda 1994 {published data only}
- Roveda S, Colombo P. Clinical controlled trial on therapeutical bioequivalence and tolerability of Serenoa repens oral capsules 160 mg or rectal capsules 640 mg [Sperimentazione clinica controllata sulla bioequivalenza terapeutica e sulla tollerabilita dei prodotti a base di Serenoa repens in capsule da 160 mg o capsule rettali da 640 mg]. Archivio di Medicina Interna 1994;46(2):61‐75. [Google Scholar]
Shi 2008 {published data only}
- Shi R, Xie Q, Gang X, Lun J, Life C, Pantuck A, et al. Effect of saw palmetto soft gel capsule on lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized trial in Shanghai, China. The Journal of Urology 2008;179:610‐5. [DOI] [PubMed] [Google Scholar]
Sökeland 1997 {published data only}
- Sökeland J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome. British Journal of Urology International 2000;86:439‐42. [DOI] [PubMed] [Google Scholar]
- Sökeland J, Albrecht J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome [Kombination aus Sabal‐ und Urticaestrakt vs. finasterid bei BPH (Stad. I bis II nach Alken); Vergleich der therapeutischen wirksamkeit in einer einjahrigen doppelblindstudie]. Urologe A 1997;36(4):327‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tasca 1985 {published data only}
- Tasca A, Barulli M, Cavazzana A, Zattoni F, Artibani W, Pagano F. Treatment of obstructive symptomatology caused by prostatic adenoma with an extract of Serenoa repens. Double‐blind clinical study vs placebo [Trattamento della sintomatologia ostruttiva da adenoma prostatico con estratto di Serenoa repens. Studio clinico in doppio cieco vs. placebo]. Minerva Urologica e Nefrologica 1985;37:87‐91. [PUBMED: 2413558] [PubMed] [Google Scholar]
Willetts 2003 {published data only}
- Willetts KE, Clements MS, Champion S, Ehsman S, Eden JA. Serenoa repens extract for benign prostate hyperplasia: a randomized controlled trial. British Journal of Urology International 2003;92:267‐70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adriazola Semino 1992 {published data only}
- Adriazola Semino M, Lozano Ortega JL, Garcia Cobo E, Tejeda Banez E, Romero Rodriguez F. Symptomatic treatment of benign hypertrophy of the prostate. Comparative study of prazosin and Serenoa repens [Tratamiento sintomatico de la hipertrofia benigna de prostata. Estudio comparativo entre prazosin y Serenoa repens]. Archivos Espanoles de Urologia 1992;45(3):211‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Aliaev 2009 {published data only}
- Aliaev IG, Apolikhin OI, Mazo EB, Vinarov AZ, Lokshin KL, Medvedev AA, et al. Efficacy and safety of prostamol‐Uno in patients with chronic abacterial prostatitis [article in Russian] [ЭФФЕКТИВНОСТЬ И БЕЗОПАСНОСТЬ ПРОСТАМОЛА‐УНО У БОЛЬНЫХ ХРОНИЧЕСКИМ АБАКТЕРИАЛЬНЫМ ПРОСТАТИТОМ]. Urologiia 2006;1:47‐50. [PubMed] [Google Scholar]
Al‐Shukri 2000 {published data only}
- Al‐Shukri SH, Deschaseaux P, Kuzmin IV. Early urodynamic effects of the lipido‐sterolic extract of Serenoa repens (Permixon®) in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Prostate Cancer and Prostatic Diseases 2000;3:195‐9. [DOI] [PubMed] [Google Scholar]
Authié 1987 {published data only}
- Authié D, Cauquil J. [Appréciation de l'efficacité de Permixon en pratique quotidienne: étude multicentrique]. Comptes Rendus de Thérapeutique et de Pharmacologie Clinique 1987;5(56):4‐13. [Google Scholar]
Comar 1986 {published data only}
- Comar OB, Rienzo A. Mepartricin versus Serenoa repens: double‐blind study in 20 cases of benign prostate hypertrophy. Rivista Italiana di Biologia e Medicina 1986;6(2):122‐5. [Google Scholar]
Di Silverio 1992 {published data only}
- Siverio F, D'Eramo G, Lubrano C, Flammia GP, Sciarra A, Palma E, et al. Evidence that Serenoa repens extract displays an antiestrogenic activity in prostatic tissue of benign prostatic hypertrophy patients. European Urology 1992;21(4):309‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gerber 1998 {published data only}
- Gerber GS, Zagaja GP, Bales GT, Chodak GW, Contreras BA. Saw palmetto (Serenoa repens) in men with lower urinary tract symptoms: effects on urodynamic parameters and voiding symptoms. Urology 1998;51(6):1003‐7. [DOI] [PubMed] [Google Scholar]
Giannakopoulos 2002 {published data only}
- Giannakopoulos X, Baltogiannis D, Giannakis D, Tasos A, Sofikitis N, Charalabopoulos K, et al. The lipidosterolic extract of Serenoa repens in the treatment of benign prostatic hyperplasia: a comparison of two dosage regimens. Advances in Natural Therapy 2002;19(2):285‐96. [DOI] [PubMed] [Google Scholar]
Grasso 1995 {published data only}
- Grasso M, Montesano A, Buonaguidi A, Castelli M, Lania C, Rigatti P, et al. Comparative effects of alfuzosin versus Serenoa repens in the treatment of symptomatic benign prostatic hyperplasia. Archivos Españoles de Urología 1995;48(1):97‐103. [MEDLINE: ] [PubMed] [Google Scholar]
Pavone 2010 {published data only}
- Pavone C, Abbadessa D, Taraintino ML, Oxenius I, Laganà A, Lupo A, et al. [Associazione di Serenoa repens, Urtica dioica e Pinus pinaster. Sicurezza ed efficacia nel trattamento dei sintomi del basso tratto ruinario. Studio prospettico su 320 pazienti]. Urologia 2010;77(1):43‐51. [PubMed] [Google Scholar]
Pecoraro 2004 {published data only}
- Pecoraro S, Annecchiarico A, Gambardella M‐C, Sepe G. Efficacy of pretreatment with Serenoa repens on bleeding associated with transurethral resection of prostate [Effetto del pretrattamento con serenoa repens sul sanguinamento associato a resezione transuretrale della prostata]. Minerva Urologica e Nefrologica 2004;56:73‐8. [PubMed] [Google Scholar]
Popa 2005 {published data only}
- Popa G, Hägele‐Kaddour H, Walther C. [Symptomatische wirksamkeit eines sabal‐urtica‐kombinationspräparats in der therapie des benignen prostatasyndroms: ergebnisse einer plazebokontrollierten doppelblindstudie]. MMW Fortschritte der Medizin 2005;147(Supp 3):103‐8. [PubMed] [Google Scholar]
Sinescu 2011 {published data only}
- Sinescu I, Geavlete P, Multescu R, Gangu C, Miclea F, Coman I, et al. Long‐term efficacy of Serenoa repens treatment in patients with mild and moderate symptomatic benign prostatic hyperplasia. Urologia Internationalis 2011;86:284‐9. [DOI: 10.1159/000322645] [DOI] [PubMed] [Google Scholar]
Sivkov 2001 {published data only}
- Sivkov A, Köhler S, Engelmann U, Medvedev A, Lopatkin N. Long‐term efficacy and safety of a combination of sabal and urtica extracts in LUTS ‐ a placebo‐controlled, double‐blind, multicenter trial. Urologe A 2001;40(Suppl 1):S19. [Google Scholar]
Stepanov 1999 {published data only}
- Stepanov VN, Siniakova LA, Sarrazin B, Raynaud JP. Efficacy and tolerability of the lipidosterolic extract of Serenoa repens (Permixon®) in benign prostatic hyperplasia: a double‐blind comparison of two dosage regimens. Advances in Therapy 1999;16(5):231‐41. [PubMed] [Google Scholar]
Strauch 1994 {published data only}
- Strauch G, Perles P, Vergult G, Gabriel M, Gibelin B, Cummings S, et al. Comparison of finasteride (Proscar®) and Serenoa repens (Permixon®) in the inhibition of 5‐alpha reductase in healthy male volunteers. European Urology 1994;26(3):247‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vela‐Navarrete 2003 {published data only}
- Vela‐Navarrete R, Garcia Cardoso JV, Barat A, Manzarbeitia F, Lopez Farre A. BPH and inflammation: pharmacological effects of Permixon on histological and molecular inflammatory markers. Results of a double blind pilot clinical assay. European Urology 2003;44:549‐55. [DOI] [PubMed] [Google Scholar]
Vela‐Navarrete 2005 {published data only}
- Vela‐Navarrete R, Escribano‐Burgos M, Lopez Farre A, Garcia‐Cardoso J, Manzarbeitia F, Carrasco C. Serenoa repens treatment modifies BAX/BCL‐2 index expression and CASPASE‐3 activity in prostatic tissue from patients with benign prostatic hyperplasia. The Journal of Urology 2005;173:507‐10. [DOI] [PubMed] [Google Scholar]
Veltri 2002 {published data only}
- Veltri RW, Marks LS, Miller CM, Bales WD, Fan J, MacAiran ML, et al. Saw palmetto alters nuclear measurements reflecting DNA content in men with symptomatic BPH: evidence for a possible molecular mechanism. Urology 2002;60:617‐22. [PUBMED: 12385921] [DOI] [PubMed] [Google Scholar]
Vinarov 2010 {published data only}
- Vinarov AZ, Aliaev IuG, Apolikhin OI, Mazo EB, Darenkov SP, Demidko IuL, et al. Results of three‐year clinical study of prostamol Uno efficacy and safety in patients with initial symptoms of prostatic adenoma and risk of its progression [Russian]. Urologiia 2010;6:3‐10. [21433319] [PubMed] [Google Scholar]
Weisser 1997 {published data only}
- Weissser H, Behnke, Helpap B, Bach D, Krieg M. Enzyme activities in tissue of human benign prostatic hyperplasia after three months' treatment with the Sabal serrulata extract IDS 89 (Strogen) or placebo. European Urology 1997;31(1):97‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Abrams 1979
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. British Journal of Urology 1979;51(2):129‐34. [DOI] [PubMed] [Google Scholar]
Bales 1999
- Bales G, Christiano AP, Kirsh E, Gerber GS. Phytotherapeutic agents in the treatment of lower urinary tract symptoms: a demographic analysis of awareness and use at the University of Chicago. Urology 1999;54:86‐9. [DOI] [PubMed] [Google Scholar]
Barnes 2002
- Barnes PM, Powell‐Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Advance data from vital and health statistics of the National Center for Health Statistics 2004;27(343):1‐19. [PubMed] [Google Scholar]
Berry 1984
- Berry SL, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. The Journal of Urology 1984;132:474‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boyle 2004
- Boyle P, Robertson C, Lowe F, Roehrborn C. Updated meta‐analysis of clinical trials of Serenoa repens extract in the treatment of symptomatic benign prostatic hyperplasia. British Journal of Urology International 2004;93(6):751‐6. [DOI] [PubMed] [Google Scholar]
Buck 1996
- Buck AC. Phytotherapy for the prostate. British Journal of Urology 1996;78(3):325‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buck 2004
- Buck AC. Is there a scientific basis for the therapeutic effects of Serenoa repens in benign prostatic hyperplasia? Mechanisms of action. The Journal of Urology 2004;172:1792. [DOI] [PubMed] [Google Scholar]
Campbell‐Walsh Urology 2010
- Roehrborn CG. Chapter 92 ‐ Benign prostatic hyperplasia: etiology, pathophysiology, epidemiology, and natural history. In: Kavoussi LR, Novick AC, Partin AW, Peters CA editor(s). Campbell‐Walsh Urology. 10th Edition. Vol. 3, Philadelphia: Elsevier Saunders, 2010. [Google Scholar]
Christensen 1990
- Christensen MM, Bruskewitz RC. Clinical manifestations of benign prostatic hyperplasia and the indications for therapeutic intervention. Urology Clinics of North America 1990;17:509‐16. [MEDLINE: ] [PubMed] [Google Scholar]
Cochrane Handbook 2011
- Higgins JPT, Altman DG, Stern JAC. Chapter 8: Assessing risk of bias in included studies. In:Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration (Available from www.cochrane‐handbook.org (accessed 4 March 2011) 2011.
Dedhia 2008
- Dedhia RC, McVary KT. Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. The Journal of Urology 2008;179:2119‐25. [DOI] [PubMed] [Google Scholar]
Di Silverio 1993
- Silverio F, Flammia GP, Sciarra A, Caponera M, Mauro M, Buscarini M, et al. Plant extracts in benign prostatic hyperplasia. Minerva Urologica e Nefrologica 1993;45:143‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Indentifying relevant studies for systematic reviews. BMJ 1994;312:944‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dreikorn 1990
- Dreikorn K, Richter R, Schönhöfer PS. Conservative nonhormonal treatment of patients with benign prostatic hyperplasia. Urologe A 1990;29(1):8‐16. [PUBMED: 1690486] [PubMed] [Google Scholar]
GRADEPro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows., 2008.
Habib 2004
- Habib FK, Wyllie MG. Not all brands are created equal: a comparison of selected components of different brands of Serenoa repens extract. Prostate Cancer and Prostatic Diseases 2004;7:195‐200. [DOI] [PubMed] [Google Scholar]
Lavori 1992
- Lavori PW. Clinical trials in psychiatry: should protocol deviation censor patient data?. Neuropsychopharmacology 1992;6:39‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Lepor 1996
- Lepor H, Williford WO, Barry MJ, Brawer MK, Dixon CM, Gormley G, et al. The efficacy of terazosin and finasteride, or both in benign prostatic hyperplasia. New England Journal of Medicine 1996;335:533‐9. [DOI] [PubMed] [Google Scholar]
Lowe 1996
- Lowe FC Ku JC. Phytotherapy in treatment of benign prostatic hyperplasia: a critical review. Urology 1996;48:12‐20. [DOI] [PubMed] [Google Scholar]
Maccagnano 2006
- Maccagnano C, Salonia A, Briganti A, Teillac P, Schulman C, Montorsi F, et al. A critical analysis of Permixon™ in the treatment of lower urinary tract symptoms due to benign prostatic enlargement. European Urology 2006;5 suppl:430‐40. [Google Scholar]
Marwick 1995
- Marwick C. Growing use of medicinal botanicals forces assessment by drug regulators. JAMA 1995;273:607‐9. [MEDLINE: ] [PubMed] [Google Scholar]
McConnell 1994
- McConnell JD, Barry MJ, Bruskewitz RC. Benign prostatic hyperplasia: diagnosis and treatment. Agency for Health Care Policy and Research. Clinical practice guideline. Quick reference guide for clinicians 1994;8:1‐17. [PUBMED: 7507389] [PubMed] [Google Scholar]
McConnell 2003
- McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The long‐term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. New England Journal of Medicine 2003;349:2387‐98. [DOI] [PubMed] [Google Scholar]
McGuire 1987
- McGuire E. Detrusor response to obstruction. NIH Publication No. 87‐2881 1987; Vol. 221.
Meigs 2001
- Meigs JB, Mohr B, Barry MJ, Collins MM, McKinlay JB. Risk factors for clinical benign prostatic hyperplasia in a community‐based population of healthy aging men. Journal of Clinical Epidemiology 2001;54:935‐44. [DOI] [PubMed] [Google Scholar]
Platz 2002
- Platz EA, Smit E, Curhan GC, Nyberg Jr LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002;59:877‐83. [DOI] [PubMed] [Google Scholar]
Roehrborn 2001
- Roehrborn CG, Malice M‐P, Cook TJ, Girman CJ. Clinical predictors of spontaneous acute urinary retention in men with LUTS and clinical BPHL a comprehensive analysis of the pooled placebo groups of several large studies. Urology 2001;58:210‐6. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Urologic Diseases in American 2007
- Wei JT, Calhoun EA, Jacobsen SJ. Chapter 2, Benign Prostatic Hyperplasia. In: Litwin MS, Saigal CS editor(s). Urologic Diseases in America. Washington, DC: US Government Publishing Office, 2007:45‐69. [NIH Publication No. 07‐5512] [Google Scholar]
Vacherot 2000
- Vacherot F, Azzouz M, Gil‐Diez‐de‐Medina S, Colombel M, Taille A, Lefrere Belda MA, et al. Induction of apoptosis and inhibition of cell proliferation by the lipido‐sterolic extract of Serenoa repens (LSESr, Permixon®) in benign prostatic hyperplasia. The Prostate 2000;45:507. [DOI] [PubMed] [Google Scholar]
Weisser 1996
- Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha‐reductase activity in human benign prostatic hyperplasia. The Prostate 1996;28:300. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Tacklind 2009
- Tacklind J, MacDonald R, Rutks I, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No.: CD001423. [DOI: 10.1002/14651858.CD001423.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilt 2000
- Wilt TJ, Ishani A, Stark G, MacDonald R, Lau J, Mulrow C, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001423] [DOI] [PubMed] [Google Scholar]
Wilt 2002
- Wilt T, Ishani A, Mac Donald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001423] [DOI] [PubMed] [Google Scholar]


