Significance
The expression of E-cadherin has been implicated in tumor metastasis, often as a tumor suppressor, but also as a promoter of growth and metastasis. We have shown that the functional activity of E-cadherin at the cell surface is often modified in response to environmental factors, and have developed monoclonal antibodies (mAbs) that activate E-cadherin adhesive function. These activating mAbs inhibit the metastasis of endogenous genetically driven mammary tumors in mice, and we dissect the process to show that these mAbs work at multiple steps of the metastatic cascade, including local invasion, intravasation, cell survival, and extravasation into the target organ. Thus, the functional state of E-cadherin is an important determinant of metastatic potential beyond whether the gene is expressed.
Keywords: E-cadherin, E-cadherin-positive tumors, MMTV-PyMT breast cancer model, circulating tumor cells, breast cancer metastasis
Abstract
E-cadherin is a tumor suppressor protein, and the loss of its expression in association with the epithelial mesenchymal transition (EMT) occurs frequently during tumor metastasis. However, many metastases continue to express E-cadherin, and a full EMT is not always necessary for metastasis; also, positive roles for E-cadherin expression in metastasis have been reported. We hypothesize instead that changes in the functional activity of E-cadherin expressed on tumor cells in response to environmental factors is an important determinant of the ability of the tumor cells to metastasize. We find that E-cadherin expression persists in metastatic lung nodules and circulating tumor cells (CTCs) in two mouse models of mammary cancer: genetically modified MMTV-PyMT mice and orthotopically grafted 4T1 tumor cells. Importantly, monoclonal antibodies that bind to and activate E-cadherin at the cell surface reduce lung metastasis from endogenous genetically driven tumors and from tumor cell grafts. E-cadherin activation inhibits metastasis at multiple stages, including the accumulation of CTCs from the primary tumor and the extravasation of tumor cells from the vasculature. These activating mAbs increase cell adhesion and reduce cell invasion and migration in both cell culture and three-dimensional spheroids grown from primary tumors. Moreover, activating mAbs increased the frequency of apoptotic cells without affecting proliferation. Although the growth of the primary tumors was unaffected by activating mAbs, CTCs and tumor cells in metastatic nodules exhibited increased apoptosis. Thus, the functional state of E-cadherin is an important determinant of metastatic potential beyond whether the gene is expressed.
The E-cadherin epithelial cell adhesion protein is a tumor suppressor that has an important role in tumor metastasis (1), but targeting it for therapeutic intervention is difficult because of its complicated role. The loss of expression of E-cadherin during the epithelial mesenchymal transition (EMT) is often thought to promote metastasis by allowing the dissociation and invasion of cancer cells (2–4). However, loss of E-cadherin expression is an oversimplification because many metastases still contain high levels of E-cadherin, and epithelial cells expressing E-cadherin can become invasive and metastasize without undergoing a full EMT in cancers (5–8). Moreover, adherent clusters of circulating mammary tumor cells are more metastatic than individual tumor cells, and E-cadherin is involved in collective cell migration behaviors that facilitate invasion and metastasis (9, 10). A positive role for E-cadherin in metastasis resulting from its enhancement of cell survival has also been reported recently (11).
How E-cadherin controls invasion and metastasis when its expression is maintained on tumor cells in not well understood. An important possibility is that functional regulation of its adhesive state at the cell surface in response to the microenvironment controls epithelial tumor cell behavior. Indeed, previous work on both Xenopus C-cadherin and human E-cadherin provided evidence for the regulation of cadherin adhesion activity independent of cell surface expression levels (12, 13). E-cadherin adhesive activity is regulated at the cell surface by an inside-out mechanism probably involving allosteric regulation of the homophilic adhesive bond, analogous to integrin regulation (14–16). These findings relied on the discovery of activating monoclonal antibodies (mAbs) for cadherins that rapidly increase cell adhesion and reduce cell movement. Therefore, activation of E-cadherin function at the cell surface with mAbs could be a potential approach to controlling tumor metastasis.
Activating mAbs for human E-cadherin were used to examine the role of E-cadherin regulation of metastasis, using a model mammary cell line, 4T1, expressing human E-cadherin (17). Nonetheless, to ensure that this finding was not unique to the 4T1 cell grafting model of metastasis, in the present study, we endeavored to examine the role of E-cadherin activation on the development and metastasis of endogenous genetically driven mammary tumors, the MMTV-PyMT model, which entails natural tumor progression as well as potential tumor cell heterogeneity (18).
We also wished to explore the stages in metastasis controlled by E-cadherin regulation, as well as the detailed mechanisms that contribute at various stages. E-cadherin is thought to prevent the initial dissociation of epithelial cells from the original tumor mass, and loss of cell-cell adhesion and cell junctions allows cells to invade surrounding tissues and migrate to distant sites. However, it has been found that dynamic regulation of E-cadherin is also required for collective cell migration in normal development and tumor growth (19, 20). Furthermore, E-cadherin has been found to reduce cell proliferation through contact inhibition and signaling, and may even stimulate proliferation in some contexts (21–24). Therefore, given the multiple functions of E-cadherin, it is important to determine the stage of metastatic progression affected by E-cadherin activation. In this study, we tested whether E-cadherin activation by mAbs affects tumor cell dissemination and entry into the circulation, the stability of tumor cells in the circulation, or the exit of tumor cells from the circulation and colonization at the distal sites. We also investigate the contributions of cell adhesion, migration, and invasion, as well as regulation of cell proliferation and apoptosis.
Results
Effects of E-Cadherin Activating mAbs on the Growth and Metastasis of Genetically Driven Spontaneous Mouse Mammary Cancer.
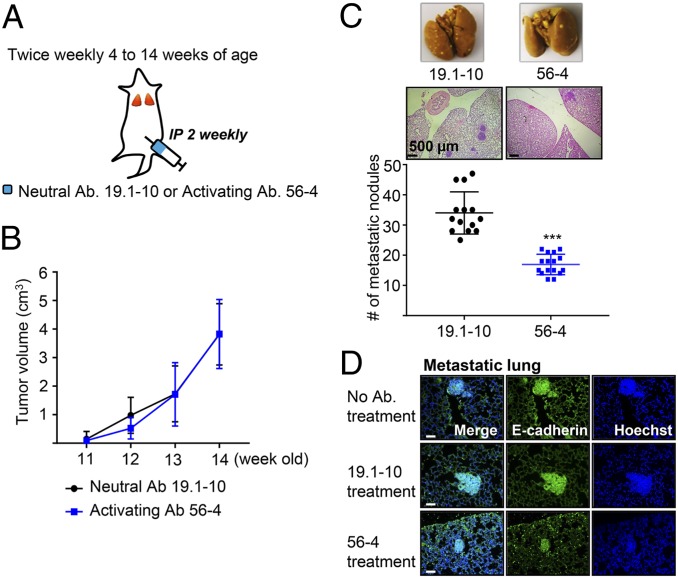
MMTV-PyMT mice develop highly invasive mammary tumors that metastasize spontaneously to the lung (18, 25, 26). We began our analysis in the luminal MMTV-PyMT invasive ductal carcinoma model, as it retains E-cadherin expression during growth, invasion, dissemination, and metastatic colonization. To study effects on endogenous mouse tumors, we first had to generate rabbit mAbs that recognize and activate mouse E-cadherin similar to our previous activating mAbs to human E-cadherin (SI Appendix, Fig. S1) (14, 17). Hybridomas positive for mouse E-cadherin binding in ELISA were screened using a functional assay (14); in this case, colo205 cells expressing mouse E-cadherin instead of human E-cadherin. Two hybridomas producing activating mAbs, 18-5 and 56-4, were obtained, as well as a control neutral mAb 19.1-10 that binds E-cadherin but does not activate adhesion. Female MMTV-PyMT or control mice were treated twice weekly with 56-4 activating mAb (5 mg/kg) or 19.1-10 neutral mAb from 4 to 14 wk of age (Fig. 1A). At 14 wk of age, all mAb-treated mice developed tumors in all 10 mammary glands (SI Appendix, Fig. S2A), and there was no difference in the growth in size of the primary tumor in the mammary gland for activating versus neutral mAb (Fig. 1B and SI Appendix, Fig. S2B). In contrast, the number of metastatic lung nodules was significantly reduced with treatment with 56-4 activating mAb compared with 19.1-10 neutral mAb (Fig. 1C). Expression of E-cadherin was observed in both metastatic lungs (Fig. 1D) and primary tumors (SI Appendix, Fig. S2C) treated with activating or neutral mAb. Immunofluorescence staining secondary Ab alone showed that the injected Abs were present in primary tumors and metastatic lungs (SI Appendix, Fig. S2D). Although the total amount of E-cadherin–positive cells in metastases in mice treated with E-cadherin activating mAb was reduced because of the decreased size of the metastases (Fig. 1C), most of the tumor cells in the lung still expressed high levels of E-cadherin. This indicates that metastatic cells had not escaped the action of the mAb simply because they lost expression. Together, these findings show that progression and metastasis of E-cadherin-positive tumors is controlled by the activity state of E-cadherin at the cell surface.
Fig. 1.
E-cadherin activation inhibits tumor metastasis in the MMTV-PyMT mouse model of breast cancer. (A) Schema of MMTV-PyMT breast cancer mouse model study. The MMTV-PyMT or FVB control female mice (Fig. 2) received i.p. injections of either 19.1-10 neutral E-cadherin-specific mAb or 56-4 E-cadherin activating mAb twice weekly. (B) All palpable masses were measured weekly, using external calipers. (C) Metastatic nodules counted in Bouin’s fluid fixation (Top) and H&E staining (Middle) of the lung from 14‐wk‐old MMTV-PyMT mice. (B and C, n = 14–16) ***P < 0.001 compared with neutral Ab-treated control. (D) Representative microscopic images of immunofluorescence staining for E-cadherin in metastases of MMTV-PyMT mice. (Scale bars, 50 μm.)
E-Cadherin Activation Reduces the Number of Circulating Tumor Cells Arising from Mammary Tumors.
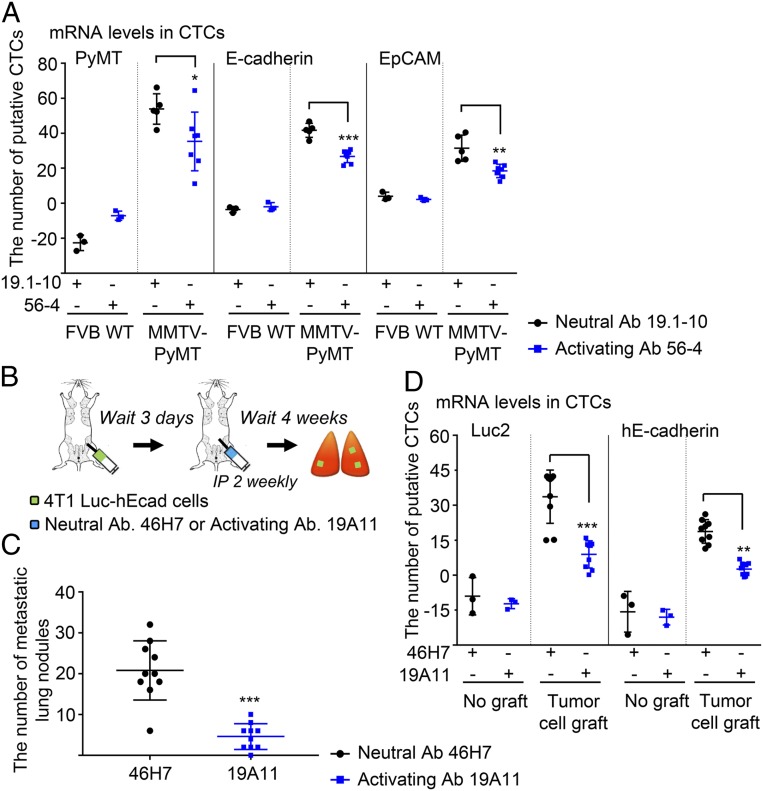
We next wished to determine what stage in the metastatic cascade is affected by E-cadherin activation. Metastasis of cells is thought to involve many steps/events (2–4), but for experimental purposes, it can be divided into two major stages: dissemination from the primary tumor and entry of tumor cells into the circulation, and movement of cells from the circulation into the distal site and seeding of the target organ. Intravasation is a critical step in the development of distant metastases, and detection of circulating tumor cells (CTCs) in blood can be a predictor of response to metastatic spread of carcinoma (27). To test whether the number of disseminated tumor cells is decreased by activating mAb, CTCs were detected by amplified specific DNA fragments of the PyMT gene, as well as epithelial cell markers using quantitative real-time PCR. The numbers of CTCs were calculated from the expression of mRNA levels compared with cultured Py2T cells, a tumor cell line derived from PyMT tumors. Treatment with activating mAb significantly decreased total mRNA levels of PyMT, E-cadherin, and EpCAM expression compared with neutral mAb treatment (Fig. 2A). Therefore, E-cadherin activation seems to reduce the number of CTCs generated from endogenous PyMT mammary tumors.
Fig. 2.
Circulating tumor cells are reduced by E-cadherin activation in the breast cancer models. mRNA levels for several tumor markers were analyzed by qRT-PCR, and the estimated number of CTCs based on levels of mRNA expression in cultured Py2T or 4T1 cells. (A) MMTV-PyMT model; CTCs in the peripheral blood were detected by mRNA levels (FVB wild-type, n = 3; MMTV-PyMT, n = 5 to 7) *P < 0.05; **P < 0.01; ***P < 0.001 compared with neutral Ab-treated control. (B–D) 4T1 tumor cell grafted metastatic mouse model study. Mouse epithelial 4T1 Luc2 cells expressing human E-cadherin (4T1 Luc-hE) were injected into mammary fat pads of BALB/c mice. After 3 d, the mice were given i.p. injections of either 46H7 neutral or 19A11 activating mAb twice weekly until the end of the experiments. (B) Schema. (C) Quantification of metastatic tumor nodules (n = 10) ***P < 0.001 compared with neutral Ab-treated control. (D) CTCs were detected by mRNA levels of Luc2 and hE-cadherin expression in 4T1 Luc-hE orthotopic grafted mouse model based on levels of expression in cultured cells (no graft group, n = 3; tumor cell graft group, n = 8 to 9). **P < 0.01; ***P < 0.001 compared with neutral Ab-treated control in tumor cell grafted group.
To be able to experimentally manipulate stages of metastasis, we needed to use a cell grafting model of tumorigenesis and metastasis. Therefore, we used the hE-cadherin- and luciferase-expressing 4T1 mammary cancer cell model similar to our previous study. Briefly, 4T1-Luc2_Puro cells were transfected with pcDNA3 plasmid containing WT human E-cadherin by electroporation. The cells were selected with neomycin, and the expression of hE-cadherin levels was confirmed at a level very similar to endogenous mouse E-cadherin in the previous study (17). We first tested whether activating mAb affected the number of CTCs associated with formation of metastasis in 4T1 Luc2-hE cells grafted into the mammary fat pad (Fig. 2B). The number of metastatic lung nodules was significantly reduced by activating mAb to human E-cadherin (Fig. 2C and SI Appendix, Fig. S3A), even though there was no detectable difference in growth of primary tumor (SI Appendix, Fig. S3B), consistent with our previous findings of a reduction in the numbers of metastatic cells in the lung (17). Treatment with 19A11 activating mAb significantly decreased the mRNA levels of luc2 and hE-cadherin in CTC fraction obtained from blood compared with 46H7 neutral mAb (Fig. 2D). Since activating mAbs did not affect the mRNA levels of E-cadherin in cell lines 4T1 and Py2T in vitro, changes in the E-cadherin mRNA levels were not due to activating mAbs (SI Appendix, Fig. S4). The numbers of CTCs were calculated from the mRNA levels compared with cultured 4T1 Luc2-hE cells. Along with the MMTV-PyMT model findings, these data show that the inhibition of distant metastasis in the presence of E-cadherin activating mAb is associated with a decrease in the number of CTCs.
Activation of E-Cadherin Suppresses Extravasation and Metastatic Colonization into the Lung Parenchyma.
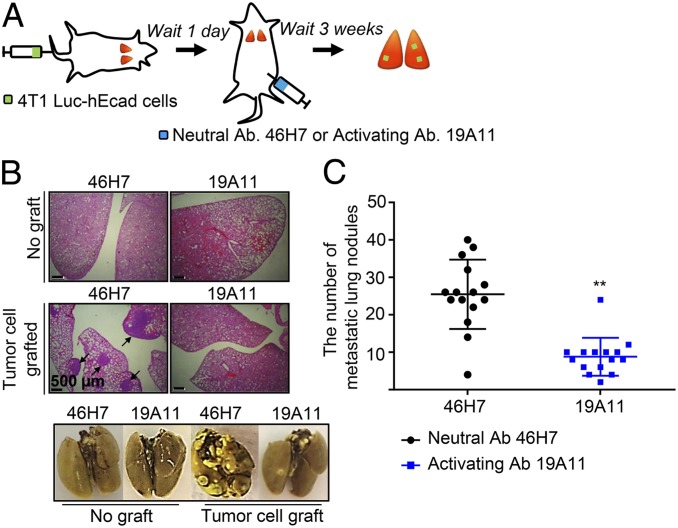
CTCs in the blood or lymphatic circulation reach a capillary bed of a distal organ and invade through the endothelial cells of the blood vessel (extravasation) (2–4, 27). To investigate whether E-cadherin activation affects the extravasation from the vasculature, metastasis was induced by injecting tumor cells directly into the tail vein. 4T1 Luc-hE cells were injected i.v., and the mice were treated with either activating or neutral mAb (Fig. 3A). The activating mAb significantly inhibited the large number of metastasic lung nodules resulting from i.v. injection (Fig. 3 B and C). These results suggest that the activity state of E-cadherin on the cell surface also controls the later stage process of CTC extravasation from the circulation and target organ seeding.
Fig. 3.
E-cadherin activation reduces the metastatic colonization from circulation. (A) Schema. Mouse epithelial 4T1 Luc-hE cells were injected into the tail-vein of BALB/c mice. One day after inoculation, the mice were treated with i.p. injections of either 46H7 neutral or 19A11 activating mAb twice weekly until the end of the experiments. (B) Representative microscopic images of H&E stained sections (Top) or lungs fixed with Bouin’s (Bottom). (C) Quantification of metastatic tumor nodules (n = 15). (Scale bar, 500 μm.) **P < 0.01 compared with neutral Ab-treated control group.
Cellular Mechanisms Underlying Inhibition of Tumor Cell Invasion by E-Cadherin Activation.
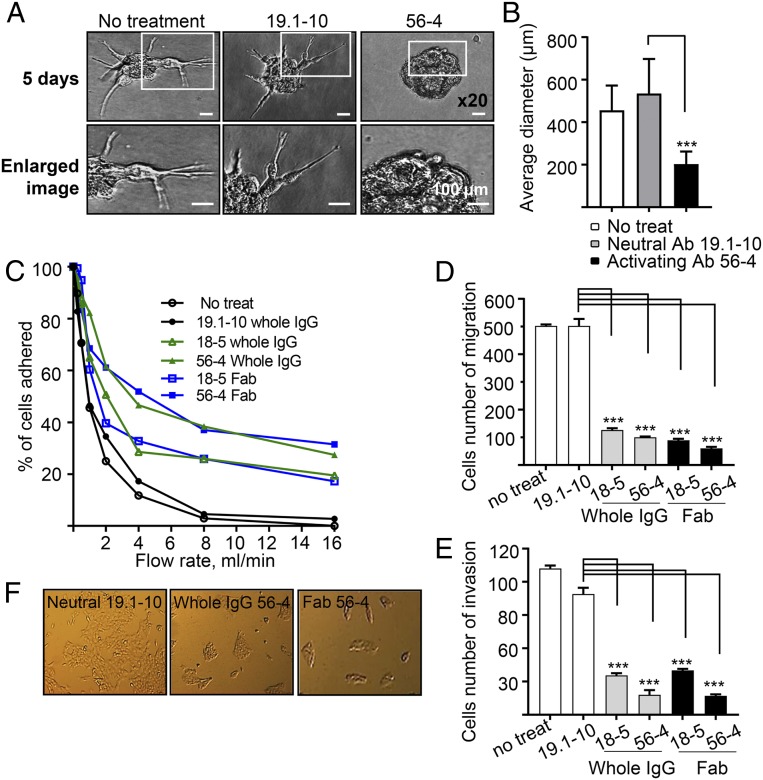
The loss of E-cadherin expression has been thought to allow malignant tumor cells to dissociate from the primary tumor mass and invade the extracellular matrix and surrounding stroma (2–4). To mimic the three-dimensional (3D) tumor environment, we analyzed whether E-cadherin activation affects tumor cell invasion in a 3D mammary tumor model in vitro. We isolated primary tumors from MMTV-PyMT mice and generated tumor spheroids embedded into a 3D extracellular matrix (SI Appendix, Fig. S5A). Numerous cell protrusions were present when treated with neutral mAb, but activating mAb significantly decreased protrusion and invasion into the surrounding matrix and maintained the spheroid morphology (Fig. 4A). In the presence of 56-4 activating mAb, the number of protrusions from spheroids was significantly reduced (Fig. 4B). We also examined the effects of activating mAb on invasion of cultured tumor cell lines grown in 3D spheroids using both Py2T (derived from PyMT tumors) (26) and parental 4T1 cells. When cultured for 5 d, Py2T cells were highly protrusive and migratory with treatment of neutral mAb. In contrast, activating mAb significantly reduced invasion (SI Appendix, Fig. S5 B and C). Similarly, invasion of 4T1 cell spheroids was significantly inhibited with activating mAb treatment compared to neutral mAb (SI Appendix, Fig. S5 D and E). Thus, enhancement of E-cadherin functional activity may contribute to suppression of the invasion of carcinoma cells.
Fig. 4.
E-cadherin activating mAbs inhibit invasiveness and migration and enhance cell adhesion of PyMT primary spheroids and in vitro cell cultures. (A) MMTV-PyMT tumor-derived spheroids were mixed with a suspension of 1–2 spheroids/µl with a 3D extracellular matrix and incubated for 5 d in the presence of mAbs. Representative image of organoid spheroids and enlarged views (Bottom). (Scale bars, 100 µm.) (B) Quantification of A. Calculation of invasion as a function of the longest invasive distance emanating from the spheroid. Average of the longest invasive distance (µm) per spheroid (n = 30 spheroids per group). ***P < 0.001 compared with neutral Ab treatment. (C–F) 4T1 cells. (C) For cell adhesion assay, activating mAbs and Fab fragments stimulated adhesion of cells to pure E-cadherin substrate. 4T1 cells were untreated, pretreated with 3 µg/mL neutral mAb, 19.1-10, or activating mAbs 18-5 or 56-4 for 2 h, and cell adhesion strength to E-cadherin-coated capillary tubes was evaluated using increasing laminar flow to determine the force required to detach cells. (D) Migration. (E) Invasion assay of cells invading through a basement membrane. The cells were treated with of 3 µg/mL neutral mAb, 19.1-10, or activating mAbs 18-5 or 56-4 for 24 h. (F) Epithelial morphology. 4T1 cells were treated with of 3 µg/mL neutral mAb, 19.1-10, or activating mAb 56-4 for 48 h. ***P < 0.001 compared with neutral Ab treatment.
To better understand the mechanisms by which activating Abs inhibit tumor cells from escaping from the primary tumor into the bloodstream, we evaluated their effects on adhesion, migration, and invasiveness of the tumor cells in vitro cell culture. E-cadherin activating Abs significantly increased the strength of attachment to purified E-cadherin protein of both mouse mammary tumor cell lines 4T1 (Fig. 4C) and Py2T (SI Appendix, Fig. S6A) compared with neutral mAb treatment. Monovalent Fab fragment had similar activities, demonstrating that the mAbs act through allosteric effect rather than antigen crosslinking (14). Cell migration was assessed using movement of cells through uncoated transwell filters, and was significantly suppressed by treatment of whole IgG and Fab activating Abs compared with neutral mAb in both cell lines 4T1 (Fig. 4D and SI Appendix, Fig. S6F) and Py2T (SI Appendix, Fig. S6 B and C). Invasion through an ECM was assessed by movement of cells through a matrigel-coated transwell filter, and both whole IgG and Fab activating Abs inhibited the invasion of 4T1 cells (Fig. 4E and SI Appendix, Fig. S6G) and Py2T cells (SI Appendix, Fig. S6 D and E). In addition, we noted that activating mAbs and Fabs triggered a more compact appearance of 4T1 monolayers, consistent with a more epithelial morphology (Fig. 4F). Overall, these data show that the four essential components of the cancer metastatic process (adhesion, detachment, migration, and invasion) are regulated by E-cadherin activity state at the cell surface.
E-Cadherin Activation Triggers Apoptosis of Tumor Cells in the Circulation.
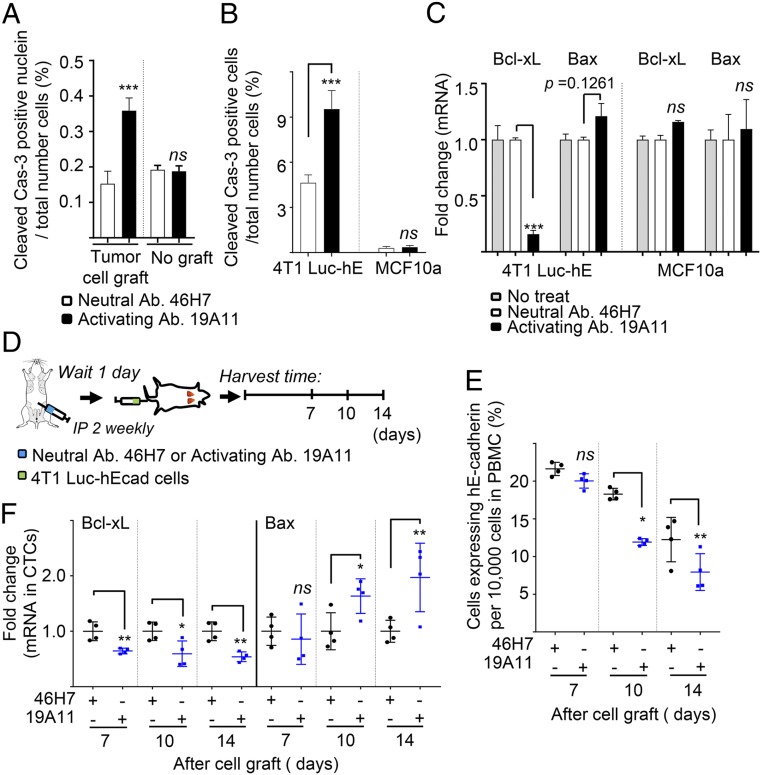
Resistance to anoikis has been shown to promote metastasis (28), and this phenomenon may be important for the survival of tumor cells in the circulation. The role of E-cadherin in apoptosis is complex, as loss of its expression has been reported to both increase (29–31) and decrease apoptosis in different circumstances (32–34). Therefore, we asked whether activating Abs might affect apoptosis of circulating and metastasizing tumor cells in the 4T1 orthotopic grafting model. Quantitative analysis of TUNEL-positive cells demonstrated a significant increase of almost 5-fold in lungs treated with activating mAb compared with neutral mAb treatment (SI Appendix, Fig. S7A). Apoptosis was confirmed using cleaved caspase-3 as a specific apoptotic marker. An increased population of cleaved caspase-3-positive cells was observed only in tumor lesions of the metastatic lungs treated with the activating mAb, while apoptosis was not changed by Ab treatment in normal lung tissue without tumor cell transplantation (Fig. 5A and SI Appendix, Fig. S7B). Treatment with activating mAb also significantly increased the percentage of cleaved caspase-3-positive 4T1 Luc-hE cells in cell culture (Fig. 5B) compared with the neutral mAb. Activating mAb decreased the mRNA level of antiapoptotic marker Bcl-xL (Fig. 5C). Apoptosis of MCF10a cells, a nontumor mammary cell line, was very low compared with 4T1 Luc-hE cells, and not altered by mAb treatment (Fig. 5 B and C). These results suggest that E-cadherin activating mAb increases cancer cell-specific apoptosis.
Fig. 5.
E-cadherin activation increases cancer cell-specific apoptosis. Sections of lung from neutral or activating mAb-treated mice described in Fig. 2B were examined for cleaved caspase-3 by immunofluorescence (A). Cells were measured in at least 10,000 cells, and each percentage represents the average of three randomly chosen fields of 1 sample (×10; n = 4 per group; no graft, n = 3). (B and C) 4T1 Luc-hE and MCF10a cells in culture were treated with 46H7 neutral or 19A11 activating mAb for 24 h. (B) Immunofluorescence staining for cleaved caspase-3 was tested in the 4T1 Luc-hE and MCF10a cells. Each percentage represents the average of three randomly chosen fields of 1 sample (×10; n = 3 per group). (C) Total RNA was prepared and analyzed for expression of the indicated transcripts by qRT-PCR, using specific primers. ***P < 0.001 compared with neutral Ab treatment. (D–F) Schema (D). Mouse was treated with i.p. injections of either 46H7 neutral or 19A11 activating mAb twice weekly until the end of the experiments. One day after beginning the treatment, 4T1 Luc-hE cells were i.v. injected into the tail vein and whole-blood was collected at the indicated times. CTCs expressing hE-cadherin were sorted by FACS. (E) Percentage of CTCs expressing hE-cadherin in blood. Population of cells expressing hE-cadherin per 10,000 cells was measured in peripheral blood mononuclear cells (PBMC) of each mouse by flow cytometry (n = 4). (F) mRNA expression of Bcl-xL (Left) and Bax (Right) in CTCs isolated by hE-cadherin. The levels were normalized by mRNA expression of hE-cadherin, and the fold change was assessed with the control group treated with neutral mAb 46H7 at each point. *P < 0.05; **P < 0.01; ***P < 0.001 compared with neutral Ab treatment. ns, not significant.
To determine whether tumor cells undergo apoptosis in the bloodstream, we examined CTCs. First, we determined whether activating mAbs affect the rate of clearance of 4T1 cells from the circulation. Activating or neutral mAb was injected intraperitoneally (i.p.) the day before injection of 4T1 Luc-hE cells into the tail vein, and the CTCs were isolated and counted using qRT-PCR for the luciferase marker at the indicated points (SI Appendix, Fig. S8A). Although ∼30,000 cells were injected into the tail vein, only about 90 CTCs were detected after 3 h in all cases, and the numbers decreased slowly over time (SI Appendix, Fig. S8B). We speculate that the rapid loss of circulating cells might result from cancer cells being cleared via lymph nodes and/or removal by the immune cells. The number of CTCs was reduced by treatment of activating mAb at later times, between 7 and 10 d (SI Appendix, Fig. S8B). Thus, over longer times, tumor cells were cleared more from the circulation when E-cadherin is activated.
We then asked whether E-cadherin activating mAbs stimulated apoptosis of tumor cells in the circulation in this time frame, by measuring the levels of mRNA for apoptotic markers (Fig. 5D). Because Bax and Bcl-xL expression can be regulated in myeloid-derived suppressor cells (35), we first sorted 4T1 cells expressing human E-cadherin by FACS. Before the FACS, flow cytometry showed that 19A11 activating mAb decreased the percentage of cells expressing hE-cadherin (Fig. 5E), similar to the results of mRNA level for the orthotopic graft shown in Fig. 2D. mRNA levels of apoptotic markers were measured by qRT-PCR in the sorted cells, and normalized to total mRNA. In the presence of 19A11 activating mAb, the level of Bax mRNA was significantly increased (Fig. 5 F, Right), but Bcl-xL mRNA was significantly reduced (Fig. 5 F, Left).
A previous study found no effect of activating mAbs on cell proliferation in the primary tumor, consistent with the lack of effect on primary tumor size (17). In the present study, activating mAbs seemed to have little or no obvious effect on proliferation in the metastatic nodules in the lung, based on immunostaining of phospho-histone-H3 (SI Appendix, Fig. S9A). In addition, mRNA level of the proliferation marker Ki67 in sorted CTCs, measured by qRT-PCR, was not affected by treatment with 19A11 (SI Appendix, Fig. S9B). Therefore, it is unlikely that E-cadherin activating Abs inhibit metastasis via effects on cell proliferation, but instead may do so, at least in part, by inducing cancer cell-specific apoptosis in the bloodstream.
Taken together, this study describes the importance of the functional down-regulation of E-cadherin expressed on tumor cells in the metastatic process, involving changes in cell-cell adhesion, local invasion, intravasation, cell survival, and extravasation (SI Appendix, Fig. S10).
Discussion
This study demonstrates that activation of E-cadherin adhesion at the cell surface with mAbs inhibits metastasis in spontaneously arising tumors. The PyMT model of mammary cancer is a widely studied genetically driven endogenous tumor model that progresses in stages while exhibiting cell heterogeneity typical of tumors (18, 25). The responsiveness of these tumors to E-cadherin activating mAbs shows that metastasizing tumor cells do not need to undergo an EMT and lose E-cadherin expression, as has been widely believed. Indeed, we observe that all stages of tumor cells in this model, including the primary tumor, the CTCs, and the distal lung metastases, retain E-cadherin expression. Nor is the promotion of metastasis by E-cadherin, as recently reported (11), simply a fixed property of E-cadherin expression. We hypothesize instead that E-cadherin activity may be down-regulated by factors in the tumor microenvironment to functionally reduce cell adhesion and facilitate the metastatic process. Regulation of cadherin-mediated adhesion by growth factors is well known to occur in morphogenetic processes during development, and tumor metastasis could mimic these normal developmental processes (24, 36). Indeed, similar activating mAbs to Xenopus C-cadherin inhibit tissue morphogenesis by increasing cell adhesion, and a similar effect on tumor morphogenesis may occur during metastasis (12, 13).
Activating E-cadherin inhibits the process of metastasis at multiple stages (SI Appendix, Fig. S10). It has been believed for a long time that E-cadherin acts as a metastasis suppressor by inhibiting the initial dissociation of cells from the primary tumor and thereby reducing the initial steps of tumor cell invasion (3, 37). Our findings are consistent with this model in part because activating mAbs suppress tumor cells in the circulation in both the endogenous PyMT and 4T1 cell grafting models (Fig. 2). Moreover, we observe that activating mAbs significantly inhibit invasiveness of tumor cells in tumor spheroids derived from PyMT primary mammary tumors (Fig. 4). However, activating mAbs also suppress later stages of metastasis, because they inhibit metastatic colonization of lungs by cells directly injected into the circulation. The activating mAbs likely inhibit extravasation through their inhibition of morphogenetic movements, but could also affect the seeding and growth of the distal metastatic nodules in the lung (Fig. 3). Importantly, we observe that the activating mAbs also seem to have direct effects on the CTCs by increasing their clearance from the bloodstream (perhaps due to apoptosis, see next paragraph), and it is possible that this effect could partially explain both how the mAbs reduce the numbers of CTCs arising from tumors and the number of metastatic nodules arising from cells injected into the bloodstream. Therefore, the original model for how E-cadherin affects metastasis at initial stages of tumor dissemination is greatly oversimplified, and we conclude that it functions instead at multiple stages of the metastatic cascade.
E-cadherin cell surface activity affects tumor cell behavior by several mechanisms, consistent with its effects on multiple processes in the metastatic cascade. The activating mAbs were identified by their ability to switch on E-cadherin-mediated adhesion in the nonadhesive colo205 cell line, but they also enhance the adhesive strength of epithelial tumor cells that already appear to be adhesive (SI Appendix, Fig. S1). These kinds of changes in adhesive strength are normally not readily observable by examining tumor tissues and are likely overlooked in most studies on metastasis. The activating mAbs allow us to test the roles of adhesive strength directly. E-cadherin activating mAbs also suppress cell invasion and migration in tumor-derived spheroids and in cell monolayer cultures (Fig. 4 and SI Appendix, Figs. S5 and S6). This is most likely due to their effects on cell adhesion, as stronger adhesive bonds should prevent cells from dissociating from the tumor spheroid or cell monolayer.
Activating mAbs also seemed to induce the apoptosis of tumor cells. Although the activating mAbs did not reduce the size of the primary tumor, they did stimulate the clearance of CTCs from the circulation (Fig. 2). Loss of CTCs in the circulation can be explained, at least partially, by stimulation of apoptosis, since CTCs exhibited associated changes in the expression of pro- and antiapoptotic markers. Activating mAbs also induced the expression of apoptotic markers in lung metastatic nodules and in cultured tumor cells without effects on cell proliferation, indicating that increased apoptosis is likely the important cell property affecting growth of metastases by the mAbs (Fig. 5 and SI Appendix, Figs. S7 and S9).
The role of E-cadherin in regulating apoptosis of epithelial cells or tumor cells is complex. Studies have reported E-cadherin expression both stimulating and inhibiting apoptosis in different conditions (11, 29–34). In normal epithelial tissues, E-cadherin adhesion ought to stabilize the cells and prevent apoptosis, but an important property of tumor cells is their resistance to apoptosis, or anoikis when cells detach from their adhesions. Tumor cells may regain their sensitivity to apoptosis when treated with E-cadherin activating mAbs. Indeed, we find that cultured 4T1 tumor cells are highly sensitive to activating mAb-induced apoptosis, while untransformed mammary epithelial cells, MCF10A, are not (Fig. 5 B and C). Activating mAbs may induce tumor cell apoptosis due to their effects on adhesion or on one of the signaling pathways regulated by E-cadherin, including the hippo signaling pathway (22, 38), the Wnt pathway (24, 39–41), the small GTPases, Rac and Rho, or PI3Kinase signaling (42). We do not yet know how activating mAbs affect these pathways.
In summary, activation of E-cadherin at the cell surface with mAbs inhibits the metastatic progression of endogenous, genetically driven mouse mammary tumors. It affects metastasis at multiple stages, and it works via multiple cellular mechanisms. Although additional preclinical and clinical trials are needed to confirm this approach, the metastatic inhibitory effect on the administration of E-cadherin-targeted mAbs raises the possibility that these Abs can be translated into clinics.
Materials and Methods
Generation of Activating Rabbit mAbs to Mouse E-Cadherin.
Hybridoma cell lines were generated from rabbits immunized with the purified extracellular domain of mouse E-cadherin. E-cadherin-positive hybridomas in ELISA were then screened in a functional assay, similar to one performed previously for mouse anti-human E-cadherin (14), for their ability to activate adhesion of colo205 cell expressing mouse E-cadherin. Hybridomas producing activating mAbs 18-5 and 56-4 were obtained; a hybridoma line producing a neutral mAb 19.1-10 also was obtained that binds E-cadherin but does not activate colo205 adhesion.
Mouse Experiments and In Vivo Treatments with Abs.
FVB MMTV-Polymavirus middle T antigen (MMTV-PyMT) breeders were obtained from The Jackson Laboratory. All mice were housed and bred under specific pathogen‐free conditions at Seattle Children's Research Institute, and all animal studies are governed through protocols approved by the Institutional Animal Care and Use Committee. Mice were treated twice weekly with neutral mAbs, 19.1-10, or E-cadherin-specific mAb 56-4 by i.p. injection.
For 4T1 tumor cell grafting metastatic mouse model studies, 4T1 Luc2-expressing hE-cadherin (4T1 Luc-hE) cells described in ref. 17 were injected into the mammary fat pads of BALB/c mice. To determine the ability of tumor cells to metastasize from bloodstream (or circulation) and colonize the lung, 4T1 Luc-hE were also injected via the tail vein.
Details of mouse experiments with generation of Abs, detection of CTCs by real-time PCR, and flow cytometric analyses are available in SI Appendix, Supplementary Materials and Methods. Materials and methods for histological analysis, immunofluorescence staining, TUNEL, isolation of CTCs, FACS analysis, transwell migration/invasion, 3D cell culture, adhesion assay, laminar flow adhesion assay, and statistical analyses are described in SI Appendix, Supplementary Materials and Methods.
Data Availability.
All data discussed in the paper are shown in the figures of the paper. Raw data will be made available by individual request to the B.M.G. laboratory.
Supplementary Material
Acknowledgments
This research is funded by NIH Grant R01CA207115. The Py2T cell line was a gift from Dr. Gerhard Christofori (University of Basel, Switzerland).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918167117/-/DCSupplemental.
References
- 1.Bracke M. E., Van Roy F. M., Mareel M. M., The E-cadherin/catenin complex in invasion and metastasis. Curr. Top. Microbiol. Immunol. 213, 123–161 (1996). [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Kang Y., Massagué J., Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 118, 277–279 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Valastyan S., Weinberg R. A., Tumor metastasis: Molecular insights and evolving paradigms. Cell 147, 275–292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukholm I. K., Nesland J. M., Børresen-Dale A. L., Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients [seecomments]. J. Pathol. 190, 15–19 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Lou Y., et al. , Epithelial-mesenchymal transition (EMT) is not sufficient for spontaneous murine breast cancer metastasis. Dev. Dyn. 237, 2755–2768 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Hollestelle A., et al. , Loss of E-cadherin is not a necessity for epithelial to mesenchymal transition in human breast cancer. Breast Cancer Res. Treat. 138, 47–57 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Querzoli P., et al. , An immunohistochemically positive E-cadherin status is not always predictive for a good prognosis in human breast cancer. Br. J. Cancer 103, 1835–1839 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung K. J., Gabrielson E., Werb Z., Ewald A. J., Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155, 1639–1651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung K. J., Ewald A. J., A collective route to metastasis: Seeding by tumor cell clusters. Science 352, 167–169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padmanaban V., et al. , E-cadherin is required for metastasis in multiple models of breast cancer. Nature 573, 439–444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brieher W. M., Gumbiner B. M., Regulation of C-cadherin function during activin induced morphogenesis of Xenopus animal caps. J. Cell Biol. 126, 519–527 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong Y., Brieher W. M., Gumbiner B. M., Analysis of C-cadherin regulation during tissue morphogenesis with an activating antibody. J. Cell Biol. 144, 351–359 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrova Y. I., Spano M. M., Gumbiner B. M., Conformational epitopes at cadherin calcium-binding sites and p120-catenin phosphorylation regulate cell adhesion. Mol. Biol. Cell 23, 2092–2108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shashikanth N., et al. , Allosteric regulation of E-cadherin adhesion. J. Biol. Chem. 290, 21749–21761 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiden S. L., Petrova Y. I., Gumbiner B. M., Microtubules inhibit E-cadherin adhesive activity by maintaining phosphorylated p120-catenin in a colon carcinoma cell model. PLoS One 11, e0148574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrova Y. I., Schecterson L., Gumbiner B. M., Roles for E-cadherin cell surface regulation in cancer. Mol. Biol. Cell 27, 3233–3244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantozzi A., Christofori G., Mouse models of breast cancer metastasis. Breast Cancer Res. 8, 212 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamir E. R., et al. , Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. J. Cell Biol. 204, 839–856 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai D., et al. , Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 157, 1146–1159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagotto F., Gumbiner B. M., Cell contact-dependent signaling. Dev. Biol. 180, 445–454 (1996). [DOI] [PubMed] [Google Scholar]
- 22.Kim N. G., Koh E., Chen X., Gumbiner B. M., E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl. Acad. Sci. U.S.A. 108, 11930–11935 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrais M., Chen X., Perez-Moreno M., Gumbiner B. M., E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol. Biol. Cell 18, 2013–2025 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendonsa A. M., Na T. Y., Gumbiner B. M., E-cadherin in contact inhibition and cancer. Oncogene 37, 4769–4780 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldmeier L., Meyer-Schaller N., Diepenbruck M., Christofori G., Py2T murine breast cancer cells, a versatile model of TGFβ-induced EMT in vitro and in vivo. PLoS One 7, e48651 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guy C. T., Cardiff R. D., Muller W. J., Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol. Cell Biol. 12, 954–961 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micalizzi D. S., Maheswaran S., Haber D. A., A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 31, 1827–1840 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y. N., Koo K. H., Sung J. Y., Yun U. J., Kim H., Anoikis resistance: An essential prerequisite for tumor metastasis. Int. J. Cell Biol. 2012, 306879 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witta S. E., et al. , Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 66, 944–950 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Lu M., et al. , E-cadherin couples death receptors to the cytoskeleton to regulate apoptosis. Mol. Cell 54, 987–998 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Sasaki C. Y., Lin Hc., Passaniti A., Expression of E-cadherin reduces bcl-2 expression and increases sensitivity to etoposide-induced apoptosis. Int. J. Cancer 86, 660–666 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Galaz S., et al. , Loss of E-cadherin mediated cell-cell adhesion as an early trigger of apoptosis induced by photodynamic treatment. J. Cell. Physiol. 205, 86–96 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Fouquet S., et al. , Early loss of E-cadherin from cell-cell contacts is involved in the onset of Anoikis in enterocytes. J. Biol. Chem. 279, 43061–43069 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Peluso J. J., Pappalardo A., Fernandez G., E-cadherin-mediated cell contact prevents apoptosis of spontaneously immortalized granulosa cells by regulating Akt kinase activity. Biol. Reprod. 64, 1183–1190 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Hu X., et al. , Deregulation of apoptotic factors Bcl-xL and Bax confers apoptotic resistance to myeloid-derived suppressor cells and contributes to their persistence in cancer. J. Biol. Chem. 288, 19103–19115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gumbiner B. M., Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Christofori G., Semb H., The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem. Sci. 24, 73–76 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Gumbiner B. M., Kim N. G., The Hippo-YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 127, 709–717 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gumbiner B. M., Propagation and localization of Wnt signaling. Curr. Opin. Genet. Dev. 8, 430–435 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Nelson W. J., Nusse R., Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303, 1483–1487 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heuberger J., Birchmeier W., Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb. Perspect. Biol. 2, a002915 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yap A. S., Kovacs E. M., Direct cadherin-activated cell signaling: A view from the plasma membrane. J. Cell Biol. 160, 11–16 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper are shown in the figures of the paper. Raw data will be made available by individual request to the B.M.G. laboratory.