Significance
Cyanobacteria harbor a photosynthetic apparatus related to plant chloroplasts. The lipid compositions of the thylakoids that harbor the photosynthetic complexes in cyanobacteria and chloroplasts are highly similar. Chloroplasts contain triacylglycerol (storage oil) and wax esters; the latter are composed of phytol derived from chlorophyll and fatty acids (phytyl esters). However, the existence of these lipids in cyanobacteria in general remained unclear. Here we show that the cyanobacterium Synechocystis contains triacylglycerol and phytyl esters. A mutant, Δslr2103, was generated, which lacked these two lipids but showed no obvious growth defect. The slr2103 gene encodes a diacylglycerol acyltransferase different from known enzymes of triacylglycerol synthesis in bacteria. This pathway can be employed to produce oil for biotechnological applications in cyanobacteria.
Keywords: cyanobacteria, triacylglycerol, wax, acyltransferase
Abstract
Cyanobacteria are unicellular prokaryotic algae that perform oxygenic photosynthesis, similar to plants. The cells harbor thylakoid membranes composed of lipids related to those of chloroplasts in plants to accommodate the complexes of photosynthesis. The occurrence of storage lipids, including triacylglycerol or wax esters, which are found in plants, animals, and some bacteria, nevertheless remained unclear in cyanobacteria. We show here that the cyanobacterium Synechocystis sp. PCC6803 accumulates both triacylglycerol and wax esters (fatty acid phytyl esters). Phytyl esters accumulate in higher levels under abiotic stress conditions. The analysis of an insertional mutant revealed that the acyltransferase slr2103, with sequence similarity to plant esterase/lipase/thioesterase (ELT) proteins, is essential for triacylglycerol and phytyl ester synthesis in Synechocystis. The recombinant slr2103 enzyme showed acyltransferase activity with phytol and diacylglycerol, thus producing phytyl esters and triacylglycerol. Acyl-CoA thioesters were the preferred acyl donors, while acyl-ACP (acyl carrier protein), free fatty acids, or galactolipid-bound fatty acids were poor substrates. The slr2103 protein sequence is unrelated to acyltransferases from bacteria (AtfA) or plants (DGAT1, DGAT2, PDAT), and therefore establishes an independent group of bacterial acyltransferases involved in triacylglycerol and wax ester synthesis. The identification of the gene slr2103 responsible for triacylglycerol synthesis in cyanobacteria opens the possibility of using prokaryotic photosynthetic cells in biotechnological applications.
Triacylglycerol (TAG) is the most important storage lipid in many organisms. Plant TAG represents the largest source of oil for human consumption, biotechnological applications, and biofuels. Oleaginous eukaryotic microalgae are increasingly considered as feedstocks for the production of oils for food and industrial applications (1, 2). However, oil yield from microalgae is oftentimes low, and most strains accumulate oil only under specific stress conditions.
Oil is stored in lipid droplets in the cytosol of plants, animals, and fungi. Lipid droplets contain nonpolar lipids, in particular TAG, enclosed by a phospholipid monolayer membrane (3). In plant seeds, TAG is predominantly synthesized by the transfer of a fatty acyl group from acyl-CoA or from a phospholipid onto diacyl-glycerol by acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) or phospholipid:diacylglycerol acyltransferase (PDAT), respectively (4–8). In addition to the storage in lipid droplets in the cytosol, plant chloroplasts accumulate nonpolar lipids in plastoglobules that are surrounded by a galactolipid monolayer (9). Plastoglobules contain TAG, carotenoids, tocopherol, and fatty acid phytyl esters (10). In Arabidopsis, phytyl esters, which are chloroplastic wax esters containing phytol, are synthesized during chlorotic stress (11, 12). Two acyltransferases (PES1, PES2) of the esterase/lipase/thioesterase (ELT) family were found to synthesize phytyl esters from phytol, which is derived from chlorophyll breakdown, and fatty acids from lipid turnover (13). The ELT enzymes PES1/PES2 from Arabidopsis and PYP1 from tomato show broad substrate specificities for the synthesis of phytyl esters and xanthophyll esters, respectively (13, 14).
According to the endosymbiont theory, plant chloroplasts are derived from an ancient cyanobacterium via endosymbiosis, suggesting that many molecular and structural characteristics of chloroplasts are of cyanobacterial origin (15, 16). For example, the cytosol of Synechocystis sp. PCC6803 and Nostoc punctiforme were shown to contain lipid droplets similar to plastoglobules in plant chloroplasts and lipid droplets in the cytosol of eukaryotic cells (17, 18). Additional evidence for the potential occurrence of TAG was obtained for filamentous cyanobacteria of the Nostocales. Nostoc commune is capable of producing a lipid comigrating with TAG after labeling with radioactive glycerol (19), and a lipid comigrating with TAG was identified in lipid droplets isolated from Nostoc punctiforme (18). TAGs were identified in the thermophilic Nostocales species Mastigocladus and Tolypothrix (20). However, evidence for the existence of TAG in nonfilamentous, nonthermophilic cyanobacteria such as Synechocystis is lacking (1). TAG accumulation has been reported for different nonphotosynthetic Gram-positive (Mycobacterium, Streptomycetes) and Gram-negative (Acinetobacter, Pseudomonas) bacteria (21). An acyltransferase essential for TAG and wax ester synthesis (WS/DGAT, AtfA-type) was isolated from Acinetobacter baylyi (22). Orthologs of AtfA represent the only known acyltransferases involved in TAG synthesis in bacteria (23).
To unravel whether cyanobacteria harbor a pathway for TAG synthesis, nonpolar lipids were isolated from Synechocystis and characterized by direct infusion mass spectrometry (MS). A candidate acyltransferase for the synthesis of TAG was identified based on sequence similarity with Arabidopsis PES1/PES2. Characterization of the corresponding Synechocystis mutant and of the recombinant gene product revealed that Synechocystis indeed contains bona fide TAG, and that a cyanobacterial PES1/PES2-like acyltransferase exists that establishes a different class of bacterial genes involved in phytyl/wax ester and TAG synthesis.
Results
Identification of an ELT-Like Acyltransferase in Cyanobacteria.
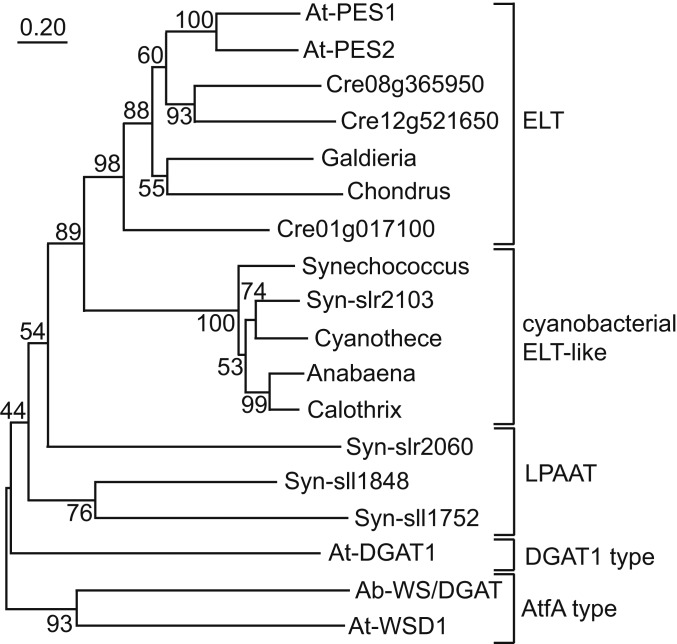
ELT sequences of plants are characterized by the presence of an N-terminal hydrolase and a C-terminal acyltransferase domain (13). ELT proteins with the two-domain structure are absent from cyanobacterial genomes. Protein BLAST searches with the C-terminal, acyltransferase part of Arabidopsis PES2 (amino acids 401 to 704) revealed the presence of one related sequence (slr2103) and three less similar acyltransferase-like sequences (sll1848, slr2060, sll1752) in the Synechocystis genome (Fig. 1). Two of the sequences (sll1848, slr2060) were previously characterized as lysophosphatidic acid acyltransferases (LPAAT) (24). Further cyanobacterial slr2103-like sequences were found by BLAST searches in the genomes of Cyanothece (Oscillatoriales) and Anabaena and Calothrix (Nostocales; Fig. 1). In contrast, three PES2-like sequences with two domains each were retrieved in green algae (Chlamydomonas reinhardtii), and one PES2-like sequence each in red algae (Galdieria and Chondrus). A phylogenetic tree was constructed with the C-terminal acyltransferase portions (PES2 and related two-domain proteins) of the plant and green and red algae, the cyanobacterial acyltransferases (slr2103-like sequences), and with Arabidopsis diacylglycerol acyltransferase (DGAT1). Furthermore, the Acinetobacter wax synthase/DGAT sequences (bacterial AtfA-type) and the related WSD1 sequence from Arabidopsis involved in wax ester synthesis were included. The sequences were clustered into five groups: a plant-type ELT group containing the C-terminal sequences of Arabidopsis PES1, PES2 and the related proteins from green and red algae, a group of ELT-like cyanobacterial acyltransferases including slr2103, a group of the three distantly related LPAAT-like sequences from Synechocystis, and Arabidopsis DGAT1 and the two AtfA-type sequences. The slr2103 sequences are much closer related to the acyltransferase domain of Arabidopsis ELT sequences compared with Arabidopsis DGAT1 or the AtfA-type sequences, indicating that they establish a different group of bacterial acyltransferases.
Fig. 1.
Phylogenetic relationship of lipid acyltransferases from cyanobacteria, plants, and green and red algae. Protein sequences were aligned with ClustalW, and a phylogenetic tree constructed using the Neighbor Joining method with 1,000 bootstrap repetitions (Mega 10.0.5). For plant ELT sequences, only the C-terminal acyltransferase-like parts were considered. A. baylyi (ADP1) Ab-WS/DGAT, AF529086.1; Anabaena sp. (CA = ATCC 33047) WP_066380359; Arabidopsis thaliana At-PES1 (amino acids 401–704), At-PES2 (401-701), At-DGAT1, At-WSD1; Calothrix brevissima WP_096644248; C. reinhardtii Cre01g017100 (591-911), Cre08g365950 (411-692), Cre12g521650 (631-873); Chondrus crispus XP_005713590 (601-965); Cyanothece sp. (PCC 7425) WP_012629067; Galdieria sulphuraria XP_005702844 (561-921); Synechococcus sp. (NKBG042902) WP_030007836; and Synechocystis sp. (PCC 6803) slr2103, sll1848, slr2060, sll1752.
Generation of a Δslr2103 Deletion Mutant.
A Synechocystis deletion mutant (Δslr2103) was generated by inserting a kanamycin resistance cassette into the ORF slr2103 (SI Appendix, Fig. S1). An isogenic mutant line was isolated after restreaking the cells on kanamycin-containing medium. Growth of Δslr2103 mutant cells on BG-11 medium as well as on N-deficient medium was not compromised (SI Appendix, Fig. S2). When grown in liquid BG-11 medium or under stress conditions (darkness and NaCl), the chlorophyll a contents of Δslr2103 cells were slightly reduced, whereas the amounts of carotenoids remained similar to WT (SI Appendix, Fig. S3). The quantum yield of photosystem II determined by fluorescence measurements was decreased to 0.31 in Δslr2103 cells compared with 0.34 in WT (SI Appendix, Fig. S3). Therefore, the deletion of the gene slr2103 had only minor consequences for growth and photosynthesis in Synechocystis.
Fatty Acid Phytyl Esters Accumulate in Synechocystis in an slr2103-Dependent Manner.
Previously, fatty acid phytyl esters were tentatively found in extremely low amounts in Synechococcus (25). To confirm their existence in cyanobacteria, phytyl esters were enriched by solid phase extraction and analyzed by quadrupole time-of-flight MS (Q-TOF MS). Different phytyl esters were clearly identified by the masses of their parental ammonium adduct ions, which gave rise to fatty acid ammonium ions after fragmentation (SI Appendix, Fig. S4). The amounts of phytyl esters in Synechocystis WT cells are very low, with 16:0-phytol, 18:1-phytol, 18:2-phytol, and 18:3-phytol representing the most abundant forms (Fig. 2).
Fig. 2.
Fatty acid phytyl esters in Synechocystis WT and Δslr2103 mutant. Synechocystis cells were grown in liquid BG-11 medium (control) or under stress conditions (3 d of darkness/with 0.5 M NaCl; N deprivation) or in the presence of phytol. Phytyl esters were quantified by Q-TOF MS. (A) Total fatty acid phytyl esters. (B) Fatty acid phytyl ester composition. Acyl groups are indicated as number of C atoms: number of double bonds. Means of three to four replicas ± SD. *Significant differences to the WT grown under the same conditions. #Significant differences to the WT grown under control conditions, respectively (Student t test, P < 0.05).
It has previously been shown that phytyl esters accumulate in plant chloroplasts during abiotic stress conditions, including N deprivation, drought, or extended darkness, as well as after feeding exogenous phytol to the plants (11, 12). Degradation of chlorophyll and membrane lipids under stress results in the release of free phytol and fatty acids, which are converted into phytyl esters and TAG in plants. Growth of Synechocystis cells under N deprivation did not result in the accumulation of phytyl esters (Fig. 2). This can be explained because in contrast to plants, cyanobacteria harbor light-harvesting complexes composed of phycobilisomes that contain tetrapyrroles such as phycoerythrobilin or phycocyanobilin instead of chlorophyll. Thus, degradation of phycobilisomes during N deprivation in Synechocystis does not cause phytol accumulation (26). We therefore chose the combination of darkness and salt stress to stimulate chlorophyll degradation. Exposure of Synechocystis to darkness results in the halt of chlorophyll synthesis (27). Salt stress disturbs the ability of cyanobacteria to take up carbon from the medium, forcing the cells to metabolize intracellular carbon (28). The combination of dark and salt stress is thus expected to cause the degradation of thylakoid lipids and chlorophyll. Indeed, Synechocystis cells accumulated fatty acid phytyl esters by more than twofold during exposure to darkness/high salt conditions (Fig. 2B). Next, we tested whether the cells can take up exogenous phytol and use it for phytyl ester synthesis. Feeding of phytol to the cells stimulated an even higher increase in phytyl esters of up to fourfold (Fig. 2B). Therefore, under salt/dark treatment and during phytol feeding, all forms of phytyl esters increased to similar extents, and in addition, low amounts of 16:1-phytol and 18:0-phytol accumulated.
The phytyl ester content in Δslr2103 cells grown under control conditions was reduced to 1 to 2 nmol OD750−1. The amount of phytyl esters in Δslr2103 did not increase during salt/dark stress or after phytol feeding. Thus, phytyl esters were about eightfold or 10-fold higher in WT compared with Δslr2103 under salt/dark stress or phytol feeding conditions, respectively. These results demonstrate that slr2103 represents the major enzyme for phytyl ester synthesis in Synechocystis.
The slr2103 Gene Is Essential for TAG Synthesis in Synechocystis.
Previous studies on the accumulation of TAG in cyanobacteria were mostly inconclusive (1, 18, 19). TAG was only identified in filamentous, thermophilic cyanobacteria of the order Nostocales (20). To address the question of TAG accumulation in cyanobacteria, nonpolar lipids were isolated from Synechocystis cells by solid phase extraction and subjected to Q-TOF MS analysis. Several peaks with m/z values corresponding to ammonium adducts of TAGs with different acyl groups were identified (Fig. 3A). MS/MS fragmentation resulted in the formation of diacyl-glycerol ions with the neutral losses of fatty acid ammonia adducts (SI Appendix, Fig. S5). Scanning for different TAGs by MS/MS experiments revealed the presence of ∼14 molecular species, with tri-16:0 TAG and 16:0-16:0-18:3 TAG representing the most abundant forms (Fig. 3A).
Fig. 3.
Triacylglycerol accumulation in Synechocystis. (A) Separation of nonpolar lipids from Synechocystis WT and Δslr2103 mutant by TLC. Lipids were stained with primuline. FFA, free fatty acids. The arrow indicates TAG in Synechocystis WT. (B) TAG content and (C) molecular species composition of Synechocystis WT and Δslr2103 mutant. TAG was quantified by Q-TOF MS. Means of three to four replicas ± SD. Asterisks indicate significant differences to the WT (Student t test, P < 0.05).
To study the role of the gene slr2103 in TAG synthesis, nonpolar lipids from Synechocystis WT and the Δslr2103 mutant were analyzed by TLC or Q-TOF MS. TLC separation revealed the presence of a lipid in WT comigrating with TAG. This band was absent from the Δslr2103 mutant. The total amount of TAG in WT cells was ∼5 nmol OD750−1, and it was about 15-fold lower in Δslr2103 with ∼0.3 nmol OD750−1 (Fig. 3). All molecular species found in WT were reduced to background levels in the Δslr2103 mutant. TAG was also quantified in cells exposed to salt/dark stress or N deprivation. The amount of TAG in WT cells was strongly reduced to 26.5 ± 3.9% or 12.1 ± 2.0% under salt/dark stress and N deprivation, respectively. These results demonstrate that different molecular species of TAG are synthesized in Synechocystis, and that TAG production depends on slr2103.
Decrease in the Number of Lipid Droplets in Δslr2103 Mutant Cells.
Plastoglobules in chloroplasts accumulate phytyl esters, TAG, carotenoids, and tocopherol (10, 13). Similarly, lipid droplets in cyanobacteria presumably also contain phytyl esters and TAG (18). To study the effect of the decrease in phytyl ester and TAG synthesis in Δslr2103 cells, lipid droplets were observed by transmission electron microscopy (Fig. 4A and SI Appendix, Fig. S6). The number of lipid droplets per cell cross section in the mutant was reduced to about 50% of WT (SI Appendix, Fig. S6). The residual number of lipid droplets in the mutant can be explained by the presence of other nonpolar lipids (carotenoids, tocopherols) and proteins in the lipid droplets (10). Therefore, deficiency in slr2103 affects phytyl ester and TAG accumulation and results in a reduced lipid droplet number per cell cross section.
Fig. 4.
The number of lipid droplets in Synechocystis Δslr2103 is decreased. Synechocystis WT and Δslr2103 mutant were grown in BG-11 medium and the cells observed by transmission electron microscopy.
The slr2103 Protein Harbors Phytyl Ester Synthase Activity.
The slr2103 gene was introduced into Escherichia coli cells. After induction of protein expression, protein accumulation was observed by SDS gel electrophoresis. The Coomassie stained gel showed a strong band at ∼32 kDa, in the range of the calculated weight of the slr2103 His tag protein (34.0 kDa). Immunoblot analysis with anti-His tag antibodies revealed a clear band corresponding to the 32-kDa band observed by Coomassie staining (SI Appendix, Fig. S7).
To study the capacity for phytyl ester synthesis, phytol was added to the slr2103-expressing cells. The synthesis of phytyl esters was followed by Q-TOF MS. While E. coli cells expressing slr2103 in the absence of phytol did not accumulate phytyl esters, phytol feeding resulted in the production of large amounts of phytyl esters. The most abundant phytyl esters were 18:1-phytol and 16:0-phytol, followed by 16:1-phytol, 17:0c-phytol, 14:0-phytol, and 19:0c-phytol (Fig. 5A). Therefore, the slr2103 gene encodes a protein with fatty acid phytyl ester synthesis activity.
Fig. 5.
The slr2103 protein harbors fatty acid phytyl ester and diacylglycerol acyltransferase synthase activity. (A) E. coli cells expressing Synechocystis slr2103 were grown in the presence of phytol. Phytyl esters were purified and quantified by Q-TOF MS. (Left) Total phytyl esters. (Right) Molecular species composition. 17:0c, cis-9,10-methylenehexadecanoic acid; 19:0c, cis-11,12-methyleneoctadecanoic acid. (B) Membrane proteins were isolated from E. coli cells expressing slr2103 and employed for acyltransferase assays with phytol and different acyl donors. (C) E. coli cells expressing slr2103 were grown with dioctanoin (di8:0). TAG was isolated and quantified by Q-TOF MS. (Left) Total TAG. (Right) Molecular species. (D) Membrane proteins from E. coli expressing slr2103 were used for DGAT assays with dioctanoin and different acyl donors. (E) Acyltransferase reactions for phytyl ester and TAG synthesis by Synechocystis slr2103. Mean and SD of three to four replicas (Student t test, *P < 0.05).
Next, membrane proteins were isolated from slr2103-expressing cells and employed for acyltransferase assays with phytol and different acyl donors. The phytyl ester synthase activity of slr2103 with 16:0-CoA was higher than of the empty vector control, but activities with 16:0-ACP (acyl carrier protein) or 16:0 free fatty acids were very low (Fig. 5B). Furthermore, we tested the hypothesis of whether lipid-bound fatty acids (i.e., 16:3 or 18:3 bound to spinach monogalactosyldiacylglycerol [MGDG]) can be directly transferred to phytol. However, the phytyl ester synthase activity with MGDG-bound fatty acids was extremely low. Therefore, slr2103 showed phytyl ester synthase activity with 16:0-CoA, while other potential acyl donors were poor substrates.
To determine the capacity for TAG synthesis, slr2103-expressing E. coli cells were incubated in the presence of dioctanoin (di8:0). This short chain diacylglycerol was used because long chain diacylglycerols are barely water-soluble and are therefore poor substrates. Dioctanoin was quickly taken up and used for TAG synthesis in slr2103-expressing cells (Fig. 5C). The E. coli fatty acids 14:0, 16:1, 16:0, 18:1, and 18:0 were predominantly employed for TAG synthesis by slr2103.
DGAT assays were performed with membrane proteins from E. coli expressing slr2103 using dioctanoin and different acyl donors. 16:0-CoA was by far the best substrate of slr2103, with an activity of up to 300 nmol⋅min−1⋅mg−1 protein (Fig. 5D). Other putative acyl donors (16:0-ACP, 16:0 free fatty acid, 16:3 or 18:3 derived from MGDG) showed activities similar to the empty vector control.
In conclusion, slr2103 shows highest acyltransferase activity with acyl-CoAs. The acyl groups incorporated into phytyl esters and TAGs after feeding of phytol or dioctanoin represent fatty acids typical for E. coli. Two different acyl acceptors are employed by slr2103 (i.e., long chain alcohols: phytol, wax ester synthase activity) and diacylglycerol (dioctanoin, DGAT activity; Fig. 5E).
Discussion
We show here that the nonfilamentous cyanobacterium Synechocystis contains an ORF, slr2103, encoding an acyltransferase capable of synthesizing phytyl esters and TAG. The slr2103 sequence is unrelated to AtfA-type acyltransferases, which is the only family of enzymes involved in TAG synthesis in bacteria known to date. Instead, slr2103 is related to the ELT family of acyltransferases from plants. ELT enzymes are involved in fatty acid phytyl ester and fatty acid xanthophyll ester synthesis in the chloroplasts of plants (13, 14). After the endosymbiont theory, the slr2103 sequence presumably represents the evolutionary origin for the ELT proteins. ELT proteins are specific for plants, green algae, and red algae (Fig. 1), but absent from animals and fungi. They consist of a hydrolase domain followed by an acyltransferase domain, and contain an N-terminal transit sequence for targeting to the chloroplast. The finding that the Synechocystis acyltransferase slr2103 harbors TAG and phytyl ester synthesis activities demonstrates that the hydrolase domain is not essential for activity.
The acyl composition of phytyl esters and TAG in Synechocystis is dominated by the presence of high amounts of 18:1, 18:2, 18:3, and 16:0 (Figs. 2 and 3). This fatty acid pattern reflects the total fatty acid composition of the membrane lipids of Synechocystis, which mostly contains 16:0, 18:1, 18:2, and 18:3 (29). Therefore, it is likely that the fatty acids in TAG and phytyl esters are originally derived from membrane lipids.
The amount of TAG is reduced to background levels in the Δslr2103 mutant under control or stress conditions, indicating that slr2103 is essential for TAG synthesis. On the other hand, the Δslr2103 mutant still contains about 50% of phytyl esters compared with WT, when grown under control conditions. This residual, low amount of phytyl esters might be derived from the activity of other acyltransferase-like enzymes, or even from chemical esterification of phytol with free fatty acids. The finding that the phytyl ester content during salt/dark stress or phytol supplementation is much more strongly decreased in Δslr2103 compared with WT indicates that slr2103 is the major acyltransferase involved in phytyl ester production under these conditions.
In vitro acyltransferase activity of recombinant slr2103 protein with dioctanoin was much higher compared with phytol. We employed a detergent (CHAPS) in the enzyme assays, but it is still possible that phytol was poorly dissolved compared with dioctanoin. DGAT assays using dipalmitin (di16:0) instead of dioctanoin showed lower activity, in accordance with the scenario that lipids containing long chain acyl groups are poorly soluble. Therefore, from the enzyme activity data (Fig. 5), it is difficult to conclude whether phytol or diacylglycerol is the preferred substrate of slr2103.
The acyltransferase slr2103 showed higher phytyl ester and TAG synthesis activity with acyl-CoA than with acyl-ACP (Fig. 5). Cyanobacteria harbor a type II fatty acid synthase, giving rise to the production of acyl-ACP thioesters similar to plant chloroplasts (30). The acyltransferases involved in membrane lipid synthesis in cyanobacteria prefer acyl-ACP, rather than acyl-CoA substrates (24, 30). In contrast, cyanobacteria also harbor acyl-CoA thioesters, as the initial steps of fatty acid synthesis are CoA-dependent and cyanobacteria contain a short-chain acyl-CoA-dependent pathway of polyhydroxyalcanoate synthesis (30, 31). Furthermore, cyanobacteria harbor CoA-dependent fatty acid modifying enzymes (e.g., the aldehyde-forming acyl-CoA reductase) (32). Therefore, in addition to the acyl-ACP pool used for membrane lipid synthesis, a separate acyl-CoA pool might exist in cyanobacteria important for the synthesis of low abundant nonpolar lipids such as phytyl esters and TAG.
In plants, phytyl esters and TAG accumulate during stress, taking up fatty acids from membrane lipids and phytol from chlorophyll breakdown (13). Similarly, salt/dark stress results in the increase in phytyl esters, but not TAG, in Synechocystis (Fig. 2). It is possible that TAG production is increased in Synechocystis during other growth conditions. The finding that the gene slr2103, which is involved in phytyl ester and TAG ester synthesis in Synechocystis, is related to PES1/PES2 of plants indicates that the pathway of conversion of lipid and chlorophyll breakdown products into nonpolar lipids with subsequent storage in plastoglobules/lipid droplets is presumably derived from the cyanobacterial progenitor of chloroplasts.
Plants are the largest source for global TAG production for human nutrition and biotechnological applications. In the past, numerous strategies were developed to produce TAG in eukaryotic microalgae, including green and red algae. The eukaryotic microalgae harbor a TAG synthesis pathway similar to plants, and TAG accumulation is most often dependent on chlorotic stress. The identification of the TAG synthesis pathway in cyanobacteria such as Synechocystis provides the means for developing a strategy of employing prokaryotic photosynthetic organisms for oil synthesis. The pathway of TAG synthesis in cyanobacteria is different from plants and eukaryotic algae. In addition, cyanobacteria are prokaryotic cells that can easily be grown and are amenable to genetic engineering to increase the capacity for oil production.
Materials and Methods
Growth of Synechocystis and Generation of Δslr2103 Deletion Mutant.
Synechocystis sp. PCC 6803 (glucose tolerant strain) was grown photomixotrophically in liquid or on solidified BG-11 medium (33) supplied with 5 mM glucose at 28 °C in incessant light (30 µmol m−2⋅s−1). Precultures of 50 mL were grown up to an OD750 of 0.6 and used to inoculate 100-mL cultures in BG-11 medium. Cells were grown to an OD750 of 0.6 and NaCl added to a final concentration of 0.5 M, and the cells grown in darkness for 3 d (salt/dark stress). For nitrogen deprivation, cells were harvested and resuspended in nitrogen-free medium to an OD750 of 0.6 (or in nitrogen-replete medium for control), and the cells were grown for 3 d. Alternatively, 10 µL of a serial dilution of cells was spotted on solid BG-11 medium (control, in the light), BG-11 with 0.5 M NaCl (salt/dark stress, grown in darkness), or BG-11 lacking nitrogen (with light) and the cells grown for 10 d.
The Δslr2103 deletion mutant of Synechocystis was generated by homologous recombination. The 5′ flanking sequence of slr2103 was amplified from genomic DNA, using oligonucleotides bn2263 and bn2264 (containing NcoI sites; SI Appendix, Table S1), and cloned into pJET1.2 (pJ-left-slr2103). In analogy, the 3′ flanking region was amplified with oligonucleotides bn2265 (harboring an MluI site) and bn2266 and ligated into pJET1.2 (pJ-right-slr2103). The construct pJ-nptII harbors the kanamycin resistance cassette nptII (amplified with oligonucleotides bn1116 and bn1117, introducing MluI and NcoI sites) cloned in pJET1.2. The 5′ flanking sequence and the nptII gene were released using NcoI/HindIII and NcoI (partial digestion)/MluI, respectively. HindIII is a restriction site in the pJET1.2 cloning vector. The two fragments were ligated in one step into the vector pJ-right-slr2103 (opened with MluI, HindIII), resulting in the knock-out construct pJ-Δslr2103-kan. The circular knock-out plasmid was transferred into Synechocystis PCC 6803 cells (34). Transformed cells were selected by restreaking on BG-11 medium with increasing kanamycin concentrations (30 µg⋅mL−1 final). The successful integration of the kanamycin cassette into the gene slr2103 was confirmed by PCR of genomic DNA.
Expression of slr2103 in E. Coli and Substrate Feeding.
The gene slr2103 was amplified by PCR using the primers bn3268 and bn3269, introducing SacI and PstI sites. The PCR product was ligated into the vector pQE-80L (Qiagen), and this construct was transferred into E. coli BL21(AI) cells (Thermo Fisher). E. coli cells were grown in LB medium at 37 °C to OD600 of 0.6 and then cooled to 16 °C, and 0.5 mM isopropyl-β-D-thiogalactopyranoside and 0.1% (wt/vol) l-arabinose were added for induction of expression at 16 °C overnight.
For substrate feeding experiments, the temperature was raised to 30 °C and the cells grown for 3 h in the presence of 3.3 mM phytol (Chemimpex, Wood Dale, IL) or 30 µM diacylglycerol (dioctanoin, di8:0, Larodan, Sweden) in the presence of 0.1% (wt/vol) CHAPS. Cells were harvested by centrifugation, the pellet washed once with water, and lipids extracted.
Acyltransferase Assays.
E. coli cells expressing slr2103 were harvested by centrifugation at 1,000 × g for 12 min. The pellet was washed and suspended in 4 mL buffer (1 mM EDTA, 200 mM sucrose, 100 mM Tris⋅HCl at pH 7.4). The cells were homogenized with a Precellys homogenizer (Bertin Technologies), using glass beads. The extract was centrifuged at 4,000 × g for 2 min. The supernatant was centrifuged at 35,000 × g for 45 min to obtain the membrane fraction. The pellet was resuspended and protein concentration measured with bicinchoninic acid. For enzyme assays (total volume, 200 µL), 400 µg membrane protein were incubated with 50 µM acyl donor (16:0-CoA, synthesized according to ref. 35; 16:0 free fatty acid [Merck KGaA, Darmstadt, Germany]; monogalactosyldiacylglycerol from spinach; Larodan) and 200 µM acyl acceptor (phytol, dioctanoin) in assay buffer (20 mM MgCl2, 0.1% CHAPS, 100 mM Tris⋅HCl at pH 7.4, 1.25 mg⋅mL−1 BSA, 10 mM Na orthovanadate). Lipid substrates were dissolved in a minimal volume of ethanol and directly added to the assay. After mixing, the assay was incubated for 20 min at 35 °C. The reaction was terminated and lipids extracted by adding 1 mL chloroform/methanol (2:1).
Lipid Analysis.
Cells were harvested from 100 mL Synechocystis culture by centrifugation and washed with BG-11 medium. The pellet was extracted three times with chloroform/methanol (1:2) (36). The extracts were combined and internal standards added. For fatty acid phytyl ester measurements, 17:0-phytol was used, which was synthesized from 17:0 and phytol (11). Tri-17:0 TAG and tri-17:1 TAG were used as internal standards for TAG quantification (Larodan). After addition of 1 mL of 300 mM ammonium acetate and 1 mL chloroform, extracts were centrifuged for phase separation. The organic phase was harvested and dried under a nitrogen stream.
The lipids were dissolved in hexane and applied to silica solid phase extraction columns (Macherey & Nagel, Düren) equilibrated with hexane (37). After washing the column with hexane, phytyl esters and TAGs were eluted with hexane/diethyl ether (99:1) and hexane/diethyl ether (92:8), respectively.
Synechocystis lipids were separated by TLC on silica plates (Silica 60 Durasil, Macherey & Nagel), using hexane/diethyl ether/acetic acid (70:30:1). The lipid bands were stained with primuline and observed under UV light. For lipid isolation, silica material from the plates was extracted with chloroform/methanol (2:1).
Phytyl esters and TAG were measured by Q-TOF MS. Lipids were dissolved in chloroform/methanol/300 mM ammonium acetate (300:665:35) (38). The samples were infused at 1 µL⋅min−1 into the HPLC-Chip Cube MS interface of the Agilent 6530 Series Accurate-Mass Q-TOF mass spectrometer (Agilent, Böblingen). Lipids were quantified by neutral loss scanning, and the amounts calculated based on internal standards (13).
Measurements of Chlorophyll and Photosynthetic Quantum Yield.
Cell pellets of Synechocystis were extracted with 1 mL cold methanol. After centrifugation, the supernatant was collected. Methanol extraction was repeated until the pellet turned blue. The chlorophyll contents were calculated after measuring the absorbances at 470, 665, and 720 nm, according to the equation: chlorophyll a (µg⋅mL−1) = 12.9447 * (A665 – A720) (39).
Chlorophyll fluorescence of Synechocystis cells in liquid BG-11 medium was measured by pulse-amplified modulation fluorometry (Junior PAM, Heinz Walz, Effeltrich, Germany). Synechocystis cells were dark adapted for 60 min before the measurements. Quantum yield of PSII was calculated according to the equation: (Fm−F)/Fm, where Fm and F are the fluorescence emission of dark-adapted cells under measuring light and after applying a saturating light pulse, respectively (40).
Transmission Electron Microscopy.
For comparative ultrastructural analysis, cells of Synechocystis WT and Δslr2103 mutant were collected by centrifugation and resuspended in 8% agarose. After solidification, the agarose was cut into blocks of ∼1 mm3 and used for combined conventional and microwave-assisted fixation, dehydration, and resin embedding, as defined in SI Appendix, Table S2. Sectioning and ultrastructural analysis were performed as described (41).
Data Availability Statement.
All data discussed in the paper are available in the main text and SI Appendix.
Supplementary Material
Acknowledgments
We thank Mathias Brands, Payal Patwari, and Jill Romer (Institute of Molecular Physiology and Biotechnology of Plants; University of Bonn) for their help with enzyme assays, and Claudia Riemey (IPK Gatersleben) for technical assistance in transmission electron microscopy. Funding was provided by University of Bonn.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915930117/-/DCSupplemental.
References
- 1.Hu Q., et al. , Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 54, 621–639 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Liu B., Benning C., Lipid metabolism in microalgae distinguishes itself. Curr. Opin. Biotechnol. 24, 300–309 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Martin S., Parton R. G., Lipid droplets: A unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7, 373–378 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Zhang M., Fan J., Taylor D. C., Ohlrogge J. B., DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 21, 3885–3901 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlqvist A., et al. , Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. U.S.A. 97, 6487–6492 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou J., et al. , The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. 19, 645–653 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Routaboul J. M., Benning C., Bechtold N., Caboche M., Lepiniec L., The TAG1 locus of Arabidopsis encodes for a diacylglycerol acyltransferase. Plant Physiol. Biochem. 37, 831–840 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Hobbs D. H., Lu C., Hills M. J., Cloning of a cDNA encoding diacylglycerol acyltransferase from Arabidopsis thaliana and its functional expression. FEBS Lett. 452, 145–149 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Austin J. R. 2nd, Frost E., Vidi P. A., Kessler F., Staehelin L. A., Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18, 1693–1703 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tevini M., Steinmüller D., Composition and function of plastoglobuli: II. Lipid composition of leaves and plastoglobuli during beech leaf senescence. Planta 163, 91–96 (1985). [DOI] [PubMed] [Google Scholar]
- 11.Ischebeck T., Zbierzak A. M., Kanwischer M., Dörmann P., A salvage pathway for phytol metabolism in Arabidopsis. J. Biol. Chem. 281, 2470–2477 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Gaude N., Bréhélin C., Tischendorf G., Kessler F., Dörmann P., Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. Plant J. 49, 729–739 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Lippold F., et al. , Fatty acid phytyl ester synthesis in chloroplasts of Arabidopsis. Plant Cell 24, 2001–2014 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariizumi T., et al. , Identification of the carotenoid modifying gene PALE YELLOW PETAL 1 as an essential factor in xanthophyll esterification and yellow flower pigmentation in tomato (Solanum lycopersicum). Plant J. 79, 453–465 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Schimper A. F. W., Ueber die Entwickelung der Chlorophyllkörner und Farbkörper (Botanische Zeitung, 1883) pp. 105–120. [Google Scholar]
- 16.Mereschkowsky C. S., Über Natur und Ursprung der Chromatophoren im Pflanzenreiche. Biologisches Centralblatt 25, 593–604 (1905). [Google Scholar]
- 17.van de Meene A. M. L., Hohmann-Marriott M. F., Vermaas W. F. J., Roberson R. W., The three-dimensional structure of the cyanobacterium Synechocystis sp. PCC 6803. Arch. Microbiol. 184, 259–270 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Peramuna A., Summers M. L., Composition and occurrence of lipid droplets in the cyanobacterium Nostoc punctiforme. Arch. Microbiol. 196, 881–890 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taranto P. A., Keenan T. W., Potts M., Rehydration induces rapid onset of lipid biosynthesis in desiccated Nostoc commune (Cyanobacteria). Biochim. Biophys. Acta 1168, 228–237 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Řezanka T., Lukavský J., Siristova L., Sigler K., Regioisomer separation and identification of triacylglycerols containing vaccenic and oleic acids, and α- and γ-linolenic acids, in thermophilic cyanobacteria Mastigocladus laminosus and Tolypothrix sp. Phytochemistry 78, 147–155 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Alvarez H. M., Steinbüchel A., Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 60, 367–376 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Kalscheuer R., Steinbüchel A., A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 278, 8075–8082 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Röttig A., Steinbüchel A., Acyltransferases in bacteria. Microbiol. Mol. Biol. Rev. 77, 277–321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weier D., Müller C., Gaspers C., Frentzen M., Characterisation of acyltransferases from Synechocystis sp. PCC6803. Biochem. Biophys. Res. Commun. 334, 1127–1134 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Lütke-Brinkhaus F., Weiss G., Kleinig H., Prenyl lipid formation in spinach chloroplasts and in a cell-free system of Synechococcus (Cyanobacteria): Polyprenols, chlorophylls, and fatty acid prenyl esters. Planta 163, 68–74 (1985). [DOI] [PubMed] [Google Scholar]
- 26.Li H., Sherman L. A., Characterization of Synechocystis sp. strain PCC 6803 and deltanbl mutants under nitrogen-deficient conditions. Arch. Microbiol. 178, 256–266 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Wu Q., Vermaas W. F. J., Light-dependent chlorophyll a biosynthesis upon chlL deletion in wild-type and photosystem I-less strains of the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol. Biol. 29, 933–945 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Marin K., Stirnberg M., Eisenhut M., Krämer R., Hagemann M., Osmotic stress in Synechocystis sp. PCC 6803: Low tolerance towards nonionic osmotic stress results from lacking activation of glucosylglycerol accumulation. Microbiology 152, 2023–2030 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto T., et al. , Cloning of ω 3 desaturase from cyanobacteria and its use in altering the degree of membrane-lipid unsaturation. Plant Mol. Biol. 26, 249–263 (1994). [DOI] [PubMed] [Google Scholar]
- 30.Lem N. W., Stumpf P. K., In vitro fatty acid synthesis and complex lipid metabolism in the cyanobacterium, Anabaena variabilis: II. Acyl transfer and complex lipid formation. Plant Physiol. 75, 700–704 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taroncher-Oldenburg G., Nishina K., Stephanopoulos G., Identification and analysis of the polyhydroxyalkanoate-specific β-ketothiolase and acetoacetyl coenzyme A reductase genes in the cyanobacterium Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol. 66, 4440–4448 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin F., Das D., Lin X. N., Marsh E. N. G., Aldehyde-forming fatty acyl-CoA reductase from cyanobacteria: Expression, purification and characterization of the recombinant enzyme. FEBS J. 280, 4773–4781 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Rippka R., Deruelles J., Waterbury J. B., Herdman M., Stanier R. Y., Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiol. 111, 1–61 (1979). [Google Scholar]
- 34.Proels R., Stable transformation of cyanobacterium Synechocystis sp. Bio Protoc. 4, e1286 (2014). [Google Scholar]
- 35.Sánchez M., Nicholls D. G., Brindley D. N., [The relationship between palmitoyl-coenzyme A synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria]. Biochem. J. 132, 697–706 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bligh E. G., Dyer W. J., A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- 37.Vom Dorp K., et al. , Remobilization of phytol from chlorophyll degradation is essential for tocopherol synthesis and growth of Arabidopsis. Plant Cell 27, 2846–2859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welti R., et al. , Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 277, 31994–32002 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Zavrel T., Sinetova M. A., Červený J., Measurement of chlorophyll a and carotenoids concentration in cyanobacteria. Bio Protoc. 5, e1467 (2015). [Google Scholar]
- 40.Schreiber U., Schliwa U., Bilger W., Continuous recording of photochemical and nonphotochemical quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62 (1986). [DOI] [PubMed] [Google Scholar]
- 41.Daghma D. S., Kumlehn J., Melzer M., The use of cyanobacteria as filler in nitrocellulose capillaries improves ultrastructural preservation of immature barley pollen upon high pressure freezing. J. Microsc. 244, 79–84 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper are available in the main text and SI Appendix.