Significance
Emerging evidence indicates that the receptor for growth-hormone–releasing hormone (GHRH-R) is involved in a wide spectrum of extra-pituitary activities, including tumor growth and inflammation. This study elucidates the molecular mechanism of inflammatory processes in acute ocular inflammation mediated by GHRH-R. In human ciliary epithelial cells, the expression of the GHRH-R gene is elevated after lipopolysaccharide insult through the phosphorylation of NF-κB. The activation of GHRH-R stimulates activity of the JAK2/STAT3 pathway, leading to cytokine and chemokine production. In a rat model of endotoxin-induced uveitis, and in human ciliary epithelial cells, antagonists targeting the GHRH-R/JAK2/STAT3–signaling axis alleviate partially the inflammatory responses, supporting a potential therapeutic approach to acute ocular inflammation.
Keywords: GHRH-R, inflammation, JAK2/STAT3 pathway, uveitis, LPS
Abstract
Ocular inflammation is a major cause of visual impairment attributed to dysregulation of the immune system. Previously, we have shown that the receptor for growth-hormone–releasing hormone (GHRH-R) affects multiple inflammatory processes. To clarify the pathological roles of GHRH-R in acute ocular inflammation, we investigated the inflammatory cascades mediated by this receptor. In human ciliary epithelial cells, the NF-κB subunit p65 was phosphorylated in response to stimulation with lipopolysaccharide (LPS), resulting in transcriptional up-regulation of GHRH-R. Bioinformatics analysis and coimmunoprecipitation showed that GHRH-R had a direct interaction with JAK2. JAK2, but not JAK1, JAK3, and TYK2, was elevated in ciliary body and iris after treatment with LPS in a rat model of endotoxin-induced uveitis. This elevation augmented the phosphorylation of STAT3 and production of proinflammatory factors, including IL-6, IL-17A, COX2, and iNOS. In explants of iris and ciliary body, the GHRH-R antagonist, MIA-602, suppressed phosphorylation of STAT3 and attenuated expression of downstream proinflammatory factors after LPS treatment. A similar suppression of STAT3 phosphorylation was observed in human ciliary epithelial cells. In vivo studies showed that blocking of the GHRH-R/JAK2/STAT3 axis with the JAK inhibitor Ruxolitinib alleviated partially the LPS-induced acute ocular inflammation by reducing inflammatory cells and protein leakage in the aqueous humor and by repressing expression of STAT3 target genes in rat ciliary body and iris and in human ciliary epithelial cells. Our findings indicate a functional role of the GHRH-R/JAK2/STAT3–signaling axis in acute anterior uveitis and suggest a therapeutic strategy based on treatment with antagonists targeting this signaling pathway.
Intraocular inflammation is a common ophthalmic complication that can lead to visual impairment. Uveitis is an intraocular inflammation occurring primarily in the uveal tract, which includes the iris, ciliary body, and choroid. Patients with uveitis suffer from symptoms of eye redness, pain, photophobia, excessive tearing, and blurred vision (1). If left untreated, uveitis can cause irreversible ocular tissue damage and eventually impaired vision. As a sight-threatening disease, uveitis is estimated to account for ∼10 to 15% of blindness in developed countries (2). Currently, corticosteroids are the main therapeutic agents for the treatment of uveitis although they may cause side effects such as cataracts, glaucoma, stomach ulcers, and osteoporosis (3, 4). Novel therapeutic approaches are needed.
Endotoxin-induced uveitis (EIU) is a widely used animal model for studying inflammatory cascades during pathogenesis of infectious uveitis (5). Acute ocular inflammation is initiated in rodents by lipopolysaccharide (LPS), which generates symptoms mimicking those of human anterior uveitis, such as miosis, iris hyperemia, destruction of blood-ocular barrier, and massive influx of immune cells into the aqueous humor (6). Previous studies using this animal model have identified that some cytokines, such as TNFα and IL-6, actively participate in the pathogenesis of ocular inflammation (7, 8). Nevertheless, the underlying mechanisms controlling the production of these cytokines remain elusive.
Signaling of growth hormone-releasing hormone (GHRH) is emerging as an important participant in mediating tumorigenesis and serial inflammatory processes (9, 10). As a hypothalamic neuropeptide, GHRH modulates a wide spectrum of physiological events, including reproduction, body growth, gastrointestinal function, and immune responses. GHRH and its receptor (GHRH-R) are expressed in immune cells such as leukocytes, thymocytes, and macrophages (10–13). Moreover, growing evidence indicates that the GHRH-signaling pathway actively participates in the progression of autoimmune diseases. The suppression of this signaling pathway reduces inflammation. For example, mice deficient in GHRH-R expression are less susceptible to develop inflammatory responses in an experimental model of autoimmune encephalomyelitis (11). It was also reported that GHRH-R antagonists significantly lower the prostate weight and decrease the level of inflammatory cytokines in a model of benign prostatic hyperplasia (14). Subsequent study in the mouse demonstrated that GHRH-R antagonists inhibited proliferation of prostatic epithelial cells induced by chronic inflammation and thus alleviated autoimmune prostatitis (15).
In addition to chronic inflammation, a previously unknown role of GHRH-R in acute inflammation was reported in our earlier work (10). We have shown an elevation of GHRH-R specifically in the ciliary and iris epithelial cells 24 h after treatment with LPS. Blocking the activity of GHRH-R with GHRH-R–specific antagonist alleviates significantly the LPS-induced ocular inflammation, as manifested by a reduction in infiltration of inflammatory cells and accumulation of protein and proinflammatory cytokines in the aqueous humor (10). However, the signaling mechanisms of this inflammatory cascade remain to be determined. In the present study, we investigated the signaling axis of GHRH-R and found evidence showing how GHRH-R is up-regulated after stimulation of LPS in human ciliary epithelial cells and the contribution of a noncanonical signaling axis of GHRH-R that involves JAK2 and STAT3 in acute ocular inflammation.
Results
LPS Activates Expression of GHRH-R through Transcriptional Regulation of NF-κB Subunit p65 in Human Ciliary Epithelial Cells.
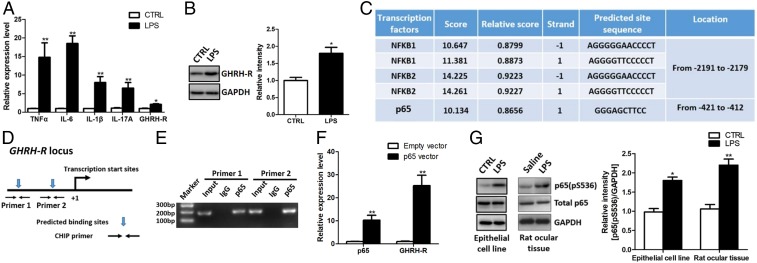
Our previous study showed that GHRH-R expression is up-regulated in ciliary epithelium of the eye in adult rats during LPS-induced inflammation (10). In the present study we first utilized human nonpigmented ciliary epithelial cells to verify whether this pattern of changes in GHRH-R expression can be observed in a corresponding human cell type. Consistent with previous findings in rats, we found that these epithelial cells are susceptible to a LPS insult. LPS at the dose of 100 ng/mL triggered dramatic up-regulation of TNFα, IL-6, IL-1β, and IL-17A in 24 h (Fig. 1A). Furthermore, both RT-PCR and Western blot analyses showed a concomitant increase in the level of GHRH-R (Fig. 1 A and B). Previous work has shown that LPS binds directly to its receptor TLR4 and initiates the activation of multiple transcription factors including NF-κB, AP-1, and IRF3 (16). By using the bioinformatics tool JASPAR we examined the potential interactions of these transcription factors with GHRH-R promoter and identified two potential binding sites in the promoter sequence of a GHRH-R gene with high scores for NF-κB family, but not for AP-1 and IRF3 (Fig. 1C). We designed primers covering these predicted binding sites (Fig. 1D) for chromatin immunoprecipitation (ChIP) assays. The results show that the NF-κB subunit p65 pulled down DNA fragments from predicted binding sites in the GHRH-R promoter region (Fig. 1E). Furthermore, the ectopic expression of NF-κB subunit p65 dramatically increased the messenger RNA (mRNA) level for GHRH-R in human ciliary epithelial cells (Fig. 1F), indicating that p65 is a positive regulator of GHRH-R expression. We also examined the phosphorylation status of p65 after LPS challenge and found that LPS could induce a significant increase in p65 phosphorylation in human ciliary epithelial cells and in ciliary body and iris of rats (Fig. 1G). These results indicate that LPS enhances expression of GHRH-R in ciliary epithelial cells by phosphorylating NF-κB that binds to the promoter sequence of GHRH-R.
Fig. 1.
NF-κB subunit p65 transcriptionally activates GHRH-R expression in human ciliary epithelial cells. (A) RT-PCR results showed that LPS treatment elevated the RNA levels of inflammation-related factors in human ciliary epithelial cells (n = 5). (B) GHRH-R was up-regulated in human ciliary epithelial cells after LPS treatment (n = 5). (C) Bioinformatics prediction of putative binding sites for the NF-κB family in the GHRH-R promoter region. (D) Schematic illustration of the primer design in the ChIP assay. (E) ChIP assay showed that p65 antibody pulled down the DNA fragments from the GHRH-R promoter region (n = 3). (F) RT-PCR showed that ectopic expression of p65 activated GHRH-R expression (n = 5). (G) LPS treatment induced p65 phosphorylation in vitro and in vivo (n = 5). *P < 0.05; **P < 0.01.
GHRH-R Physically Interacts with JAK2.
To elucidate the downstream signaling of GHRH-R, we used two independent bioinformatics tools (PrePPI and FpClass) to screen for the possible protein partners that may interact with the receptor (Fig. 2A). We identified eight common candidate genes. Among them, JAK2 is of special interest due to its biological importance in modulating a variety of immune responses (17). Coimmunoprecipitation (co-IP) assay verified that JAK2 successfully pulled down the GHRH-R protein from lysates of human ciliary epithelial cells, as well as from lysates of rat ocular tissues (Fig. 2B), showing that this mutual interaction is highly conserved across different species. Moreover, immunohistochemical results showed a colocalization of JAK2 with GHRH-R in the rat ciliary epithelium and posterior epithelium of the iris after LPS treatment (Fig. 2C and SI Appendix, Fig. S1), confirming the spatial correlation of JAK2 and GHRH-R in these ocular tissues.
Fig. 2.
GHRH-R physically interacts with JAK2. (A) The bioinformatics prediction of potential GHRH-R protein partners. (B) A co-IP assay showed that JAK2 antibody pulled down GHRH-R protein in the human ciliary epithelial cells and rat ciliary body and iris. (C) Immunofluorescence staining showed that JAK2 was colocalized with GHRH-R in the rat ciliary body and iris. AC, anterior chamber; PC, posterior chamber. (Scale bar, 50 µm.)
JAK2/STAT3-Signaling Pathway in Rats Is Activated with EIU.
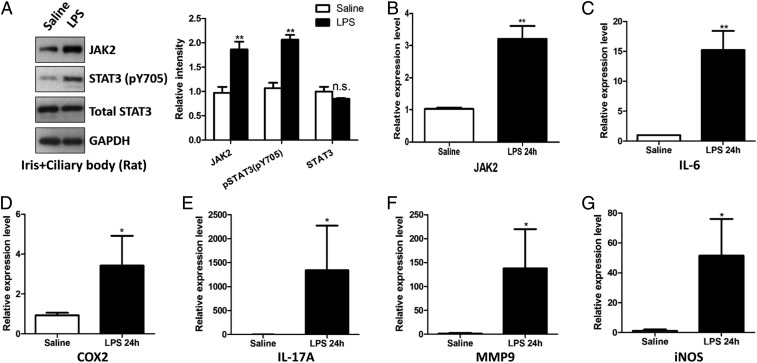
As an important immunomodulatory molecule, JAK2 can recruit and phosphorylate STAT3, which is known as the master transcription factor of inflammatory genes (18). We examined these JAK2/STAT3-signaling cascades in rats induced with EIU. Western blotting demonstrated that protein levels of JAK2 and phosphorylated STAT3 were elevated in the ciliary body and iris 24 h after treatment with LPS (Fig. 3A). The RNA levels of JAK2 and downstream target genes of STAT3, including IL-6, COX2, IL-17A, iNOS, and MMP9, were all increased significantly in these ocular tissues (Fig. 3 B–G), indicating the that JAK2/STAT3-signaling pathway was constitutively active during LPS-induced acute ocular inflammation.
Fig. 3.
Activation of the JAK2/STAT3-signaling pathway in animals with EIU. (A) Western blotting results showed that the JAK2/STAT3 pathway was activated 24 h after LPS treatment. (B) RT-PCR analysis showed an increased mRNA level of JAK2 after LPS treatment. (C–G) RT-PCR results showed that selected targets downstream of STAT3 were elevated in expression after LPS treatment. *P < 0.05; **P < 0.01; n.s., not significant; n = 6.
The Effects of Modulating GHRH-R Activities in the Signaling Pathway of JAK2/STAT3.
Given that GHRH-R/JAK2 may potentiate gene transcription of proinflammatory factors through phosphorylation of STAT3, we next examined the effect of modulating GHRH-R on activity of STAT3 in human ciliary epithelial cells. Western blotting showed that the GHRH-R agonist MR-409 (1 µM) potentiated phosphorylation of STAT3 while its antagonist, MIA-602 (1 µM), inhibited it (Fig. 4 A and B). A rescue experiment using the JAK inhibitor Ruxolitinib (at 1 µM) demonstrated that blocking JAK activity significantly suppressed STAT3 phosphorylation and that this inhibition was significantly attenuated by activating GHRH-R with MR-409 (Fig. 4C). Since the phosphorylation of STAT3 is tightly linked to its transcriptional activity, we examined whether suppression of GHRH-R activity could alleviate the expression of STAT3 target genes induced by LPS in an explant culture of ciliary body and iris isolated from the eye of adult rats. The ocular tissues were prepared after perfusion of the animal with sterile saline to remove most cells from the blood vessels. These tissues were cultured on a membrane in the upper chamber of a transwell, whereas LPS (100 ng/mL) and the GHRH-R antagonist MIA-602 (1 µM) were added to the medium in the lower chamber (Fig. 5A). RT-PCR analysis showed that LPS alone triggered increased expression of STAT3-targeted genes, which include IL-6, IL-17A, COX2, iNOS, and MMP9. MIA-602 attenuated elevation of all these proinflammatory mediators (Fig. 5 B–F). These results are consistent with our previous in vivo findings showing that the GHRH-R antagonist alleviates experimentally induced ocular inflammation by reducing production and secretion of cytokines in ciliary body and iris (10), and our results also demonstrate that GHRH-R mediates the expression of proinflammatory mediators through the JAK2/STAT3-signaling pathway.
Fig. 4.
The effect of GHRH-R activities on modulating STAT3 phosphorylation. (A) The GHRH-R agonist MR-409 (1 µM) increased phosphorylation of STAT3 in human ciliary epithelial cells. (B) The GHRH-R antagonist MIA-602 (1 µM) suppressed STAT3 phosphorylation. (C) MR-409 attenuated the Ruxolitinib-mediated inhibition on STAT3. *P < 0.05; **P < 0.01; n.s., not significant; n = 4.
Fig. 5.
The GHRH-R antagonist MIA-602 attenuates the expression of downstream targets of STAT3 in explant culture of ciliary body and iris. (A) Schematic illustration of the explant culture system. LPS and MIA-602 were added to the lower chamber. (B–F) MIA-602 repressed the RNA levels of selected downstream targets of STAT3, which include IL-6, COX2, IL-17A, MMP9, and iNOS. *P < 0.05; **P < 0.01; n = 6.
JAK Inhibitor Suppresses LPS-Induced Ocular Inflammation.
To test whether the blocking of the JAK2/STAT3 pathway alleviates ocular inflammation induced by LPS, we investigated antiinflammatory effects of Ruxolitinib, a potent inhibitor of JAK, including JAK2, in adult rats with EIU. Western blotting confirmed that Ruxolitinib at the dose of 16 mg/kg significantly suppressed phosphorylation of p65 and STAT3 induced by LPS in ciliary body and iris, whereas the effect on JAK2 was not significant (Fig. 6A). The suppression was more significant for STAT3 than for p65, as phosphorylated STAT3 was effectively inhibited at a low dose (8 mg/kg) of Ruxolitinib, but such an effect was not observed for p65 at this dosage. Moreover, the level of GHRH-R was reduced significantly after treatment with Ruxolitinib. Oral administration of Ruxolitinib reduced dose-dependently the elevation in number of inflammatory cells and total protein level in the aqueous humor 24 h after LPS treatment (Fig. 6 B and C). These effects were further confirmed by histological examination, which showed a substantial reduction in infiltrating immune cells and accumulation of protein in the aqueous humor after treatment with Ruxolitinib (SI Appendix, Fig. S2). However, these antiinflammatory actions were partial, as there was no obvious reduction in cellular infiltrates in the cornea, limbus, and vitreous body. RT-PCR analysis showed that treatment with Ruxolitinib reduced expression of STAT3 target genes, which included IL-6, IL-17A, COX2, and iNOS, in ciliary body and iris (Fig. 6 D–H). Similar effects of Ruxolitinib were also observed in human ciliary epithelial cells in which phosphorylation of STAT3 induced by LPS was virtually abolished by Ruxolitinib and expression of proinflammatory mediators regulated by STAT3 was highly suppressed (SI Appendix, Fig. S3). As Ruxolitinib is known to inhibit other kinases including JAK1, JAK3, and TYK2, we examined the expression level of these genes in ciliary body and iris. The results showed that LPS did not cause a significant change in the expression of JAK1, JAK3, and TYK2 (SI Appendix, Fig. S4). These expression patterns are different from JAK2, which is markedly elevated, and support that JAK2 is likely the major mediator and primary target of Ruxolitinib in this inflammatory process.
Fig. 6.
The JAK2 inhibitor Ruxolitinib suppresses ocular inflammation in animals with EIU. (A) Ruxolitinib suppressed STAT3 phosphorylation in rats with EIU. (B) LPS-induced cell infiltration was suppressed dose-dependently by Ruxolitinib. (C) LPS-induced protein exudation in the aqueous humor was reduced by Ruxolitinib. (D–H) Ruxolitinib repressed the RNA levels of selected STAT3 downstream targets. *P < 0.05; **P < 0.01; n.s., not significant; n = 6.
Discussion
In this study we elucidate the signaling mechanism of GHRH-R in mediating the inflammatory cascades during LPS-induced acute ocular inflammation. The major findings include the following: 1) LPS elevates expression of GHRH-R in human ciliary epithelial cells; 2) the downstream effector of LPS/TLR4, NF-κB, binds directly to the promoter regions of the GHRH-R gene and positively modulates gene transcription; 3) GHRH-R physically interacts with JAK2 and potentiates STAT3 phosphorylation; 4) the GHRH-R antagonist MIA-602 alleviates elevation of proinflammatory mediators regulated by STAT3 in explant culture of ciliary body and iris after treatment with LPS; and 5) blocking of JAK2 activity with Ruxolitinib reduces cell infiltration and protein exudation in the aqueous humor, along with down-regulation of proinflammatory mediators in the ocular tissues during inflammation. We conclude that ciliary epithelial cells are capable of responding directly to LPS and producing proinflammatory factors and that this inflammatory response is mediated by the TLR4/NF-κB/GHRH-R/JAK2/STAT3– signaling axis. A summary diagram depicting these signaling events is shown in Fig. 7.
Fig. 7.
Summary diagram showing the signaling cascades mediated by GHRH-R in LPS-induced acute inflammation in ciliary epithelial cells.
Our earlier study has shown that GHRH-R is elevated specifically in the epithelium of ciliary body and iris after a LPS insult, which is associated with production of proinflammatory factors that causes influxes of protein and inflammatory cells from the blood vessels into the aqueous humor (10). These inflammatory reactions are likely caused by an activation of LPS receptor TLR4, which has been shown to express on the ciliary and iris epithelial cells, and the antigen-presenting cells in the stroma (19). Activation of TLR4 in resident macrophages and other antigen-presenting cells in the tissues and the systemic circulation triggers phosphorylation of NF-κB, which is translocated into the nucleus and switches on gene expression of proinflammatory factors (8, 20, 21). In the present study we show that, in addition to the immune cells, human ciliary epithelial cells alone, without the influence of other antigen-presenting cells, are able to respond to LPS by phosphorylating the NF-κB subunit p65 and up-regulating expression of proinflammatory factors, playing a role similar to other antigen-presenting cells. Using co-IP, we show further a direct interaction of phosphorylated NF-κB with promoter regions of the GHRH-R gene. Overexpression of p65-NF-κB results in an increased expression of GHRH-R, demonstrating the signaling mechanism that up-regulates GHRH-R after LPS insult in the ciliary epithelial cells.
Another major finding is that we have defined the signaling cascades that mediate GHRH-R and production of proinflammatory factors. Our earlier study has shown that expression of GHRH is also elevated specifically in the ciliary body and iris after LPS treatment (10), which probably stimulates the GHRH-R on the epithelial cells and the downstream cascades through an autocrine and/or paracrine action. This regulatory mechanism has been demonstrated in cancer cell lines that express high levels of GHRH-R, in which knocking down of GHRH causes a substantial inhibition of cell growth that is reversed with exogenous GHRH (22). We show in the present study that GHRH-R signals directly through JAK2/STAT3 to mediate the inflammatory responses in human ciliary epithelial cells. The signaling mechanism is supported by the findings that JAK2 is a protein partner of GHRH-R in human ciliary epithelial cells and is colocalized with GHRH-R in the epithelial cells of ciliary body and iris. Additional supports are obtained from human ciliary epithelial cells in which the GHRH-R agonist triggers phosphorylation of STAT3, while suppression of receptor activity with antagonist reduces the phosphorylation in ciliary epithelial cells. Phosphorylation of STAT3 is suppressed by pharmacological inhibition of JAK2, which can be reverted by the GHRH-R agonist, demonstrating a role of these protein partners in the regulation of STAT3 activities. Functional analysis in explant cultures of ciliary body and iris shows that blocking of GHRH-R with antagonist reduces significantly the elevated expression of proinflammatory genes known to be regulated by STAT3. Furthermore, analysis in animals with EIU confirms that the elevated level of GHRH-R recruits and activates JAK2 that promotes phosphorylation of STAT3 in ciliary body and iris. This phosphorylated transcription factor switches on expression of proinflammatory genes such as IL-6, COX2, IL-17A, iNOS, and MMP9, which generates another wave of production of proinflammatory mediators (Fig. 7). The expression of these STAT3-regulated genes is suppressed by treatment with JAK inhibitor, which is accompanied by a reduction of expression of GHRH-R, supporting strongly the contribution of the GHRH-R/JAK2/STAT3–signaling axis in acute inflammation in anterior segments of the eye. Stimulation of GHRH-R is known to activate the adenyl cyclase/cAMP/PKA pathway that leads eventually to the synthesis and release of growth hormone (23), which likely underlies the secretion of growth hormone from the ciliary body and iris into the aqueous humor as reported in our earlier study (10). It remains to be determined whether this cAMP-dependent mechanism may regulate the JAK2/STAT3 pathway in the production of proinflammatory factors.
In this study we focused on the GHRH-R–signaling pathway, but did not rule out the contribution of other molecules like IL6 and its receptor that signal through JAK2/STAT3 in ocular inflammation. IL6 and its downstream pathway have been well documented in acute inflammation (24). Other relevant receptors including leptin, thrombopoietin, and angiotensin receptor 1 may also play important roles (25–27). However, the role of GHRH-R in acute inflammation remains poorly understood. Our results have characterized a noncanonical role of the GHRH-R–signaling pathway in mediating inflammation in the eye. The actions of GHRH in enhancing phosphorylation of STAT3 and JAK2 have been reported in HeLa cancer cells (28). However, how GHRH activates STAT3 phosphorylation is unknown since GHRH-R is not a kinase and apparently it cannot directly potentiate STAT3 phosphorylation. Here we show that the regulation of STAT3 is mediated through JAK2 that binds directly to GHRH-R. As other kinases like EGFR and Src participate in the phosphorylation of STAT3, we narrowed down the range of signaling molecules through bioinformatics analysis and identified JAK2 as a partner of GHRH-R. In another study the GHRH-R antagonist MIA-602 has been shown to effectively suppress in vivo and in vitro proliferation of gastric cancer through inhibiting PAK1-mediated STAT3 signaling (29). Apart from malignant cells, the antagonist also attenuates STAT3 phosphorylation in mesenchymal stem cells derived from mouse bone marrow (30). Our results provide a insight into this signaling cascade, demonstrating a role of JAK2 in mediating the function of GHRH-R signaling in inflammatory responses of the ciliary epithelial cells.
Ruxolitinib has been shown to protect EIU and has demonstrated strong inhibitory effects against JAK1 and JAK2 (with IC50 of 3.3 and 2.8 nM, respectively), moderate inhibitory activity against TYK2 (with an IC50 of 19 nM), and minimal inhibitory activity against JAK3 (with an IC50 of 428 nM). Thus, in the in vivo model, Ruxolitinib is likely inhibiting selectively JAK1 and JAK2 in the eye. We have conducted RT-PCR to examine the expression of JAK1/2/3 and TYK2 in control animals and animals with EIU. We found that only JAK2, but not the other kinases, is up-regulated in the ciliary body and iris after LPS treatment, supporting that JAK2 is likely the primary target of Ruxolitinib in this antiinflammatory action. We have demonstrated further that JAK2 mediates the STAT3 activity in the production of proinflammatory factors through a direct interaction with GHRH-R and that STAT3 phosphorylation and cytokine production are regulated by GHRH-R activities. Although JAK2 is likely the primary target, we cannot rule out the contribution of other kinases targeted by Ruxolitinib. Ruxolitinib has been approved by the Food and Drug Administration for the treatment of myelofibrosis and polycythemia vera (31). A previous report has shown that Ruxolitinib exerts antiinflammatory effects through interruption of the IL-17 signaling, playing a potential role in infectious and noninfectious uveitis (32). In line with this finding, a reduction in gene expression of IL-17A is found after treatment with Ruxolitinib in the iris and ciliary body. Furthermore, a recent report has demonstrated a case of using another JAK inhibitor, Tofacitinib, in the treatment of refractory uveitis and scleritis (33), supporting the potential application of JAK inhibitors in the treatment of ocular inflammation.
These findings demonstrate the potential of targeting the GHRH-R/JAK2/STAT3 axis as a therapeutic approach to the treatment of acute uveitis. In fact, we have shown in this study that blocking this signaling axis with GHRH-R antagonist attenuates the expression of inflammatory mediators regulated by STAT3 in explant cultures of ciliary body and iris. Moreover, pharmacological inhibition of JAK function in animals with EIU suppresses phosphorylation of STAT3 in ciliary body and iris, reducing expression of proinflammatory factors, accumulation of inflammatory cells, and leakage of protein into the aqueous humor. These findings support the contribution of GHRH-R/JAK2/STAT3 signaling to the pathogenesis of acute uveitis. Inhibition of JAK activity also suppresses expression of p65 NF-κB, suggesting a role of JAK in regulating NF-κB activity. Furthermore, blocking of JAK activity is more effective in improving infiltration of immune cells into the aqueous humor, but less so in reducing the exudation of protein from the ocular vessels. Similar effects are observed in rats with EIU after treatment with the GHRH-R antagonist MIA-602 (10), suggesting that the GHRH-R/JAK2/STAT3 signaling contributes to recruitment and mobilization of macrophages and leukocytes from the systemic circulation into the aqueous humor. This specific action could be caused by a reduced production of cytokines and chemokines from the ciliary epithelium due to blocking of GHRH-R signaling. Another possibility is that the GHRH-R antagonist or JAK inhibitor reduces motility of the immune cells, which also up-regulates expression of GHRH-R when they accumulate in the aqueous humor (10). The latter view is supported by early studies, which show that GHRH accelerates wound healing and tissue repair by stimulating the migration of wound-associated fibroblasts (34). In addition, GHRH reduces cell adhesion and promotes migration of prostate cancer cells (35), whereas GHRH-R antagonist impairs motility of endometrial cancer cells (36). As GHRH-R is expressed ubiquitously in a wide spectrum of immune cells, including macrophages, leukocytes, and lymphocytes (37), it is possible that GHRH-R may promote the migration of immune cells toward the aqueous humor, causing further production of proinflammatory mediators and thus augmenting the inflammation.
In conclusion, the present study elucidates the molecular mechanisms that regulate expression of GHRH-R in LPS-induced ocular inflammation and outlines a noncanonical signaling pathway of GHRH-R in controlling production of proinflammatory factors in ciliary epithelial cells. Our study reveals a previously uncharacterized role of the GHRH-R/JAK2/STAT3 axis in acute ocular inflammation and demonstrates that blocking of this signaling axis is effective in improving inflammatory responses in experimentally induced acute anterior uveitis. We also provide evidence that the ciliary epithelial cells play a role of immune surveillance in anterior segments of the eye similar to that of other antigen-presenting cells.
Materials and Methods
Cell Culture and Cell Transfection.
The human nonpigmented ciliary epithelial cells (HNPCEpiC), which were isolated from human ciliary body, were purchased from ScienCell Research Laboratories. Isolation efficiency is characterized by immunofluorescence with antibodies specific to epithelial markers cytokeratin-18 and -19. HNPCEpiC are confirmed to be negative for HIV-1, hepatitis B virus, hepatitis C virus, mycoplasma, bacteria, yeast, and fungi. To avoid spontaneous cellular senescence, we limited the use of cells within six passages. The HNPCEpiC were maintained in Epithelial Cell Medium (ScienCell) with 2% fetal bovine serum (FBS), 1% epithelial cell growth supplement (ScienCell), and 1% penicillin/streptomycin. The transfection using Lipofectamine 2000 was conducted according to the manufacturer’s instructions (Life Technologies). Briefly, the cells were seeded onto six-well plates and then transfected with 2 μg GFP-RelA (Addgene, no. 23255) plasmid plus 1 μL Lipofectamine 2000. Two days post transfection, the cells were collected for the indicated experiments.
Peptides and Chemicals.
The GHRH-R antagonist MIA-602 and the GHRH agonist MR-409 were synthesized by the A.V.S. laboratory as previously described (10, 29, 38). The JAK2 inhibitor Ruxolitinib was purchased from ApexBio. These peptides and chemicals were dissolved in dimethylsulfoxide (DMSO) (Sigma-Aldrich) and used in the final concentration of 1 μM. LPS (from Salmonella enterica serotype typhimurium) was purchased from Sigma-Aldrich (L2262). LPS was dissolved in pyrogen-free saline and used in the final concentration of 100 ng/mL.
EIU Model and Drug Treatment.
All of the animal studies were conducted in accord to the guidelines of Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Ophthalmic and Vision Research. Ethics approval was obtained from the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong. Male Sprague-Dawley rats weighing 210 to 250 g were obtained from the Laboratory Animal Service Center of the Chinese University of Hong Kong. They were housed in a 12:12-h light:dark cycle room and were allowed to freely access food and water. EIU was induced by a footpad injection of LPS at the dose of 1 mg/kg following our previous investigations (10, 19). The JAK2 inhibitor Ruxolitinib was suspended in 25 µL DMSO and then resuspended in 475 µL distilled water. Ruxolitinib was fed intragastrically into the rat at the dose of 8 mg/kg or 16 mg/kg. The rats were fed with Ruxolitinib twice at 2 and 6 h after LPS injection. The rats were euthanized at 24 h post injection. The ciliary body and iris tissues were collected for RT-PCR and Western blotting analysis.
Hematoxylin and Eosin Staining.
After deep anesthesia, the rats were perfused intracardially with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. The eyes were collected and further fixed in 10% formalin for 24 h at 4 °C. The eyes were embedded in paraffin, and 5-μm sections of the tissues were cut along the pupil-optic nerve position. After deparaffinization and rehydration, the sections were stained with hematoxylin and eosin.
Ocular Tissue Explant Culture.
The rats were anesthetized and then perfused with 150 mL sterile saline to remove the peripheral blood cells. The iris and ciliary body were isolated from the eyeball and cultured on a membrane with a pore size of 0.4 μm in a transwell (Corning) for 24 h. The tissues were cultured in the upper chamber and maintained in RPMI medium 1640 (Gibco) plus 10% FBS and 1% penicillin/streptomycin in the lower chamber. LPS and the GHRH-R antagonist MIA-602 were added into the lower chamber at the final concentration of 100 ng/mL and 1 μM, respectively.
Western Blotting.
The cells and tissues were lysed in radioimmunoprecipitation assay buffer and resolved by 12% sodium dodecyl sulfate/polyacrylamide gel electrophoresis. The protein was stained by using antibody against GHRH-R (1:1,000, ab28692), JAK2 (1:200, sc390539), Phospho-Stat3 (1:1,000, CST9145), STAT3 (1:1,000, S21320), p65 (1:1,000, sc372), p65-Ser536 (1:500, YP0191), and GAPDH (1:200,000, AM4300). The expression levels were visualized under enhanced chemiluminescence and quantified by the software ImageJ.
Real-Time PCR.
The total RNA was extracted by RNA Extraction Kit (Favorgen Biotech) and converted into complementary DNA (cDNA) by a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) following the manufacturer’s instructions. The SYBR Green PCR Master Mix (Applied Biosystems) was used for the quantitation of target genes. The relative fold changes of indicated genes were calculated the using 2-ΔΔCT method. The primer sequences are listed in SI Appendix, Table S1.
Bioinformatics Analysis.
The 4-kb DNA sequence of human GHRH-R gene promoter was obtained from the UCSC Genome Browser (https://genome.ucsc.edu/). The DNA sequence was analyzed by the online database JAPAR (http://jaspar.genereg.net/) to identify the potential transcription factor-binding sites. The candidate protein partners of GHRH-R were predicted by two online bioinformatics programs: PrePPI (http://honig.c2b2.columbia.edu/preppi/) and FpClass (http://dcv.uhnres.utoronto.ca/FPCLASS/ppis/).
ChIP.
The ChIP assay was conducted as previously described (39). Briefly, after cross-linking with 1% formaldehyde, the cells were lysed by cold immunoprecipitation (IP) buffer (150 mM NaCl, 50 mM Tris⋅HCl, 5 mM ethylenediaminetetraacetic acid [EDTA]), 0.5% Nonidet P-40, 1% Triton X-100), and the nuclear pellets were isolated by centrifugation. After sonication, 1 μg p65 antibody (sc372) was incubated with the chromatin samples overnight at 4 °C with gentle shaking. The chromatin samples were then incubated with protein G beads (GE Healthcare) at 4 °C for 2 h. After several washes with cold IP buffer, 10% Chelex 100 slurry (Bio-Rad) was added to the beads and then boiled for 10 min. After centrifugation, the supernatant was harvested for PCR analysis. The ChIP primer sequences were designed by Primer 3 software (http://primer3.ut.ee/) and are listed in SI Appendix, Table S1.
Co-IP.
The cells and tissues were lysed by cold lysis buffer (1% Triton X-100, 50 mM Tris-7.5, 1 mM EDTA, 150 mM NaCl, and protease inhibitors). After sonication and centrifugation, the supernatant from cell lysates was collected and incubated with 1 μg JAK2 antibody (sc390539) overnight at 4 °C. The washed protein G beads were incubated with the lysates at 4 °C for 2 h. After incubation, the beads were washed by cold lysis buffer. The buffer was removed, and protein loading buffer was added to the beads. The beads and protein-loading buffer were boiled, and the supernatants were subjected to Western blotting analysis.
Immunostaining.
Before performing the immunostaining, slides were processed with antigen retrieval in Tris-EDTA Buffer (1 mM EDTA Solution, 10 mM Tris Base, 0.05% Tween 20, pH 9.0) at 110 °C for 10 min. After washing with distilled water, the slides were incubated with 10% normal goat serum in PBS with 0.01% Triton X-100. Primary antibodies against GHRH-R (ab28692, 1:80), TYK2 (sc5271, 1:100), JAK1 (sc376996,1:100), and JAK2 (sc390539, 1:100) were diluted in 1% normal goat serum and incubated with the slides overnight at 4 °C. Following incubation with secondary antibody, the fluorescence signals were captured by confocal microscopy (FV300, Olympus).
Cell Counting and Protein Concentration Measurement of Aqueous Humor.
The experiments were conducted as previously described (10). Aqueous humor was obtained from the anterior chambers with insulin syringe and subsequently subjected to 10-fold dilution with PBS. The number of cells was counted by using a hemocytometer under a microscope. The remaining undiluted aqueous humor was centrifuged at 2,500 × g for 10 min at 4 °C, and the protein concentration of supernatant was measured by the Bio-Rad assay kit.
Statistical Analysis.
Experimental data are expressed as mean ± SEM and analyzed by Prism 5. Mann–Whitney U tests were used for paired comparisons. Kruskal–Wallis tests were used to compare multiple groups. The differences were considered to be statistically significant when P < 0.05.
Data Availability.
All data discussed in this paper are available in the main text and SI Appendix.
Supplementary Material
Acknowledgments
This work was supported in part by the Research Grant Council General Research Fund (project no. CUHK14113815) (to S.O.C.). The work of A.V.S. was supported by the Medical Research Service of the Veterans Affairs Department.
Footnotes
Competing interest statement: A.V.S. is listed as co-inventor on the patents on GHRH agonists and antagonists that are assigned to the University of Miami and the Veterans Administration.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904532117/-/DCSupplemental.
References
- 1.Caspi R. R., A look at autoimmunity and inflammation in the eye. J. Clin. Invest. 120, 3073–3083 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durrani O. M., et al. , Degree, duration, and causes of visual loss in uveitis. Br. J. Ophthalmol. 88, 1159–1162 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee F. F., Foster C. S., Pharmacotherapy of uveitis. Expert Opin. Pharmacother. 11, 1135–1146 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Jabs D. A., et al. , Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am. J. Ophthalmol. 130, 492–513 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum J. T., McDevitt H. O., Guss R. B., Egbert P. R., Endotoxin-induced uveitis in rats as a model for human disease. Nature 286, 611–613 (1980). [DOI] [PubMed] [Google Scholar]
- 6.Okumura A., Mochizuki M., Endotoxin-induced uveitis in rats: Morphological and biochemical study. Jpn. J. Ophthalmol. 32, 457–465 (1988). [PubMed] [Google Scholar]
- 7.Bucolo C., Cuzzocrea S., Mazzon E., Caputi A. P., Effects of cloricromene, a coumarin derivative, on endotoxin-induced uveitis in Lewis rats. Invest. Ophthalmol. Vis. Sci. 44, 1178–1184 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Smith J. R., Hart P. H., Williams K. A., Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol. Cell Biol. 76, 497–512 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Barabutis N., Schally A. V., Growth hormone-releasing hormone: Extrapituitary effects in physiology and pathology. Cell Cycle 9, 4110–4116 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Qin Y. J., et al. , Antagonist of GH-releasing hormone receptors alleviates experimental ocular inflammation. Proc. Natl. Acad. Sci. U.S.A. 111, 18303–18308 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikushima H., Kanaoka M., Kojima S., Cutting edge: Requirement for growth hormone-releasing hormone in the development of experimental autoimmune encephalomyelitis. J. Immunol. 171, 2769–2772 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Weigent D. A., Blalock J. E., Immunoreactive growth hormone-releasing hormone in rat leukocytes. J. Neuroimmunol. 29, 1–13 (1990). [DOI] [PubMed] [Google Scholar]
- 13.Guarcello V., Weigent D. A., Blalock J. E., Growth hormone releasing hormone receptors on thymocytes and splenocytes from rats. Cell. Immunol. 136, 291–302 (1991). [DOI] [PubMed] [Google Scholar]
- 14.Rick F. G., et al. , Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc. Natl. Acad. Sci. U.S.A. 108, 3755–3760 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popovics P., Schally A. V., Salgueiro L., Kovacs K., Rick F. G., Antagonists of growth hormone-releasing hormone inhibit proliferation induced by inflammation in prostatic epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 114, 1359–1364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gay N. J., Gangloff M., Structure and function of Toll receptors and their ligands. Annu. Rev. Biochem. 76, 141–165 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Villarino A. V., Kanno Y., Ferdinand J. R., O’Shea J. J., Mechanisms of Jak/STAT signaling in immunity and disease. J. Immunol. 194, 21–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H., Pardoll D., Jove R., STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J. L., et al. , Growth hormone-releasing hormone receptor mediates cytokine production in ciliary and iris epithelial cells during LPS-induced ocular inflammation. Exp. Eye Res. 181, 277–284 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Kawai T., Akira S., The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 11, 373–384 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Heiligenhaus A., Thurau S., Hennig M., Grajewski R. S., Wildner G., Anti-inflammatory treatment of uveitis with biologicals: New treatment options that reflect pathogenetic knowledge of the disease. Graefes Arch. Clin. Exp. Ophthalmol. 248, 1531–1551 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Barabutis N., Schally A. V., Knocking down gene expression for growth hormone-releasing hormone inhibits proliferation of human cancer cell lines. Br. J. Cancer 98, 1790–1796 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schally A. V., Varga J. L., Engel J. B., Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat. Clin. Pract. Endocrinol. Metab. 4, 33–43 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Garbers C., Aparicio-Siegmund S., Rose-John S., The IL-6/gp130/STAT3 signaling axis: Recent advances towards specific inhibition. Curr. Opin. Immunol. 34, 75–82 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Horiuchi M., et al. , Stimulation of different subtypes of angiotensin II receptors, AT1 and AT2 receptors, regulates STAT activation by negative crosstalk. Circ. Res. 84, 876–882 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Kurihara T., et al. , Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Invest. Ophthalmol. Vis. Sci. 47, 5545–5552 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Abella V., et al. , Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 13, 100–109 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Siejka A., Schally A. V., Barabutis N., Activation of Janus kinase/signal transducer and activator of transcription 3 pathway by growth hormone-releasing hormone. Cell. Mol. Life Sci. 67, 959–964 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gan J., et al. , Growth hormone-releasing hormone receptor antagonists inhibit human gastric cancer through downregulation of PAK1-STAT3/NF-κB signaling. Proc. Natl. Acad. Sci. U.S.A. 113, 14745–14750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma Q., et al. , Profound actions of an agonist of growth hormone-releasing hormone on angiogenic therapy by mesenchymal stem cells. Arterioscler. Thromb. Vasc. Biol. 36, 663–672 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verstovsek S., et al. , Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 363, 1117–1127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guedes M. C., Borrego L. M., Proença R. D., Roles of interleukin-17 in uveitis. Indian J. Ophthalmol. 64, 628–634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paley M. A., Karacal H., Rao P. K., Margolis T. P., Miner J. J., Tofacitinib for refractory uveitis and scleritis. Am. J. Ophthalmol. Case Rep. 13, 53–55 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dioufa N., et al. , Acceleration of wound healing by growth hormone-releasing hormone and its agonists. Proc. Natl. Acad. Sci. U.S.A. 107, 18611–18615 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muñoz-Moreno L., Bajo A. M., Prieto J. C., Carmena M. J., Growth hormone-releasing hormone (GHRH) promotes metastatic phenotypes through EGFR/HER2 transactivation in prostate cancer cells. Mol. Cell. Endocrinol. 446, 59–69 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Wu H. M., et al. , Growth hormone-releasing hormone antagonist inhibits the invasiveness of human endometrial cancer cells by down-regulating twist and N-cadherin expression. Oncotarget 8, 4410–4421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weigent D. A., Lymphocyte GH-axis hormones in immunity. Cell. Immunol. 285, 118–132 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Zarandi M., et al. , Synthesis and structure-activity studies on novel analogs of human growth hormone releasing hormone (GHRH) with enhanced inhibitory activities on tumor growth. Peptides 89, 60–70 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Nelson J. D., Denisenko O., Bomsztyk K., Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat. Protoc. 1, 179–185 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in this paper are available in the main text and SI Appendix.