Significance
New drugs targeting multiple Plasmodium parasite life stages are desperately needed to combat malaria drug resistance. The antihistamine clemastine inhibits multiple parasite forms with previously unknown molecular targets. We utilized a parallel chemoproteomic strategy to interrogate the mechanism of action of clemastine based on the global thermal and chemical protein denaturation profiling and found that clemastine binds to and destabilizes the essential Plasmodium chaperonin TRiC/CCT. Treatment with clemastine affects the levels and morphology of the major TRiC substrate tubulin during Plasmodium asexual reproduction. Our discovery that clemastine selectively modulates Plasmodium TRiC over its human counterpart encourages future efforts to target parasite protein folding as a route to malaria control.
Keywords: Plasmodium, chemoproteomic, TRiC/CCT, clemastine, malaria
Abstract
The antihistamine clemastine inhibits multiple stages of the Plasmodium parasite that causes malaria, but the molecular targets responsible for its parasite inhibition were unknown. Here, we applied parallel chemoproteomic platforms to discover the mechanism of action of clemastine and identify that clemastine binds to the Plasmodium falciparum TCP-1 ring complex or chaperonin containing TCP-1 (TRiC/CCT), an essential heterooligomeric complex required for de novo cytoskeletal protein folding. Clemastine destabilized all eight P. falciparum TRiC subunits based on thermal proteome profiling (TPP). Further analysis using stability of proteins from rates of oxidation (SPROX) revealed a clemastine-induced thermodynamic stabilization of the Plasmodium TRiC delta subunit, suggesting an interaction with this protein subunit. We demonstrate that clemastine reduces levels of the major TRiC substrate tubulin in P. falciparum parasites. In addition, clemastine treatment leads to disorientation of Plasmodium mitotic spindles during the asexual reproduction and results in aberrant tubulin morphology suggesting protein aggregation. This clemastine-induced disruption of TRiC function is not observed in human host cells, demonstrating a species selectivity required for targeting an intracellular human pathogen. Our findings encourage larger efforts to apply chemoproteomic methods to assist in target identification of antimalarial drugs and highlight the potential to selectively target Plasmodium TRiC-mediated protein folding for malaria intervention.
Malaria is a life-threatening disease that impacts hundreds of millions of people every year. The Plasmodium parasite that causes malaria has a complex life cycle that involves infection of the host liver followed by the blood stage of infection. Sporozoites, the parasite form transmitted by Anopheles mosquitoes, transform within hepatocytes and then undergo rapid expansion to generate thousands of merozoites that are released into the bloodstream (1, 2). The population bottleneck at the liver stage of infection is an ideal step for therapeutic intervention as it would prevent the release of parasites that invade erythrocytes (3, 4). Compounds with activity against multiple parasite life stages have value as agents that could both prevent and treat malaria.
Recent advances in phenotypic screens have revealed hundreds of compounds that inhibit different stages of Plasmodium in cell-based models (5–12). Unfortunately, target identification remains a key challenge for leveraging these molecules to interrogate biological processes, or to advance drug leads. Genetic approaches to dissect drug resistance mutations in Plasmodium have been successful at linking some parasite inhibitors to their molecular targets, such as imidazopyrazine to phosphatidylinositol 4-kinase (13) and febrifugine to prolyl-tRNA synthetase (14). However, this approach requires parasites to circumvent drug inhibition through mutation, which does not occur if compounds have multiple targets, bind to heterooligomeric protein complexes, or target essential processes in which mutations are not well tolerated. While these are ideal attributes for an antimalarial agent, they hinder mechanism of action studies that in turn facilitate drug development. Mass spectrometry-based proteomics methods, including the stability of proteins from rates of oxidation (SPROX) (15) and thermal proteome profiling (TPP) (16), are attractive approaches to unveil those evasive drug targets. These techniques provide global insights into small molecule–protein interactomes by quantitatively measuring ligand-induced protein stability changes. Recently, TPP identified possible Plasmodium targets for the conventional malaria drugs quinine and mefloquine, highlighting the unique capability of proteomic strategies to direct hypotheses concerning the antiparasitic mechanisms of action (17).
We previously identified the over-the-counter antihistamine clemastine as an inhibitor of blood-stage Plasmodium falciparum and liver-stage Plasmodium berghei parasites (6). Another FDA-approved antihistamine astemizole has also been shown to inhibit blood-stage Plasmodium load in mice (18). Intriguingly, Plasmodium parasites do not encode a protein with significant homology to the histamine H1 receptor, making the antiparasitic mechanism of action of these antihistamines elusive. Here we utilize two chemoproteomic strategies, the TPP (16) and SPROX (15) techniques, to identify parasite protein targets of clemastine. The thermal and chemical denaturation data generated using these techniques revealed a unique ability of clemastine to target the delta subunit of the P. falciparum TCP-1 ring complex or chaperonin containing TCP-1 (TRiC/CCT) and to destabilize all eight subunits of TRiC. TRiC, an essential chaperone consisting of eight homologous proteins, plays a pivotal role in folding tubulin and actin (19–21). Our functional studies with immunofluorescence microscopy indicate that clemastine disrupts microtubule organization as well as reduces intraparasitic tubulin levels in Plasmodium, consistent with observations after TRiC is genetically suppressed in yeast (22–24), Drosophila (25), and Caenorhabditis elegans (26). Importantly, our proteomic data and functional studies indicate that clemastine selectively targets Plasmodium TRiC over its human counterpart, highlighting a species variability that may be readily exploited by small molecules. Thus, our application of integrated proteomics methods uncovered the ability of clemastine to target P. falciparum TRiC to inhibit blood- and liver-stage malaria parasites. This parasite-selective mechanism of action for the compound encourages future efforts to disrupt protein folding as a means to combat malaria.
Results
Chemoproteomics Identify Clemastine Molecular Targets in P. falciparum.
Previous work identified clemastine as a dual-stage inhibitor of blood-stage P. falciparum and liver-stage P. berghei parasites (6). A chemoproteomics approach utilizing TPP and SPROX was employed to identify clemastine-binding proteins in P. falciparum lysate. This biological source was used for analysis since it can be obtained in high yields, and methods exist to isolate the parasites from within host erythrocytes. Synchronized parasite cultures were grown to the schizont stage (38 to 44 h postinfection [hpi]), followed by saponin treatment to remove host erythrocyte proteins. The TPP and SPROX techniques were then employed in duplicate to identify protein targets of clemastine (Fig. 1A and Dataset S1). In principle, ligand-induced changes of a protein’s chemical and thermal denaturation properties can be determined by SPROX and TPP, respectively. Such changes are detected by measuring shifts in the chemical and thermal denaturation curves of a protein in the presence of the ligand (Fig. 1A). To reduce false positives, only targets that were identified in both replicates and by both methods were considered hits. To evaluate the degree and significance of the shift observed upon thermal or chemical perturbation, as well as their reproducibility, we generated volcano plots to illustrate the global data distribution (Fig. 1 B and C and SI Appendix, Table S1). Importantly, the Plasmodium TRiC delta subunit was the only protein that satisfied our stringent selection criteria in both TPP and SPROX. This protein is one of eight different subunits that constitute an important protein folding nanomachine termed TRiC (or CCT). Intriguingly, two additional TRiC subunits (TCP-1 beta and theta) also showed significantly lower melting temperatures in the presence of clemastine (Fig. 1D). The other five TRiC subunits also showed a clemastine-induced decrease in melting temperature, despite not satisfying our stringent TPP selection criteria (Fig. 1D). Conversely, the thermal denaturation behavior of human TRiC subunits were not significantly altered in human Huh7 liver cell lysate after treatment with clemastine (SI Appendix, Fig. S1 and Dataset S1). The SPROX analysis revealed a clemastine-induced thermodynamic stabilization of Plasmodium TCP-1 delta, suggesting a binding interaction with clemastine (Fig. 1E). Based on the transition midpoint shift observed in SPROX, the Kd value for clemastine binding to the Plasmodium TRiC delta subunit is estimated to be ∼12 μM (SI Appendix). The peptide probe (TDMDNTVVVKDYNSMDR) that detected this ligand-induced stabilization mapped to the apical loop of TCP-1 delta (SI Appendix, Fig. S2) which is crucial for substrate protein folding and encapsulation (27, 28). In addition to this hit peptide, another 34 methionine-containing probes from the eight TRiC subunits were assayed. However, the transition midpoint shifts of the denaturation data (ΔC1/2 values) of the majority of those peptides did not satisfy our SPROX selection criteria (Fig. 1F). Thus, our integrated chemoproteomics approach using TPP and SPROX sheds light on the mechanism of action of clemastine’s antimalarial activity—clemastine targets Plasmodium TRiC via binding to the delta subunit.
Fig. 1.
Parallel mass spectrometry methods identify P. falciparum TRiC/CCT as clemastine target in malaria parasites. (A) Schematic representation of TPP and SPROX analyses. Hits are selected based on thermal or chemical stability changes in the presence of clemastine (Clem). (B) TPP analysis of clemastine-treated P. falciparum lysate. The dotted horizontal and vertical lines represent two of the three criteria used for hit selection. A third criterion (SI Appendix) was also applied. (C) SPROX analysis of clemastine-treated P. falciparum lysate. The dotted horizontal and vertical lines represent the two criteria used for hit selection. In B and C, DMSO-treated parasite lysate was used as a negative control. Proteins that satisfied (red) or did not satisfy (gray) all of the selection criteria are shown. The light and dark shaded data points (both red and gray) represent those proteins and peptides that were assayed in one and two replicates, respectively. The total Z-score (diff) and log (Diffprob) values are in units of SDs from the mean determined by global analyses of the overall dataset in each experiment. (D) Thermal denaturation curves of P. falciparum TRiC subunits in the presence (red) or absence (blue) of clemastine. Three of eight TRiC subunits (TCP-1 beta, delta, and theta) satisfied the selection criteria (solid lines). (E) Chemical denaturation curves of the TCP-1 delta hit peptide (TDMDNTVVVKDYNSMDR) in the presence (red) or absence (blue) of clemastine. (F) Chemical denaturation curves of a TCP-1 beta nonhit peptide (IMVANTPMDTDKIK) in the presence (red) or absence (blue) of clemastine. The data in D–F represent the average ± SD from two biological replicates.
Clemastine Inhibits Multiple Plasmodium Life Stages.
Clemastine has not previously been implicated as a TRiC-binding compound and only a few drugs have been proposed to inhibit human or yeast TRiC activity (29–31). Among the reported inhibitors, HSF1A (Fig. 2A) has been shown to modulate yeast and mammalian TRiC via direct binding to the chaperonins (31). Here we performed standard blood-stage P. falciparum and liver-stage P. berghei growth assays to compare clemastine and HSF1A anti-Plasmodium activity. At 10 μM clemastine completely inhibited parasite loads in blood and liver cells while HSF1A exhibited activities comparable to the negative control dimethyl sulfoxide (DMSO) (Fig. 2 B and C and SI Appendix, Fig. S3 C and F). Neither clemastine nor HSF1A exhibited cytotoxicity in human Huh7 hepatocytes under the assay conditions (SI Appendix, Fig. S3G). To test the correlation between parasite inhibition by clemastine and its binding to Plasmodium TRiC, expression of TCP-1 genes throughout P. falciparum blood stage and P. berghei liver stage was evaluated. The asexual blood stage involves the cycling of parasites from the ring (0 to 24 hpi), trophozoite (24 to 36 hpi), and schizont (36 to 48 hpi) forms (32). All eight P. falciparum TRiC subunits are expressed throughout these stages based on publicly available data (33) (SI Appendix, Fig. S4A). We next determined the clemastine EC50s with drug administration at each stage, and found that EC50 values ranged from 1 to 4 μM. Drug addition at the schizont stage exhibited a slightly increased EC50 (3.9 ± 0.54 μM) when compared to the ring (1.4 ± 0.07 μM, P = 0.01) or trophozoite (2.3 ± 0.33 μM, P = 0.08) stages (Fig. 2D and SI Appendix, Fig. S3 A and B). In addition, clemastine administered at the late-ring stage did not prohibit trophozoite development or interfere with the parasite size, while drug treatment at the late-trophozoite stage caused a delayed development and inhibited parasite growth (SI Appendix, Fig. S5). A similar efficacy study was completed using the P. berghei liver-stage model system where the parasite establishes an infection (2 to 4 hpi), transforms (4 to 24 hpi), and then replicates (24 to 48 hpi) before exiting the host cell (4, 34). Similarly, all eight P. berghei TRiC subunits are coexpressed throughout the liver stage based on our RNA-seq analysis (35) (SI Appendix, Fig. S4B). The clemastine EC50s ranged from 3.3 to 9.3 μM when administered at various time points during liver cell infection (Fig. 2E and SI Appendix, Fig. S3 D and E). Consistent with our P. falciparum blood-stage study, there was a slight decrease in clemastine liver-stage efficacy when administered at 24 hpi (9.3 ± 1.1 μM) when compared to 0 hpi (5.0 ± 0.53 μM, P = 0.004). These data demonstrate that the clemastine target is present throughout blood-stage P. falciparum and liver-stage P. berghei infection, and further suggest that clemastine primarily targets the mature stages.
Fig. 2.
Clemastine but not HSF1A inhibits multiple Plasmodium life stages. (A) Structures of clemastine (Clem) and a human TRiC inhibitor HSF1A. (B) Relative P. falciparum load in erythrocytes after addition of 10 μM clemastine (gray bar), 10 μM HSF1A (white bar), or DMSO (black bar) at 40 hpi (schizont). The parasite loads were measured after a 42-h incubation. (C) Relative P. berghei load in Huh7 liver cells at 48 hpi after addition of 10 μM clemastine (gray bar), 10 μM HSF1A (white bar), or DMSO (black bar) at 0 hpi. (D) Dose–response curves for clemastine inhibition of blood-stage P. falciparum when administered at the ring (R, black circles) or schizont (S, red circles) stages. Assay schematic shown above plot with R (ring), T (trophozoite), and S (schizont) developmental stages at indicated hours postinfection (hpi). Broken line indicates parasite reinvasion. (E) Dose–response curves for clemastine inhibition of liver-stage P. berghei when administered at 0 (black circles) or 24 (red circles) hpi in Huh7 cells. Assay schematic shown above plot. Data shown as the average ± SEM of three separate experiments.
Clemastine Interferes with Microtubule Biogenesis during P. falciparum Mitosis.
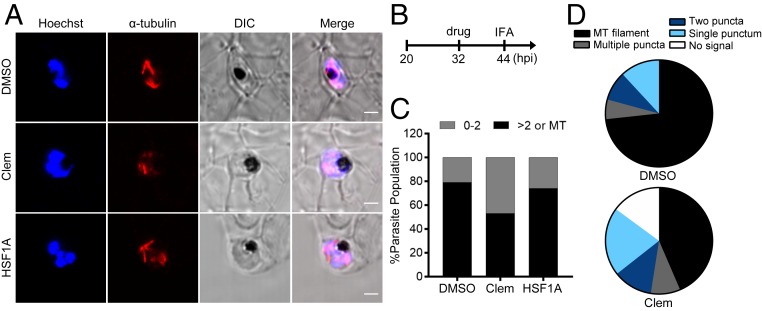
The most well-established TRiC substrates are the cytoskeletal proteins, actin and tubulin, whose proper folding require the chaperonin machinery to reach their functional native states (36). Genetic depletion of TRiC subunits in different model organisms results in a reduction in tubulin levels as well as cytoskeletal disorganization, which in turn disrupts cell motility and division (23, 25, 26, 36, 37). To test if clemastine targets Plasmodium TRiC and interferes with mitotic spindle assembly during Plasmodium schizogony (asexual reproduction stage), microtubule morphologies were probed with confocal z-sectioning after α-tubulin staining. To optimize this method, microtubule structure was scrutinized in the presence of the DMSO negative control at various time points during the intraerythrocytic cycle. Extended microtubules projecting from microtubule organizing centers were observed to align with parasite chromosomes in the middle of schizogony (Fig. 3A and Movie S1), in line with previous reports (38, 39). The microtubule phenotype was next compared to parasites after administrating clemastine or HSF1A at the late-trophozoite stage (32 hpi) for 12 h. In these assays, TRiC function was evaluated after a shorter clemastine incubation when compared to our parasite growth assays (12 h versus 42 to 72 h). Therefore, though we observed an aberrant microtubule morphology with 3 μM clemastine, we used 10 μM (approximately threefold EC50) to achieve a more complete inhibition of Plasmodium TRiC during the mature stages. To efficiently describe and compare tubulin phenotypes, parasites were classified into those having 0 to 2 tubulin puncta (early-stage morphologies) versus those showing multiple tubulin puncta and/or defined microtubule filaments (late-stage morphologies). While no difference was observed between DMSO and HSF1A treatment (Fig. 3A and Movie S3), clemastine reduced the percentage of parasites showing late-stage morphologies by 32 ± 5.5% (mean ± SEM) at 44 hpi (Fig. 3 B and C). This phenotype was further classified into parasites without tubulin signal, single punctum, two puncta, multiple puncta, and microtubule filaments to provide a finer description of tubulin morphologies. After clemastine treatment, more parasites lacking tubulin signal and fewer with filamentous structures were detected (Fig. 3D). For clemastine-treated parasites containing microtubule filaments, many exhibited disoriented microtubules with lower signal intensity when compared to the negative control (Fig. 3A and Movie S2). Additionally, parasites scored as having multiple tubulin puncta exhibited less puncta, indicating stymied microtubule biogenesis during P. falciparum mitosis. To test the specificity of this phenotype for clemastine, a different antimalarial drug pyrimethamine with a known mechanism of action as a dihydrofolate reductase inhibitor was tested. Parasites treated with pyrimethamine (fourfold EC50 concentration) did not exhibit delayed microtubule biogenesis or abnormal tubulin morphology under the assay conditions (SI Appendix, Fig. S6). Furthermore, we probed active Plasmodium mitochondria and parasite viability using the JC-1 stain, which allows for the ratiometric measurement of the mitochondrial membrane potential (40–42). Importantly, clemastine-treated P. falciparum parasites showed JC-1 red-to-green ratios comparable to that of the DMSO control (SI Appendix, Fig. S7). Additionally, no reduction in parasite numbers was detected after clemastine treatment, suggesting that the observed abnormal microtubule morphology was not a general phenotype induced by parasite death (SI Appendix, Fig. S8). These observations support the proposal that clemastine targets Plasmodium TRiC and hinders microtubule biogenesis during mitosis, which precedes parasite death. No clemastine effect was observed at the late-trophozoite stage, as the majority of parasites did not generate complex microtubule structures during the early blood stage (SI Appendix, Fig. S9).
Fig. 3.
Clemastine but not HSF1A interferes with microtubule biogenesis during P. falciparum mitosis. (A) Representative confocal z-sections of P. falciparum-infected erythrocytes after treatment with 10 μM clemastine, 10 μM HSF1A, or 0.1% DMSO at 32 hpi for 12 h (n > 30). Cells were stained with Hoechst (blue) and anti–α-tubulin antibody (red) to visualize parasite nuclei and microtubule structures, respectively. Data are representative of two to three biological replicates. (B) Assay schematic showing drug administration and cell fixation for immunofluorescence assays (IFAs). (C) Parasites were scored as 0 to 2 tubulin puncta (0 to 2, gray bar) versus containing multiple tubulin puncta or microtubule filaments (>2 or MT, black bar), which showed clemastine treatment reduced late-stage morphologies by 32 ± 5.5% (mean ± SEM). Representative data of two to three biological replicates are shown. (D) Tubulin phenotypes were quantified for DMSO- and clemastine-treated parasites. Clemastine-treated parasites showed higher numbers with aberrant microtubule filaments or no tubulin signal when compared to the negative control. DIC, differential interference contrast. (Scale bar, 2 μm.)
TRiC is an essential protein folding chaperone in all eukaryotes including humans. The fact that clemastine showed no adverse effect on Huh7 cell viability and has been approved as an over-the-counter drug suggests that clemastine does not interfere with human tubulin folding. Herein, the effect of clemastine on human mitotic spindle formation was evaluated using our phenotypic assay. In agreement with our cytotoxicity studies, mitotic spindle orientation, microtubule density, and the tubulin signal intensity were the same in DMSO- and clemastine-treated Huh7 cells throughout mitosis (SI Appendix, Fig. S10). This result highlights the species-specific effect of clemastine on Plasmodium microtubule biogenesis.
Clemastine Causes Aberrant Microtubule Morphology and Tubulin Depletion in P. falciparum.
The observed reduction in late-stage microtubule morphologies with clemastine treatment could be explained by slow parasite development, which would cause delayed tubulin phenotypes. To address this possibility, microtubule morphology throughout an infection time course was monitored using confocal z-sectioning. At the onset of schizogony (42 hpi), a reduction in late-stage tubulin morphologies was consistently observed with clemastine treatment (Fig. 4 A, C, and D). When evaluated at 50 hpi, the majority of parasites in the negative control had completed schizogony and exhibited multiple tubulin puncta well aligned with each segregated chromosome (Fig. 4B). However, 27 to 61% of clemastine-treated parasites still showed fewer than two tubulin puncta (Fig. 4 B–D). We next examined phenotypes with clemastine administration at time points before folded tubulins are expected to accumulate. Previous transcriptomic analyses have shown that α- and β-tubulins begin to express at the late-ring stage and are most up-regulated after the mid- to late-trophozoite stage during the intraerythrocytic cycle (33, 43–45). Clemastine added at the early-trophozoite stage (26 hpi) showed similar decreases in the percentage of parasites having late-stage morphologies in both early and late schizonts (SI Appendix, Fig. S11 A–C). But a more dramatic reduction in the numbers of parasites harboring complex microtubule structures was observed with drug treatment at the onset of tubulin expression (20 hpi) (SI Appendix, Fig. S11 D–F). In this experiment, many parasites with tubulin signal exhibited larger tubulin puncta, aberrant microtubule morphologies, such as microtubule disorientation, or thick microtubule bundles (SI Appendix, Fig. S12 and Movies S4–S7). Taken together, these phenotypes agree with tubulin aggregation in parasites due to the presence of the drug. In addition, fewer parasites with segregated chromosomes were observed, and some exhibited strong Hoechst signals without clearly defined borders of separated nuclei (Movie S2).
Fig. 4.
Clemastine causes aberrant microtubule morphology and α-tubulin reduction in P. falciparum. (A and B) Representative confocal z-sections of P. falciparum-infected erythrocytes after treatment with 10 μM clemastine or 10 μM HSF1A at 30 hpi for 12 h (A) or for 20 h (B). Cells were stained with Hoechst (blue) and anti–α-tubulin antibody (red) to visualize parasite nuclei and microtubule structures, respectively. Clemastine influences tubulin at various time points during schizogony. Data are representative of two to three biological replicates. (C) Assay schematic showing drug addition and cell fixation for immunofluorescence assays (IFAs) at 42 or 50 hpi. (D) Parasites were scored as 0 to 2 tubulin puncta (0 to 2, gray bar) versus containing multiple tubulin puncta or microtubule filaments (>2 or MT, black bar), which showed clemastine treatment reduced late-stage morphologies at both treatment windows (32 ± 6% reduction at 42 hpi and 33 ± 14% reduction at 50 hpi) (mean ± SEM). Representative data of two to three biological replicates are shown. (E) Assay schematic showing drug addition and cell harvesting for Western blot analyses. (F) α-Tubulin levels in P. falciparum after treatment with 10 μM clemastine, 10 μM HSF1A, or 0.1% DMSO at 20 hpi (Top) or 26 hpi (Bottom). The α-tubulin band intensities were normalized to the Ponceau S signals of each lane and then normalized to the DMSO control (red numbers). Representative data of three biological replicates for each condition are shown.
Genetic depletion of TRiC subunits in other eukaryotes results in α- and β-tubulin reduction, presumably due to degradation of misfolded tubulins (25, 26, 37). Our microscopy study suggested a similar phenotype as many parasites had no or low tubulin signals after clemastine treatment when compared to the DMSO control. Tubulin levels were further evaluated by immunoblot analysis. We observed a 14 ± 2% (mean ± SEM) reduction in tubulin in P. falciparum with 10 μM clemastine added at the early-trophozoite stage (26 hpi) for 18 h. This reduction increased to 59 ± 11% (mean ± SEM) when clemastine was added at the late-ring stage (20 hpi, 24 h incubation) (Fig. 4 E and F). The dependence of the degree of tubulin reduction with drug administration time was in accordance with TRiC expression levels in P. falciparum.
Discussion
Target identification remains a major hurdle for progressing bioactive compounds discovered in phenotypic screens as chemical probes or therapeutic agents. Here, we identified Plasmodium TRiC/CCT as a molecular target of clemastine using chemoproteomic methods. While previous genetic evidence has shown that all eight TRiC subunits are essential in P. falciparum (20, 46), they were not known to be druggable as a Plasmodium TRiC-binding molecule has not been previously reported. Our functional studies further correlated clemastine treatment with tubulin reduction and the disruption of tubulin folding in the parasites. Given that TRiC is crucial for cytoskeletal protein folding, clemastine might intervene in numerous other fundamental cellular processes and lead to general protein aggregation in multiple Plasmodium life stages.
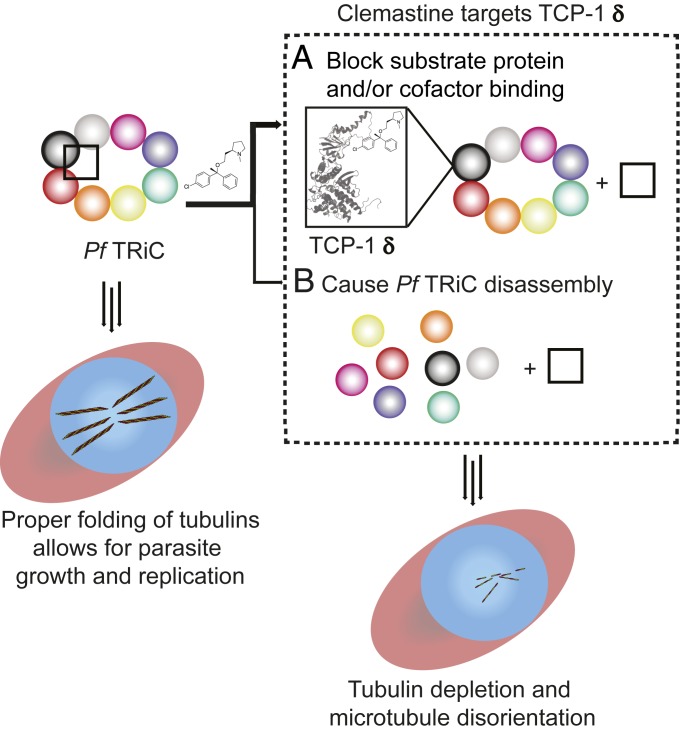
Our analysis demonstrated that clemastine destabilizes the P. falciparum TRiC complex, as all of the TRiC subunits thermally denatured at a lower temperature in the presence of the ligand. While this reduced thermal stability was detected, it does not discern if parasite inhibition stems from ligand-induced complex disassembly or from blocking TRiC client protein binding (Fig. 5). To test these possibilities, we utilized a regulatable TCP-1 theta (another TRiC subunit) P. falciparum line that was readily available (20) to examine parasite sensitivity to clemastine as a function of TCP-1 theta expression. However, no clemastine hypersensitivity (EC50 shift) was observed when TCP-1 theta was down-regulated (SI Appendix, Fig. S13). Accordingly, parasite inhibition by clemastine may not derive from general complex disassembly. Of note, the thermal denaturation behavior of TRiC is such that all eight subunits precipitate together, both in the presence and absence of clemastine (Fig. 1D). A previous TPP study has demonstrated that subunits of protein complexes coprecipitate and yield similar melting curves (47). Thus, the simultaneous melting curve shift of the TRiC subunits induced by clemastine suggests that clemastine disrupts protein–protein interactions involving the eight TRiC subunits and other TRiC-binding proteins. In fact, a similar phenomenon was observed with the human TRiC inhibitor, HSF1A, which has been shown to disrupt the protein–protein interaction between bovine TRiC and HSF1 (31). Thus, the strong thermostability change (4.4 °C shift on average) induced by clemastine may be attributed to the disrupted protein–protein interactions between Plasmodium TRiC and its associated proteins.
Fig. 5.
Mechanistic model of Plasmodium parasite inhibition by clemastine. Clemastine binding to Plasmodium TCP-1 delta (homology model based on PDB ID: 4V81.1.L) blocks the binding of TRiC substrates (A) and/or destabilizes the heterooligomeric complex TRiC (B) (TRiC subunits represented by different colored circles; TRiC substrate proteins and/or cofactors represented by a black square). As a result, tubulin as well as other TRiC client proteins are not folded properly, which leads to parasite inhibition during the blood and liver stages. Genetic evidence with the conditional TCP-1 theta (another TRiC subunit) knockdown parasite strain (SI Appendix, Fig. S13) favors the first model (A, thick arrow). Observed tubulin depletion and dysregulation in the presence of clemastine support the link between drug binding to TCP-1 delta and TRiC inhibition in Plasmodium parasites.
Our SPROX data verified the TPP analysis and further identified the TRiC delta subunit as stabilized in the presence of clemastine, suggesting a binding interaction that disrupts the association of the Plasmodium TRiC with other proteins (e.g., folding substrates and/or accessory proteins). This result is consistent with the proposal that clemastine binds to the apical domain of TCP-1 delta to block cytoskeletal protein folding and inhibit Plasmodium parasite growth (Fig. 5 and SI Appendix, Fig. S2). In this scenario, clemastine would not interact with TCP-1 theta and therefore no drug hypersensitivity would be expected with the partial knockdown of the theta subunit. This model is also supported by the observation that TCP-1 theta was not selected as a hit in our SPROX study. Notably, both actin and tubulin bind to the TRiC delta subunit but may not bind to the theta subunit (48–50). However, it is not known how TCP-1 theta reduction affects the level and composition of the Plasmodium TRiC complex, and how this in turn interferes with substrate folding. Thus far, biochemical studies using purified Plasmodium TRiC subunits have not been reported. The P. falciparum TCP-1 genes are highly AT-rich (69 to 73%) and contain multiple sequence elements with >10 consecutive AT base pairs, making the molecular cloning a challenging task. Additionally, the stability of TCP-1 proteins may require the presence of protein cofactors, further confounding possible biochemical studies to validate direct compound binding. Thus, while our current data are most consistent with the model that clemastine inhibits Plasmodium TRiC through blocking protein substrate binding, future experiments are necessary to precisely correlate substrate binding and/or general complex disassembly in the presence of clemastine with parasite inhibition.
Disrupting such protein–protein interactions might cause a thermodynamic destabilization in other TRiC subunits. However, the magnitude of this destabilization is expected to be small (<1 kcal/mol) and within the experimental error of SPROX, given the stoichiometry and concentration of the TRiC subunits in the parasite lysate (SI Appendix). Therefore, it is not surprising that the SPROX data on other TRiC subunits did not reveal such a destabilization. In contrast, it has been previously established that the TPP protein precipitation reaction is highly sensitive to protein quaternary structures (47). Thus, the sensitivity of TPP coupled with the true thermodynamic measurements of protein stability that SPROX affords provided molecular details concerning drug mechanism of action as well as reduced false positives. Moreover, the chemical denaturation data generated by SPROX enabled the clemastine binding affinity to the Plasmodium TRiC delta subunit to be calculated. It is noteworthy that the determined clemastine Kd value (Kd ∼ 12 μM) is consistent with our clemastine EC50s (1 to 10 μM) for Plasmodium parasite inhibition.
The opportunity to chemically disrupt Plasmodium over human TRiC despite a 74 to 86% amino acid sequence similarity is underscored by clemastine and HSF1A: two molecules with species selectivity to TRiC without any structure–activity relationship optimization. Importantly, the identified clemastine-binding peptide in the delta subunit has sequence variability with the human homolog, but is highly conserved in various Plasmodium species (SI Appendix, Fig. S14). This peptide maps to the apical protrusion that forms a built-in lid for substrate folding and encapsulation (27, 28). A previous biochemical study showed that the built-in lid in mammalian TRiC allosterically mediates conformational changes between different subunits and is required for efficient substrate folding (27). It is thus likely that clemastine binding to this domain prevents substrate protein binding to Plasmodium TRiC. However, more in-depth studies are needed to dissect the clemastine binding interface and the conformational changes induced by drug binding to further support this model. Our discovery that clemastine selectively modulates Plasmodium TRiC over the human counterpart encourages future efforts to target parasite protein folding as a route to malaria control.
It is known that targeting TRiC in various model organisms renders accumulation of misfolded client proteins and generates proteotoxic stress that is detrimental to cells. It is thus reasonable to hypothesize that clemastine-induced P. falciparum TRiC disassembly increases reliance on the ubiquitin–proteasome pathway to alleviate proteotoxic stress. However, we found that the overall level of K48-linked polyubiquitin remained unaltered in clemastine-treated parasites (SI Appendix, Fig. S15). One explanation for this result is that proteasomal activity increases as an endeavor to maintain protein homeostasis. Comprehensive studies on antimalarial proteasome inhibitors have provided an avenue to fight against artemisinin resistance and prevent malaria transmission (51, 52). Screening proteasome inhibitors that synergize with a Plasmodium TRiC inhibitor, such as clemastine, may lay the foundation for novel drug combination therapies to better control malaria. Additionally, these molecules would be valuable for probing the interplay between TRiC and the ubiquitin–proteasome pathway in Plasmodium. For example, the proteasomal inhibitors could be used to evaluate polyubiquitination of Plasmodium TRiC client proteins in targeted mass spectrometry-based proteomics analyses, which would help elucidate the downstream molecular mechanisms underlying clemastine-induced parasite inhibition.
In principle, increased TRiC molecules may help stabilize client proteins or other proteins as an opportunistic folding event under the drug-induced stress condition (36). Interestingly, an in vivo transcriptome analysis of >1,000 P. falciparum isolates from acute malaria patients revealed a positive correlation between artemisinin resistance and Plasmodium TRiC expression (53). As an extremely potent fast-acting antimalarial drug, artemisinin triggers general oxidative and proteotoxic stresses and subverts principle cellular processes in malaria parasites (52, 54). It is thus possible that targeting Plasmodium TRiC could provide a strategy to combat artemisinin resistance. Notably, TCP-1 delta, which we identified as the major clemastine target in our study, showed the strongest correlation with artemisinin resistance among other TRiC components (53).
Our liver-stage P. berghei and previously reported blood-stage P. falciparum RNA-seq data have revealed coexpression of the eight TRiC subunits (33, 43–45). A recent biochemical study further demonstrated that P. falciparum TCP-1 theta was encompassed in a ∼1-MDa protein complex, suggesting the existence of the TRiC complex in malaria parasites (20). In addition to the conserved essential role of the heterohexadecamer TRiC complex, TRiC subunits as monomers, homooligomers, and complexes with two or more subunits may have independent activities to help maintain homeostasis and/or prevent proteotoxicity (20, 36). Intriguingly, an adapted RNA-seq study showed an increased expression of TCP-1 delta when compared to other TCP-1 subunits in P. falciparum 3D7 (55). The fact that clemastine targets P. falciparum TCP-1 delta may thus be advantageous as it could have multiple mechanisms of action: hindering the functions of the canonical heterohexadecamer TRiC complex and inhibiting TCP-1 delta activity in other oligomeric states.
In conclusion, we have shown that parallel chemoproteomic strategies can be utilized to reveal the molecular targets of small molecules that inhibit the intracellular pathogen that causes malaria. Our approach discovered that clemastine binds to the delta subunit of the essential Plasmodium chaperonin TRiC, which in turn leads to aberrant microtubule morphologies in the parasites. The abrogation of microtubule filaments in parasites is species selective, as it was not observed in mammalian cells. Our results highlight the untapped potential to selectively target protein folding in Plasmodium as a route to advance agents that inhibit multiple stages of the Plasmodium life cycle.
Materials and Methods
A more detailed description of the materials and methods can be found in SI Appendix. Parasite proteins were extracted from P. falciparum 3D7 strain for proteomic profiling. Following TPP and SPROX analyses, protein samples were labeled for quantitative mass spectrometry to determine the clemastine targets in P. falciparum. The compound EC50s were determined using P. falciparum 3D7 parasites and P. berghei ANKA parasites for the blood and liver stages, respectively. Microtubule structures in drug-treated parasites and human liver cells were examined using immunofluorescence assays and confocal z-sectioning. The intraparasitic tubulin levels after drug treatments were assessed by Western blot analysis. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (56) via the PRIDE (57) partner repository with the dataset identifier PXD014308.
Supplementary Material
Acknowledgments
This work was supported by the NIH (DP2AI138239 to E.R.D. and R21-AI130406-01A1 to M.C.F.). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The following parasite strain was obtained through Biodefense and Emerging Infections Research Resources Repository, National Institute of Allergy and Infectious Diseases, NIH: P. falciparum, strain 3D7, MRA-102, contributed by Daniel J. Carucci. We are grateful to the Duke Proteomics Core and the Duke Light Microscopy Core facilities. We thank Daniel E. Goldberg and Eva Istvan for providing the TCP-1 theta P. falciparum conditional knockdown strain. We also thank Ana Rodriguez and Sandra Gonzalez from the New York University Insectary for providing Plasmodium-infected mosquitoes as well as members of the E.R.D. laboratory for critical reading of this manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: MS data generated in this work have been uploaded to the ProteomeXchange Consortium via the PRIDE partner repository (PXD014308).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913525117/-/DCSupplemental.
References
- 1.Cowman A. F., Healer J., Marapana D., Marsh K., Malaria: Biology and disease. Cell 167, 610–624 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Prudêncio M., Rodriguez A., Mota M. M., The silent path to thousands of merozoites: The Plasmodium liver stage. Nat. Rev. Microbiol. 4, 849–856 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Kappe S. H., Vaughan A. M., Boddey J. A., Cowman A. F., That was then but this is now: Malaria research in the time of an eradication agenda. Science 328, 862–866 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Vaughan A. M., Kappe S. H. I., Malaria parasite liver infection and exoerythrocytic biology. Cold Spring Harb. Perspect. Med. 7, a025486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brancucci N. M., Goldowitz I., Buchholz K., Werling K., Marti M., An assay to probe Plasmodium falciparum growth, transmission stage formation and early gametocyte development. Nat. Protoc. 10, 1131–1142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derbyshire E. R., Prudêncio M., Mota M. M., Clardy J., Liver-stage malaria parasites vulnerable to diverse chemical scaffolds. Proc. Natl. Acad. Sci. U.S.A. 109, 8511–8516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamo F. J., et al. , Thousands of chemical starting points for antimalarial lead identification. Nature 465, 305–310 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Guiguemde W. A., et al. , Chemical genetics of Plasmodium falciparum. Nature 465, 311–315 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucantoni L., Fidock D. A., Avery V. M., Luciferase-based, high-throughput assay for screening and profiling transmission-blocking compounds against Plasmodium falciparum gametocytes. Antimicrob. Agents Chemother. 60, 2097–2107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meister S., et al. , Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334, 1372–1377 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth A., et al. , A comprehensive model for assessment of liver stage therapies targeting Plasmodium vivax and Plasmodium falciparum. Nat. Commun. 9, 1837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka T. Q., et al. , A quantitative high throughput assay for identifying gametocytocidal compounds. Mol. Biochem. Parasitol. 188, 20–25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara C. W., et al. , Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504, 248–253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller T. L., et al. , Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 8, 311–317 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strickland E. C., et al. , Thermodynamic analysis of protein-ligand binding interactions in complex biological mixtures using the stability of proteins from rates of oxidation. Nat. Protoc. 8, 148–161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez Molina D., et al. , Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Dziekan J. M., et al. , Identifying purine nucleoside phosphorylase as the target of quinine using cellular thermal shift assay. Sci. Transl. Med. 11, eaau3174 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Chong C. R., Chen X., Shi L., Liu J. O., Sullivan D. J. Jr, A clinical drug library screen identifies astemizole as an antimalarial agent. Nat. Chem. Biol. 2, 415–416 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Yaffe M. B., et al. , TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature 358, 245–248 (1992). [DOI] [PubMed] [Google Scholar]
- 20.Spillman N. J., Beck J. R., Ganesan S. M., Niles J. C., Goldberg D. E., The chaperonin TRiC forms an oligomeric complex in the malaria parasite cytosol. Cell Microbiol. 19, e12719 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olshina M. A., Baumann H., Willison K. R., Baum J., Plasmodium actin is incompletely folded by heterologous protein-folding machinery and likely requires the native Plasmodium chaperonin complex to enter a mature functional state. FASEB J. 30, 405–416 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinh D. B., Drubin D. G., A yeast TCP-1-like protein is required for actin function in vivo. Proc. Natl. Acad. Sci. U.S.A. 91, 9116–9120 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X., Sullivan D. S., Huffaker T. C., Two yeast genes with similarity to TCP-1 are required for microtubule and actin function in vivo. Proc. Natl. Acad. Sci. U.S.A. 91, 9111–9115 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miklos D., et al. , Primary structure and function of a second essential member of the heterooligomeric TCP1 chaperonin complex of yeast, TCP1 beta. Proc. Natl. Acad. Sci. U.S.A. 91, 2743–2747 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palumbo V., et al. , Misato controls mitotic microtubule generation by stabilizing the TCP-1 tubulin chaperone complex [corrected]. Curr. Biol. 25, 1777–1783 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saegusa K., et al. , Caenorhabditis elegans chaperonin CCT/TRiC is required for actin and tubulin biogenesis and microvillus formation in intestinal epithelial cells. Mol. Biol. Cell 25, 3095–3104 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reissmann S., Parnot C., Booth C. R., Chiu W., Frydman J., Essential function of the built-in lid in the allosteric regulation of eukaryotic and archaeal chaperonins. Nat. Struct. Mol. Biol. 14, 432–440 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira J. H., et al. , Structure of the human TRiC/CCT subunit 5 associated with hereditary sensory neuropathy. Sci. Rep. 7, 3673 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan X., et al. , Trivalent arsenic inhibits the functions of chaperonin complex. Genetics 186, 725–734 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassiouni R., et al. , Chaperonin containing TCP-1 protein level in breast cancer cells predicts therapeutic application of a cytotoxic peptide. Clin. Cancer Res. 22, 4366–4379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neef D. W., et al. , A direct regulatory interaction between chaperonin TRiC and stress-responsive transcription factor HSF1. Cell Rep. 9, 955–966 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg D. E., Cowman A. F., Moving in and renovating: Exporting proteins from Plasmodium into host erythrocytes. Nat. Rev. Microbiol. 8, 617–621 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Bártfai R., et al. , H2A.Z demarcates intergenic regions of the plasmodium falciparum epigenome that are dynamically marked by H3K9ac and H3K4me3. PLoS Pathog. 6, e1001223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayabalasingham B., Bano N., Coppens I., Metamorphosis of the malaria parasite in the liver is associated with organelle clearance. Cell Res. 20, 1043–1059 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toro-Moreno M., Sylvester K., Srivastava T., Posfai D., Derbyshire E. R., RNA-seq analysis illuminates the early stages of Plasmodium liver infection. bioRxiv:10.1101/870030 (10 December 2019). [DOI] [PMC free article] [PubMed]
- 36.Brackley K. I., Grantham J., Activities of the chaperonin containing TCP-1 (CCT): Implications for cell cycle progression and cytoskeletal organisation. Cell Stress Chaperones 14, 23–31 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grantham J., Brackley K. I., Willison K. R., Substantial CCT activity is required for cell cycle progression and cytoskeletal organization in mammalian cells. Exp. Cell Res. 312, 2309–2324 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Gerald N., Mahajan B., Kumar S., Mitosis in the human malaria parasite Plasmodium falciparum. Eukaryot. Cell 10, 474–482 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahajan B., et al. , Centrins, cell cycle regulation proteins in human malaria parasite Plasmodium falciparum. J. Biol. Chem. 283, 31871–31883 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Peatey C. L., et al. , Mitochondrial membrane potential in a small subset of artemisinin-induced dormant plasmodium falciparum parasites in vitro. J. Infect. Dis. 212, 426–434 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Lee Y. Q., et al. , A high-content phenotypic screen reveals the disruptive potency of quinacrine and 3′,4′-dichlorobenzamil on the digestive vacuole of Plasmodium falciparum. Antimicrob. Agents Chemother. 58, 550–558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathore S., et al. , Disruption of a mitochondrial protease machinery in Plasmodium falciparum is an intrinsic signal for parasite cell death. Cell Death Dis. 2, e231 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otto T. D., et al. , New insights into the blood-stage transcriptome of Plasmodium falciparum using RNA-Seq. Mol. Microbiol. 76, 12–24 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bozdech Z., et al. , The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 1, E5 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Llinás M., Bozdech Z., Wong E. D., Adai A. T., DeRisi J. L., Comparative whole genome transcriptome analysis of three Plasmodium falciparum strains. Nucleic Acids Res. 34, 1166–1173 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M., et al. , Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 360, eaap7847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan C. S. H., et al. , Thermal proximity coaggregation for system-wide profiling of protein complex dynamics in cells. Science 359, 1170–1177 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Kalisman N., Schröder G. F., Levitt M., The crystal structures of the eukaryotic chaperonin CCT reveal its functional partitioning. Structure 21, 540–549 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leitner A., et al. , The molecular architecture of the eukaryotic chaperonin TRiC/CCT. Structure 20, 814–825 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llorca O., et al. , Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature 402, 693–696 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Aminake M. N., Arndt H. D., Pradel G., The proteasome of malaria parasites: A multi-stage drug target for chemotherapeutic intervention? Int. J. Parasitol. Drugs Drug Resist. 2, 1–10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tilley L., Straimer J., Gnädig N. F., Ralph S. A., Fidock D. A., Artemisinin action and resistance in Plasmodium falciparum. Trends Parasitol. 32, 682–696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mok S., et al. , Drug resistance. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science 347, 431–435 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paloque L., Ramadani A. P., Mercereau-Puijalon O., Augereau J. M., Benoit-Vical F., Plasmodium falciparum: Multifaceted resistance to artemisinins. Malar. J. 15, 149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vignali M., et al. , NSR-seq transcriptional profiling enables identification of a gene signature of Plasmodium falciparum parasites infecting children. J. Clin. Invest. 121, 1119–1129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deutsch E. W., et al. , The ProteomeXchange Consortium in 2017: Supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 54, D1100–D1106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Riverol Y., et al. , The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 47, D442–(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.