Significance
There is increasing evidence that CD8+ regulatory T cells (CD8 Treg) can modulate the immune response in the context of autoimmune disease, infection, and cancer. Here we asked whether this regulatory mechanism might control the host response to transplanted heart allografts. We report that CD8 Treg that recognize Qa-1, the murine homolog of HLA-E, strongly inhibits host production of antibodies specific for the donor organ. Since the production of antidonor antibodies is currently a major barrier to successful transplantation, identification of this Qa-1/HLA-E-dependent pathway may pave the way to approaches to this pressing clinical problem.
Keywords: Qa-1, CD8 Treg, Ab-mediated rejection, follicular helper T cell, HLA-E
Abstract
Induction of longstanding immunologic tolerance is essential for survival of transplanted organs and tissues. Despite recent advances in immunosuppression protocols, allograft damage inflicted by antibody specific for donor organs continues to represent a major obstacle to graft survival. Here we report that activation of regulatory CD8 T cells (CD8 Treg) that recognize the Qa-1 class Ib major histocompatibility complex (MHC), a mouse homolog of human leukocyte antigen-E (HLA-E), inhibits antibody-mediated immune rejection of heart allografts. We analyzed this response using a mouse model that harbors a point mutation in the class Ib MHC molecule Qa-1, which disrupts Qa-1 binding to the T cell receptor (TCR)–CD8 complex and impairs the CD8 Treg response. Despite administration of cytotoxic T lymphocyte antigen 4 (CTLA-4) immunoglobulin (Ig), Qa-1 mutant mice developed robust donor-specific antibody responses and accelerated heart graft rejection. We show that these allo-antibody responses reflect diminished Qa-1–restricted CD8 Treg-mediated suppression of host follicular helper T cell-dependent antibody production. These findings underscore the critical contribution of this Qa-1/HLA-E-dependent regulatory pathway to maintenance of transplanted organs and suggest therapeutic approaches to ameliorate allograft rejection.
Modulation of the host immune system into a more receptive environment for organ transplants is an essential element for successful transplantation. Identification of immune regulatory receptors, such as cytotoxic T lymphocyte antigen 4 (CTLA-4), as well as regulatory T cells, such as FoxP3+ CD4 T cells (CD4 Treg), has allowed promising therapeutic approaches. Additional progress may come from emerging evidence that, like the CD4 T cell lineage, CD8 T cells are divisible into an effector subset that targets pathogens and a regulatory subset that subdues antibody responses and excessive inflammatory reactions (1).
During the progression of tissue-specific autoimmune diseases including celiac disease and multiple sclerosis, activation of pathogenic CD4 T cells is accompanied by expansion of regulatory CD8 T cells (CD8 Treg) that recognize peptides presented by major histocompatibility complex (MHC) class Ia on self-reactive CD4 T cells (2). Activation of these CD8 Treg, which may account for <5% of CD8 T cells and display a characteristic genetic signature, may be essential for maintenance of self-tolerance. Studies of systemic autoimmune disease in murine models have identified a second set of CD8 Treg that recognize self-peptides associated with the class Ib MHC product termed Qa-1 (1, 3).
Qa-1 is a nonclassical MHC gene product in the mouse that is the homolog of human leukocyte antigen-E (HLA-E) in man. Unlike classical MHC genes, which have extensive polymorphisms, expression of murine Qa-1 and the human HLA-E genes is essentially limited to two alleles (1). Similar to other class I MHC molecules, Qa-1 is expressed as a heterodimer in association with β2m at the cell surface that presents peptides to T cells. These Qa-1–peptide (pQa-1) complexes, which are expressed by activated T cells, B cells, and dendritic cells, are recognized by the T cell receptor (TCR) as well as the inhibitory NKG2A receptor (1, 4). TCR engagement by pQa-1 promotes CD8 T cell activation and lytic activity, whereas NKG2A/CD94 engagement by pQa-1 transduces inhibitory signals. Analysis of Qa-1–restricted CD8 Treg has been accelerated by the production and analysis of mice that carry a point mutation in the Qa-1 gene (5–8). A single amino acid substitution in Qa-1 at pos 227 (D→K) selectively disrupts binding of Qa-1 to the TCR on CD8 cells, but spares the binding of pQa-1 to the NKG2A receptor. Mice that carry this point mutation display defective CD8 regulatory cell responses and develop a lethal systemic lupus erythematosus (SLE)-like autoimmune disease marked by generation of pathogenic autoantibodies (3).
Solid organ transplantation represents a potential life-saving therapy for patients with end-stage organ failure. Unfortunately, long term survival of allograft transplants has not substantially improved over the past several decades, in part due to antibody-mediated allograft rejection (9). Despite considerable study, the immunologic basis of antibody-mediated graft rejection is not well understood, and systemic therapeutic approaches to ameliorate this mode of graft attack have not been fully developed. Here, we asked whether Qa-1–restricted CD8 Treg might normally dampen the production of anti-allograft antibodies and associated organ rejection. We find that Qa-1–restricted CD8 Treg suppression of follicular T helper cell (TFH) expansion and associated donor-specific antibody production allow long-term survival of heart grafts in this murine model.
Results and Discussion
Qa-1–Restricted CD8 Treg Inhibit Germinal Center Response in Cardiac Transplantation.
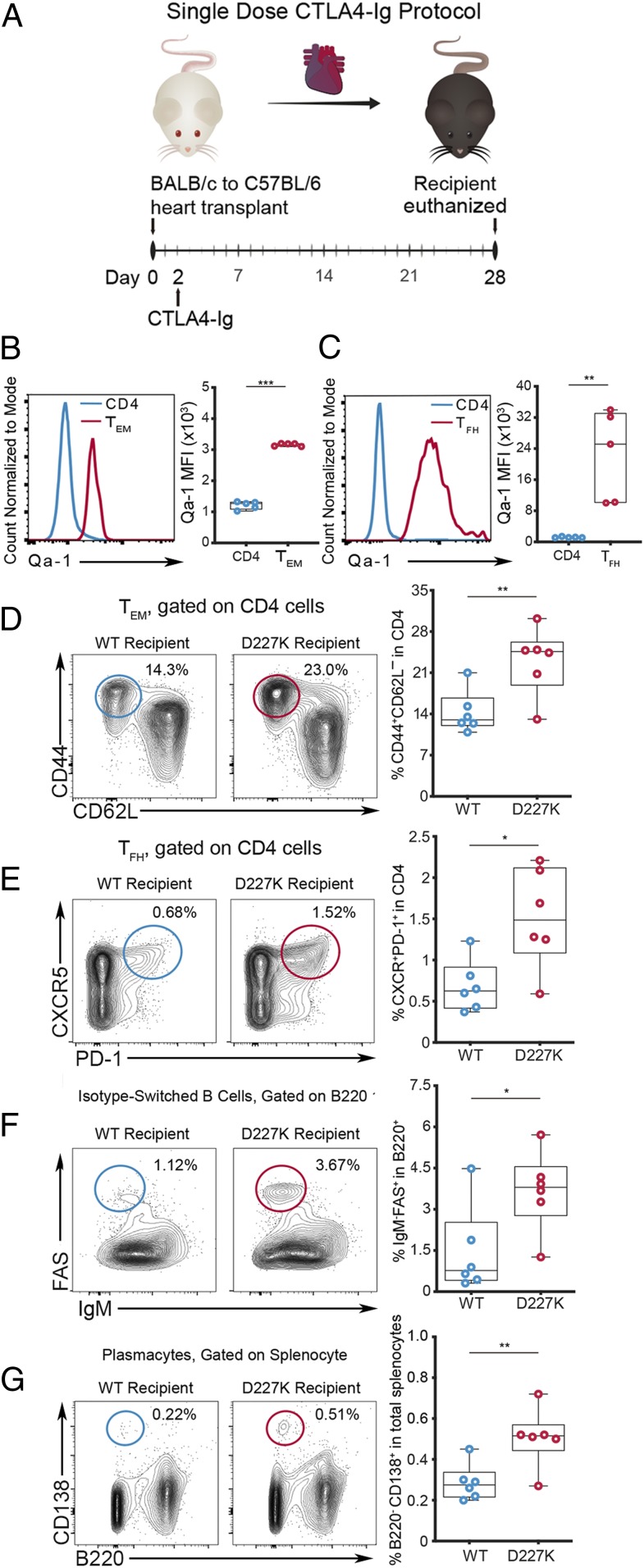
To determine whether activated CD4 T cells in a murine heart transplant model are regulated by Qa-1–restricted CD8 Treg, we initially examined Qa-1 expression by CD4 helper T cells in murine graft recipients. Briefly, heart allografts from BALB/c donor mice were heterotopically transplanted into C57BL/6 (Qa-1.WT; wild type [WT]) recipient mice followed by a single intraperitoneal (i.p.) injection CTLA4 immunoglobulin (Ig) (250 μg on day 2) as previously described (10, 11) (Fig. 1A). Flow cytometric analysis of recipient spleen at day 28 posttransplantation showed significantly increased Qa-1 expression by effector memory CD4 T cells (TEM: CD44+CD62L–CD4+) (Fig. 1B) and especially TFH cells (PD-1+CXCR5+CD4+) (Fig. 1C) compared to the whole CD4 T cell population. As noted in previous studies of activated and memory CD4 T cells (3), expression of Qa-1 by TFH cells was considerably higher than that displayed by TEM or the total CD4 T cell population. Heart transplantation induced increased expression of Qa-1 by CD4 TEM, TFH, germinal center (GC) B cells but not by plasmacytes (SI Appendix, Fig. S1). These data suggest that these lymphocyte subsets might be more susceptible to CD8 Treg-mediated suppression after graft transplantation, and that mobilization of Qa-1–restricted CD8 Treg might represent a potentially effective therapeutic approach.
Fig. 1.
Genetic disruption of pQa-1–TCR interaction enhances TFH and B cell responses upon heart transplantation. (A) Single-dose CTLA4-Ig heart transplant protocol using BALB/c heart allograft and C57BL/6 (WT) recipient. Comparison of Qa-1 MFI by total CD4 (blue) and (B) CD4 TEM (CD62L–CD44+CD4+) (red) and (C) TFH (PD-1+CXCR5+CD4+) (red) in recipient spleens at day 28 post heart transplantation (n = 5; unpaired t test). Analysis of lymphocyte subsets frequency including TEM (D) and TFH (E), as well as isotype-switched germinal center B cells (IgM−FAS+B220+) (F), and plasma cells (B220−CD138+) (G) in spleens of BALB/c heart allograft recipients under single-dose CTLA4-Ig protocol at day 28 (n = 6/group; unpaired t test). MFI: mean fluorescence intensity. *P < 0.05; **P < 0.01; ***P < 0.001.
Next, we examined the potential consequences of Qa-1 up-regulation by CD4+ TEM and TFH. In principal, Qa-1 expression might promote CD4 T cell survival by engagement of the NKG2A receptor and avoidance of destruction by natural killer (NK) cells and CD8 T cells (12) (SI Appendix, Fig. S2A). Alternatively, up-regulated pQa-1 complexes might engage TCR on Qa-1–restricted CD8 Treg and potentiate graft survival through targeting of allo-responsive CD4 T cells (SI Appendix, Fig. S2B). To distinguish between these alternatives, we used the B6.Qa-1.D227K (D227K) knock-in mouse (3, 5), which carries a Qa-1 point mutation that specifically interrupts pQa-1 by CD8 Treg (SI Appendix, Fig. S2C). As a result, the failure of CD8 Treg to recognize and target activated Qa-1+ CD4 T cells may “release the brakes” on TFH cell expansion and promote increased antibody responses in D227K recipients. To test this hypothesis, we transplanted BALB/c hearts into WT and D227K mice along with a single dose of CTLA4-Ig. Analysis of splenic CD4 T cells and B cells in D227K recipients indicated that these hosts contained significantly higher numbers and proportions of TEM (P < 0.001) and TFH (P < 0.01) compared to WT controls (Fig. 1 D and E). Increased levels of TFH cells in D227K mice were accompanied by increased GC B cells that expressed a class-switched Ig isotype (B220+Fas+IgM–, P < 0.05) (Fig. 1F) along with increased antibody (Ab)-producing plasmacytes (B220–CD138+, P < 0.01) (Fig. 1G).
In summary, these data suggest that CD8 Treg recognition of pQa-1 expressed by TFH cells may inhibit the Qa-1+ TFH cell response and associated B cell antibody response to the allograft.
Qa-1–Restricted CD8 Treg Inhibit Donor-Specific Ab Generation and Graft Pathology.
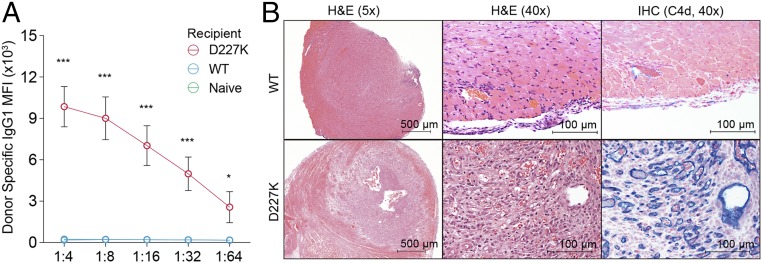
The increase in splenic antibody-producing cells in D227K recipients opened the possibility of increased production of donor-specific antibodies. Since donor-specific antibodies bind epitopes presented by mismatched organ’s MHC, we used donor splenocytes as a surrogate for organ allografts for in vitro analysis (13, 14). We therefore measured donor-specific antibodies in the sera of WT and D227K recipient mice after incubation with BALB/c donor cells and detection of surface-bound Ab with secondary anti-mouse IgG1. This analysis revealed that D227K recipients had developed an 11.2-fold greater level of circulating donor-specific antibodies (P < 0.001) compared with WT recipients (Fig. 2A).
Fig. 2.
Donor-specific Ab generation and graft pathology in D227K hosts. (A) Donor-specific antibody in heart allograft recipients (n = 6 for D227K and WT recipients and 3 for naïve WT mice; two-way ANOVA test, followed by Tukey’s multiple comparison test). (B) H&E (hematoxylin and eosin) staining and immunohistochemistry with C4d (blue) staining of cardiac allografts WT recipients (above) and D227K recipients (below) at day 28 posttransplantation with the single-dose CTLA4-Ig protocol. *P < 0.05; ***P < 0.001.
Histological examination of heart grafts that had been implanted in WT and D227K recipients showed evidence of acute rejection and lymphocyte infiltration as well as a wide range of hemorrhage and myocyte injury (Fig. 2B). Notably, graft injury and pathology were extremely severe in D227K recipients, as judged by distortion of structure, severe tissue edema, and hemorrhages. Nearly all arterioles in D227K recipient grafts contained multiple lymphocytic infiltrates and were thrombosed, consistent with severe inflammation. Allograft deposition of C4d, a key diagnostic criterion of Ab-mediated rejection that depends on complement activation (15), was also robust. Allografts implanted into D227K hosts displayed a diffuse and widespread C4d staining along endothelial cells. In contrast, allografts implanted into WT mice contained extremely sparse or no detectable C4d deposits (Fig. 2B). These results indicate that heart allografts implanted into D227K recipients underwent severe antibody-mediated rejection and were resistant to tolerance induction, despite the CTLA-4 Ig treatment protocol.
CD8 Treg-Mediated Immune Suppression Is Critical for Long-Term Graft Survival.
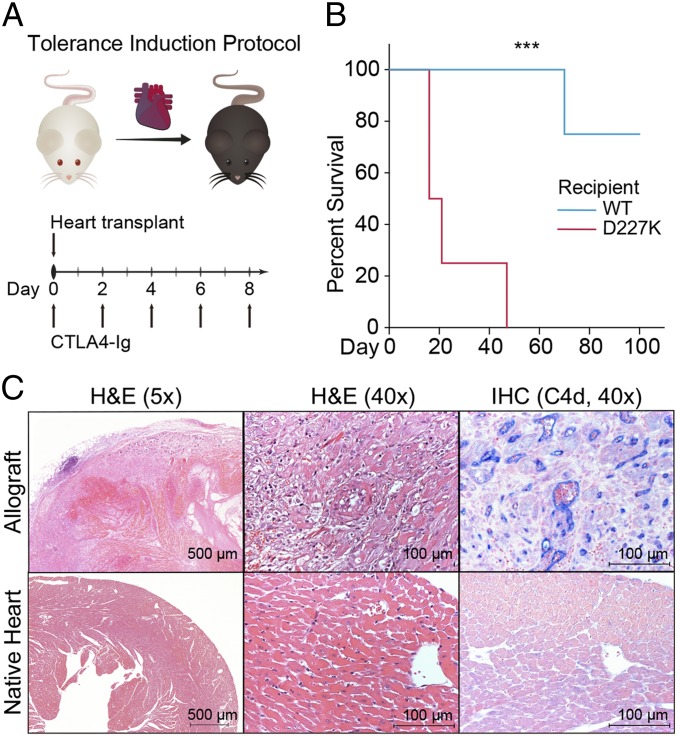
High doses of CTLA4-Ig can induce graft tolerance in models of a fully MHC-mismatched murine heart transplantation. To define the clinical consequences of the sharply increased antibody-mediated immunity in D227K hosts, we examined allograft survival of D227K and WT recipients after this tolerance induction protocol (Fig. 3A). We reasoned that, in contrast to long-term graft survival in WT recipients, D227K hosts might reject grafts despite the high dose of CTLA4-Ig. Indeed, while grafts in all WT recipients but one survived >100 d (mean survival time: 93.8 d), grafts in D227K recipients were rejected in 25 d (P < 0.001) (Fig. 3B).
Fig. 3.
CD8 Treg activity is essential for the survival of heart grafts. (A) Tolerance induction protocol. (B) Kaplan-Meier survival curve of heart allografts (mean survival time: 25 and 93.8 d for D227K and WT recipients respectively, P < 0.001) (n = 4 per group; log-rank test). (C) H&E staining and immunohistochemistry with C4d (blue) staining of the allograft (above) and the native heart (below) of D227K recipient at the time of allograft rejection. ***P < 0.001.
Since D227K mice begin to generate autoantibodies after 4 to 6 mo of life (3), we used transplanted D227K recipients that were less than 3 mo old. Nevertheless, to rule out a potential contribution of endogenous autoantibodies to the host allograft response, we reexamined both native host heart and transplanted heart of D227K recipients at rejection (Fig. 3A). Although the rejected allograft heart showed evidence of injury and complement deposition, the native host hearts revealed no sign of injury nor C4d deposition that might signify autoantibody attack (Fig. 3B). These data, taken together, indicate that D227K but not WT recipients display severe donor-specific antibody-mediated rejection even after a high dose CTLA4-Ig regimen.
CD8 Treg Recognition of Qa-1 Results in Inhibition of the CD4 Response In Vitro and In Vivo.
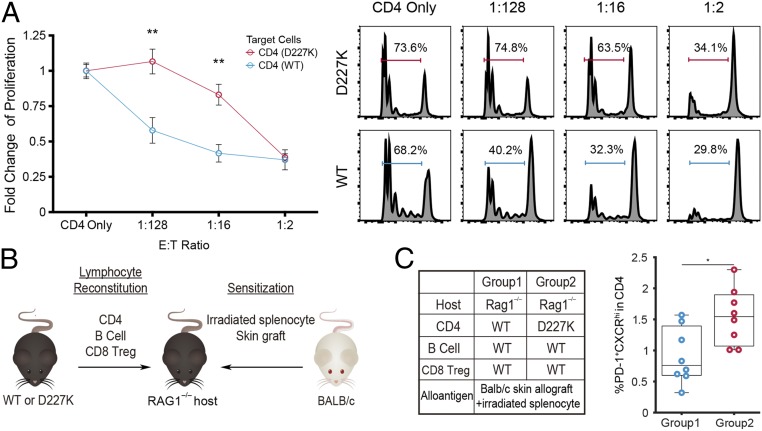
We used an in vitro assay to detect inhibition of Qa-1–expressing CD4 T cells by Qa-1–restricted CD8 T cells. CD4 Treg-depleted CD4 T cells (Tconv; CD4+CD25–) from either naïve WT or D227K mice were labeled with Celltrace Violet and incubated with fluorescence-activated cell sorting (FACS)-sorted CD8 Treg (CD44+CXCR5+CD8+) isolated from naïve WT mice (Fig. 4A). Proliferation of WT CD4 T cells but not D227K CD4 T cells was significantly inhibited by CD8 Treg in a dose-dependent fashion (Fig. 4A).
Fig. 4.
Recognition of Qa-1–peptide on CD4 T cells by CD8 Treg leads to suppression of CD4 T cell expansion in vitro and in vivo. (A) In vitro suppression analysis using CD8 Treg [CD44+CXCR5+CD8+] sorted from naïve WT mice as effector cells, and CD25–CD4+ T cells sorted from naïve D227K or WT mice as target cells. Proliferation was measured by tracing CellTrace Violet, with representative gating at each effector to target cell ratio shown on the right (n = 6 and 8 for D227K and WT target cell donors respectively, two-way ANOVA test followed by Tukey’s multiple comparison test). The data shown are representative of two independent experiments. (B) In vivo suppression assay using RAG1−/− hosts with adoptive cell transfer of B cells, CD4 T cells, and CD8 Treg. (C) Percentage (%) of TFH in CD4 cells from host spleens at day 10 after adoptive transfer (n = 8 per group; unpaired t test) in group 2 compared to group 1. *P < 0.05; **P < 0.01.
Differentiation of TFH cells from CD4 T cell precursors requires an interaction with activated dendritic cells and follicular B cells (16). We transferred CD4 Tconv from WT or D227K donors into allografted B6.RAG1−/− hosts along with CD8 Treg isolated from WT donors (Fig. 4 B, Left). Each group of B6.RAG1−/− recipients was also reconstituted with WT B cells to promote TFH differentiation (17). By 10 d after adoptive transfer, we noted a significant increase of TFH cells only in hosts that had received D227K CD4 Tconv, but not in hosts given WT Tconv (Fig. 4B, group 2 vs. group 1).
This study demonstrates that disruption of Qa-1 recognition by CD8 Treg results in antibody-mediated rejection of allografts associated with dysregulated TFH expansion and activation of robust donor-specific antibody responses. These findings extend previous studies of regulatory CD8 T cells that are programmed to monitor class Ib (Qa-1) MHC expressed by activated TFH cells and to inhibit excessive and dysregulated TFH induction of antibody responses (1). The human homolog of murine Qa-1 is HLA-E. Unlike the high polymorphism of other HLA loci, both murine Qa-1 and human HLA-E genes are essentially biallelic. Currently, the association of HLA-E alleles with antibody-mediated graft rejection is not well understood. There is emerging evidence that the two HLA-E alleles (HLA-E*1:01 and HLA-E*1:03) may present discrete peptide repertoires to CD8 T cell clones (18, 19). The ability of these distinct peptide sets to differentially engage the TCRs of conventional and regulatory CD8 T cells may underlie the disparate correlation of the two HLA-E alleles with allograft dysfunction (20, 21). Identification of the peptide sets presented by Qa-1/HLA-E recognized by CD8 Treg may allow clinical approaches to organ transplantation based on mobilization of HLA-E-restricted CD8 Treg.
Materials and Methods
Mice.
C57BL/6, BALB/c, and B6.129S7-Rag1 <tm1Mom> mice were purchased from The Jackson Laboratories. Generation of B6.Qa-1.D227K (D227K) mice has been previously described (5). All murine strains in this study were maintained in specific pathogen-free (SPF) conditions at the Brigham and Women’s Hospital animal facility in accordance with federal, state, and institutional guidelines. The study protocol was approved by the Brigham and Women’s Hospital Institutional Animal Care and Use Committee (IACUC). Mice were sex and age matched (8 to 12 wk old).
Heterotopic Heart Transplantation.
Fully vascularized hearts from BALB/c mice were heterotopically transplanted to the abdominal cavity of recipient C57BL/6 (WT) or B6.Qa-1.D227K (D227K) mice using a microsurgical technique previously described (22). For the single-dose CTLA4-Ig protocol, 250 μg of CTLA4-Ig (Belatacept) was intravenously (i.v.) administered at day 2 posttransplantation, and recipients were killed on day 28 for the analysis. For the tolerance induction protocol, a single dose of 500 μg of CTLA4-Ig (Bioxcell, # BE0099) was injected i.v. on day 0, followed by 250 μg CTLA4-Ig injections on days 2, 4, 6, and 8. Mice were killed if the allograft was rejected, confirmed by cessation of a palpable heart beating, or if the allograft reached predetermined clinical end point set at day 100.
Immunophenotyping.
The anti–CD4-allophycocyanin (APC)/Cy7 (RM4-5, #100414), anti–CD8α-PE/Cy7 (53-6.7, #100722), anti–CD44-FITC (IM7, #103022), anti–CD44-APC/Cy7 (IM7, #103027), anti–CD62L-PERCP (MEL-14, #104432); anti–CXCR5-APC (L138D7, #145506); anti–CXCR5-PERCP (L138D7); anti–CD25-PE (PC61, #102008); anti–FAS-PE (SA367H8, #152607); anti–B220-PERCP (RA3-6B2, #103235); anti–IgM-APC/Cy7 (RMM-1, #406516) were purchased from Biolegend. The anti–CD138-APC (2Q1484, #558626) and anti–Qa-1-BV510 (6A8.6F10.1A6, #744386) were purchased from BD Biosciences. The anti–PD-1-eFluor450 (RMP1-30, #48–9981-82) was purchased from eBioscience. FACS Canto II (BD Biosciences) was used for flow cytometry and the result was analyzed with FlowJo version 10 (Flowjo LLC).
Donor-Specific Antibody Measurement.
For the single-dose CTLA4-Ig protocol, serum was collected from each euthanized recipient at day 28. A 100 μL of serum was serially diluted with phosphate-buffered saline (PBS) followed by incubation with 1 × 106 BALB/c donor splenocytes diluted in 100 μL PBS at a volume for 30 min. After washing with FACS buffer (1.0% bovine serum albumin and 0.02% sodium azide in PBS), cells were stained with phycoerythrin (PE)/Cy7-conjugated anti-CD4 antibody (Biolegend, Clone: RM4-5, # 100527) and fluorescein isothiocyanate (FITC)-conjugated secondary anti-mouse IgG1 antibody (BD Biosciences, Clone: A85-1, #553443).
Histology.
Histology slide preparation of murine hearts was performed by InvivoEx Company. C4d staining was similarly performed by InvivoEx using the anti-mouse C4d antibody (Hycult Biotech, #HP8033) and the Vector Blue Alkaline Phosphatase Substrate Kit (Vector Laboratories, #SK-5300).
Cell Sorting.
CD25–CD4+ T cells were obtained through magnetic-activated cell sorting (MACS)-based sorting. In brief, using a mouse CD4 T cell isolation kit (Miltenyi Biotec, #130–104-454), CD4 T cells were stained with PE-conjugated anti-CD25 (Miltenyi Biotec, #130–118-678) followed by anti-PE microbead (Miltenyi Biotec, #130–048-801) to deplete CD25+CD4+ T cells for in vivo and in vitro analyses. For isolation of CD44+CXCR5+CD8+ T cells (CD8 Tregs), splenocytes harvested from naïve C57BL/6 mouse were first enriched by MACS-based sorting using a mouse CD8 T cell isolation kit (Miltyenyi Biotec, #130–104-075). CD8 T cells were stained with FITC-conjugated anti-CD44 antibody (IM7, #103005), APC-conjugated anti-CXCR5 antibody (L138D7, #145505) and PE/Cy7-conjugated CD8a (53-6.7, #100721) before FACS sorting with MoFlo Astrios system (Beckman Coulter).
In Vitro Suppression Assay.
The 5.0 × 104 CD4 target cells (CD4+CD25–) were isolated from naïve WT or D227K mice and labeled with CellTrace Violet (Invitrogen, #C34557). Each group of CD4 target cells was cultured in the absence or presence of CD8+CXCR5+CD44+ T (CD8 Tregs) sorted from naïve C56BL/6 mice at the indicated ratios and activated with 1 μg/mL anti-CD3ε antibody (Invitrogen, 45–2C11, #16–0031-85), 1 μg/mL anti-CD28 antibody (Invitrogen, 37.51, #16–0281-85), and 200 IU of IL-2 (Peprotech, #200–02) for 72 h. Proliferation indices were calculated and presented as mean fold changes. CD4 target cell populations were analyzed with CellTrace label using Flowjo v10. The mean percentage of proliferated population of each sample was divided by the mean percentage of proliferated populations from samples cultured with target CD4+ T cells only (positive control).
Adoptive Cell Transfer.
The 1.0–1.5 cm2 skin grafts from BALB/c donors were transplanted onto the dorsal trunks of RAG1−/− mice 72 h prior to adoptive cell transfer. FACS-sorted CD8+CXCR5+CD44+ T cells (0.25 × 106 cells) and MACS-sorted B cells (5.0 × 106 cells) from C57BL/6 mice along with either MACS-sorted C57BL/6 (WT) CD4+CD25– T cells or B6.Qa-1.D227K (D227K) CD4+CD25– T cells (2.5 × 106 cells) were transferred into RAG1−/− hosts. On the day of the adoptive cell transfer, irradiated BALB/c splenocytes (10 × 106 cells) were i.p. injected into RAG1−/− hosts. Mice were killed at day 10 after adoptive transfer for immunophenotyping.
Statistical Analyses.
Statistical analyses were performed using Prism 8.2.1 (GraphPad Software). P values < 0.05 were considered statistically significant (* = <0.05, ** = <0.01, *** = <0.001).
Data Availability.
All protocols and reagents are detailed in the manuscript. Data are available upon request from the authors.
Supplementary Material
Acknowledgments
We thank the Harvard Immunology Division Flow Cytometry Core for assistance with FACS sorting. This research was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institute of Allergy and Infectious Diseases (NIAID) of the NIH under award numbers T32DK007527 (J.Y.C.), R01AI134842 (J.P.A.), and R01AI037562 and R01AI048125 (H.C.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918950117/-/DCSupplemental.
References
- 1.Nakagawa H., Wang L., Cantor H., Kim H. J., New insights into the biology of CD8 regulatory T cells. Adv. Immunol. 140, 1–20 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Saligrama N., et al. , Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 572, 481–487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H. J., Verbinnen B., Tang X., Lu L., Cantor H., Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 467, 328–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Montfoort N., et al. , NKG2A blockade potentiates CD8 T cell immunity induced by cancer vaccines. Cell 175, 1744–1755.e15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L., Kim H. J., Werneck M. B., Cantor H., Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc. Natl. Acad. Sci. U.S.A. 105, 19420–19425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez Arias D. A., et al. , Disruption of CD8+ Treg activity results in expansion of T follicular helper cells and enhanced antitumor immunity. Cancer Immunol. Res. 2, 207–216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holderried T. A., Lang P. A., Kim H. J., Cantor H., Genetic disruption of CD8+ Treg activity enhances the immune response to viral infection. Proc. Natl. Acad. Sci. U.S.A. 110, 21089–21094 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu L., et al. , Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity 26, 593–604 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loupy A., Lefaucheur C., Antibody-mediated rejection of solid-organ allografts. N. Engl. J. Med. 379, 1150–1160 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Corry R. J., Winn H. J., Russell P. S., Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 16, 343–350 (1973). [DOI] [PubMed] [Google Scholar]
- 11.Sula Karreci E., et al. , Brief treatment with a highly selective immunoproteasome inhibitor promotes long-term cardiac allograft acceptance in mice. Proc. Natl. Acad. Sci. U.S.A. 113, E8425–E8432 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leavenworth J. W., Tang X., Kim H. J., Wang X., Cantor H., Amelioration of arthritis through mobilization of peptide-specific CD8+ regulatory T cells. J. Clin. Invest. 123, 1382–1389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J., et al. , Tracing donor-MHC class II reactive B cells in mouse cardiac transplantation: Delayed CTLA4-ig treatment prevents memory alloreactive B-cell generation. Transplantation 100, 1683–1691 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kühne L., et al. , Renal allograft rejection, lymphocyte infiltration, and de novo donor-specific antibodies in a novel model of non-adherence to immunosuppressive therapy. BMC Immunol. 18, 52 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry G. J., et al. , The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J. Heart Lung Transplant. 32, 1147–1162 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Vinuesa C. G., Linterman M. A., Yu D., MacLennan I. C., Follicular helper T cells. Annu. Rev. Immunol. 34, 335–368 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Haynes N. M., et al. , Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 179, 5099–5108 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Rolle A., Meyer M., Calderazzo S., Jager D., Momburg F., Distinct HLA-E peptide complexes modify antibody-driven effector functions of adaptive NK cells. Cell Rep. 24, 1967–1976.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Strong R. K., et al. , HLA-E allelic variants. Correlating differential expression, peptide affinities, crystal structures, and thermal stabilities. J. Biol. Chem. 278, 5082–5090 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Guberina H., et al. , Association of high HLA-E expression during acute cellular rejection and numbers of HLA class I leader peptide mismatches with reduced renal allograft survival. Immunobiology 222, 536–543 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Di Cristofaro J., et al. , HLA-E(*)01:03 allele in lung transplant recipients correlates with higher chronic lung allograft dysfunction occurrence. J. Immunol. Res. 2016, 1910852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai S., et al. , Prolonged mouse cardiac graft cold storage via attenuating ischemia-reperfusion injury using a new antioxidant-based preservation solution. Transplantation 100, 1032–1040 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All protocols and reagents are detailed in the manuscript. Data are available upon request from the authors.