Significance
The bioactive eicosanoid prostaglandin (PG) E2, which is generated from PGG2 and PGH2 by prostaglandin E synthases, is known to alter the phenotype of macrophages in the tumor microenvironment. Here, we show that a cytochrome P450 enzyme, Cyp2c44, interferes with this pathway by decreasing PGG2 and PGH2 levels. The deletion of Cyp2c44 in a genetic mouse model of breast cancer resulted in increased tumor growth and metastases that were associated with increased PGE2 levels, lymphangiogenesis, and the alternative polarization of macrophages. Moreover, inflammatory Toll-like receptor signaling in Cyp2c44-deficient macrophages was enhanced, at least partly because of the upregulation of WD repeating domain FYVE1.
Keywords: cytochrome P450, WDFY1, metastasis, lymphangiogenesis
Abstract
Arachidonic acid epoxides generated by cytochrome P450 (CYP) enzymes have been linked to increased tumor growth and metastasis, largely on the basis of overexpression studies and the application of exogenous epoxides. Here we studied tumor growth and metastasis in Cyp2c44−/− mice crossed onto the polyoma middle T oncogene (PyMT) background. The resulting PyMT2c44 mice developed more primary tumors earlier than PyMT mice, with increased lymph and lung metastasis. Primary tumors from Cyp2c44-deficient mice contained higher numbers of tumor-associated macrophages, as well as more lymphatic endothelial cells than tumors from PyMT mice. While epoxide and diol levels were comparable in tumors from both genotypes, prostaglandin (PG) levels were higher in the PyMTΔ2c44 tumors. This could be accounted for by the finding that Cyp2c44 metabolized the PG precursor, PGH2 to 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid (12-HHT), thus effectively reducing levels of effector PGs (including PGE2). Next, proteomic analyses revealed an up-regulation of WD repeating domain FYVE1 (WDFY1) in tumors from PyMTΔ2c44 mice, a phenomenon that was reproduced in Cyp2c44-deficient macrophages as well as by PGE2. Mechanistically, WDFY1 was involved in Toll-like receptor signaling, and its down-regulation in human monocytes attenuated the LPS-induced phosphorylation of IFN regulatory factor 3 and nuclear factor-κB. Taken together, our results indicate that Cyp2c44 protects against tumor growth and metastasis by preventing the synthesis of PGE2. The latter eicosanoid influenced macrophages at least in part by enhancing Toll-like receptor signaling via the up-regulation of WDFY1.
Cytochrome P450 (CYP) enzymes can metabolize polyunsaturated fatty acids (PUFAs) to biologically active signaling mediators. For example, CYP enzymes can convert arachidonic acid to hydroxyeicosatetraenoic acids and epoxyeicosatrienoic acids (EETs), which have been implicated in intracellular signaling events in numerous cell types. Perhaps most is known about the biological actions of the EETs, which possess vasodilator, anti-inflammatory, and angiogenic properties (for review, see ref. 1). Many compounds that promote angiogenesis have also been implicated in tumor development, and the same is true for the PUFA epoxides. In fact, several different tumor cell types have been shown to express CYP epoxygenases and to generate EETs, which in turn accelerate tumor cell proliferation in vitro and protect against TNFα-induced apoptosis (2–5). In addition to potentially promoting tumor angiogenesis and growth, EETs have also been linked to increased tumor metastasis (6, 7). A number of mechanisms have been proposed to explain this relationship, including the up-regulation of vascular endothelial cell growth factor (VEGF), CD44, and prometastatic matrix metalloproteinases, as well as down-regulation of the anti-angiogenic factor thrombospondin-1 and the antimetastatic genes CD82 and nm-23 (6, 7). Importantly, however, some of these data were obtained using animals that overexpressed CYP enzymes (particularly CYP2C8 or CYP2J2) or were treated with an inhibitor of the soluble epoxide hydrolase (sEH) to markedly increase EET levels or with high concentrations of 14,15-EET. Little information is available on the consequences of physiological levels of CYP activity on tumor development.
The aim of this investigation was to study the consequences of interfering with the CYP pathway in tumor growth and metastasis without the overexpression of CYP enzymes or exogenous application of high concentrations of epoxides or pharmacological inhibitors. Cyp2c44 was targeted in mice, given that it was reported to have a role in tumor growth and angiogenesis (8, 9), and more recently, Cyp2c44 expression was reported to be altered during cancer initiation at a point when arachidonic acid metabolism was dysfunctional (10). Therefore, breast cancer development was studied in Cyp2c44−/− mice crossed with mice expressing the polyoma middle T (PyMT) oncogene under the control of the mouse mammary tumor virus promoter (11).
Results
Tumor Development and Metastases in PyMT and PyMTΔ2c44 Mice.
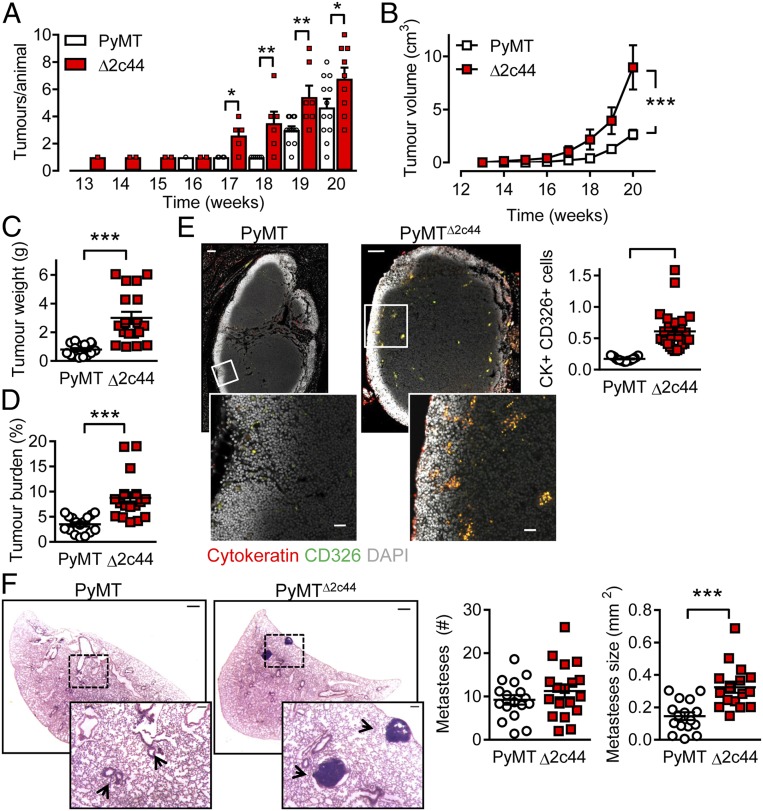
Palpable tumors developed in adult PyMT mice globally lacking Cyp2c44 (so-called PyMTΔ2c44 mice) from 13 wk of age and increased in number and size during the observation period (maximum 20 wk). However, when compared with the PyMT mice, the number of tumors that developed, as well as their size, was significantly greater in animals lacking Cyp2c44 (Fig. 1 A–D). This in itself was unexpected, as the inhibition/down-regulation of Cyp2c44 has been proposed as a strategy to treat cancer (9).
Fig. 1.
Consequences of Cyp2c44 deletion on primary tumor growth and metastases. (A) Time-dependent increase in tumor number in PyMT and PyMTΔ2c44 (Δ2c44) mice from weeks 13 to 20. (B) Tumor volume per animal/week from weeks 13 to 20. A&B n = 13 mice per group (ANOVA for repeated measures and Newman-Keuls test). (C) Total tumor weight at week 20. (D) Tumor burden (total tumor weight normalized to body weight) at week 20. (C and D) n = 16 PyMT and n = 17 PyMTΔ2c44 mice (Student’s t test). (E) Metastatic tumor cells identified using cytokeratin (red) and CD326 (green) in axillary lymph nodes from PyMT and PyMTΔ2c44 (Δ2c44) mice. (Lower) Magnifications of the areas marked by boxes. (Scale bar = 100 µm; Upper, 20 µm.) (Lower) n = 17 to 18 mice per group (Student’s t test). (F) Breast cancer metastases (H&E staining), in lungs from PyMT and PyMTΔ2c44 mice. (Scale bar = 500 µm.) (Insets) Magnifications of the areas marked by boxes; n = 16 animals per group with 5 sequential slides evaluated per sample (Student’s t test). *P < 0.05; **P < 0.01; ***P < 0.001.
Increasing CYP epoxide levels by inhibition or deletion of the sEH has been linked to escape from tumor dormancy and increased metastasis (7). Therefore, the numbers of metastases that developed in axillary lymph node and lungs from the affected 20-wk-old animals was determined. Significantly more metastases (cytokeratin+, CD326+ cells) were detected in lymph nodes from PyMTΔ2c44 than from PyMT mice (Fig. 1E). The situation was not as clear in the lung, as the total number of metastases was not different between the two genotypes (Fig. 1F). However, metastases detected in the PyMTΔ2c44 mice were markedly larger than those found in the PyMT group, in which only micrometastases were detected.
Cellular Composition of Primary Tumors from PyMT and PyMT2c44 Mice.
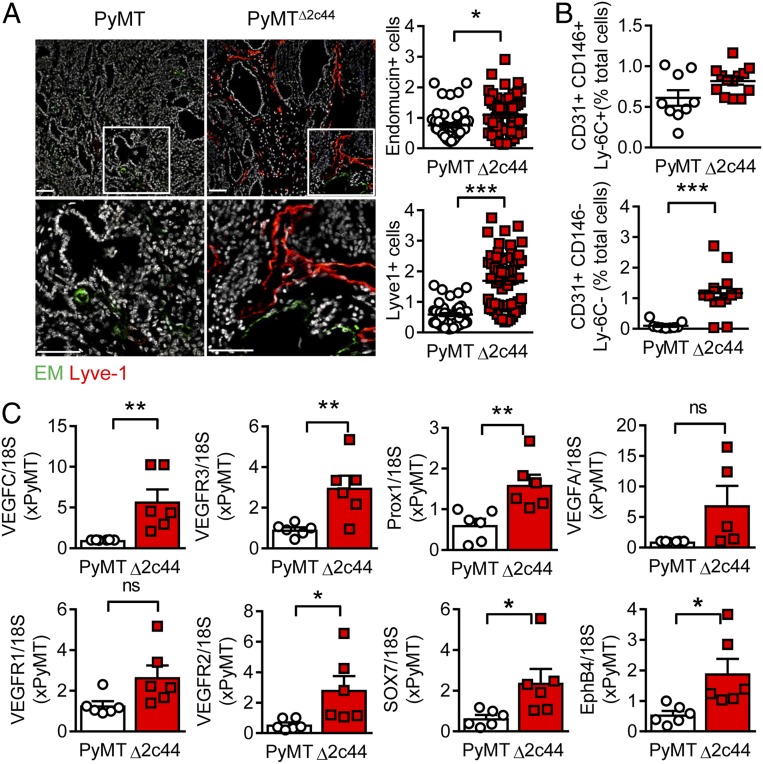
Given that metastatic potential has been partly attributed to the lymphatic system in tumors, the formation of capillaries and lymph capillaries was assessed in primary tumors. Using immunohistochemistry, it was possible to demonstrate that the vascular characteristics of the tumors differed significantly (i.e., primary tumors from PyMTΔ2c44 mice contained more Lyve1+ cells than endomucin+ cells, indicating a dominance of lymph capillaries; Fig. 2A). A similar phenomenon was detected when FACS was used to identify vascular endothelial cells (CD31+, CD146+, Ly-6C+ cells) and lymph endothelial cells (CD31+, CD146−, Ly-6C− cells) present in primary tumor digests (Fig. 2B). Consistent with these data, the expression of the lymph markers VEGFR3 and Prox1 was high in PyMT2c44 tumors. Although the vascular angiogenesis markers VEGFR1, VEGFR2, EphB4, and Sox7 were also higher in PyMTΔ2c44 than in PyMT tumors, the differences were relatively subtle (Fig. 2C).
Fig. 2.
Angiogenesis and lymphangiogenesis in primary tumors. Primary tumors were removed from 20-wk-old PyMT and PyMTΔ2c44 (Δ2c44) mice. (A) Vascular endothelial cells; endomucin+ (EM, green) and lymph endothelial cells (Lyve-1+, red). (Lower) Magnifications of the areas marked by boxes, n = 10 PyMT and n = 12 PyMTΔ2c44 mice with 5 sequential slides evaluated per tumor. (Scale bars = 50 µm.) (B) FACS analysis of primary tumor digests for endothelial cells (CD31+, CD146+, Ly-6C+) and lymph endothelial cells (CD31+, CD146−, Ly-6C−); n = 9 PyMT and n = 13 PyMTΔ2c44 mice. (C) Expression of lymphatic endothelial cell markers (VEGFC, VEGFR3, and Prox1) and blood endothelial cell markers (VEGFA, VEGFR1, VEGFR2, SOX7, EphB4) in primary tumors; n = 6 animals per group. ns = not significant (Student’s t test). *P < 0.05; **P < 0.01; ***P < 0.001.
The tumors also displayed differences in inflammatory cell infiltration as more tumor-associated macrophages were detected in tumors from PyMTΔ2c44 mice (Fig. 3A), whereas numbers of resident F4/80 high macrophages were comparable in tumors from both groups of animals. In addition, more tumor-associated macrophages were detected in the PyMTΔ2c44 group (Fig. 3B). One of the hallmarks of tumor-associated macrophages is the suppression of adaptive immunity (12); accordingly, the numbers of CD11b− lymphocytes, as well as both T cells (CD3+CD4+) and NKT cells (CD8+CD19+NK1.1+), and T cell markers were decreased in tumors from PyMTΔ2c44 mice versus PyMT mice. Thus, the deletion of Cyp2c44 in PyMT mice increased metastasis at the same time as promoting the immunosuppressive effects of the tumor microenvironment.
Fig. 3.
Lymphocyte infiltration into primary tumors. Primary tumors were removed from 20-wk-old PyMT and PyMTΔ2c44 (Δ2c44) mice. (A) F4/80 (green) and CD11b+ (red) staining, bar = 20 µm; n = 8 to 12 mice per group with 5 sequential slides evaluated per tumor (Student’s t test). (B) FACS-based quantification of primary tumor digests for monocytes, neutrophils, resident macrophages (Res. MO), CD45+ cells, tumor-associated macrophages (TAMs), lymphocytes, T cells, and NKT cells relative to the total cell count (total); n = 6 to 14 animals per group (Student’s t test). **P < 0.01; ***P < 0.001.
PUFA-Derived Lipid Metabolite Profiles in Primary Tumors from PyMT and PyMT2c44 Mice.
Next, the concentrations of ω-6 and ω-3 PUFA epoxides and diols were assessed in primary tumors from PyMT and PyMTΔ2c44 mice. Although some subtle but significant differences in epoxide or diol levels were detected, the knockout of Cyp2c44 could not be linked to a clear decrease in specific ω-3 (SI Appendix, Fig. S1) or ω-6 (SI Appendix, Fig. S2) PUFA epoxides. However, primary tumors from PyMTΔ2c44 mice contained significantly more prostaglandin (PG) E2, PGD2, PGF2α, 6-keto PGF1α, and PGE1 than primary tumors from PyMT mice (Fig. 4A).
Fig. 4.
Cyp2c44 and the prostaglandin profile. (A) Tumors were isolated from 20-wk-old PyMT and PyMTΔ2c44 (Δ2c44) mice and prostaglandin (PG) levels assessed by LC-MS/MS; n = 7 mice per group (Student’s t test). (B) PGE2 and 12-HHT levels in liver microsomes from wild-type (WT) and Cyp2c44−/− (−/−) mice, n = 6 independent experiments, each performed in triplicate (Student’s t test). (C) PGE2 and 12-HHT levels in microsomes from Cyp2c44-deficient (CTL) and Cyp2c44-expressing (2c44) Sf9 cells in the presence of PGH2. n = 6 independent experiments, each performed in triplicate (Student’s t test). *P < 0.05; **P < 0.005; ***P < 0.001.
Prostaglandins are produced in a two-step process involving the initial generation of PGG2 and PGH2 by cyclooxygenase (COX) enzymes, followed by an additional metabolic step involving specific prostaglandin synthases to generate the effector PG (13). COX expression is known to be elevated in breast cancer (14), and both COX1 and COX2 were detectable in all the tumors tested (SI Appendix, Fig. S3A). Although COX RNA levels were elevated in tumors from PyMTΔ2c44 versus PyMT mice, there were no consistent differences in protein expression (SI Appendix, Fig. S3B). Proteomic analyses of similarly sized primary tumors from PyMT and PyMTΔ2c44 mice (Dataset S1) also revealed no change in PGES3, whereas the expression of PGES2 was even significantly decreased in PyMTΔ2c44 tumors (SI Appendix, Fig. S3C). The increase in PG production was specific to the tumors and was not reflected by generally increased plasma PG levels (SI Appendix, Fig. S3D).
There is a precedent for crosstalk between the CYP and COX pathways, as at least one CYP enzyme (i.e., CYP2S1) was previously found to metabolize PGH2 to 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid (12-HHT), thus attenuating the generation of PGE2 (15). Cyp2c44 exerted similar effects, as 12-HHT levels were lower in tumors from PyMT2c44 mice versus their PyMT counterparts (Fig. 4A). Similarly, PGE2 levels were higher and 12-HHT levels lower in liver microsomes from Cyp2c44−/− versus wild-type mice (Fig. 4B). The reverse was the case when Cyp2c44 was overexpressed together with the cytochrome reductase in Sf9 cells, where the addition of PGH2 resulted in a decrease in PGE2 but an increase in 12-HHT production (Fig. 4C). The importance of the COX pathway for the enhanced tumor growth in PyMTΔ2c44 mice was confirmed by the fact that treating animals with celecoxib decreased tumor growth to levels detectable in the PyMT mice. Moreover, the decrease in PG levels was concomitant with a decrease in TAMs, as well as angiogenesis and lymph angiogenesis (SI Appendix, Fig. S4).
Consequences of Cyp2c44 Deletion on the PyMT Tumor Proteome.
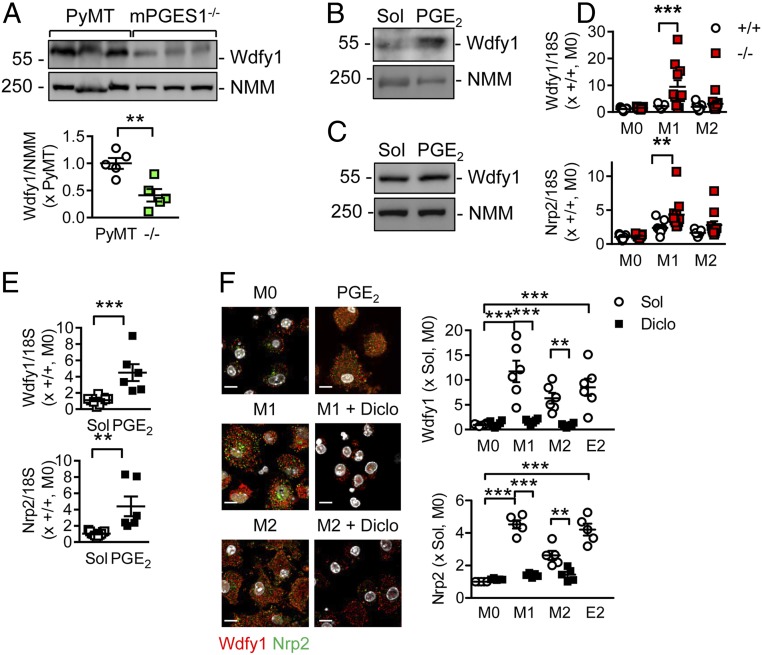
To gain insight into the molecular mechanism or mechanisms underlying the differences observed in tumor growth, proteomic analyses were performed using similarly sized primary tumors from PyMT and PyMTΔ2c44 mice (Fig. 5A and Dataset S1) (16). The proteins most altered in PyMTΔ2c44 versus PyMT mice were the hemoglobin β2 chain, which fits with the increased perfusion of the tumors, vitamin K epoxide reductase (165-fold change), and WD Repeat and FYVE domain containing 1 (WDFY1, 187-fold change vs. background in primary tumor lysates; Fig. 5B). The latter protein was also identified as the most altered protein when microsomes from the tumors were studied (159-fold increase in PyMTΔ2c44 vs. PyMT tumor microsomes; Dataset S2) (17). A comparison of the expression of different CYP enzymes in these samples revealed no marked differences (i.e., there did not seem to be a compensatory increase in the expression of another CYP enzyme to compensate for the loss of Cyp2c44). WDFY1 is also a downstream effector of neuropilin-2 (NRP2), and both proteins have been implicated in the endocytic transport of cell surface epidermal growth factor receptor (18, 19). It was possible to confirm the elevated expression of WDYF1 mRNA (Fig. 5C) and protein (Fig. 5 D and E) in tumors from PyMT2c44 mice. Consistent with the increase of WDFY1 in the PyMTΔ2c44 versus PyMT tumors, levels of NRP2 were also increased.
Fig. 5.
Link between Cyp2c44 deletion and WDFY1. (A) Volcano blot showing the proteins most altered in tumors from PyMTΔ2c44 (Δ2c44) versus PyMT mice. (B) WDFY1 expression (Log2 LFQ intensity) from the proteomics data set; n = 7 animals per group (Student’s t test). (C) WDFY1 mRNA expression from an independent series of tumors; n = 6 animals per group (Student’s t test). (D) WDFY1 and NRP protein levels normalized to nonmuscle myosin (NMM) in similarly sized primary tumors from PyMT and PyMTΔ2c44 mice; n = 6 animals per group (Student’s t test). (E) WDFY1 and NRP protein levels in similarly sized primary tumors from PyMT and PyMTΔ2c44 mice. (Insets) Magnifications of the areas marked by boxes. (Scale bar = 5 µm; Upper, bar = 2 µm.) (Lower) n = 12 animals per group (Student’s t test). *P < 0.05; ***P < 0.001.
Consequences of PGE2 on WDFY1 Expression in Macrophages.
To assess whether a change in the PG profile could be responsible for altered WDFY1 protein expression, similarly sized primary tumors were isolated from 16- to 20-wk old PyMT mice and PyMT mice lacking PGES1, and thus unable to generate PGE2. Although WDYF1 was clearly detectable in samples from the PyMT group, its expression was significantly reduced in tumors from animals lacking mPGES1 (Fig. 6A). Moreover, although the addition of PGE2 to dissociated PyMT tumors increased WDFY1 expression (Fig. 6B), it was unable to increase WDFY1 expression in cultured PyMT cells (Fig. 6C). Given that tumor-associated macrophages were highly abundant in the PyMT2c44 tumors and the tumor microenvironment influences the phenotype of macrophages, the consequences of Cyp2c44 deletion on the polarization of bone marrow-derived macrophages from wild-type and Cyp2c44−/− mice were assessed. WDFY1 and NRP2 were detectable in macrophages from wild-type and Cyp2c44−/− mice, but their expression was markedly increased in Cyp2c44-deficient macrophages treated with LPS and IFN-γ (to elicit M1 polarization). Alternative activation (M2 polarization) of wild-type and Cyp2c44−/− macrophages exerted a weaker effect (Fig. 6D). The increase in WDFY1 and NRP2 expression could be reproduced in macrophages from wild-type mice after incubation with PGE2 (Fig. 6E). Moreover, treating Cyp2c44−/− macrophages with diclofenac prevented the M1 polarization-induced increase in WDFY1 and Nrp2 expression (Fig. 6F). Macrophage polarization itself had marked effects on Cyp2c44 levels, as although the enzyme was clearly expressed in bone marrow-derived macrophages from wild-type mice, levels were significantly decreased after M1 or M2 polarization (SI Appendix, Fig. S5 A and B). In keeping with this finding, macrophage polarization also increased WDFY1 expression in cells from wild-type mice (SI Appendix, Fig. S5C), although the effects were less pronounced than in the Cyp2c44−/− macrophages.
Fig. 6.
Role of PGE2 in the regulation of WDFY1 expression. (A) WDFY1 protein expression relative to nonmuscle myosin (NMM) in similarly sized primary tumors from PyMT and PyMTxmPGES1−/− mice; n = 5 animals per group (Student’s t test). (B) WDFY1 protein expression relative to NMM in dissociated tumors, treated with solvent (Sol) or PGE2 (100 µmol/L) for 24 h. The blot shown is representative of 3 additional samples. (C) WDFY1 and NMM in cultured PyMT tumor cells, treated with solvent (Sol) or PGE2 (100 µmol/L) for 24 h. The blot shown is representative of 5 additional samples. (D) Expression of WDFY1 and NRP2 mRNA in bone marrow-derived macrophages from wild-type (+/+) and Cyp2c44−/− (−/−) mice treated with solvent (M0), LPS and IFN-γ (M1), IL-4 (M2), or PGE2 (E2; 100 µmol/L) for 24 h; n = 10 animals per group (two-way ANOVA and Newman-Keuls). (E) WDFY1 and NRP2 mRNA expression in M0 macrophages from wild-type mice treated with solvent or PGE2 (100 µmol/L); n = 6 mice per group (Student’s t test). (F) WDFY1 (red) and NRP2 (green) expression in M0, M1, and M2 polarized bone marrow-derived macrophages from Cyp2c44−/− mice treated with solvent (Sol) or diclofenac (Diclo, 40 µmol/L) or PGE2 (E2; 100 µmol/L). (Scale bar = 10 µm.) n = 6 mice per group (two-way ANOVA and Newman-Keuls). *P < 0.05; **P < 0.01; ***P < 0.001.
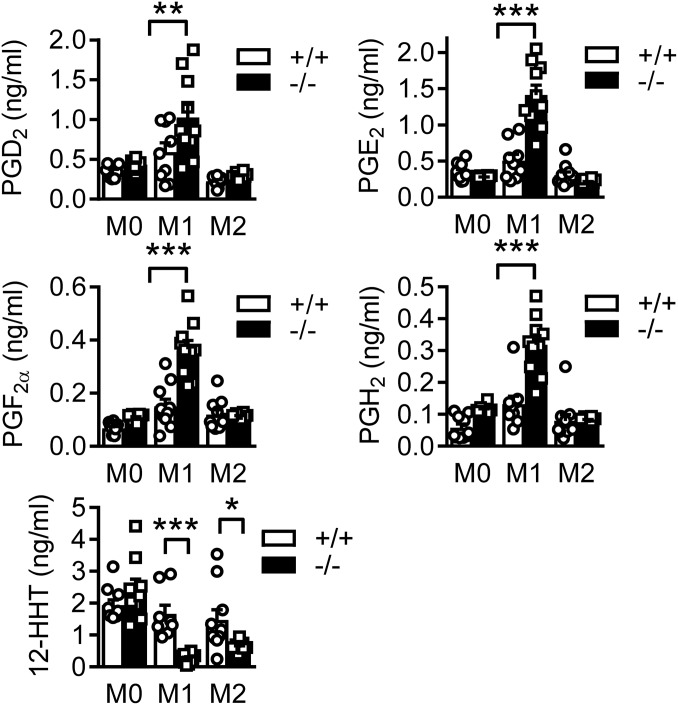
Similar to the isolated tumors, monocyte-derived macrophages from Cyp2c44−/− mice generated more PG and less 12-HHT than cells from wild-type mice, after polarization with LPS and IFN-γ (Fig. 7). Looking at markers of macrophage activation, the expression of interleukin (IL)-1β, IL-10, TNFα, and the inducible nitric oxide synthase (iNOS) were all significantly increased in M1 polarized macrophages from Cyp2c44−/− mice versus wild-type littermates (SI Appendix, Fig. S6A). Interestingly, the expression of Fizz1 (also referred to as RELMα), c-Myc, and early growth response protein 2 were also increased in M2 polarized macrophages from Cyp2c44−/− mice while arginase expression was increased in the both M1 and M2 polarized macrophages from Cyp2c44−/− mice (SI Appendix, Fig. S6B). This is consistent with the view that tumor-associated macrophages, which are expanded in primary PyMT2c44 tumors, show markers of both M1 and M2 macrophages (20).
Fig. 7.
Prostaglandin generation by bone marrow-derived macrophages. Bone marrow-derived macrophages from wild-type (+/+) and Cyp2c44−/− (−/−) mice were incubated in the presence of solvent (M0), LPS and IFN-γ (M1) or IL-4 (M2) for 24 h. Thereafter, PGD2, PGE2, PGF2α, PGH2, and 12-HHT levels in the cell supernatant were assessed by LC-MS/MS, n = 10 mice per group (two way ANOVA and Newman-Keuls). *P < 0.05; **P < 0.01; ***P < 0.001.
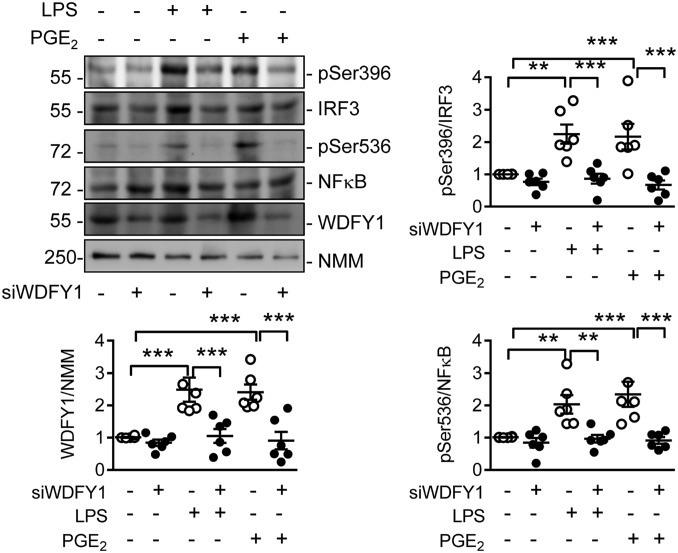
Recently, endogenous PGE2 production in macrophages was reported to amplify IL-33 production (21), which in turn can affect the numbers of myeloid-derived suppressor cells and their generation of immunosuppressive TGF-β (22, 23). Certainly, the expression of TGF-β and IL-33 was higher in macrophages lacking Cyp2c44 (SI Appendix, Fig. S6C). This profile is consistent with the lack of Cyp2c44 favoring polarization toward tumor-associated macrophages. Functionally, WDFY1 was reported to recruit the signaling adaptor TIR-domain-containing adapter-inducing IFN-β (TRIF) to Toll-like receptor (TLR) 3 and TLR4 to potentiate their signaling (24). Indeed, in human monocyte-derived macrophages, preventing the up-regulation of WDFY1 using a siRNA approach prevented the LPS- as well as the PGE2-induced phosphorylation of IFN regulatory factor 3 (IRF3) and nuclear factor (NF)-κB (Fig. 8).
Fig. 8.
Consequences of preventing WDFY1 up-regulation on TLR signaling in human monocyte-derived macrophages. Human peripheral blood-derived macrophages were transfected with a control siRNA or siRNA directed against WDFY1 (siWDFY1) for 24 h before stimulation with LPS (100 ng/mL) or PGE2 (100 µmol/L) for 60 min. The blots and graphs summarize the effectiveness of WDFY1 down-regulation and the consequences of WDFY1 down-regulation on the phosphorylation of IRF3 on Ser396 and NFκB on Ser536. NMM = non muscle myosin as loading control; n = 6 independent experiments (one-way ANOVA & Newman-Keuls). **P < 0.01; ***P < 0.001.
Discussion
The present study was aimed at determining the consequences of targeting Cyp2c44 on tumor growth and metastasis in a genetic model of breast cancer. The results revealed that in mice lacking Cyp2c44, primary tumors contained more tumor-associated macrophages, and demonstrated marked lymphangiogenesis as well as elevated expression of WDFY1, which is involved in TLR signaling. The effects went hand in hand with increased lymph node metastasis and pulmonary metastatic growth. Mechanistically, the consequences of Cyp2c44 deletion could be attributed to an increase in PG production, as the enzyme was found to metabolize PGH2 to 12-HHT, thus attenuating the production of effector PG’s, including PGE2. Further downstream, tumors lacking Cyp2c44 demonstrated a more inflammatory phenotype and elevated expression of WDFY1, which has been implicated in TLR signaling.
When the study was initially designed, it was expected that its main focus would be on a potential benefit of Cyp2c44 down-regulation in the PyMT model. This was assumed on the basis of reports linking CYP-derived EETs to angiogenesis (25, 26), tumor growth (2–5), and tumor metastasis (6). Also, sEH deletion and higher EET levels were associated with the escape from tumor dormancy in several different cancer models (7). Added to that, Cyp2c44 down-regulation in endothelial cells was reported to attenuate proliferation and angiogenesis and decrease tumor vascularization (8). However, exactly the opposite was observed, and tumor growth was accelerated in PyMTΔ2c44 compared with PyMT mice. Moreover, there was a clear increase in lymph node metastases, as well as in the development of pulmonary metastases. The more aggressive expansion of tumors in PyMTΔ2c44 mice was correlated with a marked increase in lymphangiogenesis, an effect that may, at least partially, underlie the effects on the lymph nodes and lungs, as lymphatics have been linked with metastatic potential (for reviews, see refs. 27 and 28). In addition to the effects on lymphangiogenesis, there were also differences in inflammatory cell infiltration, and significantly more tumor-associated macrophages were detected in tumors from PyMTΔ2c44 mice. The latter population was of particular interest, as these macrophages generate VEGFC (21) and can transdifferentiate into lymphatic endothelial cell clusters that join existing lymphatic vessels (22, 23). Indeed, Lyve1+/CD11b+ macrophages have been proposed to represent a lymphatic endothelial progenitor cell population (29) and associate with aberrant lymphatics in murine models of ovarian cancer (30), thus contributing to metastasis (31). Moreover, tumor-associated macrophages can suppress T cell responses, and the numbers of T cells were clearly decreased in tumors from PyMTΔ2c44 mice versus PyMT mice. Gender differences have been reported in Cyp2c44-deficient mice, at least for the sensitivity to hypoxia-induced pulmonary hypertension (32). However, it was not possible to make any statement regarding gender, as our study focused on a model of spontaneous breast cancer in which male mice develop tumors much more slowly than females (33).
Given the role of CYP enzymes in metabolizing PUFAs, the next step was to identify alterations in the fatty acid epoxide and diol profile that could influence inflammatory cells and tumor growth. There was no clear effect of Cyp2c44 deletion on the PUFA epoxides or diols assayed, a finding that is consistent with a recent report looking at the role of Cyp2c44 in the lung and heart (32). Given the well-established links among PGE2, lymphangiogenesis (34), tumor growth, and metastases (34, 35), particular attention was paid to alterations in the PG profile and PG-generating enzymes. Interestingly even though there were no consistent changes in COX, PGES expression and levels of PGG2, PGH2, and the downstream products PGF2α, PGE2, and PGD2 were all increased in primary tumors from PyMTΔ2c44 mice versus the PyMT group. There was, however, a significant decrease in levels of 12-HHT, which can be generated from PGH2 by some CYP enzymes including the thromboxane A2 synthase (36) and CYP2S1 (15, 37, 38). Cyp2c44 was found to function in a similar manner, as 12-HHT was clearly generated from PGH2 by microsomes derived from Cyp2c44-expressing Sf9 cells. The consequence of the generation of 12-HHT by Cyp2c44 was that PGE2 production was attenuated. It follows that a decrease in Cyp2c44 expression would be expected to result in the shunting of PGH2 into PG production, which fits with the marked increase in PG production in Cyp2c44-deficient animals. Indeed, the finding that PGE2 levels were increased in tumors from PyMTΔ2c44 mice goes a long way to accounting for the observed phenotype, including the increase in lymph angiogenesis. For example, PGE2 has been reported to activate the EP4 receptor on lymphatic endothelial cells and promote lymphangiogenesis (34). Preventing the increase in PGE2 levels in PyMTΔ2c44 mice with celecoxib also effectively prevented tumor tumor growth and infiltration with tumor-associated macrophages, as well as lymphangiogenesis. Interestingly, consistent with previous reports that celecoxib is metabolized by CYP enzymes, and can thus prevent the metabolism of endogenous substrates (39), we found that treating animals with the COX inhibitor also decreased epoxide generation as well as that of 12-HHT.
To further specify the anti-tumor role of Cyp2c44, protein expression was compared in similarly sized tumors from PyMT and PyMTΔ2c44 mice. One of the proteins most affected by Cyp2c44 deletion was WDFY1, which plays a role in autophagy (18, 40) and in the potentiation of TLR3 and TLR4 signaling (21). WDFY1 was identified as a downstream effector of NRP2 (18), and it was possible to confirm that the tumors from PyMTΔ2c44 mice expressed much higher levels of both proteins. WDFY1 is known to be expressed in cancer cells (19, 40), but the clear differences in infiltrating cells in the tumors from PyMTΔ2c44 versus PyMT mice indicated that the effects observed may be attributable to tumor-associated macrophages. Certainly, Cyp2c44 may be relevant for tumor-associated macrophages, as it has already been allocated a role in the resolution of inflammation (41). Indeed, macrophages lacking Cyp2c44 favored polarization toward tumor-associated macrophages and expressed more WDFY1 and NRP2 than cells isolated from wild-type mice, an effect that could be prevented by COX inhibition and mimicked by PGE2. The latter observation likely accounts for the marked decrease in WDFY1 expression in tumors from PyMT x mPGES1−/− mice. Also in macrophages, WDFY1 was linked to TLR signaling as the down-regulation of WDFY1 in macrophages attenuated the LPS-induced phosphorylation of IRF3 and NFκ-B. In the human macrophages studied, PGE2 was also able to initiate the phosphorylation of IRF3 and NFκ-B, while at first sight this was unexpected; PGE2 has been reported to elicit the phosphorylation of NFκ-B in gastric cancer cells via an EP2 receptor-dependent mechanism (42).
Cyp2c44 was chosen for this study because of previous reports indicating that the inhibition/down-regulation of Cyp2c44 may be a strategy to treat cancer (9). However, in the PyMT model studied, exactly the opposite was the case, as tumor growth, as well as metastases, were markedly increased in animals lacking Cyp2c44. A role for Cyp2c44 in cancer certainly fits with a recent study focusing on a woodchuck hepatitis virus/c-Myc model of hepatocellular carcinoma (10). In the latter model, Cyp2c44 expression was found to be inversed during the critical period of cancer initiation, at a point when inflammatory markers were high and arachidonic acid metabolism was dysfunctional (10). The authors of the latter study even proposed that Cyp2c44 is involved in the critical transition from inflammation to carcinoma. The results of the present study certainly support such a critical role of decreased Cyp2c44 expression in activated macrophages, which boosts PGE2 production and cancer development, and highlight that the lipid microenvironment within tumors plays a determinant role in immune cell function as well as the balance between angiogenesis and lymphangiogenesis to support metastasis.
Materials and Methods
All animal experiments were approved by the local government authorities (Regierungspräsidium Darmstadt: FU_1072, FU_1095). Information on animal monitoring, sample collection immunohistochemistry, tumor digestion and FACS analysis, RNA isolation and quantitative real-time PCR, insect cell (Sf-9) culture and assay of Cyp2c44 activity, monocyte isolation, small interfering RNA, immunoblotting, UPLC-MS/MS-based fatty acid metabolite profiling, proteomics, and statistical analyses is provided in SI Appendix.
Data Availability.
While most of the data are available in the manuscript and supporting information, the mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (43), with the dataset identifiers PXD017336 and PXD017303.
Supplementary Material
Acknowledgments
The authors are indebted to Katharina Engel-Herbig, Mechtild Pipenbrock, and Jana Meisterknecht for expert technical assistance. This work was supported by the Else Kröner-Fresenius-Stiftung (Else Kröner-Fresenius-Graduiertenkolleg stipend to R.K.), the Deutsche Forschungsgemeinschaft [SFB-TR 23/3 A06 and SFB 1039/2 A06 (to I.F.), B04 (to B.B.), B06 (to A.W.), SFB 815/Z1 (to I.W.); GRK 2336, and the Cardio-Pulmonary Institute (CPI), EXC 2026, Project ID: 390649896], and the Deutsche Krebshilfe (109599).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (identifiers PXD017336 and PXD017303).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921381117/-/DCSupplemental.
References
- 1.Fleming I., The pharmacology of the cytochrome P450/soluble epoxide axis in the vasculature. Pharmacol. Rev. 66, 1106–1140 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Yokose T., et al. , Immunohistochemical study of cytochrome P450 2C and 3A in human non-neoplastic and neoplastic tissues. Virchows Arch. 434, 401–411 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Jiang J. G., et al. , Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 65, 4707–4715 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Chen C., et al. , Selective inhibitors of CYP2J2 related to terfenadine exhibit strong activity against human cancers in vitro and in vivo. J. Pharmacol. Exp. Ther. 329, 908–918 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C., et al. , Cytochrome P450 2J2 is highly expressed in hematologic malignant diseases and promotes tumor cell growth. J. Pharmacol. Exp. Ther. 336, 344–355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J. G., et al. , Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res. 67, 6665–6674 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Panigrahy D., et al. , Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J. Clin. Invest. 122, 178–191 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pozzi A., et al. , The anti-tumorigenic properties of peroxisomal proliferator-activated receptor α are arachidonic acid epoxygenase-mediated. J. Biol. Chem. 285, 12840–12850 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skrypnyk N., et al. , PPARα activation can help prevent and treat non-small cell lung cancer. Cancer Res. 74, 621–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M., et al. , Dysfunction of PLA2G6 and CYP2C44-associated network signals imminent carcinogenesis from chronic inflammation to hepatocellular carcinoma. J. Mol. Cell Biol. 9, 489–503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin E. Y., et al. , Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 163, 2113–2126 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noy R., Pollard J. W., Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patrignani P., Patrono C., Aspirin and cancer. J. Am. Coll. Cardiol. 68, 967–976 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Chen E. P., Smyth E. M., COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins Other Lipid Mediat. 96, 14–20 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frömel T., et al. , Cytochrome P4502S1: A novel monocyte/macrophage fatty acid epoxygenase in human atherosclerotic plaques. Basic Res. Cardiol. 108, 319 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Wittig I., Fleming I., Consequences of Cyp2c44 deletion in the polyoma middle T (PyMT) breast cancer tumor proteome. PRIDE repository. https://www.ebi.ac.uk/pride/archive/projects/PXD017336. Deposited 30 January 2020.
- 17.Wittig I., Fleming I., Consequences of Cyp2c44 deletion on proteome of the microsomal fraction of primary polyoma middle T (PyMT) tumors. PRIDE repository. https://www.ebi.ac.uk/pride/archive/projects/PXD017303. Deposited 28 January 2020.
- 18.Dutta S., et al. , NRP2 transcriptionally regulates its downstream effector WDFY1. Sci. Rep. 6, 23588 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta S., et al. , Neuropilin-2 regulates endosome maturation and EGFR trafficking to support cancer cell pathobiology. Cancer Res. 76, 418–428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Ginderachter J. A., et al. , Classical and alternative activation of mononuclear phagocytes: Picking the best of both worlds for tumor promotion. Immunobiology 211, 487–501 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Moussai D., et al. , The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J. Invest. Dermatol. 131, 229–236 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Maruyama K., et al. , Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 115, 2363–2372 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Chemaly S., et al. , Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc. Natl. Acad. Sci. U.S.A. 106, 3958–3963 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y.-H., et al. , WDFY1 mediates TLR3/4 signaling by recruiting TRIF. EMBO Rep. 16, 447–455 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaelis U. R., et al. , Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J. 17, 770–772 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Michaelis U. R., Fleming I., From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: Epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol. Ther. 111, 584–595 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Chen Y., et al. , A meta-analysis of the relationship between lymphatic microvessel density and clinicopathological parameters in breast cancer. Bull. Cancer 100, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Semenza G. L., Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene 32, 4057–4063 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ran S., Montgomery K. E., Macrophage-mediated lymphangiogenesis: The emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) 4, 618–657 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon B. H., et al. , Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 68, 1100–1109 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Lin E. Y., et al. , Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 66, 11238–11246 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Joshi S. R., et al. , Cyp2c44 gene disruption exacerbated pulmonary hypertension and heart failure in female but not male mice. Pulm. Circ. 6, 360–368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guy C. T., Cardiff R. D., Muller W. J., Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol. Cell Biol. 12, 954–961 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandi P., et al. , PGE2 promotes breast cancer-associated lymphangiogenesis by activation of EP4 receptor on lymphatic endothelial cells. BMC Cancer 17, 11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kochel T. J., Reader J. C., Ma X., Kundu N., Fulton A. M., Multiple drug resistance-associated protein (MRP4) exports prostaglandin E2 (PGE2) and contributes to metastasis in basal/triple negative breast cancer. Oncotarget 8, 6540–6554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamberg M., Svensson J., Samuelsson B., Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc. Natl. Acad. Sci. U.S.A. 71, 3824–3828 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bui P., Solaimani P., Wu X., Hankinson O., 2,3,7,8-Tetrachlorodibenzo-p-dioxin treatment alters eicosanoid levels in several organs of the mouse in an aryl hydrocarbon receptor-dependent fashion. Toxicol. Appl. Pharmacol. 259, 143–151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madanayake T. W., Fidler T. P., Fresquez T. M., Bajaj N., Rowland A. M., Cytochrome P450 2S1 depletion enhances cell proliferation and migration in bronchial epithelial cells, in part, through modulation of prostaglandin E(2) synthesis. Drug Metab. Dispos. 40, 2119–2125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siu Y. A., Hao M. H., Dixit V., Lai W. G., Celecoxib is a substrate of CYP2D6: Impact on celecoxib metabolism in individuals with CYP2C9*3 variants. Drug Metab. Pharmacokinet. 33, 219–227 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Stanton M. J., et al. , Angiogenic growth factor axis in autophagy regulation. Autophagy 9, 789–790 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilroy D. W., et al. , CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. U.S.A. 113, E3240–E3249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lian S., et al. , Prostaglandin E2 stimulates urokinase-type plasminogen activator receptor via EP2 receptor-dependent signaling pathways in human AGS gastric cancer cells. Mol. Carcinog. 56, 664–680 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Perez-Riverol Y., et al. , The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
While most of the data are available in the manuscript and supporting information, the mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (43), with the dataset identifiers PXD017336 and PXD017303.